Abstract
Mg-chelatase catalyzes the ATP-dependent insertion of Mg2+ into protoporphyrin-IX to form Mg-protoporphyrin-IX. This is the first step unique to chlorophyll synthesis, and it lies at the branch point for porphyrin utilization; the other branch leads to heme. Using the stromal fraction of pea (Pisum sativum L. cv Spring) chloroplasts, we have prepared Mg-chelatase in a highly active (1000 pmol 30 min−1 mg−1) and stable form. The reaction had a lag in the time course, which was overcome by preincubation with ATP. The concentration curves for ATP and Mg2+ were sigmoidal, with apparent Km values for Mg2+ and ATP of 14.3 and 0.35 mm, respectively. The Km for deuteroporphyrin was 8 nm. This Km is 300 times lower than the published porphyrin Km for ferrochelatase. The soluble extract was separated into three fractions by chromatography on blue agarose, followed by size-selective centrifugal ultrafiltration of the column flow-through. All three fractions were required for activity, clearly demonstrating that the plant Mg-chelatase requires at least three protein components. Additionally, only two of the components were required for activation; both were contained in the flow-through from the blue-agarose column.
The biosynthesis of heme and chlorophyll are integral parts of chloroplast development and the acquisition of photosynthetic capacity. Both end products are required in specific but vastly different amounts and are synthesized within the chloroplast via a common tetrapyrrole biosynthetic pathway (Beale and Weinstein, 1990; Porra, 1997). The branch point for the formation of chlorophyll and heme is the use of Proto by the two enzymes that catalyze metal insertion. Ferrochelatase catalyzes the insertion of Fe2+ into Proto, whereas Mg-chelatase catalyzes the insertion of Mg2+ into Proto. Because the flux of common precursors to the respective end products is likely to be controlled by modulation of the branch-point enzymes, we were interested in the properties of these enzymes. This report describes the properties of a totally soluble enzyme system from pea (Pisum sativum L. cv Spring) chloroplasts that catalyzes the Mg-chelatase reaction.
Previous work has shown that Mg-chelatase activity requires ATP (Pardo et al., 1980; Walker and Weinstein, 1991b). Although the reaction is formally similar to the ferrochelatase reaction, insertion of a divalent cation into Proto, there is no ATP requirement for the Fe-insertion reaction. Our previous work has shown that the Mg-chelatase reaction requires at least two different protein components (Walker and Weinstein, 1991b; Walker et al., 1992). We also showed that the reaction proceeds by a two-step mechanism, involving activation followed by Mg2+ insertion; both steps require ATP (Walker and Weinstein, 1994). This hypothesis was based on the following observations. There is a lag phase in the kinetics that can be overcome by preincubation of the crude enzyme fraction with ATP before the porphyrin substrate is added. ATPγS can substitute for ATP in the preincubation, but not for the Mg2+ insertion step. The final reaction rates are enhanced if the preincubations have a higher protein concentration, suggesting protein-protein interaction in the activation step.
More recently, there have been several major developments in the study of Mg-chelatase. The most important development has come from work on the photosynthetic bacterium Rhodobacter sphaeroides, in which it was shown that Mg-chelatase activity requires three proteins (Gibson et al., 1995). This was shown by cloning and expressing the products of the bchD, bchH, and bchI genes in Escherichia coli, and combining the E. coli extracts to reconstitute Mg-chelatase activity in vitro. Similarly, in the cyanobacterium Synechocystis PCC6803, three genes were identified (chlD, chlH, and chlI), cloned, and expressed in E. coli; reconstitution of Mg-chelatase activity required all three gene products (Jensen et al., 1996a). The requirement for three subunits catalyzing an ATP-dependent metal ion insertion is analogous to the situation for Co insertion in vitamin B12 biosynthesis in Pseudomonas denitrificans (Debussche et al., 1992). Homologs for two of the bacterial Mg-chelatase genes have been identified in eukaryotic plants, olive and ch42 for bchH and bchI, respectively (Koncz et al., 1990; Hudson et al., 1993; Nakayama et al., 1995; Gibson et al., 1996; Jensen et al., 1996b). The sequence homologies between the two bacterial and plant genes and the requirement for ATP suggest that higher plants also require three subunits for Mg-chelatase activity. Evidence for this supposition comes from the finding that there are three nonallelic mutations that affect Mg-chelatase activity in isolated barley (Hordeum vulgare L.) chloroplasts (Jensen et al., 1996b), and that activity can be reconstituted by mixing chloroplast extracts from any two nonallelic mutants (Kannangara et al., 1997). Although two of the plant genes have been cloned and expressed, there is currently no system available to test whether the expressed subunits are active (Nakayama et al., 1995; Gibson et al., 1996).
An important characteristic of the prokaryotic Mg-chelatase systems is that they are completely soluble, and the derived amino acid sequences show no hydrophobic stretches capable of spanning a membrane (Gibson et al., 1995; Jensen et al., 1996a; Petersen et al., 1996). The same is true for the two plant genes that have been identified (Hudson et al., 1993; Nakayama et al., 1995; Gibson et al., 1996; Jensen et al., 1996b). Although most of our work on the in vitro characterization of the Mg-chelatase reaction has used membrane-containing chloroplast fractions, we have observed that Mg-chelatase activity can be solubilized by chloroplast lysis in buffers that contain low concentrations of MgCl2 (Walker and Weinstein, 1995). Because the higher plant system is also soluble, it is important to determine whether the observations that led to our proposal of a two-step mechanism were a function of having membranes in the system. Thus, we have continued our studies on the enzyme extracted from pea chloroplasts. The soluble enzyme system has been characterized with respect to substrate requirements, potential inhibitors, and the lag phase in the kinetics. This system has also been separated into three fractions, and the role of these fractions in activation and Mg2+ insertion has been investigated.
MATERIALS AND METHODS
Chemicals and Biochemicals
Tricine and DTT were purchased from Research Organics (Cleveland, OH). BSA and Miracloth were obtained from Calbiochem. Deutero and Mg-Deutero were purchased from Porphyrin Products (Logan, UT). ATPγS was purchased from Boehringer Mannheim. All other biochemicals were purchased from Sigma, and all organic solvents and salts were of analytical grade or better. Centrifugal ultrafiltration devices (for protein concentration and fractionation by size) were purchased from Amicon (Beverly, MA).
Plant Material and Chloroplast Isolation
Pea (Pisum sativum L. cv Spring) seeds were purchased from Asgrow (Doraville, GA). Seeds were washed with tap water to remove excess Captan fungicide and were then allowed to imbibe in water for 1.5 to 3 h. The plants were grown at about 26 to 32°C for 7 to 8 d under a 12-h light/12-h dark cycle in trays of moist vermiculite. Seedlings were harvested for chloroplast isolation between 3 and 6 h after the final light cycle started.
Intact chloroplasts were isolated from unexpanded leaflets by grinding in an isoosmotic grinding buffer (0.5 m sorbitol, 50 mm Tricine, 1 mm EDTA, 1 mm MgCl2, 1 mm DTT, and 0.1% [w/v] BSA, pH 7.8), differential centrifugation, and a final centrifugation through a Percoll pad, as previously described (Walker and Weinstein, 1991b). Remaining Percoll and BSA were removed by washing the intact plastids with grinding buffer lacking BSA.
Preparation of SPs
The intact chloroplast pellet was resuspended in a small volume of hypotonic lysis buffer (10 mm Tricine, 2 mm EDTA, 2 mm DTT, 10% [w/v] glycerol, and 0.0025% [w/v] PMSF, pH 7.85) and stored at −75°C before homogenizing. The frozen suspension was thawed and diluted to a protein concentration of about 20 mg/mL with the same buffer, then homogenized in a 10-mL Potter-Elvehjem (Wheaton, Millville, NJ) glass homogenizer to get a mixture of broken chloroplasts. The mixture was centrifuged at 280,000g for 35 min at 4°C. The pellet was discarded and the supernatant was concentrated to a protein concentration between 10 and 20 mg/mL using Centriprep 10 concentrators (Amicon). This soluble system is designated SP. A typical eight-tray preparation of pea seedlings (starting with 3.5 L of seeds) yields approximately 300 mg of SP.
Fractionation of SP on Blue-Agarose
A Cibacron Blue 3GA-agarose (4% cross-linked) column (1.5 × 7 cm, about 12-mL gel) was equilibrated with hypotonic lysis buffer. Eight milliliters of SP containing about 90 mg of protein was loaded onto the column at a flow rate of 0.2 mL/min, and the first 5 mL of effluent was discarded. The column was then washed with hypotonic lysis buffer (70 mL at 2 mL/min), and the next 15 mL was collected and designated FT. The remaining wash buffer was discarded. Then, the column was washed with 30 mL of hypotonic lysis buffer supplemented with 2 m NaCl at a flow rate of 2 mL/min. The eluate was collected and concentrated to 3 mL using Centriprep 10 concentrators. This fraction was desalted by gel filtration using a spin column and ACA 202 spectra gel (Spectrum, Los Angeles, CA). The desalted fraction was diluted to 10 mL; this fraction was designated as BB. Mg-chelatase activity of fractions FT and BB was checked using either a stopped or a continuous assay. Preparation of chloroplast fractions containing LM/S and separation of the LM and S by ultracentrifugation was as described previously (Walker et al., 1992).
Further Fractionation by Size
In some cases the FT was further fractionated by size using centrifugal ultrafiltration with membranes having different pore sizes. Low-molecular-mass components (<100 kD) were obtained in the filtrate after centrifugation (3,000g for 15 min at 4°C) of FT through a Microcon 100 concentrator (Amicon). This fraction is designated FT-lo. The higher-molecular-mass components (>100 kD) were concentrated in the retentate, but the retentate also contained contaminating low-molecular-mass components at their original concentration. To remove these low- molecular-mass components, the retentate was diluted 15-fold with hypotonic lysis buffer (without additional PMSF) and the centrifugal ultrafiltration was repeated. This process was repeated two more times, and the final retentate was resuspended in one-half the original volume (giving another 6-fold dilution). Theoretically, the overall dilution of low-molecular-mass components in the high-molecular-mass fraction was 20,000-fold, assuming no interactions. The washed high-molecular-mass fraction of FT is designated FT-hi.
Protein was determined by the method of Bradford (1976) using BSA as a standard.
Mg-Chelatase Activity Measurements
Stopped Assay
Mg-chelatase activity was measured by an adaptation of the method previously described (Walker and Weinstein, 1991b). However, because the enzyme samples no longer contained membranes, they could be conveniently incubated in 1.5-mL microcentrifuge tubes that were placed in a heating block set to 30°C and covered with two layers of aluminum foil. Samples were incubated in a total volume of 100 μL of Tricine-EDTA (10–2 mm) buffer containing 9 μm Deutero, 30 mm MgCl2, 2 mm ATP with an ATP-regenerating system (10 mm phosphocreatine/creatine kinase [2 units/mL]), 5 to 8% (w/v) glycerol, and 1 to 1.75 mm DTT, final pH, 7.85. Reactions were started by addition of samples and were allowed to proceed for 30 min with constant shaking (100 rpm on an orbital shaker) before termination of the reaction by addition of 750 μL of cold acetone. To this mixture 150 μL of cold 0.12 n NH4OH was added. The samples were vortexed and centrifuged (13,000g) for 2 min to sediment precipitated proteins. The supernatant was reserved, and the fluorescence of the product was read directly in this 75% (v/v) acetone extract. No second acetone extraction or hexane extraction was necessary.
Mg-Deutero was quantitated on a Perkin-Elmer 650-40 fluorometer calibrated with a Rhodamine block as previously described (Walker and Weinstein, 1991a). Activities are expressed as units (the number of picomoles of Mg-Deutero produced in a 30-min assay).
Continuous Assay
The continuous assay was carried out as previously described (Walker et al., 1992). In a typical assay an aliquot of the sample was thawed and added directly to a fluorescence cuvette containing the assay buffer equilibrated to 30°C. The assay buffer was exactly as described above, except that the MgCl2 concentration was reduced to 20 mm to prevent formation of turbidity. The total final incubation volume was 600 μL. In some experiments with the continuous assay, some of the plastid-derived enzyme components were preincubated for 10 min at 30°C in the presence or absence of other components required for the reaction. In these experiments Deutero was absent during the preincubation period. At the end of the preincubation period, Deutero plus the remaining components were added to the cuvette to initiate the reaction. The preincubation volumes were always less than one-half of the final volume. Unless otherwise indicated the preincubation contained 2 mm ATP (plus regenerating system, see above) and 20 mm MgCl2. For the porphyrin-concentration curves, the shutter was opened for 3 s every 60 s instead of every 30 s. Reaction rates were determined from the slope of the linear portion of the continuous assay curves. The fluorescence yield of the reaction products in aqueous buffers was sensitive to the presence or absence of protein in the assay buffer. Thus, the standard curves were constructed in buffer containing 1.5 mg/mL SP. The average deviation for duplicate measurements was 8%.
Immunoblotting
Proteins in various chloroplast fractions were separated by electrophoresis on SDS polyacrylamide (10% [w/v]) gels, according to the method of Fling and Gregerson (1986). Ten micrograms of protein from each fraction was loaded onto the gel. The separated proteins were transferred to an Immobilon-P filter (Millipore) using a semi-dry electroblotter (JKA-Biotech, Brønhøj, Denmark) according to the manufacturer's directions. One filter was probed with rabbit antibodies to a recombinant protein expressed in Escherichia coli, which was encoded by a truncated form of the ch42 gene from Arabidopsis thaliana. The other filter was probed with mouse antibodies to a recombinant protein expressed in E. coli, which was encoded by a truncated form of the olive gene from Antirrhinum majus. Both antibodies were generously provided by C. Gamini Kannangara (Carlsberg Research Laboratory, Copenhagen, Denmark). Incubation of the primary antibodies with the filters was performed according to Sambrook et al. (1989). The primary antibody was detected using secondary antibodies conjugated to alkaline phosphatase (Sigma) according to the supplier's directions.
RESULTS
Preparation and Characterization of Soluble Mg-Chelatase
We have shown that Mg-chelatase can be prepared from pea plastids in a completely soluble form by using a buffer with a low Mg concentration to wash the active components off the membranes (Walker and Weinstein, 1995). Unfortunately, this preparation required the presence of ATP and Mg2+ during enzyme preparation and storage to maintain the highest activity. Even in the presence of these components, the enzyme preparation was not very stable; 38% of the activity was lost when stored on ice for 3 h (data not shown). Ten percent (w/v) glycerol and increased DTT concentration (2 mm) vastly improved the stability of the enzyme preparation when these components were included in the chloroplast-lysis buffer and in all subsequent steps of fractionation. Compared with fresh enzyme there was only a 7% loss of activity when stored for 2 months at −75°C followed by 0°C for 5 h (data not shown). There was no loss of activity if the −75°C material was thawed and used immediately. The activities of these preparations were high; the specific activity was 1050 pmol 30 min−1 mg−1 protein when measured at a protein concentration of 7 mg/mL. As with LM/S (Walker et al., 1992), the activity versus protein-concentration curve is not linear until a threshold protein concentration between 2 and 3 mg/mL is reached in the assay (data not shown).
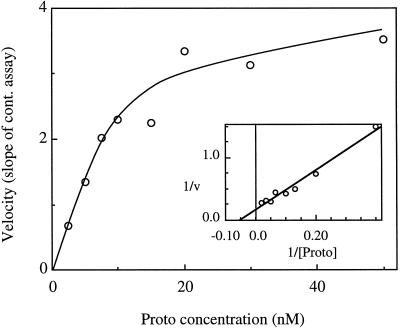
The new preparation was characterized with respect to its substrate requirements (Table I). The Km for the porphyrin turned out to be much lower than indicated by preliminary experiments (Guo, 1996). This early work used the stopped assay, and there were difficulties with achieving initial velocity conditions for Michaelis-Menten kinetics. To overcome these problems, the continuous assay was used with the following modifications: SP was preincubated with ATP and Mg2+ for 10 min at 30°C to overcome the lag period (see below). After the preincubation period the sample was diluted to the final volume with buffer containing ATP and Mg2+, and varying amounts of porphyrin were added. Slopes for the fluorescence increase with time were recorded over a 5- to 10-min period after the addition of porphyrin (the shorter time periods were required for the lower porphyrin concentrations). In Figure 1 the results of a typical experiment with Proto are shown (average Km = 13.5 nm). For most experiments the more soluble and stable porphyrin, Deutero, was used. The Km for this artificial substrate was also in the nanomolar range (Km = 7.9 nm). The Km values were determined by fitting the data to the Lineweaver-Burk double-reciprocal plot; the correlation coefficients for linearity were always greater than 0.96. Km values in the nanomolar range were confirmed by following the progress curve of substrate disappearance at several different initial concentrations of Deutero. The Km values, determined from the integrated rate equation (Segel, 1975), fell between 1 and 10 nm. These samples were also preincubated with ATP and Mg2+ before dilution and addition of porphyrin. At concentrations of Deutero greater than 500 nm, some substrate inhibition was observed. At 4 μm Deutero the velocity was 80% of the maximal velocity measured.
Table I.
Substrate requirements for soluble Mg-chelatase
The Km values for Deutero and Proto were obtained from double-reciprocal linear replots of initial velocities obtained from the continuous assay after activation (see text). The apparent Km values for ATP and Mg2+ were obtained using the linearized version of the Hill equation (Segel, 1975), with initial velocities determined using the stopped assay.
sd = 4 nm for six independent determinations.
sd = 6 for two independent determinations.
sd = 0.08 for two determinations. The Hill coefficient was 1.9 ± 0.5.
sd = 0.8 for two determinations.
Figure 1.
Mg-chelatase follows Michaelis-Menten kinetics with respect to porphyrin concentration. Initial velocities were determined from the slopes of the continuous assay after activation and subsequent addition of Proto. The assays contained 0.28 mg of SP. The preincubation volume was 40 μL. Inset, Lineweaver-Burk plot of the same data (r2 = 0.974). In this experiment the Km for Proto was 18.9 nm, and the Vmax corresponded to 794 units/mg.
The ATP concentration curve was obtained using the standard stopped assay. The ATP-regenerating system remained constant for each ATP concentration tested. The curve is sigmoidal, with a Hill coefficient of 1.9. The apparent Km was 0.35 mm, and ATP concentrations higher than 1.5 mm were slightly inhibitory. No attempt was made to differentiate ATP requirements for each stage of the reaction.
The Mg2+ concentration curve is strongly sigmoidal; the enzyme is devoid of any activity at 5 mm. The apparent Km was 14.3 mm, and the activity was almost saturated at 30 mm. However, at this concentration the enzyme solution became slightly turbid, and this turbidity interfered with the continuous assay. Therefore, the continuous assays have only 20 mm MgCl2. There was only an 8% difference in activities measured at 20 mm compared with 30 mm MgCl2 in the stopped assay.
The pH optimum was checked by diluting an aliquot of enzyme 6-fold into a buffer containing 10 mm each of the following buffers adjusted to the appropriate pH with NaOH: Tes (pKa = 7.4), Tricine (pKa = 8.1), and Gly (pKa2 = 9.8). The final pH of each reaction mixture was tested at the end of the stopped assay. There was a broad pH optimum between 7.2 and 7.9. At pH values greater than 7.9 the activity declined, with only 38% of the activity remaining at pH 8.8.
Inhibition
The inhibitory effect of the potential physiological end products or intermediates Mg-Proto, Mg-Proto monomethyl ester, and protochlorophyllide were tested with both the stopped and continuous assays. When using the stopped assay and a concentration of 260 nm for each inhibitor, there was no inhibition when 2 μm Deutero was used as the substrate in the standard assay.
When using the continuous assay, Deutero concentration curves were run in the presence or absence of the inhibitors, with the inhibitors being added after preincubation of the enzyme with ATP and Mg2+. In this set of experiments the inhibitor concentration was 50 nm (at least six times the Km for the substrate), and the substrate was varied between 5 and 50 nm. The effects of the potential inhibitors on Km and Vmax were negligible (<20%).
Fractionation of SP by Pseudo-Affinity Chromatography
We have previously reported that pea plastid Mg-chelatase can be separated into a LM and a S by centrifugation (Walker et al., 1992). Both fractions were required for activity. SP can also be separated into two fractions, FT and BB, by pseudo-affinity chromatography on blue-agarose. Both fractions were required to reconstitute activity (Table II). To avoid having to concentrate the FT, the SP was applied to the column at a fairly high protein concentration, 11 mg/mL. If there was substantial activity (>10% of the recombined activity) in the FT alone, this fraction could simply be reapplied to a smaller fresh blue-agarose column. For recovery of the active component(s) in BB, elution of the column with buffer containing ATP (up to 10 mm) could not replace elution with 2 m NaCl. FT contained 30% of the applied protein, and BB contained 50% of the applied protein.
Table II.
Fractionation of chloroplast-soluble proteins on blue-agarose
| Fraction Assayed | Mg-Chelatase Activity |
|---|---|
| units | |
| FT | 5.7 ± 1.4 |
| BB | 0.0 ± 0.0 |
| FT + BB | 320 ± 2.0 |
The soluble proteins were applied to a column of the pseudo-affinity resin Cibacron Blue 3GA-agarose in lysis buffer. The FT was collected and the column was washed with more lysis buffer. The BB was eluted with buffer containing 2 m NaCl. BB was desalted by gel filtration before assay. The following amounts of protein were used in a 200-μL assay: FT, 200 μg; and BB, 300 μg.
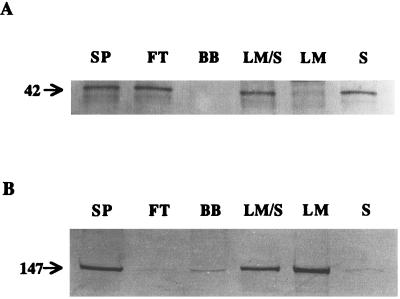
In contrast to our previous LM, both FT and BB were stable when stored separately at −75°C. The four fractions LM, S, FT, and BB were tested for the presence of two Mg-chelatase subunits by immunoblotting (Fig. 2). Antibodies to a fragment of the ch42-encoded protein (I homolog) could be used to detect the presence of the I component of Mg-chelatase in FT and S. This component was clearly absent in BB and LM. Antibodies to the olive-encoded protein (H homolog) showed the presence of the H component in BB and in LM. No H component could be seen in FT, but there appeared to be some of the H component present in the S. Antibodies to a putative eukaryotic D component were not available at this time.
Figure 2.
Immunoblots of chloroplast fractions showing the presence of Mg-chelatase subunits I and H. A, Localization of the I component; B, localization of the H component. SPs were prepared from intact chloroplasts and fractionated on a column of blue-agarose to obtain a FT and a BB. Alternatively, intact chloroplasts were lysed and the thylakoid membranes removed by centrifugation to leave an LM/S. After addition of 10 mm MgCl2, LM/S was separated into LM and S by ultracentrifugation.
Components Required for Activation
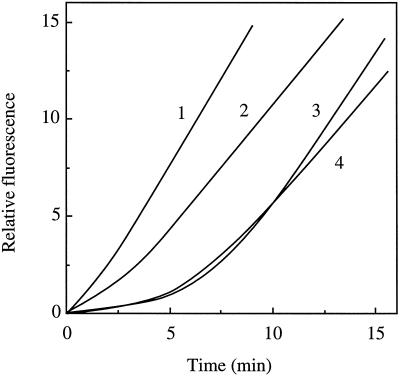
Similar to what was seen with the LM/S preparation, the Mg-chelatase activity in SP had a lag phase in the kinetics, which was observed when using the continuous assay. This lag phase was eliminated or diminished (activation) if the SP was preincubated with ATP and Mg2+ (data not shown). Unlike the LM/S system, only one of the fractions is required for the activation. As can be seen in Figure 3, when either FT (line 1) or FT plus BB (line 2) was preincubated with ATP, the lag phase was eliminated or substantially reduced compared with a direct assay (line 3) or preincubation of BB with ATP (line 4). Thus, although the chloroplast components necessary for activation were present in a single fraction, both fractions were still required for activity. In contrast to the LM/S system the activation step is not sensitive to chilling (data not shown).
Figure 3.
Continuous assay and preincubation of the blue-agarose fractions FT and BB. The fractions were preincubated in buffer containing ATP and MgCl2. After the preincubation the remaining components were added for the continuous assay. The preincubations contained: 1, FT; 2, FT plus BB; 3, no preincubation; and 4, BB. In all samples the final assay contained 338 μg of FT and 578 μg of BB. The activity for 1 is 84.5 units.
In most other ways, the SP system and the fractionated SP (FT plus BB) behave in a manner very similar to LM/S (data not shown). Higher activities were still observed when the preincubations were at a higher protein concentration compared with the assay, but this effect was not as great in the SP system. In addition to its requirement in the activation stage, ATP is also required in the Mg-insertion stage, as indicated by a loss of activity upon addition of a Glc-hexokinase ATP trap after activation. ATPγS can substitute for ATP in the activation stage, but not in the Mg2+-insertion stage.
Biochemical Rationale for Three Components in Higher-Plant Mg-Chelatase
The prokaryotes Rhodobacter sphaeroides and Synechocystis PCC6803 require three different proteins for Mg-chelatase activity (Gibson et al., 1995; Jensen et al., 1996a). Two of these proteins, products of the bchI and bchD genes, are required for activation (Willows et al., 1996). Our results with activation in the two different preparations from pea chloroplasts also predict at least a three-component system for higher plants. The fact that both fractions are required for activation in the LM/S system suggests that at least two components are required for the activation step. Because the FT is sufficient for activation, at least two components are present in this fraction. The fact that BB also is required for activity implies the existence of a third component. In combination with our immunoblot results, the fractionation and activation data allowed us to predict which fractions contained the “missing” third component. The S of LM/S contains I, as indicated by probing with the antibody to the ch42 gene product. Because the S alone does not support activation, the D component is probably in the LM along with the H component (as indicated by probing with the antibody to the H component). As indicated by the immunoblots, FT contains I. Because only FT is required for activation, D is probably also present in this fraction, which means that D is not retained by the blue-agarose column.
Previously, we have observed that the active components in the isolated LM can be recovered in a soluble form by washing this fraction with a dilute buffer (C.J. Walker and J.D. Weinstein, unpublished observations). Combined with the above information, this fact suggests a method for obtaining the D component in a separate fraction. The solubilized LM (containing D and H) was passed through a blue-agarose column. H was retained on this column and the FT was predicted to contain D. This fraction is designated LM-FT. To test this prediction, LM-FT was tested for activity in combination with the S from LM/S (which contains I) and the BB from SP (which contains H). The results are shown in Table III. None of the fractions when tested alone had greater than 1 unit of activity. Although the combination of S plus BB had significant activity, this activity was synergistically increased 2.6-fold by addition of the LM-FT. These results confirm that in chloroplasts of higher plants, Mg-chelatase requires at least three components.
Table III.
Reconstitution of activity with three chloroplast fractions obtained by combining LM/S and blue-agarose fractionation
| Protein Fractions in the Incubation | Putative Subunit Content | Mg-Chelatase Activity |
|---|---|---|
| units | ||
| S + LM-FT + BBa | I + D + H | 14.7 ± 0.6 |
| S + LM-FT | I + D | 1.4 ± 0.1 |
| S + BB | I + H | 5.7 ± 1.0 |
| BB + LM-FT | H + D | 0.0 ± 0 |
The BB fraction from the blue-agarose column contains the H component (see Fig. 2). The S fraction of LM/S contains the I component (see Fig. 2). The third component, D, was obtained from the blue-agarose FT of solubilized LM, LM-FT (see text). The following amounts of protein were used in a 200-μL assay: S, 200 μg; BB, 350 μg; and LM-FT, 27 μg.
When tested individually, these fractions had less than 1 unit of activity.
Separation of the FT Components (I and D) by Size
It is apparent from Table III that one or more of the fractions have significant cross-contamination. Thus, an easier and cleaner method was developed for separation of the three components. The I component of higher plants has a monomer molecular mass between 40 and 45 kD (Koncz et al., 1990; Nakayama et al., 1995). In R. sphaeroides this component is thought to function as a dimer (Willows et al., 1996). Although there is no information on the putative D component in higher plants, the expressed protein from R. sphaeroides purifies as a 550-kD multimer (Willows et al., 1996). Thus, these two components contained in the FT were separated by centrifugal ultrafiltration through a 100-kD cut-off filter. The filtrate (FT-lo) contains the low-molecular-mass components, and the retentate contains the high-molecular-mass components and some low-molecular-mass components. The contaminating low-molecular-mass components were removed from the retentate by repeated dilution and centrifugal ultrafiltration of the retentate FT-hi. The FT-hi and FT-lo were tested for activity in the various combinations with the BB (Table IV). Again, it is clear that three different fractions are required for activity. The best activity in the combination of any two fractions gives only 6% of the activity when all three are combined.
Table IV.
Reconstitution of activity with three fractions obtained from blue-agarose and molecular size fractionation
| Protein Fractions in the Incubation | Putative Subunit Content | Mg-Chelatase Activity |
|---|---|---|
| units | ||
| FT-lo + FT-hi + BB | I + D + H | 24.0 ± 0.3 |
| FT-lo + FT-hi | I + D | 1.4 ± 0.0 |
| FT-lo + BB | I + H | 0.0 ± 0.0 |
| BB + FT-hi | H + D | 0.3 ± 0.2 |
| FT-lo (2×)a | I | 0.0 ± 0.0 |
| FT-hi (2×)a | D | 1.2 ± 0.0 |
| BB (2×)a | H | 0.0 ± 0.0 |
The H (BB) component was retained on the column and eluted with NaCl. The I and putative D components were in the FT, and were separated from each other by centrifugation through a 100-kD cut-off membrane. The FT-hi was retained by the membrane, and the FT-lo passed through the membrane. The FT-hi fraction was repeatedly washed to remove low-molecular-mass contaminants. The following amounts of protein were used in a 200-μL assay: FT-lo (I), 25 μg; BB (H), 193 μg; and FT-hi (D), 145 μg.
When tested alone, the individual components were assayed at double the protein concentration compared with assays in combination with other fractions.
That the active components in these fractions are proteins can be seen in Table V. All three components were sensitive to protease pretreatment. In each case the activity loss was compared with a control that was also pretreated with trypsin, but in the presence of soybean trypsin inhibitor. Therefore, any nonspecific loss of activity during the pretreatment was the same in both the control and the digested samples. After the pretreatment the protease in the digested samples was inactivated by addition of the trypsin inhibitor. Therefore, continued protease activity during the enzyme assay was eliminated for all samples.
Table V.
Protease sensitivity of the three fractions required for Mg-chelatase activity
| Pretreated Fraction | Putative Subunit Content | Trypsin | Trypsin Inhibitor | Activity | Activity Loss |
|---|---|---|---|---|---|
| units | % | ||||
| FT-hi | D | +a | + | 5.8 ± 0.1 | – |
| FT-hi | D | + | –b | 0.0 ± 0.0 | 100 |
| BB | H | + | + | 8.0 ± 0.0 | – |
| BB | H | + | – | 2.5 ± 0.1 | 69 |
| FT-lo | I | + | + | 7.5 ± 0.5 | – |
| FT-lo | I | + | – | 1.6 ± 0.1 | 79 |
The individual fractions were treated with trypsin (120 units) or trypsin plus trypsin inhibitor (1200 units) for 15 min on ice. At the end of this treatment, trypsin inhibitor was added to the samples that did not already have it, then the other two fractions and the substrates were added for a standard stopped assay. The following amounts of protein were used for the protease treatments and assays: FT-hi (D), 145 μg; BB (H), 193 μg; and FT-lo (I), 16 μg.
+, Present during pretreatment.
−, Absent during pretreatment.
Subunit Requirements for Activation and Mg2+ Insertion
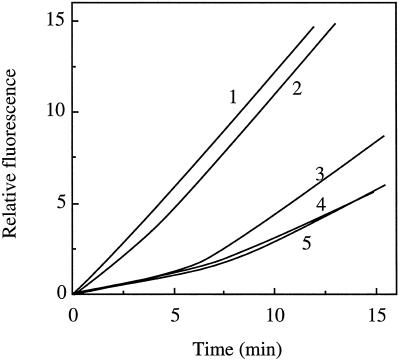
The ability to separate three active components in SP allows testing of their roles in the activation and Mg2+-insertion steps. As predicted, the two subfractions (FT-hi and FT-lo) derived from FT were necessary for activation (Fig. 4). In the control, all three subfractions were incubated in the presence of the complete substrate mixture, and a distinct lag period was observed in the reaction rate (line 3). When the three components were preincubated in the presence of ATP, this lag period was largely eliminated (line 2). This same pattern was observed when the BB subfraction was omitted from the preincubation (line 1). The other combinations in the preincubation, FT-lo and BB (line 5) or FT-hi and BB (line 4), resulted in lag phases that were similar to those of the control. Comparison of the slopes of lines 1 or 2 to the slope of line 3 indicates that preincubation not only eliminated the lag, but also increased the final rate of the reaction (1.6-fold).
Figure 4.
Activation requires preincubation of the FT-hi (D) and FT-lo (I). Fractions were preincubated in various combinations, then the remaining components were added for the continuous assay. The preincubations contained: 1, FT-hi plus FT-lo (D + I); 2, FT-hi plus FT-lo plus BB (D + I + H); 3, no preincubation; 4, FT-hi plus BB (D + H); and 5, FT-lo plus BB (I + H). In all samples the final assay contained the following amounts of protein from each fraction: FT-hi, 362 μg; FT-lo, 62 μg; and BB, 481 μg. The activity for 1 is 62 units.
It is possible to ask if, after activation, both fractions of the FT (hi and lo) are still required for the Mg2+-insertion step when subsequently combined with the BB. In this experiment FT was activated in the presence of 2 mm ATP and 30 mm MgCl2, turbidity (but not activity) from nonspecific protein aggregation was removed by ultracentrifugation (280,000g, 30 min), then FT-lo and FT-hi were immediately separated by centrifugal ultrafiltration. This separation was exactly as described in “Materials and Methods,” except that all buffers contained 2 mm ATP and 30 mm MgCl2. When either the activated FT-lo or activated FT-hi was recombined with the BB, there was no activity in a stopped assay. When all three fractions were combined, activity was restored (data not shown). A control experiment indicated that the “activated state” would have survived for at least 1 h at 4°C, which was more time than was required for the separation. Thus, even when activated, all three fractions were required for activity. We could not accurately determine whether the recombined FT-lo and FT-hi obtained from the activated FT still required activation. This limitation is because the extent of the lag is strongly dependent on the protein concentration, and there is protein loss at each step of the separation, resulting in differences in relative protein concentration of each component before and after separation.
Because it has been proposed that the H subunit is the porphyrin-binding subunit (Willows et al., 1996), we assessed the effect of preloading the H-containing fraction with Deutero. Preincubation of BB with Deutero for 5 min at 30°C, followed by addition of activated FT (preincubated with ATP as above) and the remaining buffer components for a standard continuous assay, resulted in a 32% decrease in the rate of reaction compared with when Deutero and the BB were added to the activated FT at the same time.
DISCUSSION
We have prepared a totally soluble fraction of chloroplasts that is highly active in the Mg-chelatase reaction. The stability conferred on this preparation by glycerol and extra DTT has allowed for the characterization of the soluble enzymatic activity with respect to substrate requirements, potential inhibitors, preincubation effects, and behavior during subfractionation. The new preparation with glycerol and extra DTT is as active as our previous soluble preparation, but it has the advantage of being more stable and does not require the presence of substrates to maintain the stability (Walker and Weinstein, 1995). This preparation also has about the same level of activity as that recently reported for a soluble preparation from barley (Hordeum vulgare L.) etioplasts (Kannangara et al., 1997). However, it should be noted that despite the good activity in these preparations, the activity is almost 10-fold lower than can be measured in intact pea chloroplasts (Walker et al., 1997).
We have used the SP to characterize the chelatase reaction with respect to its substrate requirements. The Km for the porphyrin was very low (8–14 nm) compared with our earlier estimates with LM/S (Walker et al., 1997) and to our initial estimates with SP (Guo, 1996). It is clear that our initial estimates were too high, because too much of the substrate was used up during the reaction. Several modifications to the assay procedure made it possible to get a more accurate value. First and foremost, the continuous assay made it possible to get an accurate initial rate. Elimination of the lag period by preincubation of SP with ATP ensured that the linear phase of the reaction occurred soon after substrate addition. This was most important for the lower porphyrin concentrations. During the actual fluorescence measurements it was important to control the excitation shutter carefully. Reaction rates were significantly higher when the shutter was opened every minute instead of every 30 s. Most likely the excitation light was causing photodestruction of the substrate, product, or both. This phenomenon may have been the cause of the substrate inhibition observed at higher substrate concentrations.
In the recent literature there are two other published porphyrin Km values for Mg-chelatase. Falbel and Staehelin (1994) report a Km for Proto of 40 nm in intact chloroplasts from greening wheat. Although this value is in reasonable agreement with our value for the soluble system, it is difficult to predict by how much the membrane barrier should increase the Km. The other report is from the in vitro reconstitution of the expressed R. sphaeroides proteins (Willows et al., 1996). In this report the ratio of subunits to each other was optimized and the initial velocities were determined from the linear portion of the continuous assay. Despite the similarity to our assay procedure, the reported Km values for Deutero and Proto were approximately 30-fold greater than that in the pea chloroplast SP. This disparity may simply reflect the different physiological needs of the different organisms or differences attributable to slightly different assay conditions.
In terms of the physiology of the pea chloroplast, one can compare the porphyrin Km of Mg-chelatase with that of ferrochelatase. The kinetic parameters of ferrochelatase have recently been determined in broken pea chloroplasts (i.e. no permeability barrier) isolated from plants grown in a manner similar to ours (Matringe et al., 1994). The Km for Deutero was 2.4 μm, 300 times greater than that of Mg-chelatase. The Vmax for ferrochelatase was 5.2 nmol min−1 mg−1 chlorophyll. For Mg-chelatase in intact pea chloroplasts the value is similar (Walker et al., 1997). Because Vmax is defined for saturating substrate values, the presence or absence of a permeability barrier is not an issue for this parameter. We recently measured a 5- to 10-fold higher flux of porphyrins through the Mg2+, compared with the Fe2+, branch of the pathway in pea chloroplasts (Walker et al., 1997). If porphyrin substrate is limiting, the difference in Km values is more than sufficient to account for the higher flux into the Mg2+ branch of the pathway. Regulation of the branch point by relative affinity for the substrate could work in addition to a mechanism of day versus night regulation, based on the demonstrated circadian rhythmicity of subunit H expression (Gibson et al., 1996; Jensen et al., 1996b).
The concentration-dependence curve for ATP is highly sigmoidal, with a Hill coefficient of 1.9 and an apparent Km of 0.35 mm. This value is in agreement with the apparent Km for the activation stage, which we measured with LM/S (Walker and Weinstein, 1994), and is twice as high as the Km for ATP with the reconstituted R. sphaeroides enzyme (Willows et al., 1996). With LM/S the shape of the curve was also sigmoidal, although this was not the case with the reconstituted R. sphaeroides enzyme. It is too early to ascribe physiological significance to the sigmoidal character of the curve. However, one of the subunits, I, has a clearly defined ATP-binding sequence (Koonin, 1993; Nakayama et al., 1995), and with the expressed R. sphaeroides protein this subunit purifies as a dimer (Willows et al., 1996). Thus, two binding sites for ATP could result in the cooperativity in ATP binding, which is indicated by the sigmoidal ATP concentration dependence. It is important to note that we have found no significant differences between the behavior of SP and LM/S with respect to ATP: ATP is required in the preincubation to overcome the lag phase, and inactivation of the reaction by addition of an ATP trap demonstrates that ATP is still required in the second stage of the reaction. As with LM/S, ATPγS will work in the first step but not in the second (Walker and Weinstein, 1994).
The Mg2+ concentration-dependence curve is also sigmoidal in shape, and demonstrates an apparent Km of 14.3 mm. For the soluble barley system, one-half maximal activity requires about 18 mm Mg (Kannangara et al., 1997). In contrast, the reconstituted expressed R. sphaeroides enzyme has a Km of 1.7 mm (Willows et al., 1996). The values from the two higher-plant systems are in reasonable agreement. We expect that Mg2+ is involved in at least three discrete roles: (a) as a substrate for the chelatase, (b) as a co-substrate with ATP, and (c) for maintenance of protein-protein interactions. This third role can be inferred from data showing that MgCl2 concentrations well above those required for binding with ATP (at least 20 mm) are required to optimize the preincubation stage of the reaction (Guo, 1996). During this stage there is no porphyrin present, so the high Mg concentration during this step cannot be required for the chelation reaction per se.
When tested on intact plastids from greening cucumber (Cucumus sativus L.) cotyledon, there was no effect on activity from the possible feedback inhibitors protochlorophyllide, Mg-Proto, and chlorophyllide (Walker and Weinstein, 1991a). To ensure that there was not a problem attributable to accessibility caused by the membranes, we retested the effects on activity of the following compounds: Mg-Proto, Mg-Proto monomethylester, and protochlorophyllide. As with intact plastids, the potential inhibitors had no significant effect on activity. Thus, at the level of modulating Mg-chelatase enzyme activity, feedback inhibition does not play a direct role in regulation of chlorophyll synthesis. It was recently shown that pretreatment of seedlings with δ-aminolevulinic acid causes an increase in the endogenous level of Mg-Proto and Mg-Proto monomethylester, and plastids from these plants had decreased Mg-chelatase levels (Averina et al., 1996). The effective plastid porphyrin concentrations were not given, and we have observed nonspecific inhibition by exogenous porphyrins (Walker and Weinstein, 1991a). Therefore, it is difficult to know if the effect is physiologically important. However, if the effect is physiological, Walker and Willows (1997) have suggested that the results can be reconciled if the buildup of intermediates inhibits the import of one or more of the Mg-chelatase subunits into the chloroplasts. Such a mechanism has been proposed for the regulation of protochlorophyllide reductase import (Reinbothe et al., 1995).
Because we know that the subunits are present at their proper physiological ratios, the unfractionated SP is a good preparation for investigating substrate requirements and the effects of inhibitors. However, to demonstrate the number of subunits involved and to investigate the individual role of each subunit, it is preferable to have a more fractionated system. Thus, we were previously able to show that a minimum of two proteins were involved in the Mg-chelatase reaction by fractionating chloroplasts into soluble and membrane-associated components (Walker and Weinstein, 1991b; Walker et al., 1992). We also showed that a minimum of two components were required for the activation step by using the fractionated LM/S system (Walker and Weinstein, 1994). We have now shown that the soluble proteins from pea chloroplasts can be resolved into three separate protein fractions that are required for activity. This resolution could be accomplished by two slightly different procedures, although both procedures rely on fractionating SP on a blue-agarose column. When inferences from the bacterial systems are combined with the results from the immunoblots, we can make tentative assignments regarding the subunit composition of each of our fractions. For the blue-agarose fractions, BB contains H, FT contains D and I, FT-lo contains I, and FT-hi contains D. For the LM/S fractions, LM contains H and D, LM-FT contains D, and S contains I. The assignments for D must be tentative, because there is no antibody available for the higher-plant D component.
That the three subunits can be separated under mild conditions implies that if the enzyme works as a complex, the interactions holding the subunits together must be very weak. However, there has been a report of a preparation of Mg-chelatase from the etioplasts of greening cucumber cotyledons in which all of the activity resided in a crude membrane fraction from the chloroplast (Lee et al., 1992). This activity was not stimulated by the addition of the soluble fraction. In this case the plastids were ruptured in an enriched medium containing high concentrations of all of the substrates, including Mg2+, ATP, and Proto (Lee et al., 1992). It is possible that this combination held the putative complex together, although it is also possible that the high Mg2+ concentration caused a nonspecific association of the individual components with the membranes. Of course, most of our fractionations have been carried out in the absence of Mg2+, and it is possible that this ion will hold the complex together. However, in the experiment in which the FT (containing I and D components) was activated in the presence of 2 mm ATP and 30 mm MgCl2, the I and D components could still be separated by the most gentle of the techniques, centrifugal ultrafiltration. Thus, if a complex is formed, something more than Mg2+ and ATP will be required to stabilize it for detection as a complex.
The availability of the fractionated enzyme system has allowed us to probe the subunit requirements for the activation and Mg2+-insertion steps. It is clear that only two of the three fractions are required for activation, as defined by elimination of the lag period upon preincubation with ATP. Preincubation of either the FT, which contains the I and D components, or the FT-lo with the FT-hi fraction, containing the I and D components, respectively, could eliminate the lag period in the kinetics. This result is entirely consistent with the results reported for the expressed R. sphaeroides subunits (Willows et al., 1996), and demonstrates another of the similarities between the two systems.
The H subunit has tentatively been identified as a porphyrin-binding subunit based on its homology to the cobN gene product, which is the corrinoid-binding protein of cobalt chelatase (Debussche et al., 1992), and its tight association with Proto during purification of the expressed R. sphaeroides protein from E. coli extracts. This role for the H subunit is consistent with the hypothesis that a product of the activation process will act on the porphyrin bound to the H subunit. Two mechanistic questions can be asked. Is the reaction facilitated if the H subunit is preloaded with porphyrin? And are both components from the activation reaction required for catalysis? A positive answer to the first question would support the hypothesis that H is the porphyrin-binding subunit. No enhancement in rate was observed when the BB fraction was preincubated with porphyrin before addition to the activated FT. This result neither supports nor negates the suggestion that the H subunit is responsible for porphyrin binding. Our approach to the second question involved separating the FT-lo (I) and FT-hi (D) fractions from each other after activation, and adding each activated component to the BB fraction (H) separately. Although we cannot rule out the possibility that an activated component could have been inactivated during the process of separation, the results would suggest that all three components are required for the Mg2+-insertion step. This result is not consistent with activation of a single catalytic subunit by the other subunit.
The soluble Mg-chelatase from pea chloroplasts is highly active and stable. For the determination of kinetic parameters, it has the advantage over a reconstituted system from expressed proteins in that the subunit ratios will more closely reflect the ratios present in vivo. The soluble system can also be conveniently separated into three fractions, each containing a single Mg-chelatase component. This fractionated system has already been useful in addressing mechanistic questions about the role of each subunit, and it will be useful in the future as a test system for the reconstitution of individually expressed eukaryotic subunits.
ACKNOWLEDGMENTS
We thank Dr. C. Gamini Kannangara (Carlsberg Research Laboratory, Copenhagen, Denmark) for providing the antibodies to the ch42- and olive-encoded proteins. We also thank Drs. Caroline J. Walker and Megen Bruce-Carver (Clemson University, Clemson, SC) for critically reading the manuscript.
Abbreviations:
- ATPγS
adenosine 5′-O-(3-thiotriphosphate)
- BB
blue-bound protein fraction that is bound to a blue-agarose column
- Deutero
deuteroporphyrin
- FT
flow-through protein fraction that does not bind to a blue-agarose column
- FT-hi
high-molecular-mass (>100 kD) fraction of FT
- FT-lo
low-molecular-mass (<100 kD) fraction of FT
- LM
membrane fraction after ultracentrifugation of LM/S
- LM-FT
protein fraction obtained from a low-salt wash of LM, which then flows through a blue-agarose column
- LM/S
chloroplast fraction that contains light membranes and soluble proteins
- Proto
protoporphyrin-IX
- S
supernatant fraction after ultracentrifugation of LM/S
- SP
soluble proteins from the chloroplast
Footnotes
This work was supported by the U.S. Department of Energy (grant no. DE-FG05-95ER20170).
LITERATURE CITED
- Averina N, Yaranskaya E, Rassadina V, Walter G. Response of magnesium chelatase activity in green pea (Pisum sativum L.) leaves to light, 5-aminolevulinic acid and dipyridyl supply. J Photochem Photobiol. 1996;36:17–22. [Google Scholar]
- Beale SI, Weinstein JD. Tetrapyrrole metabolism in photosynthetic organisms. In: Dailey HA, editor. Biosynthesis of Heme and Chlorophylls. New York: McGraw-Hill; 1990. pp. 287–391. [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Debussche L, Couder M, Thibaut D, Cameron B, Crouzet J, Blanche F. Assay, purification, and characterization of cobaltochelatase, a unique complex enzyme catalyzing cobalt insertion in hydrogenobyrinic acid a,c-diamide during coenzyme B12 biosynthesis in Pseudomonas denitrificans. J Bacteriol. 1992;174:7445–7451. doi: 10.1128/jb.174.22.7445-7451.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falbel TG, Staehelin LA. Characterization of a family of chlorophyll-deficient wheat (Triticum) and barley (Hordeum vulgare) mutants with defects in the magnesium-insertion step of chlorophyll biosynthesis. Plant Physiol. 1994;104:639–648. doi: 10.1104/pp.104.2.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fling SP, Gregerson DS. Peptide and protein molecular mass determination by electrophoresis using a high molarity Tris buffer system without urea. Anal Biochem. 1986;155:83–88. doi: 10.1016/0003-2697(86)90228-9. [DOI] [PubMed] [Google Scholar]
- Gibson LCD, Marrison JL, Leech RM, Jensen PE, Bassham DC, Gibson M, Hunter CN. A putative Mg chelatase subunit from Arabidopsis thaliana cv C24. Sequence and transcript analysis of the gene, import of the protein into chloroplasts, and in situ localization of the transcript and protein. Plant Physiol. 1996;111:61–71. doi: 10.1104/pp.111.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson LCD, Willows RD, Kannangara CG, von Wettstein D, Hunter CN. Magnesium-protoporphyrin chelatase of Rhodobacter sphaeroides: reconstitution of activity by combining the products of the bchH, -I, and -D genes expressed in Escherichia coli. Proc Natl Acad Sci USA. 1995;92:1941–1944. doi: 10.1073/pnas.92.6.1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo R (1996) Partial characterization of magnesium chelatase. MS thesis. Clemson University, Clemson, SC
- Hudson A, Carpenter R, Doyle S, Coen ES. olive: a key gene required for chlorophyll biosynthesis in Antirrhinum majus. EMBO J. 1993;12:3711–3719. doi: 10.1002/j.1460-2075.1993.tb06048.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen PE, Gibson LE, Henningsen KW, Hunter CN. Expression of the chlI, chlD, and chlH genes from the cyanobacterium Synechocystis PCC6803 in Escherichia coli and demonstration that the three cognate proteins are required for magnesium-protoporphyrin chelatase activity. J Biol Chem. 1996a;271:16662–16667. doi: 10.1074/jbc.271.28.16662. [DOI] [PubMed] [Google Scholar]
- Jensen PE, Willows RD, Petersen BL, Vothknecht UC, Stummann BM, Kannangara CG, von Wettstein D, Henningsen KW. Structural genes for Mg-chelatase subunits in barley: Xantha-f, -g and -h. Mol Gen Genet. 1996b;250:383–394. doi: 10.1007/BF02174026. [DOI] [PubMed] [Google Scholar]
- Kannangara CG, Vothknecht UC, Hansson M, von Wettstein D. Mg-chelatase: association with ribosomes and mutant complementation studies identify barley subunit Xantha-G as a functional counterpart of Rhodobacter subunit BchD. Mol Gen Genet. 1997;254:85–92. doi: 10.1007/s004380050394. [DOI] [PubMed] [Google Scholar]
- Koncz C, Mayerhofer R, Koncz-Kalman Z, Nawrath C, Reiss B, Redei GP, Schell J. Isolation of a gene encoding a novel chloroplast protein by T-DNA tagging in Arabidopsis thaliana. EMBO J. 1990;9:1337–1346. doi: 10.1002/j.1460-2075.1990.tb08248.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koonin EV. A common set of conserved motifs in a vast variety of putative nucleic acid-dependent ATPases including MCM proteins involved in the initiation of eukaryotic DNA replication. Nucleic Acids Res. 1993;21:2541–2547. doi: 10.1093/nar/21.11.2541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HJ, Ball MD, Parham R, Rebeiz CA. Chloroplast biogenesis. 65. Enzymic conversion of protoporphyrin IX to Mg-protoporphyrin IX in a subplastidic membrane fraction of cucumber etiochloroplasts. Plant Physiol. 1992;99:1134–1140. doi: 10.1104/pp.99.3.1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matringe M, Camadro J-M, Joyard J, Douce R. Localization of ferrochelatase activity within mature pea chloroplasts. J Biol Chem. 1994;269:15010–15015. [PubMed] [Google Scholar]
- Nakayama M, Masuda T, Sato N, Yamagata H, Bowler C, Ohta H, Shioi Y, Takamiya K. Cloning, subcellular localization and expression of ChlI, a subunit of magnesium-chelatase in soybean. Biochem Biophys Res Commun. 1995;215:422–428. doi: 10.1006/bbrc.1995.2481. [DOI] [PubMed] [Google Scholar]
- Pardo AD, Chereskin BM, Castelfranco PA, Franceschi VR, Wezelman BE. ATP requirement for Mg chelatase in developing chloroplasts. Plant Physiol. 1980;65:956–960. doi: 10.1104/pp.65.5.956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen BL, Møller MG, Stummann BM, Henningsen KW. Clustering of the genes with function in the biosynthesis of bacteriochlorophyll and heme in the green sulfur bacterium Chlorobium vibrioforme. Hereditas. 1996;125:93–96. [Google Scholar]
- Porra RJ. Recent progress in porphyrin and chlorophyll biosynthesis. Photochem Photobiol. 1997;65:492–516. [Google Scholar]
- Reinbothe S, Runge S, Reinbothe C, van Cleve B, Apel K. Substrate-dependent transport of the NADPH:protochlorophyllide oxidoreductase into isolated plastids. Plant Cell. 1995;7:161–172. doi: 10.1105/tpc.7.2.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambrook J, Fritsch EF, Maniatis T. Detection and analysis of proteins expressed from cloned genes. In: Irwin N, editor. Molecular Cloning: A Laboratory Manual, Vol 3. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1989. pp. 18.1–18.88. [Google Scholar]
- Segel IH. Enzyme Kinetics. New York: John Wiley & Sons; 1975. [Google Scholar]
- Walker CJ, Hupp LR, Weinstein JD. Activation and stabilization of Mg-chelatase activity by ATP as revealed by a novel in vitro continuous assay. Plant Physiol Biochem. 1992;30:263–269. [Google Scholar]
- Walker CJ, Weinstein JD. Further characterization of the magnesium chelatase in isolated developing cucumber chloroplasts. Substrate specificity, regulation, intactness and ATP requirements. Plant Physiol. 1991a;95:1189–1196. doi: 10.1104/pp.95.4.1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker CJ, Weinstein JD. In vitro assay of the chlorophyll biosynthetic enzyme Mg-chelatase: resolution of the activity into soluble and membrane-bound fractions. Proc Natl Acad Sci USA. 1991b;88:5789–5793. doi: 10.1073/pnas.88.13.5789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker CJ, Weinstein JD. The magnesium insertion step of chlorophyll biosynthesis is a two-stage reaction. Biochem J. 1994;299:277–284. doi: 10.1042/bj2990277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker CJ, Weinstein JD. Re-examination of the localization of Mg-chelatase within the chloroplast. Physiol Plant. 1995;94:419–424. [Google Scholar]
- Walker CJ, Willows RD. Mechanism and regulation of Mg-chelatase. Biochem J. 1997;327:321–333. doi: 10.1042/bj3270321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker CJ, Yu G, Weinstein JD. Comparative study of heme and Mg-protoporphyrin (monomethyl ester) biosynthesis in isolated pea chloroplasts: effects of ATP and metal ions. Plant Physiol Biochem. 1997;35:213–221. [Google Scholar]
- Willows RD, Gibson LCD, Kannangara CG, Hunter CN, von Wettstein D. Three separate proteins constitute the magnesium chelatase of Rhodobacter sphaeroides. Eur J Biochem. 1996;235:438–443. doi: 10.1111/j.1432-1033.1996.00438.x. [DOI] [PubMed] [Google Scholar]