Abstract
Cholera toxin (CT), and members of the AB5 family of toxins enter host cells and hijack the cell’s endogenous pathways to induce toxicity. CT binds to a lipid receptor on the plasma membrane (PM), ganglioside GM1, which has the ability to associate with lipid rafts. The toxin can then enter the cell by various modes of receptor-mediated endocytosis and traffic in a retrograde manner from the PM to the Golgi and the endoplasmic reticulum (ER). Once in the ER, a portion of the toxin is unfolded and retro-translocated to the cytosol so as to induce disease. GM1 is the vehicle that carries CT from PM to ER. Thus, the toxin pathway from PM to ER is a lipid-based sorting pathway, which is potentially meditated by the determinants of the GM1 ganglioside structure itself.
Keywords: cholera toxin, lipid raft, retrograde pathway, GM1, AB5 toxin, endocytosis
Introduction
Cholera toxin (CT) secreted by the gram-negative bacterium Vibrio cholerae is responsible for the massive secretory diarrhea seen in Asiatic cholera disease (Sack et al., 2004). CT, and certain other members of the AB5 family of toxins undergo a remarkable journey to enter host cells by co-opting an endogenous trafficking pathway in a process that culminates in the induction of toxicity (Lencer & Saslowsky, 2005; Spooner et al., 2006). Figure 1 In the first step of this process, CT crosses the normally impermeant mucosal epithelial barrier by binding to ganglioside GM1 located in the extracellular leaflet of the plasma membrane (PM). GM1 typifies certain membrane lipids that have the ability to self-assemble into small microdomains termed lipid rafts, and it is possible that CT-GM1 complexes must associate with such structures in order for the toxin to be efficiently internalized into the relevant endocytic pathway (Wolf et al., 1998; Wolf et al., 2002; Fujinaga et al., 2003). After internalization, CT is trafficked retrograde from the apical PM of polarized intestinal epithelia to the trans-Golgi and ultimately to the endoplasmic reticulum (ER). Once in the ER lumen, the A-subunit of CT hijacks an endogenous pathway that is meant to transport misfolded proteins from the ER to the cytosol, a process termed retro-translocation. This is how the enzymatic portion of CT ultimately gains access to the cytosol and subsequently induces toxicity by elevating cAMP levels. In this paper, we discuss our current understanding and recent developments of how the CT-GM1 complex might interact with lipid rafts in the PM so as to traffic retrograde into the ER of host cells.
Fig. 1.
General pathway for CT entry into intestinal cells via lipid rafts. CT binds to GM1 localized in lipid raft domains (green) on the apical membrane via the B-subunit and is internalized into apical early endosomes (Fujinaga et al., 2003). CT is then trafficked to the trans-Golgi network (TGN) and bypasses the Golgi apparatus on its way to the ER (Feng et al., 2004). Once inside the ER, protein disulfide isomerase (PDI) recognizes the cleaved form of the A1 chain (shown in red), where it is unfolded and dissociated from the B-subunit (in blue) (Tsai & Rapoport, 2002). The A1-chain is then retro-translocated to the cytosol, presumably by the Sec61 channel. CTcan also recycle between the Golgi and ER via anterograde and KDEL-dependent COPI-mediated pathways (dotted arrows) (Fujinaga et al., 2003). Although once in the cytosol, CT A-chain then induces chloride secretion by increasing cyclic AMP (cAMP) levels via adenylate cyclase (AC) (Spangler, 1992).
Structure and function
Members of the AB5 family of toxins, which include CT, Escherichia coli heat-labile Type I toxin (LTI) and Shiga toxin (Stx), all consist of a catalytic A chain, and a pentameric B-subunit that bind multi-valently to glycolipid receptors on the cell surface. The A-subunit of CT and LT toxins noncovalently associate with the B-pentamer, and are ADP-ribosyltransferases that constitutively activate the heterotrimeric G protein, Gs-α (Spangler, 1992); this results in the activation of adenylyl cyclase, which elevates intracellular cAMP levels and thereby induces massive chloride and water secretion in intestinal enterocytes. In contrast, the Stx A-subunit functions as an rRNA N-glycosidase, which shuts down protein synthesis and leads to cell death (O’Brien et al., 1992). The CT A-subunit contains two domains: an 11 kDa N-terminal chain (A1), which is responsible for the catalytic activity of the toxin, and an 18 kDa C-terminal chain (A2), which anchors the A-subunit to the central pore in the pentameric B-subunit. The A1 and A2 chains are in turn linked via a serine protease-containing flexible linker, which is bridged by a disulfide bond. Enzymatic cleavage at this site, which occurs after release from the bacterium in the intestinal lumen, is necessary for toxicity. Both CT and LT harbor an ER-targeting KDEL motif in the C-terminus of the A2 chain, whereas Stx lacks this motif. While all three toxins must enter the ER to gain access to the cytosol, the KDEL motif is dispensable for this activity (Lencer et al., 1995b; Johannes et al., 1997; Fujinaga et al., 2003).
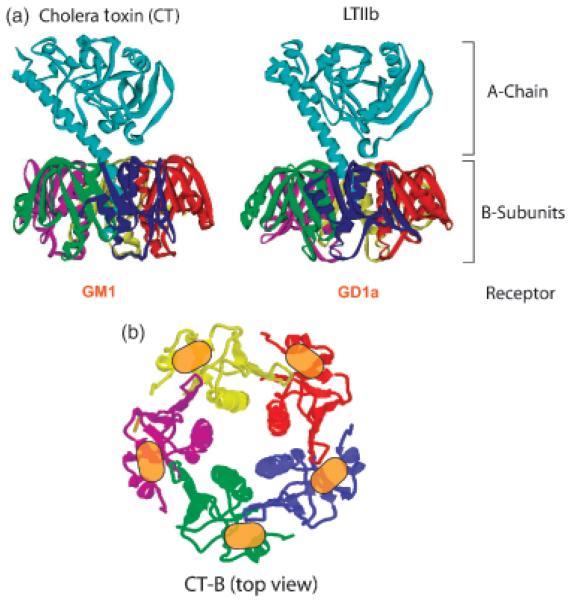
The ring-like B-subunits of CT and LT consist of five identical monomers. The assembled pentamer is capable of binding five ganglioside GM1 receptors at once (Merritt et al., 1994, 1997, 1998; Fig. 2). The CT B-subunit (CTB) binds very tightly to GM1, with Kd’s reported in the range of 5 pM to 1 nM based on surface plasmon resonance and on-cell binding measurements (Cuatrecasas, 1973; Kuziemko et al., 1996; MacKenzie et al., 1997; Dawson, 2005). The B-subunit of the E. coli Type II heat-labile toxin, LTIIb, binds ganglioside GD1a. GD1a is structurally closely related to GM1, with the exception of an additional sialic acid residue at the terminal galactose residue. The Stx B-subunit (StxB) binds with high affinity to the globoside Gb3, and possibly clusters up to fifteen molecules at a time (Ling et al., 1998). The function of the B-subunit for each of these toxins is to transport the enzymatic A chain into the ER of the host cell. Once in the ER, the A chain is unfolded and dissociated from the B-subunit by ER luminal chaperones and then retro-translocated into the cytoplasm (Tsai et al., 2002; Fujinaga et al., 2003).
Fig. 2.
Structure of CT and LTIIb toxins. (a) Three-dimensional structures of cholera toxin and the heat-labile LTIIb. The A chain noncovalently associates with the central pore of the pentameric B-subunit via the rod-like A2-chain. (b) Top view of the homopentameric ring-like structure formed by CT B-subunit. Five binding sites (orange) for the GM1 receptor are formed by mutual contribution at the interface of neighboring subunits.
Lipid rafts
Biological membranes are composed of a variety of lipids and sterols that do not heterogeneously mix, but form distinct microdomains in the plane of the bilayer. This phenomenon is readily observed in model membranes devoid of cellular material, and in live cells. In vitro, microdomain formation appears to be a function both of the chain length and degree of saturation of the hydrocarbon tails for each lipid species. Long-chain, saturated lipids are more tightly packed and have decreased rotational mobility within the hydrophobic region of the bilayer than do unsaturated lipids possessing glycerol-based acyl chains. At physiologic temperatures, these differences give rise to phase separation where long-chain, saturated lipids display gel-like properties (lo phase) while unsaturated species are more fluid (ld phase). Membrane lo microdomains composed primarily of cholesterol and highly ordered saturated glycosphingolipids and cholesterol are referred to as lipid rafts in intact cells and detergent-resistant membranes (DRMs) in biochemically isolated cell fractions. Although difficult to study, modern biophysical approaches have provided insights into the size (thought to be as small as 4-5 nm in diameter) and dynamics of lipid rafts in both model membranes and intact cells (Kenworthy et al., 2004; Mayor & Rao, 2004; Mayor & Riezman, 2004; Hancock, 2006; Kahya & Schwille, 2006; Silvius & Nabi, 2006).
Lipid rafts may provide a scaffolding for certain cellular trafficking and signaling events, effectively compartmentalizing such processes from the myriad other events occurring at or within biological membranes. The association of CT with both DRMs and lipid rafts has been well studied, as GM1 is widely viewed as a bona-fide lipid raft marker due to its propensity for such domains. One idea is that the pentameric clustering of GM1 caused by CTB binding further enhances the partitioning of GM1 (now a toxinganglioside complex) into lipid raft microdomains. This could provide the opportunity to interact with specialized host cell machinery, including that required for internalization into the retrograde pathway.
Routes of entry by cholera toxin
CT can enter cells by numerous modes of endocytosis (Orlandi & Fishman, 1998; Torgersen et al., 2001; Massol et al., 2004; Kirkham et al., 2005). Early studies found caveolae to be important in CT endocytosis (Montesano et al., 1982; Tran et al., 1987; Orlandi & Fishman, 1998), and due to the abundance of the ganglioside GM1 in caveolae (Parton, 1994), CT was considered to be a bonafide marker for caveolar endocytosis. However, it is now known that CT can enter cells by both clathrin-dependent (i.e. noncaveolar) and clathrin-independent mechanisms. (Torgersen et al., 2001; Nichols, 2002; Singh et al., 2003; Hansen et al., 2005). In studies with Caco-2 human epithelial cells devoid of caveolae, CT was not impaired in being internalized or inducing toxicity (Orlandi & Fishman, 1998;Torgersen et al., 2001). Moreover, uptake of CT by caveolae has been found to be inefficient and only a minor pathway for uptake by Caco-2 cells (Kirkham et al., 2005). This is in agreement with the apparent immobility of caveolae on the cell surface as measured by FRAP (Thomsen et al., 2002; Hommelgaard et al., 2005). However, it is important to note that the membrane compositions of different cell types vary, possibly affecting the contribution of the different endocytic pathways.
In hippocampal neurons, BHK cells and possibly HeLa cells, CT can bind GM1 and enter the cell by clathrin-mediated mechanisms (Shogomori & Futerman, 2001; Torgersen et al., 2001), but this does not occur in motor neurons or appear to be a major entry pathway for CT in certain other cell types (Massol et al., 2004; Deinhardt et al., 2006).
CT can also enter cells by a noncaveolar and nonclathrin-mediated pathway (Le & Nabi, 2003; Massol et al., 2004; Kirkham et al., 2005) (Fig. 3). The contributions of the different endocytic pathways were assessed by overexpression of dominant-negative mutants to inhibit caveolin-, clathrin- and ARF6-dependent (Naslavsky et al., 2004) pathways in monkey kidney epithelial (BSC-1) cells (Massol et al., 2004). Inhibition of any one of these pathways negligibly affected CT internalization, whereas inhibition of all three pathways completely blocked CT uptake as gauged by fluorescence imaging. However, quite surprisingly, CT function was not impaired in the latter, suggesting the possibility of an alternate caveolin-, clathrin- and Arf6-independent pathway for CT internalization that is responsible for transport to the ER and a functional response.
Fig. 3.
Modes of entry of the cholera toxin. Transport of CT across the PM can occur either by caveolar-dependent (shown in green) (Orlandi & Fishman, 1998), clathrin-dependent (in blue) (Torgersen et al., 2001) or noncaveolar/nonclathrin-mediated (CLIC) processes (Kirkham et al., 2005). All three processes are sensitive to cholesterol depletion. Following internalization, CTcan be found in early endosomes, and is trafficked to the TGN and ER.
Recent ultrastructural studies by Parton and coworkers have found that noncaveolar, nonclathrin-mediated endocytosis of CT into mouse embryonic fibroblast (MEF) cells is a major route of entry and have identified the potential carriers of this pathway (Kirkham et al., 2005). In MEFs deficient in caveolae (Cav1 − / −), transport of CT to the Golgi can occur in a caveolin-, clathrin- and dynamin-independent manner; yet, transport of the CT-GM1 complex is still cholesterol-sensitive. Morphologically, this pathway appears to have a tubular and/or ring-like appearance ranging in size from 50 to 80 nm and to nucleate from the PM. The structures are most likely GPI-AP-enriched early endosomal compartments (GEECs) which are associated with the cdc42-mediated uptake of GPI-AP and fluid phase markers (Sabharanjak et al., 2002; Kalia et al., 2006). These clathrin-independent carriers, termed CLICs, exhibit many similarities to yeast endocytic pathway due to their cholesterol dependence, caveolae and clathrin independence and enrichment of GPI-anchored proteins (Sabharanjak et al., 2002; Kirkham & Parton, 2005). Similar pathways have also been found in other lower eukaryotes and is proposed to be a primordial mode of entry into cells (Guha et al., 2003; Morgan et al., 2004; Kirkham & Parton, 2005).
Overall, our impression is that the CT-GM1 complex can enter cells via different clathrin-dependent and clathrin-independent mechanisms and this can vary by cell type, but not all endocytic pathways appear to lead to a functional response. It is possible that the critical sorting step for trafficking the CT-GM1 complex into the retrograde path-way from PM to ER occurs at the level of the PM as defined by the mechanism of endocytosis, or alternatively, it is possible that the critical sorting step occurs inside the cell, or both are equally important, perhaps dependent on a series of iterative reactions in different locations. The literature is not yet conclusive on this point.
Raft organization and CT intoxication
The roles of individual lipid raft constituents in raft dynamics and endocytosis of CT in human intestinal cells are just beginning to be understood. CT entry and induction of toxicity is dependent on the binding of toxin to lipid raft domains containing GM1 and cholesterol (Wolf et al., 1998, 2002; Fujinaga et al., 2003; Kirkham et al., 2005). Cholesterol depletion results in slower internalization and attenuated toxicity of CT (Wolf et al., 2002); however, the CT-GM1 complex appears to remain associated with DRMs. This result implies that cholesterol may be dispensable in conveying detergent insolubility to the CT-GM1 complex, but is required for associating CT-GM1 with other lipid raft components that are important in raft dynamics and trafficking. Further studies have identified the actin cytoskeleton to also play an important role in CT intoxication. Treatment of cells with F-actin-depolymerizing or -stabilizing drugs inhibits CT trafficking from the PM to the Golgi and toxin function (Badizadegan et al., 2004). The efficient trafficking of CT appears to be dependent on an intact actin cytoskeleton, wherein a physical association exists between the actin cytoskeleton and GM1-anchored CT on the cell surface. What role lipid rafts play in mediating the interaction between CT-GM1 complexes and the actin cytoskeleton remains unknown.
Sphingomylein is an integral component of lipid rafts that can have a stabilizing effect on microdomain formation via its interaction with cholesterol (Bittman et al., 1994). Sphingomyleinase reversibly converts sphingomylein to ceramide, which has a lower affinity for cholesterol (Megha & London, 2004). Recent evidence from our lab suggests that sphingomylein-rich microdomains play an important role in the toxicity of CT in human intestinal T84 cells (unpublished results). We found that treatment of the apical PM of polarized T84 cells with sphingomyleinase attenuates CT function, reduces CT internalization and induces a reorganization of apical F-actin (unpublished results). Thus, there appears to be a correlation between lipid raft structure and CT-induced toxicity. From these studies, we believe it possible that the conversion of sphingomylein to ceramide may act as a regulatory mechanism for raft-mediated functions.
Retrograde trafficking from the PM to ER
Following entry into cell, CT is transported from early endosomes to the Golgi. This latter step can be inhibited by brefeldin A, which disables COPI- and COPII-mediated vesicular transport (Richards et al., 2002). The CT-A chain, containing a KDEL motif, has been proposed to dissociate from the B-subunit in the Golgi and interact with the KDEL receptor for traffic from the Golgi to the ER in a retrograde manner (Bastiaens et al., 1996; Majoul et al., 1998; Majoul et al., 2001). However, as previously mentioned, mutational studies have shown that the KDEL motif is not needed for transport to the ER, although it probably affects the time CT dwells in the ER, thus enhancing toxin function (Lencer et al., 1995b; Fujinaga et al., 2003). Also, studies with mutant CTB harboring sulfation and glycosylation peptide motifs, which can report on Golgi and ER trafficking, respectively, demonstrate that GM1 is indeed the vehicle for retrograde transport of the CT holotoxin from the PM to the ER (Fujinaga et al., 2003). Thus, trafficking of CT from the PM to the ER appears to occur by a lipid-dependent sorting pathway, and not a protein-based pathway.
Trafficking of CT from the trans-Golgi network (TGN) to the ER bypasses the Golgi apparatus and traffics directly to the ER. In cells treated with Exo-2, a novel small molecule inhibitor that preferentially disassembles the Golgi apparatus and prevents anterograde trafficking from the ER to Golgi, CT trafficking and toxicity is unaffected, indicating that the Golgi apparatus is bypassed in transit from the TGN to the ER (Feng et al., 2003, 2004). Also, the fact that Exo-2 disperses the KDEL receptor and releases COPI coats to the cytosol, in conjunction with previous findings that CT is functional without KDEL receptor (Lencer et al., 1995b; Fujinaga et al., 2003), supports the concept that a COPI-mediated pathway is not involved for transport from trans-Golgi to ER. The observed dependence of CT on COPI-mediated transport may occur at an earlier step in the pathway from PM to Golgi, or during the recycling of CT back from the Golgi apparatus after leaving the ER. This depends on toxin binding to the KDEL receptor (Fujinaga et al., 2003).
Lipid-sorting pathway
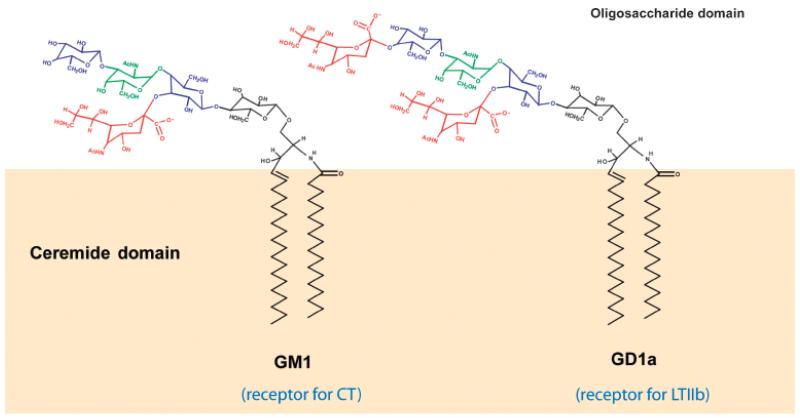
In order to induce toxicity, CT appears to require binding to gangliosides that are associated with lipid rafts. We have found that T84 cells discriminate between CT and the E. coli heat-labile toxin LTIIb, and are differentially traf-ficked such that only CT causes toxicity (Wolf et al., 1998; Fujinaga et al., 2003). Although both toxins bind to the PM via their respective ganglioside receptors [CT binds GM1, LTIIb binds GD1a - (Fig. 4)], only CTassociates with DRMs and is trafficked to the Golgi/ER to intoxicate the cell. However, in other cell lines tested, both CT and LTIIb fractionate into DRMs, enter the Golgi/ER and cause toxicity. Thus, it appears that there exists a lipid-based sorting pathway in intestinal cells that discriminates between membrane glycolipids based on ganglioside association with DRMs (and presumably lipid rafts). To theorize the basis on which such a pathway could occur, one must take into consideration the ganglioside structure. Gangliosides consist of an extracellular oligosaccharide head group and an intra-membrane ceramide domain. They are classified based on the arrangement and composition of sugars in the oligosaccharide domain. Interestingly, the ceramide domains of gangliosides can be somewhat heterogeneous, containing differential chain lengths and double bonds (Degroote et al., 2004). These factors could have a significant impact on the affinity and partitioning dynamics of various gangliosides with lipid rafts. It is the specific structure of the ceramide domain in gangliosides that we believe dictates the preferential sorting of toxin into the retrograde pathway from PM to ER.
Fig. 4.
Structures of glycosphingolipid receptors GM1 and GD1a. Gangliosides consist of a hydrophobic ceramide domain and an oligosaccharide domain by which they are classified. GM1 and GD1a, which bind to CTand LTIIb, respectively, are structurally identical in their oligosaccharide domain, with the exception of an additional sialic acid (red) present in the terminal galactose (blue) in GD1a. The ceramide domain, however, can be somewhat heterogeneous, containing degrees of unsaturation and chain length (Degroote et al., 2004). This may provide the basis of differential sorting involving lipid raft domains.
CTB-induced clustering may be another contributing factor that may help induce the endocytosis of toxin or affect its trafficking. The clustering of phospholipids by antibodies has been shown to induce partitioning into raftlike domains in lipid monolayers (Dietrich et al., 2001). Also, antibody clustering of GPI-anchored protein induces caveolar endocytosis in live cells (Parton et al., 1994). Simian virus 40 (SV40) particles can stimulate endocytotic entry into cells presumably by clustering its ganglioside receptors on the PM (Tagawa et al., 2005). Caveolae on the PM have been shown to be either in active or inactive states with respect to local cycling at the PM, and the addition of SV40 results in a doubling of the active caveolae that traffics SV40 in the cell (Pelkmans & Zerial, 2005). Likewise, the clustering of five GM1 lipids on the PM by the pentameric CT-B subunit could conceivably promote a similar internalization pathway. This idea is supported by recent studies in our group that show an impairment in the internalization and toxicity of a mutant CTB pentamer that is reduced in GM1-binding sites from 5 to either 1 or 2. As mentioned above, clustering of GM1 by CTB may promote partitioning of the toxin-ganglioside complex into lipid raft microdomains that are somehow capable of interacting with the F-actin-dependent internalization machinery. Alternatively, it is possible that CT instead exploits an existing/endogenous retrograde pathway to traffic into cells. Determining whether CT enters an endogenous pathway or an induced one is a major goal in the future.
Virus versus toxin entry into cells
The cellular trafficking of certain nonenveloped DNA viruses is quite similar to that of CT. SV40 can enter via caveolar-dependent and noncaveolar clathrin-independent mechanisms (Pelkmans et al., 2001, 2002; Norkin et al., 2002; Richards et al., 2002; Damm et al., 2005). Recent studies have shown that the primary receptors for SV40 and polyoma virus entry into cells are glycosphingolipids (Tsai et al., 2003). SV40 has been found in endocytic caveolae and in caveosomes, a unique pH neutral compartment enriched in caveolin. Although SV40 does not enter the Golgi, it is trafficked to the ER before transport to the nucleus for viral gene replication (Kartenbeck et al., 1989; Anderson et al., 1996; Pelkmans et al., 2001). It has also been shown that when CT and SV40 enter in the same caveosome, CT is sorted away in an Rab5-dependent manner and trafficked to endosomes (Pelkmans et al., 2004). This is quite interesting as both SV40 and CT bind to GM1, and begs the question of what is the nature of this cargo-dependent sorting? CT has been shown to undergo a conformational change at low pH that affects the way the B-subunit is bound to GM1 (McCann et al., 1997). It has been proposed that acidification in an acidic endosome induces a conformational change that promotes CT dissociation from caveolar domains (Pelkmans et al., 2004). Such pH-dependent events, however, may not affect the pathway taken by the toxin from PM to ER. When we examined this problem 10 years ago, we found that endosome acidification had no detectable effect on the magnitude or time course of CT-induced toxicity in intestinal T84 cells (Lencer et al., 1995a).
Summary
A wealth of elegant studies have demonstrated the ability of CT to cross the PM into the cell by several different modes of endocytosis: caveolar-dependent, clathrin-dependent or noncaveolar clathrin-independent pathways. The trafficking into cells appears to be dependent on toxin binding to the fraction of GM1 associated with lipid raft domains and involves a lipid-mediated sorting pathway. The clustering of gangliosides may be a common means for entry into the cell by stabilizing or inducing PM microdomains and promoting internalization and subsequent sorting retrograde through the secretory pathway.
Acknowledgements
These studies were supported by research grants DK48106, DK57827 and DK34854 to W.I.L.
References
- Anderson HA, Chen Y, Norkin LC. Bound simian virus 40 translocates to caveolin-enriched membrane domains, and its entry is inhibited by drugs that selectively disrupt caveolae. Mol Biol Cell. 1996;7:1825–1834. doi: 10.1091/mbc.7.11.1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badizadegan K, Wheeler HE, Fujinaga Y, Lencer WI. Trafficking of cholera toxin-ganglioside GM1 complex into Golgi and induction of toxicity depend on actin cytoskeleton. Am J Physiol Cell Physiol. 2004;287:C1453–C1462. doi: 10.1152/ajpcell.00189.2004. [DOI] [PubMed] [Google Scholar]
- Bastiaens PI, Majoul IV, Verveer PJ, Soling HD, Jovin TM. Imaging the intracellular trafficking and state of the AB5 quaternary structure of cholera toxin. EMBO J. 1996;15:4246–4253. [PMC free article] [PubMed] [Google Scholar]
- Bittman R, Kasireddy CR, Mattjus P, Slotte JP. Interaction of cholesterol with sphingomyelin in monolayers and vesicles. Biochemistry. 1994;33:11776–11781. doi: 10.1021/bi00205a013. [DOI] [PubMed] [Google Scholar]
- Cuatrecasas P. Interaction of Vibrio cholerae enterotoxin with cell membranes. Biochemistry. 1973;12:3547–3558. doi: 10.1021/bi00742a031. [DOI] [PubMed] [Google Scholar]
- Damm EM, Pelkmans L, Kartenbeck J, Mezzacasa A, Kurzchalia T, Helenius A. Clathrin- and caveolin-1-independentendocytosis: entry of simian virus 40 into cells devoid of caveolae. J Cell Biol. 2005;168:477–488. doi: 10.1083/jcb.200407113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson RM. Characterization of the binding of cholera toxin to ganglioside GM1 immobilized onto microtitre plates. J Appl Toxicol. 2005;25:30–38. doi: 10.1002/jat.1015. [DOI] [PubMed] [Google Scholar]
- Degroote S, Wolthoorn J, van Meer G. The cell biology of glycosphingolipids. Semin Cell Dev Biol. 2004;15:375–387. doi: 10.1016/j.semcdb.2004.03.007. [DOI] [PubMed] [Google Scholar]
- Deinhardt K, Berninghausen O, Willison HJ, Hopkins CR, Schiavo G. Tetanus toxin is internalized by a sequential clathrin-dependent mechanism initiated within lipid microdomains and independent of epsin1. J Cell Biol. 2006;174:459–471. doi: 10.1083/jcb.200508170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietrich C, Volovyk ZN, Levi M, Thompson NL, Jacobson K. Partitioning of Thy-1, GM1, and cross-linked phospholipid analogs into lipid rafts reconstituted in supported model membrane monolayers. Proc Natl Acad Sci USA. 2001;98:10642–10647. doi: 10.1073/pnas.191168698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng Y, Yu S, Lasell TK, et al. Exo1: a new chemical inhibitor of the exocytic pathway. Proc Natl Acad Sci USA. 2003;100:6469–6474. doi: 10.1073/pnas.0631766100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng Y, Jadhav AP, Rodighiero C, Fujinaga Y, Kirchhausen T, Lencer WI. Retrograde transport of cholera toxin from the plasma membrane to the endoplasmic reticulum requires the trans-Golgi network but not the Golgi apparatus in Exo2-treated cells. EMBO Rep. 2004;5:596–601. doi: 10.1038/sj.embor.7400152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujinaga Y, Wolf AA, Rodighiero C, et al. Gangliosides that associate with lipid rafts mediate transport of cholera and related toxins from the plasma membrane to endoplasmic reticulum. Mol Biol Cell. 2003;14:4783–4793. doi: 10.1091/mbc.E03-06-0354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guha A, Sriram V, Krishnan KS, Mayor S. Shibire mutations reveal distinct dynamin-independent and - dependent endocytic pathways in primary cultures of Drosophila hemocytes. J Cell Sci. 2003;116:3373–3386. doi: 10.1242/jcs.00637. [DOI] [PubMed] [Google Scholar]
- Hancock JF. Lipid rafts: contentious only from simplistic standpoints. Nat Rev Mol Cell Biol. 2006;7:456–462. doi: 10.1038/nrm1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen GH, Dalskov SM, Rasmussen CR, Immerdal L, Niels-Christiansen LL, Danielsen EM. Cholera toxin entry into pig enterocytes occurs via a lipid raft- and clathrin-dependent mechanism. Biochemistry. 2005;44:873–882. doi: 10.1021/bi047959+. [DOI] [PubMed] [Google Scholar]
- Hommelgaard AM, Roepstorff K, Vilhardt F, Torgersen ML, Sandvig K, van Deurs B. Caveolae: stable membrane domains with a potential for internalization. Traffic. 2005;6:720–724. doi: 10.1111/j.1600-0854.2005.00314.x. [DOI] [PubMed] [Google Scholar]
- Johannes L, Tenza D, Antony C, Goud B. Retrograde transport of KDEL-bearing B-fragment of Shiga toxin. J Biol Chem. 1997;272:19554–19561. doi: 10.1074/jbc.272.31.19554. [DOI] [PubMed] [Google Scholar]
- Kahya N, Schwille P. Fluorescence correlation studies of lipid domains in model membranes. Mol Membr Biol. 2006;23:29–39. doi: 10.1080/09687860500489099. [DOI] [PubMed] [Google Scholar]
- Kalia M, Kumari S, Chadda R, Hill MM, Parton RG, Mayor S. Arf6-independent GEECs fuse with sorting endosomes via a Rab5/PI-3′ kinase-dependent machinery. Mol Biol Cell. 2006;17:3689–3704. doi: 10.1091/mbc.E05-10-0980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kartenbeck J, Stukenbrok H, Helenius A. Endocytosis of simian virus 40 into the endoplasmic reticulum. J Cell Biol. 1989;109:2721–2729. doi: 10.1083/jcb.109.6.2721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenworthy AK, Nichols BJ, Remmert CL, Hendrix GM, Kumar M, Zimmerberg J, Lippincott-Schwartz J. Dynamics of putative raft-associated proteins at the cell surface. J Cell Biol. 2004;165:735–746. doi: 10.1083/jcb.200312170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkham M, Parton RG. Clathrin-independent endocytosis: new insights into caveolae and non-caveolar lipid raft carriers. Biochim Biophys Acta. 2005;1745:273–286. doi: 10.1016/j.bbamcr.2005.06.002. [DOI] [PubMed] [Google Scholar]
- Kirkham M, Fujita A, Chadda R, et al. Ultrastructural identification of uncoated caveolin-independent early endocytic vehicles. J Cell Biol. 2005;168:465–476. doi: 10.1083/jcb.200407078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuziemko GM, Stroh M, Stevens RC. Cholera toxin binding affinity and specificity for gangliosides determined by surface plasmon resonance. Biochemistry. 1996;35:6375–6384. doi: 10.1021/bi952314i. [DOI] [PubMed] [Google Scholar]
- Le PU, Nabi IR. Distinct caveolae-mediated endocytic pathways target the Golgi apparatus and the endoplasmic reticulum. J Cell Sci. 2003;116:1059–1071. doi: 10.1242/jcs.00327. [DOI] [PubMed] [Google Scholar]
- Lencer WI, Saslowsky D. Raft trafficking of AB5 subunit bacterial toxins. Biochim Biophys Acta. 2005;1746:314–321. doi: 10.1016/j.bbamcr.2005.07.007. [DOI] [PubMed] [Google Scholar]
- Lencer WI, Strohmeier G, Moe S, Carlson SL, Constable CT, Madara JL. Signal transduction by cholera toxin: processing in vesicular compartments does not require acidification. Am J Physiol. 1995a;269:G548–G557. doi: 10.1152/ajpgi.1995.269.4.G548. [DOI] [PubMed] [Google Scholar]
- Lencer WI, Constable C, Moe S, et al. Targeting of cholera toxin and Escherichia coli heat labile toxin in polarized epithelia: role of COOH-terminal KDEL. J Cell Biol. 1995b;131:951–962. doi: 10.1083/jcb.131.4.951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling H, Boodhoo A, Hazes B, Cummings MD, Armstrong GD, Brunton JL, Read RJ. Structure of the shiga-like toxin I B-pentamer complexed with an analogue of its receptor Gb3. Biochemistry. 1998;37:1777–1788. doi: 10.1021/bi971806n. [DOI] [PubMed] [Google Scholar]
- MacKenzie CR, Hirama T, Lee KK, Altman E, Young NM. Quantitative analysis of bacterial toxin affinity and specificity for glycolipid receptors by surface plasmon resonance. J Biol Chem. 1997;272:5533–5538. doi: 10.1074/jbc.272.9.5533. [DOI] [PubMed] [Google Scholar]
- Majoul I, Sohn K, Wieland FT, Pepperkok R, Pizza M, Hillemann J, Soling HD. KDEL receptor (Erd2p)-mediated retrograde transport of the cholera toxin A-subunit from the Golgi involves COPI, p23, and the COOH terminus of Erd2p. J Cell Biol. 1998;143:601–612. doi: 10.1083/jcb.143.3.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majoul I, Straub M, Hell SW, Duden R, Soling HD. KDEL-cargo regulates interactions between proteins involved in COPI vesicle traffic: measurements in living cells using FRET. Dev Cell. 2001;1:139–153. doi: 10.1016/s1534-5807(01)00004-1. [DOI] [PubMed] [Google Scholar]
- Massol RH, Larsen JE, Fujinaga Y, Lencer WI, Kirchhausen T. Cholera toxin toxicity does not require functional Arf6- and dynamin-dependent endocytic pathways. Mol Biol Cell. 2004;15:3631–3641. doi: 10.1091/mbc.E04-04-0283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayor S, Rao M. Rafts: scale-dependent, active lipid organization at the cell surface. Traffic. 2004;5:231–240. doi: 10.1111/j.1600-0854.2004.00172.x. [DOI] [PubMed] [Google Scholar]
- Mayor S, Riezman H. Sorting GPI-anchored proteins. Nat Rev Mol Cell Biol. 2004;5:110–120. doi: 10.1038/nrm1309. [DOI] [PubMed] [Google Scholar]
- McCann JA, Mertz JA, Czworkowski J, Picking WD. Conformational changes in cholera toxin B-subunit-ganglioside GM1 complexes are elicited by environmental pH and evoke changes in membrane structure. Biochemistry. 1997;36:9169–9178. doi: 10.1021/bi962996p. [DOI] [PubMed] [Google Scholar]
- Megha, London E. Ceramide selectively displaces cholesterol from ordered lipid domains (rafts): implications for lipid raft structure and function. J Biol Chem. 2004;279:9997–10004. doi: 10.1074/jbc.M309992200. [DOI] [PubMed] [Google Scholar]
- Merritt EA, Sarfaty S, van den Akker F, L’Hoir C, Martial JA, Hol WG. Crystal structure of cholera toxin B-pentamer bound to receptor GM1 pentasaccharide. Protein Sci. 1994;3:166–175. doi: 10.1002/pro.5560030202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merritt EA, Sarfaty S, Jobling MG, Chang T, Holmes RK, Hirst TR, Hol WG. Structural studies of receptor binding by cholera toxin mutants. Protein Sci. 1997;6:1516–1528. doi: 10.1002/pro.5560060716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merritt EA, Kuhn P, Sarfaty S, Erbe JL, Holmes RK, Hol WG. The 1.25 A resolution refinement of the cholera toxin B-pentamer: evidence of peptide backbone strain at the receptor-binding site. J Mol Biol. 1998;282:1043–1059. doi: 10.1006/jmbi.1998.2076. [DOI] [PubMed] [Google Scholar]
- Montesano R, Roth J, Robert A, Orci L. Non-coated membrane invaginations are involved in binding and internalization of cholera and tetanus toxins. Nature. 1982;296:651–653. doi: 10.1038/296651a0. [DOI] [PubMed] [Google Scholar]
- Morgan GW, Goulding D, Field MC. The single dynamin-like protein of Trypanosoma brucei regulates mitochondrial division and is not required for endocytosis. J Biol Chem. 2004;279:10692–10701. doi: 10.1074/jbc.M312178200. [DOI] [PubMed] [Google Scholar]
- Naslavsky N, Weigert R, Donaldson JG. Characterization of a nonclathrin endocytic pathway: membrane cargo and lipid requirements. Mol Biol Cell. 2004;15:3542–3552. doi: 10.1091/mbc.E04-02-0151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols BJ. A distinct class of endosome mediates clathrin-independent endocytosis to the Golgi complex. Nat Cell Biol. 2002;4:374–378. doi: 10.1038/ncb787. [DOI] [PubMed] [Google Scholar]
- Norkin LC, Anderson HA, Wolfrom SA, Oppenheim A. Caveolar endocytosis of simian virus 40 is followed by brefeldin A-sensitive transport to the endoplasmic reticulum, where the virus disassembles. J Virol. 2002;76:5156–5166. doi: 10.1128/JVI.76.10.5156-5166.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Brien AD, Tesh VL, Donohue-Rolfe A, et al. Shiga toxin: biochemistry, genetics, mode of action, and role in pathogenesis. Curr Top Microbiol Immunol. 1992;180:65–94. doi: 10.1007/978-3-642-77238-2_4. [DOI] [PubMed] [Google Scholar]
- Orlandi PA, Fishman PH. Filipin-dependent inhibition of cholera toxin: evidence for toxin internalization and activation through caveolae-like domains. J Cell Biol. 1998;141:905–915. doi: 10.1083/jcb.141.4.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parton RG. Ultrastructural localization of gangliosides; GM1 is concentrated in caveolae. J Histochem Cytochem. 1994;42:155–166. doi: 10.1177/42.2.8288861. [DOI] [PubMed] [Google Scholar]
- Parton RG, Joggerst B, Simons K. Regulated internalization of caveolae. J Cell Biol. 1994;127:1199–1215. doi: 10.1083/jcb.127.5.1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelkmans L, Zerial M. Kinase-regulated quantal assemblies and kiss-and-run recycling of caveolae. Nature. 2005;436:128–133. doi: 10.1038/nature03866. [DOI] [PubMed] [Google Scholar]
- Pelkmans L, Kartenbeck J, Helenius A. Caveolar endocytosis of simian virus 40 reveals a new two-step vesicular-transport pathway to the ER. Nat Cell Biol. 2001;3:473–483. doi: 10.1038/35074539. [DOI] [PubMed] [Google Scholar]
- Pelkmans L, Puntener D, Helenius A. Local actin polymerization and dynamin recruitment in SV40-induced internalization of caveolae. Science. 2002;296:535–539. doi: 10.1126/science.1069784. [DOI] [PubMed] [Google Scholar]
- Pelkmans L, Burli T, Zerial M, Helenius A. Caveolin-stabilized membrane domains as multifunctional transport and sorting devices in endocytic membrane traffic. Cell. 2004;118:767–780. doi: 10.1016/j.cell.2004.09.003. [DOI] [PubMed] [Google Scholar]
- Richards AA, Stang E, Pepperkok R, Parton RG. Inhibitors of COP-mediated transport and cholera toxin action inhibit simian virus 40 infection. Mol Biol Cell. 2002;13:1750–1764. doi: 10.1091/mbc.01-12-0592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabharanjak S, Sharma P, Parton RG, Mayor S. GPI-anchored proteins are delivered to recycling endosomes via a distinct cdc42-regulated, clathrin-independent pinocytic pathway. Dev Cell. 2002;2:411–423. doi: 10.1016/s1534-5807(02)00145-4. [DOI] [PubMed] [Google Scholar]
- Sack DA, Sack RB, Nair GB, Siddique AK. Cholera. Lancet. 2004;363:223–233. doi: 10.1016/s0140-6736(03)15328-7. [DOI] [PubMed] [Google Scholar]
- Shogomori H, Futerman AH. Cholera toxin is found in detergent-insoluble rafts/domains at the cell surface of hippocampal neurons but is internalized via a raft-independent mechanism. J Biol Chem. 2001;276:9182–9188. doi: 10.1074/jbc.M009414200. [DOI] [PubMed] [Google Scholar]
- Silvius JR, Nabi IR. Fluorescence-quenching and resonance energy transfer studies of lipid microdomains in model and biological membranes. Mol Membr Biol. 2006;23:5–16. doi: 10.1080/09687860500473002. [DOI] [PubMed] [Google Scholar]
- Singh RD, Puri V, Valiyaveettil JT, Marks DL, Bittman R, Pagano RE. Selective caveolin-1-dependent endocytosis of glycosphingolipids. Mol Biol Cell. 2003;14:3254–3265. doi: 10.1091/mbc.E02-12-0809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spangler BD. Structure and function of cholera toxin and the related Escherichia coli heat-labile enterotoxin. Microbiol Rev. 1992;56:622–647. doi: 10.1128/mr.56.4.622-647.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spooner RA, Smith DC, Easton AJ, Roberts LM, Lord JM. Retrograde transport pathways utilised by viruses and protein toxins. Virol J. 2006;3:26. doi: 10.1186/1743-422X-3-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tagawa A, Mezzacasa A, Hayer A, Longatti A, Pelkmans L, Helenius A. Assembly and trafficking of caveolar domains in the cell: caveolae as stable, cargo-triggered, vesicular transporters. J Cell Biol. 2005;170:769–779. doi: 10.1083/jcb.200506103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomsen P, Roepstorff K, Stahlhut M, van Deurs B. Caveolae are highly immobile plasma membrane microdomains, which are not involved in constitutive endocytic trafficking. Mol Biol Cell. 2002;13:238–250. doi: 10.1091/mbc.01-06-0317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torgersen ML, Skretting G, van Deurs B, Sandvig K. Internalization of cholera toxin by different endocytic mechanisms. J Cell Sci. 2001;114:3737–3747. doi: 10.1242/jcs.114.20.3737. [DOI] [PubMed] [Google Scholar]
- Tran D, Carpentier JL, Sawano F, Gorden P, Orci L. Ligands internalized through coated or noncoated invaginations follow a common intracellular pathway. Proc Natl Acad Sci USA. 1987;84:7957–7961. doi: 10.1073/pnas.84.22.7957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai B, Rapoport TA. Unfolded cholera toxin is transferred to the ER membrane and released from protein disulfide isomerase upon oxidation by Ero1. J Cell Biol. 2002;159:207–216. doi: 10.1083/jcb.200207120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai B, Ye Y, Rapoport TA. Retro-translocation of proteins from the endoplasmic reticulum into the cytosol. Nat Rev Mol Cell Biol. 2002;3:246–255. doi: 10.1038/nrm780. [DOI] [PubMed] [Google Scholar]
- Tsai B, Gilbert JM, Stehle T, Lencer W, Benjamin TL, Rapoport TA. Gangliosides are receptors for murine polyoma virus and SV40. Embo J. 2003;22:4346–4355. doi: 10.1093/emboj/cdg439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf AA, Jobling MG, Wimer-Mackin S, Ferguson-Maltzman M, Madara JL, Holmes RK, Lencer WI. Ganglioside structure dictates signal transduction by cholera toxin and association with caveolae-like membrane domains in polarized epithelia. J Cell Biol. 1998;141:917–927. doi: 10.1083/jcb.141.4.917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf AA, Fujinaga Y, Lencer WI. Uncoupling of the cholera toxin-G(M1) ganglioside receptor complex from endocytosis, retrograde Golgi trafficking, and downstream signal transduction by depletion of membrane cholesterol. J Biol Chem. 2002;277:16249–16256. doi: 10.1074/jbc.M109834200. [DOI] [PubMed] [Google Scholar]