Abstract
Delta opioid receptors represent a promising target for the development of novel analgesics. A number of tools have been developed recently that have significantly improved our knowledge of delta receptor function in pain control. These include several novel delta agonists with potent analgesic properties, as well as genetic mouse models with targeted mutations in the delta opioid receptor gene. Also, recent findings have further documented the regulation of delta receptor function at cellular level, which impacts on the pain-reducing activity of the receptor. These regulatory mechanisms occur at transcriptional and post-translational levels, along agonist-induced receptor activation, signaling and trafficking, or in interaction with other receptors and neuromodulatory systems. All these tools for in vivo research, as well as proposed mechanisms at molecular level, have tremendously increased our understanding of delta receptor physiology, and contribute to designing innovative strategies for the treatment of chronic pain and other diseases such as mood disorders.
Keywords: delta opioid receptor, analgesia, pain, mouse genetic model, rat, regulation, mechanism
Introduction
Morphine and other related opiates are potent analgesics widely used for pain treatment. These compounds act on opioid receptors to inhibit pain transmission and perception. The three opioid receptors, mu, delta and kappa receptors, are transmembrane G-proteins coupled receptors encoded by Oprm1, Oprd1 and Oprk1 genes, respectively (Kieffer & Gaveriaux-Ruff, 2002; Stevens, 2009). Opioid receptors are activated endogenously by the opioid peptides enkephalins, beta-endorphin and dynorphins processed from large precursor proteins encoded by Penk, Pomc and Pdyn genes (Akil et al., 1998). The opioid system plays a central role in pain control, as well as in reward (Mendez & Morales-Mulia, 2008; Rodriguez-Arias et al., 2010; Shippenberg et al., 2008) and neuroptoection (Chao & Xia, 2010; Johnson & Turner, 2010). In addition this system regulates a number of physiological functions that include respiration and gastrointestinal transit, as well as endocrine and immune systems (Kieffer & Gaveriaux-Ruff, 2002; Sauriyal et al., 2010).
Since the initial molecular cloning (Evans et al., 1992; Kieffer et al., 1992), a number of tools have been developed enabling better understanding of delta receptor function in nociception and pain. Research on delta opioid analgesia has benefited from a series of novel agonists that extend the panel of pharmacological tools available to study delta receptors in pain control. Mouse genetic models have been created, that harbor targeted mutations of the receptor. These mice represent unique tools for assessing both the endogenous pain-reducing delta tone and the implication of delta receptors in the actions of opioid as well as non-opioid analgesics. These tools will be presented here, together with novel mechanisms for the development of therapeutics strategies targeting the delta opioid receptor.
In addition, there was tremendous progress in understanding how delta receptor expression and activity are regulated, and these mechanisms heavily contribute to delta analgesia. Receptor regulation takes place at different levels, including genomic and transcriptional controls in the nucleus, post-translational events in the cytoplasm and endoplasmic reticulum, interaction of delta receptors with other receptors at the cell membrane, and processes that follow delta receptor activation within the cell, and these aspects will be discussed.
Altogether, delta opioid receptor research represents a very active field of investigation, with about 1000 publications within the last five years. This review therefore cannot be exhaustive and only recent reviews or publications are cited.
Delta opioid analgesia: from early pharmacology to novel agonists
In early opioid pharmacology, two peptidic agonists DPDPE ([2-D-penicillamin, 5-D-penicillamin]-enkephalin) (Mosberg et al., 1983) and deltorphin (Kreil et al., 1989) were most frequently used to study delta receptor function in pain control (Chang et al., 2004). However, their peptidic nature prevented their use by systemic administration in vivo. Later, the non-peptidic agonists BW373U86 and SNC80 (Bilsky et al., 1995; Chang et al., 2004), and the antagonist naltrindole (Portoghese et al., 1988) (Chang et al., 2004) proved to be more stable ligands in vivo to investigate delta receptor-mediated analgesia.
The identification of several novel delta agonists has further broadened the repertoire of molecules available to study delta receptors in vivo (Pradhan et al., 2011; Vanderah, 2010). Over the past decade, SB-235863 (Petrillo et al., 2003), DValAla-Enk (Brainin-Mattos et al., 2006), NIH 11082 (Aceto et al., 2007), AR-M100390 (Pradhan et al., 2009), ADL5859 (Le Bourdonnec et al., 2008), ADL5747 (Le Bourdonnec et al., 2009), JNJ-20788560 (Codd et al., 2009), compound 8e (Jones et al., 2009) and KNT-127 (Saitoh et al., 2011) have been developed as analgesics in preclinical models. Table 1 summarizes the analgesic effects of these novel agonists in several pain models, as well as analgesic effects of previously described reference molecules such as DPDPE, DSLET, deltorphin, SNC80 or Tan-67. Altogether, the data cited in Table 1 indicate that delta receptor activation diminishes chronic pain in the three mouse, rat and monkey species. Table 1 also shows that several distinct chronic pain models are sensitive to delta agonists, including inflammatory, neuropathic, cancer and diabetic pain. Moreover, delta receptor activation reduces hypersensitivity in heat, cold and mechanical modalities. Altogether, delta opioid agonists efficiently decrease chronic pain in many preclinical models and clinical trials will validate their translational potential to patients.
Table 1.
Analgesic effects of delta opioid agonists
| Agonist | Pain model# | Species | Pain Modality | Reference |
|---|---|---|---|---|
| DPDPE*/ DSLET | Formalin | Rat | (Obara et al., 2009) | |
| Capsaicin | Rat | (Saloman et al., 2011) | ||
| Inflammatory | Rat | M* | (Stein et al., 1989) | |
| Mouse | H* | (Hervera et al., 2009) | ||
| Rat | M | (Zhou et al., 1998) | ||
| Neuropathic | Mouse | H, M, C* | (Hervera et al., 2010) | |
| Rat | H, M | (Obara et al., 2009) | ||
| Rat | C | (Mika et al., 2001) | ||
| Cancer | Mouse | H | (Baamonde et al., 2005) | |
|
| ||||
| Deltorphin | Formalin | Mouse | (Morinville et al., 2003) | |
| Rat | (Bilsky et al., 1996) | |||
| Rat | (Cahill et al., 2001) | |||
| Rat | (Pradhan et al., 2006) | |||
| Inflammatory | Rat | H | (Fraser et al., 2000) | |
| Rat | H | (Hurley & Hammond, 2000) | ||
| Rat | H | (Cahill et al., 2003) | ||
| Rat | H | (Gendron et al., 2007a) | ||
| Rat | M | (Otis et al., 2011) | ||
| Rat | H | (Beaudry et al., 2009) | ||
| Mouse | H | (Gendron et al., 2007b) | ||
| Mouse | H | (Dubois & Gendron) | ||
| Neuropathic | Rat | C | (Mika et al., 2001) | |
| Rat | M, C | (Holdridge & Cahill, 2007) | ||
| Rat | M | (Kabli & Cahill, 2007) | ||
| Cancer | Rat | M | (Otis et al., 2011) | |
|
| ||||
| SNC80 | Formalin | Mouse | (Barn et al., 2001) | |
| Rat | (Obara et al., 2009) | |||
| GDNF hyperalgesia | Rat | M | (Joseph & Levine) | |
| NGF hyperalgesia | Rat | M | (Joseph & Levine) | |
| PGE2 hyperalgesia | Monkey | H | (Brandt et al., 2001) | |
| Rat | M | (Pacheco & Duarte, 2005) | ||
| Dynorphin A allodynia | Rat | M | (Kawaraguchi et al., 2004) | |
| Inflammatory | Monkey | H | (Brandt et al., 2001) | |
| Rat | H | (Fraser et al., 2000) | ||
| Rat | M | (Cao et al., 2001) | ||
| Rat | H | (Gallantine & Meert, 2005) | ||
| Mouse | H | (Gendron et al., 2007b) | ||
| Mouse | H, M | (Gaveriaux-Ruff et al., 2008) | ||
| Mouse | H, M | (Pradhan et al., 2009; | ||
| Pradhan et al., 2010) | ||||
| Neuropathic | Mouse | M | (Scherrer et al., 2009) | |
| Monkey | H | (Brandt et al., 2001) | ||
| Mouse | M | (Scherrer et al., 2009) | ||
| Mouse | H, M | (Gaveriaux-Ruff et al., 2011) | ||
| Rat | H, M | (Obara et al., 2009) | ||
|
| ||||
| Tan-67 | Formalin | Mouse | (Barn et al., 2001) | |
| Diabetes | Mouse | H | (Kamei et al., 1997) | |
|
| ||||
| SB-235863 | Inflammatory | Rat | H | (Petrillo et al., 2003) |
| Rat | H | (Beaudry et al., 2009) | ||
| Neuropathic | Rat | H | (Petrillo et al., 2003) | |
|
| ||||
| DValAla-Enk | Cancer | Mouse | M | (Brainin-Mattos et al., 2006) |
|
| ||||
| AR-M100390 | Inflammatory | Mouse | H, M | (Pradhan et al., 2009) |
| Mouse | H, M | (Pradhan et al., 2010) | ||
|
| ||||
| NIH 11082 | Inflammatory | Rat | M | (Aceto et al., 2007) |
|
| ||||
| ADL5859 | Inflammatory | Rat | M | (Le Bourdonnec et al., 2008) |
|
| ||||
| ADL5747 | Inflammatory | Rat | M | (Le Bourdonnec et al., 2009) |
|
| ||||
| JNJ-20788560 | Inflammatory | Rat | H | (Codd et al., 2010) |
|
| ||||
| Compound 8e | Inflammatory | Rat | n.i. | (Jones et al., 2009) |
|
| ||||
| KNT-127 | Formalin | Mouse | (Saitoh et al., 2011) | |
Visceral pain was induced by intraperitoneal acetic acid injection; inflammatory pain by injection of Complete Freund’s Adjuvant (CFA) , zymosan, yeast, or carrageenan ; neuropathic pain was induced by sciatic nerve or spinal nerve ligation; cancer pain by injection of NCTC 2472 osteosarcoma cells,
Abbreviations: C, cold: DPDPE, (D-Pen2, D-Pen5)-enkephalin; DSLET, (D-Ser2,Leu5)-enkephalin; GDNF Glial cell line-derived neurotrophic factor; H, heat; M mechanical; NGF, nerve growth factor; PGE2, prostaglandin-E2
Mouse genetic models to study delta receptor function in vivo
Delta opioid receptor knockout mice
A major step for understanding delta receptor function was the generation of mice with a targeted inactivation of the Oprd1 gene, or delta receptor knockout mice (Filliol et al., 2000)(Zhu et al., 1999). A prime finding with these mutant mice was the discovery that delta receptors plays a critical role in the control of emotional responses (Filliol et al., 2000), revealing anxiolytic and antidepressant activities of the receptor that were further confirmed by the pharmacology (Pradhan et al., 2011) (Jutkiewicz, 2006; Noble & Roques, 2007)(Javelot et al., 2010). This mouse line represents a unique genetic tool to assess both the influence of endogenous delta receptor tone on pain physiology, and the in vivo selectivity of known or new delta agonists.
Delta receptor knockout mice showed no change or only subtle alterations in their sensitivity to acute pain (Contet et al., 2006; Filliol et al., 2000; Gaveriaux-Ruff et al., 2008; Martin et al., 2003; Nadal et al., 2006; Pradhan et al., 2011) and stress-induced analgesia developed normally (Contet et al., 2006). Interestingly however, delta receptor knockout mice showed augmented neuropathic and inflammatory pain (Gaveriaux-Ruff et al., 2008; Nadal et al., 2006), suggesting that endogenous delta opioid activity alleviates chronic pain. Altogether, data from the genetic approach are in agreement with the notion that delta agonists barely modulate acute nociception, but are most efficient under conditions of persistent pain (see below). SNC80-induced analgesia was abolished in delta receptor knockout animals in a model of inflammatory hyperalgesia induced by Complete Freund’s Adjuvant (Gaveriaux-Ruff et al., 2008). These data confirmed the in vivo selectivity of the compound previously proposed by the pharmacology (Chang et al., 2004).
Interestingly, chronic treatment with tricyclic antidepressants, which produce anti-allodynic effects in a neuropathic pain model, was ineffective in delta receptor knockout mice (Benbouzid et al., 2008). These data revealed the implication of delta receptors in the analgesic effects of tricyclic anti-depressants, likely downstream from aminergic transporter systems. The implication of endogenous opioid mechanisms in this particular activity of anti-depressant drugs was specifically mediated by delta receptors, since the compounds were fully effective in mu receptor knockout animals (Bohren et al., 2010). Along this line, anti-allodynia induced by chronic beta2-agonists was blocked by the delta receptor antagonist naltrindole (Yalcin et al., 2010) suggesting an interaction between beta2-adrenergic and delta receptor systems. In the future, delta receptor knockout mice may help to reveal the interaction of delta receptor with other receptor systems in pain control.
Conditional knockout mice lacking delta opioid receptors in Nav1.8 nociceptive neurons
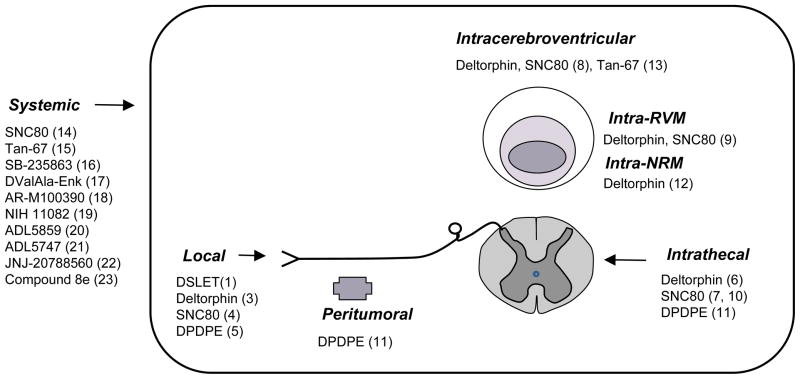
Under chronic pain, delta receptor activation produces analgesia at different sites within nociceptive circuits. Delta agonists are effective when administered systemically, intrathecally, intracerebroventricularly, or into the rostral ventromedial medulla or Nucleus Raphe Magnus (Figure 1). A potent analgesia was also obtained at the periphery where nociceptive processing is initiated, and opioid receptors of the peripheral nervous system are proposed as therapeutic targets to limit the centrally-mediated adverse effects of opiates. First trials have been promising (Stein et al., 1990) and the field of peripheral opioid receptors has gained importance lately (Hua & Cabot, 2010; Stein et al., 2009).
Figure 1.
Sites for delta opioid analgesia. This scheme summarizes sites where delta agonist administration induces analgesia in animal models of chronic pain (see table1).
*Abbreviations: DPDPE, (D-Pen2, D-Pen5)-enkephalin; DSLET, (D-Ser2,Leu5)-enkephalin; NRM, Nucleus Raphe Magnus ; RVM, Rostroventral medulla
To inactivate genes specifically in nociceptive primary afferents, a conditional knockout strategy has been reported for the cannabinoid CB1 receptor (Agarwal et al., 2007) and Nav1.7 channel (Nassar et al., 2004). The approach was based on the Cre-Lox system and used a mouse line expressing Cre recombinase in Nav1.8+ sensory neurons that include unmyelinated C and thinly myelinated Ad nociceptive neurons. The same driver Cre line was used recently to inactivate Oprd1 in these neurons and examine whether peripheral delta receptors contribute to pain control (Gaveriaux-Ruff et al., 2011). In conditional knockout mutants, analgesia induced by intraperitoneal SNC80 treatment was abolished under both inflammatory and neuropathic conditions, revealing the essential role of peripheral delta receptors in systemic delta opioid analgesia. These peripheral receptors are not necessarily sufficient to produce the full analgesic response, since delta receptors expressed in peripheral non-Nav1.8 DRG neurons, at the level of spinal cord or in the brain (Ossipov et al., 2010) may also participate in systemic delta analgesia. The results nevertheless support the notion that developing peripherally-acting delta agonists is a feasible strategy for the design of novel effective analgesics devoid of centrally-mediated side effects.
Knock-in mice expressing a functional green fluorescent delta opioid receptor
A unique genetic mouse model was developed, in order to investigate the distribution of delta receptors throughout the nervous system, and the link between receptor localization at a subcellular level and receptor function in vivo. In these mice, endogenous delta receptors are replaced by delta receptors in fusion with green fluorescent protein (DOR-eGFP) using a knock-in strategy (Scherrer et al., 2006). DOR-eGFP mice express fully functional delta receptors, which are directly visible in vivo. Fluorescent delta receptors are expressed in DRGs, spinal cord and brain (Scherrer et al., 2006, 2009,), with profiles in accordance with results from in situ hybridization (Mansour et al., 1995; Mennicken et al., 2003) and ligand binding autoradiography (Goody et al., 2002). In models of inflammatory pain, DOR-eGFP mice respond to delta agonist-induced analgesia as control wild-type mice (Pradhan et al., 2009., 2010). These animals have been instrumental in deciphering differential internalizing properties of the two delta agonists SNC80 and AR-M100390 throughout the nervous system in vivo, and elucidating short-and long-term consequences of ligand-biased receptor trafficking on analgesic responses (see details below and (Pradhan et al., 2009., 2010).
Delta receptor expression and function are regulated at different levels within the cell
A major factor contributing to delta opioid analgesia is the expression level of the receptor protein at the cell surface of neurons, and at the different sites of the pain-processing pathways. Variations in receptor density may result from sequence variations within the Oprd1 gene, transcriptional regulation, post-translational events or receptor trafficking to and from the plasma membrane.
Genetic variability
Sequence variants within the Oprd1 gene may influence delta receptor expression across individuals, and may contribute to inter-individual differences in responses to delta drugs (Lotsch & Geisslinger, 2011). In the human gene, several single nucleotide polymorphisms (SNP) have been identified (Kim et al., 2006). The T80G variant in exon 1, occurring at an allele frequency of 0.08, leads to a Phe27Cys substitution in the extracellular N-terminal domain, and has been associated with pain sensitivity (Kim et al., 2006). In heterologous expression systems, the Cys27 variant showed lower maturation efficiency, increased accumulation of receptor precursors in pre-Golgi compartment and faster constitutive internalization (Leskela et al., 2009). Other human SNP variants showed no association with pain sensitivity (Huang et al., 2008; Kim et al., 2006; Zhang et al., 2010). In the mouse Oprd1 gene, polymorphism was identified in mice selectively bred for high and low stress-induced analgesia, and later shown to differ also in basal nociception and opioid analgesia. In this study, three polymorphic sites were detected in the Oprd1 coding region (Sacharczuk et al., 2010). Among these, the C320T transition resulted in an A107V substitution in the first extracellular loop of the delta receptor protein. The C320T decreased SNC80-induced analgesia in thermal pain tests. Altogether, the identification of natural genetic variants affecting extracellular domains of the delta receptor and altering pain perception, opens the possibility of inter-individual variability in responses to delta agonists.
Transcriptional regulation
Pathological situations like chronic pain, inflammation and nerve damage were shown to induce transcriptional regulation of the delta receptor that may impact on receptor activity. Transcription factors binding to Oprd1 promoter sequences and regulating gene expression, as well as epigenetic aspects, have recently been reviewed extensively (Wei & Loh, 2011). Regulation at the transcriptional level of delta receptor expression was investigated in several models of chronic pain and appears highly variable depending on a series of factors including (i) the pain model, (ii) the receptor localization (brain, spinal cord, DRG, sciatic nerve or skin), (iii) time points considered after pain initiation, (iv) the animal strain (Herradon et al., 2008) and (v) the technique used for detecting receptor expression. Data are summarized in Table 2. In the different models of inflammatory pain, no change, up-regulation or down-regulation of delta receptor mRNA levels were reported. In models of neuropathic pain also, several regulation patterns were observed and together the data indicate that regulation at transcriptional level may be detectable in specific pain situations, and likely impact on delta receptor protein levels (Table 2). Most expression studies were performed at the level of DRG and spinal cord. Delta receptor activity, as measured by agonist-induced G protein activation, was also altered in the cingulate cortex and amygdala under conditions of inflammatory and neuropathic pain (Narita et al., 2006a,b). This particular regulation in the central nervous system was suggested to contribute to chronic pain-induced emotional dysfunction (Narita et al., 2006a,b). Finally, delta receptor expression was highly increased under two clinical pain conditions, in the skin of fibromyalgia patients (Salemi et al., 2007) as well as in hypertrophic scars (Cheng et al., 2008). Collectively the data show that delta receptor expression is regulated under experimental chronic pain, and more investigations in pain patients are needed to correlate findings in animal models with clinical situations.
Table 2.
Changes in delta opioid receptor expression in chronic pain models
| Pain model | Species | Time and region/cell type examined | mRNA or protein - technique | Change | Reference |
|---|---|---|---|---|---|
| Inflammatory pain | |||||
| CFA* into paw | rat | DRG* | mRNA Q-RT-PCR* | No | (Gendron et al., 2006) |
| CFA into paw | rat | DRG | mRNA Q-RT-PCR | No | (Puehler et al., 2004) |
| CFA into paw | rat | DRG | Protein immunohistochemistry | No | (Brack et al., 2004) |
| CFA into paw | rat | DRG | Protein immunohistochemistry | Down | (Zhang et al., 1998) |
| CFA into paw | rat | Spinal cord | mRNA Q-RT-PCR | No | (Obara et al., 2009) |
| CFA into paw | rat | Spinal cord | mRNA ISH* Protein Western blot | Up | (Cahill et al., 2003) |
| CFA into paw | rat | Spinal cord | mRNA ISH | No | (Maekawa et al., 1996) |
| CFA into paw | rat | Spinal cord | Protein radioligand binding | No | (Millan et al., 1988) |
| CFA into paw | mouse | DRG | mRNA Q-RT-PCR | Up | (Gaveriaux-Ruff et al., 2011) |
| Carrageenan into paw | rat | DRG | Protein immunohistochemistry | Down | (Ji et al., 1995) |
| Spinal cord | Down | ||||
| CFA into tail | rat | Spinal cord | mRNA Q-RT-PCR | Up | (Calza et al., 2000) |
| CFA into joint | rat | Thalamus, reticular nucleus | mRNA ISH | Down | (Neto et al., 2008) |
| Brainstem, dorsal reticular nucleus | Down | ||||
| Brainstem, parvocellular reticular nucleus | Up | ||||
| CFA into joint | rat | Spinal cord | Protein radioligand binding | Up | (Besse et al., 1992b) |
| Incision - post-operative | mouse | DRG | mRNA Q-RT-PCR | Down | (Cabanero et al., 2009) |
| Spinal cord | Down | ||||
| Intestinal, croton oil | mouse | Spinal cord | mRNA Q-RT-PCR | Up | (Pol et al., 2003) |
| Protein Western blot | |||||
| Whole gut | Up | ||||
| Brain | No | ||||
| Neuropathic pain | |||||
| CCI* | rat | DRG Spinal cord | mRNA Q-RT-PCR | Down No | (Obara et al., 2009) |
| CCI | rat | DRG | mRNA Q-RT-PCR | Down | (Herradon et al., 2008) |
| Spinal cord | No | ||||
| CCI | rat | Spinal cord | Protein immunohistochemistry | Down | (Tseng et al., 2008) |
| CCI | rat | Spinal cord | Protein western blot | No | (Holdridge & Cahill, 2007) |
| CCI, Spinal NL* | rat | Spinal cord | Protein immunohistochemistry | Down | (Stone et al., 2004) |
| CCI | rat | Spinal cord | Protein radioligand binding | Down | (Stevens et al., 1991) |
| CCI | rat | Spinal cord | Protein radioligand binding | Down | (Besse et al., 1992a) |
| CCI | mouse | DRG | mRNA Q-RT-PCR | Down | (Hervera et al., 2010) |
| Spinal cord | No | ||||
| Cuff | rat | DRG | Protein western blot | Up | (Kabli & Cahill, 2007) |
| pSNL* | mouse | DRG | mRNA Q-RT-PCR | No | (Gaveriaux-Ruff et al., 2011) |
| pSNL | mouse | DRG & spinal cord | mRNA Q-RT-PCR | No | (Pol et al., 2006) |
| Multiple sclerosis | |||||
| TMEV* infection | mouse | Spinal cord | mRNA Q-RT-PCR | Down | (Lynch et al., 2008) |
Abbreviations: CCI, chronic constriciton injury; CFA, Complete Freund’s Adjuvant; DRG, dorsal root ganglion; ISH, In Situ hybridization; NL, nerve ligation; pSNL, partial sciatic nerve ligation; Q-RT-PCR, quantitative reverse transcriptase-PCR; TMEV, Theiler’s murine encephalomyelitis virus
Post-translation regulation
A third level of regulation occurs post-translationally in receptor protein maturation and transport. Mechanisms for delta receptor folding and integration into membranes were investigated using heterologous expression systems. Tuusa et al. (2010) recently showed that delta receptor biogenesis is regulated early after translation at the level of endoplasmic reticulum by the molecular chaperone calnexin and the sarcoendoplasmic reticulum calcium ATPase SERCA2b in a calcium and ATP dependent manner. Delta receptor targeting from the endoplasmic reticulum to the cell surface was further proposed to require the golgi chaperone receptor transport protein 4 RTP4. In vitro experiments showed that this protein participates in folding of delta-mu receptor heterodimers, enhancing the trafficking of delta-mu receptor complexes from the Golgi apparatus to the cell surface and decreasing the expression of delta monomers (Decaillot et al., 2008). Future experiments will determine whether these processes occur in vivo.
Receptor activity at the cell surface
Receptor activity is also modulated at the plasma membrane, via interactions with other membrane proteins. In particular, delta receptors may associate with other GPCRs to form heterodimers or larger heteromers at the cell surface. Based on several approaches and criteria used to define potential GPCR heterodimers (see (Massotte, 2010), delta receptors have been proposed to interact with GPCRs implicated in pain control, including mu and kappa opioid receptors (Gupta et al., 2010; Kabli et al., 2010; van Rijn et al., 2010), CB1 cannabinoid receptors (see Bushlin et al., 2010) and alpha2-adrenergic receptors (see van Rijn et al., 2010). Delta receptors may also interact with chemokine receptors (Parenty et al., 2008; Pello et al., 2008) with potential consequences on inflammation, including inflammatory pain (Chen et al., 2007). These studies, mainly performed in heterologous cell recombinant systems, show new pharmacological properties for delta receptor-GPCR heterodimers (reviews (Bushlin et al., 2010; Rozenfeld & Devi, 2010; van Rijn et al., 2010). Opioid receptor heterodimers may also be involved in the activities of bi-functional opioid drugs investigated as novel classes of analgesics (Ansonoff et al., 2010; Schiller, 2010).
Receptor activation and intracellular effectors
Finally, receptor density and activity at the cell surface is tightly regulated by intracellular effectors, which engage the receptor into both signaling and trafficking processes upon activation. The best-known signaling effectors of the delta receptor are inhibitory heterotrimeric Gi/o proteins, which further modulate ion channels and second messengers leading ultimately to reduced neuronal activity (Williams et al., 2001). In addition to signaling, agonist binding to the receptor also induces phosphorylation, internalization, trafficking and redistribution of the receptor, and these events represent key mechanisms for the regulation of receptor activity (Cahill et al., 2007; Ritter & Hall, 2009). Unlike the mu opioid receptor, which rapidly recycles to the cell surface, delta receptors are targeted towards lysosomal degradation (Pradhan et al., 2009) via the Endosomal Sorting Complex Required for Transport (ESCRT) machinery, using ubiquitination-dependent or independent mechanisms (Henry et al., 2010). Other factors upstream of the ESCRT pathway also contribute to delta receptor proteolysis, including G protein-coupled receptor Associated Sorting Proteins (GASPs) shown to interact with the delta receptor (Abu-Helo & Simonin, 2010; Moser et al., 2010).
The link between agonist-induced internalization, downregulation and in vivo function of delta receptors was investigated using the DOR-eGFP mouse model (Pradhan et al., 2009., 2010). Data indicated that a single injection of a high- (SNC80) and a low (AR-M100390)-internalizing compound to DOR-eGFP mice produced equally efficient analgesia in a model of inflammatory pain. SNC80 also concomitantly induced receptor internalization throughout the nervous system, accompanied by G-protein uncoupling and acute behavioral desensitization, such that a second drug injection was inefficient. Receptor desensitization was transient and receptor responsiveness returned to basal levels after one day. In contrast, AR-M100390 induced none of the regulatory responses, since no internalization, G-protein uncoupling or acute desensitization were detectable (Pradhan et al., 2009). Interestingly further chronic treatment with the two agonists both induced the development of tolerance, but the expression of tolerance differed. The high-internalizing agonist (SNC80) induced generalized tolerance, so that all agonist effects were blunted in chronically-treated mice, and the low-internalizing agonist (AR-M100390) produced a partial tolerance that was restricted to analgesic responses, while anxiolytic or locomotor effects of delta agonists remained intact (Pradhan et al., 2010). This set of data is in accordance with previous in vitro studies showing ligand-specific conformational changes of the delta receptor (Audet et al., 2008) and definitely establish the in vivo relevance of delta receptor ligand-biased agonism in drug efficacy. The critical consequences of biased agonism at the delta receptor add further physiological support to the rapidly growing field of functional selectivity at GPCRs (Bosier & Hermans, 2007; Galandrin et al., 2007; Mailman, 2007; Vaidehi & Kenakin 2010; Zheng et al., 2010) and may be considered for the development of effective therapeutic drugs.
Functional interactions between delta and other neuromodulatory systems
An active research field is the elucidation of respective contributions of mu and delta receptors in nociceptive processing, particularly to control heat and mechanical pain modalities (Basbaum et al., 2009; Scherrer et al., 2009; Woolf, 2009), and the identification of molecular and cellular mechanisms underlying functional interactions between the two receptors. Interactions may occur either at cellular or systems levels within nociceptive pathways. This has been extensively discussed in the past (Kieffer, 1999; Smith & Lee, 2003; Zaki et al., 1996) and more recent anatomical analysis has provided evidence for potential interactions at the cellular level in vivo. Data from in situ mRNA hybridization (Mansour et al., 1995; Mennicken et al., 2003; Wang & Wessendorf, 2001), the analysis of DOR-eGFP mice (Scherrer et al., 2009), and local pharmacology in peripheral neurons (Joseph & Levine, 2010) suggest that mu and delta receptors may be co-localized in a limited number of peripheral nociceptor neurons. This co-expression opens the possibility for within-cell functional interactions in primary nociceptive processing, which may occur between receptors or their downstream signaling pathways. Delta and mu receptor co-localization in the dorsal spinal cord remains debated (Overland et al., 2009; Scherrer et al., 2009; Wang et al., 2010).
A large array of evidence indicates that delta receptor function is increased either after chronic morphine or under conditions of chronic pain. A suggested common basis for this phenomenon lies in the observation of increased delta receptor density at the cell surface in both cases, leading to higher number of receptors available for activation (Bie & Pan, 2007; Cahill et al., 2007; Morgan et al., 2009; Schramm & Honda, 2010). In the case of chronic morphine, the enhancement of delta receptor function likely involves a functional link between mu receptor activation and delta receptors, whose mechanism remains open (see above). In situations of persistent pain, other mechanisms involving bradykinin (Patwardhan et al., 2005), protease activated receptor-2 (Patwardhan et al., 2006), arachidonic acid (Rowan et al., 2009) and nerve growth Factor (Bie et al., 2010) have been shown to augment delta receptor activity. Collectively, interactions between delta receptors and several other opioid or non-opioid receptor systems have been reported to influence delta opioid analgesia, and a great diversityof GPCRs involved pain control (Pan et al., 2008; Stone & Molliver, 2009) may potentially interact with, or modulate, delta receptor analgesic activity.
Conclusion
The field of delta opioid receptor analgesia has benefited from recent contributions in pharmacology, molecular and cellular approaches. Novel selective delta agonists with potent in vivo activities are now available to strengthen approaches targeting delta receptors. The field of delta receptors and pain control may also benefit from the development of animal models more closely related to clinical pain (Finley et al., 2008). In particular the evaluation of delta receptor activity in spontaneous pain and in the emotional or affective dimensions of pain (King et al., 2009; Minami, 2009) may provide new elements for the development of delta receptor-based therapeutic strategies.
Acknowledgments
Source of funding:
The study was financially supported by the CNRS, INSERM, the Université de Strasbourg, the French ANR grants IMOP and LYMPHOPIOID, the US NIH NIDA grant #DA05010.
Footnotes
Conflict of interest: none
(1) (Obara et al., 2009), (3) (Bilsky et al., 1995., 1996),(Kabli & Cahill, 2007), (4) (Pacheco & Duarte, 2005),(Obara et al., 2009),(Joseph & Levine, 2010),(Gaveriaux-Ruff et al., 2011); (5) (Stein et al., 1989), (Zhou et al., 1998), (Hervera et al., 2009., 2010); (6) (Bilsky et al., 1996),(Cahill et al., 2001, 2003), (Mika et al., 2001), (Pradhan et al., 2006), (Gendron et al., 2007a,b), (Holdridge & Cahill, 2007), (Beaudry et al., 2009), (Dubois & Gendron, 2010),(Otis et al., 2011); (7) (Pacheco & Duarte, 2005), (Scherrer et al., 2009); (8) (Fraser et al., 2000); (9) (Hurley & Hammond, 2000); (10) (Kawaraguchi et al., 2004); (11) (Baamonde et al., 2005), (Mika et al., 2001); (12) (Ma et al., 2006); (13) (Kamei et al., 1997) ; (14) (Barn et al., 2001; Brandt et al., 2001; Gallantine & Meert, 2005; Gaveriaux-Ruff et al., 2008; Pradhan et al., 2009., 2010).. (15) (Barn et al., 2001) ; (16) (Beaudry et al., 2009; Petrillo et al., 2003) ; (17) (Brainin-Mattos et al., 2006) ; (18) (Pradhan et al., 2009., 2010) ; (19) (Aceto et al., 2007) ; (20) (Le Bourdonnec et al., 2008) ; (21) (Le Bourdonnec et al., 2009) ; (22) (Codd et al., 2010) ; (23) (Jones et al., 2009)
References
- Abu-Helo A, Simonin F. Identification and biological significance of G protein-coupled receptor associated sorting proteins (GASPs) Pharmacol Ther. 2010;126:244–250. doi: 10.1016/j.pharmthera.2010.03.004. [DOI] [PubMed] [Google Scholar]
- Aceto MD, May EL, Harris LS, Bowman ER, Cook CD. Pharmacological studies with a nonpeptidic, delta-opioid (-)-(1R,5R,9R)-5,9-dimethyl-2'-hydroxy-2-(6-hydroxyhexyl)-6,7-benzomorphan hydrochloride ((-)-NIH 11082) Eur J Pharmacol. 2007;566:88–93. doi: 10.1016/j.ejphar.2007.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agarwal N, Pacher P, Tegeder I, Amaya F, Constantin CE, Brenner GJ, et al. Cannabinoids mediate analgesia largely via peripheral type 1 cannabinoid receptors in nociceptors. Nat Neurosci. 2007;10:870–879. doi: 10.1038/nn1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akil H, Owens C, Gutstein H, Taylor L, Curran E, Watson S. Endogenous opioids: overview and current issues. Drug Alcohol Depend. 1998;51:127–140. doi: 10.1016/s0376-8716(98)00071-4. [DOI] [PubMed] [Google Scholar]
- Ansonoff MA, Portoghese PS, Pintar JE. Consequences of opioid receptor mutation on actions of univalent and bivalent kappa and delta ligands. Psychopharmacology (Berl) 2010;210:161–168. doi: 10.1007/s00213-010-1826-7. [DOI] [PubMed] [Google Scholar]
- Audet N, Gales C, Archer-Lahlou E, Vallieres M, Schiller PW, Bouvier M, et al. Bioluminescence resonance energy transfer assays reveal ligand-specific conformational changes within preformed signaling complexes containing delta-opioid receptors and heterotrimeric G proteins. J Biol Chem. 2008;283:15078–15088. doi: 10.1074/jbc.M707941200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baamonde A, Lastra A, Juarez L, Garcia V, Hidalgo A, Menendez L. Effects of the local administration of selective mu-, delta-and kappa-opioid receptor agonists on osteosarcoma-induced hyperalgesia. Naunyn Schmiedebergs Arch Pharmacol. 2005;372:213–219. doi: 10.1007/s00210-005-0013-6. [DOI] [PubMed] [Google Scholar]
- Barn DR, Caulfield WL, Cottney J, McGurk K, Morphy JR, Rankovic Z, et al. Parallel synthesis and biological activity of a new class of high affinity and selective delta-opioid ligand. Bioorg Med Chem. 2001;9:2609–2624. doi: 10.1016/s0968-0896(01)00017-7. [DOI] [PubMed] [Google Scholar]
- Basbaum AI, Bautista DM, Scherrer G, Julius D. Cellular and molecular mechanisms of pain. Cell. 2009;139:267–284. doi: 10.1016/j.cell.2009.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaudry H, Proteau-Gagne A, Li S, Dory Y, Chavkin C, Gendron L. Differential noxious and motor tolerance of chronic delta opioid receptor agonists in rodents. Neuroscience. 2009;161:381–391. doi: 10.1016/j.neuroscience.2009.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benbouzid M, Gaveriaux-Ruff C, Yalcin I, Waltisperger E, Tessier LH, Muller A, et al. Delta-opioid receptors are critical for tricyclic antidepressant treatment of neuropathic allodynia. Biol Psychiatry. 2008;63:633–636. doi: 10.1016/j.biopsych.2007.06.016. [DOI] [PubMed] [Google Scholar]
- Besse D, Lombard MC, Perrot S, Besson JM. Regulation of opioid binding sites in the superficial dorsal horn of the rat spinal cord following loose ligation of the sciatic nerve: comparison with sciatic nerve section and lumbar dorsal rhizotomy. Neuroscience. 1992a;50:921–933. doi: 10.1016/0306-4522(92)90215-n. [DOI] [PubMed] [Google Scholar]
- Besse D, Weil-Fugazza J, Lombard MC, Butler SH, Besson JM. Monoarthritis induces complex changes in mu-, delta- and kappa-opioid binding sites in the superficial dorsal horn of the rat spinal cord. Eur J Pharmacol. 1992b;223:123–131. doi: 10.1016/0014-2999(92)94830-o. [DOI] [PubMed] [Google Scholar]
- Bie B, Pan ZZ. Trafficking of central opioid receptors and descending pain inhibition. Mol Pain. 2007;3:37. doi: 10.1186/1744-8069-3-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bie B, Zhang Z, Cai YQ, Zhu W, Zhang Y, Dai J, et al. Nerve growth factor-regulated emergence of functional delta-opioid receptors. J Neurosci. 2010;30:5617–5628. doi: 10.1523/JNEUROSCI.5296-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilsky EJ, Calderon SN, Wang T, Bernstein RN, Davis P, Hruby VJ, et al. SNC 80, a selective, nonpeptidic and systemically active opioid delta agonist. J Pharmacol Exp Ther. 1995;273:359–366. [PubMed] [Google Scholar]
- Bilsky EJ, Wang T, Lai J, Porreca F. Selective blockade of peripheral delta opioid agonist induced antinociception by intrathecal administration of delta receptor antisense oligodeoxynucleotide. Neurosci Lett. 1996;220:155–158. doi: 10.1016/s0304-3940(96)13262-6. [DOI] [PubMed] [Google Scholar]
- Bohren Y, Karavelic D, Tessier LH, Yalcin I, Gaveriaux-Ruff C, Kieffer BL, et al. Mu-opioid receptors are not necessary for nortriptyline treatment of neuropathic allodynia. Eur J Pain. 2010;14:700–704. doi: 10.1016/j.ejpain.2009.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosier B, Hermans E. Versatility of GPCR recognition by drugs: from biological implications to therapeutic relevance. Trends Pharmacol Sci. 2007;28:438–446. doi: 10.1016/j.tips.2007.06.001. [DOI] [PubMed] [Google Scholar]
- Brack A, Rittner HL, Machelska H, Shaqura M, Mousa SA, Labuz D, et al. Endogenous peripheral antinociception in early inflammation is not limited by the number of opioid-containing leukocytes but by opioid receptor expression. Pain. 2004;108:67–75. doi: 10.1016/j.pain.2003.12.008. [DOI] [PubMed] [Google Scholar]
- Brainin-Mattos J, Smith ND, Malkmus S, Rew Y, Goodman M, Taulane J, et al. Cancer-related bone pain is attenuated by a systemically available delta-opioid receptor agonist. Pain. 2006;122:174–181. doi: 10.1016/j.pain.2006.01.032. [DOI] [PubMed] [Google Scholar]
- Brandt MR, Furness MS, Mello NK, Rice KC, Negus SS. Antinociceptive effects of delta-opioid agonists in Rhesus monkeys: effects on chemically induced thermal hypersensitivity. J Pharmacol Exp Ther. 2001;296:939–946. [PubMed] [Google Scholar]
- Bushlin I, Rozenfeld R, Devi LA. Cannabinoid-opioid interactions during neuropathic pain and analgesia. Curr Opin Pharmacol. 2010;10:80–86. doi: 10.1016/j.coph.2009.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabanero D, Celerier E, Garcia-Nogales P, Mata M, Roques BP, Maldonado R, et al. The pro-nociceptive effects of remifentanil or surgical injury in mice are associated with a decrease in delta-opioid receptor mRNA levels: Prevention of the nociceptive response by on-site delivery of enkephalins. Pain. 2009;141:88–96. doi: 10.1016/j.pain.2008.10.011. [DOI] [PubMed] [Google Scholar]
- Cahill CM, Morinville A, Lee MC, Vincent JP, Collier B, Beaudet A. Prolonged morphine treatment targets delta opioid receptors to neuronal plasma membranes and enhances delta-mediated antinociception. J Neurosci. 2001;21:7598–7607. doi: 10.1523/JNEUROSCI.21-19-07598.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahill CM, Morinville A, Hoffert C, O'Donnell D, Beaudet A. Up-regulation and trafficking of delta opioid receptor in a model of chronic inflammation: implications for pain control. Pain. 2003;101:199–208. doi: 10.1016/s0304-3959(02)00333-0. [DOI] [PubMed] [Google Scholar]
- Cahill CM, Holdridge SV, Morinville A. Trafficking of delta-opioid receptors and other G-protein-coupled receptors: implications for pain and analgesia. Trends Pharmacol Sci. 2007;28:23–31. doi: 10.1016/j.tips.2006.11.003. [DOI] [PubMed] [Google Scholar]
- Calza L, Pozza M, Arletti R, Manzini E, Hokfelt T. Long-lasting regulation of galanin, opioid, and other peptides in dorsal root ganglia and spinal cord during experimental polyarthritis. Exp Neurol. 2000;164:333–343. doi: 10.1006/exnr.2000.7442. [DOI] [PubMed] [Google Scholar]
- Cao CQ, Hong Y, Dray A, Perkins M. Spinal delta-opioid receptors mediate suppression of systemic SNC80 on excitability of the flexor reflex in normal and inflamed rat. Eur J Pharmacol. 2001;418:79–87. doi: 10.1016/s0014-2999(01)00934-7. [DOI] [PubMed] [Google Scholar]
- Chang K-J, Porreca F, Woods JH. The Delta Receptor. Marcel Dekker; New- York: 2004. [Google Scholar]
- Chao D, Xia Y. Ionic storm in hypoxic/ischemic stress: can opioid receptors subside it? Prog Neurobiol. 2010;90:439–470. doi: 10.1016/j.pneurobio.2009.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Geller EB, Rogers TJ, Adler MW. Rapid heterologous desensitization of antinociceptive activity between mu or delta opioid receptors and chemokine receptors in rats. Drug Alcohol Depend. 2007;88:36–41. doi: 10.1016/j.drugalcdep.2006.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng B, Liu HW, Fu XB, Sheng ZY, Li JF. Coexistence and upregulation of three types of opioid receptors, mu, delta and kappa, in human hypertrophic scars. Br J Dermatol. 2008;158:713–720. doi: 10.1111/j.1365-2133.2008.08449.x. [DOI] [PubMed] [Google Scholar]
- Codd EE, Carson JR, Colburn RW, Stone DJ, Van Besien CR, Zhang SP, et al. JNJ-20788560 [9-(8-azabicyclo[3.2.1]oct-3-ylidene)-9H-xanthene-3-carboxylic acid diethylamide], a selective delta opioid receptor agonist, is a potent and efficacious antihyperalgesic agent that does not produce respiratory depression, pharmacologic tolerance, or physical dependence. J Pharmacol Exp Ther. 2009;329:241–251. doi: 10.1124/jpet.108.146969. [DOI] [PubMed] [Google Scholar]
- Codd EE, Ma J, Zhang SP, Stone DJ, Jr, Colburn RW, Brandt MR, et al. Ex vivo delta opioid receptor autoradiography: CNS receptor occupancy of two novel compounds over their antihyperalgesic dose range. Pharmacol Biochem Behav. 2010;96 :130–135. doi: 10.1016/j.pbb.2010.04.020. [DOI] [PubMed] [Google Scholar]
- Contet C, Gaveriaux-Ruff C, Matifas A, Caradec C, Champy MF, Kieffer BL. Dissociation of analgesic and hormonal responses to forced swim stress using opioid receptor knockout mice. Neuropsychopharmacology. 2006;31:1733–1744. doi: 10.1038/sj.npp.1300934. [DOI] [PubMed] [Google Scholar]
- Decaillot FM, Rozenfeld R, Gupta A, Devi LA. Cell surface targeting of mu-delta opioid receptor heterodimers by RTP4. Proc Natl Acad Sci U S A. 2008;105:16045–16050. doi: 10.1073/pnas.0804106105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubois D, Gendron L. Delta opioid receptor-mediated analgesia is not altered in preprotachykinin A knockout mice. Eur J Neurosci. 2010;32:1921–1929. doi: 10.1111/j.1460-9568.2010.07466.x. [DOI] [PubMed] [Google Scholar]
- Evans CJ, Keith DE, Jr, Morrison H, Magendzo K, Edwards RH. Cloning of a delta opioid receptor by functional expression. Science. 1992;258:1952–1955. doi: 10.1126/science.1335167. [DOI] [PubMed] [Google Scholar]
- Filliol D, Ghozland S, Chluba J, Martin M, Matthes HW, Simonin F, et al. Mice deficient for delta- and mu-opioid receptors exhibit opposing alterations of emotional responses. Nat Genet. 2000;25:195–200. doi: 10.1038/76061. [DOI] [PubMed] [Google Scholar]
- Fraser GL, Gaudreau GA, Clarke PB, Menard DP, Perkins MN. Antihyperalgesic effects of delta opioid agonists in a rat model of chronic inflammation. Br J Pharmacol. 2000;129:1668–1672. doi: 10.1038/sj.bjp.0703248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galandrin S, Oligny-Longpre G, Bouvier M. The evasive nature of drug efficacy: implications for drug discovery. Trends Pharmacol Sci. 2007;28:423–430. doi: 10.1016/j.tips.2007.06.005. [DOI] [PubMed] [Google Scholar]
- Gallantine EL, Meert TF. A comparison of the antinociceptive and adverse effects of the mu-opioid agonist morphine and the delta-opioid agonist SNC80. Basic Clin Pharmacol Toxicol. 2005;97:39–51. doi: 10.1111/j.1742-7843.2005.pto_97107.x. [DOI] [PubMed] [Google Scholar]
- Gaveriaux-Ruff C, Karchewski LA, Hever X, Matifas A, Kieffer BL. Inflammatory pain is enhanced in delta opioid receptor-knockout mice. Eur J Neurosci. 2008;27:2558–2567. doi: 10.1111/j.1460-9568.2008.06223.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaveriaux-Ruff C, Nozaki C, Nadal X, Hever XC, Weibel R, Matifas A, et al. Genetic ablation of delta opioid receptors in nociceptive sensory neurons increases chronic pain and abolishes opioid analgesia. Pain. 2011;152:1238–1248. doi: 10.1016/j.pain.2010.12.031. [DOI] [PubMed] [Google Scholar]
- Gendron L, Lucido AL, Mennicken F, O'Donnell D, Vincent JP, Stroh T, et al. Morphine and pain-related stimuli enhance cell surface availability of somatic delta-opioid receptors in rat dorsal root ganglia. J Neurosci. 2006;26:953–962. doi: 10.1523/JNEUROSCI.3598-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gendron L, Esdaile MJ, Mennicken F, Pan H, O'Donnell D, Vincent JP, et al. Morphine priming in rats with chronic inflammation reveals a dichotomy between antihyperalgesic and antinociceptive properties of deltorphin. Neuroscience. 2007a;144:263–274. doi: 10.1016/j.neuroscience.2006.08.077. [DOI] [PubMed] [Google Scholar]
- Gendron L, Pintar JE, Chavkin C. Essential role of mu opioid receptor in the regulation of delta opioid receptor-mediated antihyperalgesia. Neuroscience. 2007b;150:807–817. doi: 10.1016/j.neuroscience.2007.09.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goody RJ, Oakley SM, Filliol D, Kieffer BL, Kitchen I. Quantitative autoradiographic mapping of opioid receptors in the brain of delta-opioid receptor gene knockout mice. Brain Res. 2002;945:9–19. doi: 10.1016/s0006-8993(02)02452-6. [DOI] [PubMed] [Google Scholar]
- Gupta A, Mulder J, Gomes I, Rozenfeld R, Bushlin I, Ong E, et al. Increased abundance of opioid receptor heteromers after chronic morphine administration. Sci Signal. 2010;3:ra54. doi: 10.1126/scisignal.2000807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry AG, White IJ, Marsh M, von Zastrow M, Hislop JN. The role of ubiquitination in lysosomal trafficking of delta-opioid receptors. Traffic. 2010;12:170–184. doi: 10.1111/j.1600-0854.2010.01145.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herradon G, Ezquerra L, Nguyen T, Wang C, Siso A, Franklin B, et al. Noradrenergic and opioidergic alterations in neuropathy in different rat strains. Neurosci Lett. 2008;438:186–189. doi: 10.1016/j.neulet.2008.03.095. [DOI] [PubMed] [Google Scholar]
- Hervera A, Leanez S, Negrete R, Pol O. The peripheral administration of a nitric oxide donor potentiates the local antinociceptive effects of a DOR agonist during chronic inflammatory pain in mice. Naunyn Schmiedebergs Arch Pharmacol. 2009;380:345–352. doi: 10.1007/s00210-009-0436-6. [DOI] [PubMed] [Google Scholar]
- Hervera A, Negrete R, Leanez S, Martin-Campos J, Pol O. The role of nitric oxide in the local antiallodynic and antihyperalgesic effects and expression of delta-opioid and cannabinoid-2 receptors during neuropathic pain in mice. J Pharmacol Exp Ther. 2010;334:887–896. doi: 10.1124/jpet.110.167585. [DOI] [PubMed] [Google Scholar]
- Holdridge SV, Cahill CM. Spinal administration of a delta opioid receptor agonist attenuates hyperalgesia and allodynia in a rat model of neuropathic pain. Eur J Pain. 2007;11 :685–693. doi: 10.1016/j.ejpain.2006.10.008. [DOI] [PubMed] [Google Scholar]
- Hua S, Cabot PJ. Mechanisms of peripheral immune-cell-mediated analgesia in inflammation: clinical and therapeutic implications. Trends Pharmacol Sci. 2010;31:427–433. doi: 10.1016/j.tips.2010.05.008. [DOI] [PubMed] [Google Scholar]
- Huang CJ, Liu HF, Su NY, Hsu YW, Yang CH, Chen CC, et al. Association between human opioid receptor genes polymorphisms and pressure pain sensitivity in females*. Anaesthesia. 2008;63:1288–1295. doi: 10.1111/j.1365-2044.2008.05760.x. [DOI] [PubMed] [Google Scholar]
- Hurley RW, Hammond DL. The analgesic effects of supraspinal mu and delta opioid receptor agonists are potentiated during persistent inflammation. J Neurosci. 2000;20 :1249–1259. doi: 10.1523/JNEUROSCI.20-03-01249.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javelot H, Messaoudi M, Garnier S, Rougeot C. Human opiorphin is a naturally occurring antidepressant acting selectively on enkephalin-dependent delta-opioid pathways. J Physiol Pharmacol. 2010;61:355–362. [PubMed] [Google Scholar]
- Ji RR, Zhang Q, Law PY, Low HH, Elde R, Hokfelt T. Expression of mu-, delta-, and kappa-opioid receptor-like immunoreactivities in rat dorsal root ganglia after carrageenan-induced inflammation. J Neurosci. 1995;15:8156–8166. doi: 10.1523/JNEUROSCI.15-12-08156.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SM, Turner SM. Protecting motor networks during perinatal ischemia: the case for delta-opioid receptors. Ann N Y Acad Sci. 2010;1198:260–270. doi: 10.1111/j.1749-6632.2010.05434.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones P, Griffin AM, Gawell L, Lavoie R, Delorme D, Roberts E, et al. N,N-Diethyl-4-[(3-hydroxyphenyl)(piperidin-4-yl)amino] benzamide derivatives: the development of diaryl amino piperidines as potent delta opioid receptor agonists with in vivo anti-nociceptive activity in rodent models. Bioorg Med Chem Lett. 2009;19:5994–5998. doi: 10.1016/j.bmcl.2009.09.072. [DOI] [PubMed] [Google Scholar]
- Joseph EK, Levine JD. Mu and delta opioid receptors on nociceptors attenuate mechanical hyperalgesia in rat. Neuroscience. 2010;171:344–350. doi: 10.1016/j.neuroscience.2010.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jutkiewicz EM. The antidepressant -like effects of delta-opioid receptor agonists. Mol Interv. 2006;6:162–169. doi: 10.1124/mi.6.3.7. [DOI] [PubMed] [Google Scholar]
- Kabli N, Cahill CM. Anti-allodynic effects of peripheral delta opioid receptors in neuropathic pain. Pain. 2007;127:84–93. doi: 10.1016/j.pain.2006.08.003. [DOI] [PubMed] [Google Scholar]
- Kabli N, Martin N, Fan T, Nguyen T, Hasbi A, Balboni G, et al. Agonists at the delta-opioid receptor modify the binding of micro-receptor agonists to the micro-delta receptor hetero-oligomer. Br J Pharmacol. 2010;161:1122–1136. doi: 10.1111/j.1476-5381.2010.00944.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamei J, Kawai K, Mizusuna A, Saitoh A, Morita K, Narita M, et al. Supraspinal delta 1-opioid receptor-mediated antinociceptive properties of (-)-TAN-67 in diabetic mice. Eur J Pharmacol. 1997;322:27–30. doi: 10.1016/s0014-2999(97)00085-x. [DOI] [PubMed] [Google Scholar]
- Kawaraguchi Y, Kawaguchi M, Takahashi M, Horiuchi T, Sakamoto T, Furuya H. Delta-opioid agonist SNC80 can attenuate the development of dynorphin A-induced tactile allodynia in rats. Anesthesiology. 2004;101:546–549. doi: 10.1097/00000542-200408000-00040. [DOI] [PubMed] [Google Scholar]
- Kieffer BL. Opioids: first lessons from knockout mice. Trends Pharmacol Sci. 1999;20:19–26. doi: 10.1016/s0165-6147(98)01279-6. [DOI] [PubMed] [Google Scholar]
- Kieffer BL, Gaveriaux-Ruff C. Exploring the opioid system by gene knockout. Prog Neurobiol. 2002;66:285–306. doi: 10.1016/s0301-0082(02)00008-4. [DOI] [PubMed] [Google Scholar]
- Kieffer BL, Befort K, Gaveriaux-Ruff C, Hirth CG. The delta-opioid receptor: isolation of a cDNA by expression cloning and pharmacological characterization. Proc Natl Acad Sci U S A. 1992;89:12048–12052. doi: 10.1073/pnas.89.24.12048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H, Mittal DP, Iadarola MJ, Dionne RA. Genetic predictors for acute experimental cold and heat pain sensitivity in humans. J Med Genet. 2006;43:e40. doi: 10.1136/jmg.2005.036079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King T, Vera-Portocarrero L, Gutierrez T, Vanderah TW, Dussor G, Lai J, et al. Unmasking the tonic-aversive state in neuropathic pain. Nat Neurosci. 2009;12:1364–1366. doi: 10.1038/nn.2407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreil G, Barra D, Simmaco M, Erspamer V, Erspamer GF, Negri L, et al. Deltorphin, a novel amphibian skin peptide with high selectivity and affinity for delta opioid receptors. Eur J Pharmacol. 1989;162:123–128. doi: 10.1016/0014-2999(89)90611-0. [DOI] [PubMed] [Google Scholar]
- Le Bourdonnec B, Windh RT, Ajello CW, Leister LK, Gu M, Chu GH, et al. Potent, orally bioavailable delta opioid receptor agonists for the treatment of pain: discovery of N,N-diethyl-4-(5-hydroxyspiro[chromene-2,4'-piperidine]-4-yl)benzamide (ADL5859) J Med Chem. 2008;51:5893–5896. doi: 10.1021/jm8008986. [DOI] [PubMed] [Google Scholar]
- Le Bourdonnec B, Windh RT, Leister LK, Zhou QJ, Ajello CW, Gu M, et al. Spirocyclic delta opioid receptor agonists for the treatment of pain: discovery of N,N-diethyl-3-hydroxy-4-(spiro[chromene-2,4'-piperidine]-4-yl) benzamide (ADL5747) J Med Chem. 2009;52:5685–5702. doi: 10.1021/jm900773n. [DOI] [PubMed] [Google Scholar]
- Le Merrer J, Plaza-Zabala A, Boca CD, Matifas A, Maldonado R, Kieffer BL. Deletion of the delta Opioid Receptor Gene Impairs Place Conditioning But Preserves Morphine Reinforcement. Biol Psychiatry. 2011 doi: 10.1016/j.biopsych.2010.10.021. In press. [DOI] [PubMed] [Google Scholar]
- Leskela TT, Markkanen PM, Alahuhta IA, Tuusa JT, Petaja-Repo UE. Phe27Cys polymorphism alters the maturation and subcellular localization of the human delta opioid receptor. Traffic. 2009;10:116–129. doi: 10.1111/j.1600-0854.2008.00846.x. [DOI] [PubMed] [Google Scholar]
- Lotsch J, Geisslinger G. Pharmacogenetics of new analgesics. Br J Pharmacol. 2011;163:447–460. doi: 10.1111/j.1476-5381.2010.01074.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch JL, Alley JF, Wellman L, Beitz AJ. Decreased spinal cord opioid receptor mRNA expression and antinociception in a Theiler's murine encephalomyelitis virus model of multiple sclerosis. Brain Res. 2008;1191:180–191. doi: 10.1016/j.brainres.2007.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma J, Zhang Y, Kalyuzhny AE, Pan ZZ. Emergence of functional delta-opioid receptors induced by long-term treatment with morphine. Mol Pharmacol. 2006;69:1137–1145. doi: 10.1124/mol.105.019109. [DOI] [PubMed] [Google Scholar]
- Maekawa K, Minami M, Masuda T, Satoh M. Expression of mu- and kappa-, but not delta-, opioid receptor mRNAs is enhanced in the spinal dorsal horn of the arthritic rats. Pain. 1996;64:365–371. doi: 10.1016/0304-3959(95)00132-8. [DOI] [PubMed] [Google Scholar]
- Mailman RB. GPCR functional selectivity has therapeutic impact. Trends Pharmacol Sci. 2007;28:390–396. doi: 10.1016/j.tips.2007.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansour A, Fox CA, Akil H, Watson SJ. Opioid-receptor mRNA expression in the rat CNS: anatomical and functional implications. Trends Neurosci. 1995;18:22–29. doi: 10.1016/0166-2236(95)93946-u. [DOI] [PubMed] [Google Scholar]
- Martin M, Matifas A, Maldonado R, Kieffer BL. Acute antinociceptive responses in single and combinatorial opioid receptor knockout mice: distinct mu, delta and kappa tones. Eur J Neurosci. 2003;17:701–708. doi: 10.1046/j.1460-9568.2003.02482.x. [DOI] [PubMed] [Google Scholar]
- Massotte D. G protein-coupled receptor heterodimerization: a molecular determinant of neuronal activity. In: Villalba ACaE., editor. G protein-coupled receptor heterodimerization: a molecular determinant of neuronal activity. Nova Science Publishers; Hauppauge: 2010. pp. 1–46. [Google Scholar]
- Mendez M, Morales-Mulia M. Role of mu and delta opioid receptors in alcohol drinking behaviour. Curr Drug Abuse Rev. 2008;1:239–252. doi: 10.2174/1874473710801020239. [DOI] [PubMed] [Google Scholar]
- Mennicken F, Zhang J, Hoffert C, Ahmad S, Beaudet A, O'Donnell D. Phylogenetic changes in the expression of delta opioid receptors in spinal cord and dorsal root ganglia. J Comp Neurol. 2003;465:349–360. doi: 10.1002/cne.10839. [DOI] [PubMed] [Google Scholar]
- Mika J, Przewlocki R, Przewlocka B. The role of delta-opioid receptor subtypes in neuropathic pain. Eur J Pharmacol. 2001;415:31–37. doi: 10.1016/s0014-2999(01)00814-7. [DOI] [PubMed] [Google Scholar]
- Millan MJ, Czlonkowski A, Morris B, Stein C, Arendt R, Huber A, et al. Inflammation of the hind limb as a model of unilateral, localized pain: influence on multiple opioid systems in the spinal cord of the rat. Pain. 1988;35:299–312. doi: 10.1016/0304-3959(88)90140-6. [DOI] [PubMed] [Google Scholar]
- Minami M. Neuronal mechanisms for pain-induced aversion behavioral studies using a conditioned place aversion test. Int Rev Neurobiol. 2009;85:135–144. doi: 10.1016/S0074-7742(09)85010-1. [DOI] [PubMed] [Google Scholar]
- Morgan MM, Ashley MD, Ingram SL, Christie MJ. Behavioral consequences of delta-opioid receptor activation in the periaqueductal gray of morphine tolerant rats. Neural Plast. 2009;2009:516328. doi: 10.1155/2009/516328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morinville A, Cahill CM, Esdaile MJ, Aibak H, Collier B, Kieffer BL, et al. Regulation of delta-opioid receptor trafficking via mu-opioid receptor stimulation: evidence from mu-opioid receptor knock-out mice. J Neurosci. 2003;23:4888–4898. doi: 10.1523/JNEUROSCI.23-12-04888.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosberg HI, Hurst R, Hruby VJ, Gee K, Yamamura HI, Galligan JJ, et al. Bis-penicillamine enkephalins possess highly improved specificity toward delta opioid receptors. Proc Natl Acad Sci U S A. 1983;80:5871–5874. doi: 10.1073/pnas.80.19.5871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moser E, Kargl J, Whistler JL, Waldhoer M, Tschische P. G protein-coupled receptor-associated sorting protein 1 regulates the postendocytic sorting of seven-transmembrane-spanning G protein-coupled receptors. Pharmacology. 2010;86:22–29. doi: 10.1159/000314161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadal X, Banos JE, Kieffer BL, Maldonado R. Neuropathic pain is enhanced in delta-opioid receptor knockout mice. Eur J Neurosci. 2006;23:830–834. doi: 10.1111/j.1460-9568.2006.04569.x. [DOI] [PubMed] [Google Scholar]
- Narita M, Kaneko C, Miyoshi K, Nagumo Y, Kuzumaki N, Nakajima M, et al. Chronic pain induces anxiety with concomitant changes in opioidergic function in the amygdala. Neuropsychopharmacology. 2006a;31:739–750. doi: 10.1038/sj.npp.1300858. [DOI] [PubMed] [Google Scholar]
- Narita M, Kuzumaki N, Narita M, Kaneko C, Hareyama N, Miyatake M, et al. Chronic pain-induced emotional dysfunction is associated with astrogliosis due to cortical delta-opioid receptor dysfunction. J Neurochem. 2006b;97:1369–1378. doi: 10.1111/j.1471-4159.2006.03824.x. [DOI] [PubMed] [Google Scholar]
- Nassar MA, Stirling LC, Forlani G, Baker MD, Matthews EA, Dickenson AH, et al. Nociceptor-specific gene deletion reveals a major role for Nav1.7 (PN1) in acute and inflammatory pain. Proc Natl Acad Sci U S A. 2004;101:12706–12711. doi: 10.1073/pnas.0404915101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neto FL, Carvalhosa AR, Ferreira-Gomes J, Reguenga C, Castro-Lopes JM. Delta opioid receptor mRNA expression is changed in the thalamus and brainstem of monoarthritic rats. J Chem Neuroanat. 2008;36:122–127. doi: 10.1016/j.jchemneu.2008.05.004. [DOI] [PubMed] [Google Scholar]
- Noble F, Roques BP. Protection of endogenous enkephalin catabolism as natural approach to novel analgesic and antidepressant drugs. Expert Opin Ther Targets. 2007;11 :145–159. doi: 10.1517/14728222.11.2.145. [DOI] [PubMed] [Google Scholar]
- Obara I, Parkitna JR, Korostynski M, Makuch W, Kaminska D, Przewlocka B, et al. Local peripheral opioid effects and expression of opioid genes in the spinal cord and dorsal root ganglia in neuropathic and inflammatory pain. Pain. 2009;141:283–291. doi: 10.1016/j.pain.2008.12.006. [DOI] [PubMed] [Google Scholar]
- Ossipov MH, Dussor GO, Porreca F. Central modulation of pain. J Clin Invest. 2010;120:3779–3787. doi: 10.1172/JCI43766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otis V, Sarret P, Gendron L. Spinal activation of delta opioid receptors alleviates cancer-related bone pain. Neuroscience. 2011;183:221–229. doi: 10.1016/j.neuroscience.2011.03.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overland AC, Kitto KF, Chabot-Dore AJ, Rothwell PE, Fairbanks CA, Stone LS, et al. Protein kinase C mediates the synergistic interaction between agonists acting at alpha2-adrenergic and delta-opioid receptors in spinal cord. J Neurosci. 2009;29 :13264–13273. doi: 10.1523/JNEUROSCI.1907-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacheco DF, Duarte ID. Delta-opioid receptor agonist SNC80 induces peripheral antinociception via activation of ATP-sensitive K+ channels. Eur J Pharmacol. 2005;512:23–28. doi: 10.1016/j.ejphar.2005.02.018. [DOI] [PubMed] [Google Scholar]
- Pan HL, Wu ZZ, Zhou HY, Chen SR, Zhang HM, Li DP. Modulation of pain transmission by G-protein-coupled receptors. Pharmacol Ther. 2008;117:141–161. doi: 10.1016/j.pharmthera.2007.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parenty G, Appelbe S, Milligan G. CXCR2 chemokine receptor antagonism enhances DOP opioid receptor function via allosteric regulation of the CXCR2-DOP receptor heterodimer. Biochem J. 2008;412:245–256. doi: 10.1042/BJ20071689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patwardhan AM, Berg KA, Akopain AN, Jeske NA, Gamper N, Clarke WP, et al. Bradykinin-induced functional competence and trafficking of the delta-opioid receptor in trigeminal nociceptors. J Neurosci. 2005;25:8825–8832. doi: 10.1523/JNEUROSCI.0160-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patwardhan AM, Diogenes A, Berg KA, Fehrenbacher JC, Clarke WP, Akopian AN, et al. PAR-2 agonists activate trigeminal nociceptors and induce functional competence in the delta opioid receptor. Pain. 2006;125:114–124. doi: 10.1016/j.pain.2006.05.007. [DOI] [PubMed] [Google Scholar]
- Pello OM, Martinez-Munoz L, Parrillas V, Serrano A, Rodriguez-Frade JM, Toro MJ, et al. Ligand stabilization of CXCR4/delta-opioid receptor heterodimers reveals a mechanism for immune response regulation. Eur J Immunol. 2008;38:537–549. doi: 10.1002/eji.200737630. [DOI] [PubMed] [Google Scholar]
- Petrillo P, Angelici O, Bingham S, Ficalora G, Garnier M, Zaratin PF, et al. Evidence for a selective role of the delta-opioid agonist [8R-(4bS*,8aalpha,8abeta, 12bbeta)]7,10-Dimethyl-1-methoxy-11-(2-methylpropyl)oxycarbonyl 5,6,7,8,12,12b-hexahydro-(9H)-4,8-methanobenzofuro[3,2-e]pyrrolo[2,3-g]iso quinoline hydrochloride (SB-235863) in blocking hyperalgesia associated with inflammatory and neuropathic pain responses. J Pharmacol Exp Ther. 2003;307:1079–1089. doi: 10.1124/jpet.103.055590. [DOI] [PubMed] [Google Scholar]
- Pol O, Palacio JR, Puig MM. The expression of delta- and kappa-opioid receptor is enhanced during intestinal inflammation in mice. J Pharmacol Exp Ther. 2003;306:455–462. doi: 10.1124/jpet.103.049346. [DOI] [PubMed] [Google Scholar]
- Pol O, Murtra P, Caracuel L, Valverde O, Puig MM, Maldonado R. Expression of opioid receptors and c-fos in CB1 knockout mice exposed to neuropathic pain. Neuropharmacology. 2006;50:123–132. doi: 10.1016/j.neuropharm.2005.11.002. [DOI] [PubMed] [Google Scholar]
- Portoghese PS, Sultana M, Takemori AE. Naltrindole, a highly selective and potent non-peptide delta opioid receptor antagonist. Eur J Pharmacol. 1988;146:185–186. doi: 10.1016/0014-2999(88)90502-x. [DOI] [PubMed] [Google Scholar]
- Pradhan AA, Siau C, Constantin A, Clarke PB. Chronic morphine administration results in tolerance to delta opioid receptor-mediated antinociception. Neuroscience. 2006;141:947–954. doi: 10.1016/j.neuroscience.2006.04.021. [DOI] [PubMed] [Google Scholar]
- Pradhan AA, Becker JA, Scherrer G, Tryoen-Toth P, Filliol D, Matifas A, et al. In vivo delta opioid receptor internalization controls behavioral effects of agonists. PLoS One. 2009;4:e5425. doi: 10.1371/journal.pone.0005425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pradhan AA, Walwyn W, Nozaki C, Filliol D, Erbs E, Matifas A, et al. Ligand-directed trafficking of the delta-opioid receptor in vivo: two paths toward analgesic tolerance. J Neurosci. 2010;30:16459–16468. doi: 10.1523/JNEUROSCI.3748-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pradhan AA, Befort K, Nozaki C, Gaveriaux-Ruff C, Kieffer BL. The delta opioid receptor: an evolving target for the treatment of brain disorders. Trens in Pharmacological Sciences. 2011 doi: 10.1016/j.tips.2011.06.008. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puehler W, Zollner C, Brack A, Shaqura MA, Krause H, Schafer M, et al. Rapid upregulation of mu opioid receptor mRNA in dorsal root ganglia in response to peripheral inflammation depends on neuronal conduction. Neuroscience. 2004;129:473–479. doi: 10.1016/j.neuroscience.2004.06.086. [DOI] [PubMed] [Google Scholar]
- Ritter SL, Hall RA. Fine-tuning of GPCR activity by receptor-interacting proteins. Nat Rev Mol Cell Biol. 2009;10:819–830. doi: 10.1038/nrm2803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Arias M, Aguilar MA, Manzanedo C, Minarro J. Preclinical evidence of new opioid modulators for the treatment of addiction. Expert Opin Investig Drugs. 2010;19 :977–994. doi: 10.1517/13543784.2010.500612. [DOI] [PubMed] [Google Scholar]
- Rowan MP, Ruparel NB, Patwardhan AM, Berg KA, Clarke WP, Hargreaves KM. Peripheral delta opioid receptors require priming for functional competence in vivo. Eur J Pharmacol. 2009;602:283–287. doi: 10.1016/j.ejphar.2008.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozenfeld R, Devi LA. Exploring a role for heteromerization in GPCR signalling specificity. Biochem J. 2010;433:11–18. doi: 10.1042/BJ20100458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacharczuk M, Lesniak A, Korostynski M, Przewlocki R, Lipkowski A, Jaszczak K, et al. A polymorphism in exon 2 of the delta-opioid receptor affects nociception in response to specific agonists and antagonists in mice selectively bred for high and low analgesia. Pain. 2010;149:506–513. doi: 10.1016/j.pain.2010.03.017. [DOI] [PubMed] [Google Scholar]
- Saitoh A, Sugiyama A, Nemoto T, Fujii H, Wada K, Oka JI, Nagase H, Yamada M. The novel delta opioid receptor agonist KNT-127 produces antidepressant-like and antinociceptive effects in mice without producing convulsions. Behav Brain Res. 2011 doi: 10.1016/j.bbr.2011.04.041. in press. [DOI] [PubMed] [Google Scholar]
- Salemi S, Aeschlimann A, Wollina U, Gay RE, Michel BA, Gay S, et al. Up-regulation of delta-opioid receptors and kappa-opioid receptors in the skin of fibromyalgia patients. Arthritis Rheum. 2007;56:2464–2466. doi: 10.1002/art.22735. [DOI] [PubMed] [Google Scholar]
- Saloman JL, Niu KY, Ro JY. Activation of peripheral delta-opioid receptors leads to anti-hyperalgesic responses in the masseter muscle of male and female rats. Neuroscience. 2011 doi: 10.1016/j.neuroscience.2011.05.062. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauriyal DS, Jaggi AS, Singh N. Extending pharmacological spectrum of opioids beyond analgesia: Multifunctional aspects in different pathophysiological states. Neuropeptides. 2010;45:175–188. doi: 10.1016/j.npep.2010.12.004. [DOI] [PubMed] [Google Scholar]
- Scherrer G, Tryoen-Toth P, Filliol D, Matifas A, Laustriat D, Cao YQ, et al. Knockin mice expressing fluorescent delta-opioid receptors uncover G protein-coupled receptor dynamics in vivo. Proc Natl Acad Sci U S A. 2006;103:9691–9696. doi: 10.1073/pnas.0603359103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherrer G, Imamachi N, Cao YQ, Contet C, Mennicken F, O'Donnell D, et al. Dissociation of the opioid receptor mechanisms that control mechanical and heat pain. Cell. 2009;137:1148–1159. doi: 10.1016/j.cell.2009.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiller PW. Bi- or multifunctional opioid peptide drugs. Life Sci. 2010;86:598–603. doi: 10.1016/j.lfs.2009.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schramm CL, Honda CN. Co-administration of delta- and mu-opioid receptor agonists promotes peripheral opioid receptor function. Pain. 2010;151:763–770. doi: 10.1016/j.pain.2010.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shippenberg TS, LeFevour A, Chefer VI. Targeting endogenous mu- and delta-opioid receptor systems for the treatment of drug addiction. CNS Neurol Disord Drug Targets. 2008;7:442–453. doi: 10.2174/187152708786927813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AP, Lee NM. Opioid receptor interactions: local and nonlocal, symmetric and asymmetric, physical and functional. Life Sci. 2003;73:1873–1893. doi: 10.1016/s0024-3205(03)00549-6. [DOI] [PubMed] [Google Scholar]
- Stein C, Millan MJ, Shippenberg TS, Peter K, Herz A. Peripheral opioid receptors mediating antinociception in inflammation. Evidence for involvement of mu, delta and kappa receptors. J Pharmacol Exp Ther. 1989;248:1269–1275. [PubMed] [Google Scholar]
- Stein C, Clark JD, Oh U, Vasko MR, Wilcox GL, Overland AC, et al. Peripheral mechanisms of pain and analgesia. Brain Res Rev. 2009;60:90–113. doi: 10.1016/j.brainresrev.2008.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein EA, Carr KD, Simon EJ. Stimulus induced feeding alters regional opiate receptor binding in the rat: an in vivo audioradiographic study. Prog Clin Biol Res. 1990;328:187–190. [PubMed] [Google Scholar]
- Stevens CW. The evolution of vertebrate opioid receptors. Front Biosci. 2009;14:1247–1269. doi: 10.2741/3306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens CW, Kajander KC, Bennett GJ, Seybold VS. Bilateral and differential changes in spinal mu, delta and kappa opioid binding in rats with a painful, unilateral neuropathy. Pain. 1991;46:315–326. doi: 10.1016/0304-3959(91)90114-D. [DOI] [PubMed] [Google Scholar]
- Stone LS, Molliver DC. In search of analgesia: emerging poles of GPCRs in pain. Mol Interv. 2009;9:234–251. doi: 10.1124/mi.9.5.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone LS, Vulchanova L, Riedl MS, Williams FG, Wilcox GL, Elde R. Effects of peripheral nerve injury on delta opioid receptor (DOR) immunoreactivity in the rat spinal cord. Neurosci Lett. 2004;361:208–211. doi: 10.1016/j.neulet.2003.12.067. [DOI] [PubMed] [Google Scholar]
- Tseng TJ, Chen CC, Hsieh YL, Hsieh ST. Influences of surgical decompression on the dorsal horn after chronic constriction injury: changes in peptidergic and delta-opioid receptor (+) nerve terminals. Neuroscience. 2008;156:758–768. doi: 10.1016/j.neuroscience.2008.08.010. [DOI] [PubMed] [Google Scholar]
- Tuusa JT, Leskela TT, Petaja-Repo UE. Human delta opioid receptor biogenesis is regulated via interactions with SERCA2b and calnexin. Febs J. 2010;277:2815–2829. doi: 10.1111/j.1742-4658.2010.07699.x. [DOI] [PubMed] [Google Scholar]
- Vaidehi N, Kenakin T. The role of conformational ensembles of seven transmembrane receptors in functional selectivity. Curr Opin Pharmacol. 2010;10:775–781. doi: 10.1016/j.coph.2010.09.004. [DOI] [PubMed] [Google Scholar]
- van Rijn RM, Whistler JL, Waldhoer M. Opioid-receptor-heteromer-specific trafficking and pharmacology. Curr Opin Pharmacol. 2010;10:73–79. doi: 10.1016/j.coph.2009.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderah TW. Delta and kappa opioid receptors as suitable drug targets for pain. Clin J Pain. 2010;26(Suppl 10):S10–15. doi: 10.1097/AJP.0b013e3181c49e3a. [DOI] [PubMed] [Google Scholar]
- Wang H, Wessendorf MW. Equal proportions of small and large DRG neurons express opioid receptor mRNAs. J Comp Neurol. 2001;429:590–600. doi: 10.1002/1096-9861(20010122)429:4<590::aid-cne6>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- Wang HB, Zhao B, Zhong YQ, Li KC, Li ZY, Wang Q, et al. Coexpression of delta- and mu-opioid receptors in nociceptive sensory neurons. Proc Natl Acad Sci U S A. 2010;107:13117–13122. doi: 10.1073/pnas.1008382107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei LN, Loh HH. Transcriptional and epigenetic regulation of opioid receptor genes: present and future. Annu Rev Pharmacol Toxicol. 2011;51:75–97. doi: 10.1146/annurev-pharmtox-010510-100605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams JT, Christie MJ, Manzoni O. Cellular and synaptic adaptations mediating opioid dependence. Physiol Rev. 2001;81:299–343. doi: 10.1152/physrev.2001.81.1.299. [DOI] [PubMed] [Google Scholar]
- Woolf CJ. Mu and delta opioid receptors diverge. Cell. 2009;137:987–988. doi: 10.1016/j.cell.2009.05.033. [DOI] [PubMed] [Google Scholar]
- Yalcin I, Tessier LH, Petit-Demouliere N, Waltisperger E, Hein L, Freund-Mercier MJ, et al. Chronic treatment with agonists of beta(2)-adrenergic receptors in neuropathic pain. Exp Neurol. 2010;221:115–121. doi: 10.1016/j.expneurol.2009.10.008. [DOI] [PubMed] [Google Scholar]
- Zaki PA, Bilsky EJ, Vanderah TW, Lai J, Evans CJ, Porreca F. Opioid receptor types and subtypes: the delta receptor as a model. Annu Rev Pharmacol Toxicol. 1996;36 :379–401. doi: 10.1146/annurev.pa.36.040196.002115. [DOI] [PubMed] [Google Scholar]
- Zhang H, Gelernter J, Gruen JR, Kranzler HR, Herman AI, Simen AA. Functional impact of a single-nucleotide polymorphism in the OPRD1 promoter region. J Hum Genet. 2010;55:278–284. doi: 10.1038/jhg.2010.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q, Schaffer M, Elde R, Stein C. Effects of neurotoxins and hindpaw inflammation on opioid receptor immunoreactivities in dorsal root ganglia. Neuroscience. 1998;85:281–291. doi: 10.1016/s0306-4522(97)00647-7. [DOI] [PubMed] [Google Scholar]
- Zheng H, Loh HH, Law PY. Agonist-selective signaling of G protein-coupled receptor: mechanisms and implications. IUBMB Life. 2010;62:112–119. doi: 10.1002/iub.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou L, Zhang Q, Stein C, Schafer M. Contribution of opioid receptors on primary afferent versus sympathetic neurons to peripheral opioid analgesia. J Pharmacol Exp Ther. 1998;286:1000–1006. [PubMed] [Google Scholar]
- Zhu Y, King MA, Schuller AG, Nitsche JF, Reidl M, Elde RP, Unterwald E, Pasternak GW, Pintar JE. Retention of supraspinal delta-like analgesia and loss of morphine tolerance in delta opioid receptor knockout mice. Neuron. 1999;24:243–252. doi: 10.1016/s0896-6273(00)80836-3. [DOI] [PubMed] [Google Scholar]