Abstract
Rationale
Ischemic cardiovascular disease represents one of the largest epidemics currently facing the aging population. Current literature has illustrated the efficacy of autologous, stem cell therapies as novel strategies for treating these disorders. The CD34+ hematopoetic stem cell has shown significant promise in addressing myocardial ischemia by promoting angiogenesis that helps preserve the functionality of ischemic myocardium. Unfortunately, both viability and angiogenic quality of autologous CD34+ cells decline with advanced age and diminished cardiovascular health.
Objective
To offset age and health-related angiogenic declines in CD34+ cells, we explored whether the therapeutic efficacy of human CD34+ cells could be enhanced by augmenting their secretion of the known angiogenic factor, sonic hedgehog (Shh).
Methods and Results
When injected into the border zone of mice following acute myocardial infarction (AMI), Shh-modified CD34+ cells (CD34Shh) protected against ventricular dilation and cardiac functional declines associated with AMI. Treatment with CD34Shh also reduced infarct size and increased border zone capillary density compared to unmodified CD34 cells or cells transfected with the empty vector. CD34Shh primarily store and secrete Shh protein in exosomes and this storage process appears to be cell-type specific. In vitro analysis of exosomes derived from CD34Shh revealed that; 1) exosomes transfer Shh protein to other cell types and, 2) exosomal transfer of functional Shh elicits induction of the canonical Shh signaling pathway in recipient cells.
Conclusions
Exosome-mediated delivery of Shh to ischemic myocardium represents a major mechanism explaining the observed preservation of cardiac function in mice treated with CD34Shh cells.
Keywords: Angiogenesis, Myocardial infarction, Gene therapy, Cell Therapy, CD34 cells
Introduction
Cell-based therapies are rapidly emerging as a predominant new strategy for the treatment of various cardiovascular diseases that often involve acute or chronic ischemia. Pre-clinical and clinical studies have illustrated the beneficial effects of using human bone marrow-derived CD34+ stem cells to treat conditions involving myocardial ischemia including refractory angina1, 2 and acute myocardial infarction (AMI).3 A major benefit of using CD34+ stem cells in human patients is that the cell source is autologous; a unique characteristic that eliminates many of the inflammatory and toxicity concerns associated with non-autologous cells and/or pharmacological therapies. Although the exact nature of their therapeutic mechanism(s) is not entirely understood, it is believed that CD34+ stem cells promote the expansion of pre-existing micro-vasculature (angiogenesis) and/or stimulate the de novo development of vascular structures (vasculogenesis) in ischemic regions of the cardiac muscle.4 Recent evidence has identified a therapeutic paracrine mechanism of CD34+ cells as mediated in part by the secretion of extracellular, membrane-bound nano-vesicles known as exosomes5 that often carry proteins, RNAs, and/or microRNAs.6 Nonetheless, the use of CD34+ cells as a strategy to enhance perfusion is known to preserve and/or improve cardiac function,7-9 which will hopefully extend and improve the quality of life for the patient.
Although CD34+ cell-based therapies exhibit strong efficacy and safety profiles, the general cardiovascular health of a patient predicts both the relative availability and the therapeutic activity of the isolated cells. Health factors including smoking and alcohol abuse negatively impact circulating CD34+ cell levels.10, 11 Additionally, circulating levels of CD34+ cells serve as indicators of cardiovascular outcome since densities of CD34+ cells in the circulation are commonly inversely proportional to both age and the severity of disease,12-15 indicating a natural time-dependent decrease in the angiogenic potential of CD34+ mobilized cells. These findings suggest that as cardiovascular disease worsens, autologous CD34+ cells become less capable of providing the intended therapeutic benefit. To counteract this cellular functional decline, several attempts have been made to boost the potency of autologous cells by using combinational therapies that co-deliver known angiogenic genes and/or proteins along with stem cells.16-19 Despite these efforts, gene therapy remains a largely inefficient procedure requiring large doses of DNA in order to establish measurable target gene translation that has resulted in some phase II and phase III gene therapy trials failing to show gene-mediated therapeutic benefit.20, 21
To improve delivery of the target gene while limiting the potential for adverse effects associated with off-target responses/poor gene transfer efficiency, we attempted to enhance the angiogenic quality of CD34+ cells by genetically modifying them to express the sonic hedgehog (Shh) protein. Shh is a well-established angiogenic morphogen22 and is known to play important roles in cardiac development23 and post-natal ischemic injury recovery.24-26 Specifically, AMI is a known stimulus for the induction of Shh and its signaling components including the g-protein coupled receptor patched1 and the downstream Gli transcription factors.25 Importantly, post-natal induction of the Shh pathway has been shown to be protective against the tissue damage and cell death associated with ischemia since blockade of this pathway worsens outcome following experimental ischemia in rodents.24
Accordingly, this study tested the hypothesis that Shh-modified human CD34+ cells provide enhanced functional benefit in the setting of AMI and that direct cellular modification provides a means to counteract age and disease related declines in the therapeutic potency of autologous cell therapy. Furthermore, these experiments also evaluated whether exosomes derived from Shh-modified CD34+ cells take part in this process.
Methods
Detailed methods are provided in the on-line data supplement.
Animal Models
Mice used in this study were obtained from The Jackson Laboratories (Bar Harbor, ME) and surgical procedures and animal care protocols were approved by the Northwestern University Animal Care and Use Committee. AMI injuries were induced (described previously27) in 8-week old male nude/J or NOD-SCID mice. Human CD34+ cells were delivered as 2 - 10μl injections (on either side of the ligation) and contained either; 1) a sub-therapeutic dose of 2.5×104 (25K) cells/mouse, or 2) a therapeutic dose of 5.0×104 (50K) cells/mouse. Treatment groups included 1) Saline (n=16); 2) 25K unmodified CD34 cells (CD34NM) (n=8); 3) 25K CD34 cells transfected with an empty vector (CD34EV) (n=7); 4) 25K CD34 cells transfected with an Shh-coding vector (CD34Shh) (n=13); 5) 25K CD34NM and 200ng Shh protein (n=7); or 6) 50K CD34NM (n=9).
Results
Expression of Shh in Modified CD34+ Cells
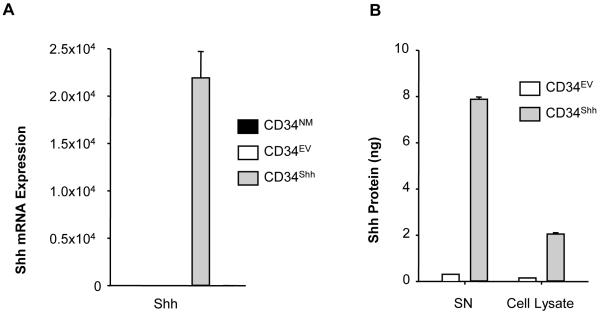
Following gene modification, both Shh mRNA and protein expression levels were assessed using quantitative polymerase chain reaction and ELISA, respectively. As compared to both CD34NM and CD34EV cells, CD34Shh cells exhibited substantially higher levels of Shh mRNA (Figure 1A). Importantly, mRNA levels of Shh (Figure 1A) compared between CD34EV and CD34NM cells, were not different indicating that modification alone failed to influence Shh expression. In terms of Shh protein (Figure 1B), both intracellular and extracellular (secreted) compartments of CD34Shh cells expressed significantly higher levels of Shh when compared to CD34EV cells. These results indicate that gene modification of CD34+ cells with the AMAXA system is effective and resulted in enhanced cellular Shh protein translation and secretion.
Figure 1. Shh Production is Increased in CD34Shh.
A. Assessment of Shh mRNA in cells 48 hours after modification using quantitative RT-PCR (qRT-PCR). CD34Shh show robust up-regulation of Shh mRNA as compared to CD34NM and CD34EV. Levels of mRNA were normalized to 18S RNA and are plotted relative to CD34EV. Bars depicting Shh mRNA for CD34NM and CD34EV treatments are present but non-visible due to low expression. Data is presented as the mean ± SE where N=4. B. Assessment of Shh protein production in modified CD34+ cells using a Shh ELISA. Shh is effectively up-regulated in supernatant (SN) and CD34Shh lysates when compared to CD34EV. The bars indicate the absolute Shh amount derived from 1 million cells. Data are presented as the mean ± SE where N=3.
Additionally, fluorescence-activated cell sorting analysis with a panel of hematopoetic stem and non-stem cell surface antigens was used to evaluate whether gene modification or the presence of excess Shh acutely affected the CD34+ stem cell antigen profile. Analysis of the antigens CD34, CD45, CD38, CD41, Lin, CD133 and CD117 failed to reveal differences in antigen expression between CD34NM, CD34EV and CD34Shh at 24 hours post-modification indicating that neither modification or excess Shh acutely impacts the antigenic identity of the cells (Online Figure I).
CD34Shh Significantly Enhances Preservation of Cardiac Function after AMI
Following gene modification, cells were injected into the ischemic border zone of immune-compromised mice to assess whether modification with Shh could provide superior functional preservation and/or improvement. To provide the largest possible window for detection of potential functional differences between the various treatment groups, a sub-threshold, non-therapeutic dose (2.5×104 cells/mouse) of CD34NM was used. This sub-threshold dose failed to provide any functional benefit as compared to mice receiving only saline following AMI (Online Figure IIA). Additionally, ejection fraction (EF), fractional shortening (FS), left ventricular end-systolic and diastolic volumes were not different when compared between saline-, CD34NM- and CD34EV-treated mice at 4 weeks post-MI (Online Figure IIA). Conversely, doubling the cell dose to 5.0×104 CD34NM cells did provide functional benefit over saline indicating; 1) the CD34+ cells used did possess therapeutic activity (Online Figure IIA) and, 2) the functional response was dose dependent.
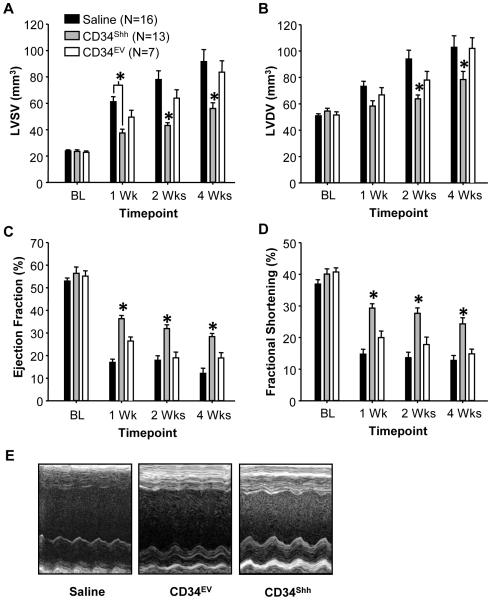
Interestingly, mice treated with 2.5×104 CD34Shh exhibited robust improvements in both EF and FS and also displayed decreased systolic and diastolic ventricular dilation as compared to all control mice (Figure 2A-E). Significantly improved cardiac function induced by CD34Shh was detected as early as one week post-AMI and persisted to post-AMI weeks two and four. These findings suggest that modification with Shh improves the therapeutic potency of CD34+ cells over CD34NM.
Figure 2. Treatment with Shh Gene-Modified CD34 Cells (CD34Shh) Improves Functional Recovery from AMI.
CD34Shh, even when administered in a sub-therapeutic dose, provide superior protection against the functional deficits associated with permanent AMI as early as 1 week and lasting out to 4 weeks post-AMI. CD34Shh protect against AMI-induced increases in left ventricular end systolic (A) and diastolic volumes (B). Using serial echocardiography, treatment with CD34Shh preserves cardiac function to a greater extent than control cells as assessed by ejection fraction (C) and fractional shortening (D). E. Representative midventricular, long-axis M-mode images taken from treated animals at 2 weeks post-infarction. * represents statistical significance (p<0.05) assessed by Two-Way Repeated Measures ANOVA with the post-hoc Holm-Sidak test. Bars on all graphs indicate group mean ± SE.
CD34Shh Reduce Infarct Size and Increase Border Zone Capillary Density
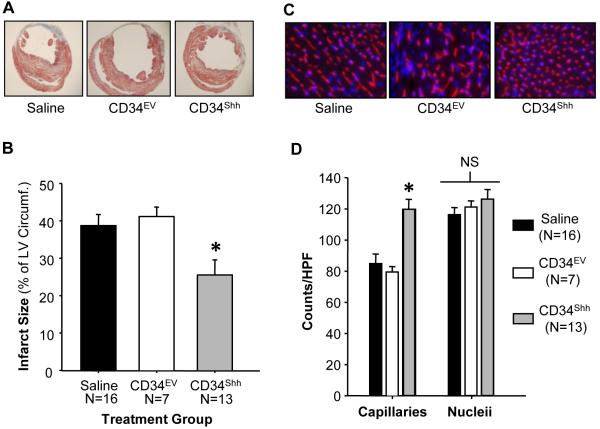
Following sacrifice and tissue harvest at the four week time point, hearts from CD34Shh treated mice exhibited significantly reduced infarct dimensions as compared to all other treatments (Figures 3A and 3B). Additionally, using immunofluorescent detection of BS-1 lectin staining, capillary density was assessed within the infarct border zone of all hearts. Density of capillaries within the infarct border zone was significantly increased in hearts of mice treated with CD34Shh as compared to control groups (Figures 3C, D). Additionally, infarct size and capillary density (compared between CD34NM- and CD34EV-treated mice) were not different (Online Figure IIB and C), further validating the non-therapeutic nature of the sub-threshold dose of CD34NM.
Figure 3. Reduced Infarct Size and Increased Border Zone Capillary Density in Hearts of Mice Receiving CD34Shh.
A. Representative cross-sectional Masson-Trichrome stained histological sections depicting the infarct zone in mice receiving either saline or sub-therapeutic doses of CD34EV or CD34Shh. B. Graph depicting the quantification of the infarct length relative to the entire left ventricular circumference which is significantly reduced in animals receiving CD34Shh. Bars indicate group means ± SE. C. Representative immunofluorescence images taken within the infarct border zone of mice treated with saline, CD34EV or CD34Shh. Capillaries were stained with BS-lectin-Alexa-555 (red) and nuclei were counterstained with DAPI (blue). D. Graph depicting the quantification of border zone capillary number across treatments presented as the number of isolectin B4 positive capillaries and DAPI stained nucleii per high power field (HPF). Bars represent group means ± SE. * represents statistical significance (p<0.05) assessed by One-Way ANOVA with the post-hoc Holm-Sidak test.
CD34Shh Exhibit Enhanced Post-Injection Myocardial Retention
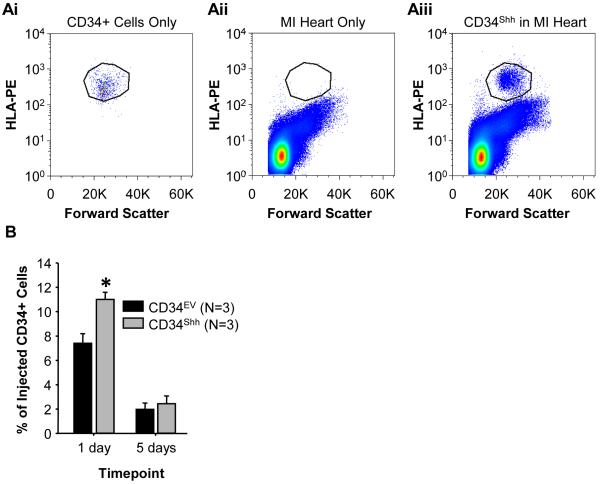
To address how the delivery of Shh via CD34Shh influences cardiac function, we sought to determine if modification affects the intra-myocardial retention of cells following direct cardiac injection. To evaluate this parameter, a sub-threshold dose of either CD34Shh or CD34EV was injected into the ischemic border zone of hearts of NOD-SCID mice following AMI. At 1 and 5 days post cell administration, hearts were harvested, enzymatically digested and then stained with antibodies to HLA-ABC to quantify the viable human cells remaining in the mouse heart at each time point. As seen in Figure 4, modification of CD34+ cells with Shh significantly improved the retention of live human cells in the myocardium at 24 hours post-injection. In contrast, no difference between cell types was observed at 5 days post-injection perhaps reflecting a time-dependent loss of HLA antigenicity or continued clearance of cells.
Figure 4. Improved Short-Term Myocardial Retention of CD34Shh.
Enzymatic digestion and subsequent fluorescence-activated cell sorting analysis of HLA-ABC positive cells from CD34Shh and CD34EV treated mice hearts. A. Representative examples of FACS analysis for (i) CD34+ cells alone or, (ii) AMI cardiac tissue alone or, (iii) AMI cardiac tissue injected with CD34Shh. CD34+ cells were detected by staining digested cardiac tissue with HLA-PE antibodies. B. Quantification of the number of live CD34+ cells recovered from digested hearts as a fraction of the original intra-myocardially injected cell number (i.e. 2.5×104 cells). * represents P<0.05 using One-way repeated measures ANOVA and the post-hoc Holm-Sidak test. Bars represent group means ± SE where n=3 animals per group per time point.
Co-therapy of CD34NM with Shh Protein Does Not Improve Cardiac Function
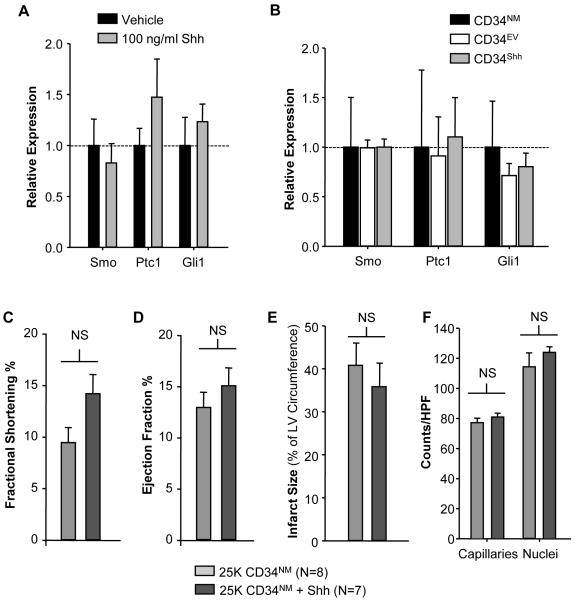
To understand the mechanisms behind the functional benefits observed with CD34Shh, we explored whether canonical Shh pathway genes are induced in CD34+ cells following treatment with Shh protein or genetic modification. As seen in Figure 5A, treatment of CD34NM with recombinant Shh (100ng/ml) for 24 hours failed to stimulate the up-regulation of mRNAs of Shh pathway components. Additionally, modification also failed to induce expression changes in Shh pathway components (Figure 5B). Lastly, several growth factors implicated in the paracrine, angiogenic activity of stem cells were also not regulated following Shh modification (data not shown) suggesting that activation of the canonical Shh signaling pathway in CD34+ cells is unlikely to be involved in the therapeutic benefit observed with CD34Shh.
Figure 5. Co-administration of CD34NM with Shh Protein Fails to Promote Cardiac Functional Preservation.
A. CD34NM cultured in complete media in the presence of 100ng/ml of recombinant Shh for 24 hours fails to show mRNA up-regulation of canonical Shh pathway components (Smoothened receptor (Smo), Patched1 receptor (Ptc1), and Gli1 transcription factor (Gli1)). B. Assessment of mRNA regulation of various Shh pathway components between CD34NM, CD34EV and CD34Shh using qRT-PCR. All comparisons are normalized to 18S mRNA and values for each transcript are normalized to the CD34NM condition as the control. C. Intramyocardial injection with the sub-therapeutic CD34NM dose and recombinant Shh protein (200ng) fails to generate improvement as compared to CD34NM with regards to fractional shortening (C) or ejection fraction (D). Both infarct size (depicted in E) and capillary density (depicted in F) are also not influenced by CD34NM plus 200ng of Shh protein. Bars on all graphs represent the group means ± SE. * represents p<0.05 assessed with a t-test.
To further explore the mechanism behind CD34Shh mediated functional preservation, we assessed whether co-therapy of the sub-therapeutic dose of CD34NM along with recombinant Shh protein (200ng/mouse) could replicate the functional benefits observed with CD34Shh. As seen in Figure 5, Shh protein co-administration along with CD34NM produced no improvements in FS or EF (Figures 5C/D) and also failed improve infarct size (Figure 5E) or border zone capillary density (Figure 5F). These findings may indicate that the functional preservation seen with CD34Shh reflects the therapeutic necessity of a short term, sustained secretion of Shh or the existence of a cellular Shh delivery process that is insufficiently replicated by the administration of a single injection of Shh protein.
CD34Shh Specifically Package Over-Expressed Shh in Secreted Exosomes
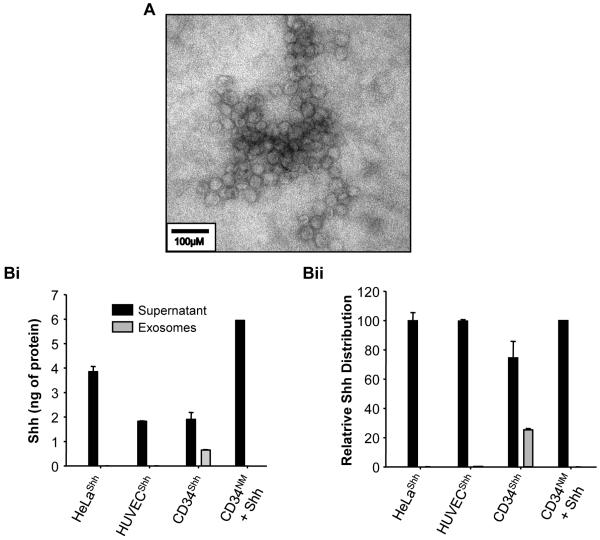
Exosomes derived from CD34NM were recently characterized and have been shown to mediate a significant component of the angiogenic capacity of these cells.5 To assess the nature by which Shh was being secreted by CD34Shh, established exosomal purification methodologies28 were used to determine whether the exosomal fraction of CD34Shh conditioned media was involved. Following purification (Figure 6A), it was determined that a significant fraction of the Shh protein secreted from CD34Shh was found to be associated with small, membrane-enclosed vesicles known as exosomes (Figure 6B). Modification of equal numbers of other cell types with Shh, such as human umbilical-vein endothelial cells (HUVECs) or HeLa cell failed to show substantial exosomal Shh deposition, indicating that this storage process appears to be largely specific to CD34+ cells (Figure 6B). Although CD34Shh secrete slightly less absolute total Shh protein (Figure 6Bi) than other modified cells types, a far greater proportion of the total Shh secreted by CD34Shh exists in the form of exosomal Shh (Figure 6Bii). Supplementation of CD34NM with recombinant Shh (50ng/ml) failed to result in exosomal deposition of the protein, indicating that genetic modification of CD34+ cells is required to exploit any potential therapeutic benefit of exosome-mediated Shh delivery.
Figure 6. CD34Shh Selectively Package Shh into Exosomes.
A. A representative transmission electron micrograph of exosomal vesicles isolated from the media of CD34Shh cultured for 48 hours post-modification. Bi. This graph represents the absolute amount of Shh present within the supernatant (culture media devoid of exosomes) and exosome fractions of modified cells (HUVECShh, HeLaShh, CD34Shh) and non-modified CD34NM (to which 50ng/ml Shh protein was added) assessed via a Shh ELISA. Supernatant and exosome samples were collected after culturing 2.5 million cells of each type for 48 hrs. Bii. This graph depicts the same data as seen in Bi although the relative amounts of Shh in the supernatant and exosomes are expressed as a percentage of the total Shh present in the media. CD34Shh deposit a large percentage of their total Shh in secreted exosomes. Bars for both Bi and Bii depict replicate means ± SEM and are representative examples of at least 3 independent experiments.
To determine whether Shh-containing exosomes derived from CD34Shh could be mediating the observed functional benefits seen in the in vivo AMI model, we assessed whether exosomes derived from modified CD34+ cells could interact with another cell type. HUVECs were chosen as the recipient cell in this experiment for two specific reasons including; 1) the well-established importance of endothelial cell signaling and function in Shh-mediated angiogenesis and, 2) the need for a recipient cell that fails to express measurable levels of Shh protein. Following treatment of starved HUVECs with CD34Shh derived exosomes for 16 hours, Shh ELISA was used to confirm that exosomes effectively transferred their Shh protein cargo to the recipient HUVECs (Online Figure IIIA).
Lastly, we assessed whether exosome-mediated transfer of Shh could functionally influence recipient cells. To do this, luciferase activity in mouse embryonic (NIH3T3) fibroblasts co-transfected with both Gli-luciferase and β-galactosidase vectors was assessed following treatment with CD34Shh-derived exosomes. Gli-reporter and β-gal transfected fibroblasts were treated with exosomes isolated from CD34Shh for 16 hours. Fibroblasts treated with exosomes derived from CD34Shh generated a modest increase in luciferase activity (Online Figure IIIB) indicating that Shh protein transfer via exosomes produced measurable signaling events in recipient cells.
Discussion
Although stem cells continue to emerge as another tool within the therapeutic armamentarium available for addressing various cardiovascular disorders involving ischemia, their use comes with unique challenges not generally observed with standard pharmacological therapies. Perhaps the principal challenge to their adoption as standard therapy relates to the current inability to normalize their inherent reparative abilities across all patients since individuals and their co-morbidities vary immensely. Patient diversity in disease burden, age and environmental factors alters the availability and therapeutic efficacy of isolated CD34+ cells.10-15 Given this serious and complex problem, we determined whether the therapeutic efficacy of CD34+ cells could be improved upon by modifying them to express an established angiogenic protein, sonic hedgehog, to circumvent age and health related declines in CD34+ cell function. The use of the AMI model for these studies was directed by our previous success using autologous CD34+ cells for the treatment of refractory angina in human patients.1
As presented in this report, CD34Shh were observed to improve functional preservation of cardiac tissue as compared to control cells. Modification with Shh enhanced the therapeutic potency beyond that observed with a sub-therapeutic dose (i.e. 2.5×104 cells) of CD34NM, indicating that CD34Shh could theoretically be used at lower doses than would be possible in their conventional form. This finding is significant given the problems associated with utilizing cell based therapies in poorly mobilizing patient populations such as those with ischemic12, 29 or diabetic30, 31 conditions. The finding that treatment with CD34Shh induces robust increases in capillary development within the infarct border zone, accompanied by reduced infarct sizes, indicates that Shh-mediated angiogenesis is the likely mechanism for the observed functional improvements although Shh-mediated trans-differentiation of resident fibroblasts to endothelial cells may also play a role. Given the abundance of information regarding the involvement of Shh-mediated repair of ischemic tissues,24, 25, 32, 33 it is not surprising that cardiac function was preserved in mice treated with CD34Shh. However, these findings do provide a number of novel insights including; 1) short term secretion of Shh appears to be required for functional benefits to occur, 2) modification with Shh improves the short-term retention of CD34+ cells, 3) CD34Shh deposit a proportionately greater amount of Shh in exosomes as compared to other Shh-modified cell types, 4) Shh-containing exosomes derived from CD34Shh are capable of transferring Shh to other cell types and, 5) exosomes containing Shh activate Shh signaling pathways in other cell types.
The observation that functional improvement requires sustained Shh secretion is derived from the finding that co-administration of a single dose of Shh protein along with CD34NM failed to replicate the in vivo functional benefits seen with CD34Shh. These results suggest that, although the injected cells appear to be of low abundance in the ischemic myocardium, their presence for at least 5 days following injection allows for the sustained secretion of Shh thus greatly enhancing the propensity for Shh-mediated angiogenic signaling. Recombinant Shh has a serum half-life of approximately 1 hour although chemical modification of these proteins can improve the half-life upwards to 12 hours with equal or greater potency.34 It remains to be determined whether, 1) direct injection of modified forms of Shh or, 2) modification of CD34+ cells with vectors expressing the improved Shh variants would provide even greater functional benefit than seen here. Nonetheless, the evidence presented here for improved cardiac function with CD34Shh (and not for CD34NM plus Shh protein) indicates that Shh-mediated angiogenic signaling events that occur between days one to five following cell injection are critical for the detection of improved functional outcomes.
This argument is strengthened when considering that CD34Shh were retained within the heart following local injection to a greater extent than control cells. Although improved retention of CD34Shh cells was not resolved at day five, a number of factors may be influencing the detection of retained cells at this time point including; 1) a loss of HLA-ABC antigenicity, 2) the general clearance of the cells over the 5 day time period, or 3) cell death. Given the difficulties of using immunohistochemical methods to quantify viable exogenous cells within cardiac tissue, we felt that enzymatic digestion of the whole heart followed by FACS analysis was a comprehensive method to quantify retention. Unfortunately, enzymatic digestion of the heart would be independently expected to cause cell death as well as potential antigen loss, suggesting that our results probably reflect an underestimate of the actual number of CD34+ cells retained in both conditions and at both time points. Further supporting the claim that sustained secretion of paracrine factors is required for the observed functional benefits of CD34Shh cells, is an elegant report that utilized programmed apoptosis of endothelial progenitor cells (EPCs) following cardiac injection to show that the presence of EPCs weeks after injection continues to contribute to the sustained improvements in cardiac function attributable to EPC function.35
One could also speculate that Shh-modification of CD34+ cells may affect adherence of cells within cardiac tissue by regulating expression of adhesion proteins and could be addressed in future experiments via cellular protein profiling. Another potential avenue of examination includes determining whether local Shh signaling processes alter the cell surface landscape of resident cardiac cells to assist in retaining therapeutic exogenous cells within that specific location.
Along these lines, attempts to determine whether Shh treatment (i.e. protein treatment or Shh-modification) acutely influenced CD34+ cells revealed no induction of endogenous Shh pathway components (Figure 5A, B) or the differentiation capacity of CD34+ cells (Online Figure I). Analysis at later time points for both assays may reveal more obvious long term effects not seen at the time points assessed in this study. With the recent characterization of a non-canonical Shh signaling pathway in endothelial cells,36, 37 it is conceivable that the direct action of Shh on CD34+ cells may also utilize this pathway. Nonetheless, delivery of CD34Shh within 24 hours of modification was clearly sufficient to provide therapeutic benefit in vivo.
Together, these results indicate that CD34Shh acted primarily as a secretory vehicle to deliver Shh to the ischemic tissue they were injected into. Although an autocrine effect of Shh on CD34Shh was not observed (as has been seen in Shh-modified mesenchymal stem cells38), both modified stem cell types provide significant protection against functional deficits associated with AMI. Given our laboratory’s recent discovery that CD34+ cells actively secrete exosomes as a major component of their paracrine-mediated reparative abilities,5 we explored whether the over-expression of Shh in modified CD34Shh would allow us to determine; 1) whether CD34Shh selectively package Shh into exosomes and, 2) whether this mechanism had any functional implications for Shh signaling in other cell types.
Fortunately, the gene modification approach allowed for the elucidation of exosomal mechanisms that, to this point, have been difficult to observe owing to their extremely small cargo load. We determined that CD34Shh divert approximately ~25% of their secreted Shh into exosomes and that this process appears to be cell-type selective given that other Shh-modified cell types such as HUVECs and HeLa cells secrete Shh almost exclusively as free protein. The observed exosomal deposition of Shh in CD34Shh doesn’t appear to result from excess protein load given that the absolute amount of protein secreted by the other cell types was generally higher. It should also be mentioned that exposing CD34NM to Shh protein did not result in its exosomal deposition, which reinforces the notion that CD34+ cells must be genetically modified in order to exploit therapeutic exosomal mechanisms.
Despite our knowledge that stem and cancer cell-derived exosomes possess angiogenic abilities independent of the cell type they are derived from,5, 39-42 the mechanistic basis of their action is still under investigation. Our finding that exosomes derived from CD34Shh physically transfer Shh to other cell types adds to this knowledge. Although emerging evidence is suggesting that exosomes also harbor mRNA and/or miRNAs that can be transferred to other cell types where they mediate expression changes in target cells,43, 44 to our knowledge, no report currently exists that depicts the ability of CD34+ cell-derived exosomes to transfer functional protein. Presumably, failure to observe exosomal-mediated protein transfer is derived from the difficulty associated with detecting low abundance proteins, however, the genetic modification procedure utilized here has allowed for the observation of this process. Although the possibility exists that Shh-containing exosomes were simply adhering to HUVECs, our methodology which included multiple vigorous cell washes followed by centrifugation, indicates that any outer cell membrane adherence that did occur was apparently a physiologically relevant association.
Our observation that exosomal Shh also activated the Shh signaling pathway in fibroblasts, evidenced by up-regulation of Gli-luciferase activity in NIH3T3 fibroblasts, supports the belief that exosomes promote meaningful communication between cells. Although the luciferase response induced by CD34Shh exosomes was modest as compared to free recombinant protein (see Online Figure IIIB), the absolute level of the response was actually very robust when compared to the amount of Shh protein actually found in CD34Shh exosomes (see Online Figure IIIA). This finding suggests that exosome-mediated activation of cell signaling may actually be more efficient than that caused by free protein. Since exosomes are membrane bound vesicles, they would be expected to protect their cargo from destructive extracellular proteases and thereby enhance cell signaling events. From a clinical standpoint, the delivery of exosomes may offer several advantages over cell delivery, a few of which include; 1) the ability to deliver a treatment that may possess a higher therapeutic potency than modified cells, 2) the avoidance of triggering potentially damaging cell clearance mechanisms following injection and, 3) potential “expansion” of therapeutic exosomes via short term culturing of isolated CD34+ cells. Current work in the laboratory is focused on further investigation of the mechanistic basis by which CD34+ cell-derived exosomes generate in vivo angiogenic responses in hind-limb and myocardial ischemia models. As our knowledge regarding these interesting cellular components expands, it is likely that the true nature of their activity will resemble a combination of multiple mechanisms that will be regulated both contextually and temporally.
Supplementary Material
Novelty and Significance.
What Is Known?
CD34+ cells are known to stimulate therapeutic neovascularization in preclinical studies as well as in phase I and II human clinical trials and the potency of CD34+ cells is greater than for unselected mononuclear cells.
Sonic hedgehog (Shh) is a well-established angiogenic morphogen capable of stimulating post-natal angiogenesis following an acute ischemic insult.
Both the number of CD34+ cells and their inherent angiogenic functionality is diminished in patients of increasing age and cardiovascular disease burden.
Exosomes are small, membrane-bound vesicles secreted from many cell types that harbor protein and nucleic acids and have lately become heavily implicated in cell-to-cell signaling mechanisms.
What New Information Does This Article Contribute?
Genetic modification of CD34+ cells with Shh imparts therapeutic efficacy to a previously determined sub-therapeutic dose of non-modified CD34+ cells following myocardial infarction.
When administered directly to ischemic myocardium, Shh-modified CD34+ cells significantly reduce both the negative anatomical and functional impact of an acute myocardial infarction as compared to non-modified and control modified cells.
The CD34+ cell type selectively deposits functional Shh protein into exosomes that can; 1) be taken up by other cell types and 2) induce Shh signaling in other cells.
The therapeutic functionality and the yield of autologous CD34+ cells are reduced in the aged and those with significant cardiovascular disease, indicating that improvement of current cell-based therapies is an important area of future study. In this study, it was discovered that the in vivo therapeutic efficacy of CD34+ cells could be improved upon through the genetic modification with the angiogenic morphogen Shh. As compared to other exosome-secreting cell types such as HUVECs or HeLa cells, CD34+ cells appear to preferentially store Shh in exosomes. Additionally, Shh containing exosomes, isolated from the culture media of genetically modified CD34+ cells, physically transfer exosomal Shh to endothelial cells and can also induce Shh specific signaling events in cultured fibroblasts. These results indicate that the exploitation of Shh gene modification in the further development of CD34+ cell/exosomal based therapies may prove beneficial in the aforementioned patient populations to treat ischemic disorders.
Acknowledgments
We thank K. Krueger for administrative assistance and D. Motlagh at Baxter Healthcare for providing the CD34+ cells used in these studies.
Funding Sources
This study was supported in part by grants from the NIH (HL-53354, HL-77428, HL-63414, HL-80137, HL95874, HLPO1-108795, HL-57516, HL-91983, and HL-105597).
Abbreviations
- AMI
Acute myocardial infarction
- CD34EV
Human CD34 cells modified with the control Empty Vector plasmid
- CD34NM
Human CD34 cells that are Non-Modified
- CD34Shh
Human CD34 cells modified with the Shh containing plasmid
- EF
Ejection fraction
- EPCs
Endothelial progenitor cells
- FS
Fractional shortening
- HUVECs
Human umbilical vein endothelial cells
- Shh
Human sonic hedgehog protein
- SN
Supernatant
Footnotes
Disclosures
D. Losordo is an employee of Baxter Healthcare.
Subject Codes: [4] Acute myocardial infarction; [87] Coronary circulation; [88] Gene therapy; [129] Angiogenesis; [142] Gene expression
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Losordo DW, Henry TD, Davidson C, Lee JS, Costa MA, Bass T, Mendelsohn F, Fortuin FD, Pepine CJ, Traverse JH, Amrani D, Ewenstein BM, Riedel N, Story K, Barker K, Povsic TJ, Harrington RA, Schatz RA. Intramyocardial, autologous CD34+ cell therapy for refractory angina. Circ Res. 2011;109:428–436. doi: 10.1161/CIRCRESAHA.111.245993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Losordo DW, Schatz RA, White CJ, Udelson JE, Veereshwarayya V, Durgin M, Poh KK, Weinstein R, Kearney M, Chaudhry M, Burg A, Eaton L, Heyd L, Thorne T, Shturman L, Hoffmeister P, Story K, Zak V, Dowling D, Traverse JH, Olson RE, Flanagan J, Sodano D, Murayama T, Kawamoto A, Kusano KF, Wollins J, Welt F, Shah P, Soukas P, Asahara T, Henry TD. Intramyocardial transplantation of autologous CD34+ stem cells for intractable angina: a phase I/IIa double-blind, randomized controlled trial. Circulation. 2007;115:3165–3172. doi: 10.1161/CIRCULATIONAHA.106.687376. [DOI] [PubMed] [Google Scholar]
- 3.Quyyumi AA, Waller EK, Murrow J, Esteves F, Galt J, Oshinski J, Lerakis S, Sher S, Vaughan D, Perin E, Willerson J, Kereiakes D, Gersh BJ, Gregory D, Werner A, Moss T, Chan WS, Preti R, Pecora AL. CD34+ cell infusion after ST elevation myocardial infarction is associated with improved perfusion and is dose dependent. Am Heart J. 2011;161:98–105. doi: 10.1016/j.ahj.2010.09.025. [DOI] [PubMed] [Google Scholar]
- 4.Jujo K, Ii M, Losordo DW. Endothelial progenitor cells in neovascularization of infarcted myocardium. J Mol Cell Cardiol. 2008;45:530–544. doi: 10.1016/j.yjmcc.2008.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sahoo S, Klychko E, Thorne T, Misener S, Schultz KM, Millay M, Ito A, Liu T, Kamide C, Agrawal H, Perlman H, Qin G, Kishore R, Losordo DW. Exosomes from human CD34+ stem cells mediate their proangiogenic paracrine activity. Circ Res. 2011;109:724–728. doi: 10.1161/CIRCRESAHA.111.253286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chaput N, Thery C. Exosomes: immune properties and potential clinical implementations. Semin Immunopathol. 2010;33:419–440. doi: 10.1007/s00281-010-0233-9. [DOI] [PubMed] [Google Scholar]
- 7.Kawamoto A, Iwasaki H, Kusano K, Murayama T, Oyamada A, Silver M, Hulbert C, Gavin M, Hanley A, Ma H, Kearney M, Zak V, Asahara T, Losordo DW. CD34-positive cells exhibit increased potency and safety for therapeutic neovascularization after myocardial infarction compared with total mononuclear cells. Circulation. 2006;114:2163–2169. doi: 10.1161/CIRCULATIONAHA.106.644518. [DOI] [PubMed] [Google Scholar]
- 8.Kawamoto A, Tkebuchava T, Yamaguchi J, Nishimura H, Yoon YS, Milliken C, Uchida S, Masuo O, Iwaguro H, Ma H, Hanley A, Silver M, Kearney M, Losordo DW, Isner JM, Asahara T. Intramyocardial transplantation of autologous endothelial progenitor cells for therapeutic neovascularization of myocardial ischemia. Circulation. 2003;107:461–468. doi: 10.1161/01.cir.0000046450.89986.50. [DOI] [PubMed] [Google Scholar]
- 9.Shintani S, Kusano K, Ii M, Iwakura A, Heyd L, Curry C, Wecker A, Gavin M, Ma H, Kearney M, Silver M, Thorne T, Murohara T, Losordo DW. Synergistic effect of combined intramyocardial CD34+ cells and VEGF2 gene therapy after MI. Nat Clin Pract Cardiovasc Med. 2006;3:S123–S128. doi: 10.1038/ncpcardio0430. [DOI] [PubMed] [Google Scholar]
- 10.Kondo T, Hayashi M, Takeshita K, Numaguchi Y, Kobayashi K, Iino S, Inden Y, Murohara T. Smoking cessation rapidly increases circulating progenitor cells in peripheral blood in chronic smokers. Arterioscler Thromb Vasc Biol. 2004;24:1442–1447. doi: 10.1161/01.ATV.0000135655.52088.c5. [DOI] [PubMed] [Google Scholar]
- 11.Pai M, Zacharoulis D, Milicevic MN, Helmy S, Jiao LR, Levicar N, Tait P, Scott M, Marley SB, Jestice K, Glibetic M, Bansi D, Khan SA, Kyriakou D, Rountas C, Thillainayagam A, Nicholls JP, Jensen S, Apperley JF, Gordon MY, Habib NA. Autologous infusion of expanded mobilized adult bone marrow-derived CD34+ cells into patients with alcoholic liver cirrhosis. Am J Gastroenterol. 2008;103:1952–1958. doi: 10.1111/j.1572-0241.2008.01993.x. [DOI] [PubMed] [Google Scholar]
- 12.Taguchi A, Matsuyama T, Moriwaki H, Hayashi T, Hayashida K, Nagatsuka K, Todo K, Mori K, Stern DM, Soma T, Naritomi H. Circulating CD34-positive cells provide an index of cerebrovascular function. Circulation. 2004;109:2972–2975. doi: 10.1161/01.CIR.0000133311.25587.DE. [DOI] [PubMed] [Google Scholar]
- 13.Valgimigli M, Rigolin GM, Fucili A, Porta MD, Soukhomovskaia O, Malagutti P, Bugli AM, Bragotti LZ, Francolini G, Mauro E, Castoldi G, Ferrari R. CD34+ and endothelial progenitor cells in patients with various degrees of congestive heart failure. Circulation. 2004;110:1209–1212. doi: 10.1161/01.CIR.0000136813.89036.21. [DOI] [PubMed] [Google Scholar]
- 14.Vasa M, Fichtlscherer S, Adler K, Aicher A, Martin H, Zeiher AM, Dimmeler S. Increase in circulating endothelial progenitor cells by statin therapy in patients with stable coronary artery disease. Circulation. 2001;103:2885–2890. doi: 10.1161/hc2401.092816. [DOI] [PubMed] [Google Scholar]
- 15.Vasa M, Fichtlscherer S, Aicher A, Adler K, Urbich C, Martin H, Zeiher AM, Dimmeler S. Number and migratory activity of circulating endothelial progenitor cells inversely correlate with risk factors for coronary artery disease. Circ Res. 2001;89:E1–E7. doi: 10.1161/hh1301.093953. [DOI] [PubMed] [Google Scholar]
- 16.Yu JX, Huang XF, Lv WM, Ye CS, Peng XZ, Zhang H, Xiao LB, Wang SM. Combination of stromal-derived factor-1alpha and vascular endothelial growth factor gene-modified endothelial progenitor cells is more effective for ischemic neovascularization. J Vasc Surg. 2009;50:608–616. doi: 10.1016/j.jvs.2009.05.049. [DOI] [PubMed] [Google Scholar]
- 17.Roncalli J, Renault MA, Tongers J, Misener S, Thorne T, Kamide C, Jujo K, Tanaka T, Ii M, Klyachko E, Losordo DW. Sonic hedgehog-induced functional recovery after myocardial infarction is enhanced by AMD3100-mediated progenitor-cell mobilization. J Am Coll Cardiol. 2011;57:2444–2452. doi: 10.1016/j.jacc.2010.11.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gao F, He T, Wang H, Yu S, Yi D, Liu W, Cai Z. A promising strategy for the treatment of ischemic heart disease: Mesenchymal stem cell-mediated vascular endothelial growth factor gene transfer in rats. Can J Cardiol. 2007;23:891–898. doi: 10.1016/s0828-282x(07)70845-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fischer KM, Cottage CT, Wu W, Din S, Gude NA, Avitabile D, Quijada P, Collins BL, Fransioli J, Sussman MA. Enhancement of myocardial regeneration through genetic engineering of cardiac progenitor cells expressing Pim-1 kinase. Circulation. 2009;120:2077–2087. doi: 10.1161/CIRCULATIONAHA.109.884403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rajagopalan S, Mohler ER, 3rd, Lederman RJ, Mendelsohn FO, Saucedo JF, Goldman CK, Blebea J, Macko J, Kessler PD, Rasmussen HS, Annex BH. Regional angiogenesis with vascular endothelial growth factor in peripheral arterial disease: a phase II randomized, double-blind, controlled study of adenoviral delivery of vascular endothelial growth factor 121 in patients with disabling intermittent claudication. Circulation. 2003;108:1933–1938. doi: 10.1161/01.CIR.0000093398.16124.29. [DOI] [PubMed] [Google Scholar]
- 21.Grossman PM, Mendelsohn F, Henry TD, Hermiller JB, Litt M, Saucedo JF, Weiss RJ, Kandzari DE, Kleiman N, Anderson RD, Gottlieb D, Karlsberg R, Snell J, Rocha-Singh K. Results from a phase II multicenter, double-blind placebo-controlled study of Del-1 (VLTS-589) for intermittent claudication in subjects with peripheral arterial disease. Am Heart J. 2007;153:874–880. doi: 10.1016/j.ahj.2007.01.038. [DOI] [PubMed] [Google Scholar]
- 22.Pola R, Ling LE, Silver M, Corbley MJ, Kearney M, Blake Pepinsky R, Shapiro R, Taylor FR, Baker DP, Asahara T, Isner JM. The morphogen Sonic hedgehog is an indirect angiogenic agent upregulating two families of angiogenic growth factors. Nat Med. 2001;7:706–711. doi: 10.1038/89083. [DOI] [PubMed] [Google Scholar]
- 23.Washington Smoak I, Byrd NA, Abu-Issa R, Goddeeris MM, Anderson R, Morris J, Yamamura K, Klingensmith J, Meyers EN. Sonic hedgehog is required for cardiac outflow tract and neural crest cell development. Dev Biol. 2005;283:357–372. doi: 10.1016/j.ydbio.2005.04.029. [DOI] [PubMed] [Google Scholar]
- 24.Pola R, Ling LE, Aprahamian TR, Barban E, Bosch-Marce M, Curry C, Corbley M, Kearney M, Isner JM, Losordo DW. Postnatal recapitulation of embryonic hedgehog pathway in response to skeletal muscle ischemia. Circulation. 2003;108:479–485. doi: 10.1161/01.CIR.0000080338.60981.FA. [DOI] [PubMed] [Google Scholar]
- 25.Kusano KF, Pola R, Murayama T, Curry C, Kawamoto A, Iwakura A, Shintani S, Ii M, Asai J, Tkebuchava T, Thorne T, Takenaka H, Aikawa R, Goukassian D, von Samson P, Hamada H, Yoon YS, Silver M, Eaton E, Ma H, Heyd L, Kearney M, Munger W, Porter JA, Kishore R, Losordo DW. Sonic hedgehog myocardial gene therapy: tissue repair through transient reconstitution of embryonic signaling. Nat Med. 2005;11:1197–1204. doi: 10.1038/nm1313. [DOI] [PubMed] [Google Scholar]
- 26.Straface G, Aprahamian T, Flex A, Gaetani E, Biscetti F, Smith RC, Pecorini G, Pola E, Angelini F, Stigliano E, Castellot JJ, Jr., Losordo DW, Pola R. Sonic hedgehog regulates angiogenesis and myogenesis during post-natal skeletal muscle regeneration. J Cell Mol Med. 2009;13:2424–2435. doi: 10.1111/j.1582-4934.2008.00440.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Iwakura A, Shastry S, Luedemann C, Hamada H, Kawamoto A, Kishore R, Zhu Y, Qin G, Silver M, Thorne T, Eaton L, Masuda H, Asahara T, Losordo DW. Estradiol enhances recovery after myocardial infarction by augmenting incorporation of bone marrow-derived endothelial progenitor cells into sites of ischemia-induced neovascularization via endothelial nitric oxide synthase-mediated activation of matrix metalloproteinase-9. Circulation. 2006;113:1605–1614. doi: 10.1161/CIRCULATIONAHA.105.553925. [DOI] [PubMed] [Google Scholar]
- 28.Thery C, Amigorena S, Raposo G, Clayton A. Isolation and characterization of exosomes from cell culture supernatants and biological fluids. Curr Protoc Cell Biol. 2006 doi: 10.1002/0471143030.cb0322s30. Chapter 3:Unit 3 22. [DOI] [PubMed] [Google Scholar]
- 29.Eizawa T, Murakami Y, Matsui K, Takahashi M, Muroi K, Amemiya M, Takano R, Kusano E, Shimada K, Ikeda U. Circulating endothelial progenitor cells are reduced in hemodialysis patients. Curr Med Res Opin. 2003;19:627–633. doi: 10.1185/030079903125002379. [DOI] [PubMed] [Google Scholar]
- 30.Fadini GP, Miorin M, Facco M, Bonamico S, Baesso I, Grego F, Menegolo M, de Kreutzenberg SV, Tiengo A, Agostini C, Avogaro A. Circulating endothelial progenitor cells are reduced in peripheral vascular complications of type 2 diabetes mellitus. J Am Coll Cardiol. 2005;45:1449–1457. doi: 10.1016/j.jacc.2004.11.067. [DOI] [PubMed] [Google Scholar]
- 31.Fadini GP, Sartore S, Albiero M, Baesso I, Murphy E, Menegolo M, Grego F, Vigili de Kreutzenberg S, Tiengo A, Agostini C, Avogaro A. Number and function of endothelial progenitor cells as a marker of severity for diabetic vasculopathy. Arterioscler Thromb Vasc Biol. 2006;26:2140–2146. doi: 10.1161/01.ATV.0000237750.44469.88. [DOI] [PubMed] [Google Scholar]
- 32.Asai J, Takenaka H, Kusano KF, Ii M, Luedemann C, Curry C, Eaton E, Iwakura A, Tsutsumi Y, Hamada H, Kishimoto S, Thorne T, Kishore R, Losordo DW. Topical sonic hedgehog gene therapy accelerates wound healing in diabetes by enhancing endothelial progenitor cell-mediated microvascular remodeling. Circulation. 2006;113:2413–2424. doi: 10.1161/CIRCULATIONAHA.105.603167. [DOI] [PubMed] [Google Scholar]
- 33.Kusano KF, Allendoerfer KL, Munger W, Pola R, Bosch-Marce M, Kirchmair R, Yoon YS, Curry C, Silver M, Kearney M, Asahara T, Losordo DW. Sonic hedgehog induces arteriogenesis in diabetic vasa nervorum and restores function in diabetic neuropathy. Arterioscler Thromb Vasc Biol. 2004;24:2102–2107. doi: 10.1161/01.ATV.0000144813.44650.75. [DOI] [PubMed] [Google Scholar]
- 34.Pepinsky RB, Shapiro RI, Wang S, Chakraborty A, Gill A, Lepage DJ, Wen D, Rayhorn P, Horan GS, Taylor FR, Garber EA, Galdes A, Engber TM. Long-acting forms of Sonic hedgehog with improved pharmacokinetic and pharmacodynamic properties are efficacious in a nerve injury model. J Pharm Sci. 2002;91:371–387. doi: 10.1002/jps.10052. [DOI] [PubMed] [Google Scholar]
- 35.Ziebart T, Yoon CH, Trepels T, Wietelmann A, Braun T, Kiessling F, Stein S, Grez M, Ihling C, Muhly-Reinholz M, Carmona G, Urbich C, Zeiher AM, Dimmeler S. Sustained persistence of transplanted proangiogenic cells contributes to neovascularization and cardiac function after ischemia. Circ Res. 2008;103:1327–1334. doi: 10.1161/CIRCRESAHA.108.180463. [DOI] [PubMed] [Google Scholar]
- 36.Renault MA, Roncalli J, Tongers J, Thorne T, Klyachko E, Misener S, Volpert OV, Mehta S, Burg A, Luedemann C, Qin G, Kishore R, Losordo DW. Sonic hedgehog induces angiogenesis via Rho kinase-dependent signaling in endothelial cells. J Mol Cell Cardiol. 2010;49:490–498. doi: 10.1016/j.yjmcc.2010.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chinchilla P, Xiao L, Kazanietz MG, Riobo NA. Hedgehog proteins activate proangiogenic responses in endothelial cells through non-canonical signaling pathways. Cell Cycle. 2010;9:570–579. doi: 10.4161/cc.9.3.10591. [DOI] [PubMed] [Google Scholar]
- 38.Ahmed RP, Haider KH, Shujia J, Afzal MR, Ashraf M. Sonic hedgehog gene delivery to the rodent heart promotes angiogenesis via iNOS/netrin-1/PKC pathway. PLoS One. 2010;5:e8576. doi: 10.1371/journal.pone.0008576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Martinez MC, Andriantsitohaina R. Microparticles in angiogenesis: therapeutic potential. Circ Res. 2011;109:110–119. doi: 10.1161/CIRCRESAHA.110.233049. [DOI] [PubMed] [Google Scholar]
- 40.Grange C, Tapparo M, Collino F, Vitillo L, Damasco C, Deregibus MC, Tetta C, Bussolati B, Camussi G. Microvesicles released from human renal cancer stem cells stimulate angiogenesis and formation of lung premetastatic niche. Cancer Res. 2011;71:5346–5356. doi: 10.1158/0008-5472.CAN-11-0241. [DOI] [PubMed] [Google Scholar]
- 41.Taverna S, Flugy A, Saieva L, Kohn EC, Santoro A, Meraviglia S, De Leo G, Alessandro R. Role of exosomes released by chronic myelogenous leukemia cells in angiogenesis. Int J Cancer. 2011;130:2033–43. doi: 10.1002/ijc.26217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vrijsen KR, Sluijter JP, Schuchardt MW, van Balkom BW, Noort WA, Chamuleau SA, Doevendans PA. Cardiomyocyte progenitor cell-derived exosomes stimulate migration of endothelial cells. J Cell Mol Med. 2010;14:1064–1070. doi: 10.1111/j.1582-4934.2010.01081.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Skog J, Wurdinger T, van Rijn S, Meijer DH, Gainche L, Sena-Esteves M, Curry WT, Jr., Carter BS, Krichevsky AM, Breakefield XO. Glioblastoma microvesicles transport RNA and proteins that promote tumour growth and provide diagnostic biomarkers. Nat Cell Biol. 2008;10:1470–1476. doi: 10.1038/ncb1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kuwabara Y, Ono K, Horie T, Nishi H, Nagao K, Kinoshita M, Watanabe S, Baba O, Kojima Y, Shizuta S, Imai M, Tamura T, Kita T, Kimura T. Increased microRNA-1 and microRNA-133a levels in serum of patients with cardiovascular disease indicate myocardial damage. Circ Cardiovasc Genet. 2011;4:446–454. doi: 10.1161/CIRCGENETICS.110.958975. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.