Abstract
Aim
Lumbricus terrestris (earthworm) erythrocruorin (LtEc) is a naturally occurring extracellular hemoglobin (Hb) with high molecular weight (3.6 MDa), low autoxidation rate, and limited nitric oxide (NO) dioxygenation activity. These properties make LtEc an potential candidate for use as red blood cell (RBC) substitute, i.e. Hb-based oxygen carrier (HBOC). Previous studies have shown that small amounts of LtEc can be safely transfused into mice, rats, and hamsters without eliciting major side-effects. Therefore, this study was designed to understand oxygen (O2) transports to tissues and systemic/microvascular hemodynamics by LtEc during anemic conditions.
Main methods
Hamsters fitted with dorsal window chambers were hemodiluted to 18% hematocrit (Hct) using 6 g/dL dextran 70 kDa (Dex70). Hemodilution was then continued to 11% Hct using 10 g/dL LtEc, 6 g/dL Dex70 or 10 g/dL human serum albumin (HSA). Blood pressure, heart rate, blood gas parameters, and microvascular hemodynamics, microvascular blood flow, functional capillary density (FCD), intravascular pO2 and perivascular pO2 were studied.
Key findings
LtEc maintained blood pressure without inducing vasoconstriction, while increasing microvascular perfusion and FCD relative to Dex70 and HSA. LtEc increased blood O2 carrying capacity, maintained systemic and microvascular parameters without decreasing arteriolar diameter or increasing vascular resistance with during extreme anemia. LtEc transfusion effects in blood flow and O2 carrying capacity increased O2 delivery compared to conventional plasma expanders.
Significance
LtEc or synthetic molecules that replicate the characteristics of LtEc, could be an effective O2 carrier with potential to be used in transfusion medicine to prevent tissue anoxia resulting from severe anemia.
Keywords: Erythrocruorin, hemodynamics, hemodilution, anemia, tissue oxygenation, functional capillary density, albumin
INTRODUCTION
The clinical need for an artificial oxygen (O2) carrying solution is highlighted by the short shelf-life, frequent shortages, and storage-induced damage associated with the use of allogeneic red blood cells (RBCs) in transfusion medicine (Spinella, et al. 2011). Numerous attempts have been made to develop a synthetic and safe hemoglobin (Hb)-based O2 carrier (HBOC), but these attempts failed during clinical trials (Spahn 2000). The first attempts at transfusing cell-free human Hb (HbA) failed when the HbA tetramer was shown to quickly dissociate in the blood stream, extravasate through the blood vessel wall, and cause kidney failure (Brandt, et al. 1951,Miller and McDonald 1951,Savitsky, et al. 1978). The next generation of HBOCs prevented tetramer dissociation by intramolecularly crosslinking the HbA tetramer (HemAssist, Baxter, Irvine, CA)(Schubert, et al. 2002) or polymerizing Hb tetramers with glutaraldehyde; PolyHeme, Northfield, Evanston, IL)(Gould, et al. 2002) and Hemopure OPK Biotech, Boston, MS (Winslow 2000). Unfortunately, these HBOCs were shown to scavenge nitric oxide (NO) and cause hypertension/vasoconstriction by interfering with endothelial smooth muscle NO signaling (Doherty, et al. 1998,Rohlfs, et al. 1998).
The latest generation of HBOCs proposes to resolve the aforementioned problems by increasing the molecular weight (i.e. size) of polymerized Hbs (Matheson, et al. 2002), or encapsulating Hb tetramers within large diameter vesicles (Arifin and Palmer 2005). Based on the effects of Hb molecular size that we have previously observed, HBOCs with relatively large molecular weights (i.e. large molecular radii) do not leak into the perivascular space and have increased vascular retention times (Cabrales, et al. 2009,Cabrales, et al. 2010). Inspired by these results, we have investigated the use of the naturally occurring large extracellular erythrocruorin (i.e. mega Hb) from the common earthworm, Lumbricus terrestris (LtEc), which also has a high molecular weight (3.6 MDa) (Royer, et al. 2006,Strand, et al. 2004). Since earthworms lack RBCs, LtEc has naturally adapted to prevent the problems associated with acellular HBOCs (Hirsch, et al. 1997). LtEc is a complex structure, it has 144 globin subunits and 36 linker proteins that are extremely stable (Sharma, et al. 1996). LtEc also has a lower rate of oxidation than mammalian Hbs, with a positive redox potential (Eo = 112 mV compared to Eo = −50 mV for HbA) (Harrington, et al. 2007). In addition, LtEc and other erythrocruorins have an extremely low rate of NO scavenging (Elmer, et al. 2012). Hirsch et al. have shown that LtEc may be safe when injected into mice and rats without any noticeable side effects (Hirsch, et al. 1997).
We have also previously shown that LtEc can be easily highly purified via tangential flow filtration (TFF) and, when injected into hamsters in an experimental model that magnifies vasoactivity, LtEc did not produce any adverse reactions (Elmer, et al. 2012). In this study, 6g/dL dextran 70 kDa was used to induce an extreme anemic state in order to magnify the effects of LtEc in O2 transport when most of the hamster native RBCs had been removed from the circulation (11% hematocrit, Hct). Our main goals were to (1) assess the contribution of LtEc to tissue O2 delivery, (2) determine if LtEc induces hypertension and/or vasoconstriction, and (3) establish the circulatory half-life and pharmacokinetics of LtEc. Overall, LtEc improved systemic and microvascular parameters compared to conventional plasma expanders during extreme hemodilution, going beyond increasing O2 carrying capacity to increase blood flow, while avoiding vasoconstriction and oxidation.
METHODS
Earthworm Preparation
For each round of purification, 1,000 Canadian nightcrawlers (Lumbricus terrestris) were purchased from Wholesale Bait Company (Hamilton, OH). Worms were rinsed in tap water to remove dirt, and then 1 L batches of worms were extensively washed with 20-30 L of tap water to remove as much mucus as possible. A blender was used to homogenize the worms (puree mode for ~10 seconds) and the homogenate was immediately centrifuged at 3,716 g for 40 min at 4°C. Solid debris was discarded and the cloudy red supernatant was centrifuged at 18,000 g for 20 min at 10-15°C. The clear red supernatant (~2.0 L per 1,000 worms) was then put through filter paper to remove any remaining large particles.
Purification of LtEc
The earthworm homogenate was passed through two 0.22 μm TFF cartridges in parallel (1050 cm2 surface area, Spectrum Labs, Rancho Dominguez, CA) at 450 mL/min until the majority of the sample volume was transmitted through the filter. The filter pores clogged several times during the filtration process (indicated by a clear filtrate) and were subsequently cleaned with deionized water. The red retentate of the 0.22 μm filter was stored at −80°C for future analysis. The 0.22 μm filtrate (1.8-2.0 L) was then concentrated on two 500 kDa TFF cartridges at 450 mL/min down to a final volume of approximately 200 mL. The 500 kDa retentate was then diafiltered by diluting it to 2 L with buffer and then concentrating it down to 200 mL ten times. The retentate was diluted with 20 mM Tris buffer (pH 7.0) during the first eight rounds of diafiltration and modified lactated Ringer’s buffer (115 mM NaCl, 4 mM KCl, 1.4 mM CaCl2, 13 mM NaOH, 12.25 mM N-acetyl cysteine, 0.3% sodium lactate, pH 7.0) during the last two rounds of diafiltration. During the final round of diafiltration, the retentate was concentrated to 20-50 mL and sterilized by passing it through a 0.45 μm syringe filter. The purified LtEc was then stored at −80°C until needed. After each round of purification, all filters were rinsed and soaked in 0.2 M NaOH for 1 hour, then rinsed with distilled water and stored at 4°C.
Estimation of Yield and Methemoglobin Level
The Hb concentration and the percent of oxidized Hb (i.e. methemoglobin [metHb]) was measured using the cyanomethemoglobin method (Crosby, et al. 1954). Before quantification, each sample was centrifuged at 10,000 g for 5 minutes to remove any debris. The concentration of each sample was then multiplied by its volume to obtain the mass of Hb in each filtrate or retentate sample.
Oxygen equilibrium curve
Oxygen equilibrium curves for the LtHb and the Hamster blood were obtained by deoxygenation of O2-equilibrated samples in a Hemox buffer at 37°C, using a Hemox Analyzer (TCS Scientific Corporation, New Hope, PA). The Hemox buffer pH was adjusted to match the arterial blood pH of the animals using Tris and BisTris buffers. Tris and BisTris buffers were prepared by titrating the reagents with HCI before adjusting the pH of the solutions to keep Cl− ions concentration equal to the buffer at the pH values.
Window Chamber Animal Preparation
Animal handling and care followed the NIH Guide for the Care and Use of Laboratory Animals and the experimental protocol was approved by the local animal care committee. Studies were performed in 55 - 65 g male Golden Syrian Hamsters (Charles River Laboratories, Boston, MA) fitted with a dorsal skinfold window chamber. The hamster window chamber model is widely used for microvascular studies without anesthesia. The complete surgical technique has been described previously (Endrich, et al. 1980). Arterial and venous catheters filled with heparinized saline solution (30 IU/mL) were implanted into the carotid and jugular vessels. Catheters were tunneled under the skin, exteriorized at the dorsal side of the neck, and securely attached to the window frame.
Inclusion Criteria
Animals were considered suitable for experiments if systemic parameters were as follows: heart rate (HR) > 340 beats/min, mean arterial blood pressure (MAP) > 80 mm Hg, systemic Hct > 45%, and arterial O2 partial pressure (pAO2) > 50 mm Hg. Additionally, animals with signs of low perfusion, inflammation, edema, or bleeding in their microvasculature were excluded from the study.
Systemic Parameters
MAP and HR were monitored continuously (MP150, Biopac System Inc., Santa Barbara, CA). Hct was measured from centrifuged arterial blood samples taken in heparinized capillary tubes. Hb content was determined spectrophotometrically (B-Hemoglobin, Hemocue, Stockholm, Sweden). Arterial blood was collected in heparinized glass capillaries (50 μL) and immediately analyzed for pO2, pCO2, base excess (BE), and pH (Rapidlab 248, Bayer, Norwood, MA). Arterial Hb saturations were measured using a IL482 CO-Oximeter (Instrumentation Laboratory, Lexington, MA).
Experimental Setup
The unanesthetized animal was placed in a restraining tube with a longitudinal slit from which the window chamber protruded, then fixed to the microscopic stage for transillumination with the intravital microscope (BX51WI, Olympus, New Hyde Park, NY). Animals were given 20 minutes to adjust to the tube environment and images were obtained using a CCD camera (4815, COHU, San Diego, CA). Measurements were carried out using a 40× (LUMPFL-WIR, numerical aperture 0.8, Olympus) water immersion objective.
Microhemodynamics
Arteriolar and venular blood flow velocities were measured using the photodiode cross-correlation method (Photo-Diode/Velocity, Vista Electronics, San Diego, CA) (Intaglietta, et al. 1975). The measured centerline velocity (V) was corrected according to blood vessel size to obtain the mean RBC velocity (Lipowsky and Zweifach 1978). A video image-shearing method was used to measure blood vessel diameter (D) (Intaglietta and Tompkins 1973). Blood flow (Q) was calculated from the measured values as Q = π × V (D/2)2. Blood vessel wall shear stress (WSS) was defined by WSS = WSR × η, where WSR is the blood vessel wall shear rate given by 8VD−1, and η is the microvascular blood viscosity or plasma viscosity. Microvascular blood flow was determined by the perfusion pressure, vascular resistance, and blood viscosity. In a rigid tube, changes in apparent fluid viscosity would lead to parallel changes in resistance and reciprocal changes in flow. Microvascular resistance (MR) was calculated as the ratio of blood pressure and microvascular volumetric blood flow (MR = MAP / average microvascular Q). Microvascular hindrance (MH) was calculated as the ratio of MR and blood viscosity (MH = MR / η).
Functional Capillary Density (FCD)
Functional capillaries, defined as capillary segments that have RBC transit of at least one RBC in a 60 second period in 10 successive microscopic fields, were assessed in a region of 0.46 mm2. FCD (cm−1) is calculated as the total length of RBC perfused capillaries divided by the area (0.46 mm2).
Microvascular pO2 and microvascular O2 saturations
pO2 measurements were made using phosphorescence quenching microscopy (PQM) (Torres Filho and Intaglietta 1993). PQM is based on the O2 dependent quenching of phosphorescence emitted by albumin-bound metalloporphyrin complex after pulsed light excitation. PQM is independent of the dye concentration. Perivascular pO2 was measured in tissue regions between functional capillaries. PQM allows for precise localization of the pO2 measurements without subjecting the tissue to injury. Intravascular Hb O2 saturations were calculated using the O2 equilibrium curves measured and the intravascular pO2s measured with the PQM. The O2 equilibrium curves were measured at arterial blood pH as described above.
Isovolemic Hemodilution
Acute anemia was induced by two isovolemic hemodilution steps. This protocol was described in detail in a previous publication (Cabrales, et al. 2004,Cabrales, et al. 2010). Briefly, the volume of each exchange-hemodilution step was calculated as a percentage of the animal’s blood volume (BV), estimated as 7% of body weight (BW). The acute anemic state was induced by lowering the Hct to 18% in two progressive isovolemic hemodilution steps using 6 g/dL dextran 70 kDa (Pharmacia, Uppsala, Sweden). The first exchange was 40% of the BV and second exchange was 35% of the BV, respectively. After hemodilution to 18% Hct, the animals were randomly divided into three experimental groups: Dex70 (further hemodiluted with 6 g/dL dextran 70 kDa to 11% Hct), HSA (further hemodiluted with 10% HSA to 11% Hct), or LtEc (further hemodiluted with 10% LtEc to 11% Hct). The properties of the test solutions are shown in Table 1. The viscosity of LtEc was higher than undiluted blood (4.2 cP) and the dextran 70 kDa and HSA solutions. In contrast, dextran 70 kDa and HSA solutions have higher COP compared to LtEc, which is also lower than the COP of plasma serum (18 mm Hg). The O2 affinity and cooperativity of LtEc (P50 = 28 mm Hg and n =3.7) are similar to hamster blood (P50 = 32 mm Hg and n = 2.9).
TABLE 1.
Physical properties of transfusion solutions.
| Solution properties |
|||||
|---|---|---|---|---|---|
| g/dL | Viscosity* CP |
COP mmHg |
P50 mmHg |
n | |
| Plasma Expander | |||||
| Dextran 70 kDa (Dex70) | 6.0 | 2.8 | 48 | -- | -- |
| Human serum albumin (HSA) | 10.0 | 1.6 | 44 | -- | -- |
|
| |||||
| Hb based 02 carriers LtEc |
10.0 | 8.2 | 6 | 28.0 | 3.7 |
Shear rate of 160 s−1 at 37°C
Oxygen Delivery and Extraction. The microvascular methodology used in our studies allows a detailed analysis of O2 supply to the tissues. Calculations were made using equations 1 and 2 (Cabrales, et al. 2010):
| (1) |
| (2) |
Where, RBCHb is the Hb encapsulated inside RBCs, total Hb - plasma Hb [gHb/dlblood], PlasmaHb is the extracellular Hb [gHb/dlblood], γ is the O2 carrying capacity of saturated Hb [1.34 mlO2/gHb], SA is the arteriolar blood O2 saturation and ŠA is the arteriolar extracellular Hb O2 saturation, the subscript (A-V) indicates the arteriolar/venular differences, and Q is the microvascular flow rate. O2 saturations were calculated using the measured pO2 and oxygen equilibrium curve.
Pharmacokinetic Analysis of LtEc
LtEc pharmacokinetics was studied in hamsters fitted with the dorsal window chamber (described in the animal preparation section) after an exchange transfusion of 40% of the estimated BV. Pharmacokinetic parameters were determined for total LtEc in the plasma using a non-compartmental method (NCOMP Pharmacokinetics Analysis Software, Philadelphia, PA). At each time point (0.5, 1, 2, 4, 8, 12, 24, and 48 h) after the exchange transfusion, a 50 μL aliquot of blood was collected, and plasma was obtained by centrifugation. The plasma Hb and metHb concentration were measured via the cyanomethemoglobin method (Sakata, et al. 1982).
Statistical Analysis
Results are presented as the mean ± standard deviation and the values are presented relative to the baseline. Data between time points in the same group were analyzed using analysis of variance for repeated measures (ANOVA, Kruskal-Wallis test) and post hoc analyses with the Dunn’s Multiple Comparison, when appropriate. Statistics were calculated using Prism 4.01 (GraphPad, San Diego, CA) and differences were considered statistically significant if P < 0.05.
RESULTS
Eighteen animals were included in the study. All animals tolerated the entire protocol without any visible signs of discomfort. Animals were assigned to the following experimental hemodilution groups: Dex70 (n=6; 61.7±6.2 g); HSA (n=6; 63.6±4.6 g); and LtEc (n=6; 65.7±4.7 g). Systemic data at baseline and during moderate hemodilution (18% Hct) were calculated by combining values from all of the experimental groups (n=18). All animals passed the Grubbs’ test, meaning that all the measured values at baseline were within a similar population (P<0.05). Similarities between groups at baseline and moderate hemodilution (18% Hct) were also statistically verified between groups (P>0.30).
Biophysical Properties of Solutions and Blood
Dex70 and HSA have higher COP values (44 and 48 mm Hg) compared to LtEc (6 mm Hg), and the viscosity of LtEc is 3 and 5 times greater than Dex70 and HSA. Blood rheological properties and COP after hemodilution are presented in Table 2. LtEc showed higher blood and plasma viscosity compared to Dex70 and HSA. However, the plasma COPs for all the study groups were similar, and not different from baseline. Interestingly, no pathophysiological effects were observed in the hamsters transfused with LtEc. Even after 1 hour post infusion of LtEc, very few leukocytes and macrophages were observed in the microcirculation. In addition, the urine collected from each animal’s bladder after the experiments showed no signs of LtEc.
TABLE 2.
Blood chemistry and rheological parameters.
| Extreme hemodilution |
|||||
|---|---|---|---|---|---|
| Baseline | Hemodilution | Dex70 | HSA | LtEc | |
| n | 18 | 18 | 6 | 6 | 6 |
| Hct, % | 48.4 ± 0.9 | 18.5 ± 0.7† | 11.2 ± 0.3†‡ | 11.3 ± 0.5†‡ | 11.6 ± 0.7†‡ |
| Total Hb, g/dl | 14.6 ± 0.4 | 5.8 ± 0.4† | 3.6 ± 0.3†‡ | 3.7 ± 0.4†‡
|
6.1 ± 0.5†§ |
| Plasma Hb, g/dl | 2.7 ± 0.4 | ||||
|
| |||||
| paO2, mmHg | 58.8 ± 5.6 | 78.3 ± 6.1† | 116.5 ± 7.4†‡ | 99.6 ± 4.7†‡ | 97.2 ± 7.8†‡§ |
| paCO2, mmHg | 53.3 ± 5.1 | 46.3 ± 5.8† | 36.2 ± 6.4†‡ | 40.6 ± 5.0† | 45.1 ± 4.8† |
| PHa | 7.336 ± 0.020 | 7.381 ± 0.024 | 7.387 ± 0.028 | 7.364 ± 0.027 | 7.372 ± 0.029 |
| BEa, mmol | 2.6 ± 1.7 | 1.0 ± 1.1 | −2.0 ± 1.2†‡ | −0.7 ± 0.9†‡ | 0.8 ± 0.9§ |
|
| |||||
| Blood Vise., cP | 4.2 ± 0.2 | 2.3 ± 0.2† | 2.0 ± 0.2†
|
2.7 ± 0.2†§ | |
| Plasma Vise., cP | 1.2 ± 0.1 | l.6 ± 0.1† | l.4 ± 0.2†
|
2.0 ± 0.2†§ | |
| COP, mm Hg | 18 ± 2 | 16 ± 2 | 18 ± 2 | 16 ± 2 | |
Values are means ± SD. Baseline and hemodilution included all the animals. No significant differences were detected between baseline or hemodilution values between the experimental groups.
Hct, hematocrit; Hb, hemoglobin; PaO2, arterial partial O2 pressure; PaCO2, arterial partial pressure of CO2; BEa, arterial base excess.
P<0.05 compared to baseline
P<0.05 compared to MH
P<0.05 compared to Dex70.
Blood Gas Parameters
Blood gas parameters are also presented in Table 2. After extreme hemodilution with Dex70, HSA, and LtEc, the Hct decreased to 11% following the experimental protocol. Total Hb concentration also decreased in the Dex70 and HSA groups (Hb = 3.6-3.7 g/dL), but the Hb concentration in the LtEc group (6.1 ± 0.5 g/dL) was slightly higher than at moderate hemodilution (5.8 ± 0.4 g/dL). Arterial pO2 increased significantly relative to baseline and moderate hemodilution for all groups, but only the LtEc group had arterial pCO2 levels similar to moderate hemodilution. Hemodilution with LtEc increased arterial pO2 compared to baseline and moderate hemodilution, however lower than hemodilution with Dex70. Arterial blood pH was not significantly different after hemodilution with any of the test solutions. Blood BE was significantly decreased after hemodilution with Dex70 and HSA compared to baseline, but no significant differences in blood BE were observed in the LtEc group.
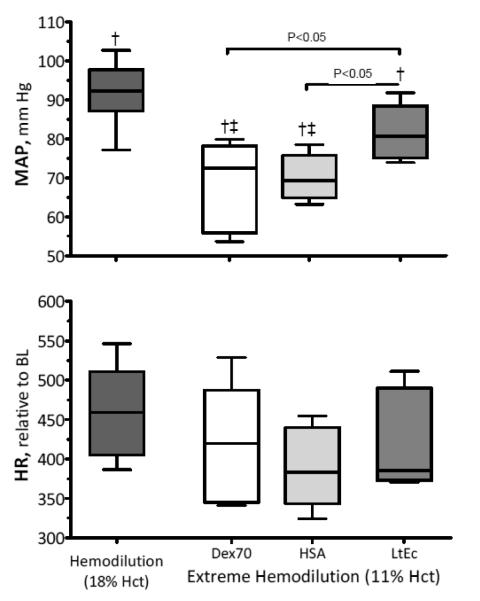
MAP and HR
Changes in MAP and HR are presented in Figure 1. The MAP after hemodilution with LtEc was lower relative to baseline, but not significantly different from moderate hemodilution at 18% Hct. The additional O2 carrying capacity provided by LtEc maintained MAP after hemodilution, while the MAP values of the Dex70 and HSA groups significantly decreased relative to baseline and moderate hemodilution. No significant changes in HR were observed in any of the test groups.
FIGURE 1.
Mean arterial pressure (MAP) and heart rate (HR) after extreme hemodilution with LtEc, HSA and Dex70. The broken line represents the baseline level. †, P < 0.05 relative to baseline; ‡, P<0.05 compared to Dex70; §, P<0.05 compared to HSA.
Microvascular Hemodynamic Measurements
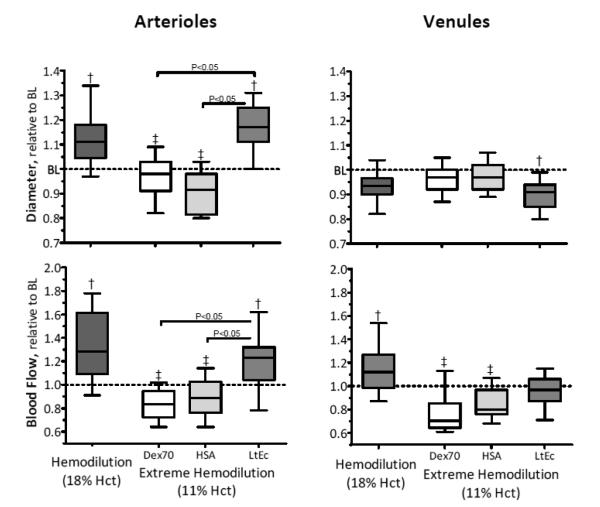
Changes in microvessel diameter and blood flow are shown in Figure 2, absolute values are listed in the figure legend. For the Dex70 and HSA groups, arteriolar diameters significantly decreased relative to moderate hemodilution. In contrast, the arteriolar diameters in animals transfused with LtEc were significantly higher than baseline and the Dex70 and HSA groups. Venular diameters for Dex70 and HSA were not significantly different from baseline, but the venular diameter of the LtEc group was significantly lower relative to baseline. The blood flow in the arterioles and venules were significantly lower for the Dex70 and HSA groups relative to baseline. In addition, the venular blood flow of the Dex70 and HSA groups was significantly lower than at moderate hemodilution, but venule blood flow was maintained after hemodilution with LtEc. On the other hand, arteriolar blood flow in the LtEc group was significantly higher than Dex70, HSA, and baseline, resulting from the significant increase in arteriolar diameter.
FIGURE 2.
Relative changes to baseline in arteriolar and venular hemodynamics after extreme hemodilution with LtEc, HSA and Dex70. The broken line represents the baseline level. †, P < 0.05 relative to baseline; ‡, P<0.05 compared to Dex70; §, P<0.05 compared to HSA. Diameters (μm, mean ± SD) at baseline in each animal group were as follows: LtEc (arterioles (A): 62.1 ± 7.2, n = 48; venules (V): 62.6 ± 5.8, n = 46); HSA (A: 61.9 ± 9.1, n = 42; V: 63.6 ± 7.2, n = 46); and Dex70 (A: 62.7 ± 8.6, n = 48; V: 64.8 ± 7.4, n = 46). n = number of small blood vessels studied. RBC velocities (mm/s, mean ± SD) at baseline in each animal group were as follows (data was not presented in the figure, but used in the calculation of blood flow): LtEc (A: 4.3 ± 0.9; V: 1.9 ± 0.7); HSA (A: 4.3 ± 1.0; V: 2.0 ± 0.8); Dex70 (A: 4.4 ± 0.8; V: 2.1 ± 0.7). Blood flow (nL/s, mean ± SD) at baseline in each animal group were as follows: LtEc (A: 12.7 ± 5.3; V: 5.4 ± 2.1); HSA (A: 12.4 ± 4.2; V: 5.7 ± 2.1); Dex70 (A: 12.0 ± 4.7; V: 6.2 ± 2.4).
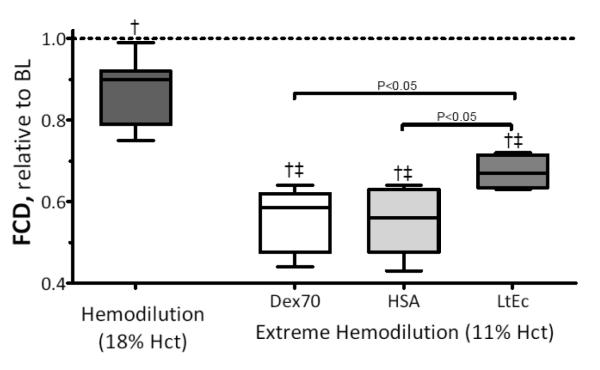
Changes in FCD are presented in Figure 3. The FCD was significantly reduced in all experimental groups compared to baseline and 18% Hct. However, the FCD of the LtEc group was significantly higher relative to the Dex70 and HSA groups. Arteriolar and venular shear stress and microvascular resistance and hindrance are also presented in Table 3. There were no significant differences in the wall shear rate of the LtEc, Dex70, and HSA groups, but wall shear stress was significantly higher for LtEc compared to Dex70 and HSA. Assuming that microvascular blood pressure is proportional to MAP, the estimated microvascular resistance of the LtEc group (62% of baseline) was significantly lower than the HSA (68% of baseline) and Dex70 (77% of baseline) groups. Vascular hindrance (i.e. the contribution of vascular geometry to blood flow) for LtEc (96% of baseline) was similar to baseline, but significantly higher in the Dex70 (146% of baseline) and HSA (147% of baseline) groups. These results confirm that LtEc does not induce a vasoconstrictive response upon infusion. This also shows that extreme hemodilution with the plasma expanders used in this study increases peripheral vascular resistance in order to maintain O2 supply to vital organs.
FIGURE 3.
Functional capillary density (FCD) after extreme hemodilution with LtEc, HSA and Dex70. †, P < 0.05 relative to baseline; ‡, P<0.05 compared to 18% Hct. FCD (capillaries per unit of area, cm−1) at baseline for LtEc were 112 ± 11, Dex70 were 114 ± 12 and for HSA were 109 ± 10, respectively.
TABLE 3.
Pharmacokinetics of LtEc.
| LtHb | |
|---|---|
| CL, mL h−1 | 3.36 |
| MRT, h | 23.2 |
| Vss, mL | 78.1 |
| Terminal K, h−1 | 0.045 |
| Terminal T1/2, h | 14.5 |
| Cmax, , Mg h−1 | 3.40 |
CL, plasma clearance; MRT, mean residence time; Vss volume of distribution at steady-state, terminal k, terminal slope; terminal T1/2, terminal half-life; Cmax, concentration maximal.
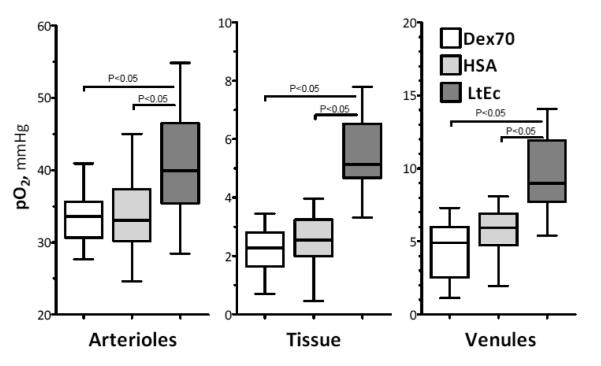
Microvascular Oxygen Tensions
Arteriolar, venular and perivascular pO2s are presented in Figure 4. Arteriolar pO2 was significantly higher in the LtEc group than the Dex70 and HSA groups. The LtEc group also had significantly higher perivascular and venular pO2 than the Dex70 and HSA groups.
FIGURE 4.
Intravascular and perivascular partial pressure of oxygen after moderate hemodilution and extreme hemodilution with LtEc, HSA and Dex70. Intravascular and perivascular PO2s for the tissues comprising the hamster window model are 51.8 mm Hg for arterioles, 32.7 mm Hg for venules and 21.7 mm Hg for perivascular areas.
Microvascular Oxygen Delivery
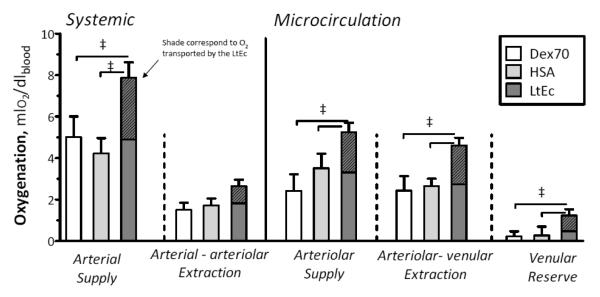
Oxygen transport parameters were calculated using the ex vivo P50 of LtEc. The O2 delivery and extraction values are presented in Figure 5. LtEc provided superior O2 delivery and maintained high O2 extraction. Arterial (systemic) and microvascular O2 delivery were significantly higher for LtEc compared to the Dex70 and HSA groups. Arterial to arteriolar O2 extraction was not different between the groups. However, the arteriolar to venular (microcirculation) O2 extraction was significantly higher for LtEc compared to the Dex70 and HSA groups. These results show that LtEc is able to maintain O2 delivery after hemodilution compared to the plasma expanders used in this study.
FIGURE 5.
Arterial oxygen delivery and extraction after extreme hemodilution with LtEc, HSA and Dex70. Oxygen transport is not directly measurable; however, it can be calculated using the measured parameters. Hatched portion of bar indicated the amount of oxygen transported by the LtEc.
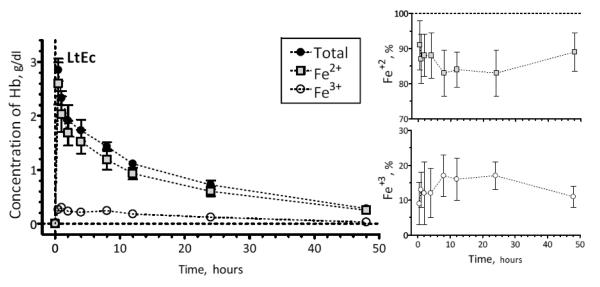
Pharmacokinetics of LtHb
Four animals were used for the pharmacokinetic study (n=4; 65.7 ± 6.7 g) presented in Figure 6 and Table 3. The initial dose of LtEc was 189 ± 52 mg and the maximum plasma concentration (Cmax) was 3.36 ± 0.61 g/dL. The volume of distribution and retention time of LtEc indicates a mean residence time of 23.2 ± 1.2 hours. MetHb levels remained lower than 20% during the entire observation period. The measurements of reduced (Fe2+) and oxidized (Fe3+, metHb) LtEc show that LtEc is able to resist oxidation in vivo. Therefore, most of the transfused LtEc remained in the bloodstream in the O2 transporting form (Fe2+) a full day after the initial transfusion.
FIGURE 6.
LtEc pharmacokinetics after a 40% exchange transfusion.
DISCUSSION
The principal result of this study is that naturally occurring extracellular megaHb complex defines the properties to generate a new generation of safe and efficacious HBOC, which can be advantageous in extreme anemic conditions when blood is not available. In addition, if highly purified LtEc were to be used as HBOC, it may avoid many costly synthetic steps necessary in order to modify mammalian Hb into HBOC. Our results show that LtEc effectively maintains systemic hemodynamics, preserves blood gas parameters, and delivers O2 during extreme anemia (11% Hct). LtEc improved O2 delivery to the tissues and sustained two-fold higher perivascular pO2 values and 50% higher oxygenation than the plasma expander groups (see Figures 4 and 5). These improvements in O2 delivery are mostly due to the O2 affinity and binding equilibrium of LtEc, which is similar to RBCs. Earthworm Hb has evolved during the course of natural selection to possess unique structural and functional properties that impart its efficacy as a naturally occurring extracellular O2 carrier. LtEc possesses a unique structure in the form of a megaHb complex that meets the molecular size requirement that we have previously defined for synthetic polymerized bovine Hb to minimize side effects of safe HBOCs (Cabrales, et al. 2009,Cabrales, et al. 2010). However, LtEc includes additional structural and oxidative stability properties, that appear to be essential for the development of efficacious extracellular HBOCs.
Our current results show that when extreme anemic states are induced by hemodilution with LtEc, hemodynamics and oxygenation are superior compared to plasma expanders (HSA & dextran 70 kDa). The systemic hemodynamics benefits of LtEc were paralleled with positive microvascular changes, where blood flow and FCD were significantly greater for LtEc compared to plasma expanders. Hemodilution decreased the Hct and dramatically reduced blood viscosity; however, when LtEc was used to further reduce the Hct, it increased plasma and blood viscosity relative to HSA or Dex70. The increase in blood viscosity with LtEc did not increase blood pressure, and on the contrary LtEc decreased microvascular resistance. In addition, the hemodynamic changes and the pharmacokinetic results indicate that LtEc can be used as a potential HBOC, since it improves blood flow, O2 delivery and has a longer circulation time than commercially developed HBOCs (Elmer, et al. 2010,Vandegriff, et al. 2006).
The infusion of current commercially developed HBOCs results in vasoconstriction, which is evident as a rapid rise in peripheral vascular resistance and blood pressure, commonly associated with bradycardia, decreasing cardiac output and perfusion to vital organs (Rohlfs, et al. 1998). Paradoxically, microvascular resistance was lower for LtEc relative to HSA and Dex70, even though LtEc has a significantly higher viscosity than HSA and Dex70. Consequently, the group hemodiluted with LtEc had higher plasma viscosities than both of the plasma expander groups. It has been shown that higher high plasma viscosity increases shear stress on the endothelial vessel walls, thereby inducing mechanotransduction responses that activate endothelial NO production, causing relaxation of the smooth muscle and increasing blood vessel diameter (Tsai, et al. 2005). This phenomenon has been previously observed with high viscosity plasma expanders (Tsai, et al. 2005), and may be one of the mechanisms that preserves vascular tone after hemodilution with LtEc. Hemodilution with low viscosity plasma expanders decreased vascular resistance, but, on the other hand, it increased vascular hindrance, which accounts for the geometric contribution of the vasculature to the resistance, suggesting generalized vasoconstriction, whereas the hemodilution with LtEc reduced vascular resistance and preserved vascular hindrance. LtEc acted as a viscogenic O2 carrier, maintaining both shear stress and oxygenation, without vasoconstriction, leading to improved transmission of central pressure to the microcirculation to preserve FCD. In a similar extreme hemodilution study, a commercially available polymerized bovine Hb (Oxyglobin, OPK Biotech Inc., Cambridge, MA) developed for veterinarian use reduced the FCD to 40% of baseline, and increased vascular resistance (Tsai 2001). Consequently, hemodilution with Oxyglobin reduced O2 delivery to the surrounding tissues (Tsai 2001). This result was found to be due to vasoconstriction, which lowers microvascular blood flow, capillary pressure and FCD (Cabrales, et al. 2004). These adverse effects are not only due to the extracellular nature of the HBOC, since non-O2 carrier plasma expanders, such as HSA and Dex70, decreased FCD, blood flow, and capillary pressure (Cabrales, et al. 2004). In contrast, hemodilution with LtEc maintained microvascular hemodynamics and FCD, which preserved oxygenation and wash out of metabolic by products.
Compared to previous work by Hirsch et al. (Hirsch, et al. 1997), this study is the first to use LtEc at a concentration capable of significantly increasing O2 transport, as verified by our results. In situations where bulk O2 transport capacity is required, LtEc can serve a potentially safe and efficacious HBOC. The acid-base balance at 11% Hct after extreme hemodilution with LtEc was not different from baseline (48% Hct), while the plasma expanders used in this study showed negative acid-base balances. Additionally, the arterial PO2 of animals hemodiluted with plasma expanders was drastically increased, suggesting significant adjustments in the respiratory system, in an attempt to improve oxygenation, which only increases the dissolved O2 within the plasma having minimal effects in the O2 transported by the blood. O2 transport calculations show that LtEc increased the arterial O2 transport by 60% compared to conventional plasma expanders, and similarly at the microcirculatory level, LtEc increased O2 transport by at least 50% compared to HSA and Dex70. The O2 transport similarities between LtEc and RBCs suggest that each species contribution to O2 transport is proportional to their concentration. Therefore, the physiological O2 affinity of LtEc preserves LtEc’s O2 transport benefits along the entire cardiovascular system. In this study, the perivascular pO2 was low due to the limited O2 carrying capacity of the hemodiluted blood. Although, LtEc showed higher perivascular pO2 compared to plasma expanders resulting from its higher O2 carrying capacity. The O2 transport benefits of LtEc may be magnified by the hamster respiratory gas exchange characteristics and their Hb O2 affinity with a P50 of 32 mm Hg. Moreover, hamsters are fossorial animals, which explains their low arterial PO2 at baseline, since their respiratory exchange is adapted to their burrow environment with a greater buffering capacity from higher HC03 and decreased sensitivity to blood pH (Tomasco, et al. 2010). LtEc meets the requirements of a HBOC and/or O2 carrier plasma expander as demonstrated by its lack of acute toxicity, long retention time and reduced oxidation. The pharmacokinetic data show that LtEc has a longer circulation time than most other HBOCs (13-17 hrs) (Elmer, et al. 2010,Vandegriff, et al. 2006). The in vivo oxidation rate of LtEc appears to be quite low, since no significant increases in metLtEc (Fe3+ form) were observed over a period of 48 hours. In contrast, HBOCs in clinical trials have report levels of Fe3+ higher than 40% in less than 24 hours after infusion (Siegel, et al. 1997). By resisting oxidation, LtEc is able to maintain O2 delivery for long periods of time and reduce oxidative stress induced by the presence of Fe3+ in the bloodstream. Additionally, the results obtained with LtEc led to the hypothesis that its molecular size and stability (structural and oxidative) are important characteristics that allow it to function as a non-toxic HBOC in vertebrates. The importance our results during hemodilution with LtEc are many fold, since vasoconstriction is perceived to be the critical barrier hampering the commercial development of HBOCs. Regardless of the exact mechanism for vasoconstriction, ultrapure naturally extracellular LtEc did not produce vasoconstriction. Finally, it is important to mention that none of the animals showed significant changes in behavior following transfusion of LtEc. LtEc appears to be safe in hamsters, but future work will be aimed to establish any potential side-effects and immunogenicity.
Conclusion
This study shows that LtEc is an effective HBOC which does not elicit any significant vasoconstriction or hypertension, and it is able to effectively and safely deliver O2 during extreme anemia. The large molecular size, O2 binding characteristics, high viscosity, stability, and high circulation half-life, contribute to LtEc superior O2 delivery. Thus seem to be essecial properties required for the next generation HBOC. Additionally, highly purified LtEc might have the potential to be used as a HBOC, since it does not require costly synthetic modification steps and appears to avoid the vasoconstriction and hypertension regarded at mayor limitations of current HBOCs. While these results are promising, much more work will have to be done before synthetic molecules inspired in LtEc or purified LtEc can enter clinical trials. Transfusion of higher LtEc concentrations must be explored to determine immunogenicity and clearance mechanism of LtEc. All extracellular Hbs are known to deplete NO, thus detailed in vitro and in vivo studies must be done to determine the exact nature of the interactions between LtEc and NO. Nonetheless, in light of our current results, LtEc characteristics should be further study to be able to incorporate these properties in the new generation of O2 carriers.
Supplementary Material
ACKNOWLEDGEMENTS
This work was partially supported by Bioengineering research grant R24-HL64395, Program project P01-HL071064, and grants R01-HL52684, R01-HL62354, R01-HL078840 and R01-DK070862. The authors thank Froilan P. Barra and Cynthia Walser for surgical preparation of the animals.
Funding Sources: This work was partially supported by Bioengineering research grant R24-HL64395, Program project P01-HL071064, and grants R01-HL52684, R01-HL62354, R01-HL078840 and R01-DK070862.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure statement: The authors have no conflicts of interest.
References
- Arifin DR, Palmer AF. Polymersome encapsulated hemoglobin: a novel type of oxygen carrier. Biomacromolecules. 2005;6(4):2172–81. doi: 10.1021/bm0501454. [DOI] [PubMed] [Google Scholar]
- Brandt JL, Frank NR, Lichtman HC. The effects of hemoglobin solutions on renal functions in man. Blood. 1951;6(11):1152–8. [PubMed] [Google Scholar]
- Cabrales P, Sun G, Zhou Y, Harris DR, Tsai AG, Intaglietta M, Palmer AF. Effects of the molecular mass of tense-state polymerized bovine hemoglobin on blood pressure and vasoconstriction. J Appl Physiol. 2009;107(5):1548–58. doi: 10.1152/japplphysiol.00622.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabrales P, Tsai AG, Intaglietta M. Microvascular pressure and functional capillary density in extreme hemodilution with low- and high-viscosity dextran and a low-viscosity Hb-based O2 carrier. Am J Physiol Heart Circ Physiol. 2004;287(1):H363–73. doi: 10.1152/ajpheart.01039.2003. [DOI] [PubMed] [Google Scholar]
- Cabrales P, Zhou Y, Harris DR, Palmer AF. Tissue oxygenation after exchange transfusion with ultrahigh-molecular-weight tense- and relaxed-state polymerized bovine hemoglobins. Am J Physiol Heart Circ Physiol. 2010;298(3):H1062–71. doi: 10.1152/ajpheart.01022.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crosby WH, Munn JI, Furth FW. Standardizing a method for clinical hemoglobinometry. U S Armed Forces Med J. 1954;5(5):693–703. [PubMed] [Google Scholar]
- Doherty DH, Doyle MP, Curry SR, Vali RJ, Fattor TJ, Olson JS, Lemon DD. Rate of reaction with nitric oxide determines the hypertensive effect of cell-free hemoglobin. Nat Biotechnol. 1998;16(7):672–6. doi: 10.1038/nbt0798-672. [DOI] [PubMed] [Google Scholar]
- Elmer J, Buehler PW, Jia Y, Wood F, Harris DR, Alayash AI, Palmer AF. Functional comparison of hemoglobin purified by different methods and their biophysical implications. Biotechnol Bioeng. 2010;106(1):76–85. doi: 10.1002/bit.22659. [DOI] [PubMed] [Google Scholar]
- Elmer J, Zorc K, Rameez S, Zhou Y, Cabrales P, Palmer AF. Hypervolemic infusion of Lumbricus terrestris erythrocruorin purified by tangential-flow filtration. Transfusion. 2012 doi: 10.1111/j.1537-2995.2011.03523.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endrich B, Asaishi K, Gotz A, Messmer K. Technical report--a new chamber technique for microvascular studies in unanesthetized hamsters. Res Exp Med (Berl) 1980;177(2):125–34. doi: 10.1007/BF01851841. [DOI] [PubMed] [Google Scholar]
- Gould SA, Moore EE, Hoyt DB, Ness PM, Norris EJ, Carson JL, Hides GA, Freeman IH, DeWoskin R, Moss GS. The life-sustaining capacity of human polymerized hemoglobin when red cells might be unavailable. J Am Coll Surg. 2002;195(4):445–52. doi: 10.1016/s1072-7515(02)01335-2. discussion 52-5. [DOI] [PubMed] [Google Scholar]
- Harrington JP, Kobayashi S, Dorman SC, Zito SL, Hirsch RE. Acellular invertebrate hemoglobins as model therapeutic oxygen carriers: unique redox potentials. Artif Cells Blood Substit Immobil Biotechnol. 2007;35(1):53–67. doi: 10.1080/10731190600974491. [DOI] [PubMed] [Google Scholar]
- Hirsch RE, Jelicks LA, Wittenberg BA, Kaul DK, Shear HL, Harrington JP. A first evaluation of the natural high molecular weight polymeric Lumbricus terrestris hemoglobin as an oxygen carrier. Artif Cells Blood Substit Immobil Biotechnol. 1997;25(5):429–44. doi: 10.3109/10731199709118932. [DOI] [PubMed] [Google Scholar]
- Intaglietta M, Silverman NR, Tompkins WR. Capillary flow velocity measurements in vivo and in situ by television methods. Microvasc Res. 1975;10:165–79. doi: 10.1016/0026-2862(75)90004-7. [DOI] [PubMed] [Google Scholar]
- Intaglietta M, Tompkins WR. Microvascular measurements by video image shearing and splitting. Microvasc Res. 1973;5(3):309–12. doi: 10.1016/0026-2862(73)90042-3. [DOI] [PubMed] [Google Scholar]
- Lipowsky HH, Zweifach BW. Application of the “two-slit” photometric technique to the measurement of microvascular volumetric flow rates. Microvasc Res. 1978;15:93–101. doi: 10.1016/0026-2862(78)90009-2. [DOI] [PubMed] [Google Scholar]
- Matheson B, Kwansa HE, Bucci E, Rebel A, Koehler RC. Vascular response to infusions of a nonextravasating hemoglobin polymer. J Appl Physiol. 2002;93(4):1479–86. doi: 10.1152/japplphysiol.00191.2002. [DOI] [PubMed] [Google Scholar]
- Miller JH, McDonald RK. THE EFFECT OF HEMOGLOBIN ON RENAL FUNCTION IN THE HUMAN. The Journal of Clinical Investigation. 1951;30(10):1033–40. doi: 10.1172/JCI102522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohlfs RJ, Bruner E, Chiu A, Gonzales A, Gonzales ML, Magde D, Magde MD, Jr., Vandegriff KD, Winslow RM. Arterial blood pressure responses to cell-free hemoglobin solutions and the reaction with nitric oxide. J Biol Chem. 1998;273(20):12128–34. doi: 10.1074/jbc.273.20.12128. [DOI] [PubMed] [Google Scholar]
- Royer WE, Jr., Sharma H, Strand K, Knapp JE, Bhyravbhatla B. Lumbricus erythrocruorin at 3.5 A resolution: architecture of a megadalton respiratory complex. Structure. 2006;14(7):1167–77. doi: 10.1016/j.str.2006.05.011. [DOI] [PubMed] [Google Scholar]
- Sakata M, Yoshida A, Haga M. Methemoglobin in blood as determined by double-wavelength spectrophotometry. Clin Chem. 1982;28(3):508–11. [PubMed] [Google Scholar]
- Savitsky JP, Doczi J, Black J, Arnold JD. A clinical safety trial of stroma-free hemoglobin. Clin Pharmacol Ther. 1978;23(1):73–80. doi: 10.1002/cpt197823173. [DOI] [PubMed] [Google Scholar]
- Schubert A, O’Hara JF, Jr., Przybelski RJ, Tetzlaff JE, Marks KE, Mascha E, Novick AC. Effect of diaspirin crosslinked hemoglobin (DCLHb HemAssist) during high blood loss surgery on selected indices of organ function. Artif Cells Blood Substit Immobil Biotechnol. 2002;30(4):259–83. doi: 10.1081/bio-120006118. [DOI] [PubMed] [Google Scholar]
- Sharma PK, Kuchumov AR, Chottard G, Martin PD, Wall JS, Vinogradov SN. The role of the dodecamer subunit in the dissociation and reassembly of the hexagonal bilayer structure of Lumbricus terrestris hemoglobin. J Biol Chem. 1996;271(15):8754–62. doi: 10.1074/jbc.271.15.8754. [DOI] [PubMed] [Google Scholar]
- Siegel JH, Fabian M, Smith JA, Costantino D. Use of recombinant hemoglobin solution in reversing lethal hemorrhagic hypovolemic oxygen debt shock. J Trauma. 1997;42(2):199–212. doi: 10.1097/00005373-199702000-00005. [DOI] [PubMed] [Google Scholar]
- Spahn DR. Current status of artificial oxygen carriers. Adv Drug Deliv Rev. 2000;40(3):143–51. doi: 10.1016/s0169-409x(99)00046-0. [DOI] [PubMed] [Google Scholar]
- Spinella PC, Sparrow RL, Hess JR, Norris PJ. Properties of stored red blood cells: understanding immune and vascular reactivity. Transfusion. 2011;51(4):894–900. doi: 10.1111/j.1537-2995.2011.03103.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strand K, Knapp JE, Bhyravbhatla B, Royer WE., Jr. Crystal structure of the hemoglobin dodecamer from Lumbricus erythrocruorin: allosteric core of giant annelid respiratory complexes. J Mol Biol. 2004;344(1):119–34. doi: 10.1016/j.jmb.2004.08.094. [DOI] [PubMed] [Google Scholar]
- Tomasco IH, Del Rio R, Iturriaga R, Bozinovic F. Comparative respiratory strategies of subterranean and fossorial octodontid rodents to cope with hypoxic and hypercapnic atmospheres. J Comp Physiol B. 2010;180(6):877–84. doi: 10.1007/s00360-010-0465-y. [DOI] [PubMed] [Google Scholar]
- Torres Filho IP, Intaglietta M. Microvessel pO2 measurements by phosphorescence decay method. Am J Physiol. 1993;265(34):H1434–H8. doi: 10.1152/ajpheart.1993.265.4.H1434. [DOI] [PubMed] [Google Scholar]
- Tsai AG. Influence of cell-free hemoglobin on local tissue perfusion and oxygenation after acute anemia after isovolemic hemodilution. Transfusion. 2001;41(10):1290–8. doi: 10.1046/j.1537-2995.2001.41101290.x. [DOI] [PubMed] [Google Scholar]
- Tsai AG, Acero C, Nance PR, Cabrales P, Frangos JA, Buerk DG, Intaglietta M. Elevated plasma viscosity in extreme hemodilution increases perivascular nitric oxide concentration and microvascular perfusion. Am J Physiol Heart Circ Physiol. 2005;288(4):H1730–9. doi: 10.1152/ajpheart.00998.2004. [DOI] [PubMed] [Google Scholar]
- Vandegriff KD, Malavalli A, Minn C, Jiang E, Lohman J, Young MA, Samaja M, Winslow RM. Oxidation and haem loss kinetics of poly(ethylene glycol)-conjugated haemoglobin (MP4): dissociation between in vitro and in vivo oxidation rates. Biochem J. 2006;399(3):463–71. doi: 10.1042/BJ20060809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winslow RM. alphaalpha-crosslinked hemoglobin: was failure predicted by preclinical testing? Vox Sang. 2000;79(1):1–20. doi: 10.1159/000031200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.