Abstract
Background
The antiretroviral drug efavirenz (EFV) and the antimalarial artemisinin-based combination therapy (ACT) artemether-lumefantrine (AL) are commonly co-administered to treat HIV and malaria. EFV is a known inducer of cytochrome P450 3A4, which converts artemether to dihydroartemisinin (DHA) that is also active and metabolizes longer acting lumefantrine (LR). A study in healthy volunteers was completed to address the concern that EFV impacts AL pharmacokinetics (PK).
Methods
Adults received AL (80/480 mg BID) for 3-days prior to and during EFV co-administration (600 mg daily for 26-days) with intensive PK for artemether, DHA, and LR conducted after the last AL dose for each period. EFV PK was evaluated with and without AL. PK parameters were estimated using non-compartmental methods.
Results
Twelve subjects completed the two-period study. PK exposure for artemether, DHA, and LR [as estimated by the area under the concentration time curve (AUClast)] decreased or trended toward decrease with EFV, compared to when administered alone [−51% (p=0.084), −46% (p=0.005), and −21% (p=0.102), respectively]. Day 7 LR levels, previously deemed predictive of treatment success, were 46% lower (p=0.002) with EFV, but the LR half-life was unchanged. EFV PK exposure was minimally altered following AL co-administration [AUC0–24h decreased by 17% (p=0.034)].
Conclusions
Exposure to DHA, but not LR, was significantly lower during EFV-AL co-administration compared to that during administration of AL alone. These findings may have implications for the treatment efficacy of AL, particularly in children. However, the observed modest changes probably do not warrant dosage adjustment during co-administration of AL with EFV.
Keywords: efavirenz, artemether, dihydroartemisinin, lumefantrine, pharmacokinetic, drug-drug interaction
Introduction
Malaria and human immunodeficiency virus (HIV) infection are two of the most pernicious infectious diseases in the developing world. Malaria is caused by Plasmodium parasites and transmitted by the female Anopheles mosquito1. The World Health Organization (WHO) estimated that in 2010, 216 million episodes of malaria led to 655,000 deaths worldwide, with 86% of these deaths in children less than 5 years of age2. HIV infects over 30 million persons world-wide, with the bulk of HIV and malaria co-infection residing in sub-Saharan Africa3. HIV co-infection rates have been reported to be 11% in children with malaria undergoing hospital admission in certain regions of sub-Saharan Africa4. HIV infection increases the frequency and severity of malaria. In children, HIV infection leads to an increased risk of severe malaria and malarial treatment failure compared to HIV-uninfected children5. Although the availability of antiretroviral therapy (ART) has expanded in Africa, multiple complicating factors must be considered in the context of malaria co-infection, including the potential for clinically important drug-drug interactions between ART and antimalarial therapy.
Artemisinin-based combination therapy (ACT) is recommended by WHO as first line for uncomplicated falciparum malaria. Artemisinin, originating in China from the plant qinghao (Artemisia annua L. or sweet wormwood), is a potent antimalarial, responsible for rapid decline of parasite burden and resolution of clinical symptoms6. Due to the short elimination half-life (typical 1–3hr) of artemisinins7, 8, recommendations for the treatment of uncomplicated malaria advocate use of artemisinins in combination with long-acting partner drugs, including lumefantrine (LR) which has an elimination half-life of 3–6 days9, to ensure elimination of residual parasites and to diminish selection for artemisinin resistance. Artemether-lumefantrine (AL) is one of the most widely used ACTs in the world and adopted in 48 countries as first line therapy for uncomplicated falciparum malaria, including patients with HIV co-infection10. True treatment failure (recrudescence) is uncommon with multiple studies showing the drug is effective11–13. However, for patients within whom pharmacokinetic (PK) exposure to AL may be reduced (e.g. in the context of enzyme inducers or pregnancy), recrudescence rates may increase and in the context of pregnancy have been reported to be higher.14.
ACT pharmacology is complex, and significant drug-drug interactions with ART, which may impact on outcomes, are expected. Artemether (ARM) is metabolized by the CYP enzymes including CYP2B6 and CYP3A4 to active dihydroartemisinin (DHA), which is further metabolized (via glucuronidation and/or oxidation) to inactive products 9, 15, 16. Metabolism of LR is also mediated by CYP3A417. Efavirenz (EFV), one of commonly prescribed ART, is increasingly used for HIV in sub-Sahara Africa in setting endemic for malaria. EFV induces cytochrome P450 (CYP) 3A4 and 2B6 activity18, 19. To assess interactions between AL and EFV, we performed a standard drug-drug PK interaction evaluation in healthy adult volunteers.
Methods
General study design
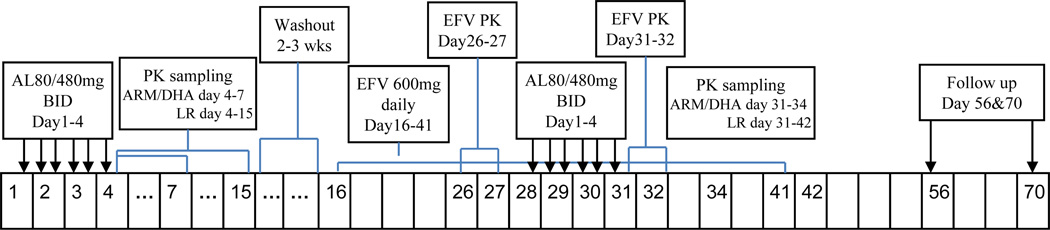
This was an open label two-period crossover study in healthy adults (Figure 1). Subjects were recruited based on the following inclusion criteria: (a) age 21–60 years; (b) within 20% (+/−) of ideal body weight; (c) weight at least 50 kg; and (d) screening laboratory tests that were normal or deemed not clinically significant by the study physician. Female subjects required a negative pregnancy test and agreed to two forms of birth control. Exclusion criteria included: (a) use of illicit drugs or alcohol; (b) use of drugs known to inhibit or induce CYP enzymes or known to be substrates of CYP enzymes; (c) pregnancy or breast-feeding; (d) history of acute or chronic illness, including diabetes, hypertension, coronary artery disease, psychiatric illness, renal or hepatic impairment, hypokalemia, hypomagnesemia or hypercholesteremia; and (e) family history of QTc prolongation or conditions known to prolong the QTc interval, such as cardiac arrhythmias, bradycardia or severe heart disease.
Fig. 1.
Study scheme. AL, artemether/lumefantrine; EFV, efavirenz; BID, twice daily.
The study was approved by the University of California, San Francisco Committee on Human Research and conducted at the Clinical Research Center (CRC), San Francisco General Hospital. All subjects read and signed the informed consent before participation. Enrolled subjects received 6 doses of co-formulated AL (Coartem®, Novartis), 80/480 mg twice daily (study days 1–4). Dose 1 was given on day 1 in the evening to permit the 6th dose to be given in the morning on Day 4 and permit intensive PK sampling during the day. PK sampling on day 4–7 was for ARM/DHA and day 4–15 for LR. This regimen was followed by a washout period of at least 2 weeks (following study day 15), which in turn was followed by a 26-day course of EFV 600 mg once daily (study days 16–41) The washout period did not count toward the sequence of study days, (thus EFV started on study day 16). EFV was initially administered alone (study days 16–27) and then given concomitantly with a second 6-dose course of AL 80/480 mg twice daily (study days 28–31). Subjects were instructed to take all AL doses with food to optimize absorption and asked to record the time of dose and food intake in a diary. Subjects were not allowed to take alcohol and any medications known to modulate CYP enzymes during the study period. Study personnel contacted subjects by phone or e-mail before each visit to evaluate medication/dietary compliance and address questions. Study participants were admitted into the CRC the night before all intensive PK collections, and were discharged after a 24-hour blood collection.
Subject safety monitoring
A physical examination, complete blood count with differential (CBC), comprehensive metabolic chemistry panel (CMP, including serum electrolytes, glucose, blood urea nitrogen, creatinine, and liver function tests), fasting lipid panel, and electrocardiogram (ECG) were performed before enrollment and on the last day of the study. A serum HIV antibody test and urine tests for drugs of abuse were carried out at the screening visit. Female subjects had a pregnancy test within 48 hours before receiving study drugs. Additional laboratory and safety tests (CBC, CMP, lipid panel and ECG) were done following completion of each 3 day course of AL and at the day 10 of EFV administration. Standard audiometry tests were conducted at baseline, during the wash-out, and following completion of the second course of AL. All subjects returned for repeat laboratory tests 2 and 4 weeks following study completion.
Compliance was monitored through review of patient diaries and pill counts and subjects were asked to recount any untoward effects during study participation. All adverse events were classified according to the Division of AIDS Table for Grading the Severity of Adult Adverse Experiences20. Follow-up of serious adverse events was at the discretion of study physicians.
Pharmacokinetic sample collection and processing
Intensive serial PK sampling for ARM, DHA, and LR was conducted on study day 4 (AL alone) and 31 (with co-administration of EFV). Blood samples were collected before and then 0.5. 1, 2, 4, 6, 8, 12, 24, 48, and 72 hr following the sixth AL dose (study days 4 and 31) for determination of ARM, DHA and LR plasma concentrations. Blood samples were collected at 96, 120, 168, 216, and 264 hr for analysis of LR only. Steady state PK sampling for analyses of plasma levels of EFV alone (study day 26) and during co-administration of AL (study day 31) was conducted prior to and 1, 2, 4, 6, 8, 12, and 24 hr following EFV administration (Figure 1).
Blood samples (6 mL) were drawn into heparin and EDTA-containing tubes for subsequent analyses of ARM/DHA/LR and EFV, respectively. ARM/DHA/LR tubes were immediately placed on ice and centrifuged at 800g for 10 minutes at 4 °C. The resulting plasma was split into aliquots and kept at −70 °C until analysis.
Plasma samples analysis
ARM and DHA were analyzed from 500 µL plasma samples using a liquid chromatography tandem mass spectrometry method, as previously described21. The lower limit of quantification (LLOQ) was 2.0 ng/mL and the calibration range was 2.0-200 ng/mL. During sample analysis, the coefficient of variation (CV%) for quality control (QC) samples ranged from 6.4 to 10.1 for DHA and 6.9 to 10.2 for ARM. LR concentration was determined from 200 µL plasma samples using a high performance liquid chromatography-ultraviolet detection (HPLC-UV) method with a linear calibration range of 50–10,000 ng/mL, as previously described22. The CV% range during analysis was 5.6 to 11.7%. EFV concentrations were determined with an HPLC-UV method using reserpine as the internal standard. Briefly, a 200 µL plasma sample was mixed with 100 µL reserpine (0.5 µg/mL in 25% acetonitrile) followed by vortex mixing with 700 µL acetonitrile to precipitate proteins, then 700 µL of the supernatant was dried and reconstituted with 200 µL mobile phase solvent, and 40 µL was injected onto a Waters Symmetry shield C8 column (3.9x150 mm, 5 µm) and eluted with a mixture (55:45) of 25 mM sodium phosphate (pH 6.9) and acetonitrile in isocratic mode at a flow rate of 0.7 mL/min. The detector was a photodiode-array detector set at λ= 247 nm. The LLOQ was 100 ng/mL. The CV during the analysis was less than 8% at all QC levels.
Pharmacokinetic and statistical data analysis
All PK parameters for ARM, DHA, and LR were estimated using non-compartmental analysis via the linear up-log down trapezoidal rule in conjunction with first-order input using WinNonlin 5.2.1® (Pharsight Corporation, Mountain View, CA, USA). Samples below the LLOQ were treated as missing data except for the pre-dose drug concentration, which was set at 0 if below LLOQ. For the antimalarial compounds the area under the plasma concentration versus time curve (AUC) was calculated as AUC from 0 to the last sampling or measurable time point (AUC0-last) and from 0 to time infinity (AUC0-∞). For EFV, the AUC was estimated under steady state conditions and thus the AUC from 0 time to the end of the dosing interval was estimated (AUC0–24hr). For ARM, DHA and LR, the extrapolated area to ∞ for AUC0-∞ was determined by dividing the last measured concentration by the terminal elimination rate constant (lambda z or k). Extrapolation to infinity was only carried out if there were at least 3 measurable concentrations following the peak concentration. Lambda z (k) was estimated using the program’s “best fit” feature combined with fine tuning manually in some cases. Sample size was selected to test the hypothesis that EFV co-administration would reduce LR exposure (AUC) by 40% with an 80% power and 5% significance level. All reported PK parameters except for tmax were compared between groups using Wilcoxon signed-rank test calculated with R-2.14.1 for two group comparisons. For the purpose of paired analysis, specific PK parameters for ARM and DHA were only reported if sufficient data for both PK study periods (Day 4 and 31) were available.
Results
Demographics and Safety
Seventeen subjects were enrolled, and 12 completed the study. Reasons for withdrawal were a) central nervous system (CNS) disturbances with EFV dosing (n=1), b) incorrect study drug dosing and withdrawal by the study physician (n=1), c) rash following AL administration (n=1) and d) elective study discontinuation for personal reasons (n=2). The 12 participants included 10 males and 2 females with a mean age of 36 years (range, 24–53 years) and mean height, weight, and body mass index (BMI) of 176 cm (165–192 cm), 79.5 kg (64.7–92.5 cm), and 26 (21–30 kg/m2), respectively.
Adverse events reported during the study were mild and transient. One subject developed a rash three days following AL initiation (leading to withdrawal), a second subject experienced CNS disturbances and a third subject experienced a mild change in audiology results at study completion compared to baseline; a change deemed of borderline clinical significance. Laboratory tests and ECG results were all within normal limits.
Pharmacokinetic Results
The impact of EFV on ARM and DHA disposition
PK results for ARM, DHA and LR are summarized in Table 1. A trend toward a decrease in most exposure estimates during co-administration was evident. Most notably, a non-significant trend toward a lower AUClast [−51% (p=0.084)] was observed, with t1/2 decreasing by 44% (P<0.05). The estimated reduction was not statistically significant but was large enough to possibly be considered clinically important. Due to more rapid conversion of ARM to DHA during co-administration with EFV, more data points fell below LLOQ (2ng/mL). AUClast could not be estimated in two subjects due to insufficient concentration data points above LLOQ. Moreover, since estimation of AUC0-∞ requires at least three data points defining the terminal elimination phase of the curve, sufficient data for AUC0-∞ was only available in 7 of the 12 subjects during coadministration with EFV. Paired analysis for ARM parameters was possible in 10 of 12 subjects for AUClast and 7 of 12 subjects for AUC0-∞.
Table 1.
PK parameters for artemether, DHA, and LR after administration of AL (Coartem®) alone and in combination with EFV (n=12)
| AL GM; 90%CI |
AL+EFV GM; 90%CI |
Change % (p-value), n |
|
|---|---|---|---|
| ARM | |||
| Cmax (ng/mL) | 21.2 (15.2 to 35.0) | 16.8 (12.0 to 35.7) | −21 (0.359), 10* |
| Tmax (hr) | 0.79 (0.50 to 1.00) | 0.50 (0.50 to 1.25) | |
| AUClast (hr•ng/mL) | 59.5 (40.8 to 128) | 29.4 (23.8 to 76.6) | −51 (0.084), 10* |
| AUC0-∞ (hr•ng/mL) | 98.6 (60.2 to 198) | 65.1 (41.3 to 123) | −34 (0.156), 7** |
| t1/2 (hr) | 5.16 (2.08 to 9.23) | 2.88 (1.23 to 5.87) | −44 (0.016), 7** |
| DHA | |||
| Cmax (ng/mL) | 59.8 (50.3 to 83.6) | 36.8 (31.2 to 54.8) | −38 (0.021), 12 |
| Tmax (hr) | 1.00 (1.00 to 2.00) | 1.00 (0.50 to 1.75) | |
| AUClast (hr•ng/mL) | 171 (146 to 236) | 91.8 (82.4 to 141) | −46 (0.005), 12 |
| AUC0-∞ (hr•ng/mL) | 187 (157 to 259) | 114 (99.9 to 156) | −39 (0.010), 11** |
| t1/2 (hr) | 1.89 (1.31 to 3.74) | 1.65 (1.21 to 2.46) | −13 (0.032), 11** |
| LF | |||
| Cmax (µg/mL) | 11.6 (9.5 to 17.4) | 12.1 (10.5 to 16.4) | 4 (1.000), 11*** |
| Tmax (hr) | 2.00 (2.00 to 6.00) | 6.00 (0.50 to 6.00) | |
| AUClast (hr•µg/mL) | 418 (339 to 639) | 331 (270 to 503) | −21 (0.102), 11*** |
| AUC0-∞ (hr•µg/mL) | 473 (384 to 716) | 367 (300 to 541) | −22 (0.067), 11*** |
| t1/2 (hr) | 108 (81.5 to 144) | 114 (85.9 to 152) | 9 (0.638), 11*** |
Note: CI, confidence intervals; GM, geometric means; Tmax and t1/2 presented as median (P25, P75) and P is percentile; Statistical comparisons between two periods utilized Wilcoxon signed-rank test with 2-tailed distribution.
10 of the 12 subjects were used for comparison due to insufficient data in AL+EFV;
3 subjects for artemether and 1 subject for DHA were excluded from comparison due to insufficient data for extrapolation to infinity.
one subject was excluded because samples were only collected up to 24 hr in the second phase (AL + EFV).
In contrast to results for ARM, EFV had a significant impact on DHA exposure as measured by changes in Cmax, AUClast, AUC0-∞ and t1/2 which decreased, compared to exposure without co-administration, by 38% (p=0.021), 46% (p=0.005), 39% (p=0.010) and from 1.89 to 1.65 hrs (p=0.032), respectively. The DHA to ARM AUC ratio (representing conversion kinetics for ARM to DHA) was not significantly altered by co-administration (median AUClast ratio is 3.47 for AL alone versus 3.88 for AL+EFV, p=0.684). Mean (± SD) plasma concentration-time profiles of ARM and DHA are represented in Figure 2 insert.
Fig. 2.
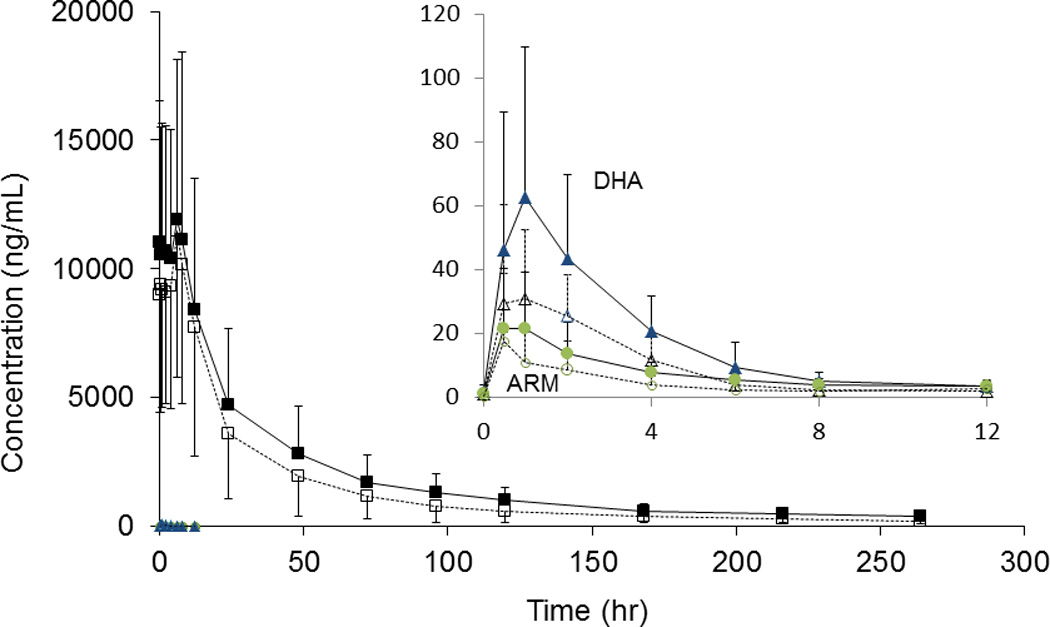
Mean plasma concentration versus time profile for artemether, dihydroartemisinin, and lumefantrine after AL administration alone (solid line) and with EFV (dash line). The insert is a blow-up figure for ARM (circle) /DHA (triangle). Error bar represented standard deviation (SD). Ideal PK time was used as X-axis,
Effects of efavirenz on lumefantrine disposition
Day 7 LR levels are commonly used to predict therapeutic outcome and are represented by the LR values collected 120 hrs following the last dose. Upon co-administration of AL with EFV, LR exposure at 120 hrs (Day 7) decreased 46% from 1020 ±478 (AL alone) to 554 ±432 ng/mL (AL+EFV), (p=0.002). Other PK parameters were not altered significantly (Cmax, t1/2, AUClast, and AUC0-∞) (P>0.05). Specifically, AUClast and AUC0-∞ were decreased by 21% (p=0.102) and 22% (p=0.067), respectively (Table 1). One subject was excluded from paired analysis of LR PK parameters, as samples were available only up to 24 hrs during the second study period. Two subjects were excluded from paired analysis of day 7 LR levels as the concentration was not available at the time point in one period. The mean (± SD) plasma concentration-time profile of LR is shown in Figure 2.
Effects of artemether-lumefantrine on efavirenz disposition
For EFV, PK parameters (Cmax, t1/2, and AUC0–24) did not change to clinically significant levels with AL coadministration. Specifically, the AUC0–24 decreased by 17% (p=0.034) (Table 2), suggesting no important effect on EFV PK exposure. The mean (± SD) plasma concentration-time profile of EFV is shown in Figure 3.
Table 2.
PK parameters for EFV after administration of EFV alone and in combination with AL (Coartem®).
| EFV GM; 90%CI |
EFV + AL GM; 90%CI |
Change % (p-value), n |
|
|---|---|---|---|
| Cmax (µg/mL) | 4.06 (3.58 to 4.92) | 3.69 (3.26 to 4.64) | −9 (0.622), 12 |
| Tmax (hr) | 4.0 (2.0 to 6.0) | 2.0 (2.0 to 3.5) | |
| AUC0–24 (hr•µg/mL) | 56.9 (49.0 to 72.7) | 47.2 (39.5 to 68.0) | −17 (0.034), 12 |
| t1/2 (hr) | 22.4 (16.5 to 35.9) | 17.1 (12.6 to 35.1) | −24 (0.083), 11 |
Note: CI, confidence intervals; GM, geometric means; Tmax and t1/2 presented as median (P25, P75). Wilcoxon signed-rank test with 2-tailed distribution was used for p-value calculation.
Fig. 3.
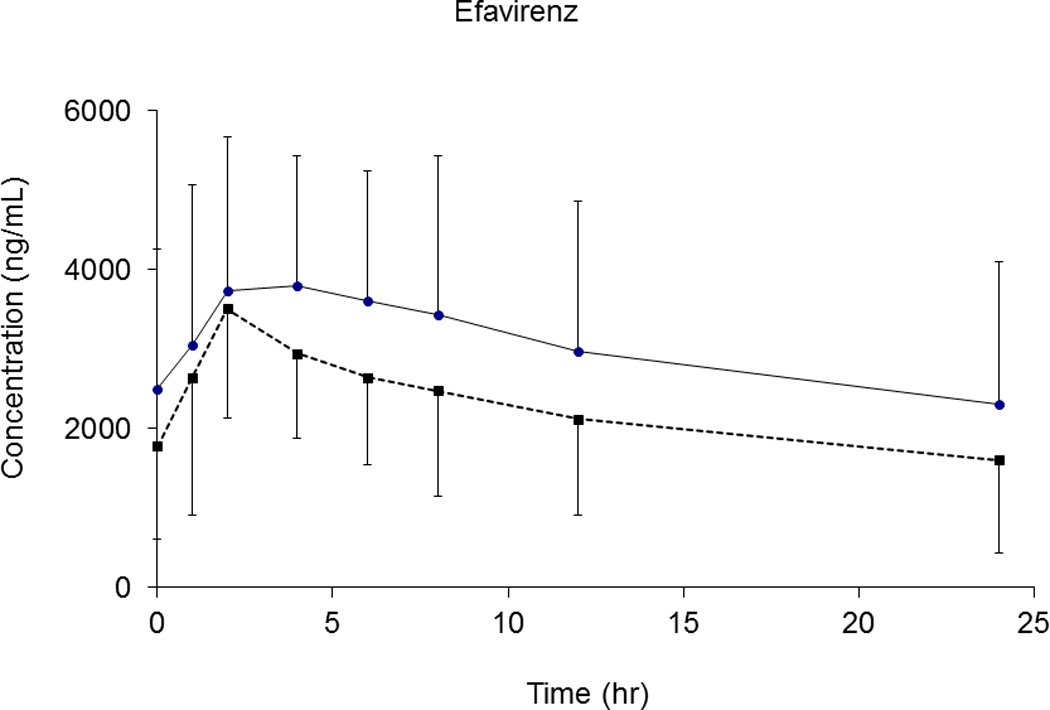
Mean plasma concentration versus time profile for efavirenz after EFV administration alone (solid line) and with AL (dash line). Error bar represented standard deviation (SD).
Discussion
The WHO recommends ACT for the treatment of uncomplicated falciparum malaria. Although resistance has been suggested due to reports of prolonged parasite clearance23, ACTs consistently provide excellent efficacy for the treatment of uncomplicated malaria11–13, 24, 25. However, ACT efficacy has not been systematically studied in the context of co-administration of drugs subject to clinically relevant drug-drug interactions and the impacts of such drugs on the ACT PKs has been little studied. This is the first study evaluating the effect of the widely prescribed antiretroviral drug EFV on the disposition of AL in HIV seronegative healthy adults where the conditions for this intensive PK study were carefully controlled. The most concerning finding was a 50% reduction in the AUC of DHA, the active metabolite of ARM, during exposure to EFV and the significant decrease in Day 7 levels for LR. Most recently, a separate study carried out in HIV infected patients in Uganda has reported similar findings26. Significant decreases in exposure to artemisinins may be particularly relevant given that ACT treatment for uncomplicated malaria is for only three days. Both ARM and DHA have potent antimalarial effects and contribute to rapid clearance of parasites. Diminished concentrations due to drug-drug interactions may alter the time course or extent of parasite elimination.
We found that, when AL was given with EFV, all exposure parameters for DHA decreased significantly, compared to administration alone, results consistent with the study in Uganda26. Results for ARM did not achieve statistical significance, however a trend toward diminished exposure during co-administration with EFV was evident: Pseudomedian percent reduction of AUClast (n=10) with 90% confidence interval for ARM is −43%(−66, −0.05), p=0.084; in comparison, the study in Uganda reported a 79% reduction (n=22) with a median ratio of 0.1 (0.03, 2.3), p<0.01, a higher magnitude but wider range. Two out of 12 subjects in our study versus 8 out of 30 in their study were excluded from analysis of AUClast for ARM due to ARM concentration below LLOQ, results at least partially attributable to the induction of metabolism.
For LR, the most notable finding was the significant 46% decrease in day 7 concentrations with EFV co-administration, which is in contrast to the marginal effect on LR AUC (−22%, p=0.067). AUC is the preferred parameter for assessing overall PK exposure. For field studies, measurement of Day 7 levels offers a practical estimate of exposure. Due to the widespread use of Day 7 monitoring in clinical trials, we reported findings for both Day 7 and AUC.
EFV is a known inducer of CYP metabolism, especially of CYP3A4 and 2B618, 19, 27, 28, suggesting ARM and LR exposure should decrease during co-administration. In contrast, DHA is primarily glucuronidated16 and EFV is not known to induce glucuronidation. Thus, the decrease in DHA exposure observed in this study is difficult to explain. One possibility would be a change in conversion of ARM to DHA. Since the ratio of DHA and ARM AUCs was unaltered, it is possible that absorption of ARM may have been altered due to gut CYP3A4 induction resulting in a decrease in plasma ARM level and thus a decrease in DHA level. Interestingly, our results for DHA are consistent with other reports showing EFV co-administration results in significant reduction in exposure to other UGT substrates, including pravastatin and the active deacetyl metabolite of norgestimate28, 29.
Interactions between AL and other drugs have been reported. The CYP 3A4 inhibitor ketoconazole increases exposure to ARM, DHA and LR by up to 2 fold30. From our study with lopinavir/ritonavir (LPV/r)31, LR exposure was increased by 2–3 fold in healthy adults. Moreover, our field work has revealed a lower re-infection rate when AL is co-administrated with LPV/r due to the extended elimination of LR32. Grapefruit juice, which inhibits gut CYP3A4, increased exposure of ARM by 2–3 fold33. Interaction with other enzyme inducers has been investigated. For NVP, clinical studies have yielded conflicting results with one report showing that NVP caused no significant change in LR AUC in non-malaria infected subjects26. In addition, one South African study reported a paradoxical increase in LR exposure with NVP34.
Although definitive studies on the relationship between artemisinin PK exposure and parasite clearance kinetics are lacking, an association between exposure and parasite clearance has been best described by White, et al, whereby higher ARM and DHA AUC significantly decreased parasite clearance time, while LR AUC was less predictive9. Current guidelines from the WHO for treatment of uncomplicated malaria emphasize the need for 3 days of adequate ACT exposure to ensure elimination of parasites35. For LR, the change in day 7 levels may be of interest given that day 7 levels are increasingly considered predictive of clinical outcomes in terms of recrudescence (rare) or malaria re-infection rates. Of note, only one subject exhibited an LR day 7 level below 175 ng/mL, a threshold previously associated with the risk for treatment failure36.
The results from this study may be particularly relevant for children and pregnant women, who suffer the greatest burden from malaria infection, and for whom AL dosing may already be suboptimal. Of the 216 million malaria cases reported annually, 90% occur in sub-Saharan African, with the vast majority of cases affecting young children. In turn, up to 25% of pregnant women exhibit evidence of placental malaria and/or peripheral parasitemia37–40. Both groups exhibit atypical pharmacokinetics due to metabolic maturation in children or induction in pregnant women, resulting in lower than ideal concentrations of ACT components41, 42 Dosing for these populations has been largely extrapolated from non-pregnant adult data and adjusted for body weight, with guidelines ignoring developmental or physiological changes on drug disposition43, 44. This omission is underscored by prior experience with sulfadoxine-pyrimethamine (SP), with children consistently prescribed lower than necessary doses, a practice that may have contributed to SP resistance and “loss” of this previously effective regimen45. For AL in particular, most evidence points to a lowering of exposure for LR in children41 and pregnant women46 with conflicting results for the artemisinins47, 48.
Limitations of this study include that only 12 subjects were included and this was done in malaria uninfected participants as was done in the study by Byakika-Kibwika26. This interaction needs to be confirmed in malaria infected adults and children requiring use of AL for treatment.
In summary, we report a decrease in the PK exposure of active DHA and day 7 LR when AL is co-administered with EFV. Although only a non-significant trend toward diminished ARM exposure was observed, overall results for ARM and DHA suggest that further studies are warranted to decipher the clinical relevance of this interaction, especially when EFV and AL are to be co-administered to children and pregnant women.
Acknowledgments
This work was supported by an unrestricted grant from Novartis pharmaceutical Co. (Basel, Switzerland), and by NIH/NIAID International Maternal Pediatric Adolescent AIDS Clinical Trials (IMPAACT) Group (grant number 1U01AI068632), ACTG (U01 AI068636), and UCSF-GIVI Center for AIDS Research (CFAR). The study sponsors did not place any restriction on statements made in the final article. We thank Kira Freeman as the study coordinator and Dr. John Kornak at CTSI UCSF for his help with statistical analysis, and thank Dr. Gilbert Lefevre at Novartis Pharmaceutical Co. for reviewing the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
No other conflicts reported.
References
- 1.Centers for Disease Control and Prevention. Malaria biology. Available at: http://www.cdc.gov/malaria/about/biology/index.html.
- 2.World Health Organization. World Malaria Report 2011. Available at: http://www.who.int/malaria/world_malaria_report_2011/en/index.html.
- 3.World Health Organization. Global epidemic, HIV/AIDS, data and statistics. Available as powerpoint slides at: http://www.who.int/hiv/data/en/.
- 4.Rogerson SR, Gladstone M, Callaghan M, et al. HIV infection among paediatric in-patients in Blantyre, Malawi. Trans. R. Soc Trop Med Hyg. 2004;98:544–552. doi: 10.1016/j.trstmh.2003.12.011. [DOI] [PubMed] [Google Scholar]
- 5.Kamya MR, Kigonya CN, McFarland W. HIV infection may adversely affect clinical response to chloroquine therapy for uncomplicated malaria in children. AIDS. 2001;15(9):1187–1188. doi: 10.1097/00002030-200106150-00019. [DOI] [PubMed] [Google Scholar]
- 6.Webster HK, Lehnert EK. Chemistry of artemisinin: an overview. Trans R Soc Trop Med Hyg. 1994;88(s1):27–29. doi: 10.1016/0035-9203(94)90467-7. [DOI] [PubMed] [Google Scholar]
- 7.Mordi MN, Mansor SM, Navaratnam V, et al. Single dose pharmacokinetics of oral artemether in healthy Malaysian volunteers. Br. J. Clin. Pharmacol. 1997;43:363–365. doi: 10.1046/j.1365-2125.1997.00573.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hong X, Liu C, Huang X, et al. Pharmacokinetics of dihydroartemisinin in Artekin tablets for single and repeated dosing in Chinese healthy volunteers. Biopharm. Drug Dispos. 2008;29:237–244. doi: 10.1002/bdd.607. [DOI] [PubMed] [Google Scholar]
- 9.White NJ, van Vugt M, Ezzet F. Clinical Pharmacokinetics and pharmacodynamics of Artemether-lumefantrine. Clin. Pharmacokinet. 1999;37:106–125. doi: 10.2165/00003088-199937020-00002. [DOI] [PubMed] [Google Scholar]
- 10.World Health Organization. Malaria treatment guidelines. Available at: http://www.who.int/malaria/diagnosis_treatment/treatment/en/index.html.
- 11.Bouchaud O, Muehlberger N, Parola P, et al. Therapy of uncomplicated falciparum malaria in Europe: MALTHER - a prospective observational multicentre study. Malar J. 2012;11:212. doi: 10.1186/1475-2875-11-212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Makanga M, Krudsood S. The clinical efficacy of artemether/lumefantrine (Coartem®) Malar J. 2009;8(Suppl 1):S5. doi: 10.1186/1475-2875-8-S1-S5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alecrim MG, Lacerda MV, Mourao MP, et al. Successful treatment of Plasmodium falciparum malaria with a six-dose regimen of artemether-lumefantrine versus quinine-doxycycline in the Western Amazon region of Brazil. Am J Trop Med Hyg. 2006;74:20–25. [PubMed] [Google Scholar]
- 14.McGready R, Tan SO, Ashley EA, et al. A randomised controlled trial of artemether-lumefantrine versus artesunate for uncomplicated plasmodium falciparum treatment in pregnancy. PLoS Med. 2008;5:e253. doi: 10.1371/journal.pmed.0050253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Navaratnam V, Mansor SM, Sit NW, et al. Pharmacokinetics of artemisinin-type compounds. Clin Pharmacokinet. 2000;39:255–270. doi: 10.2165/00003088-200039040-00002. [DOI] [PubMed] [Google Scholar]
- 16.Ilett KF, Ethell BT, Maggs JL, et al. Glucuronidation of dihydroartemisinin in vivo and by human liver microsomes and expressed UDP-glucuronosyltransferases. Drug Metab and Dispos. 2002;30:1005–1012. doi: 10.1124/dmd.30.9.1005. [DOI] [PubMed] [Google Scholar]
- 17.Hatz C, Soto J, Nothdurft HD, et al. Treatment of acute uncomplicated falciparum malaria with artemether-lumefantrine in nonimmune populations: a safety, efficacy, and pharmacokinetic study. Am J Trop Med Hyg. 2008;78:241–247. [PubMed] [Google Scholar]
- 18.Hariparsad N, Nallani SC, Sane RS, et al. Induction of CYP3A4 by efavirenz in primary human hepatocytes: comparison with rifampin and Phenobarbital. J Clin Pharmacol. 2004;44:1273–1281. doi: 10.1177/0091270004269142. [DOI] [PubMed] [Google Scholar]
- 19.Robertson SM, Maldarelli F, Natarajan V, et al. Efavirenz induces CY P2B6-mediated hydroxylation of bupropion in healthy subjects. J Acquir Immune Defic Syndr. 2008;49:513–519. doi: 10.1097/QAI.0b013e318183a425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Division of AIDS Table for Grading the Severity for Adult and Pediatric Adverse Events. Published in December 2004. Available at: http://www.niaid.nih.gov/LabsAndResources/resources/DAIDSClinRsrch/Documents/daidsaegradingtable.pdf.
- 21.Huang L, Jayewardene AL, Li X, et al. Development and validation of a high-performance liquid chromatography-tandem mass spectrometry method for determination of artemether and its active metabolite dihydroartemisinin in human plasma. J Pharm Biomed Ana. 2009;50:959–965. doi: 10.1016/j.jpba.2009.06.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huang L, Lizak PS, Jayewardene AL, et al. A modified method for determination of lumefantrine in human plasma by HPLC-UV and combination of protein precipitation and solid-phase extraction: Application to a pharmacokinetic study. Anal Chem Insights. 2010;5:15–23. doi: 10.4137/aci.s4431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Noedl H, Se Y, Sriwichai S, et al. Artemisinin Resistance in Cambodia: A Clinical Trial Designed to Address an Emerging Problem in Southeast Asia. Clin Infect Dis. 2010;51:E82–E89. doi: 10.1086/657120. [DOI] [PubMed] [Google Scholar]
- 24.Dorsey G, Gasasira AF, Machekano R, et al. The impact of age temperature, and parasite density on treatment outcomes from antimalarial clinical trials in Kampala, Uganda. Am J Trop Med Hyg. 2004;71:531–536. [PubMed] [Google Scholar]
- 25.Zongo I, Dorsey G, Rouamba N, et al. Artemether-lumefantrine versus amodiaquine plus sulfadoxine-pyrimethamine for uncomplicated falciparum malaria in Burkina Faso: a randomised non-inferiority trial. Lancet. 2007;369:491–498. doi: 10.1016/S0140-6736(07)60236-0. [DOI] [PubMed] [Google Scholar]
- 26.Byakika-Kibwika P, Lamorde M, Mayito J, et al. Significant pharmacokinetic interactions between artemether/lumefantrine and efavirenz or nevirapine in HIV-infected Ugandan adults. J Antimicrob Chemother. 2012 Jun; doi: 10.1093/jac/dks207. Epub doi: 10.1093/jac/dks207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pozniak AL, Boffito M, Russell D, et al. A novel probe drug interaction study to investigate the effect of selected antiretroviral combinations on the pharmacokinetics of a single oral dose of maraviroc in HIV-positive subjects. Br J Clin Pharmacol. 2008;65(S1):54–59. doi: 10.1111/j.1365-2125.2008.03136.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gerber JG, Rosenkranz SL, Fichtenbaum CJ, et al. Effect of efavirenz on the pharmacokinetics of simvastatin, atorvastatin, and pravastatin - Results of AIDS clinical trials group 5108 study. J Acquir Immune Defic Syndr. 2005;39:307–312. doi: 10.1097/01.qai.0000167156.44980.33. [DOI] [PubMed] [Google Scholar]
- 29.Sevinsky H, Eley T, Persson A, et al. The effect of efavirenz on the pharmacokinetics of an oral contraceptive containing ethinyl estradiol and norgestimate in healthy HIV-negative women. Antivir Ther. 2011;16:149–156. doi: 10.3851/IMP1725. [DOI] [PubMed] [Google Scholar]
- 30.Lefevre G, Carpenter P, Souppart C, et al. Pharmacokinetics and electrocardiographic pharmacodynamics of artemether-lumefantrine (Riamet) with concomitant administration of ketoconazole in healthy subjects. Br. J. Clin.Pharmacol. 2002;54:485–492. doi: 10.1046/j.1365-2125.2002.01696.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.German P, Parikh S, Lawrence J, et al. Lopinavir/ritonavir affects pharmacokinetic exposure of artemether/lumefantrine in HIV-uninfected healthy volunteers. J. Acquir Immune. Defic. Syndr. 2009;51:424–429. doi: 10.1097/QAI.0b013e3181acb4ff. [DOI] [PubMed] [Google Scholar]
- 32.Achan J, Kahuru A, Ikilezi G, et al. Significant reduction in risk of malaria among HIV+ children receiving lopinavir/ritonavir-based ART compared to NNRTI-based ART a randomised open- label trial. 19th CROI 5–8 March 2012, Seattle; Oral Abstract 26. Available at www.retroconference.org/2012b/Abstracts/43194.htm. [Google Scholar]
- 33.Van Agtmael MA, Gupta V, van der Graaf CAA, et al. The effect of grapefruit juice on the time-dependent decline of artemether plasma lavels in healthy subjects. Clin. Pharmacol. Ther. 1999;66:408–414. doi: 10.1053/cp.1999.v66.a101946. [DOI] [PubMed] [Google Scholar]
- 34.Kredo T, Mauff K, Van der Walt JS, et al. The interaction between artemether-lumefantrine and nevirapine-based antiretroviral therapy in HIV-1 infected patients. Antimicrob Agents Chemother. 2011;55:5616–5623. doi: 10.1128/AAC.05265-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Guidelines for treatment of malaria. World Health Organization. (2nd edition) 2010 [PubMed]
- 36.Price RN, Uhlemann AC, van Vugt M, et al. Molecular and pharmacological determinants of the therapeutic response to artemether-lumefantrine in multidrug-resistant Plasmodium falciparum malaria. Clin. Infect. Dis. 2006;42:1570–1577. doi: 10.1086/503423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Snow RW, Craig M, Deichmann U, et al. Estimating mortality, morbidity and disability due to malaria among Africa's non-pregnant population. Bulletin of the World Health Organization. 1999;77:624–640. [PMC free article] [PubMed] [Google Scholar]
- 38.Guyatt HL, Snow RW. The epidemiology and burden of Plasmodium falciparum-related anemia among pregnant women in sub-Saharan Africa. Am J Trop Med Hyg. 2001;64:36–44. doi: 10.4269/ajtmh.2001.64.36. [DOI] [PubMed] [Google Scholar]
- 39.Steketee RW, Nahlen BL, Parise ME, et al. The burden of malaria in pregnancy in malaria-endemic areas. Am J Trop Med Hyg. 2001;64:28–35. doi: 10.4269/ajtmh.2001.64.28. [DOI] [PubMed] [Google Scholar]
- 40.World Health Organization. WHO Global Malaria Program: pregnant women and infants. 2007 September 20, 2009]; Available from: http://www.who.int/malaria/pregnantwomenandinfants.html.
- 41.Mwesigwa J, Parikh S, McGee B, et al. Pharmacokinetics of artemether-lumefantrine and artesunate-amodiaquine in children in Kampala, Uganda. Antimicrob Agents Chemother. 2010;54:52–59. doi: 10.1128/AAC.00679-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tarning J, McGready R, Lindegardh N, et al. Population pharmacokinetics of lumefantrine in pregnant women treated with artemether-lumefantrine for uncomplicated Plasmodium falciparum malaria. Antimicrob. Agents and Chemother. 2009;53:3837–3846. doi: 10.1128/AAC.00195-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bartelink IH, Rademaker CM, Schobben AF, et al. Guidelines on paediatric dosing on the basis of developmental physiology and pharmacokinetic considerations. Clin Pharmacokinet. 2006;45:1077–1097. doi: 10.2165/00003088-200645110-00003. [DOI] [PubMed] [Google Scholar]
- 44.Strolin Benedetti M, Baltes EL. Drug metabolism and disposition in children. Fundam Clin Pharmacol. 2003;17:281–299. doi: 10.1046/j.1472-8206.2003.00140.x. [DOI] [PubMed] [Google Scholar]
- 45.Barnes KI, Little F, Smith PJ, et al. Sulfadoxine-pyrimethamine pharmacokinetics in malaria: pediatric dosing implications. Clin Pharmacol Ther. 2006;80:582–596. doi: 10.1016/j.clpt.2006.08.016. [DOI] [PubMed] [Google Scholar]
- 46.McGready R, Stepniewska K, Lindegardh N, et al. The pharmacokinetics of artemether and lumefantrine in pregnant women with uncomplicated falciparum malaria. Eur J Clin Pharmacol. 2006;62:1021–1031. doi: 10.1007/s00228-006-0199-7. [DOI] [PubMed] [Google Scholar]
- 47.McGready R, Phyo AP, Rijken M, et al. Artesunate/dihydroartemisinin pharmacokinetics in acute falciparum malaria in pregnancy: absorption, bioavailability, disposition and disease effects. Br J Clin Pharmacol. 2012;73:467–477. doi: 10.1111/j.1365-2125.2011.04103.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rijken MJ, McGready R, Phyo AP, et al. The pharmacokinetics of dihydroartemisinin and piperaquine in pregnant and non-pregnant women with uncomplicated falciparum malaria. Antimicrob Agents Chemother. 2011;55:5500–5506. doi: 10.1128/AAC.05067-11. [DOI] [PMC free article] [PubMed] [Google Scholar]