Abstract
The functional avidity is determined by exposing T-cell populations in vitro to different amounts of cognate antigen. T-cells with high functional avidity respond to low antigen doses. This in vitro measure is thought to correlate well with the in vivo effector capacity of T-cells. We here present the multifaceted factors determining and influencing the functional avidity of T-cells. We outline how changes in the functional avidity can occur over the course of an infection. This process, known as avidity maturation, can occur despite the fact that T-cells express a fixed TCR. Furthermore, examples are provided illustrating the importance of generating T-cell populations that exhibit a high functional avidity when responding to an infection or tumors. Furthermore, we discuss whether criteria based on which we evaluate an effective T-cell response to acute infections can also be applied to chronic infections such as HIV. Finally, we also focus on observations that high-avidity T-cells show higher signs of exhaustion and facilitate the emergence of virus escape variants. The review summarizes our current understanding of how this may occur as well as how T-cells of different functional avidity contribute to antiviral and anti-tumor immunity. Enhancing our knowledge in this field is relevant for tumor immunotherapy and vaccines design.
1. Introduction
CD8 T cells play a critical role in antiviral immunity, and a large number of studies in both human and mice indicate that antigen-specific CD8 T cells are directly involved in not only the control of viral replication, but also tumor growth [1–25]. Especially CD8 T-cell immunity against HIV replication, and thus the prevention of the disease progression, is well documented. This is primarily based on the following observations: (a) depleting CD8 T cells in the macaque model of AIDS leads to a loss of control of virus replication [4, 5, 26], (b) HIV-specific T-cell responses can be detected in previously virus exposed but presently uninfected individuals [27–30], and (c) a higher numbers of polyfunctional T cells are found in individuals with nonprogressive infection or in the so-called “elite controllers” [9–11], although there is a long-term debate as to whether this is a cause or a consequence of viral control [31]. Polyfunctional T cells characteristically show high IL-2 expression and strong ability to upregulate granzyme B and perforin. They have a high proliferative capacity and are superior in controlling HIV infection. Furthermore, it has been demonstrated by the whole genome analyses that HLA class I alleles are the genetic factors most strongly associated with nonprogressive infection [8, 15–22, 32, 33].
The aforementioned polyfunctionality is a well-established important indicator for the ability of T cells to control a virus infection. However, this parameter does not reflect the ability of how a T-cell or a population of T cells responds to a specific antigen. Instead, the polyfunctionality is usually assessed upon exposing T cells to peptide-MHC [pMHC] ligands at close to saturating concentration. In this situation, it can be that T cells show similar cytokine response patterns, although T cells might respond significantly different upon exposure to limited or physiologically relevant amounts of a ligand in vivo and in vitro.
In contrast, the functional avidity is a biological measure that describes how well a T-cell responds in vitro to a given concentration of a ligand. By definition, T cells with high functional avidity respond in in vitro tests to very low antigen doses, while T cells of lower functional avidity require higher amounts of antigen before they mount an immune response similar to that of high-avidity T cells. The functional avidity can be considered as a quantitative determinant of the activation threshold of a T-cell clone. In vivo, T cells of high and low avidity are exposed to similar antigen doses, but numerous correlations exist between the functional avidity and the effectiveness of an antiviral immune response, some of which will be discussed later in this paper. Of note, ex vivo studies have shown that distinct T-cell functions (e.g., proliferation, cytokines production, etc.) are triggered with different thresholds [34–37].
The purpose of this paper is to provide information on what distinguishes the functional avidity from other parameters used to describe the ability of T cells to recognize antigen and to summarize the known factors that determine the functional avidity of T cells as well as the functional avidity maturation of a T-cell population. The latter refers to increases of the overall functional avidity with which a polyclonal population responds to antigen. Moreover, we suggest that combining functional avidity assessment and polyfunctional analysis might lead to better predictions concerning the ability of a T-cell population to control a chronic infection than when both tests are independently performed. Finally, we will critically discuss the general consensus that high-avidity CD8 T-cell responses are always better in controlling virus infections by presenting evidence that this might not be the case in chronic infections, particularly during HIV infection.
2. Factors Impacting the Functional Avidity of a T-Cell Clone
The functional avidity inversely correlates with the antigen dose that is needed to trigger a T-cell response. It is determined by ex vivo quantification of biological functions such as IFN-γ production, cytotoxic activity (ability to lyse target cells), or proliferation. The concentration needed to induce a half-maximum response (EC50) is often used to describe the functional avidity of T cells. In particular, it can be used to describe how monoclonal but also antigen-specific polyclonal T-cell populations respond to antigen stimulation.
The functional avidity of a T-cell clone (Figure 1) is primarily impacted by (a) the affinity of the TCR for the pMHC-complex, that is, the strength of the interaction between the TCR and pMHC [38, 39], (b) expression levels of the TCR and the CD4 or CD8 coreceptors, and (c) the distribution and composition of signaling molecules [40, 41] as well as expression levels of molecules that attenuate T-cell function and TCR signaling.
Figure 1.
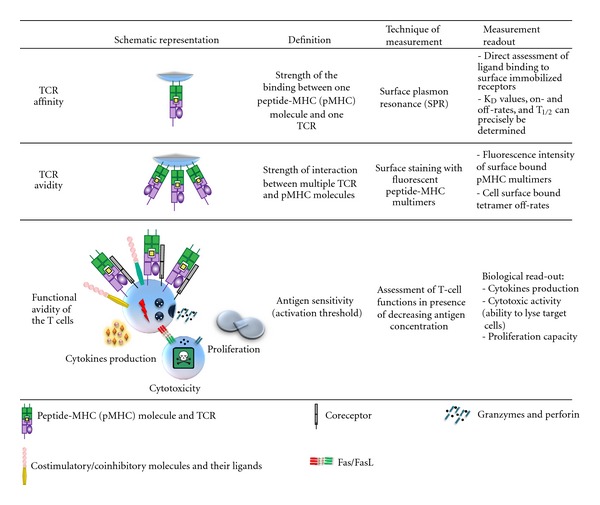
Schematic representation, definition, technique of measurement and readout of TCR affinity and functional avidity.
2.1. Affinity, Avidity, and Functional Avidity of a T-Cell Clone
The terms affinity, avidity, and functional avidity are often incorrectly interchangeably used. The TCR affinity (Figure 1) refers to the physical strength of the monomeric interaction between the TCR and a pMHC-complex [42, 43]. The dissociation constant (KD) for different pMHC-TCR pairs have been determined by surface plasmon resonance [44]. Several reports indicate that a lower KD and thus a stronger interaction lead to a better T-cell response [45, 46]. Another parameter that has been shown to influence the T-cell response efficacy is the half-life (t 1/2) value of the interaction between the TCR and the pMHC-complex; longer t 1/2 times also result in more potent T-cell stimulation [45, 47, 48]. It still remains controversial which of these two parameters, the KD or the t 1/2 value, offers a better prediction of how T cells respond to antigen stimulation [48]. Low KD values result from slow off-rates and/or rapid on-rates. Thus, pMHC-TCR interactions, which have a long t 1/2 time, usually also show a low KD value. This relationship may in parts explain why both KD and the t 1/2 time have been reported to correlate with the efficiency of T-cell activation. However, both low KD and long t 1/2 values are thought to permit completion of intracellular signaling cascades leading to T-cell activation [42, 49].
However, a clean biochemical determination of KD values and t 1/2 times is rather complicated, and it requires the availability of soluble pMHC-complexes and a soluble form of the TCR. Moreover, it needs to be considered that the binding kinetics can significantly vary depending on whether the interaction is measured with soluble or membrane-bound ligands [50]. Alternatively, a more practical but less precise way to assess the strength of pMHC-TCR interaction is to stain living T cells with pMHC-multimers. Binding kinetics can then be determined by measuring fluorescent intensity of cell-surface bound multimers [51–53]. As the latter involves binding of a ligand via multiple interactions (i.e., the pMHC-multimers bind to more than one TCR), such measurements are best described by the term avidity, which is normally used to refer to the strength of multimeric receptor-ligand engagement (Figure 1).
In contrast to the physical parameters affinity and avidity, the functional avidity describes how well a T-cell responds to antigens. Though all of the three parameters correlate in most cases, that is, high-affinity T cells often have a high functional avidity, this does not need to be the case. There are several factors besides the antigen recognition ability of the TCR that can impact the T-cell response. In principle, T cells could express a high-affinity TCR, but due to other factors, for example, inhibitory molecules, it might show a very weak response to antigen stimulation. Thus, determining the functional avidity is not only often more practical, but is also the only one out of the three parameters that actually describes the functional outcome of the stimulation.
Notably, the level of TCR expression impacts the functional avidity. Even though T cells are functional when they express very little TCR, it has been shown that reduced expression levels go along with decreased ability to respond to antigen [54]. T cells with reduced TCR expression levels are impaired in their proliferative capacity and in their ability to secrete IL-2 and IFN-γ [55].
2.2. Coreceptor Expression Impacts the Functional Avidity of a T-Cell Clone
CD4 and CD8 coreceptors bind to the MHC classes II and I, respectively [56], and stabilize the pMHC-TCR interaction. This is for instance illustrated by the fact that pMHC-multimers bearing a mutation in the CD8 binding site bind less efficiently to T cells [57], and this is particularly prominent when a TCR binds with low-affinity to a pMHC-complex [58–60]. The importance of the coreceptor engagement for pMHC-binding to the TCR is also underlined by observations that antibodies against the CD8 coreceptor can decrease or block the extent to which pMHC-multimers bind to a TCR. There are even antibody clones enhancing the binding [61]. This might occur by inducing a conformation that facilitates better binding of the coreceptor to the MHC. This antibody enhancement or blockade is even more critical when a TCR engages a low-affinity ligand [61]. Moreover, coreceptor engagement supports TCR signal transduction by bringing Lck in close proximity to the TCR complex [62, 63]. Reducing this coreceptor engagement of Lck lowers the TCR sensitivity to antigen stimulation and thus decreases induction of effector function [39, 64]. However, the enhancement of the coreceptor MHC binding is more critical for low than high-affinity T-cell clones. Thus, high-affinity pMHC-TCR interactions are in contrast to low-affinity binding characterized by a relative CD8-independence for both T-cell proliferation and cytotoxicity as well as for multimer binding [57, 58, 60].
The aforementioned observations indicate how critically the presence or absence of the coreceptor impacts the ability of T cells to respond to a pMHC-complex. In addition, several examples indicate the extent to which variations in coreceptor expression levels or binding ability to MHC molecules impact T-cell function. In mice, downregulation of CD8 expression and reduced ability of T cells to respond to antigen have been seen following Listeria monocytogenes or Vaccinia virus infection [65]. Moreover, there is a polymorphism in the α3 domain of the HLA-A*68 molecule resulting in weak binding of the CD8 coreceptor to the MHC. When the HLA-A*68 sequence is artificially altered to restore CD8 binding, then this altered molecule is recognized by TCRs that fail to respond to normal HLA-A*68. Thus, restoring coreceptor engagement rescues cytokine production and T-cell proliferation, even though the pMHC-TCR interaction itself remains unchanged [66, 67]. Moreover, self-antigen-specific T cells can downregulate CD8 expression to reduce their functional avidity and thereby their autoreactive potential [68].
2.3. Alterations in TCR Signaling Can Impact the Functional Avidity
TCR signaling is initiated when Lck (Src family tyrosine kinase) phosphorylates immunoreceptor tyrosine-based activation motifs (ITAM) within the CD3ζ molecule. This provides a docking site for ZAP-70, which in turn gets phosphorylated by Lck [69]. Activated ZAP-70 leads to the recruitment and phosphorylation of linker for activation of T-cell (LAT) and SH2-domain-containing leukocyte protein of 76 kDa (SLP-76). This initiates a signaling cascade that leads to Ca2+ mobilization as well as to the activation of the mitogen-activated protein kinase (MAPK) signaling pathway [70, 71].
Different lines of evidence indicate that T cells can adjust or tune the sensitivity of their signaling apparatus indicating that functional avidity is not fixed [72]. In the thymus, TCR-signaling sensitivity is thought to be augmented by a micro RNA (miR181a), and this occurs via targeting multiple phosphatases that otherwise inhibit the TCR signal [73, 74]. This goes along with observations that T cells respond to ligands in the thymus to which they much less effectively respond in the periphery [75, 76]. Furthermore, TCR signal transduction is thought to be fine tuned by inhibitory molecules such as CD5 [77]. CD5 expression levels presumably correlate with the strength with which a T-cell clone responded to its positive selecting ligand [77]. CD5 is an immune-tyrosine-based inhibition motif-bearing receptor that could antagonize overt TCR activation in peripheral T cells [78] and has been shown to be involved in peripheral tolerance by adjusting T-cell reactivity [79]. Along those lines, higher antigen sensitivity is determined by the superior ability of high-avidity T cells to achieve threshold levels of CD3ζ phosphorylation through increasing the amount of activated Lck [80, 81].
If and how these and other pathways impact the functional avidity of T cells need to be better determined. However, the ability of peripheral T cells to alter their antigen reactivity has been reported. Deprivation from MHC molecules has been shown to increase CD4 T-cell reactivity [82], but also the opposite effect has been observed [83].
3. Factors Impacting the Functional Avidity at the Population Level
The overall functional avidity of a heterogeneous oligoclonal T-cell population that forms during an infection [84–88] is primarily impacted by the ratio of recruited clones with high versus low functional avidity. Thus, the stimulatory potency and the range of functional avidity that antigen-presenting cells [APC] recruit during an immune response strongly impact the avidity of the emerging T-cell population. Whether or not an APC is able to recruit not only T cells with high but also low functional avidity is critically impacted by the net level of costimulatory and inhibitory molecules, but also by the magnitude of antigen presentation.
3.1. Impact of Costimulatory and Inhibitory Molecules
The interaction between T cells and DC involves several molecular contacts between not only costimulatory but also inhibitory molecules. Expression levels of these molecules can modulate the T-cell activation threshold which in turn impacts the functional avidity of the emerging T-cell population; that is, if the threshold is for a particular reason very high, then only T-cell clones which have a rather high functional avidity will be activated and the overall functional avidity of the merging population will be high.
An example for the modulation of T-cell activation thresholds is the CD70/CD27 mediated costimulation which enhances the response to low-affinity ligands [89]. Moreover, it has been shown that APCs which express higher levels of B7.1, but also ICAM and LFA3, induce T cells with higher functional avidity. Those were shown to proliferate more vigorously, produce more cytokines, and kill target cells more efficiently in both primary and secondary T-cell responses [90, 91]. Similar observations were made using a combination of B7 costimulations and α-CTLA-4 anti-body-mediated blockade [92].
On the other hand, APCs can express molecules that induce negative signals in CD8 T cells such as PD-L1. The expression of such inhibitory receptors is mostly driven by persisting antigen stimulation in chronic infections or tumors [93, 94].
3.2. Antigen Doses and Antigen Presentation
Several in vitro and in vivo studies indicate that antigen exposure influences the antigen sensitivity of the emerging T-cell population [95–97]. It was shown that CD8 T cells expanded by low doses of peptide successfully lyse target cells expressing less antigen and mediated increased viral clearance than CD8 T cells stimulated with high peptide doses [97]. DC presenting different densities of pMHC-complexes had distinct influence on the functional avidity of responding CD8 T cells in immunized mice. In particular, low antigen doses were associated with high avidity and higher capacity of recall responses to recognize melanoma cells [96]. Thus, the ligand density that is presented during an infection and during T-cell priming can impact what types of T cells emerge [40], which seems to be particularly important in the context of vaccination [97].
The amount of pMHC-complexes presented by an APC critically depends on the stability of the individual pMHC-complex. Surface pMHC-turnover rates also impact which types of T-cell clones become activated [98, 99]. Interestingly, DCs seem to be able to present pMHC-complexes much longer than other cells, which likely supports their nonredundant role in initiating T-cell responses [100].
More recently, the impact of the peptide dose on CD8 T-cell avidity has been investigated in melanoma patients vaccinated with different doses of Melan-A/MART-1 peptide. Melan-A-specific CD8 T cells from patients vaccinated with low peptide doses had functionally high-avidity T cells with low CD8 dependency. In particular, they showed enhanced degranulation and cytotoxic activities and lower levels of CD8 expression [101]. These observations facilitated the development of new immunotherapy approaches against cancer and chronic infections.
4. Functional Avidity Maturation
The functional avidity of a T-cell population often increases during the course of an immune response and following pathogen reexposure [102]. Along with that, enhancement in pMHC-multimer binding has been reported [103]. Two principle mechanisms have been shown to contribute to the avidity maturation phenomenon. Clonal remodeling in the population of antigen-specific T cells occurs massively in primary infections [104], recall responses [105, 106], and for instance during persisting infection like CMV in humans [107]. During this remodeling, the progeny of T-cell clones with high functional avidity become more prevalent. In primary infections, the differences in expansion length between T-cell clones with high or low functional avidity account for this phenomenon [104]. However, the mechanisms driving clonal remodeling in secondary infections are less clear, but it is likely caused by antigen competition between high and low-affinity T cells clones [108] and possibly by alterations in the T-cell stimulation threshold.
Moreover, several lines of evidence suggest that even T cells expressing the same TCR can differ in their functional avidity and that the latter depends on the state of differentiation of T cells. For instance, it has been shown that during an LCMV infection, the functional avidity of TCR transgenic T cells increases [81]. Moreover, it has been observed that memory T cells can exhibit a higher functional avidity than that of naïve T cells [109, 110]. Several mechanisms have been proposed to contribute to the maturation of the functional T-cell avidity at the clonal T-cell level. Those include (1) the formation of clusters that comprise several TCRs and other molecules able to reinforce the immunological synapses and changes in the cholesterol content of the membrane contribute to such differences [111–113] and (2) the optimization of the signal transduction machinery, for example, by increasing the amount and the basal phosphorylation levels of signaling molecules [81, 114]. Moreover, it has been shown that the expression of Lck correlates with the production of IFN-γ, whereby minor increases in Lck expression lead to major increases in IFN-γ production [57]. In contrast, it has also been reported that the functional avidity can decrease [65] or remain similar (as seen for OT-1 TCR transgenic T cells [104]) during an infection, and whether or not memory T cells are truly more sensitive than naïve T cells remains controversial.
Overall, functional avidity maturation allows faster virus clearance/control at the time of antigen reencounter and a progressive acquisition of coreceptor binding and costimulatory signal independency [81, 115, 116]. In the context of peripheral tolerance, however, the continuous exposure to antigen in the context of molecular mimicry might lead to affinity maturation which in turn may result in autoimmunity [72]. Besides this consideration, it has also been shown that autoreactive T cells undergo limited avidity maturation [117].
In contrast to these processes that follow acute infection, the dynamics in the T-cell population in chronic infections appear to be different. We recently demonstrated that HIV-specific CD8 T cells undergo a massive TCR renewal for instance following a virus rebound [84]. Interestingly, it has been observed that changes in TRBV populations overtime go along with a loss of low-avidity T cells clones or more generally speaking an increase in functional avidity [118]. These observations will be discussed in more details in the final sections of this paper.
5. The Functional Avidity of T Cells as a Correlate of Immune Protection
There is a general consensus that higher functional avidity CD8 T-cell responses are of higher efficacy to eliminate cancer cells and to clear acute virus infections, a notion that is supported by a large number of reports [103, 106, 119–121].
It was for instance shown that high functional avidity Tax-specific CD8 T-cell lines—which use a very diverse TCR repertoire—are superior in their ability to eliminate HTLV-1-infected cells than low-avidity Tax-specific CD8 T cells. These cells were also able to recognize a latent Tax level (detectable only by RT-PCR) produced by adult T-cell leukemia cells (ATLs), thus possibly leading to the prevention of HTLV-1 infection [122].
For tumors, high functional avidity T cells mediate better T-cell responses [119], though it needs to be said that tolerance-enforcing mechanisms effectively remove high-avidity self- and tumor-antigen reactive T cells [123, 124]. Thus, tumor-reactive T cells will in most cases have a lower functional avidity than what can be observed during acute infections.
Low antigen expression and absence of inflammatory and costimulatory signals may be partially responsible for the low immunogenicity of many tumor cells. The presence of higher-avidity CD8 T cells may be particularly relevant to overcome the tolerance to tumor antigens. This may be achieved by defining a combination of adjuvants and by regulating antigen doses in vaccines [97, 125, 126]. Indeed, higher-avidity T cells are preferentially triggered leading to a more rapid and effective target-cell elimination [14, 26, 97, 119, 127–129]. Consistently, in both humans and mice models, induction of higher-avidity CD8 T-cell responses promoted more efficient tumor rejection [101, 130] and earlier target cell lysis in the context of viral infection, reducing viral burden more effectively than low-avidity CD8 T cells. In addition, this activity does not depend on the frequency of antigen-specific CD8 T cells [131].
There are also several studies correlating the presence of certain MHC alleles with differences in the diversity and functional avidity of T-cell clones that emerge during tumors or chronic infections. For example, H-2Kbm8 mice express an H-2K allele that differs in four amino acids from H-2Kb. Compared to C57BL/6 mice, the H-2Kbm8 mice generate a different repertoire of high-avidity herpes simplex virus- (HSV-) specific T cells which correlates with a higher resistance to HSV infection [14].
Similarly, C57BL/6 mice are less susceptible to respiratory syncytial virus (RSV) infection than BALB/c mice. Among the many possible explanations, it has been observed that the H-2d alleles induce the generation of immunodominant antigen-specific CD8 T-cell populations that use a more restricted TCR repertoire, and that is less efficient in lysing target cells than what can be observed in C57BL/6 mice. This results in continuous T-cell stimulation and thus in cytokine-mediated immunopathology [132].
Furthermore, the strongest genetic association of the ability to effectively control HIV infections points at the MHC locus [32] and at the presence of certain MHC molecules such as HLA-B*57 [133]. In line with the earlier mentioned, the latter is thought to strongly impact the repertoire and the quality of HIV-specific T cells and thus enabling an enhanced virus control.
In contrast to these observations, the relevance of high- and low-avidity T cells in chronic virus infections and established tumors [86, 134, 135] remains to be determined, in particular, since some studies have challenged the superiority of high-avidity CD8 T cells [136, 137]. Indeed, low-avidity T cells (1) might better distinguish between tumors overexpressing self-antigens and healthy self-tissue [136] and (2) might during chronic viral infections and tumors be less sensitive to activation-induced cell death (AICD) [129, 138], senescence and exhaustion, leading to protracted survival of functionally-competent T cells, and (3) are less likely to induce viral or tumor escape [26, 134, 139].
In the context of chronic-controlled infections, such as CMV and EBV, the T-cell responses to immunodominant antigens have a more diverse repertoire with higher TCR avidity than that of subdominant clonotypes. However, they are also more prone to senescence [140]. In addition, in vitro stimulation at high antigen concentrations induces higher AICD [138] and more pronounced inhibition of proliferation in high-avidity than in low-avidity T cells [129]. Finally, we [36] and others [141] have shown that high-avidity CD8 T cells express higher levels of the T-cell exhaustion marker PD-1 than those of low-avidity CD8 T cells.
In addition, in the context of tumor immunity, skin depigmentation is considered as a good prognosis indicator in melanoma patients, since it is a sign of immune activation against tumor/self-antigens, and a high frequency of CD8 T cells was observed in depigmentated tissue from patients [137]. These were MC1R HLA-A2-specific, and despite harboring low functional avidity, they were cytolytic and produced IFN-γ and granzyme B [137]. Interestingly, immunization of mice tolerant to the hemagglutinin (HA) antigen and bearing a renal carcinoma overexpressing HA led to the expansion of low-avidity HA-specific CD8 T cells. These could only target tumor cells expressing high antigen doses, thus allowing the destruction of HA-over-expressing tumors but not healthy pancreatic cells [136].
However, it is worth mentioning that depending on the biology of the pathogen, T cells endowed with different functions and tropism are required, and, thus, a generalization of the features of a universally efficient T-cell response is complicated or may be not possible.
6. Functional Avidity as a Correlate of Control in HIV Infection
With regard to HIV infection, contrasting conclusions on the relationship between functional avidity and virus control have been reported [142–146]. Some studies indicated that protective HIV-specific CD8 T-cell responses (e.g., those observed in HIV-infected patients with nonprogressive infection) were of high functional avidity and mediated superior variants recognition [86, 135, 143, 147, 148]. In these studies, high-avidity CD8 T cells were not only mostly polyfunctional, endowed with potent virus suppressive activity, increased cross-reactivity, and associated with low levels of virus replication [86, 135, 147], but were also characterized by an increased T-cell turnover and senescence [86].
However, most of these studies focused on HIV-specific CD8 T-cell responses directed against only one epitope, and analyses were performed on single clones derived from T-cell expansion which may not reflect the in vivo/ex vivo profile of T cells [38, 39]. In addition, since the majority of studies reporting correlations between functional avidity and virus control are cross-sectional and not prospective studies in unselected populations, it is not possible to determine causality between avidity and virus control.
More recently, it was shown that gag-specific and HLA-B-restricted CD8 T-cell responses, usually associated with virus control [16, 149], have higher functional avidity than nef-, pol-, and env-specific CD8 T-cell responses [147]. Also, both gag-specific and HLA-B-restricted CD8 T-cell responses were of higher functional avidity in controllers than in noncontrollers. Finally, consistently with the above-mentioned studies, protective T-cell responses against KK10 and KF11 (restricted by HLA-B*2705 and B*5701, resp.) had higher functional avidity than all the other HLA-B-restricted epitopes [147].
Conversely, other studies indicated that the functional avidity of HIV-specific CD8 T cells is not different between patients with progressive or nonprogressive chronic infection or between gag- and other HIV-specific CD8 T cells [150] and also that uncontrolled virus replication seen in progressive HIV infection occurs despite the presence of high-avidity HIV-specific CD8 T-cell responses [142–146, 151, 152]. In this regard, we previously showed that polyfunctional virus-specific CD8 T-cell responses in the context of chronic viral infections were predominantly of low functional avidity [36]. In addition, when the avidity of two different CD8 T-cell clonotypes recognizing one HLA-B*35-restricted Pol epitope was analyzed, a 3fold difference in t 1/2 for HLA-multimer interaction was found. In contrast to the clone of lower affinity, the one with higher affinity did not show cytotoxic activity, cytokine production, or proliferative capacity following stimulation with the cognate antigen [153]. Furthermore, CD8 T cells transduced with a high-affinity TCR showed greater binding activity toward the specific multimer, but impaired cytotoxicity [153].
Finally, it was also reported that high functional avidity T-cell responses preferentially led to viral escape, T-cell clonal exhaustion, and senescence [26, 86, 134, 139, 141, 154]. Indeed, CD8 T cells have distinct ability to select for escape mutations for the same epitope depending on HLA restriction. The HLA restriction which confers higher avidity for the epitope induces a substantially higher level of sequence variation and clonal turnover which in turn leads to faster viral escape [134] and T-cell senescence [86]. However, to some extent, the emergence of viral variants escaping recognition from higher avidity T-cell responses may also be interpreted as an argument to support the efficacy of high-avidity T cells against HIV. Furthermore, the specificity of CD8 T-cell responses is critical, since cells directed toward highly variable regions may nonetheless not be able to mediate virus control. In case, they might only cause the emergence of virus escape variants. Moreover, TCR avidity correlates with PD-1 expression levels, and, consistently, high-avidity CD8 T cells displayed an impaired survival in in vitro culture at low levels of antigen stimulation. In vivo, subdominant clonotypes not only are of low functional avidity and express lower PD-1 levels than those of dominant clonotypes, but also respond more efficiently to variant epitopes, thus displaying a greater capacity of cross-recognition [141]. Although increased PD-1 expression might also be interpreted as a marker of increased activation of higher avidity T cells [155], in the context of chronic infection such as HIV, PD-1 is predominantly considered as a marker of exhausted cells [156].
Although large-scale longitudinal studies are needed to further elucidate the dynamic relationships between functional avidity, immunodominance, and viral escape, the aforementioned information suggest that lower functional avidity T-cell responses might be more suitable in the context of HIV infection.
7. Functional Avidity of T Cells in Acute HIV Infection
A better understanding of the immune response during primary HIV infection (PHI) is of particular relevance, since HIV-specific CD8 T cells in PHI are temporally associated with the initial control of virus replication [2].
Of interest, Lichterfeld and colleagues suggested that high-avidity HIV-specific CD8 T-cell responses are present during early infection (defined as HIV seroconversion within 6 months) but are then selectively depleted overtime [118]. To our knowledge, there is no previous study addressing the issue of the functional avidity of HIV-specific CD8-T cells in a cohort of HIV-infected patients with very early acute infection (based on stringent criteria of enrollment).
However, we recently had the opportunity to investigate the functional avidity of HIV-specific CD8 T-cell responses in a true PHI cohort (i.e., presence of an acute clinical syndrome, a negative HIV antibody test, a positive test for HIV RNA in plasma, and presence of fewer than three positive bands in a Western blot) [157]. In this context, we observed that the functional avidity of HIV-specific CD8 T-cell responses was significantly lower in PHI than in chronic infection and remained low after several years of antiretroviral therapy (Vigano and Harari, unpublished observation). Conversely, we noted (Figure 2) an increase in the functional avidity of HIV-specific CD8 T cells in patients experiencing a virus rebound following treatment interruption [84].
Figure 2.
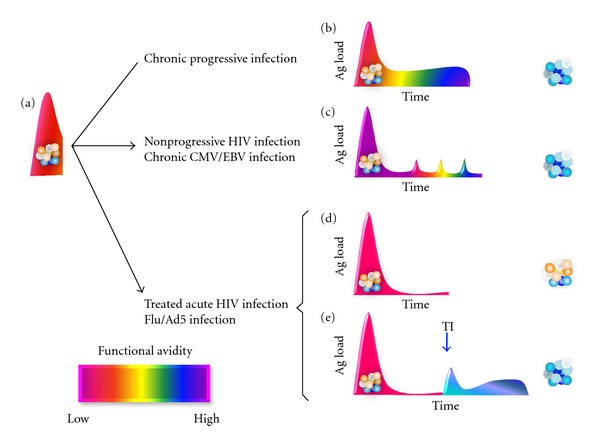
Proposed Model of the relationships between antigen exposure and functional avidity of T cells. Functional avidity of virus-specific CD8 T cells during (a) acute infection and then translation after transition to the chronic phase for (b) uncontrolled virus infection (such as progressive HIV infection) or (c) controlled but persistent virus infection (such as nonprogressive HIV, cytomegalovirus [CMV], or Epstein-Barr virus [EBV], or (d) after virus clearance (such as influenza [Flu] or adenovirus [Ad5], or early treatment of acute HIV infection). (e) Increase in the functional avidity of HIV-specific CD8 T cells of patients treated during acute infection who interrupted the antiretroviral therapy [TI] and experienced a virus rebound.
These observations might be explained by two nonmutually exclusive mechanisms: first, the progressive selection of clones with higher functional avidity and, on the other hand, the recruitment of new clones with higher functional avidity. The potential combination of these mechanisms would induce a modification of the TCR repertoire [84, 103, 106, 107, 118].
8. Perspectives and Hypothesis
Detailed monitoring of phenotypes and functional characteristics of T cells in different viral infection has strongly augmented our understanding of the relationship between viral infections and the immune response they induce. We recently made thorough comparisons of the types of T cells responding to infections that the immune system rapidly clears (Influenza (Flu) or Adenovirus (Ad5)), or infections caused by CMV and EBV as well as HIV infections in the acute and chronic phase. We saw that the functional avidity of T cells specific to Flu and Ad5 was similar to that of T cells in the acute phase of HIV infection. In contrast, significantly higher functional avidities of T cells were noticed in the chronic progressive and nonprogressive HIV infection, but interestingly those were comparable to the functional avidity seen during chronic CMV and EBV infections. Furthermore, when patients were treated during acute infection but experienced a virus rebound following treatment interruption (TI), the functional avidity of HIV-specific CD8 T cells increased (AH and SV unpublished observations).
9. Summary
Functional T-cell avidity is a critical attribute of antiviral and antitumoral immunity. The strength of interaction between the TCR and pMHC-molecule, expression levels of the coreceptors, as well as signaling particularities are pivotal in determining the functional avidity of a T-cell clone.
It is well established that T-cell populations can over the course of infection or upon multiple exposure to infections or infectious exacerbation undergo significant changes in the ability to recognize cognate antigen. The later is at a first glance somewhat surprising as T cells, unlike B cells, express a fixed TCR and cannot undergo somatic hypermutation. Predominantly, the clonal composition impacts the functional avidity of the T-cell population and avidity maturation. In addition to the reinforcement of the immunological synapses through the formation of TCR clusters, the optimization of the signal transduction machinery further contributes to avidity maturation. However, the understanding of mechanisms underlying this phenomenon is still limited, and further studies need to be undertaken to better understand how all these possible variations and likely many yet unknown ones impact the functional avidity of T cells when responding to different types of infections.
In the context of viral infections, functional avidity maturation allows faster virus clearance by recall T-cell responses. However, the role of high versus low functional avidity T cells in chronic viral infections such as HIV remains unclear. Here, it needs to be considered that high-avidity T cells exhibit greater T-cell exhaustion and lead to rapid emergence of escape variants suggesting a pivotal role of low-avidity T cells.
Studies to better delineate the factors influencing the functional avidity of T-cell responses are relevant in order to allow fine tuning of the profile of vaccine-induced T cells. We consider that the goal of vaccination or immunotherapy against acute infections and to induce pathogen clearance should be the induction of high-avidity T cells, since such cells most effectively eliminate infected cells. Conversely, when pathogen clearance cannot be achieved, then the ultimate goal is to provide durable control of a persistent pathogen. A vaccine that deals with such a situation should be designed to generate low-avidity T cells, since those might be more suitable to generate a pool of long-lasting effector T cells in a situation of chronic infection.
Authors' Contribution
D. Zehn and A. Harari equally contributed to the paper.
References
- 1.Zhang N, Bevan M. CD8+ T cells: foot soldiers of the immune system. Immunity. 2011;35(2):161–168. doi: 10.1016/j.immuni.2011.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Koup RA, Safrit JT, Cao Y, et al. Temporal association of cellular immune responses with the initial control of viremia in primary human immunodeficiency virus type 1 syndrome. Journal of Virology. 1994;68(7):4650–4655. doi: 10.1128/jvi.68.7.4650-4655.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Borrow P, Lewicki H, Hahn BH, Shaw GM, Oldstone MBA. Virus-specific CD8+ cytotoxic T-lymphocyte activity associated with control of viremia in primary human immunodeficiency virus type 1 infection. Journal of Virology. 1994;68(9):6103–6110. doi: 10.1128/jvi.68.9.6103-6110.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jin X, Bauer DE, Tuttleton SE, et al. Dramatic rise in plasma viremia after CD8+ T cell depletion in simian immunodeficiency virus-infected macaques. Journal of Experimental Medicine. 1999;189(6):991–998. doi: 10.1084/jem.189.6.991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schmitz JE, Kuroda MJ, Santra S, et al. Control of viremia in simian immunodeficiency virus infection by CD8+ lymphocytes. Science. 1999;283(5403):857–860. doi: 10.1126/science.283.5403.857. [DOI] [PubMed] [Google Scholar]
- 6.Moore CB, John M, James IR, Christiansen FT, Witt CS, Mallal SA. Evidence of HIV-1 adaptation to HLA-restricted immune responses at a population level. Science. 2002;296(5572):1439–1443. doi: 10.1126/science.1069660. [DOI] [PubMed] [Google Scholar]
- 7.Pereyra F, Addo MM, Kaufmann DE, et al. Genetic and immunologic heterogeneity among persons who control HIV infection in the absence of therapy. Journal of Infectious Diseases. 2008;197(4):563–571. doi: 10.1086/526786. [DOI] [PubMed] [Google Scholar]
- 8.Hersperger AR, Pereyra F, Nason M, et al. Perforin expression directly ex vivo by HIV-specific CD8 T-cells is a correlate of HIV elite control. PLoS pathogens. 2010;6(5) doi: 10.1371/journal.ppat.1000917.e1000917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Harari A, Zimmerli SC, Pantaleo G. Cytomegalovirus (CMV)-specific cellular immune responses. Human Immunology. 2004;65(5):500–506. doi: 10.1016/j.humimm.2004.02.012. [DOI] [PubMed] [Google Scholar]
- 10.Harari A, Dutoit V, Cellerai C, Bart PA, Du Pasquier RA, Pantaleo G. Functional signatures of protective antiviral T-cell immunity in human virus infections. Immunological Reviews. 2006;211:236–254. doi: 10.1111/j.0105-2896.2006.00395.x. [DOI] [PubMed] [Google Scholar]
- 11.Wherry EJ, Blattman JN, Murali-Krishna K, Van Der Most R, Ahmed R. Viral persistence alters CD8 T-cell immunodominance and tissue distribution and results in distinct stages of functional impairment. Journal of Virology. 2003;77(8):4911–4927. doi: 10.1128/JVI.77.8.4911-4927.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hersperger AR, Martin JN, Shin LY, et al. Increased HIV-specific CD8+ T-cell cytotoxic potential in HIV elite controllers is associated with T-bet expression. Blood. 2011;117(14):3799–3808. doi: 10.1182/blood-2010-12-322727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Reichstetter S, Kwok WW, Kochik S, Koelle DM, Beaty JS, Nepom GT. MHC-peptide ligand interactions establish a functional threshold for antigen-specific T cell recognition. Human Immunology. 1999;60(7):608–618. doi: 10.1016/s0198-8859(99)00038-5. [DOI] [PubMed] [Google Scholar]
- 14.Messaoudi I, Guevara Patiño JA, Dyall R, LeMaoult J, Nikolich-Žugich J. Direct link between mhc polymorphism, T cell avidity, and diversity in immune defense. Science. 2002;298(5599):1797–1800. doi: 10.1126/science.1076064. [DOI] [PubMed] [Google Scholar]
- 15.Pereyra F, Jia X, McLaren PJ, Telenti A, De Bakker PIW, Walker BD. The major genetic determinants of HIV-1 control affect HLA class I peptide presentation. Science. 2010;330(6010):1551–1557. doi: 10.1126/science.1195271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Klepiela P, Leslie AJ, Honeyborne I, et al. Dominant influence of HLA-B in mediating the potential co-evolution of HIV and HLA. Nature. 2004;432(7018):769–774. doi: 10.1038/nature03113. [DOI] [PubMed] [Google Scholar]
- 17.Chopera DR, Mlotshwa M, Woodman Z, et al. Virological and immunological factors associated with HIV-1 differential disease progression in HLA-B*58:01-positive individuals. Journal of Virology. 2011;85(14):7070–7080. doi: 10.1128/JVI.02543-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Peretz Y, Marra O, Thomas R, et al. Relative contribution of HIV-specific functional lymphocyte subsets restricted by protective and non-protective HLA alleles. Viral Immunology. 2011;24(3):189–198. doi: 10.1089/vim.2010.0117. [DOI] [PubMed] [Google Scholar]
- 19.Payne RP, Kløverpris H, Sacha JB, et al. Efficacious early antiviral activity of HIV Gag- and Pol-specific HLA-B*2705-restricted CD8+ T cells. Journal of Virology. 2010;84(20):10543–10557. doi: 10.1128/JVI.00793-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Migueles SA, Sabbaghian MS, Shupert WL, et al. HLA B*5701 is highly associated with restriction of virus replication in a subgroup of HIV-infected long term nonprogressors. Proceedings of the National Academy of Sciences of the United States of America. 2000;97(6):2709–2714. doi: 10.1073/pnas.050567397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Carrington M, O’Brien SJ. The Influence of HLA Genotype on AIDS. Annual Review of Medicine. 2003;54:535–551. doi: 10.1146/annurev.med.54.101601.152346. [DOI] [PubMed] [Google Scholar]
- 22.Martin MP, Gao X, Lee JH, et al. Epistatic interaction between KIR3DS1 and HLA-B delays the progression to AIDS. Nature Genetics. 2002;31(4):429–434. doi: 10.1038/ng934. [DOI] [PubMed] [Google Scholar]
- 23.Ahmadzadeh M, Johnson LA, Heemskerk B, et al. Tumor antigen-specific CD8 T cells infiltrating the tumor express high levels of PD-1 and are functionally impaired. Blood. 2009;114(8):1537–1544. doi: 10.1182/blood-2008-12-195792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Day CL, Kaufmann DE, Kiepiela P, et al. PD-1 expression on HIV-specific T cells is associated with T-cell exhaustion and disease progression. Nature. 2006;443(7109):350–354. doi: 10.1038/nature05115. [DOI] [PubMed] [Google Scholar]
- 25.Urbani S, Amadei B, Tola D, et al. PD-1 expression in acute hepatitis C virus (HCV) infection is associated with HCV-specific CD8 exhaustion. Journal of Virology. 2006;80(22):11398–11403. doi: 10.1128/JVI.01177-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.O’Connor DH, Allen TM, Vogel TU, et al. Acute phase cytotoxic T lymphocyte escape is a hallmark of simian immunodeficiency virus infection. Nature Medicine. 2002;8(5):493–499. doi: 10.1038/nm0502-493. [DOI] [PubMed] [Google Scholar]
- 27.Pérez CL, Hasselrot K, Bratt G, Broliden K, Karlsson AC. Induction of systemic HIV-1-specific cellular immune responses by oral exposure in the uninfected partner of discordant couples. AIDS. 2010;24(7):969–974. doi: 10.1097/qad.0b013e328337aff8. [DOI] [PubMed] [Google Scholar]
- 28.Hasselrot K. Genital and oral mucosal immune response against HIV-1 in exposed uninfected individuals. Critical Reviews in Immunology. 2009;29(5):369–377. doi: 10.1615/critrevimmunol.v29.i5.10. [DOI] [PubMed] [Google Scholar]
- 29.Hasselrot K, Bratt G, Hirbod T, et al. Orally exposed uninfected individuals have systemic anti-HIV responses associating with partners’ viral load. AIDS. 2010;24(1):35–43. doi: 10.1097/QAD.0b013e3283329853. [DOI] [PubMed] [Google Scholar]
- 30.Erickson AL, Willberg CB, McMahan V, et al. Potentially exposed but uninfected individuals produce cytotoxic and polyfunctional human immunodeficiency virus type 1-specific CD8+ T-cell responses which can be defined to the epitope level. Clinical and Vaccine Immunology. 2008;15(11):1745–1748. doi: 10.1128/CVI.00247-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Betts MR, Harari A. Phenotype and function of protective T cell immune responses in HIV. Current Opinion in HIV and AIDS. 2008;3(3):349–355. doi: 10.1097/COH.0b013e3282fbaa81. [DOI] [PubMed] [Google Scholar]
- 32.Pereyra F, Jia X, McLaren PJ, Telenti A, De Bakker PIW, Walker BD. The major genetic determinants of HIV-1 control affect HLA class I peptide presentation. Science. 2010;330(6010):1551–1557. doi: 10.1126/science.1195271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fellay J, Shianna KV, Ge D, et al. A whole-genome association study of major determinants for host control of HIV-1. Science. 2007;317(5840):944–947. doi: 10.1126/science.1143767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Porgador A, Yewdell JW, Deng Y, Bennink JR, Germain RN. Localization, quantitation, and in situ detection of specific peptide- MHC class I complexes using a monoclonal antibody. Immunity. 1997;6(6):715–726. doi: 10.1016/s1074-7613(00)80447-1. [DOI] [PubMed] [Google Scholar]
- 35.Betts MR, Price DA, Brenchley JM, et al. The functional profile of primary human antiviral CD8+ T cell effector activity is dictated by cognate peptide concentration. Journal of Immunology. 2004;172(10):6407–6417. doi: 10.4049/jimmunol.172.10.6407. [DOI] [PubMed] [Google Scholar]
- 36.Harari A, Cellerai C, Enders FB, et al. Skewed association of polyfunctional antigen-specific CD8 T cell populations with HLA-B genotype. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(41):16233–16238. doi: 10.1073/pnas.0707570104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Langenkamp A, Casorati G, Garavaglia C, Dellabona P, Lanzavecchia A, Sallusto F. T cell priming by dendritic cells: thresholds for proliferation, differentiation and death and intraclonal functional diversification. European Journal of Immunology. 2002;32(7):2046–2054. doi: 10.1002/1521-4141(200207)32:7<2046::AID-IMMU2046>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 38.Schamel WWA, Arechaga I, Risueño RM, et al. Coexistence of multivalent and monovalent TCRs explains high sensitivity and wide range of response. Journal of Experimental Medicine. 2005;202(4):493–503. doi: 10.1084/jem.20042155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cawthon AG, Lu H, Alexander-Miller MA. Peptide requirement for CTL activation reflects the sensitivity to CD3 engagement: correlation with CD8αβ versus CD8αα expression. Journal of Immunology. 2001;167(5):2577–2584. doi: 10.4049/jimmunol.167.5.2577. [DOI] [PubMed] [Google Scholar]
- 40.Viola A, Lanzavecchia A. T cell activation determined by T cell receptor number and tunable thresholds. Science. 1996;273(5271):104–106. doi: 10.1126/science.273.5271.104. [DOI] [PubMed] [Google Scholar]
- 41.Valitutti S, Müller S, Dessing M, Lanzavecchia A. Different responses are elicited in cytotoxic T lymphocytes by different levels of T cell receptor occupancy. Journal of Experimental Medicine. 1996;183(4):1917–1921. doi: 10.1084/jem.183.4.1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stone JD, Chervin AS, Kranz DM. T-cell receptor binding affinities and kinetics: impact on T-cell activity and specificity. Immunology. 2009;126(2):165–176. doi: 10.1111/j.1365-2567.2008.03015.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zehn D, King C, Bevan MJ, Palmer E. TCR signaling requirements for activating T cells and for generating memory. Cellular and Molecular Life Sciences. 2012;69(10):1565–1575. doi: 10.1007/s00018-012-0965-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Alam SM, Travers PJ, Wung JL, et al. T cell-receptor affinity and thymocyte positive selection. Nature. 1996;381(6583):616–620. doi: 10.1038/381616a0. [DOI] [PubMed] [Google Scholar]
- 45.Holler PD, Lim AR, Cho BK, Rund LA, Kranz DM. CD8-T cell transfectants that express a high affinity T cell receptor exhibit enhanced peptide-dependent activation. Journal of Experimental Medicine. 2001;194(8):1043–1052. doi: 10.1084/jem.194.8.1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tian S, Maile R, Collins EJ, Frelinger JA. CD8+ T cell activation is governed by TCR-peptide/MHC affinity, not dissociation rate. Journal of Immunology. 2007;179(5):2952–2960. doi: 10.4049/jimmunol.179.5.2952. [DOI] [PubMed] [Google Scholar]
- 47.Matsui K, Boniface JJ, Steffner P, Reay PA, Davis MM. Kinetics of T-cell receptor binding to peptide/I-E(k) complexes: correlation of the dissociation rate with T-cell responsiveness. Proceedings of the National Academy of Sciences of the United States of America. 1994;91(26):12862–12866. doi: 10.1073/pnas.91.26.12862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.La Gruta NL, Doherty PC, Turner SJ. A correlation between function and selected measures of T cell avidity in influenza virus-specific CD8+ T cell responses. European Journal of Immunology. 2006;36(11):2951–2959. doi: 10.1002/eji.200636390. [DOI] [PubMed] [Google Scholar]
- 49.Mckeithan TW. Kinetic proofreading in T-cell receptor signal transduction. Proceedings of the National Academy of Sciences of the United States of America. 1995;92(11):5042–5046. doi: 10.1073/pnas.92.11.5042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Huang J, Zarnitsyna VI, Liu B, et al. The kinetics of two-dimensional TCR and pMHC interactions determine T-cell responsiveness. Nature. 2010;464(7290):932–936. doi: 10.1038/nature08944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Holmberg K, Mariathasan S, Ohteki T, Ohashi PS, Gascoigne NRJ. TCR binding kinetics measured with MHC class I tetramers reveal a positive selecting peptide with relatively high affinity for TCR. Journal of Immunology. 2003;171(5):2427–2434. doi: 10.4049/jimmunol.171.5.2427. [DOI] [PubMed] [Google Scholar]
- 52.Yee C, Savage PA, Lee PP, Davis MM, Greenberg PD. Isolation of high avidity melanoma-reactive CTL from heterogeneous populations using peptide-MHC tetramers. Journal of Immunology. 1999;162(4):2227–2234. [PubMed] [Google Scholar]
- 53.Naeher D, Daniels MA, Hausmann B, Guillaume P, Luescher I, Palmer E. A constant affinity threshold for T cell tolerance. Journal of Experimental Medicine. 2007;204(11):2553–2559. doi: 10.1084/jem.20070254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Labrecque N, Whitfield LS, Obst R, Waltzinger C, Benoist C, Mathis D. How much TCR does a T cell need? Immunity. 2001;15(1):71–82. doi: 10.1016/s1074-7613(01)00170-4. [DOI] [PubMed] [Google Scholar]
- 55.Hofmann M, Radsak M, Rechtsteiner G, et al. T cell avidity determines the level of CTL activation. European Journal of Immunology. 2004;34(7):1798–1806. doi: 10.1002/eji.200425088. [DOI] [PubMed] [Google Scholar]
- 56.König R. Interactions between MHC molecules and co-receptors of the TCR. Current Opinion in Immunology. 2002;14(1):75–83. doi: 10.1016/s0952-7915(01)00300-4. [DOI] [PubMed] [Google Scholar]
- 57.Choi EML, Chen JL, Wooldridge L, et al. High avidity antigen-specific CTL identified by CD8-independent tetramer staining. Journal of Immunology. 2003;171(10):5116–5123. doi: 10.4049/jimmunol.171.10.5116. [DOI] [PubMed] [Google Scholar]
- 58.Holler PD, Kranz DM. Quantitative analysis of the contribution of TCR/pepMHC affinity and CD8 to T cell activation. Immunity. 2003;18(2):255–264. doi: 10.1016/s1074-7613(03)00019-0. [DOI] [PubMed] [Google Scholar]
- 59.Khan N, Cobbold M, Cummerson J, Moss PAH. Persistent viral infection in humans can drive high frequency low-affinity T-cell expansions. Immunology. 2010;131(4):537–548. doi: 10.1111/j.1365-2567.2010.03326.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Laugel B, Van Den Berg HA, Gostick E, et al. Different T cell receptor affinity thresholds and CD8 coreceptor dependence govern cytotoxic T lymphocyte activation and tetramer binding properties. Journal of Biological Chemistry. 2007;282(33):23799–23810. doi: 10.1074/jbc.M700976200. [DOI] [PubMed] [Google Scholar]
- 61.Daniels MA, Jameson SC. Critical role for CD8 in T cell receptor binding and activation by peptide/major histocompatibility complex multimers. Journal of Experimental Medicine. 2000;191(2):335–346. doi: 10.1084/jem.191.2.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Xu H, Littman DR. A kinase-independent function of Lck in potentiating antigen-specific T cell activation. Cell. 1993;74(4):633–643. doi: 10.1016/0092-8674(93)90511-n. [DOI] [PubMed] [Google Scholar]
- 63.Thome M, Germain V, DiSanto JP, Acuto O. The p56(lck) SH2 domain mediates recruitment of CD8/p56(lck) to the activated T cell receptor/CD3/ξ complex. European Journal of Immunology. 1996;26(9):2093–2100. doi: 10.1002/eji.1830260920. [DOI] [PubMed] [Google Scholar]
- 64.Irie HY, Ravichandran KS, Burakoff SJ. CD8β chain influences CD8α chain-associated Lck kinase activity. Journal of Experimental Medicine. 1995;181(4):1267–1273. doi: 10.1084/jem.181.4.1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Xiao Z, Mescher MF, Jameson SC. Detuning CD8 T cells: down-regulation of CD8 expression, tetramer binding, and response during CTL activation. Journal of Experimental Medicine. 2007;204(11):2667–2677. doi: 10.1084/jem.20062376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gostick E, Cole DK, Hutchinson SL, et al. Functional and biophysical characterization of an HLA-A* 6801-restricted HIV-specific T cell receptor. European Journal of Immunology. 2007;37(2):479–486. doi: 10.1002/eji.200636243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wooldridge L, Lissina A, Vernazza J, et al. Enhanced immunogenicity of CTL antigens through mutation of the CD8 binding MHC class I invariant region. European Journal of Immunology. 2007;37(5):1323–1333. doi: 10.1002/eji.200636765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Schonrich G, Kalinke U, Momburg F, et al. Down-regulation of T cell receptors on self-reactive T cells as a novel mechanism for extrathymic tolerance induction. Cell. 1991;65(2):293–304. doi: 10.1016/0092-8674(91)90163-s. [DOI] [PubMed] [Google Scholar]
- 69.Acuto O, Bartolo VD, Michel F. Tailoring T-cell receptor signals by proximal negative feedback mechanisms. Nature Reviews Immunology. 2008;8(9):699–712. doi: 10.1038/nri2397. [DOI] [PubMed] [Google Scholar]
- 70.Daniels MA, Teixeiro E, Gill J, et al. Thymic selection threshold defined by compartmentalization of Ras/MAPK signalling. Nature. 2006;444(7120):724–729. doi: 10.1038/nature05269. [DOI] [PubMed] [Google Scholar]
- 71.Luik RM, Lewis RS. New insights into the molecular mechanisms of store-operated Ca2+ signaling in T cells. Trends in Molecular Medicine. 2007;13(3):103–107. doi: 10.1016/j.molmed.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 72.Anderton SM, Wraith DC. Selection and fine-tuning of the autoimmune T-cell repertoire. Nature Reviews Immunology. 2002;2(7):487–498. doi: 10.1038/nri842. [DOI] [PubMed] [Google Scholar]
- 73.Ebert PJ, Jiang S, Xie J, Li QJ, Davis MM. An endogenous positively selecting peptide enhances mature T cell responses and becomes an autoantigen in the absence of microRNA miR-181a. Nature immunology. 2009;10(11):1162–1169. doi: 10.1038/ni.1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Garcia Z, Pradelli E, Celli S, Beuneu H, Simon A, Bousso P. Competition for antigen determines the stability of T cell-dendritic cell interactions during clonal expansion. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(11):4553–4558. doi: 10.1073/pnas.0610019104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Davey GM, Schober SL, Endrizzi BT, Dutcher AK, Jameson SC, Hogquist KA. Preselection thymocytes are more sensitive to T cell receptor stimulation than mature T cells. Journal of Experimental Medicine. 1998;188(10):1867–1874. doi: 10.1084/jem.188.10.1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Pircher H, Hoffmann Rohrer U, Moskophidis D, Zinkernagel RM, Hengartner H. Lower receptor avidity required for thymic clonal deletion than for effector T-cell function. Nature. 1991;351(6326):482–485. doi: 10.1038/351482a0. [DOI] [PubMed] [Google Scholar]
- 77.Azzam HS, Grinberg A, Lui K, Shen H, Shores EW, Love PE. CD5 expression is developmentally regulated by T cell receptor (TCR) signals and TCR avidity. Journal of Experimental Medicine. 1998;188(12):2301–2311. doi: 10.1084/jem.188.12.2301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bamberger M, Santos AM, Gonçalves CM, et al. A new pathway of CD5 glycoprotein-mediated T cell inhibition dependent on inhibitory phosphorylation of fyn kinase. Journal of Biological Chemistry. 2011;286(35):30324–30336. doi: 10.1074/jbc.M111.230102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hawiger D, Masilamani RF, Bettelli E, Kuchroo VK, Nussenzweig MC. Immunological unresponsiveness characterized by increased expression of CD5 on peripheral T cells induced by dendritic cells in vivo. Immunity. 2004;20(6):695–705. doi: 10.1016/j.immuni.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 80.Sharma SK, Alexander-Miller MA. Increased sensitivity to antigen in high avidity CD8+ T cells results from augmented membrane proximal T-cell receptor signal transduction. Immunology. 2011;133(3):307–317. doi: 10.1111/j.1365-2567.2011.03440.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Slifka MK, Whitton JL. Functional avidity maturation of CD8+ T cells without selection of higher affinity TCR. Nature Immunology. 2001;2(8):711–717. doi: 10.1038/90650. [DOI] [PubMed] [Google Scholar]
- 82.Bhandoola A, Tai X, Eckhaus M, et al. Peripheral expression of self-MHC-II influences the reactivity and self-tolerance of mature CD4+ T cells: evidence from a lymphopenic T cell model. Immunity. 2002;17(4):425–436. doi: 10.1016/s1074-7613(02)00417-x. [DOI] [PubMed] [Google Scholar]
- 83.Štefanoví I, Dorfman JR, Germain RN. Self-recognition promotes the foreign antigen sensitivity of naive T lymphocytes. Nature. 2002;420(6914):429–434. doi: 10.1038/nature01146. [DOI] [PubMed] [Google Scholar]
- 84.Miconnet I, Marrau A, Farina A, et al. Large TCR diversity of virus-specific CD8 T cells provides the mechanistic basis for massive TCR renewal after antigen exposure. Journal of Immunology. 2011;186(12):7039–7049. doi: 10.4049/jimmunol.1003309. [DOI] [PubMed] [Google Scholar]
- 85.Schaubert KL, Price DA, Frahm N, et al. Availability of a diversely avid CD8+ T cell repertoire specific for the subdominant HLA-A2-restricted HIV-1 Gag p2419-27 epitope. Journal of Immunology. 2007;178(12):7756–7766. doi: 10.4049/jimmunol.178.12.7756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Almeida JR, Price DA, Papagno L, et al. Superior control of HIV-1 replication by CD8+ T cells is reflected by their avidity, polyfunctionality, and clonal turnover. Journal of Experimental Medicine. 2007;204(10):2473–2485. doi: 10.1084/jem.20070784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Douek DC, Betts MR, Brenchley JM, et al. A novel approach to the analysis of specificity, clonality, and frequency of HIV-specific T cell responses reveals a potential mechanism for control of viral escape. Journal of Immunology. 2002;168(6):3099–3104. doi: 10.4049/jimmunol.168.6.3099. [DOI] [PubMed] [Google Scholar]
- 88.Janbazian L, Price DA, Canderan G, et al. Clonotype and repertoire changes drive the functional improvement of HIV-specific CD8 T cell populations under conditions of limited antigenic stimulation. Journal of Immunology. 2012;188(3):1156–1167. doi: 10.4049/jimmunol.1102610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.van Gisbergen KPJM, Klarenbeek PL, Kragten NAM, et al. The costimulatory molecule CD27 maintains clonally diverse CD8+ T cell responses of low antigen affinity to protect against viral variants. Immunity. 2011;35(1):97–108. doi: 10.1016/j.immuni.2011.04.020. [DOI] [PubMed] [Google Scholar]
- 90.Yang S, Hodge JW, Grosenbach DW, Schlom J. Vaccines with enhanced costimulation maintain high avidity memory CTL. Journal of Immunology. 2005;175(6):3715–3723. doi: 10.4049/jimmunol.175.6.3715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Oh S, Hodge JW, Ahlers JD, Burke DS, Schlom J, Berzofsky JA. Selective induction of high avidity CTL by altering the balance of signals from APC. Journal of Immunology. 2003;170(5):2523–2530. doi: 10.4049/jimmunol.170.5.2523. [DOI] [PubMed] [Google Scholar]
- 92.Hodge JW, Chakraborty M, Kudo-Saito C, Garnett CT, Schlom J. Multiple costimulatory modalities enhance CTL avidity. Journal of Immunology. 2005;174(10):5994–6004. doi: 10.4049/jimmunol.174.10.5994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Sharpe AH, Wherry EJ, Ahmed R, Freeman GJ. The function of programmed cell death 1 and its ligands in regulating autoimmunity and infection. Nature Immunology. 2007;8(3):239–245. doi: 10.1038/ni1443. [DOI] [PubMed] [Google Scholar]
- 94.Blackburn SD, Shin H, Haining WN, et al. Coregulation of CD8+ T cell exhaustion by multiple inhibitory receptors during chronic viral infection. Nature Immunology. 2009;10(1):29–37. doi: 10.1038/ni.1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Rees W, Bender J, Teague TK, et al. An inverse relationship between T cell receptor affinity and antigen dose during CD4+ T cell responses in vivo and in vitro. Proceedings of the National Academy of Sciences of the United States of America. 1999;96(17):9781–9786. doi: 10.1073/pnas.96.17.9781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Bullock TNJ, Mullins DW, Engelhard VH. Antigen density presented by dendritic cells in vivo differentially affects the number and avidity of primary, memory, and recall CD8+ T cells. Journal of Immunology. 2003;170(4):1822–1829. doi: 10.4049/jimmunol.170.4.1822. [DOI] [PubMed] [Google Scholar]
- 97.Alexander-Miller MA, Leggatt GR, Berzofsky JA. Selective expansion of high- or low-avidity cytotoxic T lymphocytes and efficacy for adoptive immunotherapy. Proceedings of the National Academy of Sciences of the United States of America. 1996;93(9):4102–4107. doi: 10.1073/pnas.93.9.4102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Levitsky V, Zhang QJ, Levitskaya J, Masucci MG. The life span of major histocompatibility complex-peptide complexes influences the efficiency of presentation and immunogenicity of two class I-restricted cytotoxic T lymphocyte epitopes in the Epstein-Barr virus nuclear antigen 4. Journal of Experimental Medicine. 1996;183(3):915–926. doi: 10.1084/jem.183.3.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Galea I, Stasakova J, Dunscombe MS, Ottensmeier CH, Elliott T, Thirdborough SM. CD8+ T-cell cross-competition is governed by peptide-MHC class I stability. European Journal of Immunology. 2012;42(1):256–263. doi: 10.1002/eji.201142010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Zehn D, Cohen CJ, Reiter Y, Walden P. Extended presentation of specific MHC-peptide complexes by mature dendritic cells compared to other types of antigen-presenting cells. European Journal of Immunology. 2004;34(6):1551–1560. doi: 10.1002/eji.200324355. [DOI] [PubMed] [Google Scholar]
- 101.Lövgren T, Baumgaertner P, Wieckowski S, et al. Enhanced cytotoxicity and decreased CD8 dependence of human cancer-specific cytotoxic T lymphocytes after vaccination with low peptide dose. Cancer Immunology, Immunotherapy. 2012;61(6):817–826. doi: 10.1007/s00262-011-1140-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Von Essen MR, Kongsbak M, Geisler C. Mechanisms behind functional avidity maturation in T cells. Clinical and Developmental Immunology. 2012;2012:8 pages. doi: 10.1155/2012/163453.163453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Savage PA, Boniface JJ, Davis MM. A kinetic basis for T cell receptor repertoire selection during an immune response. Immunity. 1999;10(4):485–492. doi: 10.1016/s1074-7613(00)80048-5. [DOI] [PubMed] [Google Scholar]
- 104.Zehn D, Lee SY, Bevan MJ. Complete but curtailed T-cell response to very low-affinity antigen. Nature. 2009;458(7235):211–214. doi: 10.1038/nature07657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Bachmann MF, Speiser DE, Ohashi PS. Functional maturation of an antiviral cytotoxic T-cell response. Journal of Virology. 1997;71(8):5764–5768. doi: 10.1128/jvi.71.8.5764-5768.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Busch DH, Pamer EG. T cell affinity maturation by selective expansion during infection. Journal of Experimental Medicine. 1999;189(4):701–709. doi: 10.1084/jem.189.4.701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Day EK, Carmichael AJ, Ten Berge IJM, Waller ECP, Sissons JGP, Wills MR. Rapid CD8+ T cell repertoire focusing and selection of high-affinity clones into memory following primary infection with a persistent human virus: human cytomegalovirus. Journal of Immunology. 2007;179(5):3203–3213. doi: 10.4049/jimmunol.179.5.3203. [DOI] [PubMed] [Google Scholar]
- 108.Rechtsteiner G, Warger T, Hofmann M, Rammensee HG, Schild HJ, Radsak MP. Precursor frequency can compensate for lower TCR expression in T cell competition during priming in vivo. European Journal of Immunology. 2006;36(10):2613–2623. doi: 10.1002/eji.200636331. [DOI] [PubMed] [Google Scholar]
- 109.Pihlgren M, Dubois PM, Tomkowiak M, Sjögren T, Marvel J. Resting memory CD8+ T cells are hyperreactive to antigenic challenge in vitro. Journal of Experimental Medicine. 1996;184(6):2141–2151. doi: 10.1084/jem.184.6.2141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Curtsinger JM, Lins DC, Mescher MF. CD8+ memory T cells (CD44high, Ly-6C+) are more sensitive than naive cells (CD44low, Ly-6C−) to TCR/CD8 signaling in response to antigen. Journal of Immunology. 1998;160(7):3236–3243. [PubMed] [Google Scholar]
- 111.Alarcón B, Mestre D, Martínez-Martín N. The immunological synapse: a cause or consequence of T-cell receptor triggering? Immunology. 2011;133(4):420–425. doi: 10.1111/j.1365-2567.2011.03458.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Yokosuka T, Saito T. Dynamic regulation of T-cell costimulation through TCR-CD28 microclusters. Immunological Reviews. 2009;229(1):27–40. doi: 10.1111/j.1600-065X.2009.00779.x. [DOI] [PubMed] [Google Scholar]
- 113.Fahmy TM, Bieler JG, Edidin M, Schneck JP. Increased TCR avidity after T cell activation: a mechanism for sensing low-density antigen. Immunity. 2001;14(2):135–143. [PubMed] [Google Scholar]
- 114.Robinson AT, Miller N, Alexander DR. CD3 antigen-mediated calcium signals and protein kinase C activation are higher in CD45RO+ than in CD45RA+ human T lymphocyte subsets. European Journal of Immunology. 1993;23(1):61–68. doi: 10.1002/eji.1830230111. [DOI] [PubMed] [Google Scholar]
- 115.Schwinzer R, Siefken R, Franklin RA, Saloga J, Wonigeit K, Gelfand EW. Human CD45RA+ and CD45R0+ T cells exhibit similar CD3/T cell receptor-mediated transmembrane signaling capacities but differ in response to co-stimulatory signals. European Journal of Immunology. 1994;24(6):1391–1395. doi: 10.1002/eji.1830240623. [DOI] [PubMed] [Google Scholar]
- 116.Croft M, Bradley LM, Swain SL. Naive versus memory CD4 T cell response to antigen: memory cells are less dependent on accessory cell costimulation and can respond to many antigen- presenting cell types including resting B cells. Journal of Immunology. 1994;152(6):2675–2685. [PubMed] [Google Scholar]
- 117.Turner MJ, Jellison ER, Lingenheld EG, Puddington L, Lefrançois L. Avidity maturation of memory CD8 T cells is limited by self-antigen expression. Journal of Experimental Medicine. 2008;205(8):1859–1868. doi: 10.1084/jem.20072390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Lichterfeld M, Yu XG, Mui SK, et al. Selective depletion of high-avidity human immunodeficiency virus type 1 (HIV-1)-specific CD8+ T cells after early HIV-1 infection. Journal of Virology. 2007;81(8):4199–4214. doi: 10.1128/JVI.01388-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Dutoit V, Rubio-Godoy V, Dietrich PY, et al. Heterogeneous T-cell response to MAGE-A10254-262: high avidity-specific cytolytic T lymphocytes show superior antitumor activity. Cancer Research. 2001;61(15):5850–5856. [PubMed] [Google Scholar]
- 120.Snyder JT, Alexander-Miller MA, Berzofskyl JA, Belyakov IM. Molecular mechanisms and biological significance of CTL avidity. Current HIV Research. 2003;1(3):287–294. doi: 10.2174/1570162033485230. [DOI] [PubMed] [Google Scholar]
- 121.Berger CT, Frahm N, Price DA, et al. High-functional-avidity cytotoxic T lymphocyte responses to HLA-B-restricted gag-derived epitopes associated with relative HIV control. Journal of Virology. 2011;85(18):9334–9345. doi: 10.1128/JVI.00460-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Kitazono T, Okazaki T, Araya N, et al. Advantage of higher-avidity CTL specific for Tax against human T-lymphotropic virus-1 infected cells and tumors. Cellular Immunology. 2011;272(1):11–17. doi: 10.1016/j.cellimm.2011.10.002. [DOI] [PubMed] [Google Scholar]
- 123.McMahan RH, Slansky JE. Mobilizing the low-avidity T cell repertoire to kill tumors. Seminars in Cancer Biology. 2007;17(4):317–329. doi: 10.1016/j.semcancer.2007.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Zehn D, Bevan MJ. T cells with low avidity for a tissue-restricted antigen routinely evade central and peripheral tolerance and cause autoimmunity. Immunity. 2006;25(2):261–270. doi: 10.1016/j.immuni.2006.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Kirkwood JM, Butterfield LH, Tarhini AA, Zarour H, Kalinski P, Ferrone S. Immunotherapy of cancer in 2012. CA Cancer Journal for Clinicians. 2012;62(5):309–335. doi: 10.3322/caac.20132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.June CH. Adoptive T cell therapy for cancer in the clinic. Journal of Clinical Investigation. 2007;117(6):1466–1476. doi: 10.1172/JCI32446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Bennett MS, Ng HL, Dagarag M, Ali A, Yang OO. Epitope-dependent avidity thresholds for cytotoxic T-lymphocyte clearance of virus-infected cells. Journal of Virology. 2007;81(10):4973–4980. doi: 10.1128/JVI.02362-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Belyakov IM, Kuznetsov VA, Kelsall B, et al. Impact of vaccine-induced mucosal high-avidity CD8+CTLs in delay of AIDS viral dissemination from mucosa. Blood. 2006;107(8):3258–3264. doi: 10.1182/blood-2005-11-4374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Zeh HJ, Jr., Perry-Lalley D, Dudley ME, Rosenberg SA, Yang JC. High avidity CTLs for two self-antigens demonstrate superior in vitro and in vivo antitumor efficacy. Journal of Immunology. 1999;162(2):989–994. [PubMed] [Google Scholar]
- 130.Aranda F, Llopiz D, Díaz-Valdés N, et al. Adjuvant combination and antigen targeting as a strategy to induce polyfunctional and high-avidity T-cell responses against poorly immunogenic tumors. Cancer Research. 2011;71(9):3214–3224. doi: 10.1158/0008-5472.CAN-10-3259. [DOI] [PubMed] [Google Scholar]
- 131.Derby MA, Alexander-Miller MA, Tse R, Berzofsky JA. High-avidity CTL exploit two complementary mechanisms to provide better protection against viral infection than low-avidity CTL. Journal of Immunology. 2001;166(3):1690–1697. doi: 10.4049/jimmunol.166.3.1690. [DOI] [PubMed] [Google Scholar]
- 132.Jessen B, Faller S, Krempl CD, Ehl S. Major histocompatibility complex-dependent cytotoxic T lymphocyte repertoire and functional avidity contribute to strain-specific disease susceptibility after murine respiratory syncytial virus infection. Journal of Virology. 2011;85(19):10135–10143. doi: 10.1128/JVI.00816-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Košmrlj A, Read EL, Qi Y, et al. Effects of thymic selection of the T-cell repertoire on HLA class I-associated control of HIV infection. Nature. 2010;465(7296):350–354. doi: 10.1038/nature08997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Leslie A, Price DA, Mkhize P, et al. Differential selection pressure exerted on HIV by CTL targeting identical epitopes but restricted by distinct HLA alleles from the same HLA supertype. Journal of Immunology. 2006;177(7):4699–4708. doi: 10.4049/jimmunol.177.7.4699. [DOI] [PubMed] [Google Scholar]
- 135.Almeida JR, Sauce D, Price DA, et al. Antigen sensitivity is a major determinant of CD8+ T-cell polyfunctionality and HIV-suppressive activity. Blood. 2009;113(25):6351–6360. doi: 10.1182/blood-2009-02-206557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Morgan DJ, Kreuwel HTC, Fleck S, Levitsky HI, Pardoll DM, Sherman LA. Activation of low avidity CTL specific for a self epitope results in tumor rejection but not autoimmunity. Journal of Immunology. 1998;160(2):643–651. [PubMed] [Google Scholar]
- 137.Wankowicz-Kalinska A, Mailliard RB, Olson K, et al. Accumulation of low-avidity anti-melanocortin receptor 1 (anti-MC1R) CD8+ T cells in the lesional skin of a patient with melanoma-related depigmentation. Melanoma Research. 2006;16(2):165–174. doi: 10.1097/01.cmr.0000198452.03957.73. [DOI] [PubMed] [Google Scholar]
- 138.Anderton SM, Radu CG, Lowrey PA, Ward ES, Wraith DC. Negative selection during the peripheral immune response to antigen. Journal of Experimental Medicine. 2001;193(1):1–11. doi: 10.1084/jem.193.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Iglesias MC, Almeida JR, Fastenackels S, et al. Escape from highly effective public CD8+ T-cell clonotypes by HIV. Blood. 2011;118(8):2138–2149. doi: 10.1182/blood-2011-01-328781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Price DA, Brenchley JM, Ruff LE, et al. Avidity for antigen shapes clonal dominance in CD8+ T cell populations specific for persistent DNA viruses. Journal of Experimental Medicine. 2005;202(10):1349–1361. doi: 10.1084/jem.20051357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Conrad JA, Ramalingam RK, Smith RM, et al. Dominant clonotypes within HIV-specific T cell responses are programmed death-1highand CD127lowand display reduced variant cross-reactivity. Journal of Immunology. 2011;186(12):6871–6885. doi: 10.4049/jimmunol.1004234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Miura T, Brumme CJ, Brockman MA, et al. HLA-associated viral mutations are common in human immunodeficiency virus type 1 elite controllers. Journal of Virology. 2009;83(7):3407–3412. doi: 10.1128/JVI.02459-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Migueles SA, Laborico AC, Imamichi H, et al. The differential ability of HLA B*5701+ long-term nonprogressors and progressors to restrict human immunodeficiency virus replication is not caused by loss of recognition of autologous viral gag sequences. Journal of Virology. 2003;77(12):6889–6898. doi: 10.1128/JVI.77.12.6889-6898.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Migueles SA, Laborico AC, Shupert WL, et al. HIV-specific CD8+ T cell proliferation is coupled to perforin expression and is maintained in nonprogressors. Nature Immunology. 2002;3(11):1061–1068. doi: 10.1038/ni845. [DOI] [PubMed] [Google Scholar]
- 145.Draenert R, Verrill CL, Tang Y, et al. Persistent recognition of autologous virus by high-avidity CD8 T cells in chronic, progressive human immunodeficiency virus type 1 infection. Journal of Virology. 2004;78(2):630–641. doi: 10.1128/JVI.78.2.630-641.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Bailey JR, Williams TM, Siliciano RF, Blankson JN. Maintenance of viral suppression in HIV-1-infected HLA-B*57+ elite suppressors despite CTL escape mutations. Journal of Experimental Medicine. 2006;203(5):1357–1369. doi: 10.1084/jem.20052319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Berger CT, Frahm N, Price DA, et al. High-functional-avidity cytotoxic T lymphocyte responses to HLA-B-restricted gag-derived epitopes associated with relative HIV control. Journal of Virology. 2011;85(18):9334–9345. doi: 10.1128/JVI.00460-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Mothe B, Llano A, Ibarrondo J, et al. Ctl responses of high functional avidity and broad variant cross-reactivity are associated with hiv control. PLoS One. 2012;7(1) doi: 10.1371/journal.pone.0029717.e29717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Kiepiela P, Ngumbela K, Thobakgale C, et al. CD8+ T-cell responses to different HIV proteins have discordant associations with viral load. Nature Medicine. 2007;13(1):46–53. doi: 10.1038/nm1520. [DOI] [PubMed] [Google Scholar]
- 150.Chen DY, Balamurugan A, Ng HL, Cumberland WG, Yang OO. Epitope targeting and viral inoculum are determinants of Nef-mediated immune evasion of HIV-1 from cytotoxic T lymphocytes. Blood. 2012;120(1):100–111. doi: 10.1182/blood-2012-02-409870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Keane NM, Roberts SG, Almeida C-AM, et al. High-avidity, high-IFNγ-producing CD8 T-cell responses following immune selection during HIV-1 infection. Immunology and Cell Biology. 2012;90(2):224–234. doi: 10.1038/icb.2011.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Chen H, Ndhlovu ZM, Liu D, et al. TCR clonotypes modulate the protective effect of HLA class I molecules in HIV-1 infection. Nature Immunology. 2012;13(7):691–700. doi: 10.1038/ni.2342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Ueno T, Tomiyama H, Fujiwara M, Oka S, Takiguchi M. Functionally impaired HIV-specific CD8 T cells show high affinity TCR-ligand interactions. Journal of Immunology. 2004;173(9):5451–5457. doi: 10.4049/jimmunol.173.9.5451. [DOI] [PubMed] [Google Scholar]
- 154.Kamari Y, Werman-Venkert R, Shaish A, et al. Differential role and tissue specificity of interleukin-1α gene expression in atherogenesis and lipid metabolism. Atherosclerosis. 2007;195(1):31–38. doi: 10.1016/j.atherosclerosis.2006.11.026. [DOI] [PubMed] [Google Scholar]
- 155.Chomont N, El-Far M, Ancuta P, et al. HIV reservoir size and persistence are driven by T cell survival and homeostatic proliferation. Nature Medicine. 2009;15(8):893–900. doi: 10.1038/nm.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Wherry EJ, Ha SJ, Kaech SM, et al. Molecular signature of CD8+ T cell exhaustion during chronic viral infection. Immunity. 2007;27(4):670–684. doi: 10.1016/j.immuni.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 157.Rizzardi GP, Harari A, Capiluppi B, et al. Treatment of primary HIV-1 infection with cyclosporin A coupled with highly active antiretroviral therapy. Journal of Clinical Investigation. 2002;109(5):681–688. doi: 10.1172/JCI14522. [DOI] [PMC free article] [PubMed] [Google Scholar]


