Abstract
Circulating 25-OHD levels vary among human populations. Only limited information regarding determinants of these measures is available in infants and children, particularly in minority groups at greatest risk for vitamin D deficiency. We identified demographic determinants of circulating 25-OHD in a large cohort of minority children, and now extend our studies to examine potential roles of vitamin D binding protein (DBP) as a determinant of 25-OHD levels. Serum DBP level and common single nucleotide polymorphisms (SNPs) at positions 432 and 436 in the GC gene, encoding DBP, were examined. We confirmed self-reported ancestry using ancestry informative markers (AIMs), and included quantitative AIMs scores in the analysis. The multivariate model incorporated previously identified demographic and nutritional determinants of 25-OHD in this cohort, as well as GC SNPs and circulating DBP.
Genetic variants in GC differed by self-reported ancestry. The 1f allele (D432/T436) was enriched in African Americans, occurring in 71%. Homozygosity for the 1f allele (DDTT) occurred in 53% of African Americans but only 6% of Caucasians and 13% of Hispanics. Circulating DBP was significantly correlated with 25-OHD. GC SNPs were associated with both circulating DBP and 25-OHD. It appears that progressive substitution of lysine for threonine at the 436 position results in lower circulating 25-OHD. Multivariate analysis revealed that genetic variance in GC significantly contributes to circulating DBP as well as 25-OHD. Moreover, the effect of GC SNPs on 25-OHD are evident after adjusting for their effects on circulating DBP. Thus in young children genetic variance of the common GC T436K SNP affects circulating levels of the DBP protein, which in turn affects circulating 25-OHD. However, GC genotype also affects circulating 25-OHD independently of its effect on circulating DBP. These findings provide data which may be important in the interpretation of vitamin D status in children of varying ancestral backgrounds.
Keywords: Vitamin D, Vitamin D Binding Protein, Single Nucleotide Polymorphisms, Nutrition, Pediatrics, Population Studies
INTRODUCTION
Recently, a great deal of attention has been directed toward identifying determinants of circulating levels of vitamin D metabolites in humans [1, 2]. There is sparse information regarding such measures in young children. Even less information is available for minority children, a group widely considered to be at higher risk for vitamin D deficiency. In a large urban cohort of infants and toddlers from the northeast US (n>750), we reported on demographic, nutritional, and biochemical variables associated with circulating vitamin D metabolite levels [3]. Our findings indicated that significantly greater 25-OHD levels in this cohort were present in children of younger age, with lesser skin pigmentation, in those receiving formula feeds, during the summer or fall, and in association with lower circulating PTH levels.
As there has been substantial concern in urban communities related to risk of vitamin D deficiency, we have extended this analysis to identify whether ancestral background or specific genetic effects may also serve as determinants of the circulating 25-OHD level. Indeed our cohort is genetically heterogeneous, but largely comprised of Hispanic and African American families. These groups are generally perceived to be at increased risk for developing overt skeletal manifestations of vitamin D deficiency, namely rickets [4]. Further analysis of our cohort data therefore focuses on the potential role of common variants in genes identified to be determinants of circulating 25-hydroxyvitamin D (25-OHD). Others have used similar approaches to the identification of genetic determinants of circulating (25-OHD) levels in adult cohorts. Among these, the common single nucleotide polymorphisms (SNPs) in the GC gene encoding vitamin D binding protein (DBP) have been established as strong determinants of circulating 25-OHD in several genome wide association studies [5–8] and in other cohorts [9–11], as well, but all have been limited largely to adults. To address the substantial admixture present in our cohort, we derived quantitative scores of African, European, or Native American ancestry. These scores, based upon a validated set of more than 100 ancestry informative markers (AIMs) were used as a covariate in our extended analyses of 25-OHD levels and their genetic determinants. This study focuses on the common missense SNPs (the D432E and T436K variants) in the GC gene encoding biochemically polymorphic DBP species. These variants have previously been shown to be strongly associated with circulating 25-OHD. In addition we looked at circulating GC levels in individuals in the cohort, in an effort to clarify the relationships between GC genotype and circulating DBP concentrations, and the potential contribution of each of these factors to the circulating concentrations of 25-OHD. Finally, we have constructed a multivariate model to examine these effects taking into account the nutritional and demographic variables established in our initial study [3].
METHODS
Subjects and Study Design
Over 750 healthy children were recruited during well-child visits to one of four neighborhood health clinics in a mid-sized northeastern US urban community (New Haven, CT). Recruitment was limited to subjects 6 – 36 months old, the age range in which we have most commonly observed nutritional rickets [4], and were products of pregnancies greater than 28 weeks gestational age. Children were excluded from the study if there was any prior history of vitamin D or mineral metabolic disorders or if medications known to affect vitamin D metabolism (e.g., systemic glucocorticoids, pharmacologic vitamin D metabolites, or vitamin D supplements in excess of 400 IU/d) were being administered. The study was approved by the Yale University Human Investigation Committee and written informed consent was obtained from the appropriate parent or guardian.
Data Collection
Self-reported ancestry was determined by asking for the origin of the four grandparents of each subject. When 3 grandparents were similarly categorized as Hispanic, Caucasian, or African American, the subject was reported to be of that ancestry, and if there were not three grandparents of the same group, then the ancestry was designated as “other,” based on the likely admixture.
Skin type assessment employed the Fitzpatrick skin pigmentation scale [12]. One of four grades of pigmentation was recorded, based on comparison with a color chart simultaneously examined at the interview. A blood sample was obtained for collection of DNA and serum for measurement of vitamin D metabolites, and other measures as reported previously [3].
Analytical Methods
Genotype analysis
The p.D432E (rs7041) and p.T436K (rs4588) GC SNPs were genotyped with phase assignment based on allele-specific amplification of the p.T436K site followed by restriction endonuclease digestion of the p.D432E site, as described before [9]. Allele-specific amplification was carried out in a 20μL reaction mixture containing 1× PCR buffer (Qiagen), 0.2 mM each of dNTPs, 50 ng genomic DNA, 0.5U HotStarTaq™ (Qiagen) and 0.3–1 μM of the following primers: 5'-GGCATGTTTCACTTTCTGATCTC-3' (forward), 5'-ACCAGCTTTGCCAGTACCG-3' (wild-type reverse) and 5'-GCAAAGTCTGAGTGCTTGTTATGCAGCTTTGCCAGTTGCT-3' (mutant reverse). The underlined bases in the primer sequences are mismatched nucleotides introduced to avoid cross priming. After the initial DNA denaturation and HotStarTaq™ activation at 95°C for 15min, the amplification went through 35 cycles of denaturation at 94°C for 20 sec, annealing at 58°C for 20 sec and extension at 72°C for 20 sec with an increment of 1 sec after each subsequent cycle and a final extension at 72°C for 5 min at the finish. Eight microliters of the amplified products were run in a 2% NuSieve gel containing ethidium bromide and then visualized using UV illumination. The p.T436K wild-type allele produced a 246bp band and the mutant allele a 270bp band. Another 8 μl of amplicon was digested with HaeIII (New England BioLabs) at 37°C overnight and the digested products were analyzed by repeat electrophoresis in 2% NuSieve gel. The DNA bands containing the wild-type p.D432E allele remained unchanged while those with p.D432E mutant allele were cut, producing 221bp fragments.
Given the absence of any recombinants between the two polymorphic loci, assignment of a diplotype for each subject, based on the three haplotype alleles – wildtype (electrophoretic variant 1f), mutant 432E (elecrophoretic variant 1s), and mutant 436K (electrophoretic variant 2) – was unambiguous.
Estimation of ancestry proportions using Ancestry Informative Markers (AIMs)
Ancestry proportions were estimated with a panel of 107 AIMs. These markers are distributed throughout the genome, and were selected based on their high allele frequency differences between European, Native American and African populations. This panel has been used in previous admixture studies in African American and Hispanic populations [13–16]. Genotyping was carried out at the Biomedical Genomics Center of the University of Minnesota. Individual ancestry proportions were estimated with the program ADMIXMAP, which is a general purpose program for modeling population admixture with genotype data, based on a combination of Bayesian and classical methods. For this study, the samples were modeled as formed by admixture between European, Native American and African populations. Allele frequency data from unadmixed individuals were used as a prior distribution for the parental allele frequencies. More information about ADMIXMAP can be found at http://homepages.ed.ac.uk/pmckeigu/admixmap/.
Biochemical assays
Serum 25-OHD was measured by radioimmunoassay kit methodology (DiaSorin, Stillwater, MN). Results of samples analyzed in our assay for serum 25-OHD are consistently found to agree with the mid-range of outcomes of those using this assay and participating in the international DEQAS standardization system [17]. The inter- and intra-assay coefficients of variation for the 25-OHD assay in our hands are 9.6% and 6.6 %, respectively. Serum parathyroid hormone (PTH) was measured as described [3].
Immunonephelometry was performed with a Behring Nephelometer (Behring Diagnostics Inc, Westwood, MA) as described by Wians et al [18]. Briefly, a working Gc (DBP) standard solution (400 μg/mL) was prepared by manually diluting a 1 mg/mL solution of purified DBP protein (Calbiochem, La Jolla, CA) 1:2.5 with N-Diluent (Behring Diagnostics Inc). The working standard solution and patients' serum samples were assayed in duplicate with rabbit antihuman DBP antibody (Ab) (Dako, Carpinteria, CA), supplementary precipitation reagent (Suppl.R.“P”/SRP, Behring Diagnostics Inc). Light scattered by Ag-Ab complexes was measured in light-scattering intensity units or bits. DBP concentration was calculated by automated data reduction by using the logit-log function and linear regression analysis. All samples were assayed in duplicate. Interassay variation was 6.8%. An estimate of free circulating 25-OHD was calculated from the measured total 25-OHD level, and the serum level of DBP, using a formula derived for this purpose and previously reported affinity constants of 25-OHD for DBP and albumin [19]. We assumed a fixed level of the serum albumin concentration for all subjects, as previously validated for calculation of serum free testosterone [20].
Statistical analysis
Descriptive statistics were used to summarize the data. Chi-square tests were performed to test the distribution of genotype among different ancestral groups, especially between African Americans and others. Pearson correlation was used to evaluate the associations among 25-OHD, DBP and AIMs scores. Analysis of variance and/or regression were performed to assess the relative contributions of AIMs score, DBP concentration and GC genotype to the circulating 25-OHD level. Multivariate regression analysis was performed to determine specific significant genetic determinants of circulating 25-OHD. Analyses were adjusted for demographic and nutritional factors found to be significantly associated with 25-OHD as determined previously [3]. We applied a similar model, adding the interaction of GC genotype and 25-OHD to explore the role of genotype with respect to the relationship of 25-OHD to PTH levels. We used a 0.05 significance level and two-sided tests in all cases without adjustments for repeated tests. SAS (version 9.2) was employed for all analyses.
RESULTS
GC variants and Self-reported Ancestry
Consistent with our expectations from earlier reports, there was a significant association between self-reported ancestry and GC alleles. Table 1 shows the frequency of each diplotype, haplotype, and individual SNP variants at the D432E and T436K positions in the DBP protein. African Americans were significantly enriched with the DT (1f) allele (71%, P<0.001), and the DDTT diplotype (homozygotes for the 1f allele; 53%, P < 0.001), compared to the others. Homozygosity for the D432 allele at position rs7041 was greatest in African Americans (71%, P < 0.001), and homozygosity for the T allele at position rs4588 was also greatest in this group (77%, P = 0.015). Among DDTT- identified individuals, African Americans were the most frequently identified group compared with Hispanics (P < 0.001)
Table 1.
Frequency of rs7041/rs4588_diplotypes, haplotypes, and alleles of the GC gene among self-reported ancestral groups
| CAUCASIAN | HISPANIC | AFRICAN AMERICAN | OTHER | ||
|---|---|---|---|---|---|
| N (%) | N (%) | N(%) | N (%) | ||
| Gc Diplotypes (rs7041 + rs4588) | |||||
| Diplotypes | 1f-1f (DDTT) ttcc | 1 (5.9) | 66 (13.1) | 96 (52.8) | 10 (13.9) |
| 1f-1s (DETT) gtcc | 5 (29.4) | 172 (34.1) | 38 (20.9) | 24 (33.3) | |
| 1f-2 (DDTK) ttca | 4 (23.5) | 75 (14.9) | 29 (15.9) | 13 (18.1) | |
| 1s-1s (EETT) ggcc | 5 (29.4) | 91 (18.1) | 6 (3.3) | 12 (16.7) | |
| 1s-2 (DETK) gtca | 2 (11.8) | 82 (16.3) | 9 (5.0) | 10 (13.9) | |
| 2-2 (DDKK) ttaa | 0 (0) | 18 (3.6) | 4 (2.2) | 3 (4.2) | |
| Allele | 1f (DT) tc | (32.4) | (37.6) | (71.1) | (39.6) |
| 1s (ET) gc | (50) | (43.3) | (16.2) | (40.3) | |
| 2 (DK) ta | (17.6) | (19.1) | (12.6) | (20.2) | |
| D432E (rs7041) | |||||
| Genotypes | (EE) gg | 5 (29.4) | 91 (18.1) | 6 (3.3) | 12 (16.7) |
| (ED) gt | 7 (41.2) | 254 (50.4) | 47 (25.8) | 34 (47.2) | |
| (DD) tt | 5 (29.4) | 159 (31.6) | 129 (70.9) | 26 (36.1) | |
| Allele (amino acid) | (E) g | (50) | (43.3) | (16.2) | (40.3) |
| (D) t | (50) | (56.8) | (83.8) | (59.7) | |
| T436K (rs4588) | |||||
| Genotypes | (TT) cc | 11 (64.7) | 329 (65.3) | 140 (76.9) | 46 (63.9) |
| (TK) ca | 6 (35.3) | 157 (31.2) | 38 (20.9) | 23 (31.9) | |
| (KK) aa | 0 (0) | 18 (3.6) | 4 (2.2) | 3 (4.2) | |
| Allele (amino acid) | (T) c | (82.4) | (80.9) | (87.4) | (79.9) |
| (K) a | (17.6) | (19.2) | (12.6) | (20.1) | |
Ancestry Assessment by Ancestry-Informative Markers (AIMs)
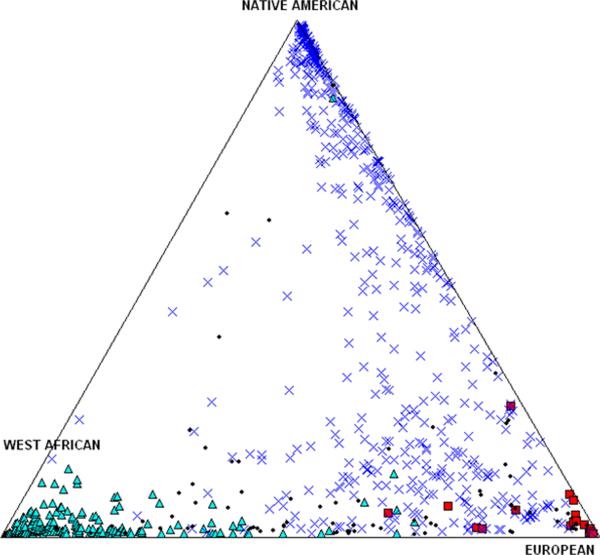
In order to quantitatively analyze the effect of ancestry in this analysis we determined individual admixture proportions with the program ADMIXMAP, as described above. Figure 1 depicts the individual admixture proportions estimated for each subject, classified (by color) according to self-reported ancestry. As expected, self-reported African Americans showed, on average, a high proportion of African ancestry, but some variation is evident in the extent of European contributions. Similarly, self-reported Caucasians primarily had European ancestry, although there was also evidence of African and Native American contributions in some individuals. Finally, self-reported Hispanics had the broadest distribution of individual ancestry proportions. On average, self-reported Hispanics showed a relatively high Native American ancestry, but there is considerable dispersion in the relative Native American, European and African contributions in this group.
Figure 1.
Each individual is represented by symbols indicating their self-reported ancestry (X = Hispanic, Δ = African-American, □ = Caucasian, • = Other). Subjects without 3 grandparents identified as Hispanic, African-American, or Caucasian were identified as “Other.” The positions of the symbols in this triangular plot indicate relative proportions of Native American, European and West African genomic heritage, as determined by a validated panel of ancestral informative markers. The vectorial plot sums three scores for each individual, which sum to 100%. A detailed description is provided in Supplementary Figure 1.
Correlations with Circulating 25-OHD
We next performed a correlative analysis to assess the associations between 25-OHD, circulating DBP, and the African and European admixture proportions derived above. (As the three parental scores sum to 1.00, only two of the three AIMs scores need be included in the analysis; Table 2). Pearson correlations indicate that 25-OHD levels are positively correlated with circulating DBP (R = 0.25, P < 0.001); the regression of this relationship is shown in Figure 2, and is defined by the equation: 25-OHD (nmol/L) = 37.3 + 0.138 DBP (mg/L). Circulating 25-OHD was positively correlated with European ancestry (R = 0.19, P < 0.001), and modestly correlated with African ancestry in an inverse manner (R = −0.09, P = 0.016).
Table 2.
Pearson's correlations among circulating levels of vitamin D metabolites, DBP, and AIMs scores
| DBP | AIM West African | AIM European | AIM Native American | |
|---|---|---|---|---|
| 25-OHD | 0.250* | −0.088* | 0.186* | −0.062 |
| DBP | 0.003 | 0.018 | −0.016 | |
| AIM West African | −0.311* | −0.687* | ||
| AIM European | −0.477* |
P < 0.05
Figure 2.
Regression of circulating 25-OHD level (Y axis) and circulating DBP level (X-axis). 95% confidence intervals are shown in the dotted lines. The regression equation is defined as 25-OHD (nmol/L) = 37.3 + 0.138 DBP (mg/L).
GC Genotype and Circulating DBP Concentrations
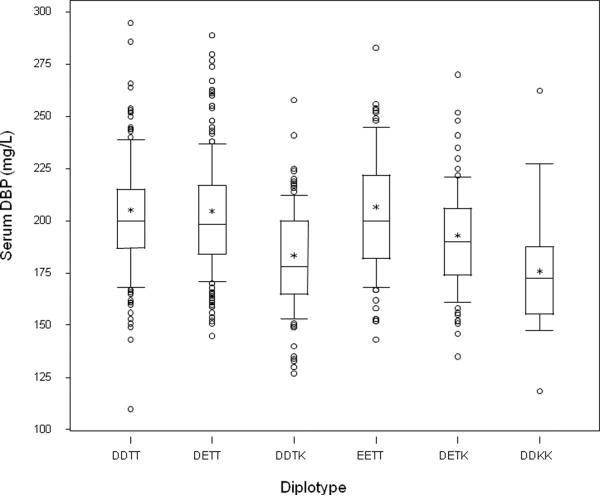
We then examined the impact of GC diplotype (e.g. possible combinations of rs7041 and rs4588 variants) on the circulating DBP level. Mean (± standard deviation) DBP for the entire cohort was 197 ± 29 mg/L. There was no effect of vitamin D intake or season of sampling on circulating DBP levels. As shown in Figure 3, circulating DBP levels (mean ± standard deviation) were greatest in subjects with the EETT diplotype (homozygotes for the allele 1s) (204 ± 31 mg/L), and values were similar for the DDTT (homozygotes for the allele 1f) and DETT (1f/1s heterozygotes) diplotypes (203 ± 30 and 202 ± 27 mg/L, respectively). However, individuals with the 436 K variant (GC2allele) had lower circulating DBP, with DETK (1s/2 heterozygotes) (191 ± 25 mg/L) > DDTK (1f/2 heterozygotes) (181 ± 25 mg/L) > DDKK (homozygous for the 2 allele) (173 ± 32 mg/L).
Figure 3.
Circulating DBP levels in the 6 GC diplotypes (DDTT, DETT, DDTK, EETT, DETK, DDKK) identified from the common variants at rs7041 and rs4588. ANOVA revealed significant differences among groups (P < 0.001) and pairwise comparison testing indicating that diplotypes homozygous for the T allele were no different (P > 0.65). Groups with a K allele present differed from those without a K allele (P < 0.002). The 25th, 50th, and 75th centiles for serum DBP values are represented by the bottom, middle line, and top of the box, respectively. The lower and upper whiskers extend to the 10th and 90th centiles, respectively. The mean value for the population is represented by an asterisk. Circles represent values less than the 10th or greater than 90th centile.
Thus when examining the effect of the SNPs independently there is no effect of the rs7041 (D432E) polymorphism on circulating DBP, but in contrast we find a significant effect of the rs4588 (T436K) marker, with progressive substitution of the K allele for the T allele resulting in lower DBP levels (P < 0.001). The adjusted mean (± standard deviation) of serum DBP levels for the TT, TK and KK haplotypes are 203 ± 29, 185 ± 25 and 173 ± 32 mg/L, respectively. Pairwise comparisons revealed highly significant differences between TT and TK (P < 0.001), and TT and KK groups (P < 0.001), with a near significant difference between TK and KK (P = 0.077). The possibility of a D/E effect (resulting in an increase in circulating DBP) in the setting of the TK heterozygote is of interest in that DBP levels for the DETK diplotype (1s/2 heterozygote) are greater than for the DDTK genotype (1f/2 heterozygote) (P = 0.019), whereas D/E changes in the setting of TT homozgosity at position 436 are not (P > 0.5).
GC Genotypes and 25-OHD
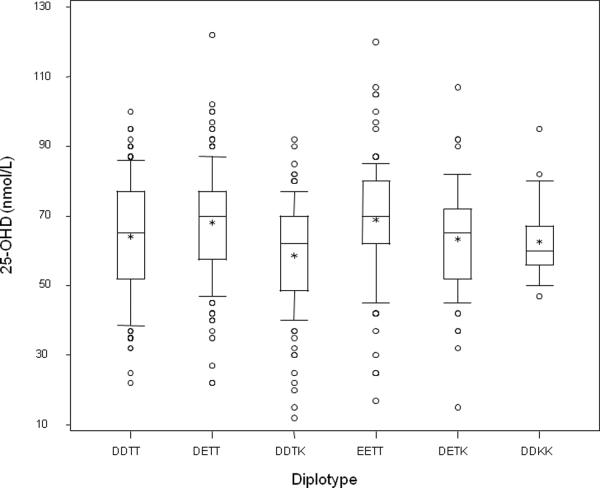
We then examined whether GC genotype served as a determinant of circulating 25-OHD, as has been described in several adult populations, and in genome wide association studies [5–11]. The observed circulating 25-OHD levels by diplotype are shown in Figure 4. Circulating 25-OHD (mean ± standard deviation) is greatest in diplotypes with no T->K substitution at position 436: DDTT (1f/1f) (66.5 ± 34.4 nmol/L), DETT (1f/1s) (68.2 ± 15.2 nmol/L), and EETT (1s/1s) (69.0 ± 17.5 nmol/L); lesser values for 25-OHD were evident in the setting of the presence of the 436 K (2) allele: DDTK (1f/2) (58.7 ± 15.7 nmol/L), DETK (1s/2) (63.4 ± 14.5 nmol/L), and DDKK (2/2) (62.7 ± 11.3 nmol/L). Effects of both rs7041 (D432E) and rs4588 (T436K) polymorphisms on 25-OHD were investigated after adjustment for circulating DBP and AIMs scores. We found no effect of the rs7041 (D432E) polymorphism on circulating 25-OHD (p = 0.53), but a significant effect of the rs4588 (T436K) SNP (P = 0.002).
Figure 4.
Circulating 25-OHD level in the 6 GC diplotypes (DDTT, DETT, DDTK, EETT, DETK, DDKK) identified from the common variants at rs7041 and rs4588. ANOVA revealed differences among groups (P = 0.008). The 25th, 50th, and 75th centiles for serum 25-OHD values are represented by the bottom, middle line, and top of the box, respectively. The lower and upper whiskers extend to the 10th and 90th centiles, respectively. The mean value for the population is represented by an asterisk. Circles represent values less than the 10th or greater than 90th centile.
Moreover, we calculated putative free 25-OHD levels based on the circulating serum DBP, assuming a fixed serum albumin level of 4.3 g/L, and affinity constants as previously reported [19, 20]. Free 25-OHD was strongly correlated with total circulating 25-OHD (R = 0.93, P < 0.0001). In contrast to the effects of genotypes on total 25-OHD, a comparable analysis revealed no effect of genotype on the free 25-OHD (P > 0.2). We also explored the functional role of 25-OHD with respect to the relationship of total or free 25-OHD to circulating PTH as an entire group, and among different GC genotypes. Indeed both total and free 25-OHD were (inversely) correlated with PTH (R = −0.11 and −0.12 respectively, P = 0.0018), but there was no effect of GC genotype on this relationship (P > 0.6).
These analyses indicated significant effects of GC genotype on both the circulating DBP and on the circulating 25-OHD. Moreover, as shown in Figure 2, circulating DBP is related to 25-OHD. Thus it must be considered whether the genotype effect serves as a determinant of 25-OHD entirely because of the genotype effects on circulating DBP, or whether the influence of the genotype may be greater than that explained by a relationship with DBP concentration. We therefore examined the magnitude of these separate effects by multivariate analysis, and demonstrated that only part of the genotype effect on circulating 25-OHD can be attributed to the concomitant effect on DBP levels, suggesting that the effect may be mediated by other mechanisms as well. To explore a more complete model, our multivariate analysis adjusted for the demographic and nutritional variables we previously identified as significant correlates of 25-OHD, including age, season, daily vitamin D intake, and degree of skin pigmentation. Data from this analysis are shown in Table 3. In the complete model, DBP diplotype, circulating DBP, and European ancestry are all significant determinants of the circulating 25-OHD level. Age, estimated vitamin D intake, and season of sampling remain as significant determinants of 25-OHD when diplotype, circulating DBP, and ancestry are included in the analysis. The effect of genotype on circulating 25-OHD levels persists after correction of 25-OHD for circulating DBP level, which is also affected by genotype (Figure 3). The specific effect of diplotype on the 25-OHD level is explained by variance of the rs4588 (T436K) SNP; the means of serum 25-OHD (± standard deviation) are 67.8 ± 24, 62.7 ± 11, and 60.9 ± 15 nmol/L for TT, TK and KK, respectively (Figure 5).
Table 3.
Multivariate analysis of determinants of 25-OHD
| Determinant | Estimator | Direction | P |
|---|---|---|---|
| DBP | 0.1078a | Increases with DBP | <0.001 |
| T436K haplotype | −7.3, −3.3 (TT is reference) | TT>TK>KK | 0.025 |
| Age | −0.2676b | Decreases with age | <0.001 |
| Skin Type | −0.3469 | 0.738 | |
| Vitamin D intake | 0.03164c | Increases with vitamin D intake | <0.001 |
| Season of sampling | −5.7492 | Summer/Fall > Winter/Spring | <0.001 |
| West African AIM score | −0.4204 | 0.88 | |
| European AIM score | 7.8962d | Increases with European ancestry | 0.001 |
The R2 for the model described is 0.25.
For every mg/L increase in DBP, there is a 0.1078 nmol/L increase in 25-OHD.
For every month of age, there is a 0.2676 nmol/L decrease in 25-OHD, or annual decrease of 3.211 nmol/L.
Increasing dietary intake of vitamin D by 100 IU daily increases 25-OHD by 3.164 nmol/L.
The AIM score for each ancestry group can range between 0 and 1. The estimated 25-OHD level in individuals with European AIM score of 1 is 7.8962 nmol/L greater than those with a European AIM score of 0 (no European ancestry).
Figure 5.

Circulating 25-OHD levels in the three T436K (rs4588) haplotypes. The genotype differences are explained by substitution of the T residue with a K residue. Pairwise comparison testing indicates TT > TK (P = 0.024); the TT vs KK assessement approaches significance (P = 0.072), as the KK group is relatively rare. The 25th, 50th, and 75th centiles for serum 25-OHD values are represented by the bottom, middle line, and top of the box, respectively. The lower and upper whiskers extend to the 10th and 90th centiles, respectively. The mean value for the population is represented by an asterisk. Circles represent values less than the 10th or greater than 90th centile.
DISCUSSION
The current study extends our investigation of an urban cohort of minority infants and children to include an analysis of some genetic determinants for circulating 25-OHD. In particular we have focused on diplotypes of common SNPs (rs7041 and rs4588) of the GC gene, encoding DBP, which have been shown to be strong predictors of circulating 25-OHD in adults.. We have shown that individuals self-identified as African American have a significantly higher proportion (53%) of the wildtype diplotype (DDTT; 1f homozygosity) compared to those of Hispanic ethnicity (13%), as posited by Speekaert et al [21]. The DDTT diplotype was evident in 6% of Caucasians, and 14% of the “Other” or mixed ethnicity groups, but the numbers of subjects was substantially smaller than in the African American and Hispanic identified groups. Indeed, the frequency of the other possible diplotypes ranged between 2% and 21% among the African American subjects. Moreover, the DT (1f) allele occurred in 71% of African Americans, but only 32–40% of the others (Table 1). The ET (1s) and DK (2) alleles were much less common in African Americans (16% and 13%, respectively). These data confirm that our sample reflects the genetic variance by reported ancestry as shown by others [9, 10]. Our analysis of AIMs scores among the categories of self-reported ancestry verifies that the self-reporting of ancestry is relatively consistent with validated genetic markers of the specific ancestral background.
We identified a strong correlation between ancestry and circulating 25-OHD. Greater African AIMs scores were correlated with lower 25-OHD levels, while greater European AIMs scores were correlated with higher 25-OHD levels. Although the AIMs scores are necessarily intercorrelated (as all 3 scores for an individual must sum to 1.0), admixture resulted in relatively large variance for Native American AIMs scores (see Figure 1), such that use of this score alone did not reveal significant correlations with the 25-OHD levels. Interestingly, none of the AIMs scores correlated with the circulating DBP level. Yet, as expected, 25-OHD levels were strongly correlated with the circulating DBP level (Table 2 and Figure 2).
The GC genotype was also a strong determinant of circulating DBP level (P < 0.001, Figure 3). The largest effect appears to be related to the T436K SNP, with TT individuals having the highest mean circulating DBP level, followed by TK heterozygotes, and KK homozygotes having the lowest values for this measure. The circulating DBP levels do not differ by D/K status at position 432 in the diplotypes homozygous for the T allele at position 436, however there is a slight increase in the DETK (1s/2 heterozygote) as compared to the DDTK (1f/2 heterozygote) diplotype. The substitution of a lysine (K) for the threonine (T) at position 436 eliminates an O-glycosylation site from the molecule, and there is evidence that loss of glycosylation may affect DBP half-life.. In addition, there may be trans effects, generated by a D->E substitution at the nearby 432 position that influence the extent of O-glycosylation at the 436 position, although this appears to be of significance only in the setting of double heterozygosity, consistent with the 436 allele being the major determinant. It is not known how such changes in the DBP molecule would affect its serum concentration, but the substitutions could result in altered rates of transcription, changes in mRNA stability, or as some have suggested, clearance of the protein itself [2].
Genotype is also a strong determinant of the circulating 25-OHD level (P = 0.005, Figure 4). As with effects on circulating DBP, the major effect appears to be associated with the T436K SNP. 25-OHD level decreases with progressive substitution of threonine (T) residues at position 436 with lysine (K) residues (Figure 5), such that 25-OHD levels are ordered TT > TK > KK, as with DBP.
We used multivariate regression to determine whether the genotype effect on DBP could account for the effects on circulating 25-OHD. We included all significant determinants of the circulating 25-OHD level available in the current analysis (436 T/K haplotype, circulating DBP level, and the West African/European AIMs scores), together with the demographic and nutritional variables previously found to be significant determinants of circulating 25-OHD [3] (age, degree of skin pigmentation, daily vitamin D intake, and season of sampling). Our regression model indicates that genotype is a significant determinant of circulating DBP and 25-OHD, an effect that is, at least in part, independent of possible direct DBP effects on 25-OHD. Overall, the estimated magnitude of the observed predictors for 25OHD are greatest for genotype, ancestry, and season, all of which have relatively larger estimated effects than age and circulating DBP level.
In the currently developed model with GC genotype incorporated as a determinant of the circulating 25-OHD level, the effect of daily vitamin D supplementation is moderately impressive in this age group. Quantitatively, a daily vitamin D intake of 100 IU may account for a 3.16 nmol/L increase in the circulating 25-OHD level. However, the relative importance of the T436K genetic effect appeared greater still. Mean circulating 25-OHD in the TT genotype group was 3.3 ± 1. 5 nmol/L greater than that for the TK genotype, and 7.3 ± 4.1 nmol/L greater than in the KK group (P = 0.025). Thus progressive substitution of the T allele correlates with the circulating 25-OHD level.
Our findings are consistent with the notion that DBP concentration and diplotype influence the total circulating reservoir of 25-OHD, but the regulated compartment may be free 25-OHD. Indeed recent examination of bioavailability of vitamin D metabolites by Chun et al [22], ascertained by mathematical modeling, suggests that the DBP variants studied here have differences in binding affinity for both 25-OHD and 1,25(OH)2D. In the context of our finding that total, but not free, 25-OHD is determined by GC diplotype, it appears that homeostatic mechanisms favor regulating circulating levels of free 25-OHD, independent of genotype. Moreover Chun et al predict that the TK variants have an ordered effect on affinity for 25-OHD, in keeping with the effects on circulating levels of this metabolite we observed. It is of interest that these GC SNPs are not situated within the vitamin D binding site of the protein [23], suggesting that secondary or higher order structure may be important in determining the binding affinity. It should also be considered that GC genotype may influence 25-OHD levels beyond its effects on DBP concentration: As DBP serves as the ligand for the megalin/cubulin complex that mediates entry of bound 25-OHD into renal tubular cells [24], the protein may also be involved in regulating bioavailability of the active metabolite, 1,25(OH)2D. Other mechanisms worth further investigation include the possibility that genotype may affect DBP clearance from the circulation, binding affinities with other cell surface receptors related to tissue delivery [25], metabolizing hydroxylase enzymes, or the vitamin D receptor itself.
Supplementary Material
ACKNOWLEDGEMENTS
We wish to acknowledge the support and cooperation of the following individuals who made this project possible: Drs. Elizabeth Bailey and Mary Ellen Flaherty-Hewitt at the Hospital of St.Raphael, Drs. Robert Windom and Steven Updegrove at the Hill Health Center, Dr. Brian Forsyth of the Yale-New Haven Hospital Primary Care Clinic, Dr. Semeon Tsalbins of the Fair Haven Health Center, all in New Haven, CT. We thank Dr. Francisca Herreros and Ms. Esther Torrealba-Fox for their dedicated assistance, and Dr. Hong-Yu Zhao for his advice and fruitful discussion. Finally we thank the children of New Haven and their families for their generosity in participating in the study.
These studies were carried out with the support of the Gerber Foundation, and made possible by the Yale Center for Clinical Investigation (supported by CTSA UL1 RR024139 from the National Center for Research Resources of the NIH), and the Dairy Farmers of Canada (DECC, LF, and BY-LW). Support for data analysis was provided in part by the Thrasher Research Fund Award 02829-4 (to TOC) and the National Institute of Child Health and Development of the NIH, (ARRA Award 5RC1HD063562 (to TOC).
Funding Sources: These studies were carried out with the support of the Gerber Foundation, and made possible by the Yale Center for Clinical Investigation (supported by CTSA UL1 RR024139 from the National Center for Research Resources of the NIH), and the Dairy Farmers of Canada (DECC, LF, and BY-LW). Support for data analysis was provided in part by the Thrasher Research Fund Award 02829-4 (TOC) and the National Institute of Child Health and Development of the NIH (ARRA Award 5RC1HD063562 (to TOC).
Footnotes
DISCLOSURE PAGE All authors state that they have no conflict of interest.
REFERENCES
- 1.Whiting SJ, Calvo MS. Nutrition and lifestyle effects on vitamin D status. In: Feldman D, Pike JW, Adams JS, editors. Vitamin D. 3rd ed. Academic Press; London: 2011. pp. 979–1007. [Google Scholar]
- 2.Bouillon R. The vitamin D binding protein DBP. In: Feldman D, Pike JW, Adams JS, editors. Vitamin D. 3rd ed. Academic Press; London: 2011. pp. 57–72. [Google Scholar]
- 3.Carpenter TO, Herreros F, Zhang JH, Ellis BK, Simpson C, Torrealba-Fox E, Kim GJ, Savoye M, Held NA, Cole DE. Demographic, dietary, and biochemical determinants of vitamin D status in inner-city children. Am J Clin Nutr. 2012 Jan;95(1):137–46. doi: 10.3945/ajcn.111.018721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.DeLucia MC, Mitnick ME, Carpenter TO. Nutritional rickets with normal circulating 25-hydroxyvitamin D: a call for reexamining the role of dietary calcium intake in North American infants. J Clin Endocrinol Metab. 2003 Aug;88(8):3539–45. doi: 10.1210/jc.2002-021935. [DOI] [PubMed] [Google Scholar]
- 5.Wjst M, Altmüller J, Braig C, Bahnweg M, André E. A genome-wide linkage scan for 25-OH-D(3) and 1,25-(OH)2-D3 serum levels in asthma families. J Steroid Biochem Mol Biol. 2007 Mar;103(3–5):799–802. doi: 10.1016/j.jsbmb.2006.12.053. [DOI] [PubMed] [Google Scholar]
- 6.Engelman CD, Meyers KJ, Ziegler JT, Taylor KD, Palmer ND, Haffner SM, Fingerlin TE, Wagenknecht LE, Rotter JI, Bowden DW, Langefeld CD, Norris JM. Genome-wide association study of vitamin D concentrations in Hispanic Americans: the IRAS family study. J Steroid Biochem Mol Biol. 2010 Oct;122(4):186–92. doi: 10.1016/j.jsbmb.2010.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang TJ, Zhang F, Richards JB, Kestenbaum B, van Meurs JB, Berry D, Kiel DP, Streeten EA, Ohlsson C, Koller DL, Peltonen L, Cooper JD, O'Reilly PF, Houston DK, Glazer NL, Vandenput L, Peacock M, Shi J, Rivadeneira F, McCarthy MI, Anneli P, de Boer IH, Mangino M, Kato B, Smyth DJ, Booth SL, Jacques PF, Burke GL, Goodarzi M, Cheung CL, Wolf M, Rice K, Goltzman D, Hidiroglou N, Ladouceur M, Wareham NJ, Hocking LJ, Hart D, Arden NK, Cooper C, Malik S, Fraser WD, Hartikainen AL, Zhai G, Macdonald HM, Forouhi NG, Loos RJ, Reid DM, Hakim A, Dennison E, Liu Y, Power C, Stevens HE, Jaana L, Vasan RS, Soranzo N, Bojunga J, Psaty BM, Lorentzon M, Foroud T, Harris TB, Hofman A, Jansson JO, Cauley JA, Uitterlinden AG, Gibson Q, Jarvelin MR, Karasik D, Siscovick DS, Econs MJ, Kritchevsky SB, Florez JC, Todd JA, Dupuis J, Hypponen E, Spector TD. Common genetic determinants of vitamin D insufficiency: a genome-wide association study. Lancet. 2010 Jul 17;376(9736):180–8. doi: 10.1016/S0140-6736(10)60588-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ahn J, Yu K, Stolzenberg-Solomon R, Simon KC, McCullough ML, Gallicchio L, Jacobs EJ, Ascherio A, Helzlsouer K, Jacobs KB, Li Q, Weinstein SJ, Purdue M, Virtamo J, Horst R, Wheeler W, Chanock S, Hunter DJ, Hayes RB, Kraft P, Albanes D. Genome-wide association study of circulating vitamin D levels. Hum Mol Genet. 2010 Jul 1;19(13):2739–45. doi: 10.1093/hmg/ddq155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gozdzik A, Zhu J, Wong BY, Fu L, Cole DE, Parra EJ. Association of vitamin D binding protein (VDBP) polymorphisms and serum 25(OH)D concentrations in a sample of young Canadian adults of different ancestry. J Steroid Biochem Mol Biol. 2011 Nov;127(3–5):405–12. doi: 10.1016/j.jsbmb.2011.05.009. [DOI] [PubMed] [Google Scholar]
- 10.Engelman CD, Fingerlin TE, Langefeld CD, Hicks PJ, Rich SS, Wagenknecht LE, Bowden DW, Norris JM. Genetic and environmental determinants of 25-hydroxyvitamin D and 1,25-dihydroxyvitamin D levels in Hispanic and African Americans. J Clin Endocrinol Metab. 2008 Sep;93(9):3381–8. doi: 10.1210/jc.2007-2702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lauridsen AL, Vestergaard P, Hermann AP, Brot C, Heickendorff L, Mosekilde L, Nexo E. Plasma concentrations of 25-hydroxy-vitamin D and 1,25-dihydroxy-vitamin D are related to the phenotype of Gc (vitamin D-binding protein): a cross-sectional study on 595 early postmenopausal women. Calcif Tissue Int. 2005 Jul;77(1):15–22. doi: 10.1007/s00223-004-0227-5. [DOI] [PubMed] [Google Scholar]
- 12.Fitzpatrick TB. The validity and practicality of sun-reactive skin types I through VI. Arch Dermatol. 1988 Jun;124(6):869–71. doi: 10.1001/archderm.124.6.869. [DOI] [PubMed] [Google Scholar]
- 13.Yaeger R, Avila-Bront A, Abdul K, Nolan PC, Grann VR, Birchette MG, Choudhry S, Burchard EG, Beckman KB, Gorroochurn P, Ziv E, Consedine NS, Joe AK. Comparing genetic ancestry and self-described race in African Americans born in the United States and in Africa. Cancer Epidemiol Biomarkers Prev. 2008 Jun;17(6):1329–38. doi: 10.1158/1055-9965.EPI-07-2505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fejerman L, John EM, Huntsman S, Beckman K, Choudhry S, Perez-Stable E, Burchard EG, Ziv E. Genetic ancestry and risk of breast cancer among U.S. Latinas. Cancer Res. 2008 Dec 1;68(23):9723–8. doi: 10.1158/0008-5472.CAN-08-2039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lai CQ, Tucker KL, Choudhry S, Parnell LD, Mattei J, Garcia-Bailo B, Beckman K, Burchard EG, Ordovas JM. Population admixture associated with disease prevalence in the Boston Puerto Rican health study. Hum Genet. 2009 Mar;125(2):199–209. doi: 10.1007/s00439-008-0612-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kumar R, Seibold MA, Aldrich MC, Williams LK, Reiner AP, Colangelo L, Galanter J, Gignoux C, Hu D, Sen S, Choudhry S, Peterson EL, Rodriguez-Santana J, Rodriguez-Cintron W, Nalls MA, Leak TS, O'Meara E, Meibohm B, Kritchevsky SB, Li R, Harris TB, Nickerson DA, Fornage M, Enright P, Ziv E, Smith LJ, Liu K, Burchard EG. Genetic ancestry in lung-function predictions. N Engl J Med. 2010 Jul 22;363(4):321–30. doi: 10.1056/NEJMoa0907897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hyppönen E, Turner S, Cumberland P, Power C, Gibb I. Serum 25-hydroxyvitamin D measurement in a large population survey with statistical harmonization of assay variation to an international standard. J Clin Endocrinol Metab. 2007 Dec;92(12):4615–22. doi: 10.1210/jc.2007-1279. [DOI] [PubMed] [Google Scholar]
- 18.Wians FH, Jr., Lin W, Brown LP, Schiodt FV, Lee WM. Immunonephelometric quantification of group-specific component protein in patients with acute liver failure. Liver Transpl Surg. 1997 Jan;3(1):28–33. doi: 10.1002/lt.500030104. [DOI] [PubMed] [Google Scholar]
- 19.Powe CE, Ricciardi C, Berg AH, Erdenesanaa D, Collerone G, Ankers E, Wenger J, Karumanchi SA, Thadhani R, Bhan I. Vitamin D binding protein modifies the vitamin D-bone mineral density relationship. J Bone Miner Res. 2011 Jul;26(7):1609–16. doi: 10.1002/jbmr.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vermeulen, Verdonck L, Kaufman JM. A Critical Evaluation of Simple Methods for the Estimation of Free Testosterone in Serum. J Clin Endocrinol Metab. 1999 Oct;84(10):3666–3672. doi: 10.1210/jcem.84.10.6079. [DOI] [PubMed] [Google Scholar]
- 21.Speeckaert M, Huang G, Delanghe JR, Taes YE. Biological and clinical aspects of the vitamin D binding protein (Gc-globulin) and its polymorphism. Clin Chim Acta. 2006 Oct;372(1–2):33–42. doi: 10.1016/j.cca.2006.03.011. [DOI] [PubMed] [Google Scholar]
- 22.Chun RF, Peercy BE, Adams JS, Hewison M. Vitamin D binding protein and monocyte response to 25-hydroxyvitamin D and 1,25-dihydroxyvitamin D: analysis by mathematical modeling. PLoS One. 2012;7(1):e30773. doi: 10.1371/journal.pone.0030773. Epub 2012 Jan 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Verboven C, Rabijns A, De Maeyer M, Van Baelen H, Bouillon R, De Ranter C. A structural basis for the unique binding features of the human vitamin D-binding protein. Nat Struct Biol. 2002 Feb;9(2):131–6. doi: 10.1038/nsb754. [DOI] [PubMed] [Google Scholar]
- 24.Nykjaer A, Dragun D, Walther D, Vorum H, Jacobsen C, Herz J, Melsen F, Christensen EI, Willnow TE. An endocytic pathway essential for renal uptake and activation of the steroid 25-(OH) vitamin D3. Cell. 1999 Feb 19;96(4):507–15. doi: 10.1016/s0092-8674(00)80655-8. [DOI] [PubMed] [Google Scholar]
- 25.Chun RF, Lauridsen AL, Suon L, Zella LA, Pike JW, Modlin RL, Martineau AR, Wilkinson RJ, Adams J, Hewison M. Vitamin D-binding protein directs monocyte responses to 25-hydroxy- and 1,25-dihydroxyvitamin D. J Clin Endocrinol Metab. 2010 Jul;95(7):3368–76. doi: 10.1210/jc.2010-0195. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.