Abstract
Social and emotional behavior are known to be sensitive to both developmental iron deficiency and monoamine oxidase A (MAOA) gene polymorphisms. In this study, male rhesus monkey infants deprived of dietary iron in utero (ID) were compared to iron sufficient (IS) controls (n=10/group). Half of each group had low MAOA activity genotypes and half had high MAOA activity genotypes. A series of social response tests were conducted at 3 to 14 months of age. MAOA genotype influenced attention to a video of aggressive behavior, emotional expression (fear grimace and sniff) in the social intruder test, social actions (displacement, grooming) in the social dyad test, and aggressive responses to a threatening picture. Interactions between MAOA and prenatal ID were seen in response to the aggressive video, in temperament ratings, in affiliative behavior in the social dyad test, in cortisol response in the social buffering test, and in response to a social intruder and to pictures with social and nonsocial themes. In general the effects of ID were dependent on MAOA genotype in terms of both direction and size of the effect. Nutrition/genotype interactions may shed new light on behavioral consequences of nutritional deprivation during brain development.
Keywords: monkey, rhesus, nonhuman primate, iron, anemia, MAOA polymorphism, social behavior, infant
Introduction
Iron deficiency (ID) is the most common single nutrient deficiency worldwide. Iron deficiency anemia (IDA) is most prevalent during the 3d trimester of pregnancy (33.9% in US) and in infants 12–18 months of age (18.0% in US) (CDC, 2011). Behavioral consequences of ID and IDA in 6–12 month old infants are well documented (Lozoff, 2007). Early observations of altered emotional response and delayed motor development were followed by large, systematic international studies linking infant IDA with later IQ and school performance deficits even when anemia was corrected with supplements. Most recently, long term followup of infants with chronic iron deficiency showed internalizing and externalizing behavior problems at 5 years of age and in early adolescence (11–14 years of age) (Corapci et al., 2011). This effect was hypothesized to originate in affective and social interaction changes associated with infant ID (Armony-Sivan et al., 2011, Chang et al., 2011, Corapci et al., 2011, Lozoff et al., 2008), and 3d trimester ID (Tamura et al., 2002, Wachs et al., 2005). However, the externalizing behavior problems were persistent when activity during infancy had been low, suggesting that other factors were in play to create susceptibility to the long term consequences of ID for behavioral competence.
Externalizing behavior problems are one of the areas of research associated with MAOA polymorphisms in humans. In particular, interaction of low MAOA transcription polymorphisms with environmental factors such as early abuse and deprivation (Derringer et al., 2010, Enoch et al., 2010, Kinnally et al., 2009) has been found to promote occurrence of conduct disorder, antisocial behavior, and violent behavior. Other developmental stressors such as poor nutrition and exposure to toxic agents have not yet been studied for their ability to interact with MAOA polymorphisms to alter these important aspects of human behavior.
We have established a nonhuman primate model to examine the effects of 3d trimester iron deficiency on infant behavioral development in the rhesus monkey that uncovered a strong affective component to the syndrome produced (Golub et al., 2007, Golub et al., 2006a, Golub et al., 2006b). In addition we have been able to access information from colony wide genotyping for monoamine oxidase A (rhMAO-A-LPR) promoter polymorphisms (Newman et al., 2005) at the California National Primate Research Center (CNPRC). In the current experiment, we study a potential interaction of prenatal iron deficiency with MAOA polymorphism in male rhesus monkeys.
The focus for this interaction study was response to social challenge of monkeys 3 to14 months of age. In addition to the extensive literature linking MAOA polymorphisms to aspects of social behavior in humans, there is also a small literature in nonhuman primates (Karere et al., 2009, Kinnally et al., 2010, Newman et al., 2005), This work complements other research effort on the influence of serotonin and other monoamine neurotransmitters on modulation of social behavior in nonhuman primates (Fairbanks et al., 1999, Fairbanks et al., 2001, Manuck et al., 2003). Here we included a series of structured social challenges in a more extensive one-year test battery. In addition, we included noninvasive measures of brain monoamines (CSF monoamine metabolites and PET scans for striatal dopamine D2 receptor binding (DRD2)). CSF monoamines are influenced by developmental iron deficiency in monkeys (Coe et al., 2009), and by MAOA polymorphisms in humans (Aklillu et al., 2009, Jonsson et al., 2000, Zalsman et al., 2005). DRD2 receptors numbers are altered in iron deficiency in rats (Beard et al., 2003, Unger et al., 2007, Unger et al., 2008) and in MAOA-deficient transgenic mice (Levant et al., 2001).
Materials and methods
Assurance of compliance with animal codes
All procedures followed the Guide for the Care and Use of Laboratory Animals of the National Research Council. CNPRC is accredited by the Association for Accreditation of Laboratory Animal Care. Protocols for this project were approved prior to implementation by the UC Davis Institutional Animal Care and Use Committee (IACUC).
Animals and animal care
The nonhuman primate model for diet-induced third trimester iron deficiency has been described previously (Golub et al., 2006), including information on caging, environmental control and diet composition. Briefly, time-mated rhesus monkeys were assigned to iron sufficient (100 ppm iron, IS group; n=10) or iron deficient (10 ppm iron, ID group; n=10) purified diets at 35–45 days gestation. Animal selection utilized CNPRC electronic records. Breeders were screened for prior reproductive history and the two groups were balanced as much as possible for age, weight and parity. Dams within the same group were housed in pairs. Fetal sex was also determined at this time (Jimenez & Tarantal, 2003) and only pregnancies with a male fetus were selected for the study. Subjects were limited to the same sex to increase power by decreasing intragroup variability due to sex differences. Males were chosen because they were more sensitive to some behavioral effects of prenatal iron deficiency in previous studies.
Ultrasound exams were conducted at gestation day (GD) 90 to confirm fetal viability. After birth, nonpurified iron sufficient diets (Purina Lab Diet #5037) were provided, and, additionally, dams and infants in the ID group were given supplemental iron in the early postnatal period. Two mother-infant pairs from the same group were housed together in a double cage in indoor colony rooms limited to subjects of this experiment. Weanlings were re-paired with a new peer based upon the availability of an age appropriate companion. Eight out of 10 pairs were comprised of one IS and one ID infant; one pair contained two IS infants and one contained two ID infants. All but five of the infants were of Indian descent; the five Indian-Chinese hybrids, ranging from 12.5% to 37.5% Chinese, were in both the ID group (n=3) and IS group (n=2).
Genotyping
As previously described (Karere et al., 2009, Kinnally et al., 2010) genomic DNA was isolated from lymphocytes from routine blood samples obtained at 3 months of age from most rhesus infants in the colony. Genotyping of the MAOA length polymorphic region used primers designed from the published sequence for rhesus. Genotypes were determined with STrand software and confirmed by direct sequencing. CNPRC colony population genetics (Kinnally et al., 2009) indicate that 57% of male infants are hemizygous for VNTR polymorphisms with 4, 5 or 6 repeats, associated with high MAOA transcription (Newman et al., 2005), while 43% have the low MAOA activity polymorphism (7 repeats). These proportions are similar to human populations. In the IS group, 5 infants had the low MAOA activity genotype and 5 had the high MAOA activity genotype. The genotypes were also balanced within the ID group (low-MAOA, n=4; hi-MAOA, n=5). Weanling cagemate pairs consisted of both mixed (low-MAOA/hi-MAOA, n=4) and identical (low-MAOA/low-MAOA, n=2; hi-MAOA/hi-MAOA, n=3) genotypes. One infant had an ambiguous genotype (6.5 repeats) and was therefore excluded from analyses of diet*MAOA interactions.
Assessment schedule
A summary of the social assessments presented in this report is provided in Table 1. The complete schedule of assessments, including nonsocial and nonbehavioral evaluations, is provided in Supporting Information, Table S1.
Table 1.
Social Assessment and Nonbehavioral Assessment Schedule
Summary of assessments by age, and corresponding apical variables, for social tests contained in this report. See Supporting Information, Table S1, for a complete list of assessments throughout the study.
| Age | Sessions | Assessment | Apical variable |
|---|---|---|---|
|
| |||
| Day of birth | 1 | CBC, blood samples for iron status and lymphocyte assay, morphometrics | Body weight, hemoglobin |
| 3–4 months | 1 day | Biobehavioral assessment Adult nonsocial/aggression video Temperament rating |
Time looking at nonsocial and aggressive video segments Rating on 16 temperament scales |
| 4 months | 1 | CSF sample | 5HIAA, HVA |
| 6 months | 1 | CBC, blood samples for iron status, morphometrics | Body weight, hemoglobin |
| 7–8 months | 2 | Social buffering | Change in plasma cortisol |
| 8–12 months | 1–2/week | Social dyadic interaction | Affiliation behavior |
| 11–12 months | 2 | Social intruder | Proximity to intruder |
| 12 months | 1 | CBC, blood samples for iron status, morphometrics | Body weight, hemoglobin |
| 12–14 months | 3 | Picture-elicited aggression | Fear, aggressive behavior |
| 18–24 months | 1 | PET scan | DRD2 binding potential |
| 22.5 months | 1 | CBC, blood samples for iron status, morphometrics | Body weight, hemoglobin |
Growth, hematology and iron status evaluations
The infants were evaluated at birth, 6 months, 12 months and 22.5 months of age for growth (body weight and morphometrics), hematology, and iron status parameters (ferritin, plasma iron, ZPP, TIBC, %Tf saturation). These data will be reported in detail separately.
CSF monoamine metabolites
Cerebrospinal fluid samples (0.5 mL) were obtained by cisternal puncture at 4 months of age using ketamine (10 mg/kg) and medetomidine (20–40 μg/kg) reversed with atipamezole (20–40 μg/kg). Samples were frozen until analysis when a 50 μL aliquot of CSF was used for HPLC analysis. Perchloric acid (50 μL) and internal standard 3,4-dihydroxybenzylamine (DHBA; 10 μL) were added to each aliquot, and then samples were passed through a 0.2 μm micro-Sephadex column to remove endogenous substrates. Samples were loaded onto a refrigerated ESA model 542 autosampler and 10 μL of sample was injected onto an ESA MD-150 narrow-bore HPLC column (150 × 2 mm; ESA Inc). The mobile phase consisted of 75 mM sodium phosphate, 1.7 mM 1-octanesulfonic acid, 25 μM ethylenediaminetetracetic acid, 7.0 μM triethylamine, and 10% v/v acetonitrile in a volume of 2 L (pH 3.0). Once separated, compounds were measured with a coulometric detector (ESA model 5300, guard cell potential, +400 mV; working cell potentials, −174 mV and 350 mV). The neurotransmitter metabolite peak areas were integrated using EZ Chrom Elite software and quantified against known standards.
PET scans for dopamine D2 receptor
Animals were anesthetized with ketamine (10 mg/kg i.m.), intubated and connected to isoflurane anesthesia for imaging utilizing the first generation microPET scanner, P4 (Siemens Preclinical Imaging). [11C]-Raclopride was synthesized at the Center for Molecular and Genomic Imaging. Approximately 2.0 mCi of [11C]-Raclopride was injected 5 seconds after the start of the scan. Dynamic PET scans were acquired for 90 minutes, then a 57Co transmission scan was acquired. Listmode data were histogrammed into dynamic frames as follows: 10 frames of 60 seconds, 5 frames of 120 seconds, 4 frames of 300 seconds, and 5 frames 600 seconds. Attenuation sinograms were generated from forward projecting the smoothed segmentated transmission images. Images were reconstructed with a 3DRP reconstruction protocol utilizing attenuation and scatter corrections. Data analysis was performed using Inveon Research Workplace. Regions of interest (caudate, putamen) were drawn on the PET images of the striatum (left and right). Cerebellum was used as a reference tissue. Kinetic modeling determined the binding potential of raclopride in the striatum. Scans from two infants could not be used due to technical problems.
Video of Adult Male from the Biobehavioral Assessment
The biobehavioral assessment has been described previously (Golub et al., 2006, Golub et al., 2009). Briefly, most 3–4 month old infants in the CNPRC colony, including the infants in this study, were separated from their home environments for 25 h and given several successive assessments including response to videos of adult monkeys, and a rating of temperament. For the adult video test, a 10 min video of an adult male monkey which contained segments showing aggressive displays was shown on a monitor 1 m from a test cage with a clear plexiglass front holding the infant. Looking directed at the monitor was scored for frequency and direction, and species-typical behaviors related to fear and aggression were scored for frequency from videorecordings made during the session. The temperament of each infant was rated on a scale of 1 to 7 (1 being total absence and 7 being extremely large amount) in 16 different categories by a trained observer at the conclusion of the 25 h test period. The definitions of the temperament categories are in Supporting Information, Table S4. Additional tests not involving social stimulation (home cage observations, human intruder, plasma cortisol responsiveness, response to novel object in the holding cage, and visual novelty preference) are not reported here.
Dyadic social interaction
This multiple session, round-robin approach to social dyadic testing helps remove the influence of different partners in evaluating social interaction. The test was conducted as a 10 min session once or twice a week for a total of 20 sessions between the ages of 8 and 12 months of age. It evaluated the ability of the monkeys to regulate their social interactions, primarily play (Golub et al., 2005). The monkey infant was paired with all other monkey infants (excluding its cagemate) currently in the experiment in a round robin manner. Most monkeys had eight play partners. Three monkeys had more than 20 sessions (22, 23, 27) to accommodate the need for play partners. Behavior was coded during the session by observers trained for reliability using the Observer software (Version 5.0, Noldus Information Technology, Wageningen, The Netherlands). The ethogram included five states (duration recorded): four affiliative social behaviors (rough and tumble play, chase play, cling and grooming) and a nonsocial state. Frequencies of individual social behaviors (lipsmack, displace, mount, fear grimace, threat, sniff, genital inspection as well as motor stereotypies) were also recorded. Initiator and recipient of each behavior were recorded. The same state durations were used in the data set for both members of the dyad. For analysis, values were summed across all sessions, and adjusted to 20 for infants with >20 sessions. Derived variables included percent active affiliation (play/play+cling).
Social/nonsocial intruder
This test of anxiety and impulsivity is based on previous work with adult monkeys (Bethea et al., 2004, Fairbanks et al., 1999, Manuck et al., 2003). It was comprised of two sessions 15 to 20 min in length conducted between 11 and 12 months of age. The subject in its home cage was separated by a metal grate from an adjacent cage. The social intruder (unfamiliar young adult male rhesus) or nonsocial intruder (remote control car followed by a familiar (apple) and unfamiliar (pineapple) fruit) were placed in the adjacent cage. Behavior was scored by observers trained for reliability using The Observer software. Behavioral evaluation focused on approach and proximity to the intruder. The subject was either in close proximity or far proximity to the intruder at all times; the first occurrence of “near proximity” was used as the latency to approach the intruder. Other ethogram behaviors included touching and sniffing at the metal grate; pacing; freezing; aggressive behaviors such as lunge, hit, threaten; and distress behaviors such as yawn, scratch, shake, stereotypy, fear grimace and lipsmack.
Social buffering
This test evaluates the ability of a familiar cagemate to mitigate the stressfulness of a novel environment (Winslow et al., 2003). The test consisted of two 30 min sessions conducted one week apart in 7–8 month old infants, one with the subject alone in a standard cage located in an unfamiliar workroom and one with the cagemate in a second novel workroom. The order of sessions (alone and with cagemate) was balanced for each group. Sessions were video recorded for coding using established ethograms on The Observer. After each session an 0.5 mL blood sample was obtained for analysis of cortisol by RIA (Golub et al., 2009).
Picture elicited emotional response
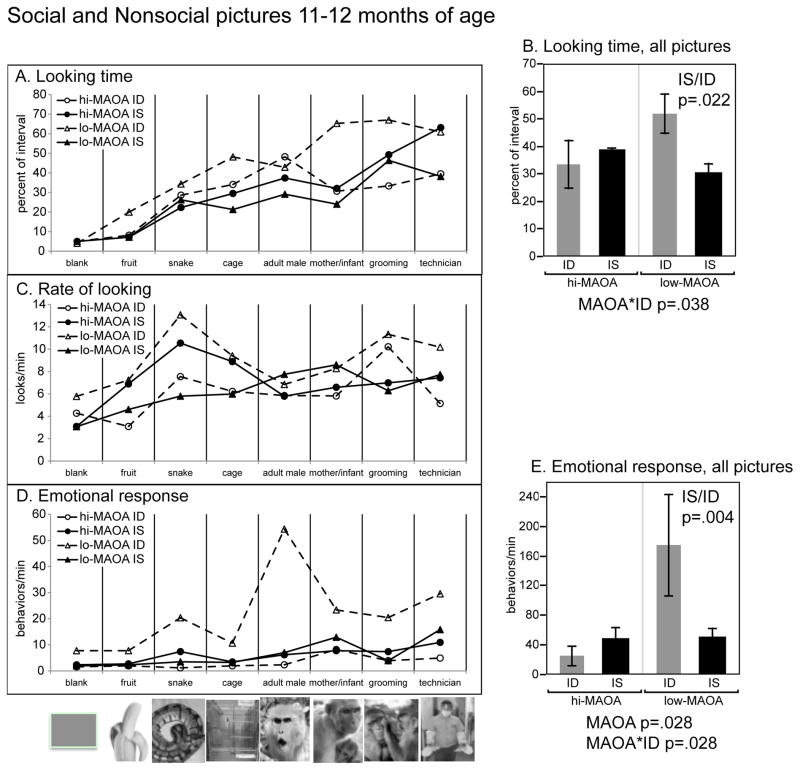
This test evaluates vocalizations and facial expressions when viewing either threatening/frightening images or familiar/neutral images (Kyes et al., 1995). Infants 12 to 14 months of age viewed a brief (11.5 min) slide show on three consecutive days. The eight slides (see Figure 4) were: a plain light green colored slide; fruit (apple slice and half peeled banana); a snake; a cage (identical to the home cage); an adult male monkey with open mouth stare; a mother and infant monkey; two monkeys grooming; and a technician dressed in protective clothing wearing leather gauntlet/handcatching gloves. The pictures were prepared as a Powerpoint slide show. Three slide orders were used for each monkey, randomized across sessions. Each slide was displayed for 30 sec followed by a 1 min interslide interval of a black screen. The test cage had a plexiglass front and each session was video recorded for later coding using The Observer. Time looking at the monitor, activity, and stereotypy were scored for duration and an ethogram was used to score the incidence of individual vocalizations and facial expressions.
Figure 4.
Diet*MAOA interactions on test of picture elicited emotional response. See Figure 2 for formatting conventions.
Statistical analysis
Most variables were analyzed by ANOVA using general linear models (JMP9.0, SAS, Carey, NC). Effects evaluated were diet group (iron deprived, ID; iron sufficient, IS), MAOA genotype category (hi-MAOA, low-MAOA expression) and diet*MAOA interaction. One infant in the study had an ambiguous MAOA VNTR (6.5 repeats). Due to lack of empirical data on MAOA expression with this polymorphism, this infant (in the ID group) was excluded from data analyses including the MAOA variable. In addition to ANOVA, planned comparisons were conducted comparing ID and IS groups within each MAOA genotype group. Because the infants were tested in two yearly cohorts, cohort was examined for association with major endpoints and included as a covariate when p<.10.
Each test was structured to focus on a different aspect of social-emotional responsiveness and included one or two “apical” variables related to the purpose of the test. Most tests also included coded behavioral observation using an ethogram. To limit the statistical tests performed, and resulting potential for false positive results, individual items in the ethograms were not evaluated unless effects were seen on apical variables. Additionally, components of the ethogram were grouped into composite scores.
Results
Postnatal growth and health
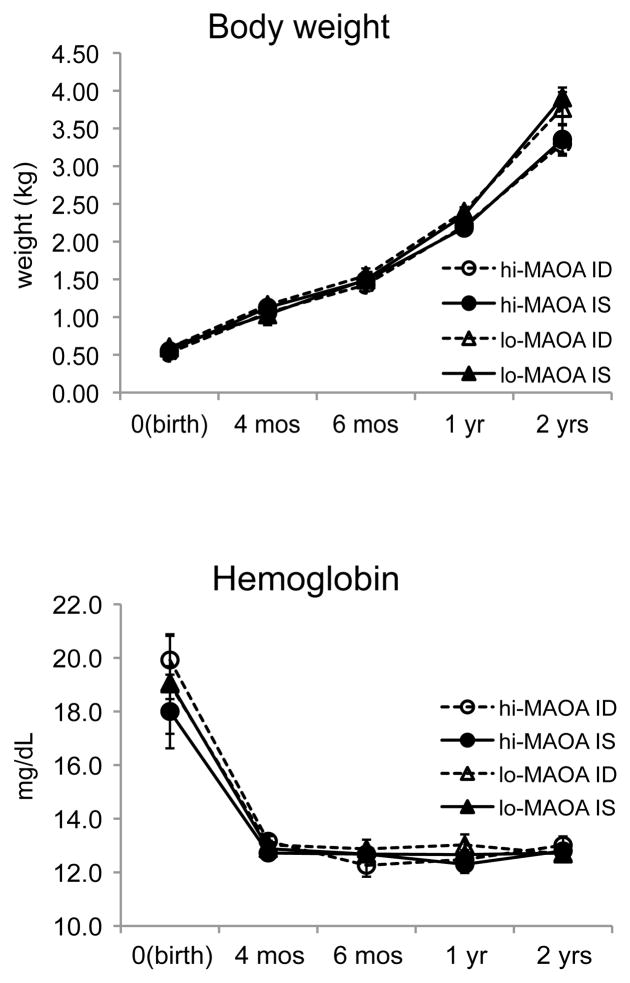
As indicated in Figure 1, prenatally iron deprived infants were not growth retarded or anemic during the evaluation period. More details on growth, iron status, and hematology will be provided separately. These endpoints were not found to reflect MAOA genotype or interactions of diet with MAOA genotype, with the exception of head circumference at birth (corrected for gestational age) which was lower in the ID than in the IS subgroup of the low MAOA category.
Figure 1.
Normal growth and hematology through two years of life as represented with body weight and plasma hemoglobin.
CSF monoamine metabolites
No effects of MAOA, diet, or diet*MAOA interactions were found for the CSF analysis. Although peaks for the three monoamine transmitters and their metabolites were examined, only 5HIAA and HVA had quantifiable values. Values were very similar across individuals and groups. Data are provided in Supporting Information, Table S2.
PET for D2 receptor in striatum
Binding potential measure for dopamine D2 receptors (DRD2) in right and left caudate and putamen did not show significant diet or diet*MAOA interaction, while the MAOA effect in the two-way ANOVA was marginally significant (.05<p<.10) for right side values. Discarding the diet variable from the ANOVA, low-MAOA infants had significantly lower DRD2 binding potentials than the hi-MAOA group for right caudate F(1,15)=4.76, p=0.045 and right putamen F(1,15)=5.11, p=0.039. The mean difference was close to significant for left caudate (p=0.10) and left putamen (p=0.052). Binding potentials are in Supporting Information, Table S3.
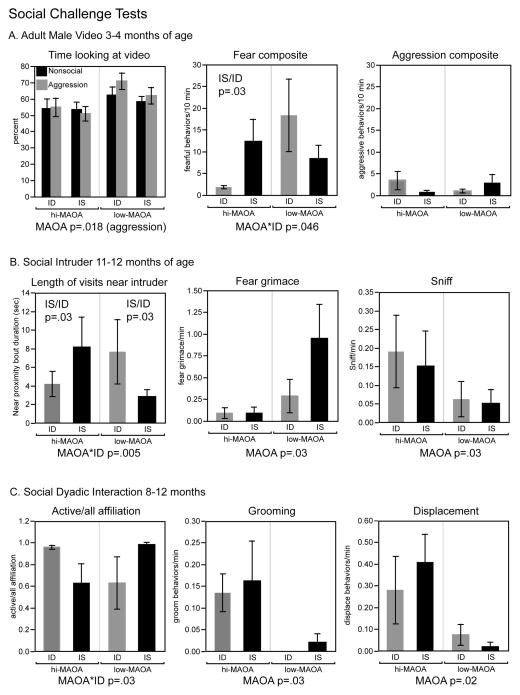
Response to video of adult male
Under the two conditions of the test, a video of an adult male showing both nonsocial and aggressive behaviors, ANOVA showed a strong effect of the MAOA variable on the amount of time spent facing toward the monitor (F(1,15)=6.98, p=0.018) and away from the monitor (F(1,15)=14.79, p=0.002) during the aggressive segments of the video (Figure 2A). A third category, “out of view”, was used if the infant’s face was positioned such that gaze direction could not be determined. There were no effects on the “out of view” category. The low-MAOA group spent more time facing the monitor than the hi-MAOA group. Discrete behaviors were combined into an index of aggressive behaviors (cage shake, threat, bark) and fearful behaviors (self-clasp, fear grimace, scream). These indices were higher during the aggressive than the nonsocial segments of the video (aggressive: F(1,19)=7.44, p=0.013; fearful: (F1,19)=8.80, p=0.008). MAOA genotype did not significantly affect either of these indices during either the nonsocial or aggressive segments. Diet group did not influence the time facing the monitor or the aggressive behavior indices. Notably, little aggressive behavior was seen in these young infants; less than half of the infants demonstrated more than one aggressive behavior during the aggression segment. However, a diet*MAOA interaction was seen for the fearful behavior index during aggressive video segments (F(1,15)=4.74, p=0.046) Planned comparisons between ID and IS infants within genotype category were not significant. The hi-MAOA ID infants showed the least fearful behavior while the low-MAOA ID infants had higher emotional scores than the low-MAOA IS infants.
Figure 2.
MAOA-ID interactions on three social challenge tests. Main effects and interaction from ANOVA are shown under each graph. Post hoc comparisons of ID and IS groups within genotypes are shown above the bars. Mean ± sem are shown.
The infants responded to the aggressive segments of the videos with behaviors related to fearful displays in adults. An interaction pattern suggested that hi-MAOA infants, particularly the hi-MAOA ID infants demonstrated the least fearful behaviors while low-MAOA ID infants demonstrated the most fearful behaviors.
Temperament ratings
There were no main effects of MAOA or diet group on temperament ratings (data not shown), although several ANOVA suggested a potentially relevant effect size (p= 0.05<p<0.10). Significant diet*MAOA interactions were found for two descriptors, “fearful” (F(1,15)=7.07,p=0.018) and “slow” (F(1,15)=4.89, p=0.043). A marginal interaction was also seen for “confident” (F(1,15)=4.01, p=0.064). For the “fearful” descriptor, planned comparisons showed ID and IS infants differed only within the hi-MAOA genotype category, with the ID infants having lower “fearful” ratings (p=0.006). The complementary pattern was seen for the “confident” scores, with hi-MAOA ID group having the highest “confident” scores, significantly higher for the ID infants in the hi-MAOA vs. low-MAOA genotypes (p=0.04). For the “slow rating” the direction of the ID effect was different within the two MAOA categories, with higher “slow” rating within the hi-MAOA category and a lower rating with the low-MAOA category, but planned pairwise (ID/IS) comparisons were not significant. Description of the behaviors used for rating these temperaments are provided in the Supporting Information Table S4.
Taken together, the data from the 3–4 month old infants indicated that the hi-MAOA ID infants were generally less fearful, more confident and slower moving than IS infants with the same genotype. In the low-MAOA group, the opposite pattern was seen.
Social Intruder
An apical endpoint for this test was time spent in half of the cage closest to the intruder. Effects were not significant for total time spent near the stranger, but significant diet*MAOA interaction was seen for the average duration of each visit to that side of the cage (F(1, 12)=11.43, p=0.005)(Figure 2B). For the hi-MAOA genotype, the ID group had shorter visits to the side of the cage near the stranger than the IS group (p=0.026). For the low-MAOA genotype, ID infants had longer visit to the side of the cage near the stranger than IS infants (p=0.029).
Analysis of individual social behaviors such as sniff and present to groom as well as facial expressions and vocalizations (Figure 2B) illustrated that in general hi-MAOA infants had more positive social responses (sniff, lipsmack, present), and less negative social responses (threat, fear grimace), although not all reached statistical significance: fear grimace, F(1, 15)=5.47, p=0.034; sniff, F(1,14)=5.96, p=0.028, lipsmack p=0.052, threat p=0.14). There were no diet effects or diet*MAOA interactions for these variables.
For purposes of comparison, a test with nonsocial intruders (remote control toy, novel fruit) was also conducted. This test, previously used for adult monkeys living in an outdoor environment, was not effective in indoor-reared, test-experienced infants in our sample. Only about 1/3 of the infants made any response to the mechanical toy intruder. ANOVA did not demonstrate differences between groups in latency to inspect, touch and eat the familiar or novel fruit.
Overall the data from intruder tests in the year-old infants indicated positive social response to the stranger in the hi-MAOA group, and less regulated approach to the stranger in the ID infants of the low-MAOA group.
Social dyadic interaction
This test allowed assessment of direct physical social interactions. The main focus was affiliative behavior (play, cling). (Infants at this age do not display aggressive actions). Effects were not seen on individual affiliative behavior or the sum of all affiliative behaviors. However the ratio of the duration of active (play) to all (play and cling) affiliative behavior demonstrated a diet*MAOA interaction (F(1,15)=5.89, p=0.028). Neither of the planned comparisons was significant. Data shown in Figure 2C indicate the interaction was generated by difference in direction of ID and IS means under the two MAOA genotype categories. ID infants in the hi-MAOA genotype demonstrated active affiliate behavior (play) almost exclusively, while IS counterparts demonstrated about 60% active affiliative behavior. In contrast, the active/passive affiliation was less in the ID than the IS infants from the low-MAOA genotype group. The difference in the ratio of active to passive affiliation was determined by both more play and less cling in the individual infants with higher ratios.
An MAOA effect was seen for the number of grooming episodes (F(1,15)=5.97, p=.027). Hi-MAOA infants spent more time grooming than low-MAOA infants. Vocalizations were not scored for this test but facial expressions (threat, fear grimace, lipsmack) were not affected by diet or MAOA. Of the additional discrete physical interaction behaviors observable in this situation (displace, sniff, genital inspect, mount) an effect of MAOA was seen for displace (approach and replace an infant who moves away upon approach) (F(1,15)=6.60, p=0.021). Hi-MAOA infants initiated more displacements than low-MAOA infants. As in social video and confined stranger tests, infants showed very little behavior that could be classified as aggression-related; fifteen of the 20 infants averaged <1 threat per session. Vocalizations were not scored for this test but facial expressions (threat, fear grimace, lipsmack) were not affected by diet or MAOA.
The MAOA genotype appeared as an important determinant of physical social interaction in this test with the hi-MAOA group showing more grooming and more displacements than the low-MAOA group. The ID influence was on the use of active vs. passive affiliation (play vs. cling) and was modified by MAOA genotype. The ID infants with the hi-MAOA genotype showed more active affiliation (play) while the ID infants with the low-MAOA genotype showed less active affiliation than corresponding IS infants with the same genotype.
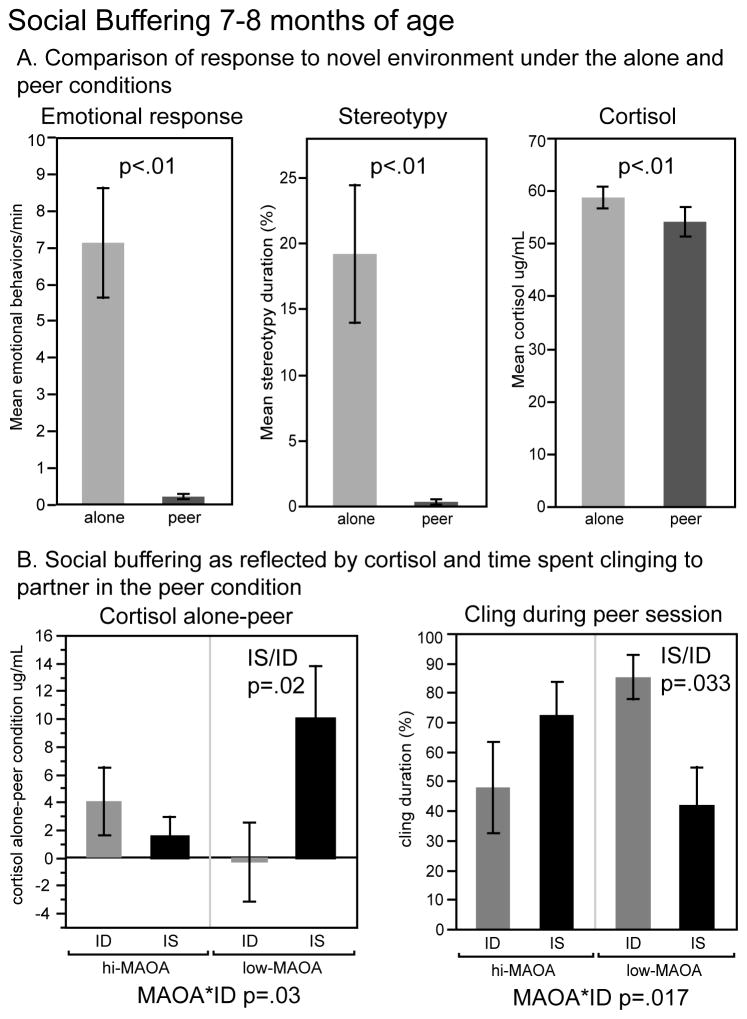
Social buffering
Figure 3A shows the social buffering effect in all subjects as reflected in reduction in emotional responses (crouch, scream, threat, bark), stereotypy, and plasma cortisol. The apical variable was the difference between plasma cortisol after the alone and peer sessions in the novel environment (Figure 3B). A diet*MAOA interaction was seen for this variable (F(1,15)=5.73, p=0.030). ID infants showed less social buffering than IS infants within the low MAOA genotype category (p=0.023). A diet*MAOA interaction was also seen for partner clinging (F(1,15)=7.20, p=0.017), which was higher in the ID than the IS infants within the low-MAOA group (p=0.033).
Figure 3.
Monkeys were placed in a novel environment either alone or with their cagemate for the Social Buffering test. A. Response to the novel environment in the alone and peer conditions is shown for all infants. B. Effects on social buffering as reflected in a decrease in plasma cortisol between the alone and peer conditions, and in the duration of clinging during the peer condition. See Figure 2 for formatting conventions.
Picture-elicited emotional response
As illustrated in Figure 4, response to the eight pictures differed markedly in terms of looking durations (RMANOVA, picture, p<0.0001), looking frequencies (RMANOVA, p=0.003), as well as emotional response to the pictures (RMANOVA, p=0.03). Response to the pictures was also affected by diet*MAOA interactions. In general the low-MAOA ID group was more responsive to the pictures with social-emotional content.
For looking durations (Figure 4A), response to the inanimate pictures was generally less than to the social pictures and the technician picture. A significant diet*MAOA interaction was seen across all pictures during the first exposure (session 1: F(1,15)=5.20, p=0.038)(Figure 4B). The low-MAOA ID group had significantly higher looking durations than the low-MAOA IS group across all pictures (p=0.022). The interaction was also significant for the three social pictures as a group (grooming, aggressive male, mother/infant: F (1,15)=5.81, p=0.029, low-MAOA ID vs. IS p=0.041), and for the technician picture: diet*MAOA F(1,15)=5.19, p=0.039, low-MAOA ID vs. IS, p=0.066. As seen in Figure 4B the difference between the means of the ID and IS groups were in the opposite direction in the hi-MAOA group. However this difference was not significant in planned pairwise testing.
For looking frequency (Figure 4C), the snake picture elicited the highest rates of looking (with a corresponding lower duration of each look). A significant diet*MAOA interaction was seen for this picture (F(1,15)=4.87, p=0.043) with a significant pairwise comparison between the low-MAOA ID and IS groups (p=0.048). A diet*MAOA interaction was also seen for the cage picture, but pairwise comparisons were not significant. For all pictures, looking frequency was highest in the low-MAOA ID infants.
For looking habituation (session 1–3), an diet*MAOA interaction was seen across all pictures (F(1,15)=4.96, p=0.042, data not shown). Habituation was greater in the low-MAOA ID vs. IS group (p=0.034), perhaps reflecting the greater initial duration of looking in the low-MAOA ID group. No interaction was seen for individual pictures.
The apical variable for this test was the frequency of display of emotional (fear and aggressive) behaviors (Figure 4D). These composite indexes were evaluated for the first exposure of the pictures, session 1. In RMANOVA across all pictures, the effect of picture on the combined index of fear and aggressive behaviors did not reach significance (F(1,15)=2.90, p=0.070) (Figure 4E). There was an effect of MAOA (F(1,15)=5.93, p=0.028), and a diet*MAOA interaction (F(1,15)=5.90, p=0.028) for the combined index. In addition the MAOA effect was independently shown for the aggression index F(1,15)=4.97, p=0.041, and the diet*MAOA interaction was shown for the fear index (F(1,15)=4.89, p=0.043) (data not shown). For both interactions, the low-MAOA ID and IS groups differed in planned comparisons (fear, p=0.010, fear+aggression, p=0.004).
For individual pictures, the emotional response diet*MAOA interaction was significant for the grooming picture (F(1,15)=4.79, p=0.045, low-MAOA ID/IS p=0.037), and the snake picture (F(1,15)=6.68, p=0.021, low-MAOA ID/IS p=0.001). For the technician picture, the MAOA main effect was significant (F(1,15)=9.77, p=0.007). This MAOA effect was due primarily to aggressive behaviors (F(1,15)=9.34, p=0.008).
Stereotypy (data not shown) was also examined as a possible response to emotion-eliciting slides. Twelve of 20 subjects showed no stereotypy (n=9) or only 1 incidence of stereotypy (n=3) during the slide presentations. These infants were distributed evenly between the ID (n=6) and IS (n=6) groups. In monkeys exhibiting stereotypies during testing, the lowest incidence of stereotypy occurred for the snake (10%) and technician (13%) slides, while the highest occurred for the blank slide (22%) and the empty cage slide (24%), suggesting that stereotypy was suppressed rather than aggravated by emotion-eliciting slides. There was no diet or MAOA effect, and no interaction, for the stereotypy endpoint.
Discussion
This study provides both an insight into behaviors affected by MAOA polymorphisms in normal infants, and a new potentially important environmental interaction, with developmental nutrition. It also brings some focus to a sensitive early developmental period for gene-environment interactions, the fetal period.
A main effect of MAOA genotype was seen in several socio-emotional tests (Figure 5). In general the low-MAOA infants may be described as having less emotional regulation and/or more emotional responsiveness than the hi-MAOA group. An integrative concept might be that hi-MAOA leads to more effective regulation of behavior in challenging situations. This finding parallels a single study of human infants (Zhang et al., 2011) which found better emotional regulation reflected in gaze aversion from a threatening visual stimulus (White et al., 2009) in 6-month old infants (girls only) with high expression MAOA polymorphisms.
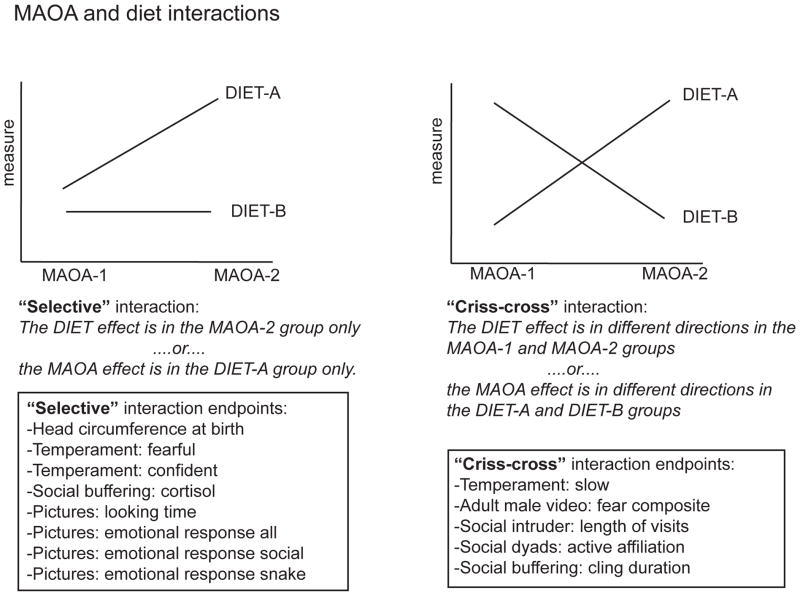
Figure 5.
Summary of types of diet*MAOA interactions, and endpoints demonstrating interactions in social-emotional testing.
In humans, both low and high MAOA transcription genotypes have both been associated with heightened risk of neurobehavioral disorders. Specifically risk for hyperactivity and conduct disorder in children was associated with low-MAOA genotype (Enoch et al., 2010), while risk for autism (Cohen et al., 2011, Tassone et al., 2011), suicide in depression (Lung et al., 2011) and impulsive violent criminal recidivism (Tikkanen et al., 2010) were associated with the hi-MAOA genotypes. These risks can be modified by sex, ethnicity, child abuse, and alcohol abuse (Armony-Sivan et al., 2011, Aslund et al., 2011, Kim-Cohen et al., 2006, Tikkanen et al., 2010). In addition to interacting with environment to increase risk of abnormal behavior, MAOA genotypes are associated with differences in personality and temperament (Buckholtz et al., 2008, Longato-Stadler et al., 2002, Tsuchimine et al., 2008). The present study extends to infancy the lifespan periods when MAOA genotypes can influence behavior in combination with environmental influences.
Prenatal ID interacted with MAOA genotype in each of the tests. The pattern of the interaction generally indicated that the effect of ID depended on genotype, either in that the ID effect was seen only or predominantly in one of the MAOA polymorphism categories (“selective” interaction), or the ID effect appeared in both MAOA groups, but the direction of the effect differed (“criss-cross” interaction) (Figure 5). Notably, of the three other studies of MAOA polymorphisms and behavior in rhesus (Karere et al., 2009, Kinnally et al., 2010, Newman et al., 2005), two also found interaction with an environmental variable, rearing environment, which influenced emotional responses in rhesus in different directions depending on genotype (Karere et al., 2009, Newman et al., 2005).
Our previous studies of behavior in infant and juvenile monkeys deprived of iron prenatally suggested a syndrome of reduced emotionality and less inhibition of behavior in novel situations. During infancy, testing indicated lower inhibitory response to novel environment (Golub et al., 2006). As juveniles, prenatal ID led to less emotionality in a novel environment, shorter latencies to respond and fewer trials with no response while learning cognitive tasks (Golub et al., 2007). This syndrome more closely paralleled the ID effect in the hi-MAOA group than the low-MAOA group of the present study. Genotyping was not available in previous studies, and both males and females were tested, so that direct comparisons cannot be made.
We examined the social-emotional response of infants in five quite different situations not expected to elicit the same type of responses or intensity of response. Emotional responsiveness was assessed as the frequency of facial expressions, vocalizations and behaviors associated with fear and aggressive displays in older monkeys (Hinde & Rowell, 1962). While these components of aggressive behavior are seen in the infants they have not matured into adult patterns of social interaction that could be called aggressive. The possible influence of MAOA genotype on aggression relevant to studies of violence and conduct disorder in humans (Buckholtz & Meyer-Lindenberg, 2008, Grigorenko et al., 2010, Taylor & Kim-Cohen, 2007) would have to be tested in adult nonhuman primates. The present study is more relevant to human findings of an association between MAOA genotype and personality/ temperament (Buckholtz et al., 2008, Fahlke et al., 2002, Huang et al., 2004, Sebastian et al., 2010, Tsuchimine et al., 2008, Williams et al., 2009). More importantly it addresses the as yet little explored area of MAOA polymorphism and infant behavior.
Monoamine systems in brain are easily proposed as the site for interacting influences of MAOA and ID but more specific mechanism hypotheses are difficult. The classification of high and low activity MAOA genotypes is based on MAOA gene transcription in in vitro systems, not enzyme activity. Attempts to evaluate functional differences in central monoamine neurotransmission with noninvasive methods in humans have not found consistent patterns (in CSF assays) (Aklillu et al., 2009, Ducci et al., 2006, Jonsson et al., 2000, Williams et al., 2003, Zalsman et al., 2005), or changes in monoamine oxidase protein levels (with PET studies) (Fowler et al., 2007). The current study did not find genotype differences in CSF monoamine metabolites at 4 months of age. Because monoamine systems as reflected in CSF change dramatically in rhesus during the first year of life (Coe et al., 2009, Higley et al., 1991, Higley et al., 1992), later sampling is needed. We also failed to find difference in DRD2 binding via PET. DRD2 binding is associated with a variety of behavioral traits in adult humans (Egerton et al., 2010, Kim et al., 2011, Zald et al., 2008), but similar work has not been done in infants and children.
As regards iron deficiency, early studies documented decreased peripheral MAO activity in iron deficient rats (Youdim & Green, 1978), but no effects of iron deficiency on CNS MAO activity have been found (Ashkenazi et al., 1982, Mackler et al., 1979, Schauer et al., 1989, Shukla et al., 1989, Youdim et al., 1980). However, monoamine neurotransmitter levels in brain are altered by developmental iron deficiency in rodents. Both MAOA and MAOB are found in brain with differential localization and function (Shih et al., 2011, Westlund et al., 1985) and metabolic disturbances secondary to iron deficiency may modify activity of both of these enzymes while MAOA polymorphisms would only influence MAOA. A change in the balance of the two could underly the differential “criss-cross” interactions of MAOA genotype and ID.
If MAOA genotype and ID interact to influence monoamine transmitter systems in emotional regulation, a brain circuit of interest would be the serotonin system in orbitofrontal cortex and its connections with amygdala and cingulate cortex. This circuit is well studied in nonhuman primates (Izquierdo & Murray, 2004, Man et al., 2011, Roberts, 2011, Way et al., 2007), in connection with MAOA polymorphism and psychopathology in humans (Gottfried et al., 2003, Lewis et al., 2011, Meyer-Lindenberg et al., 2006, Volkow et al., 1999, Winstanley et al., 2004) and also in MAOA hypomorphic mice (Bortolato et al., 2011)
An equally interesting hypothesis concerning the site of MAOA*ID interactions involves processes basic to structural brain development, like cell division and differentiation, myelination and synapse formation. This hypothesis is suggested by recent studies of MAOA influences on early brain development (Cheng et al., 2010, Wang et al., 2011) and also structural brain differences associated with MAOA polymorphisms (Meyer-Lindenberg et al., 2006). Myelination and synapse formation are processes also known to be influenced by developmental iron deficiency in rodent studies (Georgieff, 2008, Lozoff et al., 2006).
While some attention has been directed toward genetic variations in iron regulatory genes that influence the risk for developing anemia (Beutler et al., 2010, Lee et al., 2001), no information to date has been directed at genes modifying risk of iron deficient infants for developing childhood behavior disorders. The nonhuman primate model of iron deficiency offers the opportunity to extend the observation that the two common human MAOA polymorphisms provide markedly different backgrounds for adapting to the impact of environmental factors.
The reappearance of the genotype*interactions in several social tests over the first year of life in our study supports persistent trait-like effects. Limitations of the study include the small group size (n=4–5/group), and multiple statistical tests on the same groups. Low power promotes false negative results, while multiple tests promote false positives. In many cases detection of significant interactions was not followed up by significant pairwise comparisons to clarify the nature of the interaction. Replication in other samples is needed for confident generalization of influences to behavior of all young rhesus.
Supplementary Material
Acknowledgments
The authors thank and acknowledge Laura del Rosso and Laura Colander, who conducted the 3-month behavior testing, CNPRC personnel who cared for the daily well-being of the animals, and colleagues from the Brain and Behavior in Early Iron Deficiency Program Project who provided support and insight into the planning and interpretation of the study. Supported by HD39386 (B. Lozoff PI), RR019970 and RR000169.
Footnotes
The authors report no conflict of interest.
References
- Aklillu E, Karlsson S, Zachrisson OO, Ozdemir V, Agren H. Association of MAOA gene functional promoter polymorphism with CSF dopamine turnover and atypical depression. Pharmacogenet Genomics. 2009;19:267–275. doi: 10.1097/FPC.0b013e328328d4d3. [DOI] [PubMed] [Google Scholar]
- Armony-Sivan R, Kaplan-Estrin M, Jacobson SW, Lozoff B. Iron-deficiency anemia in infancy and mother-infant interaction during feeding. J Dev Behav Pediatr. 2011;31:326–332. doi: 10.1097/DBP.0b013e3181dc525d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashkenazi R, Ben-Shachar D, Youdim KA. Nutrtional deficiency and dopamine binding sites in the rat brain. Pharmacol Biochem Behav. 1982;17:43–47. doi: 10.1016/0091-3057(82)90509-3. [DOI] [PubMed] [Google Scholar]
- Aslund C, Nordquist N, Comasco E, Leppert J, Oreland L, Nilsson KW. Maltreatment, MAOA, and delinquency: sex differences in gene-environment interaction in a large population-based cohort of adolescents. Behav Genet. 2011;41:262–272. doi: 10.1007/s10519-010-9356-y. [DOI] [PubMed] [Google Scholar]
- Beard J, Erikson KM, Jones BC. Neonatal iron deficiency results in irreversible changes in dopamine function in rats. J Nutr. 2003;133:1174–1179. doi: 10.1093/jn/133.4.1174. [DOI] [PubMed] [Google Scholar]
- Bethea CL, Streicher JM, Coleman K, Pau FK, Moessner R, Cameron JL. Anxious behavior and fenfluramine-induced prolactin secretion in young rhesus macaques with different alleles of the serotonin reuptake transporter polymorphism (5HTTLPR) Behav Genet. 2004;34:295–307. doi: 10.1023/B:BEGE.0000017873.61607.be. [DOI] [PubMed] [Google Scholar]
- Beutler E, Van Geet C, te Loo DM, Gelbart T, Crain K, Truksa J, Lee PL. Polymorphisms and mutations of human TMPRSS6 in iron deficiency anemia. Blood Cells Mol Dis. 2010;44:16–21. doi: 10.1016/j.bcmd.2009.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bortolato M, Chen K, Godar SC, Chen G, Wu W, Rebrin I, Farrell MR, Scott AL, Wellman CL, Shih JC. Social Deficits and Perseverative Behaviors, but not Overt Aggression, in MAO-A Hypomorphic Mice. Neuropsychopharmacology. 2011;36:2674–2688. doi: 10.1038/npp.2011.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckholtz JW, Callicott JH, Kolachana B, Hariri AR, Goldberg TE, Genderson M, Egan MF, Mattay VS, Weinberger DR, Meyer-Lindenberg A. Genetic variation in MAOA modulates ventromedial prefrontal circuitry mediating individual differences in human personality. Mol Psychiatry. 2008;13:313–324. doi: 10.1038/sj.mp.4002020. [DOI] [PubMed] [Google Scholar]
- Buckholtz JW, Meyer-Lindenberg A. MAOA and the neurogenetic architecture of human aggression. Trends Neurosci. 2008;31:120–129. doi: 10.1016/j.tins.2007.12.006. [DOI] [PubMed] [Google Scholar]
- CDC. CDC’s Pediatric and Pregnancy Nutrition Surveillance System 2010. Center for Disease Control; 2011. http://www.cdc.gov/pednss/pnss_tables/html/pnss_national_table5.htm. [Google Scholar]
- Chang S, Wang L, Wang Y, Brouwer ID, Kok FJ, Lozoff B, Chen C. Iron-deficiency anemia in infancy and social emotional development in preschool-aged chinese children. Pediatrics. 2011;127:e927–933. doi: 10.1542/peds.2010-1659. [DOI] [PubMed] [Google Scholar]
- Cheng A, Scott AL, Ladenheim B, Chen K, Ouyang X, Lathia JD, Mughal M, Cadet JL, Mattson MP, Shih JC. Monoamine oxidases regulate telencephalic neural progenitors in late embryonic and early postnatal development. J Neurosci. 2010;30:10752–10762. doi: 10.1523/JNEUROSCI.2037-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coe CL, Lubach GR, Bianco L, Beard JL. A history of iron deficiency anemia during infancy alters brain monoamine activity later in juvenile monkeys. Dev Psychobiol. 2009;51:301–309. doi: 10.1002/dev.20365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen I, Liu X, Lewis M, Chudley A, Forster-Gibson C, Gonzalez M, Jenkins E, Brown W, Holden J. Autism severity is associated with child and maternal MAOA genotypes. Clin Genet. 2011;79:355–362. doi: 10.1111/j.1399-0004.2010.01471.x. [DOI] [PubMed] [Google Scholar]
- Corapci F, Calatroni A, Kaciroti N, Jimenez E, Lozoff B. Longitudinal evaluation of externalizing and internalizing behavior problems following iron deficiency in infancy. J Pediatr Psychol. 2011;35:296–305. doi: 10.1093/jpepsy/jsp065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derringer J, Krueger RF, Irons DE, Iacono WG. Harsh discipline, childhood sexual assault, and MAOA genotype: an investigation of main and interactive effects on diverse clinical externalizing outcomes. Behav Genet. 2010;40:639–648. doi: 10.1007/s10519-010-9358-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ducci F, Newman TK, Funt S, Brown GL, Virkkunen M, Goldman D. A functional polymorphism in the MAOA gene promoter (MAOA-LPR) predicts central dopamine function and body mass index. Mol Psychiatry. 2006;11:858–866. doi: 10.1038/sj.mp.4001856. [DOI] [PubMed] [Google Scholar]
- Egerton A, Rees E, Bose SK, Lappin JM, Stokes PR, Turkheimer FE, Reeves SJ. Truth, lies or self-deception? Striatal D(2/3) receptor availability predicts individual differences in social conformity. Neuroimage. 2010;53:777–781. doi: 10.1016/j.neuroimage.2010.06.031. [DOI] [PubMed] [Google Scholar]
- Enoch MA, Steer CD, Newman TK, Gibson N, Goldman D. Early life stress, MAOA, and gene-environment interactions predict behavioral disinhibition in children. Genes Brain Behav. 2010;9:65–74. doi: 10.1111/j.1601-183X.2009.00535.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahlke C, Garpenstrand H, Oreland L, Suomi SJ, Higley JD. Platelet monoamine oxidase activity in a nonhuman primate model of type 2 excessive alcohol consumption. Am J Psychiatry. 2002;159:2107. doi: 10.1176/appi.ajp.159.12.2107. [DOI] [PubMed] [Google Scholar]
- Fairbanks LA, Fontenot MB, Phillips-Conroy JE, Jolly CJ, Kaplan JR, Mann JJ. CSF monoamines, age and impulsivity in wild grivet monkeys (Cercopithecus aethiops aethiops) Brain Behav Evol. 1999;53:305–312. doi: 10.1159/000006601. [DOI] [PubMed] [Google Scholar]
- Fairbanks LA, Melega WP, Jorgensen MJ, Kaplan JR, McGuire MT. Social impulsivity inversely associated with CSF 5-HIAA and fluoxetine exposure in vervet monkeys. Neuropsychopharmacology. 2001;24:370–378. doi: 10.1016/S0893-133X(00)00211-6. [DOI] [PubMed] [Google Scholar]
- Fowler JS, Alia-Klein N, Kriplani A, Logan J, Williams B, Zhu W, Craig IW, Telang F, Goldstein R, Volkow ND, Vaska P, Wang GJ. Evidence that brain MAO A activity does not correspond to MAO A genotype in healthy male subjects. Biol Psychiatry. 2007;62:355–358. doi: 10.1016/j.biopsych.2006.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgieff MK. The role of iron in neurodevelopment: fetal iron deficiency and the developing hippocampus. Biochem Soc Trans. 2008;36:1267–1271. doi: 10.1042/BST0361267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golub MS, Hogrefe CE, Germann SL. Behavior of juvenile rhesus monkeys deprived of iron during fetal or infant development. J Nutr. 2007;137:979–984. doi: 10.1093/jn/137.4.979. [DOI] [PubMed] [Google Scholar]
- Golub MS, Hogrefe CE, Germann SL, Tran TT, Beard JL, Crinella FM, Lonnerdal B. Neurobehavioral evaluation of rhesus monkey infants fed cow’s milk formula, soy formula, or soy formula with added manganese. Neurotoxicol Teratol. 2005;27:615–627. doi: 10.1016/j.ntt.2005.04.003. [DOI] [PubMed] [Google Scholar]
- Golub MS, Hogrefe CE, Tarantal AF, Germann SL, Beard JL, Calatroni A. Diet-induced iron deficiency anemia and pregnancy outcome in the rhesus monkey. Am J Clin Nutr. 2006;83:647–656. doi: 10.1093/ajcn.83.3.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golub MS, Hogrefe CE, Widaman KF, Capitanio JP. Iron deficiency anemia and affective response in rhesus monkey infants. Dev Psychobiol. 2009;51:47–59. doi: 10.1002/dev.20345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottfried JA, O’Doherty J, Dolan RJ. Encoding predictive reward value in human amygdala and orbitofrontal cortex. Science. 2003;301:1104–1107. doi: 10.1126/science.1087919. [DOI] [PubMed] [Google Scholar]
- Grigorenko EL, De Young CG, Eastman M, Getchell M, Haeffel GJ, Klinteberg B, Koposov RA, Oreland L, Pakstis AJ, Ponomarev OA, Ruchkin VV, Singh JP, Yrigollen CM. Aggressive behavior, related conduct problems, and variation in genes affecting dopamine turnover. Aggress Behav. 2010;36:158–176. doi: 10.1002/ab.20339. [DOI] [PubMed] [Google Scholar]
- Higley JD, Suomi SJ, Linnoila M. CSF monoamine metabolite concentrations vary according to age, rearing, and sex, and are influenced by the stressor of social separation in rhesus monkeys. Psychopharmacology (Berl) 1991;103:551–556. doi: 10.1007/BF02244258. [DOI] [PubMed] [Google Scholar]
- Higley JD, Suomi SJ, Linnoila M. A longitudinal assessment of CSF monoamine metabolite and plasma cortisol concentrations in young rhesus monkeys. Biol Psychiatry. 1992;32:127–145. doi: 10.1016/0006-3223(92)90016-s. [DOI] [PubMed] [Google Scholar]
- Hinde R, Rowell T. Comunication by postures and facial expression in the rhesus monkey (Macaca mulatta) Proc Zool Soc London. 1962;138:1–21. [Google Scholar]
- Huang YY, Cate SP, Battistuzzi C, Oquendo MA, Brent D, Mann JJ. An association between a functional polymorphism in the monoamine oxidase a gene promoter, impulsive traits and early abuse experiences. Neuropsychopharmacology. 2004;29:1498–1505. doi: 10.1038/sj.npp.1300455. [DOI] [PubMed] [Google Scholar]
- Izquierdo A, Murray EA. Combined unilateral lesions of the amygdala and orbital prefrontal cortex impair affective processing in rhesus monkeys. J Neurophysiol. 2004;91:2023–2039. doi: 10.1152/jn.00968.2003. [DOI] [PubMed] [Google Scholar]
- Jimenez DF, Tarantal AF. Fetal gender determination in early first trimester pregnancies of rhesus monkeys (Macaca mulatta) by fluorescent PCR analysis of maternal serum. J Med Primatol. 2003;32:315–319. doi: 10.1046/j.1600-0684.2003.00041.x. [DOI] [PubMed] [Google Scholar]
- Jonsson EG, Norton N, Gustavsson JP, Oreland L, Owen MJ, Sedvall GC. A promoter polymorphism in the monoamine oxidase A gene and its relationships to monoamine metabolite concentrations in CSF of healthy volunteers. J Psychiatr Res. 2000;34:239–244. doi: 10.1016/s0022-3956(00)00013-3. [DOI] [PubMed] [Google Scholar]
- Karere GM, Kinnally EL, Sanchez JN, Famula TR, Lyons LA, Capitanio JP. What is an “adverse” environment? Interactions of rearing experiences and MAOA genotype in rhesus monkeys. Biol Psychiatry. 2009;65:770–777. doi: 10.1016/j.biopsych.2008.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JH, Son YD, Kim HK, Lee SY, Cho SE, Kim YB, Cho ZH. Association of harm avoidance with dopamine D2/3 receptor availability in striatal subdivisions: a high resolution PET study. Biol Psychol. 2011;87:164–167. doi: 10.1016/j.biopsycho.2011.02.011. [DOI] [PubMed] [Google Scholar]
- Kim-Cohen J, Caspi A, Taylor A, Williams B, Newcombe R, Craig IW, Moffitt TE. MAOA, maltreatment, and gene-environment interaction predicting children’s mental health: new evidence and a meta-analysis. Mol Psychiatry. 2006;11:903–913. doi: 10.1038/sj.mp.4001851. [DOI] [PubMed] [Google Scholar]
- Kinnally EL, Huang YY, Haverly R, Burke AK, Galfalvy H, Brent DP, Oquendo MA, Mann JJ. Parental care moderates the influence of MAOA-uVNTR genotype and childhood stressors on trait impulsivity and aggression in adult women. Psychiatr Genet. 2009;19:126–133. doi: 10.1097/YPG.0b013e32832a50a7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinnally EL, Karere GM, Lyons LA, Mendoza SP, Mason WA, Capitanio JP. Serotonin pathway gene-gene and gene-environment interactions influence behavioral stress response in infant rhesus macaques. Dev Psychopathol. 2010;22:35–44. doi: 10.1017/S0954579409990241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyes RC, Botchin MB, Kaplan JR, Manuck SB, Mann JJ. Aggression and brain serotonergic responsivity: response to slides in male macaques. Physiol Behav. 1995;57:205–208. doi: 10.1016/0031-9384(94)00219-u. [DOI] [PubMed] [Google Scholar]
- Lee PL, Halloran C, Trevino R, Felitti V, Beutler E. Human transferrin G277S mutation: a risk factor for iron deficiency anaemia. Br J Haematol. 2001;115:329–333. doi: 10.1046/j.1365-2141.2001.03096.x. [DOI] [PubMed] [Google Scholar]
- Levant B, Morgan KA, Ahlgren-Beckendorf JA, Grandy DK, Chen K, Shih JC, Seif I. Modulation of [3H]quinpirole binding at striatal D2 dopamine receptors by a monoamine oxidaseA-like site: evidence from radioligand binding studies and D2 receptor- and MAO(A)-deficient mice. Life Sci. 2001;70:229–241. doi: 10.1016/s0024-3205(01)01400-x. [DOI] [PubMed] [Google Scholar]
- Lewis PA, Rezaie R, Brown R, Roberts N, Dunbar RI. Ventromedial prefrontal volume predicts understanding of others and social network size. Neuroimage. 2011;57:1624–1629. doi: 10.1016/j.neuroimage.2011.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longato-Stadler E, af Klinteberg B, Garpenstrand H, Oreland L, Hallman J. Personality traits and platelet monoamine oxidase activity in a Swedish male criminal population. Neuropsychobiology. 2002;46:202–208. doi: 10.1159/000067806. [DOI] [PubMed] [Google Scholar]
- Lozoff B. Iron deficiency and child development. Food Nutr Bull. 2007;28:S560–571. doi: 10.1177/15648265070284S409. [DOI] [PubMed] [Google Scholar]
- Lozoff B, Beard J, Connor J, Barbara F, Georgieff M, Schallert T. Long-lasting neural and behavioral effects of iron deficiency in infancy. Nutr Rev. 2006;64:S34–43. doi: 10.1301/nr.2006.may.S34-S43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lozoff B, Clark KM, Jing Y, Armony-Sivan R, Angelilli ML, Jacobson SW. Dose-response relationships between iron deficiency with or without anemia and infant social-emotional behavior. J Pediatr. 2008;152:696–702. doi: 10.1016/j.jpeds.2007.09.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lung FW, Tzeng DS, Huang MF, Lee MB. Association of the MAOA promoter uVNTR polymorphism with suicide attempts in patients with major depressive disorder. BMC medical genetics. 2011;12:74. doi: 10.1186/1471-2350-12-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackler B, Person R, Miller LR, Finch CA. Iron deficiency in the rat: effects on phenylalanine metabolism. Pediatr Res. 1979;13:1010–1011. doi: 10.1203/00006450-197909000-00012. [DOI] [PubMed] [Google Scholar]
- Man MS, Mikheenko Y, Braesicke K, Cockcroft G, Roberts AC. Serotonin at the level of the amygdala and orbitofrontal cortex modulates distinct aspects of positive emotion in primates. Int J Neuropsychopharmacol. 2011:1–15. doi: 10.1017/S1461145711000587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manuck SB, Kaplan JR, Rymeski BA, Fairbanks LA, Wilson ME. Approach to a social stranger is associated with low central nervous system serotonergic responsivity in female cynomolgus monkeys (Macaca fascicularis) Am J Primatol. 2003;61:187–194. doi: 10.1002/ajp.10118. [DOI] [PubMed] [Google Scholar]
- Meyer-Lindenberg A, Buckholtz JW, Kolachana B, ARH, Pezawas L, Blasi G, Wabnitz A, Honea R, Verchinski B, Callicott JH, Egan M, Mattay V, Weinberger DR. Neural mechanisms of genetic risk for impulsivity and violence in humans. Proc Natl Acad Sci U S A. 2006;103:6269–6274. doi: 10.1073/pnas.0511311103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman TK, Syagailo YV, Barr CS, Wendland JR, Champoux M, Graessle M, Suomi SJ, Higley JD, Lesch KP. Monoamine oxidase A gene promoter variation and rearing experience influences aggressive behavior in rhesus monkeys. Biol Psychiatry. 2005;57:167–172. doi: 10.1016/j.biopsych.2004.10.012. [DOI] [PubMed] [Google Scholar]
- Roberts AC. The importance of serotonin for orbitofrontal function. Biol Psychiatry. 2011;69:1185–1191. doi: 10.1016/j.biopsych.2010.12.037. [DOI] [PubMed] [Google Scholar]
- Schauer R, Reuter G, Stoll S, Shukla AK. Partial purification and characterization of sialate O-acetylesterase from bovine brain. J Biochem. 1989;106:143–150. doi: 10.1093/oxfordjournals.jbchem.a122804. [DOI] [PubMed] [Google Scholar]
- Sebastian CL, Roiser JP, Tan GC, Viding E, Wood NW, Blakemore SJ. Effects of age and MAOA genotype on the neural processing of social rejection. Genes Brain Behav. 2010;9:628–637. doi: 10.1111/j.1601-183X.2010.00596.x. [DOI] [PubMed] [Google Scholar]
- Shih JC, Wu JB, Chen K. Transcriptional regulation and multiple functions of MAO genes. J Neural Transm. 2011;118:979–986. doi: 10.1007/s00702-010-0562-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shukla A, Agarwal KN, Chansuria JP, Taneja V. Effect of latent iron deficiency on 5-hydroxytryptamine metabolism in rat brain. J Neurochem. 1989;52:730–735. doi: 10.1111/j.1471-4159.1989.tb02515.x. [DOI] [PubMed] [Google Scholar]
- Tamura T, Goldenberg RL, Hou J, Johnston KE, Cliver SP, Ramey SL, Nelson KG. Cord serum ferritin concentrations and mental and psychomotor development of children at five years of age. J Pediatr. 2002;140:165–170. doi: 10.1067/mpd.2002.120688. [DOI] [PubMed] [Google Scholar]
- Tassone F, Qi L, Zhang W, Hansen RL, Pessah IN, Hertz-Picciotto I. MAOA, DBH, and SLC6A4 variants in CHARGE: a case-control study of autism spectrum disorders. Autism Res. 2011;4:250–261. doi: 10.1002/aur.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor A, Kim-Cohen J. Meta-analysis of gene-environment interactions in developmental psychopathology. Dev Psychopathol. 2007;19:1029–1037. doi: 10.1017/S095457940700051X. [DOI] [PubMed] [Google Scholar]
- Tikkanen R, Ducci F, Goldman D, Holi M, Lindberg N, Tiihonen J, Virkkunen M. MAOA alters the effects of heavy drinking and childhood physical abuse on risk for severe impulsive acts of violence among alcoholic violent offenders. Alcohol Clin Exp Res. 2010;34:853–860. doi: 10.1111/j.1530-0277.2010.01157.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuchimine S, Yasui-Furukori N, Kaneda A, Saito M, Nakagami T, Sato K, Kaneko S. Association between monoamine oxidase A (MAOA) and personality traits in Japanese individuals. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32:1932–1935. doi: 10.1016/j.pnpbp.2008.09.012. [DOI] [PubMed] [Google Scholar]
- Unger EL, Paul T, Murray-Kolb LE, Felt B, Jones BC, Beard JL. Early iron deficiency alters sensorimotor development and brain monoamines in rats. J Nutr. 2007;137:118–124. doi: 10.1093/jn/137.1.118. [DOI] [PubMed] [Google Scholar]
- Unger EL, Wiesinger JA, Hao L, Beard JL. Dopamine D2 receptor expression is altered by changes in cellular iron levels in PC12 cells and rat brain tissue. J Nutr. 2008;138:2487–2494. doi: 10.3945/jn.108.095224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang GJ. Imaging studies on the role of dopamine in cocaine reinforcement and addiction in humans. J Psychopharmacol. 1999;13:337–345. doi: 10.1177/026988119901300406. [DOI] [PubMed] [Google Scholar]
- Wachs TD, Pollitt E, Cueto S, Jacoby E, Creed-Kanashiro H. Relation of neonatal iron status to individual variability in neonatal temperament. Dev Psychobiol. 2005;46:141–153. doi: 10.1002/dev.20049. [DOI] [PubMed] [Google Scholar]
- Wang ZQ, Chen K, Ying QL, Li P, Shih JC. Monoamine oxidase A regulates neural differentiation of murine embryonic stem cells. J Neural Transm. 2011;118:997–1001. doi: 10.1007/s00702-011-0655-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Way BM, Lacan G, Fairbanks LA, Melega WP. Architectonic distribution of the serotonin transporter within the orbitofrontal cortex of the vervet monkey. Neuroscience. 2007;148:937–948. doi: 10.1016/j.neuroscience.2007.06.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westlund KN, Denney RM, Kochersperger LM, Rose RM, Abell CW. Distinct monoamine oxidase A and B populations in primate brain. Science. 1985;230:181–183. doi: 10.1126/science.3875898. [DOI] [PubMed] [Google Scholar]
- White LK, Helfinstein SM, Reeb-Sutherland BC, Degnan KA, Fox NA. Role of attention in the regulation of fear and anxiety. Dev Neurosci. 2009;31:309–317. doi: 10.1159/000216542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams LM, Gatt JM, Kuan SA, Dobson-Stone C, Palmer DM, Paul RH, Song L, Costa PT, Schofield PR, Gordon E. A polymorphism of the MAOA gene is associated with emotional brain markers and personality traits on an antisocial index. Neuropsychopharmacology. 2009;34:1797–1809. doi: 10.1038/npp.2009.1. [DOI] [PubMed] [Google Scholar]
- Williams RB, Marchuk DA, Gadde KM, Barefoot JC, Grichnik K, Helms MJ, Kuhn CM, Lewis JG, Schanberg SM, Stafford-Smith M, Suarez EC, Clary GL, Svenson IK, Siegler IC. Serotonin-related gene polymorphisms and central nervous system serotonin function. Neuropsychopharmacology. 2003;28:533–541. doi: 10.1038/sj.npp.1300054. [DOI] [PubMed] [Google Scholar]
- Winslow JT, Noble PL, Lyons CK, Sterk SM, Insel TR. Rearing effects on cerebrospinal fluid oxytocin concentration and social buffering in rhesus monkeys. Neuropsychopharmacology. 2003;28:910–918. doi: 10.1038/sj.npp.1300128. [DOI] [PubMed] [Google Scholar]
- Winstanley CA, Theobald DE, Cardinal RN, Robbins TW. Contrasting roles of basolateral amygdala and orbitofrontal cortex in impulsive choice. J Neurosci. 2004;24:4718–4722. doi: 10.1523/JNEUROSCI.5606-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youdim MB, Green AR. Iron deficiency and neurotransmitter synthesis and function. Proc Nutr Soc. 1978;37:173–179. doi: 10.1079/pns19780022. [DOI] [PubMed] [Google Scholar]
- Youdim MB, Green AR, Bloomfield MR, Mitchell BD, Heal DJ, Grahame-Smith DG. The effects of iron deficiency on brain biogenic monoamine biochemistry and function in rats. Neuropharmacology. 1980;19:259–267. doi: 10.1016/0028-3908(80)90148-3. [DOI] [PubMed] [Google Scholar]
- Zald DH, Cowan RL, Riccardi P, Baldwin RM, Ansari MS, Li R, Shelby ES, Smith CE, McHugo M, Kessler RM. Midbrain dopamine receptor availability is inversely associated with novelty-seeking traits in humans. J Neurosci. 2008;28:14372–14378. doi: 10.1523/JNEUROSCI.2423-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zalsman G, Huang YY, Harkavy-Friedman JM, Oquendo MA, Ellis SP, Mann JJ. Relationship of MAO-A promoter (u-VNTR) and COMT (V158M) gene polymorphisms to CSF monoamine metabolites levels in a psychiatric sample of caucasians: A preliminary report. Am J Med Genet B Neuropsychiatr Genet. 2005;132B:100–103. doi: 10.1002/ajmg.b.30094. [DOI] [PubMed] [Google Scholar]
- Zhang M, Chen X, Way N, Yoshikawa H, HD, Ke Z, Yu W, Chen P, He C, Chi X, Lu Z. The association between infants self-regulatory behavior and MAOA gene polymorphism. Dev Sci. 2011;14:1059–1065. doi: 10.1111/j.1467-7687.2011.01047.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.