Abstract
Background
Elevated von Willebrand Factor (VWF) plasma levels are associated with an increased risk of cardiovascular disease. A meta-analysis of genome wide association studies on VWF identified novel candidate genes, i.e. syntaxin-binding protein 5 (STXBP5) and syntaxin 2 (STX2), which are possibly involved in the secretion of VWF. We investigated whether VWF antigen levels (VWF:Ag), VWF collagen-binding activity (VWF:CB), and the risk of arterial thrombosis are affected by common genetic variations in these genes.
Methods and Results
In 463 young Caucasian subjects (males ≤ 45 years, females ≤ 55 years), who were included one to three months after a first event of arterial thrombosis, and 406 controls, we measured VWF:Ag and VWF:CB. Nine haplotype tagging SNPs of STXBP5 and STX2 were selected and subsequently analysed using linear regression with additive genetic models adjusted for age, sex and ABO blood group. The minor alleles of rs9399599 and rs1039084 in STXBP5 were associated with lower VWF plasma levels and activity, whereas the minor allele of rs7978987 in STX2 was associated with higher VWF plasma levels and activity. The minor alleles of the SNPs in STX2 were associated with a reduced risk of arterial thrombosis (rs1236:OR 0.73 [95%CI 0.59, 0.89], rs7978987:OR 0.81 [95%CI 0.65, 1.00], rs11061158:OR 0.69 [95%CI 0.55, 0.88]).
Conclusion
Genetic variability in STXBP5 and STX2 affects both VWF concentration and activity in young individuals with premature arterial thrombosis. Furthermore, in our study genetic variability in STX2 is associated with the risk of arterial thrombosis. However, at this point the underlying mechanism remains unclear.
Keywords: Von Willebrand Factor, genetics, STX2, STXBP5, cardiovascular diseases
Introduction
Since elevated von Willebrand Factor (VWF) plasma levels are associated with an increased risk of cardiovascular disease (CVD), elucidating determinants of VWF plasma levels is of great interest 1, 2. It is already known that VWF plasma levels can be influenced by both genetic 3, 4 and non-genetic factors. These factors have their effect on different stages during the lifetime of the VWF molecules, which involve many biological mechanisms. However, determinants that have been identified previously - heritability estimates for VWF levels are on average 50% - are not sufficient to explain the entire variability of VWF levels 3.
VWF has a twofold function in primary haemostasis: it initiates adherence of platelets to the injured vessel wall and subsequent platelet aggregation, especially at sites of high shear 5, 6. VWF is mainly produced by endothelial cells, but also for about 5–10% by megakaryocytes. The majority of the newly synthesized VWF proteins is directly secreted into the circulation via the constitutive pathway 7. Large and ultra-large VWF multimers are stored in Weibel Palade bodies (WPBs) of endothelial cells and alpha-granules of platelets 8, 9. These storage granules release VWF multimers upon a variety of physiologic agonists, such as hypoxia 10, epinephrine, histamine, thrombin, fibrin and vasopressin 11.
Recently, the Cohorts for Heart and Aging Research in Genomic Epidemiology (CHARGE) consortium conducted a meta-analysis of genome wide association studies (GWAS) in five large population-based cohort studies to identify new genetic determinants of VWF levels 12. Besides confirmation of previously indentified candidate genes, such as the ABO blood group gene and the VWF structural gene itself, the CHARGE consortium identified and replicated novel associations with six genetic loci, among which the STXBP5 and the STX2 genes. Syntaxin 2 (STX2) is a binding substrate for syntaxin binding protein 5 (STXBP5) and is a member of the Soluble N-ethylmaleimide-sensitive factor (NSF) Attachment protein Receptor (SNARE) protein family. These proteins drive vesicle exocytosis by fusion of granules and target membranes, a process involved in the regulation of numerous secretory events 13, such as WPB exocytosis. WPBs and alpha-granules release large amounts of VWF after endothelial cell activation, for example in atherosclerosis and subsequent arterial thrombosis. Moreover, these storage granules secrete not only VWF molecules, but also other substances, including pro-inflammatory factors, such as P-selectin, eotaxin and interleukine-8. Hence, considering the involvement of STXBP5 and STX2 in secretion of VWF and other pro-thrombotic and pro-inflammatory factors, which may in turn lead to development of atherosclerosis, these candidate genes may have a direct effect on the risk of CVD as well.
We aimed to further expand previous findings of the CHARGE consortium in an independent case-control study. Our study population is unique, since it consists of specifically young individuals with a first event of arterial thrombosis. In addition, the influence of genetics is generally more pronounced in younger individuals than in older individuals who have been exposed to potential cardiovascular risk factors for a longer period of time 14. Consequently, we have investigated the effect of common genetic variations in STXBP5 and STX2, including the 3′ and 5′ UTR-regions, on VWF plasma concentration, VWF collagen binding activity, and the risk of arterial thrombosis.
Method
Patients
The ‘Genetic risk factors for Arterial Thrombosis at young age: the role of TAFI and other Coagulation factors’ (ATTAC) study is a single-center, case-control study, described in more detail previously 15, 16. Briefly, the cases (n = 463) were defined as patients with a first event of arterial thrombosis, which were consecutively recruited one to three months after the event at the departments of cardiology, neurology and vascular surgery at the Erasmus University Medical Center. Patients were eligible for inclusion when they were 18–45 years for males and 18–55 years for females at the time of diagnosis. The cases consist of three subgroups: (1) patients with coronary heart disease (CHD), defined as either an acute myocardial infarction (AMI) or unstable angina pectoris (UAP), (2) patients with either ischemic stroke (IS) or a transient ischemic attack (TIA) and (3) patients with peripheral arterial disease (PAD). The control group (n = 409) consists of neighbours or friends of the patient, fulfilling the same age criteria and without a history of cardiovascular events. For the current study we included Caucasian individuals only.
The study was approved by the medical research board at Erasmus University Medical Center and written informed consent was obtained from all participants at inclusion.
Blood sampling
Blood was drawn one to three months after the ischemic event by venipuncture in the antecubital vein using Vacutainer system (Becton-Dickinson, Plymouth, UK). Blood for coagulation measurements was collected in 3.2% trisodium citrate (9:1 vol/vol). Citrated blood was centrifuged within 1 hour at 2000 × g for 10 min at 4°C. Plasma was additionally centrifuged at 20 000× g for 10 minutes at 4°C and stored in aliquots at −80°C. For DNA isolation blood was collected in tubes containing ethylene diaminetetraacetic acid (EDTA; Beckon Dickinson) and genomic DNA was isolated according to standard salting-out procedures and stored at 4°C for genetic analysis.
Laboratory measurements
VWF antigen (VWF:Ag) was determined with an in-house ELISA with polyclonal rabbit anti-human VWF antibodies and horseradish peroxidase conjugated anti-human VWF antibodies (DakoCytomation, Glostrop, Denmark) for catching and tagging, respectively. VWF collagen binding (VWF:CB) was measured with an in-house ELISA using type I collagen (Sigma, St. Louis, USA) for catching and horseradish peroxidase conjugated anti-human VWF antibodies for tagging. The intra-assay variation coefficients of VWF:Ag and VWF:CB were 5.7% and 5.9%, respectively.
Genotyping
The STXBP5 gene spans 182 kbps and is located in the q24 region of chromosome 6. The STX2 gene spans 50 kbp and is located in the q24.3 region of chromosome 12. We obtained data from the International HapMap project (phase II November 2008 http://www.hapmap.org) on the linkage disequilibrium (LD) pattern and selected haplotype-tagging single-nucleotide polymorphisms (ht-SNPs) using Haploview software (version 3.11; www.broad.mit.edu/mpg/haploview/index/php). For both genes blocks of haplotypes with a frequency of ≥ 0.03 were defined in order to select these ht-SNPs. We took potential functionality into consideration by preferentially selecting non-synonymous ht-SNPs or SNPs that are located in known regulatory elements. In this study, we considered only SNPs that were present in a Caucasian population. We selected 6 ht-SNPs in STXBP5 and 3 ht-SNPs in STX2, which were genotyped using Custom TaqMan Genotyping Assays (Applied Biosystems, Foster City, CA, USA). The nucleotide sequences of the primers and probes used for the assay are available upon request. Endpoint fluorescence was measured on the ABI 7900HT instrument (Applied Biosystems, Foster City, CA, USA) and clustered according to genotype using SDS 2.1 software (Applied Biosystems, Foster City, CA, USA). Genotyping was successful for each SNP in on average 96% of all subjects. Baseline characteristics of missing individuals were similar to those who were genotyped successfully.
Statistical analysis
Allele frequencies were calculated by genotype counting. For each SNP the deviation from the Hardy-Weinberg equilibrium was tested in controls by means of a Chi-squared test with one degree of freedom. The data of VWF:Ag and VWF:CB levels are approximately normally distributed and presented as mean and standard deviation (SD). We used linear regression on untransformed VWF:Ag and VWF:CB measures using additive genetic models adjusted for age, sex and ABO blood group (data are shown as unstandardized beta-coefficients representing the change in VWF:Ag or VWF:CB per minor allele with a 95% confidence interval). The relative risks of arterial thrombosis for all polymorphisms were assessed by means of a logistic regression analysis using additive genetic models adjusted for age, sex and ABO blood group (data are shown as odds ratios representing the increase in risk per minor allele with a 95% confidence interval).
Statistical analyses were performed with SPSS for Windows, version 17.0 (SPSS Inc, Chicago, USA). A two-sided value of p < 0.05 was considered statistically significant.
Results
Baseline characteristics
We included 463 cases and 406 controls of Caucasian origin, of which 188 (41%) in the arterial thrombosis group and 149 (36%) in the control group were men (table 1). Mean age was 43.1 ± 6.7 years in patients and 39.3 ± 7.7 years in controls. The arterial thrombosis group consisted of 271 (59%) patients with either AMI or UAP, 148 (32%) cases with either IS or TIA and 44 (9%) cases with PAD. As expected the prevalence of known classical cardiovascular risk factors, including positive family history, high body mass index (BMI), hypertension, diabetes, statin use and current smoking, was significantly higher in patients than in controls (table 1). The distribution of ABO blood group was similar in cases and controls (P = 0.66).
Table 1.
ATTAC population baseline characteristics
| Cases (n = 463) | Controls (n = 409) | P-value | |
|---|---|---|---|
| Demographics | |||
| Sex (% men) | 188 (41%) | 149 (36%) | |
| Age (years) | 43.1 ± 6.7 | 39.3 ± 7.7 | |
| Diagnosis | |||
| UAP/ AMI | 271 (59%) | ||
| TIA/IS | 148 (32%) | ||
| Peripheral Arterial disease | 44 (9%) | ||
| Risk Factors | |||
| Positive family history (%) | 280 (61%) | 129 (32%) | <0.0001 |
| Body Mass Index (kg/m2) | 26.6 ± 4.7 | 25.5 ± 4.3 | <0.0001 |
| Hypertension (%) | 136 (29%) | 24 (6%) | <0.0001 |
| Diabetes (%) | 39 (8%) | 3 (1%) | <0.0001 |
| Statin use (%) | 372 (81%) | 5 (1%) | <0.0001 |
| Current smoker (%) | 187 (41%) | 92 (23%) | <0.0001 |
| ABO blood group | |||
| O (%) | 202 (44%) | 186 (45%) | |
| Non-O (%) | 257 (56%) | 123 (55%) | |
Data are presented as mean ± SD or percentage for categorical variables
Mean levels of VWF:Ag and VWF:CB were significantly higher in cases (mean ± SD, 1.28 ± 0.54 IU/mL and 1.40 ± 0.57 IU/mL, respectively) than in controls (1.09 ± 0.37 IU/mL; P < 0.0001 and 1.25 ± 0.42 IU/mL; P = < 0.0001, respectively). The allele frequency distributions of all polymorphisms in controls did not deviate from Hardy-Weinberg equilibrium.
Genetic variation and VWF antigen levels
In patients, two ht-SNPs of STXBP5 showed an association with VWF:Ag: rs9399599, which is located in intron 25, and rs1039084, which is a missense mutation that encodes an amino acid substitution of asparagine into serine at position 436 (table 2). Both SNPs are in high linkage disequilibrium with rs9390459, which had the highest genome wide significance level for VWF plasma levels in the meta-analysis of the CHARGE consortium (D′ = 1.00, R2 = 0.87 for rs9399599 and D′= 0.96, R2 = 0.86 for rs1039084) (phase II November 2008 http://www.hapmap.org).
Table 2.
Effect of STXBP5 and STX2 polymorphisms on VWF:Ag and VWF:CB
| Gene | rs-number | Allele | MAF* | Controls | Cases | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||
| VWF:Ag β (95% CI) | P | VWF:CB β (95% CI) | P | VWF:Ag β (95% CI) | P | VWF:CB β (95% CI) | P | ||||
| STXBP5 | rs9399599 | T>A | 0.45 | −0.05 [−0.09, −0.002] | 0.04 | −0.03 [−0.09, 0.02] | 0.22 | −0.09 [−0.15, −0.02] | 0.007 | −0.10 [−0.17, −0.03] | 0.006 |
| rs1039084 | G>A | 0.44 | −0.05 [−0.09, 0.001] | 0.06 | −0.03 [−0.08, 0.03] | 0.33 | −0.09 [−0.15, −0.03] | 0.006 | −0.10 [−0.17, −0.03] | 0.008 | |
| rs693850 | T>C | 0.17 | −0.02 [−0.08, 0.04] | 0.59 | −0.02 [−0.09, 0.05] | 0.47 | −0.08 [−0.16, −0.002] | 0.05 | −0.11 [−0.19, −0.02] | 0.02 | |
| rs497704 | G>A | 0.04 | 0.02 [−0.11, 0.15] | 0.75 | 0.02 [−0.13, 0.18] | 0.77 | −0.06 [−0.25, 0.14] | 0.58 | −0.05 [−0.27, 0.17] | 0.66 | |
| rs127173905 | C>T | 0.25 | 0.04 [−0.01, 0.10] | 0.10 | 0.02 [−0.04, 0.09] | 0.55 | 0.02 [−0.06, 0.09] | 0.68 | 0.10 [0.01, 0.18] | 0.03 | |
| rs1765028 | C>T | 0.43 | 0.04 [−0.01, 0.08] | 0.14 | 0.03 [−0.03, 0.08] | 0.34 | 0.02 [−0.04, 0.09] | 0.46 | 0.06 [−0.01, 0.13] | 0.09 | |
| STX2 | rs1236 | T>A | 0.47 | −0.01 [−0.05, 0.04] | 0.70 | −0.001 [−0.06, 0.05] | 0.98 | 0.05 [−0.02, 0.12] | 0.19 | 0.06 [−0.02, 0.14] | 0.14 |
| rs7978987 | G>A | 0.37 | −0.02 [−0.07, 0.03] | 0.42 | 0.001 [−0.06, 0.06] | 0.98 | 0.08 [0.01, 0.16] | 0.03 | 0.10 [0.02, 0.18] | 0.02 | |
| rs11061158 | G>T | 0.26 | 0.002 [−0.05, 0.05] | 0.92 | 0.03 [−0.03, 0.09] | 0.30 | 0.02 [−0.06, 0.10] | 0.66 | 0.03 [−0.06, 0.12] | 0.54 | |
Linear regression analysis with an additive genetic model, adjusted for age, sex, and blood group. P-value is the probability that the null-hypothesis, beta-coefficient is zero after adjustment, is true. Data are represented as beta-coefficients (β) per minor allele with a 95% confidence interval (CI).
MAF = minor allele frequency
In the arterial thrombosis group the minor alleles of rs9399599 and rs1039084 were associated with lower VWF:Ag levels, also after Bonferroni correction. In the subgroup of patients with CHD only, rs9399599 was borderline significantly associated with VWF concentration (β = −0.08 [−0.15, 0.002], P = 0.06). Interestingly, the strength of association for rs1039084 increased even further (β = −0.12 [−0.20, −0.04], P = 0.004). By contrast, neither of the polymorphisms remained related to VWF:Ag levels in the subgroup of patients with IS or TIA. In the control group the effect of rs9399599 and rs1039084 on VWF:Ag levels was only borderline significant.
In STX2 only one ht-SNP showed an effect on VWF plasma levels: rs7978987, which is located in intron 9 and had a P value of 3.82 × 10−11 in the meta-analysis of the CHARGE consortium GWAS (table 2). In patients, rs7978987 was associated with higher VWF:Ag levels. After Bonferroni correction rs7978987 remained borderline significantly associated with VWF:Ag levels. In controls none of the ht-SNPs of STX2 were associated with VWF plasma levels.
In the linear regression analyses we also used an age and sex adjusted model (data not shown). The effect sizes of the polymorphisms in this model were similar to those obtained using a model which was in addition adjusted for ABO blood group.
VWF collagen binding activity
VWF collagen binding activity (VWF:CB) levels are displayed in table 2 for cases and controls, separately. Interestingly, all STXBP5 polymorphisms that were associated with VWF:Ag showed similar associations with lower VWF:CB levels in patients. None of these SNPs were associated with the VWF:CB/VWF:Ag ratio (data not shown). By contrast, rs7978987 of STX2 was associated with higher VWF:CB levels and VWF:CB/VWF:Ag ratio (β = 0.18 [0.01, 0.36], P = 0.03). In controls VWF:CB is not influenced by genetic variability in STXBP5 and STX2.
Risk of arterial thrombosis
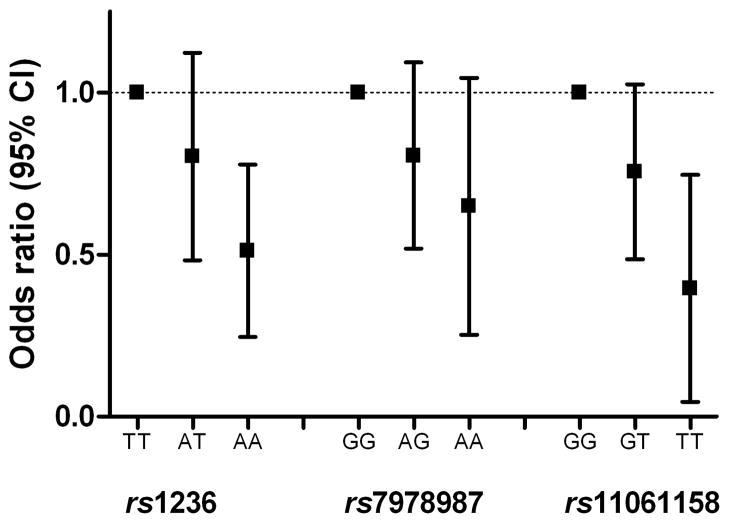
In the total population, rs1236 and rs11061158 of STX2 had a strong and significant relationship with the risk of arterial thrombosis, independent of ABO blood group and after correction for multiple testing (table 3). The minor alleles of rs1236 and rs11061158 had a protective effect on the occurrence of arterial thrombosis (figure 1). Interestingly, in the subgroup analysis of patients with CHD the minor alleles of the three polymorphisms that cover the entire genetic variation of STX2 were associated with a decreased risk of CHD. In the subgroup of patients with ischemic stroke or TIA this effect was less apparent. Genetic variants in the STXBP5 gene were not associated with the risk of arterial thrombosis.
Table 3.
Association between STXBP5 and STX2 polymorphisms and the risk of arterial thrombosis
| Gene | rs-number | Allele | Total Group (OR [95% CI]) | CHD subgroup (OR [95% CI]) | IS/TIA subgroup (OR [95% CI]) |
|---|---|---|---|---|---|
| STXBP5 | rs9399599 | T>A | 1.11 [0.91, 1.34] | 1.12 [0.88, 1.42] | 1.04 [0.79, 1.37] |
| rs1039084 | G>A | 0.99 [0.81, 1.21] | 1.02 [0.80, 1.29] | 0.87 [0.66, 1.14] | |
| rs693850 | T>C | 1.02 [0.79, 1.31] | 0.93 [0.68, 1.26] | 1.04 [0.74, 1.45] | |
| rs497704 | G>A | 0.88 [0.49, 1.56] | 0.82 [0.40, 1.66] | 0.89 [0.41, 1.96] | |
| rs127173905 | C>T | 0.99 [0.79, 1.25] | 0.96 [0.73, 1.26] | 1.13 [0.84, 1.54] | |
| rs1765028 | C>T | 1.02 [0.84, 1.25] | 1.08 [0.85, 1.37] | 1.06 [0.81, 1.40] | |
| STX2 | rs1236 | T>A | 0.72 [0.59, 0.89] | 0.64 [0.49, 0.82] | 0.76 [0.57, 1.00] |
| rs7978987 | G>A | 0.81 [0.65, 1.00] | 0.70 [0.54, 0.91] | 0.81 [0.60, 1.10] | |
| rs11061158 | G>T | 0.69 [0.55, 0.88] | 0.67 [0.50, 0.89] | 0.72 [0.52, 0.99] |
Logistic regression analysis with an additive genetic model adjusted for age, sex, and blood group. Data are represented as the increase in odds ratio per minor allele with a 95% confidence interval. OR = odds ratio. CI = confidence interval. CHD = coronary heart disease. IS/TIA = ischemic stroke or transient ischemic attack.
Figure 1.
STX2 genotypes and the risk of arterial thrombosis. Odds ratios per genotype of STX2 polymorphisms. Homozygous carriers of the common allele are used as reference category.
Discussion
In this manuscript, we show that genetic variations in STXBP5 and STX2 affect both VWF concentration and VWF collagen binding activity in a population of young individuals with a first event of arterial thrombosis. Whereas the minor alleles of rs9399599 and rs1039084 in STXBP5 were associated with lower VWF:Ag and VWF:CB levels, the minor allele of rs7978987 in STX2 was associated with higher VWF:Ag and VWF:CB levels. These findings are a further exploration of the results of the meta-analysis of the CHARGE consortium. Interestingly, the effects of the minor alleles on VWF concentration are similar to those demonstrated by the CHARGE consortium (β-coefficient −0.048 for rs9390459 and 0.033 for rs7978987 per dosage allele). As well as confirming the association with VWF plasma levels, we also found an association between these genetic variants and VWF activity. In addition, rs7978987 in STX2 was associated with a higher VWF:CB/VWF:Ag ratio, which indicates that the secreted VWF molecules are functionally more active. Since ultra large VWF multimers, which have the most haemostatic potential, are stored in WPB and alpha-granules, this finding came up our expectations.
It is noteworthy that the relationship between genetic variability in STXBP5 and STX2 and VWF plasma levels was seen especially in patients with arterial thrombosis and that this relationship was less clear in healthy controls. In healthy individuals, VWF plasma levels are determined mainly by the activity of the constitutive pathway, because the endothelium is not triggered to release the VWF molecules. Since STX2 and STXBP5 encode proteins that may be involved in the regulated secretion pathway of VWF molecules, which is only stimulated after endothelial cell activation, one would expect to find an effect of these polymorphisms not in healthy subjects, but particularly in patients who have CVD. In addition, it is known that at a higher age more atherosclerosis is present, which brings the endothelium into a mild state of activation and leads to chronic low-level stimulation of the regulatory pathway of VWF release. This may explain why a relationship between VWF:Ag levels and genetic polymorphisms in STXBP and STX2 was found in the older (mean age 60.0 years), but relatively healthy subjects of the CHARGE cohorts.
In the CHD subgroup the associations between polymorphisms and VWF levels seem more pronounced than in the IS/TIA subgroup. As ischemic stroke is a heterogeneous phenotype, caused by multiple and sometimes unknown underlying factors, the contribution of genetic variation is hard to study. While heterogeneity might be reduced by analysis by etiologic subtype, such analysis was not possible in our ATTAC study because of the limited number of patients in the subgroup of stroke17.
Unexpectedly, the minor alleles of the polymorphisms in STX2 were strongly associated with a decreased risk of arterial thrombosis. This effect seemed strongest in the CHD subgroup, where the minor alleles of all three SNPs gave a protective effect. Yet, the precise mechanisms by which these polymorphisms influence the risk of arterial thrombosis is unclear, because the minor alleles of the polymorphisms in STX2 tended to be associated with higher VWF plasma levels. As well as VWF molecules, WPBs and alpha-granules contain numerous other substances, such as P-selectin, angiopoeitin-2, osteoprotegerin, and eotoxin 313. It has also been hypothesized that not all components of the storage granules have to be present at all times per se and can even be segregated into different subgroups of substances 18. We therefore propose that the risk of CVD is reduced not by VWF itself but by other substances that are secreted by the Weibel Palade Bodies.
In conclusion, this study shows that genetic variations in STXBP5 and STX2 affect VWF antigen plasma levels and VWF collagen binding activity in young patients with premature arterial thrombosis. It remains unclear whether altered VWF levels are caused by dysfunction of the VWF secretion pathway or by another, unknown, mechanism. We also observed that genetic variability in STX2 is associated with the risk of arterial thrombosis in young individuals. Future research is required to study the functionality of the polymorphisms in more detail and to improve our understanding of the possible importance of the secretion pathway of VWF in the pathogenesis of arterial thrombosis.
Acknowledgments
The authors thank Marjolein Dieterich of the Department of Haematology of the Erasmus MC for her excellent technical assistance.
Funding sources
This work was supported by a grant of the Netherlands Heart Foundation (2007B159) and the Thrombosis Foundation Holland (2010-3).
Footnotes
Disclosures
We have none to declare.
References
- 1.Lip GY, Blann A. von Willebrand factor: a marker of endothelial dysfunction in vascular disorders? Cardiovascular research. 1997;34:255–265. doi: 10.1016/s0008-6363(97)00039-4. [DOI] [PubMed] [Google Scholar]
- 2.Martinelli I. von Willebrand factor and factor VIII as risk factors for arterial and venous thrombosis. Seminars in hematology. 2005;42:49–55. doi: 10.1053/j.seminhematol.2004.09.009. [DOI] [PubMed] [Google Scholar]
- 3.Bladbjerg EM, de Maat MP, Christensen K, Bathum L, Jespersen J, Hjelmborg J. Genetic influence on thrombotic risk markers in the elderly--a Danish twin study. J Thromb Haemost. 2006;4:599–607. doi: 10.1111/j.1538-7836.2005.01778.x. [DOI] [PubMed] [Google Scholar]
- 4.de Lange M, Snieder H, Ariens RA, Spector TD, Grant PJ. The genetics of haemostasis: a twin study. Lancet. 2001;357:101–105. doi: 10.1016/S0140-6736(00)03541-8. [DOI] [PubMed] [Google Scholar]
- 5.Ruggeri ZM, Ware J. von Willebrand factor. Faseb J. 1993;7:308–316. doi: 10.1096/fasebj.7.2.8440408. [DOI] [PubMed] [Google Scholar]
- 6.Ruggeri ZM. Old concepts and new developments in the study of platelet aggregation. J Clin Invest. 2000;105:699–701. doi: 10.1172/JCI9604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sporn LA, Marder VJ, Wagner DD. Inducible secretion of large, biologically potent von Willebrand factor multimers. Cell. 1986;46:185–190. doi: 10.1016/0092-8674(86)90735-x. [DOI] [PubMed] [Google Scholar]
- 8.Wagner DD. Cell biology of von Willebrand factor. Annu Rev Cell Biol. 1990;6:217–246. doi: 10.1146/annurev.cb.06.110190.001245. [DOI] [PubMed] [Google Scholar]
- 9.Burgess TL, Kelly RB. Constitutive and regulated secretion of proteins. Annu Rev Cell Biol. 1987;3:243–293. doi: 10.1146/annurev.cb.03.110187.001331. [DOI] [PubMed] [Google Scholar]
- 10.Pinsky DJ, Naka Y, Liao H, Oz MC, Wagner DD, Mayadas TN, Johnson RC, Hynes RO, Heath M, Lawson CA, Stern DM. Hypoxia-induced exocytosis of endothelial cell Weibel-Palade bodies. A mechanism for rapid neutrophil recruitment after cardiac preservation. J Clin Invest. 1996;97:493–500. doi: 10.1172/JCI118440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Spiel AO, Gilbert JC, Jilma B. von Willebrand factor in cardiovascular disease: focus on acute coronary syndromes. Circulation. 2008;117:1449–1459. doi: 10.1161/CIRCULATIONAHA.107.722827. [DOI] [PubMed] [Google Scholar]
- 12.Smith NL, Chen MH, Dehghan A, Strachan DP, Basu S, Soranzo N, Hayward C, Rudan I, Sabater-Lleal M, Bis JC, de Maat MP, Rumley A, Kong X, Yang Q, Williams FM, Vitart V, Campbell H, Malarstig A, Wiggins KL, Van Duijn CM, McArdle WL, Pankow JS, Johnson AD, Silveira A, McKnight B, Uitterlinden AG, Aleksic N, Meigs JB, Peters A, Koenig W, Cushman M, Kathiresan S, Rotter JI, Bovill EG, Hofman A, Boerwinkle E, Tofler GH, Peden JF, Psaty BM, Leebeek F, Folsom AR, Larson MG, Spector TD, Wright AF, Wilson JF, Hamsten A, Lumley T, Witteman JC, Tang W, O’Donnell CJ. Novel associations of multiple genetic loci with plasma levels of factor VII, factor VIII, and von Willebrand factor: The CHARGE (Cohorts for Heart and Aging Research in Genome Epidemiology) Consortium. Circulation. 2010;121:1382–1392. doi: 10.1161/CIRCULATIONAHA.109.869156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lowenstein CJ, Morrell CN, Yamakuchi M. Regulation of Weibel-Palade body exocytosis. Trends Cardiovasc Med. 2005;15:302–308. doi: 10.1016/j.tcm.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 14.Drenos F, Miller GJ, Humphries SE. Increase of plasma fibrinogen levels and variability with age in a sample of middle aged healthy men. Ann Hum Genet. 2007;71:43–53. doi: 10.1111/j.1469-1809.2006.00302.x. [DOI] [PubMed] [Google Scholar]
- 15.de Bruijne EL, Gils A, Guimaraes AH, Dippel DW, Deckers JW, van den Meiracker AH, Poldermans D, Rijken DC, Declerck PJ, de Maat MP, Leebeek FW. The role of thrombin activatable fibrinolysis inhibitor in arterial thrombosis at a young age: the ATTAC study. J Thromb Haemost. 2009;7:919–927. doi: 10.1111/j.1538-7836.2009.03350.x. [DOI] [PubMed] [Google Scholar]
- 16.Bongers TN, de Bruijne EL, Dippel DW, de Jong AJ, Deckers JW, Poldermans D, de Maat MP, Leebeek FW. Lower levels of ADAMTS13 are associated with cardiovascular disease in young patients. Atherosclerosis. 2009;207:250–254. doi: 10.1016/j.atherosclerosis.2009.04.013. [DOI] [PubMed] [Google Scholar]
- 17.Adams HP, Jr, Bendixen BH, Kappelle LJ, Biller J, Love BB, Gordon DL, Marsh EE., 3rd Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke. 1993;24:35–41. doi: 10.1161/01.str.24.1.35. [DOI] [PubMed] [Google Scholar]
- 18.Goligorsky MS, Patschan D, Kuo MC. Weibel-Palade bodies--sentinels of acute stress. Nat Rev Nephrol. 2009;5:423–426. doi: 10.1038/nrneph.2009.87. [DOI] [PubMed] [Google Scholar]