Abstract
Background & Aims
We aimed to identify risk factors for hepatocellular carcinoma (HCC) in patients with cirrhosis in the US. We performed a prospective study to identify associations between etiologies of cirrhosis and ethnicity with HCC incidence.
Methods
We used convenience sampling to select a cohort of 379 patients with cirrhosis who visited the liver clinic at the Stanford University Medical Center from 2001 to 2009 (65% male, 75% white or Hispanic, and 20% Asian). Study endpoints were HCC diagnosis by histology or noninvasive criteria, liver transplantation, or last screening without HCC. Patients were followed, with ultrasound or computed tomographic imaging analyses and measurements of serum levels of α-fetal protein approximately every 6 months, for a median time of 34 months (range, 6–99 months).
Results
The etiologies of cirrhosis in the cohort were 68% hepatitis C, 7% hepatitis B, and 25% non-viral. Forty-four patients (12%) were diagnosed with HCC during the follow-up period. Patients with cirrhosis related to viral hepatitis had a statistically significantly higher incidence of HCC than those with non-viral diseases in Kaplan-Meier analysis (P=.04). There was no statistically significant difference in HCC incidence between Asian and non-Asian patients. In a multivariate Cox proportional hazards model that included age, sex, ethnicity, etiology, and Child-Pugh-Turcotte score, viral cirrhosis was significantly associated with HCC, compared to non-viral cirrhosis (hazard ratio, 3.6; 95% confidence interval, 1.3–10.1; P=.02) but Asian ethnicity was not.
Conclusions
In a diverse cohort of patients in the US with cirrhosis, a viral etiology of cirrhosis was associated with increased incidence of HCC, but Asian ethnicity was not. These findings indicate the importance of cirrhosis etiology in determining risk for HCC.
Keywords: epidemiology, liver cancer risk factor, HCV, HBV
Introduction
Cirrhosis of any etiology is a significant risk factor for hepatocellular carcinoma (HCC).1, 2 There are several well-defined risk factors for HCC in cirrhotic patients such as male sex and increasing age and severity of cirrhosis but the importance of many other risk factors such as ethnicity and etiology of cirrhosis are not fully understood. 1, 3, 4 Cohort studies from Europe and Asia suggests significant geographic variation in HCC incidence in cirrhotic patients but there is little longitudinal data from the US.1, 5, 6 A review summarizing HCC cases per total follow-up time in cirrhotic cohorts reports annual incidence rates of 3.7% for European/US HCV cirrhosis patients and 7.1% for hepatitis C virus (HCV)-related cirrhosis patients in Japan. For hepatitis B virus (HBV)-related cirrhosis, summary annual incidence estimates are 2.2% in Europe and 4.3% in Japan and for alcoholic cirrhosis the summary rates are 1.7% for Europe and 1.8% for Japan.1 There is limited US cirrhotic cohort HCC incidence data with Kaplan-Meier estimates of annual incidence from 1.2-4.7% for 4 studies following cirrhotic patients with HCV cirrhosis, a single estimate of 4% for HBV cirrhosis, and estimates of 2.6% for 2 studies measuring incidence in NASH cirrhosis.7-12
There are several significant limitations to the current understanding of HCC risk in cirrhosis in the US. The higher HCC incidence rates observed in viral cirrhosis in Asia together with a US case-control study demonstrating an association between Asian ethnicity and HCC in cirrhosis suggest that Asian ethnicity could be an important risk factor for HCC in cirrhosis in the US.1, 13 This association has not been examined with longitudinal data in the US since US Cirrhotic cohort studies have involved few Asian patients.7-12 Summary incidence rates from Europe and Japan suggest that HCC incidence is higher in viral as compared to non-viral cirrhosis but these direct comparisons do not adjust for group differences in important risk factors and are therefore potentially biased by confounding. Several cohort studies from Europe and Japan adjust for potential confounders and report statistically significant associations between viral and non-viral etiologies and HCC.6, 14-16 The only two US studies comparing HCC incidence by etiology of cirrhosis report higher HCC rates in HCV-related cirrhosis compared to non-alcoholic steatohepatitis (NASH)-related cirrhosis but neither adjusts for significant group differences in established risk factors. 9, 11 The relationship between Asian ethnicity, etiology of cirrhosis, and HCC incidence are not well defined in the US and clarifying these associations will help characterize the highest risk patients with implications for standards and resource utilization for patient screening, anticipating transplantation needs, and understanding the mechanisms underlying HCC.
Patients and Methods
Patients
This study involves a cohort of patients prospectively enrolled between February 2001 and January 2010 at the liver clinic at Stanford University Medical Center. A total of 1,037 patients with chronic liver disease were identified during this period from a daily encounter list with selection based on availability of study staff and priority was placed on enrolling patients with HCV. Approximately 95% of those invited to enroll agreed to participate. This study includes all 379 patients from the prospective cohort meeting the following criteria: 1) cirrhosis at the time of study enrollment, 2) no history of liver transplantation, 3) no prior diagnosis of HCC and 4) no diagnosis of HCC within 6 months of consent and follow-up with screening imaging tests at least 6 months from consent. Patients diagnosed with HCC within 6 months of enrollment date were considered to have prevalent rather than incident HCC and were excluded from the study since many patients were enrolled during their first clinic visit and received their first screening imaging during the first 6 months of enrollment. Patients who had no imaging studies more than 6 months from enrollment or who had a liver transplantation within 6 months of enrollment were also excluded since any HCC cases identified within this time-period would be considered prevalent and these patients therefore had no at risk time of follow-up in which they could have developed incident HCC. This technique of excluding HCC cases or loss to follow-up within a 6-12 month window from enrollment is consistent with many prior studies of HCC incidence.10, 17 This study was approved by the Institutional Review Board at Stanford University, Stanford, CA.
Patients were considered cirrhotic if they met at least one of the following criteria prior to the date of study enrollment: 1) liver biopsy demonstrating stage 4 fibrosis, 2) history of hepatic decompensation involving ascites, hepatic encephalopathy, or variceal bleed, 3) esophageal or gastric varices as noted on prior imaging reports, 4) nodular and shrunken liver on imaging studies, 5) nodular liver on imaging studies in combination with either albumin<3 g/dL, total bilirubin>2 mg/dL, INR>1.3 or platelets<112,000/mL.
Baseline Clinical Information
Each patient was assigned a primary etiology of cirrhosis of HCV, HBV, HCV/HBV co-infection, alcohol, alpha-1 antitrypsin deficiency, autoimmune-hepatitis, primary biliary cirrhosis, primary schlerosing cholangitis, hemochromatosis, or non-alcoholic steatohepatitis (NASH)/cryptogenic based on liver clinic progress note records. Etiology was verified with past records of laboratory testings (anti-HCV, HCV RNA PCR, HBsAg, HBV DNA PCR) and risk factor questionnaire data on alcohol use history. At the time of enrollment, the most recent laboratory, radiological, and clinical data were reviewed and recorded. Model for End-Stage Liver Disease (MELD) and Child-Pugh-Turcott (CPT) scores were calculated using these data. Risk factor data were collected through individual face-to-face interviews conducted by study coordinators using a 12-page risk factor questionnaire. Ethnicity was determined by patient self-identification via interview and study questionnaire.
Follow-up and Evaluation
All patients were followed prospectively with periodic imaging and α-fetal protein (AFP) screening for HCC. Screening was performed according to clinic standard of practice with patients being scheduled for serum AFP and ultrasound or CT imaging approximately every six months. All imaging reports were reviewed and patients were considered to be at risk in study between the date of enrollment and the date of last imaging or other endpoint independent of imaging frequency. Endpoints for follow-up were diagnosis of HCC, liver transplantation without HCC on pathologic examination of explanted livers, and last imaging studies without evidence of HCC prior to either death, loss to follow-up, or the end of the study. Laboratory values and clinical notes closest to endpoints were recorded and used to calculate final MELD and CPT scores. HCC diagnosis was based on criteria by the European Association for the Study of Liver Diseases (EASL) which requires at least one of the following conditions: 1) cytologic or histologic evidence of HCC, 2) presence of hypervascular lesion greater than 2 cm in two imaging studies (CT, MRI, angiography), 3) presence of hypervascular lesion greater than 2 cm in one imaging study and AFP>200 ng/mL, 4) presence of enlarging hypervascular lesion or recurrent hypervascular lesions following chemoembolization, 5) presence of AFP>1000 ng/mL.
Statistical Analysis
Kaplan-Meier methods were used to estimate cumulative incidence of HCC. Censoring occurred at time of last negative imaging tests or time of liver transplantation without evidence of HCC by pathologic examination of liver explants. Group differences in HCC-free survival time were compared with the log-rank test. Cox-proportional hazards regression analysis methods were used to evaluate risk factors for HCC. All variables of interest were first studied in univariate analysis and the proportional hazards assumption tested with hazard and Schoenfield residuals plots and by testing the statistical significance of the interaction term of variable and time for each predictor in the final multivariate model. Statistically significant univariate variables and established risk factors were selected for multivariate analysis and non-significant variables removed with stepwise backwards selection. Statistical significance was defined with a two-tailed p-value < 0.05. Statistical analysis was completed with SAS Enterprise Guide version 4.3.
Results
Characteristics of patients at enrollment
Table 1 describes patient characteristics at baseline. The study population included 227 men (60%) and 152 women (40%) with median age at consent of 53 years. The vast majority of patients were non-Hispanic White (54%), Hispanic (21%), or Asian (20%). Etiology of cirrhosis was viral in the majority of patients: HCV for 260 (68%), HBV for 26 (7%), and HBV/HCV co-infection for 4 (1%). Among patients with chronic hepatitis C, the vast majority had never received antiviral therapy (70%) and few had achieved sustained viral response (4%). The majority (80%) of HBV cirrhosis patients were receiving anti-HBV therapy at enrollment and overall 90% of these patients were treated at some point during the study period (data not shown). Approximately half of the patients had Child class A cirrhosis (46%) and half had experienced at least one episode of clinical decompensation (data not shown).
Table 1.
Demographics and Characteristics of 379 Patients
| Age, median y (range) | 53(20-87) |
| Male gender, n(%) | 227(60) |
| Ethnicity, n(%) | |
| White | 204(54) |
| Hispanic | 81(21) |
| Asian | 77(20) |
| Black | 10(3) |
| Other | 7(2) |
| Birthplace (N=330), n(%) | |
| US | 226(68) |
| Asia | 58(18) |
| Mexico, South or Central America | 27(8) |
| Others | 19(6) |
| Body Mass Index (N=271), median(range) | 28(15-57) |
| Diabetes, n(%) | 92(24) |
| HIV, n(%) | 4(1) |
| Cirrhosis Etiology, n(%) | |
| HCV | 260(68) |
| HBV | 26(7) |
| HBV/HCV Co-Infection | 4(1) |
| Alcoholic Liver Disease | 35(9) |
| Cryptogenic or non-alcoholic steatohepatitis | 38(10) |
| Other | 16(5) |
| Childclass, n(%) | |
| A | 174(46) |
| B | 175(46) |
| C | 30(8) |
| MELD (N=355), median(range) | 10(2-36) |
| Prior anti-HCV therapy with sustained viral response, n(% of HCV patients) | 10(4) |
| Prior anti-HCV therapy without sustained viral response, n(% of HCV patients) | 67(26) |
| Never treated for HCV, n(% of HCV patients) | 184(70) |
| Anti-HBV therapy at enrollment, n(% of HBV patients) | 24(80) |
| Baseline laboratory tests, median(range) | |
| Albumin | 3.3(1.6-6.4) |
| Platelets | 95(19-333) |
| INR | 1.2(0.8-13.6) |
| Total bilirubin | 1.3(0.3-33.7) |
| Aspartate aminotransferase | 86(14-704) |
| Alanine aminotransferase | 67(5-558) |
| Creatinine | 0.9(0.5-10.2) |
| Alpha-fetoprotein >20, n(%) | 60(16) |
MELD, model for end-stage liver disease; HCV, hepatitis C virus; HBV, hepatitis B virus; INR, international normalized ratio
Follow-up and HCC incidence
Table 2 describes characteristics of patients during follow-up and of patients who developed HCC during this time period. Median time of follow-up for all patients was 32 months (range 6-99). During this time, 44(12%) developed HCC and 43 (11%) patients were censored at the time of OLT and 12 (28%) of these 43 individuals were diagnosed with HCC on explant histology. The 78 patients lost to follow-up were nearly identical to the other 301 patients who were not lost to follow-up in terms of age, sex, race, viral vs. non-viral etiology, and Child class distributions at time of consent (data not shown).
Table 2.
Characteristics at follow-up for all 379 patients
| Months of follow-up, median(range) | 32(6-99) |
| Study endpoint, n(%) | |
| HCC | 44(12) |
| Liver Transplantation | 31(8) |
| Last imaging before death | 46(12) |
| Last imaging before loss to follow-up | 78(21) |
| Last imaging before end of study | 180(47) |
| Child class, n(%) | |
| A | 143(38) |
| B | 136(36) |
| C | 99(26) |
| MELD, median(range) | 14(6-54) |
| HCC Diagnostic Criteria, n(%) | |
| Biopsy | 13(30%) |
| Imaging | 30(68%) |
| AFP>1000 | 1(2%) |
| AJCC staging at diagnosis, n(%) | I |
| T1N0M0 | 20(45) |
| T2N0M0 | 19(43) |
| T2N1M0 | 1(2) |
| T3N1M0 | 2(4) |
| T4N1M1 | 1(2) |
| Undetermined | 1(2) |
| BCLC staging at diagnosis, n(%) | |
| A | 29(66) |
| B | 13(30) |
| C | 1(2) |
| Undetermined | 1(2) |
| 3 Year Kaplan-Meier cumulative HCC incidence, % | |
| Entire cohort | 10.4 |
| HCV patients | 12.2 |
| Viral cirrhosis | 12.0 |
| Non-viral cirrhosis | 5.1 |
| Asian | 13.1 |
| Non-Asian | 9.6 |
| Child A | 5.7 |
| Child B or C | 14.8 |
HCC, hepatocellular cardinoma; MELD, model for end-stage liver disease; AJCC, American Joint Committee on Cancer; BCLC, Barcelona Clinic Liver Cancer; HCV, hepatitis C virus
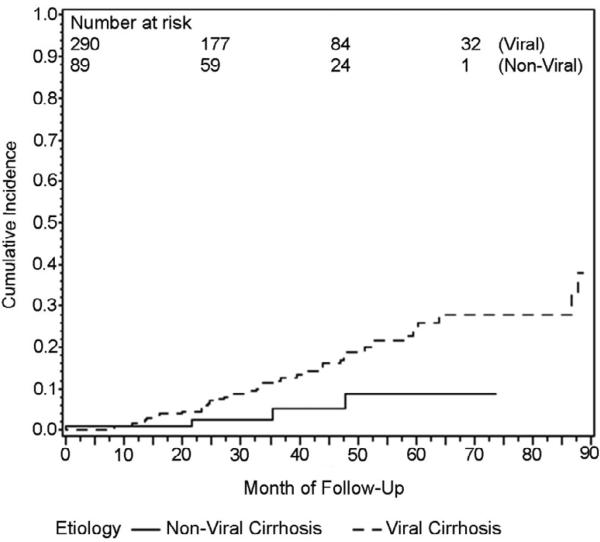
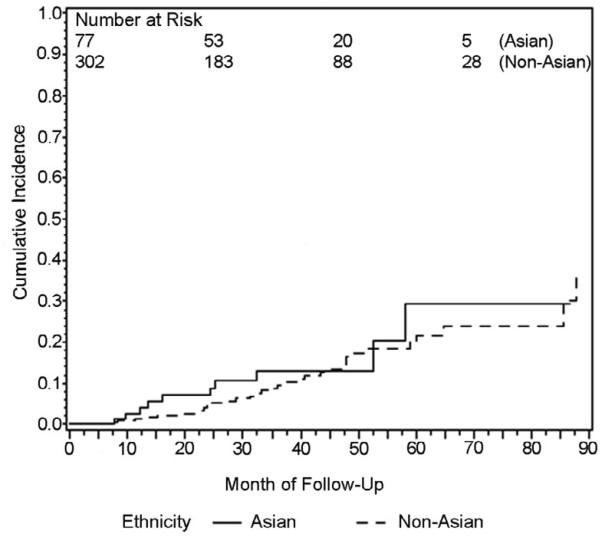
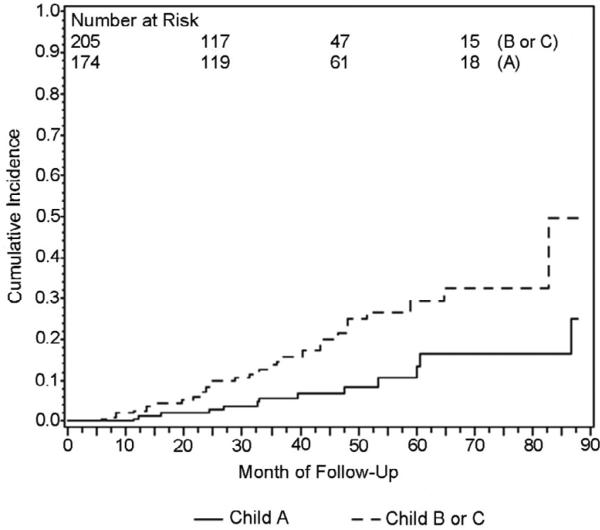
Using Kaplan-Meier methods, cumulative HCC incidence for the entire cohort was 10.4% at 3 years. When Kaplan-Meier curves were compared for patients with viral vs. non-viral liver disease, cumulative incidence at 3 years was 12.0% for viral cirrhosis and 5.1% for non-viral cirrhosis and these groups were significantly different by log-rank test, p=.04 (Figure 1). Ethnicity was analyzed as Asian and non-Asian since this was a predetermined hypothesis of interest. Cumulative incidence at 3 years was 13.1% for Asian patients and 9.6% for non-Asian patients and groups were not significantly different by log-rank test, p=.58 (Figure 2). Three year cumulative incidence was 5.7% for Child Class A and 14.8% for class B/C patients, a statistically significant difference by log rank test, p=.003 (Figure 3).
Figure 1.
Kaplan-Meier plot of cumulative HCC incidence in patients with viral and non-viral cirrhosis (Logrank p=.04)
Figure 2.
Kaplan-Meier plot of cumulative HCC incidence in patients with Asian and Non-Asian ethnicity (Logrank p=.58)
Figure 3.
Kaplan-Meier plot of cumulative HCC incidence in patients with Child class A and Child class B or C cirrhosis (Logrank p=.003)
Risk Factors for HCC
In univariate Cox proportional hazards regression, viral vs. non-viral etiology (p=.05), age as a continuous variable (p<.001), and Child score as a continuous variable (p<.001) were all statistically significantly associated with occurrence of HCC. Sex (p=.13) and Asian vs. non-Asian etiology (p=.58) were not significantly associated with HCC (data not shown).
The variables of viral vs. non-viral etiology, Asian vs. non-Asian ethnicity, age, sex, and Child score were then combined into a multivariate model to adjust for confounding variables and examine independent effects of risk factors (Table 3). In the first model developed including all five of these variables, viral vs. non-viral etiology (HR 3.6 95%CI 1.3-10.1, p=0.02), male sex (HR 2.0 95%CI=1.03-4.0, p=.04), age (for 10 year step HR=2.0 95%CI=1.5-2.8, p<.0001) and Child score (for 1 point step HR=1.4 95%CI=1.2-1.7, p=.0002) were each significantly associated with HCC but Asian ethnicity was not (p=.56). In the second model including the 4 significant variables from model 1, each was again statistically significantly associated with HCC incidence.
Table 3.
Predictors of hepatocellular carcinoma by Cox mulitvariate analysis
| Model 1 | ||
|---|---|---|
| Predictor Variable | Hazard Ratio (95%CI) | p value |
| Age (10 years) | 2.0 (1.5-2.8) | <.0001 |
| Male sex | 2.0 (1.03-4.0) | .04 |
| Viral etiology (vs. non-viral) | 3.6 (1.3-10.1) | .02 |
| CPT score (1 point) | 1.4 (1.2-1.7) | .0002 |
| Asian ethnicity (vs. non-Asian) | 1.2 (0.6-2.6) | .56 |
| Model 2 | ||
|---|---|---|
| Predictor Variable | Hazard Ratio (95%CI) | p value |
| Age (10 years) | 2.1 (1.7-2.4) | <.0001 |
| Male sex | 2.1 (1.05-4.1) | .04 |
| Viral etiology (vs. non-viral) | 3.6 (1.3-10.3) | .02 |
| CPT score (1 point) | 1.4 (1.2-1.7) | .0001 |
CPT, Child-Pugh-Turcotte; CI, confidence interval
Discussion
There is limited longitudinal data examining HCC risk factors in US cirrhotic patients. In particular, few US studies have directly compared HCC incidence between patients with different ethnicities and etiologies of cirrhosis. A US case control study and comparisons between cohort studies from Asia and Europe suggest that Asian ethnicity could be an important risk factor for HCC in cirrhosis13 but there is little prospective data involving cirrhotic Asian patients in the US or Europe.1, 5, 7-10, 12, 16 Two US studies suggest a higher rate of HCC incidence in cirrhosis caused by HCV in comparison to NASH cirrhosis but these studies did not adjust for significant baseline differences in established risk factors.9, 11
The current study helps characterize US patients at high risk for HCC by examining incidence in a prospective cohort of ethnically diverse patients with cirrhosis of all etiologies. Clarifying risk factors for HCC in cirrhosis in the US has many potential implications for clinical practice, policy, and future research. This understanding could help anticipate liver transplantation needs, determine the positive predictive value of non-diagnostic screening findings, influence utilization of resources to identify and follow-up with cirrhotic patients for screening, suggest that certain groups of patients could benefit from different frequencies or modalities of screening, provide insight into mechanisms of HCC development, and clarify which risk factors are most important for stratification and statistical adjustment in future studies.18
Forty-four cases of HCC were identified in 379 patients with a total of 1108 years of follow-up for overall annual incidence of 4.0%. Kaplan-Meier methods estimate a 3-year cumulative incidence of 10.4 % for the entire cohort and 12.2% for the HCV cohort. These rates are consistent with the high end of previous estimates of annual HCC incidence in HCV-related cirrhosis in the US ranging from 1.2-4.7%7, 9-12 and are lower than the summary estimate of 7.1% for HCV cirrhosis in Japan.1 The most likely explanation for the higher incidence rates in this study in comparison to the annual estimate of 1.2% from the largest HCV cirrhosis cohort from the US is the severity of liver disease since the present study involves a convenience sample from a tertiary care center liver clinic and includes individuals listed for transplant and those with Child class C cirrhosis.10
One notable finding from the current study involves etiology of cirrhosis. HCC incidence was statistically significantly higher in patients with viral compared to non-viral etiology of cirrhosis in Kaplan-Meier analysis, univariate Cox-proportional hazards regression, and a multivariate Cox model including and adjusting for sex, age, and baseline CPT score. To our knowledge, this is the first time a US study adjusting for baseline differences in established risk factors has demonstrated a statistically significant difference in HCC incidence between different etiologies of cirrhosis. The comparison between viral and non-viral etiologies is broad and of limited use for an individual patient with a specific cause of cirrhosis. The main significance of the finding is to highlight the importance of cirrhosis etiology in predicting HCC risk, suggesting that future studies will clarify differences between HCC incidence in HCV, HBV and major non-viral etiologies of cirrhosis in the US.
The current study also involves one of the largest longitudinal cohorts of Asian cirrhotic patients living in western nations with 77 Asian patients, 71% of whom were born in Asia. There was no difference in HCC incidence between Asian and non-Asian patients in Kaplan-Meier, univariate Cox proportional hazards regression, or multivariate Cox proportional hazards regression including and adjusting for etiology, sex, age, and consent CPT score. This observation suggests that Asian ethnicity is not a major independent risk factor for HCC in cirrhosis in the US.
Consistent with prior data, the current study observed statistically significant associations between HCC risk and higher Child score, male sex, and older age.1, 3, 4 These are all well-established risk factors and support the validity of study data and methods. The magnitude of the association with Child score is notable with an estimated hazard ratio of 1.4 for each one point increase in CPT score in multivariate Cox analysis and hazard ratio of 2.6 in univariate Cox proportional hazards analysis for class B or C in comparison with class A cirrhosis. Patients with decompensated cirrhosis are excluded from many similar European and Asian studies5, 14-17 but included here since HCC gives transplant waitlist priority in the US making HCC incidence in Child class C patients clinically relevant.2 Therefore this current study provides a unique estimate of the importance of disease severity in a population with advanced cirrhosis and the high hazard ratios suggest cirrhosis severity could be the most important risk factor for HCC in cirrhosis.
There are several significant limitations to this study. This is a convenience sample from a tertiary center liver clinic with an over-represented sampling of HCV cirrhosis patients so the study is not a good representation of any real patient population and the incidence rates for the overall cohort is of little significance. However, all patients were observed prospectively with the same screening imaging protocol and well-defined endpoints and there did not appear to be any informed censoring in terms of characteristics of patients lost to follow-up, so there is no clear bias associating any risk factor with HCC detection or diagnosis. Therefore although the subjects do not directly represent a tertiary care population they provide an accurate representation of the relationships between risk factors and HCC incidence. Another significant limitation is that study size and demographics limited the detail with which etiology and ethnicity could be analyzed. With limited numbers of HBV cirrhotic patients, etiology was divided into viral and nonviral cirrhosis for multivariate analysis, a broad comparison difficult to apply to an individual patient with a specific etiology but still highlighting an important trend in the population. Similarly, patient demographics permitted the broad comparison of HCC incidence in Asian and non-Asian patients but did not allow for a more granular analysis of ethnicity. In particular, the study population involved few patients of African descent and there continues to be very limited prospective data on HCC incidence in African-American individuals with cirrhosis.7-11 The study involves observational comparisons between groups and therefore has the potential for significant confounding. Although multivariate methods were used to adjust for the well-defined risk factors of age, sex, and CPT score, there is always the potential for unmeasured confounders such as time since infection and CPT score is not a perfect representation and adjustment for cirrhosis severity. The fact that there were only 44 observed events limits the number of variables that can be placed into a multivariate model without over-fitting and makes it difficult to study the significance of interaction terms. There are no universal criteria for noninvasive diagnosis of compensated cirrhosis and since only 29% were diagnosed with biopsy it is possible that not all patients were truly cirrhotic (data not shown).
In summary, this study has several significant implications for the understanding of HCC risk factors in US patients with cirrhosis. It highlights the strong association between CPT score and HCC incidence and the importance of carefully considering severity of cirrhosis in all observational studies of HCC in cirrhosis. Analysis by etiology demonstrates that patients with viral cirrhosis are at greater risk for HCC than those with non-viral cirrhosis and suggests that the relationships between cirrhosis etiology and HCC are clinically significant and need to be clarified. This study also provides initial longitudinal data indicating that Asian ethnicity is not a major independent risk factor for HCC in patients with cirrhosis in the US. Additional studies are needed to further assess HCC risk predictors for African Americans since the current study included few African Americans, an ethnic group with a disproportionately high HCC disease burden.
Acknowledgments
Grant Support
Stanford NIH/NCCR CTSA grant number TLI RR025742
Abbreviations
- AJCC
American Joint Committee on Cancer
- BCLC
Barcelona Clinic Liver Cancer
- CPT
Child-Pugh-Turcotte
- HBV
Hepatitis B virus
- HCC
Hepatocellular carcinoma
- HCV
Hepatitis C virus
- EASL
European Association for the Study of Liver Diseases
- MELD
model for end-stage liver disease
- NASH
non-alcoholic steatohepatitis
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Contributions--List how each author was involved with the manuscript
Robert D. Mair- study concept and design; acquisition of data; analysis and interpretation of data; drafting of the manuscript; statistical analysis
Antonia Valenzuela study concept and design; acquisition of data
Nghiem B. Ha study concept and design; acquisition of data
Walid S. Ayoub acquisition of data; review of manuscript
Tami Daugherty acquisition of data; review of manuscript
Glen A. Lutchman acquisition of data; review of manuscript
Gabriel Garcia acquisition of data; review of manuscript
Aijaz Ahmed acquisition of data; review of manuscript
Mindie H. Nguyen study concept and design; acquisition of data; analysis and interpretation of data; critical revision of the manuscript for important intellectual content; statistical analysis; study supervision
Disclosures: none
Writing Assistance: None
References
- 1.Fattovich G, Stroffolini T, Zagni I, Donato F. Hepatocellular carcinoma in cirrhosis: incidence and risk factors. Gastroenterology. 2004;127:S35–50. doi: 10.1053/j.gastro.2004.09.014. [DOI] [PubMed] [Google Scholar]
- 2.Bruix J, Sherman M. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53:1020–2. doi: 10.1002/hep.24199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.El-Serag HB. Hepatocellular carcinoma and hepatitis C in the United States. Hepatology. 2002;36:S74–83. doi: 10.1053/jhep.2002.36807. [DOI] [PubMed] [Google Scholar]
- 4.Tsai JF, Jeng JE, Ho MS, Chang WY, Hsieh MY, Lin ZY, Tsai JH. Effect of hepatitis C and B virus infection on risk of hepatocellular carcinoma: a prospective study. Br J Cancer. 1997;76:968–74. doi: 10.1038/bjc.1997.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fattovich G, Giustina G, Degos F, Tremolada F, Diodati G, Almasio P, Nevens F, Solinas A, Mura D, Brouwer JT, Thomas H, Njapoum C, Casarin C, Bonetti P, Fuschi P, Basho J, Tocco A, Bhalla A, Galassini R, Noventa F, Schalm SW, Realdi G. Morbidity and mortality in compensated cirrhosis type C: a retrospective follow-up study of 384 patients. Gastroenterology. 1997;112:463–72. doi: 10.1053/gast.1997.v112.pm9024300. [DOI] [PubMed] [Google Scholar]
- 6.Ikeda K, Saitoh S, Koida I, Arase Y, Tsubota A, Chayama K, Kumada H, Kawanishi M. A multivariate analysis of risk factors for hepatocellular carcinogenesis: a prospective observation of 795 patients with viral and alcoholic cirrhosis. Hepatology. 1993;18:47–53. [PubMed] [Google Scholar]
- 7.Gordon SC, Bayati N, Silverman AL. Clinical outcome of hepatitis C as a function of mode of transmission. Hepatology. 1998;28:562–7. doi: 10.1002/hep.510280238. [DOI] [PubMed] [Google Scholar]
- 8.Tong MJ, Hsien C, Song JJ, Kao JH, Sun HE, Hsu L, Han SH, Durazo FA, Saab S, Blatt LM. Factors associated with progression to hepatocellular carcinoma and to death from liver complications in patients with HBsAg-positive cirrhosis. Dig Dis Sci. 2009;54:1337–46. doi: 10.1007/s10620-009-0747-y. [DOI] [PubMed] [Google Scholar]
- 9.O'Leary JG, Landaverde C, Jennings L, Goldstein RM, Davis GL. Patients With NASH and Cryptogenic Cirrhosis Are Less Likely Than Those With Hepatitis C to Receive Liver Transplants. Clin Gastroenterol Hepatol. 2011 doi: 10.1016/j.cgh.2011.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lok AS, Seeff LB, Morgan TR, di Bisceglie AM, Sterling RK, Curto TM, Everson GT, Lindsay KL, Lee WM, Bonkovsky HL, Dienstag JL, Ghany MG, Morishima C, Goodman ZD. Incidence of hepatocellular carcinoma and associated risk factors in hepatitis C-related advanced liver disease. Gastroenterology. 2009;136:138–48. doi: 10.1053/j.gastro.2008.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ascha MS, Hanouneh IA, Lopez R, Tamimi TA, Feldstein AF, Zein NN. The incidence and risk factors of hepatocellular carcinoma in patients with nonalcoholic steatohepatitis. Hepatology. 2010;51:1972–8. doi: 10.1002/hep.23527. [DOI] [PubMed] [Google Scholar]
- 12.Hu KQ, Tong MJ. The long-term outcomes of patients with compensated hepatitis C virus-related cirrhosis and history of parenteral exposure in the United States. Hepatology. 1999;29:1311–6. doi: 10.1002/hep.510290424. [DOI] [PubMed] [Google Scholar]
- 13.Nguyen MH, Whittemore AS, Garcia RT, Tawfeek SA, Ning J, Lam S, Wright TL, Keeffe EB. Role of ethnicity in risk for hepatocellular carcinoma in patients with chronic hepatitis C and cirrhosis. Clin Gastroenterol Hepatol. 2004;2:820–4. doi: 10.1016/s1542-3565(04)00353-2. [DOI] [PubMed] [Google Scholar]
- 14.Kato Y, Nakata K, Omagari K, Furukawa R, Kusumoto Y, Mori I, Tajima H, Tanioka H, Yano M, Nagataki S. Risk of hepatocellular carcinoma in patients with cirrhosis in Japan. Analysis of infectious hepatitis viruses. Cancer. 1994;74:2234–8. doi: 10.1002/1097-0142(19941015)74:8<2234::aid-cncr2820740805>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 15.Tsukuma H, Hiyama T, Tanaka S, Nakao M, Yabuuchi T, Kitamura T, Nakanishi K, Fujimoto I, Inoue A, Yamazaki H, et al. Risk factors for hepatocellular carcinoma among patients with chronic liver disease. N Engl J Med. 1993;328:1797–801. doi: 10.1056/NEJM199306243282501. [DOI] [PubMed] [Google Scholar]
- 16.Velazquez RF, Rodriguez M, Navascues CA, Linares A, Perez R, Sotorrios NG, Martinez I, Rodrigo L. Prospective analysis of risk factors for hepatocellular carcinoma in patients with liver cirrhosis. Hepatology. 2003;37:520–7. doi: 10.1053/jhep.2003.50093. [DOI] [PubMed] [Google Scholar]
- 17.Chiaramonte M, Stroffolini T, Vian A, Stazi MA, Floreani A, Lorenzoni U, Lobello S, Farinati F, Naccarato R. Rate of incidence of hepatocellular carcinoma in patients with compensated viral cirrhosis. Cancer. 1999;85:2132–7. [PubMed] [Google Scholar]
- 18.Bruix J, Llovet JM. HCC surveillance: who is the target population? Hepatology. 2003;37:507–9. doi: 10.1053/jhep.2003.50142. [DOI] [PubMed] [Google Scholar]