Abstract
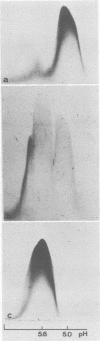
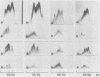
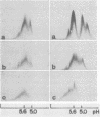
The concentration of alpha 2-macroglobulin (alpha 2-M) in human gingival sulci has been investigated in two studies: first, in gingival washings during a 21-day period of experimental gingivitis in eight human volunteers and, second, in crevicular fluid collected with filter paper strips before and after initial periodontal therapy in 11 patients. The concentration of total alpha 2-M was found to increase in the washings of the volunteers throughout the period of experimental gingivitis. In the group of patients receiving periodontal therapy, the absolute amount of alpha 2-M in the fluid showed a significant decrease after therapy. The gingival index of inflammation and the crevicular fluid flow also decreased significantly. The specific content of the inhibitor (micrograms of alpha 2-M per mg of fluid per min), however, was found to increase in the fluid with decreasing inflammation. As detected by crossed immunoelectrophoresis, the fluid collected in these patients before therapy, in the presence of severe inflammation, invariably showed peaks of both free and complexed alpha 2-M. In contrast, the fluid collected from the same sites after healing of the inflammation contained no detectable free alpha 2-M.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Attström R., Egelberg J. Emigration of blood neutrophils and monocytes into the gingival crevices. J Periodontal Res. 1970;5(1):48–55. doi: 10.1111/j.1600-0765.1970.tb01837.x. [DOI] [PubMed] [Google Scholar]
- Barrett A. J., Brown M. A., Sayers C. A. The electrophoretically 'slow' and 'fast' forms of the alpha 2-macroglobulin molecule. Biochem J. 1979 Aug 1;181(2):401–418. doi: 10.1042/bj1810401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golub L. M., Siegel K., Ramamurthy N. S., Mandel I. D. Some characteristics of collagenase activity in gingival crevicular fluid and its relationship to gingival diseases in humans. J Dent Res. 1976 Nov-Dec;55(6):1049–1057. doi: 10.1177/00220345760550060701. [DOI] [PubMed] [Google Scholar]
- Ishikawa I., Cimasoni G. Partial purification of a neutral protease from human polymorphonuclear leukocytes and its proteolytic effect on immunoglobulin G. Arch Oral Biol. 1978;23(11):933–940. doi: 10.1016/0003-9969(78)90246-7. [DOI] [PubMed] [Google Scholar]
- Kowashi Y., Jaccard F., Cimasoni G. Increase of free collagenase and neutral protease activities in the gingival crevice during experimental gingivitis in man. Arch Oral Biol. 1979;24(9):645–650. doi: 10.1016/0003-9969(79)90112-2. [DOI] [PubMed] [Google Scholar]
- Kowashi Y., Jaccard F., Cimasoni G. Sulcular polymorphonuclear leucocytes and gingival exudate during experimental gingivitis in man. J Periodontal Res. 1980 Mar;15(2):151–158. doi: 10.1111/j.1600-0765.1980.tb00269.x. [DOI] [PubMed] [Google Scholar]
- LOE H., THEILADE E., JENSEN S. B. EXPERIMENTAL GINGIVITIS IN MAN. J Periodontol. 1965 May-Jun;36:177–187. doi: 10.1902/jop.1965.36.3.177. [DOI] [PubMed] [Google Scholar]
- Laurell C. B. Quantitative estimation of proteins by electrophoresis in agarose gel containing antibodies. Anal Biochem. 1966 Apr;15(1):45–52. doi: 10.1016/0003-2697(66)90246-6. [DOI] [PubMed] [Google Scholar]
- Löe H. The Gingival Index, the Plaque Index and the Retention Index Systems. J Periodontol. 1967 Nov-Dec;38(6 Suppl):610–616. doi: 10.1902/jop.1967.38.6.610. [DOI] [PubMed] [Google Scholar]
- Mancini G., Carbonara A. O., Heremans J. F. Immunochemical quantitation of antigens by single radial immunodiffusion. Immunochemistry. 1965 Sep;2(3):235–254. doi: 10.1016/0019-2791(65)90004-2. [DOI] [PubMed] [Google Scholar]
- Mosher D. F., Wing D. A. Synthesis and secretion of alpha2-macroglobulin by cultured human fibroblasts. J Exp Med. 1976 Feb 1;143(2):462–467. doi: 10.1084/jem.143.2.462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohlsson K. Alpha1-antitrypsin and alpha2-macroglobulin. Interactions with human neutrophil collagenase and elastase. Ann N Y Acad Sci. 1975 Jun 13;256:409–419. doi: 10.1111/j.1749-6632.1975.tb36067.x. [DOI] [PubMed] [Google Scholar]
- Ohlsson K., Skude G. Demonstration and semiquantitative determination of complexes between various proteases and human alpha2-macroglobulin. Clin Chim Acta. 1976 Jan 2;66(1):1–7. doi: 10.1016/0009-8981(76)90365-x. [DOI] [PubMed] [Google Scholar]
- Schenkein H. A., Genco R. J. Gingival fluid and serum in periodontal diseases. I. Quantitative study of immunoglobulins, complement components, and other plasma proteins. J Periodontol. 1977 Dec;48(12):772–777. doi: 10.1902/jop.1977.48.12.772. [DOI] [PubMed] [Google Scholar]
- Shtacher G., Maayan R., Feinstein G. Proteinase inhibitors in human synovial fluid. Biochim Biophys Acta. 1973 Mar 23;303(1):138–147. doi: 10.1016/0005-2795(73)90155-4. [DOI] [PubMed] [Google Scholar]
- Uitto V. J., Raeste A. M. Activation of latent collagenase of human leukocytes and gingival fluid by bacterial plaque. J Dent Res. 1978 Jul-Aug;57(7-8):844–851. doi: 10.1177/00220345780570071401. [DOI] [PubMed] [Google Scholar]
- White R., Janoff A., Godfrey H. P. Secretion of Alpha-2-macroglobulin by human alveolar macrophages. Lung. 1980;158(1):9–14. doi: 10.1007/BF02713697. [DOI] [PubMed] [Google Scholar]