Summary
Methicillin-resistant Staphylococcus aureus (MRSA) is endemic in hospitals worldwide and a significant cause of morbidity and mortality. Healthcare-associated MRSA infections occur in individuals with predisposing risk factors for disease, such as surgery or presence of an indwelling medical device. By contrast, community-associated MRSA (CA-MRSA) infections often occur in otherwise healthy individuals who lack such risk factors. In addition, CA-MRSA infections are epidemic in some countries. These observations suggest that CA-MRSA strains are more virulent and transmissible than traditional hospital-associated MRSA strains. Relatively limited treatment options for CA-MRSA infections compound the problem of enhanced virulence and transmission. Although progress has been made toward understanding emergence of CA-MRSA, virulence, and treatment of infections, our knowledge in these areas remains incomplete. Here were review the most current knowledge in these areas and provide perspective on future outlook for prophylaxis and/or new therapies for CA-MRSA infections.
Introduction
Staphylococcus aureus is a leading cause of human bacterial infections worldwide.1 Severity of these infections is quite varied and can range from minor skin infections to fatal necrotizing pneumonia. The pathogen is also a human commensal organism and ~30% of healthy non-institutionalized individuals are colonized asymptomatically with S. aureus in the anterior nares.2 These observations are of note because S. aureus nasal carriage has been associated with subsequent infection.3
S. aureus has a remarkable ability to acquire resistance to antibiotics. Infections caused by antibiotic resistant S. aureus have occurred in epidemic or pandemic waves over the past 60 years.4,5 Within 10 years after penicillin was introduced for use in humans, in many cases it was no longer effective for treatment of S. aureus infections due to acquisition of plasmid-encoded beta lactamase.6 Penicillin-resistant S. aureus became pandemic throughout the late 1950s and early 1960s.7 Methicillin-resistant S. aureus (MRSA) was first reported in 1961, two years after the antibiotic was introduced to treat penicillin-resistant S. aureus.8 MRSA spread worldwide over the next several decades and is now endemic in most hospitals and healthcare facilities of industrialized countries. In the United States, MRSA is among the leading causes of death by any single infectious agent.4,9 A major concern for treatment of MRSA infections is the increasing prevalence of resistance to multiple antibiotics (multidrug resistance).
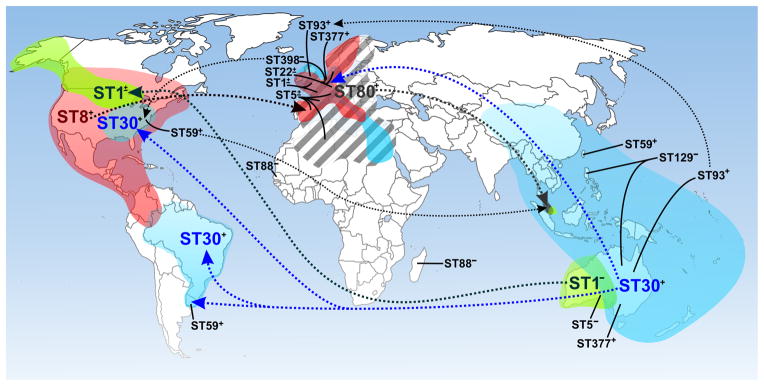
One of the most surprising recent events in the area of infectious diseases is the emergence of community-associated MRSA (CA-MRSA). In contrast to healthcare-associated MRSA (HA-MRSA) infections, for which there is a predisposing risk factor or condition, CA-MRSA infections can occur in otherwise healthy individuals,10 suggesting that CA-MRSA strains have enhanced virulence compared with traditional HA-MRSA strains. In addition to enhanced virulence, some CA-MRSA strains such as USA300 have the ability to spread readily. These characteristics are perhaps in part the reason CA-MRSA is present in many countries globally (figure 1).11,12
Figure 1. Global distribution of CA-MRSA as indicated by multilocus sequence type (ST).
Dotted lines indicated possible route of dissemination for the indicated clones. Major CA-MRSA clones are indicated by larger font and color. Colored regions are an estimate of the area in which infections have been reported for the indicated clone (not all are shown). ST1, green; ST8, red; ST30, blue; ST80, gray hatched. +, PVL-positive; −, PVL-negative; ±, combination of PVL-positive and PVL-negative strains isolated from the region.
In this Seminar we review our current understanding of CA-MRSA emergence, the basis for enhanced transmission and virulence, and provide an update on the most recent strategies for diagnosis and treatment of CA-MRSA infections.
Epidemiology and disease
Epidemiology
Since its first description in 1961, MRSA has been considered as a nosocomial pathogen and its presence in the community was uncommon. However, this concept has been reshaped dramatically over the past 15 years and CA-MRSA infections are now both prevalent and widespread (figure 1). Although MRSA infections acquired from the community were reported in the Detroit, Michigan area in 1982, these patients all had predisposing risk factors for infection, such as previous hospitalization and/or intravenous drug abuse.13 The first bona fide cases of CA-MRSA infection were reported among individuals from the Kimberley region of Western Australia in the early 1990s.14 Notably, these patients were from remote and sparsely populated areas and thus did not have close contact with individuals who had access to large medical centers. In addition, the patient MRSA isolates (later known collectively as strain WA-MRSA-1 or WA-1) were non-multidrug resistant and distinct from other MRSA strains present in Australia.14,15
During 1997–1999, four otherwise healthy children in the upper Midwestern region of the US died from sepsis and/or necrotizing pneumonia caused by MRSA.16 These infections were not acquired in a healthcare setting and the children had no risk factors for MRSA infection. This small outbreak was caused by a CA-MRSA strain later known as MW2,17 a close relative of WA-MRSA-1.15 An earlier retrospective study by Herold et al. reported an increasing prevalence of CA-MRSA infections during 1988–1995 among children in the Chicago area who had no predisposing risk factors.10 Cellulitis and abscesses were the most frequent syndromes associated with these infections, whereas those from HA-MRSA infections were most often (4 of 12 patients) bacteremias.10 In contrast to the majority of HA-MRSA isolates, most of these CA-MRSA isolates were susceptible to non beta-lactam antibiotics and therefore not multidrug resistant.10 These early outbreaks were the beginnings of what is now an epidemic of CA-MRSA in North America, especially in the US (figure1).
Whole genome sequencing of MW2 revealed a unique staphylococcal chromosomal cassette mec (SCCmec) named SCCmecIV,17,18 which harbors the mecA gene encoding resistance to methicillin (see below for more details). Unlike SCCmec elements I–III, which encode molecules that provide resistance to multiple classes of antibiotics, SCCmecIV encodes resistance only to beta-lactam antibiotics,18 accounting in part for the non-multidrug resistant phenotype of MW2. The antibiogram and gene composition of MW2 give credence to the hypothesis that this was a newly emerging MRSA clone, likely the result of SCCmecIV integrating into a methicillin-susceptible S. aureus (MSSA) strain. MW2 also contains genes encoding Panton-Valentine leukocidin (PVL), a prophage-encoded bi-component toxin that targets phagocytic leukocytes. SCCmecIV and PVL are molecular markers associated with the emergence of CA-MRSA worldwide,11 although more recent data indicates PVL is not always present among CA-MSA strains (for example, WA-MRSA-1 strains typically lack PVL).19–22 Association of PVL with CA-MRSA led to a renewed interest in this toxin and its potential role in pathogenesis and this topic is discussed in detail below.
MW2 and closely related strains, collectively known as pulsed-field type USA400 (USA400),23 multilocus sequence type (MLST or ST) 1 strains,24 were the most prominent community-associated strains in the US prior to 2001.16,25 From the early 2000s to the present, in both the scientific community and the lay press, numerous outbreaks and increasing case rates of CA-MRSA were reported. These reports described an inordinate number of skin and soft tissues infections among healthy and diverse populations including inmates in correctional facilities,26 military personnel,26 children in day-care centers,27 men-who-have-sex-with-men,28 athletes at all levels,29 Native Americans,30 and Pacific Islanders.31 Surprisingly, a new CA-MRSA clone known as USA300, a ST8 strain unrelated to the MW2/ST1 lineage, was identified in the majority of US cases, indicating the rapid replacement of USA400 in most communities.25 Although USA400 remains a prominent cause of disease in some regions of North America, USA300 is currently the leading cause of community-associated bacterial infections in the US.23,32–34
CA-MRSA is currently a human health problem in nearly all industrialized countries, albeit to varied extents. For example, PVL-positive CA-MRSA were isolated from 1–3% of all skin and soft tissue infections in France in 2000–2003,35,36 whereas the prevalence of these strains was recently reported as >50% in the US.34 In addition, the most abundant CA-MRSA strains in Europe are distinct from those in North America, Oceania, or other parts of the world (figure 1).12 Such distinctions are made in part based upon molecular typing schemes that include MLST,24 SCCmec typing,18 spa37 and/or agr typing, and pulsed-field gel electrophoresis (PFGE).23 These S. aureus typing methods have been reviewed recently.5 The change in epidemiology of MRSA–i.e., its emergence as a community pathogen–correlates with a change in the genetic organization of SCCmec. Since the late 1960s, five major MLST-defined pandemic clones of MRSA, ST5, ST8, ST22, ST36, and ST45, spread successfully in different regions of the world and caused significant nosocomial disease. With the exception of ST22, which has only been reported as SCCmecIV,38 these multidrug resistant clones originally harbored three genetically distinct SCCmec elements (I, II, and III) that, based on their relatively large size and other properties, likely have limited movement in nature. As discussed above, CA-MRSA clones identified in the US, Europe and Australia carry SCCmecIV or a recent variant, and in contrast to the historic SCCmec types, these recent recombinant elements are highly promiscuous and have moved repeatedly into diverse lineages of MSSA.39 SCCmecIV appears critical to the emergence and success of CA-MRSA because the element is smaller and much more mobile than SCCmecI–III present in HA-MRSA strains, as it is widely dispersed among numerous MRSA lineages,39 and it imparts little or no fitness cost in vitro40,41 or in vivo.42 Several other SCCmec variants and types (e.g., types V, VI, VII, and VIII) have since been identified.43–45 These SCCmec elements, although they tend to be within a similar size range, differ in fine structure and thus demonstrate the remarkable plasticity of the element.43–45
Colonization and Disease
S. aureus can be considered normal human flora because approximately a third of the population is colonized by the organism with no associated disease.46,47 Although CA-MRSA should be similar to other S. aureus strains in this regard, i.e., high prevalence of CA-MRSA infections should be reflected by correspondingly high levels of nasal colonization, most individuals in the US that are colonized by S. aureus are colonized by MSSA strains despite the higher abundance of CA-MRSA infections.2 This observation suggests CA-MRSA strains cause infection in the absence of nasal colonization. In addition to the anterior nares, the throat, axilla, groin and perirectal area, and non-intact skin are sites colonized by S. aureus and could be considered as potentially important sites for colonization by CA-MRSA.
Nasal carriage studies provide a sentinel approach to determine the burden of S. aureus in a population; historically, as a healthcare associated organism, MRSA was rarely recovered from healthy populations. In 1998, among 500 nasal swabs cultured from children and guardians in a New York City vaccination clinic, S. aureus carriage rates ranged from 35% in children to 28% in adults, but significantly, only 1 subject (0.26%) harbored MRSA.48 By comparison, a concurrent study at the University of Chicago Children’s Hospital Emergency Department reported a 2.2% MRSA colonization rate (11/500 children),49 which may reflect the higher burden of CA-MRSA in the upper Midwest region of the US at that time. MRSA carriage in Nashville, Tennessee was reported as 0.8% in 2001; however, when the authors repeated the study in 2005, they found that among 500 swabbed children, 46 or 9.2% of the population were harboring MRSA.50 These results are consistent with the changing epidemiology of MRSA and its increasing prevalence in the community. S. aureus carriers have a higher risk of infection than non-carriers and they are an important source of spread of S. aureus strains among individuals. Thus, as the proportion of CA-MRSA increases among carriage isolates so does its transmission within a population of exposed individuals. Moreover, the rapid dissemination of CA-MRSA strains and the high attack rate in outbreak settings suggest that they are more easily transmitted than other S. aureus stains.51
CA-MRSA, like all S. aureus, is transmitted by directly contacting the organism, and this usually occurs by direct skin-to-skin contact with a colonized or infected individual.52–54 However, fomites contaminated with CA-MRSA may have a role in transmission in some settings.30,32 The Centers for Disease Control and Prevention in Atlanta, Georgia have proposed five factors or “5 C’s” of MRSA transmission. These factors are Crowding, frequent skin-to-skin Contact, Compromised skin integrity, Contaminated items and surfaces, and lack of Cleanliness (www.cdc.gov/niosh/topics/mrsa/). The “5 C’s” are prevalent among the diverse populations with increased numbers of CA-MRSA infections as described above (e.g., military personnel, children in day-care centers, and etc). S. aureus, either as a part of normal flora or transmitted by contaminated objects or colonized / infected individuals, circumvents human host defense to cause infection.
The burden of staphylococcal disease has increased worldwide since the emergence of CA-MRSA.55–58 Skin and soft-tissue infections (SSTIs) constitute approximately 90% of CA-MRSA cases (table 1), and 90% of these are abscesses and/or cellulitis59 with purulent drainage.34 In essence, CA-MRSA strains can cause the same types of infections as MSSA strains (table 1). However, some CA-MRSA strains have been associated with particularly severe, invasive disease or syndromes, suggesting they are more virulent than other S. aureus strains (see below for details). These syndromes include purpura fulminans with or without Waterhouse-Friderichsen syndrome,60,61 pyomyositis and myositis,62 necrotizing fasciitis (virtually unheard of before CA-MRSA),63 osteomyelitis,64,65 and pneumonia (sometimes necrotizing) (figure 2).10,59,65–69
Table 1.
Distribution of infections caused by CA-MRSA in four observational studies.
| Author (Year of Study) | ||||
|---|---|---|---|---|
| 1 Kaplan55 (2005) | Fridkin59 (2005) | Liu124 (2008) | 1 Purcell58 (2005) | |
| Patients (N) | 2659 | 1647 | 2270 | 826 |
| SSTI (%) | 95.6 | 87 | 88 | 94.1 |
| Bone, joint (%) | 2.4 | 3 | <1 | <1 |
| Respiratory (%) | <1 | 2 | 4 | <1 |
| Bacteremia (%) | <1 | 3 | 4 | <1 |
| Urinary (%) | 0 | 4 | 2 | <1 |
| Other (%) | 1 | 4 | 2 | 4.5 |
Children
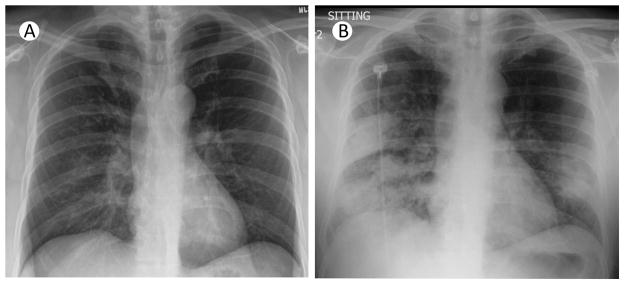
Figure 2. Rapid progression of radiographic findings in a fatal case of CA-MRSA pneumonia complicating novel H1N1 influenza A infection.
(A) Chest radiograph at initial presentation of a patient with symptoms of fevers, headache, myalgias, and non-productive cough. (B) Chest radiograph taken 24 hours later with presence of new infiltrates and signs of consolidation in the right upper lobe, right middle lobe and left lower lobe.
Virulence and pathogenesis
The ability of bacteria to cause disease in humans is due largely to evasion of innate immunity, which includes resistance to killing by phagocytic leukocytes. S. aureus produces numerous molecules–some on the cell surface and others freely secreted–that together elicit a robust inflammatory response. Inasmuch as neutrophils are a key component of the inflammatory response and the most prominent cellular defense against S. aureus infections, the pathogen has evolved means to circumvent function of these host cells. For example, S. aureus synthesizes molecules that block function of serum complement or antimicrobial peptides, and detoxify reactive oxygen species.70 In addition, S. aureus is notorious for its ability to produce secreted toxins implicated in pathogenesis, including those that are cytolytic for host cells.70 Some of the secreted toxins are produced by most S. aureus strains and are thus not unique to CA-MRSA strains. A comprehensive discussion of S. aureus virulence molecules is outside the scope of this Seminar and we refer the reader to several relatively recent reviews on the topic.70–73 Here we focus our discussion on molecules for which there is information in the context of CA-MRSA transmission, virulence and pathogenesis.
CA-MRSA virulence determinants
CA-MRSA strains cause infections in otherwise healthy people10,16 and have the capacity to cause unusually severe disease.60,63 Consistent with these observations, CA-MRSA strains are significantly more virulent than HA-MRSA strains in animal infection models.74,75 Collectively, these observations suggest CA-MRSA strains have increased virulence and capacity to evade host defenses compared to traditional HA-MRSA strains. Pronounced virulence may contribute not only to disease severity, but also to more persistent disease, which could increase chances of pathogen transmission. Despite significant progress over the past several years, the molecular basis of CA-MRSA pathogenesis remains incompletely determined.
The bi-component leukotoxin Panton-Valentine leukocidin (PVL) has been intensely investigated because the two genes encoding PVL (lukS-PV and lukF-PV) are the only ones coding for a known virulence determinant that has been epidemiologically linked to CA-MRSA infections.11 The cytolytic and biochemical properties of the PVL toxin were well established before the appearance of CA-MRSA.76,77 Although PVL was associated with community S. aureus infections caused by phage-type 80/81 in the 1950s and 1960s,7 the relatively recent epidemiological association of PVL with CA-MRSA prompted examination of the role of PVL in pathogenesis using isogenic lukS/F-PV gene deletion mutants in CA-MRSA strains. Surprisingly, many experimental studies in animal models of skin and soft tissue infection, sepsis, and pneumonia, largely have shown no effect or only minor and strain-dependent effects of PVL.78–84 Although not directly addressing a potential role of PVL in CA-MRSA virulence, an earlier study using transduced S. aureus laboratory strains reported that PVL contributed significantly to severe pneumonia in mice.85 These studies also found that direct instillation of purified PVL into lungs produced tissue injury. Another potentially intriguing finding was that PVL altered virulence gene regulation as a basis for the contribution to virulence.85 However, it was shown later that the effects attributed to PVL expressed during infection were due to an unintended genetic mutation causing defective virulence gene regulation, rather than PVL.86
The susceptibility of white blood cells to PVL in vitro can differ considerably among mammalian species76 and is potentially a caveat for the interpretation of experimental studies investigating PVL’s effect in animal infection models. For example, mouse neutrophils are more resistant to the cytolytic activity of purified PVL in vitro compared with those from rabbits or humans.76,87 Although, early studies with partially purified PVL in animal models indicate systemic effects of PVL toward leukocytes are comparable in mice and rabbits in vivo,88,89 the relative resistance of rodent neutrophils to PVL produced during an actual infection could account in part for the negative data from rodent infection models concerning PVL’s role in pathogenesis. To the extent that neutrophils largely mediate the effects of or are targets for PVL, rabbit neutrophils–which similar to those from humans are highly susceptible to PVL–may be a more appropriate model system to study the effects of PVL. Indeed, more recently PVL has been shown to contribute to severity of disease in rabbit models of pneumonia and osteomyelitis, albeit when using relatively high bacterial inocula.90,91 It seems likely that only under very specific growth conditions will PVL-positive CA-MRSA strains produce sufficient toxin to promote lysis of human neutrophils to a greater extent than the corresponding PVL-negative isogenic mutants.79,81,87 Clearly, knowledge of PVL activity in vitro is alone insufficient to resolve the question about its role in CA-MRSA pathogenesis. Although most currently available data from experimental studies indicate PVL may not be a major determinant of CA-MRSA virulence, it is still possible that the activity of PVL is largely human-specific and/or the toxin plays a role in pathogenesis under unique conditions, such as those involving host susceptibility factors, and in certain infections such as necrotizing pneumonia and osteomyelitis.90–92
While most efforts to understand CA-MRSA pathogenesis have focused on PVL, some recent studies have also addressed the possible role of other cytolytic toxins–namely alpha-toxin and phenol-soluble modulins (PSMs).82,93 Alpha-toxin (or alpha-hemolysin) is a well described pore-forming toxin that has been reported to lyse many types of host cells, including most types of leukocytes94 (although not neutrophils)95 and it has pro-inflammatory effects.94,96 PSMs are short, amphipathic and alpha-helical peptide toxins, among which those of the alpha-type stimulate and lyse neutrophils and other host cells.93 Both alpha-toxin and alpha-type PSMs have a dramatic impact on the severity of experimental CA-MRSA disease. CA-MRSA strains lacking alpha-type PSMs caused significantly reduced mortality in a murine sepsis model, and lesion size and area of dermonecrosis were decreased significantly in a murine skin infection model.93 Alpha-toxin negative CA-MRSA strains (USA300 and USA400) are essentially avirulent in experimental murine pneumonia82 and antibodies to alpha-toxin protect mice from experimental CA-MRSA pneumonia.97 By comparison, in the same models there was no difference in virulence between wild-type and isogenic lukS/F-PV-negative (PVL negative) strains81–83 and anti-PVL antibodies were non-protective.97 These studies demonstrate that alpha-toxin and alpha-type PSMs play a major role of in CA-MRSA disease and pathogenesis, and also underscore the value of parallel investigation to determine the relative contribution of each S. aureus virulence determinant to pathogenesis. This information is critical for evaluation of potential targets for prophylaxis or therapeutic agents directed against CA-MRSA.
In contrast to the PVL genes, which are encoded on a mobile genetic element (the acquisition of which is proposed to confer virulence), both alpha-toxin and PSMs are encoded in the core genome of S. aureus. Thus, observed differences in virulence between CA-MRSA and HA-MRSA strains that are attributed to these toxins must be due to differential gene expression. Accordingly, investigation of gene expression within representative subclones of S. aureus clonal complex 8 (defined by MLST),98 which includes USA300 and other closely related strains, revealed that USA300 expresses alpha-toxin, alpha-type PSMs, and other putative determinants of virulence such as secreted proteases to an exceptionally high extent.74 In addition, changes in gene expression may explain the greater success of USA300 compared to the more distantly-related USA400 strain in the US.99 These findings indicate that evolution of virulence in CA-MRSA is based, at least in part, on differential gene expression, which may include yet poorly understood rearrangements of gene regulatory networks in CA-MRSA strains.
The question then remains what distinguishes the strains of the pandemic USA300 clone from those of its less successful direct predecessor USA500, as these strains have comparable virulence in mouse infection models.74 The answer to this question may be found by examining determinants of colonization and transmissibility rather than virulence. For example, USA300 strains harbor a mobile genetic element called arginine catabolic mobile element (ACME), which may contain genes that potentially facilitate survival on human skin.42,100 ACME, which is absent from other, less successful CA-MRSA strains, may contribute to the noted greater success of USA300 by comparison. Animal models of S. aureus colonization are needed to facilitate a better understanding of current and future MRSA pandemics.
Given that a traditional opsonophagocytic vaccine is not available for S. aureus, and as there is an escalating increase in multidrug resistance among CA-MRSA strains in some areas of the world,28,101,102 novel approaches targeting virulence as a means of attenuating disease severity are under development.103 Such endeavors may be aimed for example at passive immunization using antibodies against S. aureus toxins.104 Given that all studies of PVL in animal infection models, including those that show a contribution of the toxin to virulence, demonstrate PVL-negative CA-MRSA strains retain considerable virulence, therapeutic efforts that target this toxin solely may have limited potential efficacy. The significant contribution of alpha-toxin and alpha-type PSMs to CA-MRSA virulence in animal models suggests that these molecules could be valuable targets for antitoxin-based therapeutic approaches. Clearly, any S. aureus antitoxin preparation ideally should be directed against multiple targets and will have to be designed such that functional redundancy of S. aureus virulence determinants and the relative contribution of each are taken into account.
Diagnosis
S. aureus infection is diagnosed readily by isolating the organism from cultures of blood, tissue, or pus. Such material is typically loaded with S. aureus and as organism is not fastidious it will grow in virtually any non-selective bacterial culture medium. Unless a patient has been previously treated with an effective anti-staphylococcal agent (and several days of effective therapy generally are required to render a site culture-negative), failure to culture S. aureus is strong evidence against staphylococcal infection. If S. aureus is isolated from blood or other sterile body site, specificity is essentially 100%. Isolation of S. aureus from a respiratory specimen is less specific because nasopharyngeal colonization of normal individuals is so prevalent (also the reason isolation of S. aureus from a nasal swab or throat culture is not useful for determining whether an infection at some other site is caused by S. aureus). However, in the clinical setting of pneumonia, if staphylococci are the predominant organisms that stain as Gram-positive and numerous PMNs and few or no epithelial cells are present, S. aureus infection is likely.
Standard antimicrobial susceptibility test methods such as disk diffusion, broth dilution, or automated methods accurately identify MRSA strains. A latex agglutination test that detects PBP2a, the penicillin-binding protein that mediates methicillin resistance, and nucleic acid amplification methods to detect mecA, the gene encoding PBP2a, are also available.105–107 A detailed discussion of the advantages and disadvantages of these various methods is beyond the scope of this article, except to note that sensitivity and specificity of any single test is about 95%, no test is perfect, and most clinical laboratories perform confirmatory testing. Presently, the only way to determine susceptibility to non-beta-lactam antibiotics is by standard susceptibility testing.
Susceptibility tests cannot discriminate between CA-MRSA isolates and other MRSA strains. For example, the characteristic USA300 phenotype is susceptibility to trimethoprim-sulfamethoxazole (TMP-SMX), tetracyclines, and clindamycin, but this pattern is not uniform and the organism can acquire other resistance genes.28 Moreover, each CA-MRSA lineage has a typical antibiotic resistance pattern.11,12 Although the current epidemic CA-MRSA clones are now a significant cause of healthcare-associated infections, these clones can be distinguished from traditional HA-MRSA strains by genotyping by PFGE,23 spa typing,37 or MLST,24 SCCmec typing,18 and presence of PVL genes.11 However, these typing methods have no proven value in the clinic, as selecting the appropriate treatment for infection requires careful evaluation of patient history and testing antibiotic susceptibility patterns of any recovered S. aureus isolates.
Prevention and treatment
Emergence of CA-MRSA has profoundly impacted the choice of empirical therapy for suspected staphylococcal infection, particularly common skin and soft tissue infections. Beta-lactams, which are relatively inexpensive, non-toxic, and highly effective, have been the drug of choice for treating such infections, but like HA-MRSA, CA-MRSA are broadly resistant to almost all beta-lactam antibiotics, making these an undesirable option when the prevalence of CA-MRSA strains is high. Clinical evidence supporting the efficacy of alternative agents for treatment of CA-MRSA infections is limited. The treatment of choice for cutaneous abscesses caused by S. aureus, regardless of antibiotic susceptibility, is incision and drainage.34,108–112 Antibiotic therapy provides little or no marginal benefit in most cases and is not routinely recommended unless the patient has conditions such as those listed in table 2.113
Table 2.
Situations in which to consider use of antimicrobial therapy following incision and debridement of a CA-MRSA SSTI.
| Severe or extensive disease and/or rapid progression in presence of associated cellulitis |
| Signs and symptoms of systemic illness |
| Associated comorbidities or immune suppression (DM, HIV/AIDS, neoplastic disease) |
| Extremes of age |
| Abscess in area difficult to drain |
| Associated septic phlebitis |
| Lack of response of treatment with incision and drainage alone |
Antimicrobial therapy
Inexpensive oral agents commonly recommended for treatment of CA-MRSA infections include clindamycin, long-acting tetracyclines (doxycycline and minocycline), TMP-SMX, rifampin, and fusidic acid (table 3).114–116 Clindamycin is active in vitro against 80% or more of CA-MRSA strains and has been used with success in the treatment of CA-MRSA infections, primarily SSTI.51,117,118 It is active against group A Streptococcus, a common cause of SSTI, which makes it an attractive choice for treatment of skin and soft infections, especially those not accompanied by abscess. Clindamycin resistance, however, may be increasing.119,120
Table 3.
Rates of resistance and dosing of oral agents for treatment of CA-MRSA infections.
| Antimicrobial agent | Resistance rates | Typical adult oral dosing | Comments |
|---|---|---|---|
| Clindamycin | 3–24% | 300 TID | D-test should be performed. Excellent activity against strep. Increasing resistance a concern. |
| Doxycycline Minocycline |
19–24% | 100 mg BID 100 mg BID |
Doxycycline and minocycline probably active against tetracycline resistant strains. |
| Trimethoprim-sulfamethoxazole | 0–10% | 1–2 DS (160/800 mg) BID | Low resistance rates in community, reasonable option for empiric therapy. |
| Rifampin | <1% | 600 mg QD | Should not be used alone; potential for significant drug interactions |
| Fusidic acid | <5% | 500 mg TID | Should not be used alone; limited experience in children |
| Linezolid | < 1 % | 600 mg PO BID | Expensive. |
Rates shown are for tetracycline and are likely to be < 5% or less for doxycycline and minocycline
The long-acting tetracyclines, doxycycline and minocycline, are commonly used in the treatment of CA-MRSA disease. They have enhanced anti-staphylococcal activity compared to tetracycline,121 but activity against group A Streptococcus is not well defined. Doxycycline and minocycline appear to be effective in the treatment of MRSA SSTI.122,123 Tetracyclines are not recommended for pregnant women or children under the age of eight years.
TMP-SMX is active against 90–100% of CA-MRSA isolates.10,59,124 Efficacy data of TMP-SMX for the treatment of MRSA infections are limited,125–128 but suggest that TMP-SMX is an appropriate for oral therapy of suspected CA-MRSA SSTI. Activity of TMP-SMX against group A Streptococcus is unknown and if infection with this organism is suspected, some other agent, such as clindamycin or a beta-lactam, should be used instead. TMP-SMX in not recommended for treatment of women during the third trimester of pregnancy.
Rifampin or fusidic acid may be considered for use as an adjunctive agent in combination with another active agent or used in combination with one another;114,115 neither agent should be used alone because resistance is likely to emerge during single drug therapy.129
Linezolid, an oxazolidinone, is FDA approved for the treatment of complicated SSTI and MRSA pneumonia. Clinically it is comparable to vancomycin in efficacy,130–133 and resistance so far is rare. Because linezolid is expensive and has the potential for significant toxicity, including myelosuppresion, peripheral neuropathy, optic neuritis, and lactic acidosis,134–136 it should be reserved for treatment of more serious infections when other oral agents are not an option.
Parenteral therapy
Vancomycin, for the moment, remains the first-line intravenous agent for severe MRSA infections, both CA-MRSA and HA-MRSA. Daptomycin, tigecycline, and linezolid are FDA approved for the treatment of MRSA infections, but to date no clinical trial has demonstrated superiority of any of these agents over vancomycin. Although vancomycin remains a first-line agent for treatment of MRSA infections, it is far from ideal therapy. Persistent or recurrent bacteremia during therapy,137,138 relatively high treatment failure rates,139 nephrotoxicity associated with higher doses needed to attain the recommended trough concentrations of 15–20 μg/ml,140 and emergence of non-susceptible strains141,142 are all too commonly encountered. Unfortunately, in the absence of demonstrated superiority of any single agent or combination of agents over vancomycin alone, which alternative agent(s) should be used to treat severe MRSA infections, or used for those not responding to vancomycin, is completely unknown.
Experimental agents and adjunctive therapy
Investigational agents that are under development for treatment of MRSA infections include glycopeptides derivatives, such as telavancin, dalbavancin, and oritavancin,143–145 and anti-MRSA beta-lactams, such as ceftaroline and ceftobiprole.143–145 Telavancin, dalbavancin, and oritavancin are vancomycin derivatives; all exhibit rapid, concentration-dependent killing in vitro and have good activity in vivo in animal infection models. Randomized clinical trials of these new agents for treatment of skin and soft tissue infections indicate that they are comparable, but not superior to standard therapy.
Anti-MRSA beta-lactams are active because they have high binding affinity for PBP2a. Two cephalosporin compounds, ceftobiprole and ceftaroline, are highly active against MRSA in rabbit endocarditis models146,147 and have been shown to be as effective as vancomycin for treatment of MRSA skin and soft infections.148,149 Ceftobiprole has been approved for clinical use in Canada and Switzerland. Further studies are needed to define the role of anti-MRSA beta-lactams in the therapy of MRSA infections.
The glycopeptide-derivatives and beta-lactams can only be administered parenterally and thus there is still a need for oral agents that are active against MRSA. Oxazolidinone compounds, which have good activity against MRSA and are orally bioavailable are in early stages of development.150
The facility with which S. aureus can acquire or develop resistant to antimicrobials has prompted an interest in non-traditional approaches and agents for treatment and prevention of MRSA. Among these are lysostaphin151, antimicrobial peptides,152 natural products (e.g., tea tree oil),153 and active and passive immunization against S. aureus.97,154,155 Assuming that a development program is even successful, these agents are years away from the clinic. Barriers to development are expensive cost of goods, hypersensitivity that can occur upon repeated administration of protein products, unfavorable pharmacological properties (e.g., short half-life, toxicity), and a notable lack of success in previous efforts at active and passive immunization. With regard to the latter point, there remains a significant need for a vaccine that protects against or controls S. aureus infection and this is an active area of research. A vaccine approach directed to prevent or control all S. aureus infections is perhaps unrealistic, since all humans have naturally occurring anti-staphylococcal antibodies and are protected already. This natural immunity, coupled with the ability of S. aureus to survive after uptake by phagocytosis leukocytes,156 is presumably one of the key reasons a vaccine approach directed at enhancing opsonophagocytosis has been largely unsuccessful. Factors such as environment, host innate immune status and genetics likely play a significant role in determining susceptibility to severe infection and research is ongoing in these areas. For the foreseeable future physicians will have to rely principally on currently available agents, which must be used judiciously and wisely in order to avoid their further loss from the antimicrobial armamentarium.
Concluding remarks and future outlook
S. aureus has been a cause of human disease throughout recorded history. Alexander Fleming’s discovery of penicillin in 1928 and the ensuing modern antibiotic era was perhaps largely expected to eliminate S. aureus (and other bacterial pathogens) as a leading cause of human infections. However, S. aureus has extraordinary capacity to develop resistance to antibiotics and these agents have been the impetus for waves of antibiotic resistance in the pathogen over the past 60 years.5 This problem is perplexing, because antibiotics are absolutely critical for treatment of many types of bacterial infections. One alternative approach is to develop a better understanding of the factors involved in human disease, both host- and pathogen-related, and target those. Undetermined host genetic factors are likely to be important–if not the major–determinants of susceptibility to severe staphylococcal infections. Such factors must be considered in order to have a full understanding the success of CA-MRSA. There are also bacterial factors, some related directly to virulence, that distinguish CA-MRSA strains from hospital relatives in the context of promoting pathogenesis, and such factors should be considered as targets for new therapeutics. In addition, new technologies such as high-throughput whole genome sequencing make it possible to fully understanding the evolution of strains that cause epidemics. In aggregate, this information will ultimately be important in our efforts to limit the impact of antibiotic resistant S. aureus infections in the community.
Acknowledgments
This article was supported by US Public Health Service Grant AI070289 (H.F.C.) and the Intramural Research Program of the National Institutes of Health, National Institutes of Allergy and Infectious Diseases (F.R.D. and M.O.). The authors thank Dr. Scott Kobayashi for critical review of the manuscript.
Footnotes
Author contributions
All authors participated in conception and writing of the article. Authors were assigned specific sections to write and these sections were then edited by all authors. As corresponding author, F.R.D. had the final responsibility for the decision to submit the article for publication.
Search strategy and selection criteria
We searched PubMed using the terms “CA-MRSA”, “Europe and CA-MRSA”, “Panton-Valentine Leukocidin”, and “USA300”. We selected references primarily from the past 5 years, including cross-references, although landmark and/or highly regarded references were also included. Review articles were cited where appropriate to more detail on a specific topic. We also included references based upon comments from peer reviewers.
Conflict of interest statement
H.F.C has been a consultant for Johnson and Johnson and has been a consultant and has received research grant support from Pfizer. No conflicts of interest exist for the other authors.
References
- 1.Diekema DJ, Pfaller MA, Schmitz FJ, et al. Survey of infections due to Staphylococcus species: frequency of occurrence and antimicrobial susceptibility of isolates collected in the United States, Canada, Latin America, Europe, and the Western Pacific region for the SENTRY Antimicrobial Surveillance Program, 1997–1999. Clin Infect Dis. 2001;32(Suppl 2):S114–S132. doi: 10.1086/320184. [DOI] [PubMed] [Google Scholar]
- 2.Gorwitz RJ, Kruszon-Moran D, McAllister SK, et al. Changes in the prevalence of nasal colonization with Staphylococcus aureus in the United States, 2001–2004. J Infect Dis. 2008;197:1226–34. doi: 10.1086/533494. [DOI] [PubMed] [Google Scholar]
- 3.von Eiff C, Becker K, Machka K, Stammer H, Peters G. Nasal carriage as a source of Staphylococcus aureus bacteremia. Study Group. N Engl J Med. 2001;344:11–16. doi: 10.1056/NEJM200101043440102. [DOI] [PubMed] [Google Scholar]
- 4.DeLeo FR, Chambers HF. Reemergence of antibiotic-resistant Staphylococcus aureus in the genomics era. J Clin Invest. 2009;119:2464–74. doi: 10.1172/JCI38226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chambers HF, DeLeo FR. Waves of resistance: Staphylococcus aureus in the antibiotic era. Nat Rev Microbiol. 2009;7:2464–74. doi: 10.1038/nrmicro2200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kirby WM. Extraction of a highly potent penicillin inactivator from penicillin resistant staphylococci. Science. 1944;99:452–53. doi: 10.1126/science.99.2579.452. [DOI] [PubMed] [Google Scholar]
- 7.Roundtree PM, Freeman BM. Infections caused by a particular phage type of Staphylococcus aureus. Med J Aust. 1956;42:157–61. [PubMed] [Google Scholar]
- 8.Jevons MP. “Celbenin”-resistant staphylococci. Br Med J. 1961;1:124–25. [Google Scholar]
- 9.Klevens RM, Morrison MA, Nadle J, et al. Invasive methicillin-resistant Staphylococcus aureus infections in the United States. JAMA. 2007;298:1763–71. doi: 10.1001/jama.298.15.1763. [DOI] [PubMed] [Google Scholar]
- 10.Herold BC, Immergluck LC, Maranan MC, et al. Community-acquired methicillin-resistant Staphylococcus aureus in children with no identified predisposing risk. JAMA. 1998;279:593–98. doi: 10.1001/jama.279.8.593. [DOI] [PubMed] [Google Scholar]
- 11.Vandenesch F, Naimi T, Enright MC, et al. Community-acquired methicillin-resistant Staphylococcus aureus carrying Panton-Valentine leukocidin genes: worldwide emergence. Emerg Infect Dis. 2003;9:978–84. doi: 10.3201/eid0908.030089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tristan A, Bes M, Meugnier H, et al. Global distribution of Panton-Valentine leukocidin--positive methicillin-resistant Staphylococcus aureus, 2006. Emerg Infect Dis. 2007;13:594–600. doi: 10.3201/eid1304.061316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Saravolatz LD, Pohlod DJ, Arking LM. Community-acquired methicillin-resistant Staphylococcus aureus infections: a new source for nosocomial outbreaks. Ann Intern Med. 1982;97:325–29. doi: 10.7326/0003-4819-97-3-325. [DOI] [PubMed] [Google Scholar]
- 14.Udo EE, Pearman JW, Grubb WB. Genetic analysis of community isolates of methicillin-resistant Staphylococcus aureus in Western Australia. J Hosp Infect. 1993;25:97–108. doi: 10.1016/0195-6701(93)90100-e. [DOI] [PubMed] [Google Scholar]
- 15.Coombs GW, Pearson JC, O’Brien FG, et al. Methicillin-resistant Staphylococcus aureus clones, Western Australia. Emerg Infect Dis. 2006;12:241–47. doi: 10.3201/eid1202.050454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.From the Centers for Disease Control and Prevention. Four pediatric deaths from community-acquired methicillin-resistant Staphylococcus aureus--Minnesota and North Dakota, 1997–1999. JAMA. 1999;282:1123–25. [PubMed] [Google Scholar]
- 17.Baba T, Takeuchi F, Kuroda M, et al. Genome and virulence determinants of high virulence community-acquired MRSA. Lancet. 2002;359:1819–27. doi: 10.1016/s0140-6736(02)08713-5. [DOI] [PubMed] [Google Scholar]
- 18.Ma XX, Ito T, Tiensasitorn C, et al. Novel type of staphylococcal cassette chromosome mec identified in community-acquired methicillin-resistant Staphylococcus aureus strains. Antimicrob Agents Chemother. 2002;46:1147–52. doi: 10.1128/AAC.46.4.1147-1152.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rossney AS, Shore AC, Morgan PM, et al. The emergence and importation of diverse genotypes of methicillin-resistant Staphylococcus aureus (MRSA) harboring the Panton-Valentine leukocidin gene (pvl) reveal that pvl is a poor marker for community-acquired MRSA strains in Ireland. J Clin Microbiol. 2007;45:2554–63. doi: 10.1128/JCM.00245-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.O’Brien FG, Lim TT, Chong FN, et al. Diversity among community isolates of methicillin-resistant Staphylococcus aureus in Australia. J Clin Microbiol. 2004;42:3185–90. doi: 10.1128/JCM.42.7.3185-3190.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim ES, Song JS, Lee HJ, et al. A survey of community-associated methicillin-resistant Staphylococcus aureus in Korea. J Antimicrob Chemother. 2007;60:1108–14. doi: 10.1093/jac/dkm309. [DOI] [PubMed] [Google Scholar]
- 22.Zhang K, McClure JA, Elsayed S, Tan J, Conly JM. Coexistence of Panton-Valentine leukocidin-positive and -negative community-associated methicillin-resistant Staphylococcus aureus USA400 sibling strains in a large Canadian health-care region. J Infect Dis. 2008;197:195–204. doi: 10.1086/523763. [DOI] [PubMed] [Google Scholar]
- 23.McDougal LK, Steward CD, Killgore GE, et al. Pulsed-field gel electrophoresis typing of oxacillin-resistant Staphylococcus aureus isolates from the United States: establishing a national database. J Clin Microbiol. 2003;41:5113–20. doi: 10.1128/JCM.41.11.5113-5120.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Enright MC, Day NP, Davies CE, Peacock SJ, Spratt BG. Multilocus sequence typing for characterization of methicillin-resistant and methicillin-susceptible clones of Staphylococcus aureus. J Clin Microbiol. 2000;38:1008–15. doi: 10.1128/jcm.38.3.1008-1015.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stemper ME, Brady JM, Qutaishat SS, et al. Shift in Staphylococcus aureus clone linked to an infected tattoo. Emerg Infect Dis. 2006;12:1444–46. doi: 10.3201/eid1209.051634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Aiello AE, Lowy FD, Wright LN, Larson EL. Meticillin-resistant Staphylococcus aureus among US prisoners and military personnel: review and recommendations for future studies. Lancet Infect Dis. 2006;6:335–41. doi: 10.1016/S1473-3099(06)70491-1. [DOI] [PubMed] [Google Scholar]
- 27.Adcock PM, Pastor P, Medley F, Patterson JE, Murphy TV. Methicillin-resistant Staphylococcus aureus in two child care centers. J Infect Dis. 1998;178:577–80. doi: 10.1086/517478. [DOI] [PubMed] [Google Scholar]
- 28.Diep BA, Chambers HF, Graber CJ, et al. Emergence of multidrug-resistant, community-associated, methicillin-resistant Staphylococcus aureus clone USA300 in men who have sex with men. Ann Intern Med. 2008;148:249–57. doi: 10.7326/0003-4819-148-4-200802190-00204. [DOI] [PubMed] [Google Scholar]
- 29.Begier EM, Frenette K, Barrett NL, et al. A high-morbidity outbreak of methicillin-resistant Staphylococcus aureus among players on a college football team, facilitated by cosmetic body shaving and turf burns. Clin Infect Dis. 2004;39:1446–53. doi: 10.1086/425313. [DOI] [PubMed] [Google Scholar]
- 30.Baggett HC, Hennessy TW, Rudolph K, et al. Community-onset methicillin-resistant Staphylococcus aureus associated with antibiotic use and the cytotoxin Panton-Valentine leukocidin during a furunculosis outbreak in rural Alaska. J Infect Dis. 2004;189:1565–73. doi: 10.1086/383247. [DOI] [PubMed] [Google Scholar]
- 31.Community-associated methicillin-resistant Staphylococcus aureus infections in Pacific Islanders--Hawaii, 2001–2003. Morb Mortal Wkly Rep. 2004;53:767–70. [PubMed] [Google Scholar]
- 32.Miller LG, Diep BA. Clinical practice: colonization, fomites, and virulence: rethinking the pathogenesis of community-associated methicillin-resistant Staphylococcus aureus infection. Clin Infect Dis. 2008;46:752–60. doi: 10.1086/526773. [DOI] [PubMed] [Google Scholar]
- 33.Kennedy AD, Otto M, Braughton KR, et al. Epidemic community-associated methicillin-resistant Staphylococcus aureus: recent clonal expansion and diversification. Proc Natl Acad Sci U S A. 2008;105:1327–32. doi: 10.1073/pnas.0710217105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moran GJ, Krishnadasan A, Gorwitz RJ, et al. Methicillin-resistant S. aureus infections among patients in the emergency department. N Engl J Med. 2006;355:666–74. doi: 10.1056/NEJMoa055356. [DOI] [PubMed] [Google Scholar]
- 35.Robert J, Etienne J, Bertrand X. Methicillin-resistant Staphylococcus aureus producing Panton-Valentine leukocidin in a retrospective case series from 12 French hospital laboratories, 2000–2003. Clin Microbiol Infect. 2005;11:585–87. doi: 10.1111/j.1469-0691.2005.01173.x. [DOI] [PubMed] [Google Scholar]
- 36.Del Giudice P, Blanc V, Durupt F, et al. Emergence of two populations of methicillin-resistant Staphylococcus aureus with distinct epidemiological, clinical and biological features, isolated from patients with community-acquired skin infections. Br J Dermatol. 2006;154:118–24. doi: 10.1111/j.1365-2133.2005.06910.x. [DOI] [PubMed] [Google Scholar]
- 37.Shopsin B, Gomez M, Montgomery SO, et al. Evaluation of protein A gene polymorphic region DNA sequencing for typing of Staphylococcus aureus strains. J Clin Microbiol. 1999;37:3556–63. doi: 10.1128/jcm.37.11.3556-3563.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Enright MC, Robinson DA, Randle G, et al. The evolutionary history of methicillin-resistant Staphylococcus aureus (MRSA) Proc Natl Acad Sci U S A. 2002;99:7687–92. doi: 10.1073/pnas.122108599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Daum RS, Ito T, Hiramatsu K, et al. A novel methicillin-resistance cassette in community-acquired methicillin-resistant Staphylococcus aureus isolates of diverse genetic backgrounds. J Infect Dis. 2002;186:1344–47. doi: 10.1086/344326. [DOI] [PubMed] [Google Scholar]
- 40.Lee SM, Ender M, Adhikari R, et al. Fitness cost of staphylococcal cassette chromosome mec in methicillin-resistant Staphylococcus aureus by way of continuous culture. Antimicrob Agents Chemother. 2007;51:1497–99. doi: 10.1128/AAC.01239-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Okuma K, Iwakawa K, Turnidge JD, et al. Dissemination of new methicillin-resistant Staphylococcus aureus clones in the community. J Clin Microbiol. 2002;40:4289–94. doi: 10.1128/JCM.40.11.4289-4294.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Diep BA, Stone GG, Basuino L, et al. The arginine catabolic mobile element and staphylococcal chromosomal cassette mec linkage: convergence of virulence and resistance in the USA300 clone of methicillin-resistant Staphylococcus aureus. J Infect Dis. 2008;197:1523–30. doi: 10.1086/587907. [DOI] [PubMed] [Google Scholar]
- 43.Oliveira DC, Milheirico C, de Lencastre H. Redefining a structural variant of staphylococcal cassette chromosome mec, SCCmec type VI. Antimicrob Agents Chemother. 2006;50:3457–59. doi: 10.1128/AAC.00629-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Higuchi W, Takano T, Teng LJ, Yamamoto T. Structure and specific detection of staphylococcal cassette chromosome mec type VII. Biochem Biophys Res Commun. 2008;377:752–56. doi: 10.1016/j.bbrc.2008.10.009. [DOI] [PubMed] [Google Scholar]
- 45.Zhang K, McClure JA, Elsayed S, Conly JM. Novel staphylococcal cassette chromosome mec type, tentatively designated type VIII, harboring class A mec and type 4 ccr gene complexes in a Canadian epidemic strain of methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother. 2009;53:531–40. doi: 10.1128/AAC.01118-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Graham PL, III, Lin SX, Larson EL. A U.S. population-based survey of Staphylococcus aureus colonization. Ann Intern Med. 2006;144:318–25. doi: 10.7326/0003-4819-144-5-200603070-00006. [DOI] [PubMed] [Google Scholar]
- 47.Kluytmans J, van Belkum A, Verbrugh H. Nasal carriage of Staphylococcus aureus: epidemiology, underlying mechanisms, and associated risks. Clin Microbiol Rev. 1997;10:505–20. doi: 10.1128/cmr.10.3.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shopsin B, Mathema B, Martinez J, et al. Prevalence of methicillin-resistant and methicillin-susceptible Staphylococcus aureus in the community. J Infect Dis. 2000;182:359–62. doi: 10.1086/315695. [DOI] [PubMed] [Google Scholar]
- 49.Suggs AH, Maranan MC, Boyle-Vavra S, Daum RS. Methicillin-resistant and borderline methicillin-resistant asymptomatic Staphylococcus aureus colonization in children without identifiable risk factors. Pediatr Infect Dis J. 1999;18:410–414. doi: 10.1097/00006454-199905000-00003. [DOI] [PubMed] [Google Scholar]
- 50.Creech CB, Kernodle DS, Alsentzer A, Wilson C, Edwards KM. Increasing rates of nasal carriage of methicillin-resistant Staphylococcus aureus in healthy children. Pediatr Infect Dis J. 2005;24:617–21. doi: 10.1097/01.inf.0000168746.62226.a4. [DOI] [PubMed] [Google Scholar]
- 51.Crum NF, Lee RU, Thornton SA, et al. Fifteen-year study of the changing epidemiology of methicillin-resistant Staphylococcus aureus. Am J Med. 2006;119:943–51. doi: 10.1016/j.amjmed.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 52.Methicillin-resistant Staphylococcus aureus infections among competitive sports participants--Colorado, Indiana Pennsylvania, and Los Angeles County, 2000–2003. Morb Mortal Wkly Rep. 2003;52:793–95. [PubMed] [Google Scholar]
- 53.Kazakova SV, Hageman JC, Matava M, et al. A clone of methicillin-resistant Staphylococcus aureus among professional football players. N Engl J Med. 2005;352:468–75. doi: 10.1056/NEJMoa042859. [DOI] [PubMed] [Google Scholar]
- 54.Nguyen DM, Mascola L, Brancoft E. Recurring methicillin-resistant Staphylococcus aureus infections in a football team. Emerg Infect Dis. 2005;11:526–32. doi: 10.3201/eid1104.041094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kaplan SL, Hulten KG, Gonzalez BE, et al. Three-year surveillance of community-acquired Staphylococcus aureus infections in children. Clin Infect Dis. 2005;40:1785–91. doi: 10.1086/430312. [DOI] [PubMed] [Google Scholar]
- 56.Laupland KB, Ross T, Gregson DB. Staphylococcus aureus bloodstream infections: risk factors, outcomes, and the influence of methicillin resistance in Calgary, Canada, 2000–2006. J Infect Dis. 2008;198:336–43. doi: 10.1086/589717. [DOI] [PubMed] [Google Scholar]
- 57.Mathews WC, Caperna JC, Barber RE, et al. Incidence of and risk factors for clinically significant methicillin-resistant Staphylococcus aureus infection in a cohort of HIV-infected adults. J Acquir Immune Defic Syndr. 2005;40:155–60. doi: 10.1097/01.qai.0000179464.40948.b9. [DOI] [PubMed] [Google Scholar]
- 58.Purcell K, Fergie J. Epidemic of community-acquired methicillin-resistant Staphylococcus aureus infections: a 14-year study at Driscoll Children’s Hospital. Arch Pediatr Adolesc Med. 2005;159:980–985. doi: 10.1001/archpedi.159.10.980. [DOI] [PubMed] [Google Scholar]
- 59.Fridkin SK, Hageman JC, Morrison M, et al. Methicillin-resistant Staphylococcus aureus disease in three communities. N Engl J Med. 2005;352:1436–44. doi: 10.1056/NEJMoa043252. [DOI] [PubMed] [Google Scholar]
- 60.Adem PV, Montgomery CP, Husain AN, et al. Staphylococcus aureus sepsis and the Waterhouse-Friderichsen syndrome in children. N Engl J Med. 2005;353:1245–51. doi: 10.1056/NEJMoa044194. [DOI] [PubMed] [Google Scholar]
- 61.Kravitz GR, Dries DJ, Peterson ML, Schlievert PM. Purpura fulminans due to Staphylococcus aureus. Clin Infect Dis. 2005;40:941–47. doi: 10.1086/428573. [DOI] [PubMed] [Google Scholar]
- 62.Pannaraj PS, Hulten KG, Gonzalez BE, Mason EO, Jr, Kaplan SL. Infective pyomyositis and myositis in children in the era of community-acquired, methicillin-resistant Staphylococcus aureus infection. Clin Infect Dis. 2006;43:953–60. doi: 10.1086/507637. [DOI] [PubMed] [Google Scholar]
- 63.Miller LG, Perdreau-Remington F, Rieg G, et al. Necrotizing fasciitis caused by community-associated methicillin-resistant Staphylococcus aureus in Los Angeles. N Engl J Med. 2005;352:1445–53. doi: 10.1056/NEJMoa042683. [DOI] [PubMed] [Google Scholar]
- 64.Arnold SR, Elias D, Buckingham SC, et al. Changing patterns of acute hematogenous osteomyelitis and septic arthritis: emergence of community-associated methicillin-resistant Staphylococcus aureus. J Pediatr Orthop. 2006;26:703–8. doi: 10.1097/01.bpo.0000242431.91489.b4. [DOI] [PubMed] [Google Scholar]
- 65.Buck JM, Como-Sabetti K, Harriman KH, et al. Community-associated methicillin-resistant Staphylococcus aureus, Minnesota, 2000–2003. Emerg Infect Dis. 2005;11:1532–38. doi: 10.3201/eid1110.050141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Francis JS, Doherty MC, Lopatin U, et al. Severe community-onset pneumonia in healthy adults caused by methicillin-resistant Staphylococcus aureus carrying the Panton-Valentine leukocidin genes. Clin Infect Dis. 2005;40:100–107. doi: 10.1086/427148. [DOI] [PubMed] [Google Scholar]
- 67.Gonzalez BE, Hulten KG, Dishop MK, et al. Pulmonary manifestations in children with invasive community-acquired Staphylococcus aureus infection. Clin Infect Dis. 2005;41:583–90. doi: 10.1086/432475. [DOI] [PubMed] [Google Scholar]
- 68.Hageman JC, Uyeki TM, Francis JS, et al. Severe community-acquired pneumonia due to Staphylococcus aureus, 2003–04 influenza season. Emerg Infect Dis. 2006;12:894–99. doi: 10.3201/eid1206.051141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Naimi TS, LeDell KH, Como-Sabetti K, et al. Comparison of community- and health care-associated methicillin-resistant Staphylococcus aureus infection. JAMA. 2003;290:2976–84. doi: 10.1001/jama.290.22.2976. [DOI] [PubMed] [Google Scholar]
- 70.DeLeo FR, Diep BA, Otto M. Host defense and pathogenesis in Staphylococcus aureus infections. Infect Dis Clin North Am. 2009;23:17–34. doi: 10.1016/j.idc.2008.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Rooijakkers SH, Van Strijp JA. Bacterial complement evasion. Mol Immunol. 2007;44:23–32. doi: 10.1016/j.molimm.2006.06.011. [DOI] [PubMed] [Google Scholar]
- 72.Kraus D, Peschel A. Staphylococcus aureus evasion of innate antimicrobial defense. Future Microbiol. 2008;3:437–51. doi: 10.2217/17460913.3.4.437. [DOI] [PubMed] [Google Scholar]
- 73.Foster TJ. Immune evasion by staphylococci. Nat Rev Microbiol. 2005;3:948–58. doi: 10.1038/nrmicro1289. [DOI] [PubMed] [Google Scholar]
- 74.Li M, Diep BA, Villaruz AE, et al. Evolution of virulence in epidemic community-associated methicillin-resistant Staphylococcus aureus. Proc Natl Acad Sci U S A. 2009;106:5883–88. doi: 10.1073/pnas.0900743106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Voyich JM, Braughton KR, Sturdevant DE, et al. Insights into mechanisms used by Staphylococcus aureus to avoid destruction by human neutrophils. J Immunol. 2005;175:3907–19. doi: 10.4049/jimmunol.175.6.3907. [DOI] [PubMed] [Google Scholar]
- 76.Szmigielski S, Prevost G, Monteil H, Colin DA, Jeljaszewicz J. Leukocidal toxins of staphylococci. Zentralbl Bakteriol. 1999;289:185–201. doi: 10.1016/s0934-8840(99)80105-4. [DOI] [PubMed] [Google Scholar]
- 77.Woodin AM. Staphylococcal leukocidin. In: Montje t, Kadis S, Ajl S., editors. Microbial Toxins. New York and London: Academic Press, Inc; 1970. pp. 327–55. [Google Scholar]
- 78.Tseng CW, Kyme P, Low J, et al. Staphylococcus aureus Panton-Valentine leukocidin contributes to inflammation and muscle tissue injury. PLoS ONE. 2009;4:e6387. doi: 10.1371/journal.pone.0006387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Diep BA, Palazzolo-Ballance AM, Tattevin P, et al. Contribution of Panton-Valentine leukocidin in community-associated methicillin-resistant Staphylococcus aureus pathogenesis. PLoS ONE. 2008;3:e3198. doi: 10.1371/journal.pone.0003198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Montgomery CP, Daum RS. Transcription of inflammatory genes in the lung after infection with community-associated methicillin-resistant Staphylococcus aureus: A role for Panton-Valentine Leukocidin? Infect Immun. 2009;77:2159–67. doi: 10.1128/IAI.00021-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Voyich JM, Otto M, Mathema B, et al. Is Panton-Valentine leukocidin the major virulence determinant in community-associated methicillin-resistant Staphylococcus aureus disease? J Infect Dis. 2006;194:1761–70. doi: 10.1086/509506. [DOI] [PubMed] [Google Scholar]
- 82.Bubeck Wardenburg J, Bae T, Otto M, DeLeo FR, Schneewind O. Poring over pores: alpha-hemolysin and Panton-Valentine leukocidin in Staphylococcus aureus pneumonia. Nat Med. 2007;13:1405–6. doi: 10.1038/nm1207-1405. [DOI] [PubMed] [Google Scholar]
- 83.Bubeck Wardenburg J, Palazzolo-Ballance AM, Otto M, Schneewind O, DeLeo FR. Panton-Valentine leukocidin is not a virulence determinant in murine models of community-associated methicillin-resistant Staphylococcus aureus disease. J Infect Dis. 2008;198:1166–70. doi: 10.1086/592053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Brown EL, Dumitrescu O, Thomas D, et al. The Panton-Valentine leukocidin vaccine protects mice against lung and skin infections caused by Staphylococcus aureus USA300. Clin Microbiol Infect. 2009;15:156–64. doi: 10.1111/j.1469-0691.2008.02648.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Labandeira-Rey M, Couzon F, Boisset S, et al. Staphylococcus aureus Panton-Valentine leukocidin causes necrotizing pneumonia. Science. 2007;315:1130–1133. doi: 10.1126/science.1137165. [DOI] [PubMed] [Google Scholar]
- 86.Villaruz AE, Bubeck Wardenburg J, Khan BA, et al. A point mutation in the agr locus rather than expression of the Panton-Valentine leukocidin caused previously reported phenotypes in Staphylococcus aureus pneumonia and gene regulation. J Infect Dis. 2009;200:724–34. doi: 10.1086/604728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hongo I, Baba T, Oishi K, et al. Phenol-soluble modulin alpha 3 enhances the human neutrophil lysis mediated by Panton-Valentine leukocidin. J Infect Dis. 2009;200:715–23. doi: 10.1086/605332. [DOI] [PubMed] [Google Scholar]
- 88.Grojec PL, Jeljaszewicz J. Effect of staphylococcal leukocidin on mouse leukocyte system. Zentralbl Bakteriol Mikrobiol Hyg [A] 1981;250:446–55. [PubMed] [Google Scholar]
- 89.Szmigielski S, Jeljaszewicz J, Wilczynski J, Korbecki M. Reaction of rabbit leucocytes to staphylococcal (Panton-Valentine) leucocidin in vivo. J Pathol Bacteriol. 1966;91:599–604. doi: 10.1002/path.1700910237. [DOI] [PubMed] [Google Scholar]
- 90.Cremieux AC, Dumitrescu O, Lina G, et al. Panton-valentine leukocidin enhances the severity of community-associated methicillin-resistant Staphylococcus aureus rabbit osteomyelitis. PLoS ONE. 2009;4:e7204. doi: 10.1371/journal.pone.0007204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Diep BA, Chan L, Tattevin P, et al. Panton-Valentine leukocidin determines the virulence of USA300 methicillin-resistant Staphylococcus aureus in a rabbit model of fulminant necrotizing, hemorrhagic pneumonia. Abstract No. B-1087, 49th ICAAC; San Francisco, CA. Sept 12–15, 2009. [Google Scholar]
- 92.Gillet Y, Issartel B, Vanhems P, et al. Association between Staphylococcus aureus strains carrying gene for Panton-Valentine leukocidin and highly lethal necrotising pneumonia in young immunocompetent patients. Lancet. 2002;359:753–59. doi: 10.1016/S0140-6736(02)07877-7. [DOI] [PubMed] [Google Scholar]
- 93.Wang R, Braughton KR, Kretschmer D, et al. Identification of novel cytolytic peptides as key virulence determinants for community-associated MRSA. Nat Med. 2007;13:1510–1514. doi: 10.1038/nm1656. [DOI] [PubMed] [Google Scholar]
- 94.Bhakdi S, Tranum-Jensen J. Alpha-toxin of Staphylococcus aureus. Microbiol Rev. 1991;55:733–51. doi: 10.1128/mr.55.4.733-751.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Valeva A, Walev I, Pinkernell M, et al. Transmembrane beta-barrel of staphylococcal alpha-toxin forms in sensitive but not in resistant cells. Proc Natl Acad Sci U S A. 1997;94:11607–11. doi: 10.1073/pnas.94.21.11607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Bartlett AH, Foster TJ, Hayashida A, Park PW. Alpha-toxin facilitates the generation of CXC chemokine gradients and stimulates neutrophil homing in Staphylococcus aureus pneumonia. J Infect Dis. 2008;198:1529–35. doi: 10.1086/592758. [DOI] [PubMed] [Google Scholar]
- 97.Bubeck Wardenburg J, Schneewind O. Vaccine protection against Staphylococcus aureus pneumonia. J Exp Med. 2008;205:287–94. doi: 10.1084/jem.20072208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Feil EJ, Enright MC. Analyses of clonality and the evolution of bacterial pathogens. Curr Opin Microbiol. 2004;7:308–13. doi: 10.1016/j.mib.2004.04.002. [DOI] [PubMed] [Google Scholar]
- 99.Montgomery CP, Boyle-Vavra S, Adem PV, et al. Comparison of virulence in community-associated methicillin-resistant Staphylococcus aureus pulsotypes USA300 and USA400 in a rat model of pneumonia. J Infect Dis. 2008;198:561–70. doi: 10.1086/590157. [DOI] [PubMed] [Google Scholar]
- 100.Diep BA, Gill SR, Chang RF, et al. Complete genome sequence of USA300, an epidemic clone of community-acquired meticillin-resistant Staphylococcus aureus. Lancet. 2006;367:731–39. doi: 10.1016/S0140-6736(06)68231-7. [DOI] [PubMed] [Google Scholar]
- 101.Larsen AR, Stegger M, Bocher S, et al. Emergence and characterization of community-associated methicillin-resistant Staphyloccocus aureus infections in Denmark, 1999 to 2006. J Clin Microbiol. 2009;47:73–78. doi: 10.1128/JCM.01557-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Boyle-Vavra S, Ereshefsky B, Wang CC, Daum RS. Successful multiresistant community-associated methicillin-resistant Staphylococcus aureus lineage from Taipei, Taiwan, that carries either the novel Staphylococcal chromosome cassette mec (SCCmec) type VT or SCCmec type IV. J Clin Microbiol. 2005;43:4719–30. doi: 10.1128/JCM.43.9.4719-4730.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Alksne LE, Projan SJ. Bacterial virulence as a target for antimicrobial chemotherapy. Curr Opin Biotechnol. 2000;11:625–36. doi: 10.1016/s0958-1669(00)00155-5. [DOI] [PubMed] [Google Scholar]
- 104.Otto M. Looking toward basic science for potential drug discovery targets against community-associated MRSA. Med Res Rev. 2009 doi: 10.1002/med.20160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Shore AC, Rossney AS, O’Connell B, et al. Detection of SCCmec-associated DNA Segments in multiresistant methicillin-susceptible Staphylococcus aureus (MSSA) and identification of Staphylococcus epidermidis ccrAB4 in both methicillin-resistant S. aureus (MRSA) and MSSA. Antimicrob Agents Chemother. 2008;52:4407–19. doi: 10.1128/AAC.00447-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.van Hal SJ, Stark D, Lockwood B, Marriott D, Harkness J. Methicillin-resistant Staphylococcus aureus (MRSA) detection: comparison of two molecular methods (IDI-MRSA PCR assay and GenoType MRSA Direct PCR assay) with three selective MRSA agars (MRSA ID, MRSASelect, and CHROMagar MRSA) for use with infection-control swabs. J Clin Microbiol. 2007;45:2486–90. doi: 10.1128/JCM.00139-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Zschock M, Nesseler A, Sudarwanto I. Evaluation of six commercial identification kits for the identification of Staphylococcus aureus isolated from bovine mastitis. J Appl Microbiol. 2005;98:450–455. doi: 10.1111/j.1365-2672.2004.02470.x. [DOI] [PubMed] [Google Scholar]
- 108.Lee MC, Rios AM, Aten MF, et al. Management and outcome of children with skin and soft tissue abscesses caused by community-acquired methicillin-resistant Staphylococcus aureus. Pediatr Infect Dis J. 2004;23:123–27. doi: 10.1097/01.inf.0000109288.06912.21. [DOI] [PubMed] [Google Scholar]
- 109.Llera JL, Levy RC. Treatment of cutaneous abscess: a double-blind clinical study. Ann Emerg Med. 1985;14:15–19. doi: 10.1016/s0196-0644(85)80727-7. [DOI] [PubMed] [Google Scholar]
- 110.Rajendran PM, Young D, Maurer T, et al. Randomized, double-blind, placebo-controlled trial of cephalexin for treatment of uncomplicated skin abscesses in a population at risk for community-acquired methicillin-resistant Staphylococcus aureus infection. Antimicrob Agents Chemother. 2007;51:4044–48. doi: 10.1128/AAC.00377-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Stevens DL, Bisno AL, Chambers HF, et al. Practice guidelines for the diagnosis and management of skin and soft-tissue infections. Clin Infect Dis. 2005;41:1373–406. doi: 10.1086/497143. [DOI] [PubMed] [Google Scholar]
- 112.Duong M, Markwell S, Peter J, Barenkamp S. Randomized, controlled trial of antibiotics in the management of community-acquired skin abscesses in the pediatric patient. Ann Emerg Med. 2009 doi: 10.1016/j.annemergmed.2009.03.014. [DOI] [PubMed] [Google Scholar]
- 113.Stevens DL, Bisno AL, Chambers HF, et al. Practice guidelines for the diagnosis and management of skin and soft-tissue infections. Clin Infect Dis. 2005;41:1373–406. doi: 10.1086/497143. [DOI] [PubMed] [Google Scholar]
- 114.Nathwani D, Morgan M, Masterton RG, et al. Guidelines for UK practice for the diagnosis and management of methicillin-resistant Staphylococcus aureus (MRSA) infections presenting in the community. J Antimicrob Chemother. 2008;61:976–94. doi: 10.1093/jac/dkn096. [DOI] [PubMed] [Google Scholar]
- 115.Barton MD, Hawkes M, Moore D. Guidelines for the prevention and management of community-acquired methicillin-resistant Staphylococcus aureus: A perspective for Canadian health care practitioners. Can J Infect Dis Med Microbiol. 2006;17(Suppl C):4C–24C. [PMC free article] [PubMed] [Google Scholar]
- 116.Gorwitz RJ, Jernigan DB, Powers JH, Jernigan JA Participants in the Centers for Disease Control and Prevention-Convened Experts’Meeting on Management of MRSA in the Community. Strategies for clinical management of MRSA in the community: summary of an experts’ meeting convened by the Centers for Disease Control and Prevention. 2006 Available at http://www.cdc.gov/ncidod/dhqp/pdf/ar/CAMRSA_ExpMtgStrategies.pdf.
- 117.Frank AL, Marcinak JF, Mangat PD, et al. Clindamycin treatment of methicillin-resistant Staphylococcus aureus infections in children. Pediatr Infect Dis J. 2002;21:530–534. doi: 10.1097/00006454-200206000-00010. [DOI] [PubMed] [Google Scholar]
- 118.Martinez-Aguilar G, Hammerman WA, Mason EO, Jr, Kaplan SL. Clindamycin treatment of invasive infections caused by community-acquired, methicillin-resistant and methicillin-susceptible Staphylococcus aureus in children. Pediatr Infect Dis J. 2003;22:593–98. doi: 10.1097/01.inf.0000073163.37519.ee. [DOI] [PubMed] [Google Scholar]
- 119.Braun L, Craft D, Williams R, Tuamokumo F, Ottolini M. Increasing clindamycin resistance among methicillin-resistant Staphylococcus aureus in 57 northeast United States military treatment facilities. Pediatr Infect Dis J. 2005;24:622–26. doi: 10.1097/01.inf.0000171175.76673.64. [DOI] [PubMed] [Google Scholar]
- 120.Hulten KG, Kaplan SL, Gonzalez BE, et al. Three-year surveillance of community onset health care-associated Staphylococcus aureus infections in children. Pediatr Infect Dis J. 2006;25:349–53. doi: 10.1097/01.inf.0000207404.50143.1e. [DOI] [PubMed] [Google Scholar]
- 121.Minuth JN, Holmes TM, Musher DM. Activity of tetracycline, doxycycline, and minocycline against methicillin-susceptible and -resistant staphylococci. Antimicrob Agents Chemother. 1974;6:411–14. doi: 10.1128/aac.6.4.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Ruhe JJ, Menon A. Tetracyclines as an oral treatment option for patients with community onset skin and soft tissue infections caused by methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother. 2007;51:3298–303. doi: 10.1128/AAC.00262-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Ruhe JJ, Monson T, Bradsher RW, Menon A. Use of long-acting tetracyclines for methicillin-resistant Staphylococcus aureus infections: case series and review of the literature. Clin Infect Dis. 2005;40:1429–34. doi: 10.1086/429628. [DOI] [PubMed] [Google Scholar]
- 124.Liu C, Graber CJ, Karr M, et al. A population-based study of the incidence and molecular epidemiology of methicillin-resistant Staphylococcus aureus disease in San Francisco, 2004–2005. Clin Infect Dis. 2008;46:1637–46. doi: 10.1086/587893. [DOI] [PubMed] [Google Scholar]
- 125.Cenizal MJ, Skiest D, Luber S, et al. Prospective randomized trial of empiric therapy with trimethoprim-sulfamethoxazole or doxycycline for outpatient skin and soft tissue infections in an area of high prevalence of methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother. 2007;51:2628–30. doi: 10.1128/AAC.00206-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Markowitz N, Quinn EL, Saravolatz LD. Trimethoprim-sulfamethoxazole compared with vancomycin for the treatment of Staphylococcus aureus infection. Ann Intern Med. 1992;117:390–398. doi: 10.7326/0003-4819-117-5-390. [DOI] [PubMed] [Google Scholar]
- 127.Proctor RA. Role of folate antagonists in the treatment of methicillin-resistant Staphylococcus aureus infection. Clin Infect Dis. 2008;46:584–93. doi: 10.1086/525536. [DOI] [PubMed] [Google Scholar]
- 128.Szumowski JD, Cohen DE, Kanaya F, Mayer KH. Treatment and outcomes of infections by methicillin-resistant Staphylococcus aureus at an ambulatory clinic. Antimicrob Agents Chemother. 2007;51:423–28. doi: 10.1128/AAC.01244-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Howden BP, Grayson ML. Dumb and dumber--the potential waste of a useful antistaphylococcal agent: emerging fusidic acid resistance in Staphylococcus aureus. Clin Infect Dis. 2006;42:394–400. doi: 10.1086/499365. [DOI] [PubMed] [Google Scholar]
- 130.Rubinstein E, Cammarata S, Oliphant T, Wunderink R. Linezolid (PNU-100766) versus vancomycin in the treatment of hospitalized patients with nosocomial pneumonia: a randomized, double-blind, multicenter study. Clin Infect Dis. 2001;32:402–12. doi: 10.1086/318486. [DOI] [PubMed] [Google Scholar]
- 131.Stevens DL, Herr D, Lampiris H, et al. Linezolid versus vancomycin for the treatment of methicillin-resistant Staphylococcus aureus infections. Clin Infect Dis. 2002;34:1481–90. doi: 10.1086/340353. [DOI] [PubMed] [Google Scholar]
- 132.Weigelt J, Itani K, Stevens D, et al. Linezolid versus vancomycin in treatment of complicated skin and soft tissue infections. Antimicrob Agents Chemother. 2005;49:2260–2266. doi: 10.1128/AAC.49.6.2260-2266.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Wunderink RG, Cammarata SK, Oliphant TH, Kollef MH. Continuation of a randomized, double-blind, multicenter study of linezolid versus vancomycin in the treatment of patients with nosocomial pneumonia. Clin Ther. 2003;25:980–992. doi: 10.1016/s0149-2918(03)80118-2. [DOI] [PubMed] [Google Scholar]
- 134.Apodaca AA, Rakita RM. Linezolid-induced lactic acidosis. N Engl J Med. 2003;348:86–87. doi: 10.1056/NEJM200301023480123. [DOI] [PubMed] [Google Scholar]
- 135.De Vriese AS, Coster RV, Smet J, et al. Linezolid-induced inhibition of mitochondrial protein synthesis. Clin Infect Dis. 2006;42:1111–17. doi: 10.1086/501356. [DOI] [PubMed] [Google Scholar]
- 136.Gerson SL, Kaplan SL, Bruss JB, et al. Hematologic effects of linezolid: summary of clinical experience. Antimicrob Agents Chemother. 2002;46:2723–26. doi: 10.1128/AAC.46.8.2723-2726.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Hawkins C, Huang J, Jin N, et al. Persistent Staphylococcus aureus bacteremia: an analysis of risk factors and outcomes. Arch Intern Med. 2007;167:1861–67. doi: 10.1001/archinte.167.17.1861. [DOI] [PubMed] [Google Scholar]
- 138.Khatib R, Johnson LB, Sharma M, et al. Persistent Staphylococcus aureus bacteremia: Incidence and outcome trends over time. Scand J Infect Dis. 2008:1–6. doi: 10.1080/00365540802441711. [DOI] [PubMed] [Google Scholar]
- 139.Dombrowski JC, Winston LG. Clinical failures of appropriately-treated methicillin-resistant Staphylococcus aureus infections. J Infect. 2008;57:110–115. doi: 10.1016/j.jinf.2008.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Lodise TP, Lomaestro B, Graves J, Drusano GL. Larger vancomycin doses (at least four grams per day) are associated with an increased incidence of nephrotoxicity. Antimicrob Agents Chemother. 2008;52:1330–1336. doi: 10.1128/AAC.01602-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Steinkraus G, White R, Friedrich L. Vancomycin MIC creep in non-vancomycin-intermediate Staphylococcus aureus (VISA), vancomycin-susceptible clinical methicillin-resistant S. aureus (MRSA) blood isolates from 2001–05. J Antimicrob Chemother. 2007;60:788–94. doi: 10.1093/jac/dkm258. [DOI] [PubMed] [Google Scholar]
- 142.Wang G, Hindler JF, Ward KW, Bruckner DA. Increased vancomycin MICs for Staphylococcus aureus clinical isolates from a university hospital during a 5-year period. J Clin Microbiol. 2006;44:3883–86. doi: 10.1128/JCM.01388-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Micek ST. Alternatives to vancomycin for the treatment of methicillin-resistant Staphylococcus aureus infections. Clin Infect Dis. 2007;45(Suppl 3):S184–S190. doi: 10.1086/519471. [DOI] [PubMed] [Google Scholar]
- 144.Pan A, Lorenzotti S, Zoncada A. Registered and investigational drugs for the treatment of methicillin-resistant Staphylococcus aureus infection. Recent Patents Anti-Infect Drug Disc. 2008;3:10–33. doi: 10.2174/157489108783413173. [DOI] [PubMed] [Google Scholar]
- 145.Stryjewski ME, Chambers HF. Skin and soft-tissue infections caused by community-acquired methicillin-resistant Staphylococcus aureus. Clin Infect Dis. 2008;46(Suppl 5):S368–S377. doi: 10.1086/533593. [DOI] [PubMed] [Google Scholar]
- 146.Chambers HF. Evaluation of ceftobiprole in a rabbit model of aortic valve endocarditis due to methicillin-resistant and vancomycin-intermediate Staphylococcus aureus. Antimicrob Agents Chemother. 2005;49:884–88. doi: 10.1128/AAC.49.3.884-888.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Jacqueline C, Caillon J, Le MV, et al. In vivo efficacy of ceftaroline (PPI-0903), a new broad-spectrum cephalosporin, compared with linezolid and vancomycin against methicillin-resistant and vancomycin-intermediate Staphylococcus aureus in a rabbit endocarditis model. Antimicrob Agents Chemother. 2007;51:3397–400. doi: 10.1128/AAC.01242-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Noel GJ, Strauss RS, Amsler K, et al. Results of a double-blind, randomized trial of ceftobiprole treatment of complicated skin and skin structure infections caused by gram-positive bacteria. Antimicrob Agents Chemother. 2008;52:37–44. doi: 10.1128/AAC.00551-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Parish D, Scheinfeld N. Ceftaroline fosamil, a cephalosporin derivative for the potential treatment of MRSA infection. Curr Opin Investig Drugs. 2008;9:201–9. [PubMed] [Google Scholar]
- 150.Shaw KJ, Poppe S, Schaadt R, et al. In vitro activity of TR-700, the active ingredient of the antibacterial prodrug TR-701, against linezolid resistant strains. Antimicrob Agents Chemother. 2008;52:4442–47. doi: 10.1128/AAC.00859-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Dajcs JJ, Thibodeaux BA, Hume EB, et al. Lysostaphin is effective in treating methicillin-resistant Staphylococcus aureus endophthalmitis in the rabbit. Curr Eye Res. 2001;22:451–57. doi: 10.1076/ceyr.22.6.451.5486. [DOI] [PubMed] [Google Scholar]
- 152.Lawton EM, Ross RP, Hill C, Cotter PD. Two-peptide lantibiotics: a medical perspective. Mini Rev Med Chem. 2007;7:1236–47. doi: 10.2174/138955707782795638. [DOI] [PubMed] [Google Scholar]
- 153.Stapleton PD, Shah S, Ehlert K, Hara Y, Taylor PW. The beta-lactam-resistance modifier (−)-epicatechin gallate alters the architecture of the cell wall of Staphylococcus aureus. Microbiology. 2007;153:2093–103. doi: 10.1099/mic.0.2007/007807-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Schaffer AC, Lee JC. Vaccination and passive immunisation against Staphylococcus aureus. Int J Antimicrob Agents. 2008;32(Suppl 1):S71–S78. doi: 10.1016/j.ijantimicag.2008.06.009. [DOI] [PubMed] [Google Scholar]
- 155.Shinefield H, Black S, Fattom A, et al. Use of a Staphylococcus aureus conjugate vaccine in patients receiving hemodialysis. N Engl J Med. 2002;346:491–96. doi: 10.1056/NEJMoa011297. [DOI] [PubMed] [Google Scholar]
- 156.Rogers DE, Tompsett R. The survival of staphylococci within human leukocytes. J Exp Med. 1952;95:209–30. doi: 10.1084/jem.95.2.209. [DOI] [PMC free article] [PubMed] [Google Scholar]