Abstract
Study purposes were to determine the prevalence of persistent pain in the breast; characterize distinct persistent pain classes using growth mixture modeling, and evaluate for differences among these pain classes in demographic, preoperative, intraoperative, and postoperative characteristics. In addition, differences in the severity of common symptoms and quality of life outcomes measured prior to surgery, among the pain classes, were evaluated. Patients (n=398) were recruited prior to surgery and followed for six months. Using growth mixture modeling, patients were classified into no (31.7%), mild (43.4%), moderate (13.3%), and severe (11.6%) pain groups based on ratings of worst breast pain. Differences in a number of demographic, preoperative, intraoperative, and postoperative characteristics differentiated among the pain classes. In addition, patients in the moderate and severe pain classes reported higher preoperative levels of depression, anxiety, and sleep disturbance than the no pain class. Findings suggest that approximately 25% of women experience significant and persistent levels of breast pain in the first six months following breast cancer surgery.
Keywords: breast pain, persistent postsurgical pain, risk factors, breast cancer surgery, growth mixture modeling, latent class analysis
INTRODUCTION
Prevalence rates for persistent pain following breast cancer surgery range from 25% to 60%.28 This wide range is attributed to differences in the definition of persistent pain; whether the assessments were done prospectively or retrospectively; and the measures used to assess pain. As noted by Andersen and Kehlet,2 studies that evaluated post-mastectomy pain syndrome reported a prevalence rate of approximately 25%,9,76,83 whereas studies with a wider definition of persistent pain reported a prevalence rate of 50%.1,62 In two studies, approximately 10% of patients reported severe persistent pain.23,28 Prospective, longitudinal studies, that enroll patients prior to breast cancer surgery, are needed to determine the magnitude of this problem.
Given the large number of women who will undergo breast cancer surgery worldwide, an equally important consideration is the identification of risk factors for the development of persistent pain. In a recent review of these risk factors,2 Andersen and Kehlet evaluated the evidence for preoperative, intraoperative, and postoperative risk factors. Of the sixty studies reviewed, most were retrospective and used self-report measures. In addition, about two thirds of the studies did not reflect more modern principles of surgical and adjuvant treatment for breast cancer. The authors concluded that it was difficult to draw definitive conclusions about risk factors because the heterogeneity of the studies precluded detailed comparisons across studies. Additional research is warranted that includes a more precise definition of persistent pain; as well as a comprehensive evaluation of preoperative, intraoperative, and postoperative risk factors in the same patients and prospective follow-up of these patients from prior to through at least 6 months after breast cancer surgery.
Some of the differences in prevalence rates and risk factors identified to date may be due to heterogeneity in the characterization of persistent pain phenotypes associated with breast cancer surgery. In fact, all of the studies published to date have classified patients into pain versus no pain groups based on different definitions of persistent pain. A type of latent class analysis, growth mixture modeling (GMM) facilitates the detection of underlying patterns of change in symptom severity over time and enables the identification of subgroups of patients, referred to as latent growth classes, whose symptoms share a similar trajectory over time.53–55 Recently, our research group has used (GMM) to identify subgroups of patients with distinct trajectories of depression,20 anxiety,19 and sleep disturbance.82 However, this approach has not been used to characterize persistent pain following breast cancer surgery.
Given the limitations noted above, the purposes of this prospective, longitudinal study, that recruited 398 women prior to surgery for breast cancer and followed them monthly for six months were to determine the prevalence of persistent pain in the breast; characterize distinct persistent pain phenotype(s) using GMM; and evaluate for differences among these pain classes in demographic, preoperative, intraoperative, and postoperative characteristics. In addition, differences in the severity of common symptoms and quality of life (QOL) outcomes measured prior to surgery, among the identified pain classes, were evaluated.
METHODS
Patients and Settings
This longitudinal study is part of a larger study that evaluated for neuropathic pain and lymphedema in a sample of women who underwent breast cancer surgery. Patients were recruited from Breast Care Centers located in a Comprehensive Cancer Center, two public hospitals, and four community practices. Patients were eligible to participate if they were: an adult woman (≥18 years) who would undergo breast cancer surgery on one breast; able to read, write, and understand English; agreed to participate, and gave written informed consent. Patients were excluded if they were: having breast cancer surgery on both breasts and/or had distant metastasis at the time of diagnosis. A total of 516 patients were approached to participate, 410 were enrolled in the study (response rate 79.4%)and 398 completed the study questionnaires. The major reasons for refusal were: too busy, overwhelmed with the cancer diagnosis, or insufficient time available to do baseline assessment prior to surgery.
Subjective Measures
The demographic questionnaire obtained information on age, education, ethnicity, marital status employment status, living situation, and financial status. The Karnofsky Performance Status (KPS) scale is widely used to evaluated functional status in patients with cancer and has well established validity and reliability.36,37 Patients rated their functional status using the KPS scale that ranged from 30 (I feel severely disabled and need to be hospitalized) to 100 (I feel normal; I have no complaints or symptoms). Patients were asked to indicate if they exercised on a regular basis (yes/no format).
Self-Administered Comorbidity Questionnaire (SCQ) is a short and easily understood instrument that was developed to measure comorbidity in clinical and health service research settings.67 The questionnaire consists of 13 common medical conditions that were simplified into language that could be understood without any prior medical knowledge. Patients were asked to indicate if they had the condition using a “yes/no” format. If they indicated that they had a condition, they were asked if they received treatment for it (yes/no; proxy for disease severity) and did it limit their activities (yes/no; indication of functional limitations). Patients were given the option to add two additional conditions not listed on the instrument. For each condition, a patient can receive a maximum of 3 points. Because there are 13 defined medical conditions and 2 optional conditions, the maximum score totals 45 points if the open-ended items are used and 39 points if only the closed-ended items are used. The SCQ has well-established validity and reliability and has been used in studies of patients with a variety of chronic conditions.5,12,48,67,70
Persistent pain was evaluated using the Breast Symptoms Questionnaire (BSQ). The BSQ consists of two parts. Part 1 obtained information on the occurrence of pain and the occurrence of other symptoms in the breast scar area (i.e., swelling, numbness, strange sensations, hardness). The additional symptoms that were assessed were identified in studies by Tasmuth and colleagues.78,79 If the patient had pain in the breast scar area, they completed Part 2 of the BSQ. Patients were asked to rate the intensity of their average and worst pain, in the past week, using a numeric rating scale (NRS) that ranged from 0 (no pain) to 10 (worst imaginable pain). NRS is a valid and reliable measure of pain intensity.33 Patients completed the BSQ prior to surgery and monthly for six months after surgery.
Postsurgical pain was evaluated using the Postsurgical Pain Questionnaire. Patients were asked to rate pain intensity, pain relief, and satisfaction with pain treatment in the first 24 to 48 hours after surgery. Average and worst pain were rated using a 0 to 10 NRS. Pain relief was rated on a 0% (no relief) to 100% (complete relief) rating scale. Satisfaction with pain treatment was rated on a 0 (not satisfied at all) to 10 (extremely satisfied) NRS. Patients completed this questionnaire during the month 1 study visit.
Center for Epidemiologic Studies-Depression (CES-D) scale consists of 20 items selected to represent the major symptoms in the clinical syndrome of depression. Scores can range from 0 to 60, with scores of ≥ 16 indicating the need for individuals to seek clinical evaluation for major depression. The CES-D has well established concurrent and construct validity.10,64,69 Cronbach’s alpha for the CES-D was .90.
Spielberger State-Trait Anxiety Inventories (STAI-T and STAI-S) consist of 20 items each that are rated from 1 to 4. Scores for each scale can range from 20 to 80 and higher scores indicate greater anxiety. Cuttoff scores of ≥ 31.8 and ≥ 32.2 indicate high levels of trait and state anxiety, respectively. Both inventories have well established criterion and construct validity and internal consistency reliability coefficients.3,38,72 Cronbach’s alphas for the STAI-T and STAI-S were .88 and .95, respectively.
General Sleep Disturbance Scale (GSDS) consists of 21-items designed to assess sleep disturbance in the past week. Each item was rated on a 0 (never) to 7 (everyday) NRS. The GSDS total score is the sum of the 21 items that can range from 0 (no disturbance) to 147 (extreme sleep disturbance). A GSDS total score of ≥ 43 indicates a significant level of sleep disturbance.8,26,27,50 The GSDS has well-established validity and reliability in shift workers, pregnant women, and patients with cancer and HIV.43,44,51 The Cronbach’s alpha for the GSDS total score was .86.
Lee Fatigue Scale (LFS) consists of 18 items designed to assess physical fatigue and energy.45 Each item was rated on a 0 to 10 NRS. Total fatigue and energy scores were calculated as the mean of the 13 fatigue items and the 5 energy items, with higher scores indicating greater fatigue severity and higher levels of energy. Respondents were asked to rate each item based on how they felt “right now”. The LFS has been used with healthy individuals29,45 and in patients with cancer and HIV.46,50–52 A cutoff score of ≥4.4 indicates high levels of fatigue.17 A cutoff score of ≤4.8 indicates low levels of energy.17 The LFS has well established validity and reliability. Cronbach’s alphas for fatigue and energy were .96 and .93, respectively.
Attentional Function Index (AFI) consists of 16 items designed to measure self-reported attentional function (i.e., ability to voluntarily direct and sustain attention) in patients with cancer. Each item is rated on a 0 to 10 NRS. A mean AFI score was calculated, with higher scores indicating greater capacity to direct attention and, therefore, lower levels of attentional fatigue.14,16 Based on a previously conducted analysis of the frequency distributions of AFI scores, attentional fatigue can be grouped into categories of functional status (i.e., patients who score <5.0 functioning poorly and experiencing high levels of attentional fatigue, patients who score 5.0 to 7.5 functioning moderately well and experiencing moderate levels of attentional fatigue, patients who score >7.5 functioning well and experiencing low levels of attentional fatigue.15 The AFI has established reliability and validity.16 Cronbach’s alpha for the AFI was .95.
Quality of Life-Scale-Patient Version (QOL-PV) is a 41-item instrument that measures four dimensions of QOL in cancer patients (physical well-being, psychological well-being, spiritual well-being, social well being) as well as overall QOL. Each item is scored on a 0 to 10 NRS with higher scores indicating a better QOL. The QOL-PV has established validity and reliability.24,25,58,59 Cronbach’s alpha for the QOL-PV total score was .86.
Medication Use
At each assessment, the research nurse recorded the names of the medications the patient took in the previous month.
Objective Measures
Grip strength (in kilograms), in both hands, was measured using a Jamar hydraulic hand dynamometer (Sammons Preston). This measure has been used to evaluate for changes in muscle strength in women following breast cancer surgery78 and other forms of breast cancer treatment.65 The measurement was performed with women in a standing position with the arm held in a comfortable position according to the procedures described by Spijkerman and colleagues.73 Grip strength was measured three times in each hand. If a variance of more than 20% occurred among the three readings on each hand, the test was repeated. The three readings from the affected and unaffected hands were averaged.65,73
Shoulder mobility was assessed using goniometric measurement of range of motion (ROM). While the patient was lying supine, ROM was measured, in degrees, twice on each side in four positions (i.e., flexion, abduction, internal rotation, external rotation) and these measurements were averaged.
Study Procedures
The study was approved by the Committee on Human Research at the University of California, San Francisco and by the Institutional Review Boards at each of the study sites. During the patient’s preoperative visit, a clinician explained the study to the patient, determined her willingness to participate, and introduced the patient to the research nurse. The research nurse determined eligibility and obtained written informed consent prior to surgery. After obtaining written informed consent, patients completed the enrollment questionnaires (Assessment 0). Following the completion of the questionnaires, the research nurse performed the following baseline objective measurements: height, weight, grip strength, and shoulder mobility.
The research nurse met with the patients either in their home or in the Clinical Research Center at 1, 2, 3, 4, 5, and 6 months after surgery. During each of the study visits, the women completed the study questionnaires, provided information on new and ongoing treatments, and had the objective measures done by the research nurse. Over the course of the study, patients’ medical records were reviewed for disease and treatment information.
Statistical analyses
Data were analyzed using SPSS Version 19.074 and Mplus Version 6.11.56 Descriptive statistics and frequency distributions were calculated for patients’ demographic and clinical characteristics and symptom severity scores.
Unconditional GMM with robust maximum likelihood estimation was carried out to identify latent classes of patients with distinct persistent breast pain trajectories. Breast pain scores at the Month 1 assessment (i.e., the month after surgery) were not used in the GMM analyses because the models could not be estimated reliably. Prior to conducting GMM analyses, patients who reported no pain in their affected breast for all 6 assessments (i.e., enrollment and 2, 3, 4, 5, and 6 months) were identified (N=126; 31.7%) and not included in the GMM analysis. The remaining 272 women’s ratings of worst breast pain were used in the GMM analysis. These methods are described in detail elsewhere.20 In brief, a single growth curve that represented the “average” change trajectory was estimated for the sample. Then, the number of latent growth classes that best fit the data was identified using published guidelines.35,57,80
Model fit for the GMM was assessed statistically by identifying the model with the lowest Bayesian Information Criterion (BIC). The parametric bootstrapped likelihood ratio test (BLRT) was used to evaluate whether a model with K classes fit the data better than a model with K-1 classes. In addition to using the BLRT to compare models, the Vuong-Lo-Mendell-Rubin Likelihood Ratio Test (VLMR) for the “K” versus “K-1” class models was examined. A non-significant VLMR test provides evidence that the K-class model is not better than the K-1-class model. The fourth index used to evaluate model fit was entropy, with >.80 being preferred.11,56 Finally, the best fitting model was visually inspected by plotting observed against model-predicted values to determine whether the predicted trajectories followed the empiric trajectories for the classes and to evaluate whether the predicted plots “made sense” theoretically and clinically.55
Intercepts and linear and quadratic slopes for each latent class were estimated for each model. Intercept variances were estimated for each class and were allowed to differ across classes for the two class model. However, the three and four class models could not be estimated reliably without fixing the intercept variance for two of the classes to zero. Given the relatively small sample sizes, the within-class linear and quadratic slope variances were fixed at zero, because estimation failed when they were free to vary.39,54 Without setting these slope variances to zero, the model could not be estimated due to non-positive definite covariance matrices. Mixture models are known to produce solutions at local maxima, so each model was fit with random starts to be sure that the solution for the model with the maximum log likelihood values was replicated.56 Missing data for the worst breast pain scores were accommodated by Mplus Version 6.11 through the use of Full Information Maximum Likelihood and the use of the Expectation-Maximization algorithm. This method assumes that any missing data are missing at random.54,68
Analyses of variance and Chi-square analyses were used to assess for differences in demographic, preoperative, intraoperative, and postoperative characteristics, symptom severity scores, and QOL scores at enrollment, among the GMM latent classes. Based on the recommendations of Rothman,66 adjustments were not made for multiple testing. For each assessment, the percentages of patients who reported taking a nonopioid, a nonsteroidal anti-inflammatory drug (NSAID), a weak opioid (e.g., codeine, hydrocodone), a strong opioid (e.g., morphine), an antidepressant, or an anticonvulsant were determined.
RESULTS
GMM Analysis
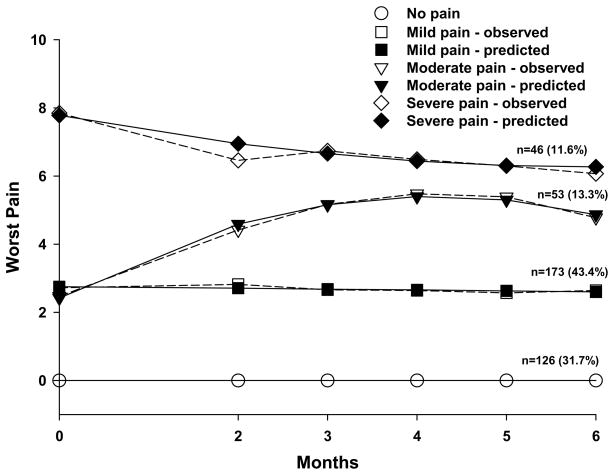
A total of 126 patients (31.7%; no pain class) did not report any breast pain for any of the six assessments. In the remaining 272 patients, three distinct latent classes of persistent breast pain were identified using GMM (Figure 1). A three-class model was selected because its BIC was smaller than the two-class and four-class models. In addition, comparisons of the other fit indices (Table 1) and the plots of the observed against model predicted values (Figure 1) supported the choice of the three class model (Table 1).
Figure 1.
Observed and estimated breast pain trajectories for patients in each of the latent classes.
Table 1.
Fit Indices for the GMM Class Solutions for the Severity of Worst Pain in the Affected Breast
| GMM Solution | LL | AIC | BIC | Entropy | VLMR (df) | BLRT (df) |
|---|---|---|---|---|---|---|
| 1-Classa | −1535.70 | 3091.40 | 3127.46 | N/A | N/A | N/A |
| 2-Class | −1513.49 | 3054.99 | 3105.47 | .50 | 204.10† (5) | 202.40† (5) |
| 3-Classb | −1493.61 | 3023.22 | 3088.12 | .66 | 74.81† (5) | 74.97† (5) |
| 4-Classc | −1485.39 | 3016.79 | 3099.72 | .57 | 14.74ns (4) | 14.57ns (4) |
ns = not significant;
p ≥ .01;
p < .001
Latent growth curve model with linear and quadratic components; Chi2= 15.84, 17 df, p = .54, CFI = 1.0, RMSEA = .00.
3-class model was selected.
4-class model could not be fit without fixing linear and quadratic slope variances to zero.
Abbreviations: GMM = Growth mixture model; LL = loglikelihood; AIC = Akaike Information Criterion; BIC = Bayesian Information Criterion; BLRT = parametric bootstrapped likelihood ratio test for K-1 (H0) vs K classes; VLMR = Vuong-Lo-Mendell-Rubin likelihood ratio test for K-1 (H0) vs K classes; N/A = not applicable; df = degrees of freedom; CFI = Comparative Fit Index; RMSEA = Root Mean Squared Error of Approximation.
As shown in Table 2, the largest group of the patients with breast pain was classified into the mild pain class (n=173, 43.1%). Of these patients, 66 reported breast pain at enrollment (mean=2.7, SD=1.3). As a class, these patients maintained a similar level of pain throughout the study. Patients in the next largest class reported moderate breast pain (n=53, 13.3%). Of these patients, 16 reported pain scores that were low at enrollment (mean=2.4, SD=1.0). As a class, their breast pain increased over time and began to decrease at the 6 month assessment. Finally, 17 patients in the severe pain class (n=46, 11.6%) had high preoperative breast pain scores (mean=8.0, SD=1.3). As a class, these patients reported breast pain that decreased only slightly during the 6 months following surgery.
Table 2.
Parameter Estimates for Predicted Growth Mixture Model Latent Classes from Six Assessments of Ratings of the Severity of Worst Pain in the Affected Breasta
| Parameter Estimatesb | Mild Pain | Moderate Pain | Severe Pain |
|---|---|---|---|
|
| |||
| N = 173 (43.1%) | N = 53 (13.3%) | N = 46 (11.6%) | |
| Mean | Mean | Mean | |
| Intercept | 2.75+ | 2.42+ | 7.78+ |
| Linear slope | −0.02 | 1.42+ | −0.50 |
| Quadratic slope | −0.001 | −0.17+ | 0.04 |
| Variancesc | |||
| Intercept | 0c | 0c | 0.90 |
| Linear slope | 0c | 0c | 0c |
| Quadratic slope | 0c | 0c | 0c |
Abbreviations: ns = not significant
p≤.005
Trajectory group sizes are for classification of individuals based on their most likely latent class probabilities.
Growth mixture model estimates were obtained with robust maximum likelihood.
Fixed at zero to aid in model convergence.
Differences in Demographic Characteristics
As shown in Table 3, no differences were found among the four pain groups in living arrangements, marital status, and employment status. However, patients in all three pain classes were significantly younger than patients in the no pain class. In addition, patients in the severe pain class were more likely to be non-White, to have a lower level of education, and to report less income than patients in the no pain and mild pain classes.
Table 3.
Differences in Demographic Characteristics Among the Breast Pain Classes
| Characteristic | No Pain (0) n=126 (31.7%) | Mild Pain (1) n=173 (43.4%) | Moderate Pain (2) n=53 (13.3%) | Severe Pain (3) n=46 (11.6%) | Statistics |
|---|---|---|---|---|---|
|
| |||||
| Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | ||
|
| |||||
| Age (years) | 58.6 (11.4) | 53.4 (11.5) | 53.4 (12.1) | 52.4 (9.4) | F=6.47, p<.0001 0>1, 2, and 3 |
|
| |||||
| Education (years) | 15.8 (2.8) | 16.0 (2.6) | 15.6 (2.2) | 14.3 (2.9) | F=5.07, p=.002 0 and 1 > 3 |
|
| |||||
| % (N) | % (N) | % (N) | % (N) | ||
|
| |||||
| Ethnicity | χ2 = 14.88, p=.002 0 and 1 < 3 |
||||
| White | 73.0 (92) | 63.7 (109) | 66.0 (35) | 41.3 (19) | |
| Non-white* | 27.0 (34) | 36.3 (62) | 34.0 (18) | 58.7 (27) | |
|
| |||||
| Lives alone | NS | ||||
| Yes | 20.8 (26) | 24.6 (42) | 26.4 (14) | 29.5 (13) | |
| No | 79.2 (99) | 75.4 (129) | 73.6 (39) | 70.5 (31) | |
|
| |||||
| Marital status | NS | ||||
| Married/Partnered | 41.3 (52) | 39.5 (68) | 37.7 (20) | 58.1 (25) | |
| Single, separated, widowed, divorced | 58.7 (74) | 60.5 (104) | 62.3 (33) | 41.9 (18) | |
|
| |||||
| Currently working for pay | NS | ||||
| Yes | 52.0 (65) | 50.9 (87) | 39.6 (21) | 34.8 (16) | |
| No | 48.0 (60) | 49.1 (84) | 60.4 (32) | 65.2 (30) | |
|
| |||||
| Total annual household income | KW, p<.0001 0 and 1 > 3 |
||||
| <$10,000 to $19,999 | 8.5 (9) | 14.4 (21) | 17.9 (7) | 39.5 (15) | |
| $20,000 to $99,000 | 48.1 (51) | 43.8 (64) | 51.3 (20) | 44.7 (17) | |
| ≥$100,000 | 43.4 (46) | 41.8 (61) | 30.8 (12) | 15.8 (6) | |
Abbreviations: NS = Not significant
Post hoc contrasts refer to non-whites
Differences in Preoperative Clinical Characteristics
The largest number of significant differences among the four pain classes was found in the preoperative characteristics (Table 4). In general, compared to patients in the no pain class, patients in the severe pain class had a lower KPS score; higher ratings of average and worst breast pain prior to surgery; decreased flexion, abduction, and external rotation in their affected arm; and were more likely to report rheumatoid arthritis. In addition, compared to the no pain class, a higher percentage of patients in all three pain classes reported numbness and hardness in the affected breast prior to surgery.
Table 4.
Differences in Preoperative Clinical Characteristics Among the Breast Pain Classes
| Characteristic | No Pain (0) n=126 (31.7%) | Mild Pain (1) n=173 (43.4%) | Moderate Pain (2) n=53 (13.3%) | Severe Pain (3) n=46 (11.6%) | Statistics |
|---|---|---|---|---|---|
|
| |||||
| Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | ||
|
| |||||
| Body mass index (kg/m2) | 27.1 (7.0) | 25.9 (5.3) | 27.6 (6.3) | 28.6 (6.3) | F=2.99, p=.031 1<3 |
|
| |||||
| Karnofsky Performance Status score | 96.2 (8.7) | 93.6 (9.3) | 89.6 (9.3) | 87.6 (14.9) | F=11.04, p<.0001 0 > 2 and 3; 1 > 3 |
|
| |||||
| Self-Administered Comorbidity Scale score | 4.0 (2.3) | 4.0 (3.0) | 4.7 (2.9) | 5.6 (3.2) | F=4.43, p=.004 0 and 1 < 3 |
|
| |||||
| Number of breast biopsies | 1.4 (0.7) | 1.6 (0.8) | 1.5 (0.8) | 1.6 (1.1) | NS |
|
| |||||
| Average breast pain | 0.0 (0.0) | 0.6 (1.1) | 0.5 (0.9) | 2.3 (3.2) | F=33.05, p<.0001 0 < 1 and 3; 1 and 2 < 3 |
|
| |||||
| Worst breast pain | 0.0 (0.0) | 1.1 (1.2) | 0.8 (1.3) | 3.2 (4.1) | F=36.00, p<.0001 0 < 1 and 3; 1 and 2 < 3 |
|
| |||||
| Number of breast symptoms | |||||
|
| |||||
| Grip strength – unaffected hand (kg) | 23.2 (5.8) | 24.3 (5.7) | 23.6 (5.7) | 23.1 (6.4) | NS |
|
| |||||
| Grip strength – affected hand (kg) | 23.1 (6.0) | 23.9 (5.2) | 23.5 (5.5) | 22.4 (6.6) | NS |
|
| |||||
| Flexion –unaffected arm | 166.7 (9.1) | 168.0 (7.4) | 164.3 (13.5) | 163.5 (11.3) | F=3.97, p=.008 1>3 |
|
| |||||
| Flexion – affected arm | 166.2 (9.5) | 167.0 (8.6) | 163.7 (13.0) | 160.0 (14.8) | F=6.16, p<.0001 0 and 1 > 3 |
|
| |||||
| Abduction – unaffected arm | 151.0 (17.4) | 152.2 (18.5) | 147.3 (18.3) | 145.7 (19.0) | NS |
|
| |||||
| Abduction – affected arm | 149.8 (19.9) | 152.3 (18.1) | 145.8 (20.0) | 137.6 (27.9) | F=6.81, p<.0001 0 and 1 > 3 |
|
| |||||
| Internal rotation – unaffected arm | 63.3 (8.6) | 63.9 (8.2) | 61.8 (9.0) | 62.0 (8.2) | NS |
|
| |||||
| Internal rotation – affected arm | 62.9 (8.4) | 63.6 (8.3) | 61.4 (11.4) | 61.7 (9.5) | NS |
|
| |||||
| External rotation – unaffected arm | 80.1 (6.2) | 79.3 (8.8) | 78.1 (7.5) | 77.3 (8.8) | NS |
|
| |||||
| External rotation – affected arm | 79.3 (8.0) | 79.4 (8.4) | 79.0 (8.3) | 74.9 (10.8) | F=3.56, p=.014 0 and 1 > 3 |
|
| |||||
| % (N) | % (N) | % (N) | % (N) | ||
|
| |||||
| Occurrence of comorbid conditions (% and number of women who reported each comorbid condition from the Self-Administered Comorbidity Questionnaire) | |||||
| Heart disease | 4.0 (5) | 2.9 (5) | 7.5 (4) | 2.2 (1) | NS |
| High blood pressure | 34.9 (44) | 24.3 (42) | 30.2 (16) | 45.7 (21) | χ2=9.21, p=.027 1 < 3 |
| Lung disease | 2.4 (3) | 3.5 (6) | 0.0 (0) | 6.5 (3) | NS |
| Diabetes | 7.1 (9) | 5.8 (10) | 2.5 (4) | 17.4 (8) | NS |
| Ulcer | 3.2 (4) | 4.0 (7) | 5.7 (3) | 2.2 (1) | NS |
| Kidney disease | 1.6 (2) | 0.6 (1) | 0.0 (0) | 0.0 (0) | NS |
| Liver disease | 3.2 (4) | 1.7 (3) | 1.9 (1) | 4.3 (2) | NS |
| Anemia | 7.9 (10) | 5.2 (9) | 13.2 (7) | 13.0 (6) | NS |
| Depression | 16.7 (21) | 22.5 (39) | 20.8 (11) | 34.8 (16) | NS |
| Osteoarthritis | 17.5 (22) | 14.5 (25) | 24.5 (13) | 19.6 (9) | NS |
| Back pain | 22.2 (28) | 27.2 (47) | 34.0 (18) | 41.3 (19) | NS |
| Rheumatoid arthritis | 1.6 (2) | 2.9 (5) | 1.9 (1) | 13.0 (6) | χ2=14.30, p=.003 0 < 3 |
|
| |||||
| Diagnosed with mastitis | NS | ||||
| Yes | 11.2 (14) | 12.3 (21) | 17.0 (9) | 7.0 (3) | |
| No | 88.8 (111) | 87.7 (150) | 83.0 (44) | 93.0 (40) | |
|
| |||||
| Exercise on a regular basis | χ2=10.22, p=.017 1 > 2 |
||||
| Yes | 65.6 (82) | 77.3 (133) | 56.6 (30) | 66.7 (30) | |
| No | 34.4 (43) | 22.7 (39) | 43.4 (23) | 33.3 (15) | |
|
| |||||
| Diagnosed with fibrocystic disease | NS | ||||
| Yes | 18.6 (22) | 22.4 (38) | 15.7 (8) | 11.4 (5) | |
| No | 81.4 (96) | 77.6 (132) | 84.3 (43) | 88.6 (39) | |
|
| |||||
| Ever breast fed | NS | ||||
| Yes | 48.0 (60) | 47.1 (81) | 49.1 (26) | 41.3 (19) | |
| No | 52.0 (65) | 52.9 (91) | 50.9 (27) | 58.7 (27) | |
|
| |||||
| Surgery to affected breast unrelated to cancer | NS | ||||
| Yes | 7.9 (10) | 14.5 (25) | 7.5 (4) | 6.5 (3) | |
| No | 92.1 (116) | 85.5 (148) | 92.5 (49) | 93.5 (43) | |
|
| |||||
| Surgery on affected arm not related to cancer | NS | ||||
| Yes | 4.0 (5) | 4.0 (7) | 1.9 (1) | 2.2 (1) | |
| No | 96.0 (121) | 96.0 (166) | 98.1 (52) | 97.8 (45) | |
|
| |||||
| Surgery on affected hand not related to cancer | NS | ||||
| Yes | 7.1 (9) | 4.0 (7) | 7.5 (4) | 2.2 (1) | |
| No | 92.9 (117) | 96.0 (166) | 92.5 (49) | 97.8 (45) | |
|
| |||||
| Injury to affected arm | NS | ||||
| Yes | 15.1 (19) | 15.0 (26) | 11.3 (6) | 15.2 (7) | |
| No | 84.9 (107) | 85.0 (147) | 88.7 (47) | 84.8 (39) | |
|
| |||||
| Injury to affected hand | NS | ||||
| Yes | 7.9 (10) | 11.6 (20) | 13.2 (7) | 4.3 (2) | |
| No | 92.1 (116) | 88.4 (153) | 86.8 (46) | 95.7 (44) | |
|
| |||||
| Prior hysterectomy | NS | ||||
| Yes | 13.5 (17) | 13.3 (23) | 13.2 (7) | 15.2 (7) | |
| No | 86.5 (109) | 86.7 (150) | 86.8 (46) | 84.8 (39) | |
|
| |||||
| Prior oophorectomy | NS | ||||
| Yes | 13.5 (17) | 8.1 (14) | 11.5 (6) | 13.0 (6) | |
| No | 86.5 (109) | 91.9 (159) | 88.5 (46) | 87.0 (40) | |
|
| |||||
| Gone through menopause | NS | ||||
| Yes | 71.0 (88) | 58.3 (98) | 67.3 (35) | 62.8 (27) | |
| No | 29.0 (36) | 41.7 (70) | 32.7 (17) | 37.2 (16) | |
|
| |||||
| Received neoadjuvant chemotherapy | NS | ||||
| Yes | 17.5 (22) | 18.6 (32) | 32.1 (17) | 17.4 (8) | |
| No | 82.5 (104) | 81.4 (140) | 67.9 (36) | 82.6 (38) | |
|
| |||||
| On hormonal replacement therapy prior to surgery | NS | ||||
| Yes | 19.0 (24) | 29 (16.9) | 20.8 (11) | 6.7 (3) | |
| No | 81.0 (102) | 83.1 (143) | 79.2 (42) | 93.3 (42) | |
|
| |||||
| Stage of disease | NS | ||||
| Stage 0 | 17.5 (22) | 20.8 (36) | 17.0 (9) | 13.0 (6) | |
| Stage 1 | 41.3 (52) | 35.8 (62) | 39.6 (21) | 34.8 (16) | |
| Stage IIA and IIB | 35.7 (45) | 35.8 (62) | 30.2 (16) | 39.1 (18) | |
| Stage IIIA, IIIB, IIIC, and IV | 5.6 (7) | 7.5 (13) | 13.2 (7) | 13.0 (6) | |
|
| |||||
| Estrogen receptor status | NS | ||||
| Positive | 84.1 (106) | 76.2 (131) | 71.7 (38) | 69.6 (32) | |
| Negative | 15.9 (20) | 23.8 (41) | 28.3 (15) | 30.4 (14) | |
|
| |||||
| Progesterone receptor status | NS | ||||
| Positive | 70.6 (89) | 70.9 (122) | 71.7 (38) | 65.2 (30) | |
| Negative | 29.4 (37) | 29.1 (50) | 28.3 (15) | 34.8 (16) | |
|
| |||||
| HER2/neu receptor positive | NS | ||||
| Yes | 12.9 (15) | 14.7 (23) | 21.3 (10) | 27.5 (11) | |
| No | 87.1 (101) | 85.3 (133) | 78.7 (37) | 72.5 (29) | |
|
| |||||
| BRCA1 and BRCA2 genetic testing | χ2=14.02, p=.029 No significant pairwise contrasts |
||||
| Positive | 4.8 (6) | 0.0 (0) | 1.9 (1) | 2.2 (1) | |
| Negative | 7.1 (9) | 13.5 (23) | 15.4 (8) | 4.3 (2) | |
| Not done | 88.1 (111) | 86.5 (147) | 82.7 (43) | 93.5 (43) | |
|
| |||||
| Used NSAID preoperatively | NS | ||||
| Yes | 4.0 (5) | 1.7 (3) | 1.9 (1) | 4.4 (2) | |
| No | 96.0 (121) | 98.3 (169) | 98.1 (52) | 95.6 (43) | |
|
| |||||
| Pain in breast prior to surgery | χ2=61.24, p<.0001 0 < 1, 2, and 3 |
||||
| Yes | 2.4 (3) | 41.1 (69) | 35.8 (19) | 43.2 (19) | |
| No | 97.6 (122) | 58.9 (99) | 64.2 (34) | 56.8 (25) | |
|
| |||||
| Swelling in affected breast | χ2=21.64, p<.0001 0 and 1 < 3 |
||||
| Yes | 3.2 (4) | 6.4 (11) | 13.2 (7) | 23.9 (11) | |
| No | 96.8 (122) | 93.6 (162) | 86.8 (46) | 76.1 (35) | |
|
| |||||
| Numbness in affected breast | χ2=18.46, p<.0001 0 and 1 < 3 |
||||
| Yes | 2.4 (3) | 3.5 (6) | 3.8 (2) | 17.4 (8) | |
| No | 97.6 (123) | 96.5 (167) | 96.2 (51) | 82.6 (38) | |
|
| |||||
| Strange sensations in affected breast | χ2=18.57, p<.0001 0 < 1 |
||||
| Yes | 12.7 (16) | 34.7 (60) | 28.3 (15) | 28.3 (13) | |
| No | 87.3 (110) | 65.3 (113) | 71.7 (38) | 71.7 (33) | |
|
| |||||
| Hardness in affected breast | χ2=15.84, p=.001 0 < 1, 2, and 3 |
||||
| Yes | 7.9 (10) | 21.4 (37) | 24.5 (13) | 30.4 (14) | |
| No | 92.1 (116) | 78.6 (136) | 75.5 (40) | 69.6 (32) | |
Abbreviations: NS = Not significant, SD = standard deviation
Differences in Preoperative Symptom Severity Scores
As shown in Table 5, significant differences in symptom severity scores were found among the four pain classes. In general, compared to the no pain class, patients in the moderate and severe pain classes reported significantly higher depression, trait anxiety, sleep disturbance, and fatigue scores, and poorer attentional function scores.
Table 5.
Differences in Preoperative Symptom and Quality of Life Scores Among the Breast Pain Classes
| Symptom and Quality of Life Scores | No Pain (0) n=126 (31.7%) | Mild Pain (1) n=173 (43.4%) | Moderate Pain (2) n=53 (13.3%) | Severe Pain (3) n=46 (11.6%) | Statistics |
|---|---|---|---|---|---|
| Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | ||
| SYMPTOM SCORES PRIOR TO SURGERY | |||||
| Center for Epidemiological Studies-Depression score | 11.1 (8.4) | 13.7 (9.0) | 15.4 (9.8) | 19.7 (13.1) | F=9.15, p<.0001 0 < 2 and 3; 1 <3 |
| Trait anxiety | 32.6 (7.8) | 35.8 (9.0) | 37.2 (10.0) | 39.1 (9.2) | F=7.22, p<.0001 0 < 1, 2, and 3 |
| State anxiety | 38.9 (13.3) | 41.6 (12.6) | 44.5 (14.3) | 47.0 (15.1) | F=4.88, p=.002 0 <3 |
| General Sleep Disturbance Scale score | 41.7 (18.5) | 49.6 (21.9) | 54.0 (19.9) | 55.8 (25.7) | F=7.34, p<.0001 0 < 1, 2, and 3 |
| Lee Fatigue Subscale score | 2.5 (2.2) | 3.1 (2.2) | 4.1 (2.5) | 3.9 (2.6) | F=7.91, p<.0001 0 < 2 and 3; 1 < 2 |
| Lee Energy Subscale score | 5.1 (2.6) | 5.0 (2.4) | 3.9 (2.1) | 5.1 (2.7) | F=3.61, p=.013 0 and 1 > 2 |
| Attentional Function Index score | 7.1 (1.8) | 6.6 (1.9) | 6.0 (2.0) | 5.7 (2.1) | F=8.29, p<.0001 0 > 2 and 3; 1 > 3 |
| QUALITY OF LIFE SUBSCALE AND TOTAL SCORES PRIOR TO SURGERY | |||||
| Physical well-being score | 8.6 (1.2) | 8.1 (1.4) | 7.4 (1.6) | 6.8 (2.3) | F=18.02, p<.0001 0 > 1, 2, and 3 1 > 2 and 3 |
| Psychological well-being score | 6.4 (1.6) | 5.6 (1.7) | 5.3 (2.0) | 4.9 (2.1) | F=10.39, p<.0001 0 > 1, 2, and 3 |
| Social well-being score | 7.8 (1.6) | 6.8 (1.8) | 6.3 (2.0) | 5.6 (2.4) | F=18.28, p<.0001 0 > 1, 2, and 3; 1 > 3 |
| Spiritual well-being score | 5.7 (1.7) | 5.7 (1.8) | 5.4 (2.0) | 6.0 (2.0) | NS |
| Total quality of life score | 7.0 (1.1) | 6.4 (1.2) | 5.9 (1.4) | 5.6 (1.6) | F=17.53, p<.0001 0 > 1, 2, and 3; 1 > 3 |
Abbreviations: NS = Not significant, SD = standard deviation
Differences in Preoperative QOL Scores
As shown in Table 5, significant differences in QOL scores were found among the four pain classes. In general, compared to the no pain class, patients in the mild, moderate, and severe pain classes reported significantly lower physical well-being, psychological well-being, social well-being, and total QOL scores.
Differences in Intraoperative Characteristics
As shown in Table 6, no significant differences were found among the four pain classes for the majority of the intraoperative characteristics. Compared to patients in the no pain class, patients in the severe pain class had a higher number of lymph nodes removed and were more likely to have had an axillary lymph node dissection (ALND).
Table 6.
Differences in Intraoperative Characteristics Among the Breast Pain Classes
| Characteristic | No Pain (0) n=126 (31.7%) | Mild Pain (1) n=173 (43.4%) | Moderate Pain (2) n=53 (13.3%) | Severe Pain (3) n=46 (11.6%) | Statistics |
|---|---|---|---|---|---|
|
| |||||
| Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | ||
|
| |||||
| Number of lymph nodes removed | 4.3 (4.7) | 6.0 (7.1) | 6.7 (6.4) | 8.1 (9.0) | F=4.13, p=.007 0 < 3 |
|
| |||||
| Number of positive lymph nodes | 0.7 (1.8) | 0.8 (2.0) | 1.1 (3.3) | 1.4 (2.9) | NS |
|
| |||||
| Number of drains placed during surgery | 0.4 (0.7) | 0.5 (0.7) | 0.6 (0.9) | 0.5 (0.6) | NS |
| % (N) | % (N) | % (N) | % (N) | ||
|
| |||||
| Type of surgery | NS | ||||
| Breast conserving | 84.1 (106) | 77.5 (134) | 75.5 (40) | 82.6 (38) | |
| Mastectomy | 15.9 (20) | 22.5 (39) | 24.5 (13) | 17.4 (8) | |
|
| |||||
| Location of cancer | NS | ||||
| Right breast | 47.6 (60) | 46.8 (81) | 49.1 (26) | 45.7 (21) | |
| Left breast | 52.4 (66) | 53.2 (92) | 50.9 (27) | 54.3 (25) | |
|
| |||||
| Surgery done on | NS | ||||
| Dominant side | 47.6 (60) | 46.8 (81) | 50.9 (27) | 41.3 (19) | |
| Nondominant side | 52.4 (66) | 53.2 (92) | 49.1 (26) | 58.7 (27) | |
|
| |||||
| Sentinal lymph node biopsy | NS | ||||
| Yes | 84.1 (106) | 82.7 (143) | 86.8 (46) | 71.7 (33) | |
| No | 15.9 (20) | 17.3 (30) | 13.2 (7) | 28.3 (13) | |
|
| |||||
| Axillary lymph node dissection | χ2=8.20, p=.042 0 < 3 |
||||
| Yes | 29.4 (37) | 38.4 (66) | 41.5 (22) | 52.2 (24) | |
| No | 70.6 (89) | 61.6 (106) | 58.5 (31) | 47.8 (22) | |
|
| |||||
| Intercostobrachial nerve sacrificed | NS | ||||
| Yes | 0.8 (1) | 4.0 (7) | 5.7 (3) | 4.3 (2) | |
| No | 10.4 (13) | 10.4 (18) | 7.5 (4) | 10.9 (5) | |
| Unable to determine | 88.8 (111) | 85.5 (148) | 86.8 (46) | 84.8 (39) | |
|
| |||||
| Intraoperative wound infiltration with local anesthetic | NS | ||||
| Yes | 66.7 (84) | 60.1 (104) | 64.2 (34) | 58.7 (27) | |
| No | 10.3 (13) | 11.0 (19) | 11.3 (6) | 8.7 (4) | |
| Unable to determine | 23.0 (29) | 28.9 (50) | 24.5 (13) | 32.6 (15) | |
|
| |||||
| Intraoperative radiation therapy | NS | ||||
| Yes | 2.4 (3) | 4.1 (7) | 7.5 (4) | 2.2 (1) | |
| No | 97.6 (123) | 95.9 (165) | 92.5 (49) | 97.8 (45) | |
|
| |||||
| Reconstruction at the time of surgery | χ2=10.31, p=.016 no significant pairwise contrasts |
||||
| Yes | 15.9 (20) | 29.1 (50) | 18.9 (10) | 13.0 (6) | |
| No | 84.1 (106) | 70.9 (122) | 81.1 (43) | 87.0 (40) | |
Abbreviations: NS = Not significant, SD = standard deviation
Differences in Postoperative Characteristics
As shown in Table 7, significant differences in a number of postoperative characteristics were found among the four pain classes. Compared to the no pain class, patients in the other three pain classes reported higher average and worst pain intensity scores in the immediate postoperative period. Compared to the no pain class, patients in the severe pain class were more likely to have had a re-excision or mastectomy in the six months following surgery. Compared to the mild pain and the severe pain classes, a higher percentage of patients in the moderate pain class had radiation therapy in the six months following surgery.
Table 7.
Differences in Postoperative Clinical Characteristics Among the Breast Pain Classes
| Characteristic | No Pain (0) n=126 (31.7%) | Mild Pain (1) n=173 (43.4%) | Moderate Pain (2) n=53 (13.3%) | Severe Pain (3) n=46 (11.6%) | Statistics |
|---|---|---|---|---|---|
|
| |||||
| Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | ||
|
| |||||
| Number of postoperative complications | 0.2 (0.5) | 0.2 (0.5) | 0.2 (0.4) | 0.3 (0.6) | NS |
|
| |||||
| Severity of average postoperative pain | 2.8 (2.1) | 3.7 (2.2) | 4.4 (2.3) | 6.5 (2.2) | F=30.81, p<.0001 0 <1, 2, and 3; 1 and 2 < 3 |
|
| |||||
| Severity of worst postoperative pain | 4.2 (2.6) | 5.0 (2.6) | 6.1 (2.4) | 7.9 (2.5) | F=23.36, p<.0001 0 < 1, 2 and 3; 1 and 2 <3 |
|
| |||||
| Amount of relief from analgesics (%) | 79.6 (26.4) | 80.3 (21.0) | 77.2 (21.6) | 73.1 (22.8) | NS |
|
| |||||
| Satisfaction with postoperative pain treatment | 9.0 (1.9) | 8.7 (1.9) | 8.2 (2.4) | 7.8 (2.7) | F=3.85, p=.01 0 > 3 |
|
| |||||
| % (N) | % (N) | % (N) | % (N) | ||
|
| |||||
| Placement of surgical drain | NS | ||||
| None | 69.0 (87) | 56.1 (97) | 62.3 (33) | 56.5 (26) | |
| Only in breast | 14.3 (18) | 20.2 (35) | 13.2 (7) | 8.7 (4) | |
| Only in axilla | 12.7 (16) | 19.1 (33) | 13.2 (7) | 28.3 (13) | |
| In both breast and axilla | 4.0 (5) | 4.6 (8) | 4.6 (8) | 6.5 (3) | |
|
| |||||
| Had a postoperative complication | NS | ||||
| Yes | 18.3 (23) | 21.5 (37) | 21.6 (11) | 26.1 (12) | |
| No | 81.7 (103) | 78.5 (135) | 78.4 (40) | 73.9 (34) | |
|
| |||||
| Had a seroma | NS | ||||
| Yes | 11.1 (14) | 11.6 (20) | 5.7 (3) | 6.5 (3) | |
| No | 88.9 (112) | 88.4 (153) | 94.3 (50) | 93.5 (43 | |
|
| |||||
| Had a hematoma | NS | ||||
| Yes | 4.8 (6) | 5.2 (9) | 3.8 (2) | 13.0 (6) | |
| No | 95.2 (120) | 94.8 (164) | 96.2 (51) | 87.0 (40) | |
|
| |||||
| Had bleeding | NS | ||||
| Yes | 0.0 (0) | 1.2 (2) | 1.9 (1) | 4.3 (2) | |
| No | 100.0 (126) | 98.8 (171) | 98.1 (52) | 95.7 (44) | |
|
| |||||
| Had a wound infection | NS | ||||
| Yes | 3.2 (4) | 4.0 (7) | 3.8 (2) | 6.5 (3) | |
| No | 96.8 (122) | 96.0 (166) | 96.2 (51) | 93.5 (43) | |
|
| |||||
| Received radiation therapy during the 6 months | χ2=12.39, p=.006 1 and 3 < 2 |
||||
| Yes | 56.3 (71) | 54.3 (94) | 75.5 (40) | 41.3 (19) | |
| No | 43.7 (55) | 45.7 (79) | 24.5 (13) | 58.7 (27) | |
|
| |||||
| Received adjuvant chemotherapy during the 6 months | NS | ||||
| Yes | 31.7 (40) | 34.7 (60) | 28.3 (15) | 39.1 (18) | |
| No | 68.3 (86) | 65.3 (113) | 71.7 (38) | 60.9 (28) | |
|
| |||||
| Received hormonal therapy during the 6 months | NS | ||||
| Yes | 45.2 (57) | 41.6 (72) | 47.2 (25) | 30.4 (14) | |
| No | 54.8 (69) | 58.4 (101) | 52.8 (28) | 69.6 (32) | |
|
| |||||
| Received biological therapy during the 6 months | NS | ||||
| Yes | 8.7 (11) | 12.7 (22) | 13.2 (7) | 6.5 (3) | |
| No | 91.3 (115) | 87.3 (151) | 86.8 (46) | 93.5 (43) | |
|
| |||||
| Received complementary therapy during the 6 months | NS | ||||
| Yes | 23.8 (30) | 28.3 (49) | 34.0 (18) | 23.9 (11) | |
| No | 76.2 (96) | 71.7 (124) | 66.0 (35) | 76.1 (35) | |
|
| |||||
| Received physical therapy during the 6 months | NS | ||||
| Yes | 9.5 (12) | 19.1 (33) | 17.0 (9) | 19.6 (9) | |
| No | 90.5 (114) | 80.9 (140) | 83.0 (44) | 80.4 (37) | |
|
| |||||
| Had breast reconstruction during the 6 months | NS | ||||
| Yes | 4.8 (6) | 10.4 (18) | 5.7 (3) | 2.2 (1) | |
| No | 95.2 (120) | 89.6 (155) | 94.3 (50) | 97.8 (45) | |
|
| |||||
| Breast cancer recurred during the 6 months | NS | ||||
| Yes | 0.0 (0) | 0.0 (0) | 0.0 (0) | 0.0 (0) | |
| No | 100.0 (126) | 100.0 (173) | 100.0 (53) | 100.0 (46) | |
|
| |||||
| Had re-excision or mastectomy during the 6 months | χ2=9.77, p=.021 0<3 |
||||
| Yes | 18.3 (23) | 31.2 (54) | 26.4 (14) | 39.1 (18) | |
| No | 81.7 (103) | 68.8 (119) | 73.6 (39) | 60.9 (18) | |
|
| |||||
| Any other surgery during the 6 months | χ2=8.34, p=.04 No significant pairwise contrasts |
||||
| Yes | 7.1 (9) | 13.9 (24) | 1.9 (1) | 13.0 (6) | |
| No | 92.9 (117) | 86.1 (149) | 98.1 (52) | 87.0 (40) | |
|
| |||||
| Evidence of metastatic disease during the 6 months | NS | ||||
| Yes | 0.0 (0) | 0.6 (1) | 0.0 (0) | 0.0 (0) | |
| No | 100.0 (126) | 99.4 (172) | 100.0 (53) | 100.0 (46) | |
Abbreviations: NS = Not significant, SD = standard deviation
Use of Analgesic Medications
For a random sample of approximately 25% of the patients in each pain class, the use of analgesic medications was evaluated. As summarized in Table 8, with the exception of antidepressants, regardless of pain class membership, a low percentage of patients reported using various types of analgesics during the six months of the study.
Table 8.
Percentage of Patients Using Analgesic Medications*
| Breast Pain Class | Enrollment | Month 1 | Month 2 | Month 3 | Month 4 | Month 5 | Month 6 |
|---|---|---|---|---|---|---|---|
| Nonopioid analgesic | |||||||
| No pain | 7.7 | 7.7 | 0.0 | 3.8 | 11.5 | 3.8 | 3.8 |
| Mild pain | 4.7 | 18.6 | 11.6 | 11.6 | 7.0 | 7.0 | 7.0 |
| Moderate pain | 0.0 | 0.0 | 0.0 | 7.1 | 14.3 | 7.1 | 7.1 |
| Severe pain | 16.7 | 50.0 | 25.0 | 33.0 | 25.0 | 25.0 | 25.0 |
| Nonsteroidal anti-inflammatory drug | |||||||
| No pain | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 3.8 | 3.8 |
| Mild pain | 4.7 | 9.3 | 7.0 | 7.0 | 7.0 | 9.3 | 9.3 |
| Moderate pain | 14.3 | 14.3 | 14.3 | 7.1 | 7.1 | 7.1 | 7.1 |
| Severe pain | 0.0 | 8.3 | 0.0 | 8.3 | 0.0 | 0.0 | 0.0 |
| Weak opioid | |||||||
| No pain | 7.7 | 7.7 | 0.0 | 3.8 | 11.5 | 3.8 | 3.8 |
| Mild pain | 4.7 | 18.6 | 9.3 | 9.3 | 4.7 | 4.7 | 4.7 |
| Moderate pain | 0.0 | 0.0 | 0.0 | 7.1 | 7.1 | 0.0 | 0.0 |
| Severe pain | 8.3 | 33.0 | 8.3 | 16.7 | 8.3 | 8.3 | 8.3 |
| Strong opioid | |||||||
| No pain | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Mild pain | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Moderate pain | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Severe pain | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Antidepressant | |||||||
| No pain | 19.2 | 19.2 | 19.2 | 19.2 | 15.4 | 15.4 | 15.4 |
| Mild pain | 20.9 | 20.9 | 20.9 | 18.6 | 20.9 | 18.6 | 16.3 |
| Moderate pain | 21.4 | 21.4 | 21.4 | 21.4 | 14.3 | 14.3 | 14.3 |
| Severe pain | 8.3 | 8.3 | 8.3 | 16.7 | 25.0 | 16.7 | 16.7 |
| Anticonvulsant | |||||||
| No pain | 3.8 | 3.8 | 3.8 | 3.8 | 3.8 | 3.8 | 3.83 |
| Mild pain | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Moderate pain | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Severe pain | 8.3 | 8.3 | 16.7 | 16.7 | 16.7 | 8.3 | 16.7 |
Medication data from a representative sample of patients from each pain class are summarized above (i.e., No pain – n = 26/126 = 21%, Mild pain – n = 43/173 = 25%, Moderate pain – n = 14/53 = 26%, and Severe pain – n = 12/46 – 26%)
DISCUSSION
This study is the first to use GMM to identify subgroups of patients with distinct persistent breast pain trajectories following breast cancer surgery. Moreover, a comprehensive list of demographic, preoperative, intraoperative, and postoperative characteristics was used, in the same sample of patients, to identify predictors of pain class membership. Over the six months of the study, 31.7% of the patients responded no to the question about having pain in their affected breast. However, and consistent with previous reports,23,28 11.6% of the patients were classified as having severe pain that persisted over the six months of the study. The largest class of patients had worst breast pain scores of approximately 3 that remained relatively constant over the study period. Based on the limited number of patients using any analgesic medications during the six months of the study, the distinct latent classes identified using GMM, are not associated with analgesic use.
Four non-modifiable demographic characteristics, namely younger age, less education, being non-white, and having a lower total annual income, were associated with being in the severe pain class. Consistent with previous studies,1,7,28,32,47,61,62,71,75,77,83 younger age was associated with a higher risk of being in all three of the pain classes identified in this study. While studies of racial/ethnic differences in experimental21 and clinical pain22,30 have produced inconsistent results, the higher percentage of non-whites in the severe pain class is consistent with one other study of breast cancer patients.23 However, this finding should be interpreted with caution because compared to White patients, non-white patients in our study were more likely to be diagnosed with more advanced disease (p=.009) and to have had an ALND (p=.04) both of which may contribute to more severe pain. While lower levels of education40,63 and lower income levels13,60 are associated with increase rates of chronic pain in the general population, in two studies of persistent pain after breast cancer surgery,41,62 no association was found between education and pain status.
In this study, detailed information was collected on a large number of preoperative characteristics (Table 4). Consistent with previous reports,6,42,81 a lower functional status and a higher comorbidity score were identified as risk factors for membership in the moderate or severe pain groups. In addition, a higher percentage of women in the severe pain group reported hypertension and rheumatoid arthritis. Findings on the association between chronic pain and arthritis are inconsistent, with some studies suggesting that the prevalence of chronic pain is lower in patients with hypertension18,31 while others suggest completely opposite results.4,49 However, since no studies have identified specific chronic conditions as being associated with the development of persistent pain in patients with breast cancer, these associations warrant confirmation in future studies.
The major modifiable preoperative risk factors identified in this study were preoperative breast pain or changes in breast sensations. Women who reported the occurrence of pain and hardness in the breast, as well as higher breast pain intensity ratings prior to surgery, were more likely to be classified into the mild, moderate, or severe pain classes. While one study found no association,62 our results confirm the finding of a previous study41 that found a relationship between preoperative breast pain and the occurrence of persistent pain following breast cancer surgery. Future studies need to identify the causes of preoperative breast pain and determine whether preoperative treatment prevents the development of persistent pain.
Consistent with previous reports, the two intraoperative predictors associated with membership in the severe pain latent class were number of lymph nodes removed1,34 and having an ALND.1,7,28 It is interesting to note that these two surgical characteristics that involve the shoulder, arm, and axilla were associated with pain groups identified based on ratings for breast pain. In our study, patients were asked to rate breast pain separate from arm pain. Future analyses will use GMM to determine latent classes of patients with distinct arm pain trajectories following breast cancer surgery as well as predictors of these latent classes. While an attempt was made to evaluate the impact of having to sacrifice the intercostobrachial nerve on pain group membership, in over 85% of the cases, the status of the nerve was not documented on the operative report.
Another modifiable risk factor identified in this study is the severity of postoperative pain. Compared to the no pain class, retrospective ratings of average and worst pain were significantly higher in mild, moderate and severe pain classes. This finding is consistent with data from two retrospective studies that found an association between higher postoperative pain scores and the development of persistent pain.75,78 While detailed information is not available on the postoperative pain regimens that were ordered for the patients in our study, our data suggest that efforts to improve postoperative pain management are needed to reduce the occurrence of persistent pain.
Two additional postoperative risk factors are worth noting. The receipt of radiation therapy (RT) in the six months following surgery was associated with membership in the moderate pain class. As shown in Figure 1, as a group, these patients’ pain increased over time and began to decrease at the 6 month assessment. The trajectory of breast pain identified for this class is consistent with the time course of RT. Most women begin RT about 2 months after surgery. The course of RT lasts approximately six weeks. The other risk factor associated with membership in the severe pain class was the need for re-excision or mastectomy in the six months after surgery. This finding suggests that repeated noxious stimulation contributes to the development of persistent pain.
In the review by Anderson and Kehlet,2 they noted the need for standardized evaluations of patients’ psychosocial status prior to surgery for breast cancer. In our prospective study, detailed evaluations of common symptoms, as well as a detailed disease specific evaluation of patients’ QOL, were done prior to surgery. Taken together, the findings in Table 5, suggest that patients with higher preoperative levels of depressive symptoms, trait anxiety, sleep disturbance, fatigue, and lower levels of attentional function are at increased risk for persistent breast pain following breast cancer surgery. Whether preoperative treatment of these symptoms would decrease the development of persistent pain requires additional investigation.
Several study limitations need to be acknowledged. While the sample size was relatively large, additional latent classes may be identified with a larger sample. In addition, with a larger sample, the number of patients in each pain class would have been larger and additional differences among the pain classes may have been identified. While the sample was representative of breast cancer patients in the United States, different latent classes and/or different risk factors may have been identified if a larger percentage of the sample was non-White, older, had more advanced disease, and/or required more extensive surgery. The differences, among the latent classes, in retrospective ratings of postoperative pain scores need to be verified in prospective studies. Finally, future studies should obtain detailed information on analgesic use in the perioperative period, as well as during long-term follow-up.
In conclusion, this study is one of the largest, prospective, longitudinal studies to evaluate the prevalence of and risk factors for persistent breast pain following breast cancer surgery. It is the first study to evaluate the majority of the subjective and objective risk factors for persistent breast pain identified by Andersen and Kehlet.2 Parallel analyses are being done to identify the phenotypic and genotypic predictors of persistent arm and shoulder pain following breast cancer surgery. Future analyses are focused on determining the pain characteristics for the three breast pain classes identified using GMM as well as genomic predictors of these classes.
Perspective.
Persistent pain is a significant problem for 25% of women following surgery for breast cancer. Severe breast pain is associated with clinically meaningful decrements in functional status and quality of life.
Acknowledgments
This study was funded by grants from the National Cancer Institute (CA107091 and CA118658). Dr. Bradley Aouizerat was funded through the National Institutes of Health (NIH) Roadmap for Medical Research Grant (KL2 RR624130). Dr. Dunn received funding from the Mount Zion Health Fund. Dr. Christine Miaskowski is an American Cancer Society Clinical Research Professor. Dr. Dhruva is funded through NIH Mentored Patient-Oriented Research Career Development Award (K23 AT005340). Dr. Langford is supported by a Department of Defense Breast Cancer Research Program Postdoctoral Fellowship. Mr. Merriman is supported by an NINR fellowship (F31 NR012604), an ACS Doctoral Degree Scholarship (DSCN-10-087), an Oncology Nursing Society Doctoral Scholarship, and a UCSF Nursing Alumni Association Scholarship. Dr. Baggott is funded by an American Cancer Society Mentored Research Scholar Award (MRSG 12-01-PCSM). This project is supported by NIH/NCRR UCSF-CTSI Grant Number UL1 RR024131. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
Footnotes
Conflicts of interest and disclosures: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Alves Nogueira Fabro E, Bergmann A, do Amaral ESB, Padula Ribeiro AC, de Souza Abrahao K, da Costa Leite Ferreira MG, de Almeida Dias R, Santos Thuler LC. Post-mastectomy pain syndrome: Incidence and risks. Breast. 2012;3:321–325. doi: 10.1016/j.breast.2012.01.019. [DOI] [PubMed] [Google Scholar]
- 2.Andersen KG, Kehlet H. Persistent pain after breast cancer treatment: A critical review of risk factors and strategies for prevention. J Pain. 2011;12:725–746. doi: 10.1016/j.jpain.2010.12.005. [DOI] [PubMed] [Google Scholar]
- 3.Bieling PJ, Antony MM, Swinson RP. The State-Trait Anxiety Inventory, Trait version: structure and content re-examined. Behav Res Ther. 1998;36:777–788. doi: 10.1016/s0005-7967(98)00023-0. [DOI] [PubMed] [Google Scholar]
- 4.Bruehl S, Burns JW, McCubbin JA. Altered cardiovascular/pain regulatory relationships in chronic pain. Int J Behav Med. 1998;5:63–75. doi: 10.1207/s15327558ijbm0501_5. [DOI] [PubMed] [Google Scholar]
- 5.Brunner F, Bachmann LM, Weber U, Kessels AG, Perez RS, Marinus J, Kissling R. Complex regional pain syndrome 1--the Swiss cohort study. BMC Musculoskelet Disord. 2008;9:92. doi: 10.1186/1471-2474-9-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Butchart A, Kerr EA, Heisler M, Piette JD, Krein SL. Experience and management of chronic pain among patients with other complex chronic conditions. Clin J Pain. 2009;25:293–298. doi: 10.1097/AJP.0b013e31818bf574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Caffo O, Amichetti M, Ferro A, Lucenti A, Valduga F, Galligioni E. Pain and quality of life after surgery for breast cancer. Breast Cancer Res Treat. 2003;80:39–48. doi: 10.1023/A:1024435101619. [DOI] [PubMed] [Google Scholar]
- 8.Carney S, Koetters T, Cho M, West C, Paul SM, Dunn L, Aouizerat BE, Dodd M, Cooper B, Lee K, Wara W, Swift P, Miaskowski C. Differences in sleep disturbance parameters between oncology outpatients and their family caregivers. J Clin Oncol. 2011;29:1001–1006. doi: 10.1200/JCO.2010.30.9104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carpenter JS, Andrykowski MA, Sloan P, Cunningham L, Cordova MJ, Studts JL, McGrath PC, Sloan D, Kenady DE. Postmastectomy/postlumpectomy pain in breast cancer survivors. J Clin Epidemiol. 1998;51:1285–1292. doi: 10.1016/s0895-4356(98)00121-8. [DOI] [PubMed] [Google Scholar]
- 10.Carpenter JS, Andrykowski MA, Wilson J, Hall LA, Rayens MK, Sachs B, Cunningham LL. Psychometrics for two short forms of the Center for Epidemiologic Studies-Depression Scale. Issues Ment Health Nurs. 1998;19:481–494. doi: 10.1080/016128498248917. [DOI] [PubMed] [Google Scholar]
- 11.Celeux G, Soromenho G. An entropy criterion for assessing the number of clusters in a mixture model. Journal of Classification. 1996;13:195–212. [Google Scholar]
- 12.Cieza A, Geyh S, Chatterji S, Kostanjsek N, Ustun BT, Stucki G. Identification of candidate categories of the International Classification of Functioning Disability and Health (ICF) for a Generic ICF Core Set based on regression modelling. BMC Med Res Methodol. 2006;6:36. doi: 10.1186/1471-2288-6-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cimmino MA, Ferrone C, Cutolo M. Epidemiology of chronic musculoskeletal pain. Best Pract Res Clin Rheumatol. 2011;25:173–183. doi: 10.1016/j.berh.2010.01.012. [DOI] [PubMed] [Google Scholar]
- 14.Cimprich B. Attentional fatigue following breast cancer surgery. Res Nurs Health. 1992;15:199–207. doi: 10.1002/nur.4770150306. [DOI] [PubMed] [Google Scholar]
- 15.Cimprich B, So H, Ronis DL, Trask C. Pre-treatment factors related to cognitive functioning in women newly diagnosed with breast cancer. Psychooncology. 2005;14:70–78. doi: 10.1002/pon.821. [DOI] [PubMed] [Google Scholar]
- 16.Cimprich B, Visovatti M, Ronis DL. The Attentional Function Index--a self-report cognitive measure. Psychooncology. 2011;20:194–202. doi: 10.1002/pon.1729. [DOI] [PubMed] [Google Scholar]
- 17.Dhruva A, Dodd M, Paul SM, Cooper BA, Lee K, West C, Aouizerat BE, Swift PS, Wara W, Miaskowski C. Trajectories of fatigue in patients with breast cancer before, during, and after radiation therapy. Cancer Nurs. 2010;33:201–212. doi: 10.1097/NCC.0b013e3181c75f2a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Diatchenko L, Anderson AD, Slade GD, Fillingim RB, Shabalina SA, Higgins TJ, Sama S, Belfer I, Goldman D, Max MB, Weir BS, Maixner W. Three major haplotypes of the beta2 adrenergic receptor define psychological profile, blood pressure, and the risk for development of a common musculoskeletal pain disorder. Am J Med Genet B Neuropsychiatr Genet. 2006;141B:449–462. doi: 10.1002/ajmg.b.30324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dunn LB, Aouizerat BE, Cooper BA, Dodd M, Lee K, West C, Paul SM, Wara W, Swift P, Miaskowski C. Trajectories of anxiety in oncology patients and family caregivers during and after radiation therapy. Eur J Oncol Nurs. 2012;16:1–9. doi: 10.1016/j.ejon.2011.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dunn LB, Cooper BA, Neuhaus J, West C, Paul S, Aouizerat B, Abrams G, Edrington J, Hamolsky D, Miaskowski C. Identification of distinct depressive symptom trajectories in women following surgery for breast cancer. Health Psychol. 2011;30:683–692. doi: 10.1037/a0024366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Edwards RR, Doleys DM, Fillingim RB, Lowery D. Ethnic differences in pain tolerance: clinical implications in a chronic pain population. Psychosom Med. 2001;63:316–323. doi: 10.1097/00006842-200103000-00018. [DOI] [PubMed] [Google Scholar]
- 22.Edwards RR, Moric M, Husfeldt B, Buvanendran A, Ivankovich O. Ethnic similarities and differences in the chronic pain experience: a comparison of african american, Hispanic, and white patients. Pain Med. 2005;6:88–98. doi: 10.1111/j.1526-4637.2005.05007.x. [DOI] [PubMed] [Google Scholar]
- 23.Fecho K, Miller NR, Merritt SA, Klauber-Demore N, Hultman CS, Blau WS. Acute and persistent postoperative pain after breast surgery. Pain Med. 2009;10:708–715. doi: 10.1111/j.1526-4637.2009.00611.x. [DOI] [PubMed] [Google Scholar]
- 24.Ferrell BR. The impact of pain on quality of life. A decade of research. Nurs Clin North Am. 1995;30:609–624. [PubMed] [Google Scholar]
- 25.Ferrell BR, Dow KH, Grant M. Measurement of the quality of life in cancer survivors. Qual Life Res. 1995;4:523–531. doi: 10.1007/BF00634747. [DOI] [PubMed] [Google Scholar]
- 26.Fletcher BS, Paul SM, Dodd MJ, Schumacher K, West C, Cooper B, Lee K, Aouizerat B, Swift P, Wara W, Miaskowski CA. Prevalence, severity, and impact of symptoms on female family caregivers of patients at the initiation of radiation therapy for prostate cancer. J Clin Oncol. 2008;26:599–605. doi: 10.1200/JCO.2007.12.2838. [DOI] [PubMed] [Google Scholar]
- 27.Garrett K, Dhruva A, Koetters T, West C, Paul SM, Dunn LB, Aouizerat BE, Cooper BA, Dodd M, Lee K, Wara W, Swift P, Miaskowski C. Differences in sleep disturbance and fatigue between patients with breast and prostate cancer at the initiation of radiation therapy. J Pain Symptom Manage. 2011;42:239–250. doi: 10.1016/j.jpainsymman.2010.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gartner R, Jensen MB, Nielsen J, Ewertz M, Kroman N, Kehlet H. Prevalence of and factors associated with persistent pain following breast cancer surgery. JAMA. 2009;302:1985–1992. doi: 10.1001/jama.2009.1568. [DOI] [PubMed] [Google Scholar]
- 29.Gay CL, Lee KA, Lee SY. Sleep patterns and fatigue in new mothers and fathers. Biol Res Nurs. 2004;5:311–318. doi: 10.1177/1099800403262142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Green CR, Ndao-Brumblay SK, Nagrant AM, Baker TA, Rothman E. Race, age, and gender influences among clusters of African American and white patients with chronic pain. J Pain. 2004;5:171–182. doi: 10.1016/j.jpain.2004.02.227. [DOI] [PubMed] [Google Scholar]
- 31.Guasti L, Zanotta D, Diolisi A, Garganico D, Simoni C, Gaudio G, Grandi AM, Venco A. Changes in pain perception during treatment with angiotensin converting enzyme-inhibitors and angiotensin II type 1 receptor blockade. J Hypertens. 2002;20:485–491. doi: 10.1097/00004872-200203000-00024. [DOI] [PubMed] [Google Scholar]
- 32.Hack TF, Cohen L, Katz J, Robson LS, Goss P. Physical and psychological morbidity after axillary lymph node dissection for breast cancer. J Clin Oncol. 1999;17:143–149. doi: 10.1200/JCO.1999.17.1.143. [DOI] [PubMed] [Google Scholar]
- 33.Jensen MP. The validity and reliability of pain measures in adults with cancer. J Pain. 2003;4:2–21. doi: 10.1054/jpai.2003.1. [DOI] [PubMed] [Google Scholar]
- 34.Johansen J, Overgaard J, Blichert-Toft M, Overgaard M. Treatment of morbidity associated with the management of the axilla in breast-conserving therapy. Acta Oncol. 2000;39:349–354. doi: 10.1080/028418600750013122. [DOI] [PubMed] [Google Scholar]
- 35.Jung T, Wickrama KAS. An introduction to latent class growth analysis and growth mixture modeling. Social and Personality Psychology Compass. 2008;2:302–317. [Google Scholar]
- 36.Karnofsky D. Performance scale. New York: Plenum Press; 1977. [Google Scholar]
- 37.Karnofsky D, Abelmann WH, Craver LV, Burchenal JH. The use of nitrogen mustards in the palliative treatment of carcinoma. Cancer. 1948;1:634–656. [Google Scholar]
- 38.Kennedy BL, Schwab JJ, Morris RL, Beldia G. Assessment of state and trait anxiety in subjects with anxiety and depressive disorders. Psychiatr Q. 2001;72:263–276. doi: 10.1023/a:1010305200087. [DOI] [PubMed] [Google Scholar]
- 39.Kreuter F, Muthen B. Analyzing criminal trajectory profiles: Briding multilevel and group-based approaches using growth mixture modeling. Journal of Quantitative Criminology. 2008;24:1–31. [Google Scholar]
- 40.Krueger AB, Stone AA. Assessment of pain: a community-based diary survey in the USA. Lancet. 2008;371:1519–1525. doi: 10.1016/S0140-6736(08)60656-X. [DOI] [PubMed] [Google Scholar]
- 41.Kudel I, Edwards RR, Kozachik S, Block BM, Agarwal S, Heinberg LJ, Haythornthwaite J, Raja SN. Predictors and consequences of multiple persistent postmastectomy pains. J Pain Symptom Manage. 2007;34:619–627. doi: 10.1016/j.jpainsymman.2007.01.013. [DOI] [PubMed] [Google Scholar]
- 42.Langford DJ, Paul SM, Tripathy D, West C, Dodd MJ, Schumacher K, Miaskowski C. Trajectories of pain and analgesics in oncology outpatients with metastatic bone pain during participation in a psychoeducational intervention study to improve pain management. J Pain. 2011;12:652–666. doi: 10.1016/j.jpain.2010.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lee KA. Self-reported sleep disturbances in employed women. Sleep. 1992;15:493–498. doi: 10.1093/sleep/15.6.493. [DOI] [PubMed] [Google Scholar]
- 44.Lee KA, DeJoseph JF. Sleep disturbances, vitality, and fatigue among a select group of employed childbearing women. Birth. 1992;19:208–213. doi: 10.1111/j.1523-536x.1992.tb00404.x. [DOI] [PubMed] [Google Scholar]
- 45.Lee KA, Hicks G, Nino-Murcia G. Validity and reliability of a scale to assess fatigue. Psychiatry Res. 1991;36:291–298. doi: 10.1016/0165-1781(91)90027-m. [DOI] [PubMed] [Google Scholar]
- 46.Lee KA, Portillo CJ, Miramontes H. The fatigue experience for women with human immunodeficiency virus. J Obstet Gynecol Neonatal Nurs. 1999;28:193–200. doi: 10.1111/j.1552-6909.1999.tb01984.x. [DOI] [PubMed] [Google Scholar]
- 47.Macdonald L, Bruce J, Scott NW, Smith WC, Chambers WA. Long-term follow-up of breast cancer survivors with post-mastectomy pain syndrome. Br J Cancer. 2005;92:225–230. doi: 10.1038/sj.bjc.6602304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.MacLean CD, Littenberg B, Kennedy AG. Limitations of diabetes pharmacotherapy: results from the Vermont Diabetes Information System study. BMC Fam Pract. 2006;7:50. doi: 10.1186/1471-2296-7-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Maixner W, Fillingim R, Kincaid S, Sigurdsson A, Harris MB. Relationship between pain sensitivity and resting arterial blood pressure in patients with painful temporomandibular disorders. Psychosom Med. 1997;59:503–511. doi: 10.1097/00006842-199709000-00007. [DOI] [PubMed] [Google Scholar]
- 50.Miaskowski C, Cooper BA, Paul SM, Dodd M, Lee K, Aouizerat BE, West C, Cho M, Bank A. Subgroups of patients with cancer with different symptom experiences and quality-of-life outcomes: a cluster analysis. Oncol Nurs Forum. 2006;33:E79–89. doi: 10.1188/06.ONF.E79-E89. [DOI] [PubMed] [Google Scholar]
- 51.Miaskowski C, Lee KA. Pain, fatigue, and sleep disturbances in oncology outpatients receiving radiation therapy for bone metastasis: a pilot study. J Pain Symptom Manage. 1999;17:320–332. doi: 10.1016/s0885-3924(99)00008-1. [DOI] [PubMed] [Google Scholar]
- 52.Miaskowski C, Paul SM, Cooper BA, Lee K, Dodd M, West C, Aouizerat BE, Swift PS, Wara W. Trajectories of fatigue in men with prostate cancer before, during, and after radiation therapy. J Pain Symptom Manage. 2008;35:632–643. doi: 10.1016/j.jpainsymman.2007.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Muthen B, Muthen LK. Integrating person-centered and variable-centered analyses: growth mixture modeling with latent trajectory classes. Alcohol Clin Exp Res. 2000;24:882–891. [PubMed] [Google Scholar]
- 54.Muthen BO. Beyond SEM: General latent variable modeling. Behaviormetrika. 2002;29:81–117. [Google Scholar]
- 55.Muthen BO. Latent variable analysis: Growth mixture modeling and related techniques for longitudinal data. In: Kaplan DW, editor. Handbook of Quantitative Methodology for the Social Sciences. Newbury Park, CA: Sage Publications; 2004. pp. 345–368. [Google Scholar]
- 56.Muthen LK, Muthen BO. Mplus User’s Guide. 6. Los Angeles, CA: Muthen & Muthen; 1998–2010. [Google Scholar]
- 57.Nylund KL, Asparouhov T, Muthen BO. Deciding on the number of classes in latent class analysis and growth mixture modeling: A Monte Carlo simulation study. Struct Equ Modeling. 2007;14:535–569. [Google Scholar]
- 58.Padilla GV, Ferrell B, Grant MM, Rhiner M. Defining the content domain of quality of life for cancer patients with pain. Cancer Nurs. 1990;13:108–115. [PubMed] [Google Scholar]
- 59.Padilla GV, Presant C, Grant MM, Metter G, Lipsett J, Heide F. Quality of life index for patients with cancer. Res Nurs Health. 1983;6:117–126. doi: 10.1002/nur.4770060305. [DOI] [PubMed] [Google Scholar]
- 60.Palmlof L, Skillgate E, Alfredsson L, Vingard E, Magnusson C, Lundberg M, Holm LW. Does income matter for troublesome neck pain? A population-based study on risk and prognosis. J Epidemiol Community Health. 2012 doi: 10.1136/jech-2011-200783. [DOI] [PubMed] [Google Scholar]
- 61.Peuckmann V, Ekholm O, Rasmussen NK, Groenvold M, Christiansen P, Moller S, Eriksen J, Sjogren P. Chronic pain and other sequelae in long-term breast cancer survivors: nationwide survey in Denmark. Eur J Pain. 2009;13:478–485. doi: 10.1016/j.ejpain.2008.05.015. [DOI] [PubMed] [Google Scholar]
- 62.Poleshuck EL, Katz J, Andrus CH, Hogan LA, Jung BF, Kulick DI, Dworkin RH. Risk factors for chronic pain following breast cancer surgery: a prospective study. J Pain. 2006;7:626–634. doi: 10.1016/j.jpain.2006.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Portenoy RK, Ugarte C, Fuller I, Haas G. Population-based survey of pain in the United States: differences among white, African American, and Hispanic subjects. J Pain. 2004;5:317–328. doi: 10.1016/j.jpain.2004.05.005. [DOI] [PubMed] [Google Scholar]
- 64.Radloff LS. The CES-D Scale: A self-report depression scale for research in the general population. Applied Psychological Measurement. 1977;1:385–401. [Google Scholar]
- 65.Ribom EL, Piehl-Aulin K, Ljunghall S, Ljunggren O, Naessen T. Six months of hormone replacement therapy does not influence muscle strength in postmenopausal women. Maturitas. 2002;42:225–231. doi: 10.1016/s0378-5122(02)00079-8. [DOI] [PubMed] [Google Scholar]
- 66.Rothman KJ. No adjustments are needed for multiple comparisons. Epidemiology. 1990;1:43–46. [PubMed] [Google Scholar]
- 67.Sangha O, Stucki G, Liang MH, Fossel AH, Katz JN. The Self-Administered Comorbidity Questionnaire: a new method to assess comorbidity for clinical and health services research. Arthritis Rheum. 2003;49:156–163. doi: 10.1002/art.10993. [DOI] [PubMed] [Google Scholar]
- 68.Schafer JL, Graham JW. Missing data: our view of the state of the art. Psychol Methods. 2002;7:147–177. [PubMed] [Google Scholar]
- 69.Sheehan TJ, Fifield J, Reisine S, Tennen H. The measurement structure of the Center for Epidemiologic Studies Depression Scale. J Pers Assess. 1995;64:507–521. doi: 10.1207/s15327752jpa6403_9. [DOI] [PubMed] [Google Scholar]
- 70.Smith SK, Zimmerman S, Williams CS, Zebrack BJ. Health status and quality of life among non-Hodgkin lymphoma survivors. Cancer. 2009;115:3312–3323. doi: 10.1002/cncr.24391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Smith WC, Bourne D, Squair J, Phillips DO, Chambers WA. A retrospective cohort study of post mastectomy pain syndrome. Pain. 1999;83:91–95. doi: 10.1016/s0304-3959(99)00076-7. [DOI] [PubMed] [Google Scholar]
- 72.Spielberger CG, Gorsuch RL, Suchene R, Vagg PR, Jacobs GA. Manual for the State-Anxiety (Form Y): Self Evaluation Questionnaire. Palo Alto, CA: Consulting Psychologists Press; 1983. [Google Scholar]
- 73.Spijkerman DC, Snijders CJ, Stijnen T, Lankhorst GJ. Standardization of grip strength measurements. Effects on repeatability and peak force. Scand J Rehabil Med. 1991;23:203–206. [PubMed] [Google Scholar]
- 74.SPSS. IBM SPSS for Windows (Version 19) Chicago, Illinois: SPSS, Inc; 2010. [Google Scholar]
- 75.Steegers MA, Wolters B, Evers AW, Strobbe L, Wilder-Smith OH. Effect of axillary lymph node dissection on prevalence and intensity of chronic and phantom pain after breast cancer surgery. J Pain. 2008;9:813–822. doi: 10.1016/j.jpain.2008.04.001. [DOI] [PubMed] [Google Scholar]
- 76.Stevens PE, Dibble SL, Miaskowski C. Prevalence, characteristics, and impact of postmastectomy pain syndrome: an investigation of women’s experiences. Pain. 1995;61:61–68. doi: 10.1016/0304-3959(94)00162-8. [DOI] [PubMed] [Google Scholar]
- 77.Swenson KK, Nissen MJ, Ceronsky C, Swenson L, Lee MW, Tuttle TM. Comparison of side effects between sentinel lymph node and axillary lymph node dissection for breast cancer. Ann Surg Oncol. 2002;9:745–753. doi: 10.1007/BF02574496. [DOI] [PubMed] [Google Scholar]
- 78.Tasmuth T, von Smitten K, Hietanen P, Kataja M, Kalso E. Pain and other symptoms after different treatment modalities of breast cancer. Ann Oncol. 1995;6:453–459. doi: 10.1093/oxfordjournals.annonc.a059215. [DOI] [PubMed] [Google Scholar]
- 79.Tasmuth T, von Smitten K, Kalso E. Pain and other symptoms during the first year after radical and conservative surgery for breast cancer. Br J Cancer. 1996;74:2024–2031. doi: 10.1038/bjc.1996.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Tofighi D, Enders CK. Identifying the correct number of classes in growth mixture models. Charlotte, NC: Information Age Publishing; 2008. [Google Scholar]
- 81.Utne I, Miaskowski C, Bjordal K, Paul SM, Jakobsen G, Rustoen T. Differences in the use of pain coping strategies between oncology inpatients with mild vs. moderate to severe pain. J Pain Symptom Manage. 2009;38:717–726. doi: 10.1016/j.jpainsymman.2009.03.005. [DOI] [PubMed] [Google Scholar]
- 82.Van Onselen C, Cooper BA, Lee K, Dunn L, Aouizerat BE, West C, Dodd M, Paul S, Miaskowski C. Identification of distinct subgroups of breast cancer patients based on self-reported changes in sleep disturbance. Support Care Cancer. 2012 doi: 10.1007/s00520-012-1381-3. [DOI] [PubMed] [Google Scholar]
- 83.Vilholm OJ, Cold S, Rasmussen L, Sindrup SH. The postmastectomy pain syndrome: an epidemiological study on the prevalence of chronic pain after surgery for breast cancer. Br J Cancer. 2008;99:604–610. doi: 10.1038/sj.bjc.6604534. [DOI] [PMC free article] [PubMed] [Google Scholar]