Abstract
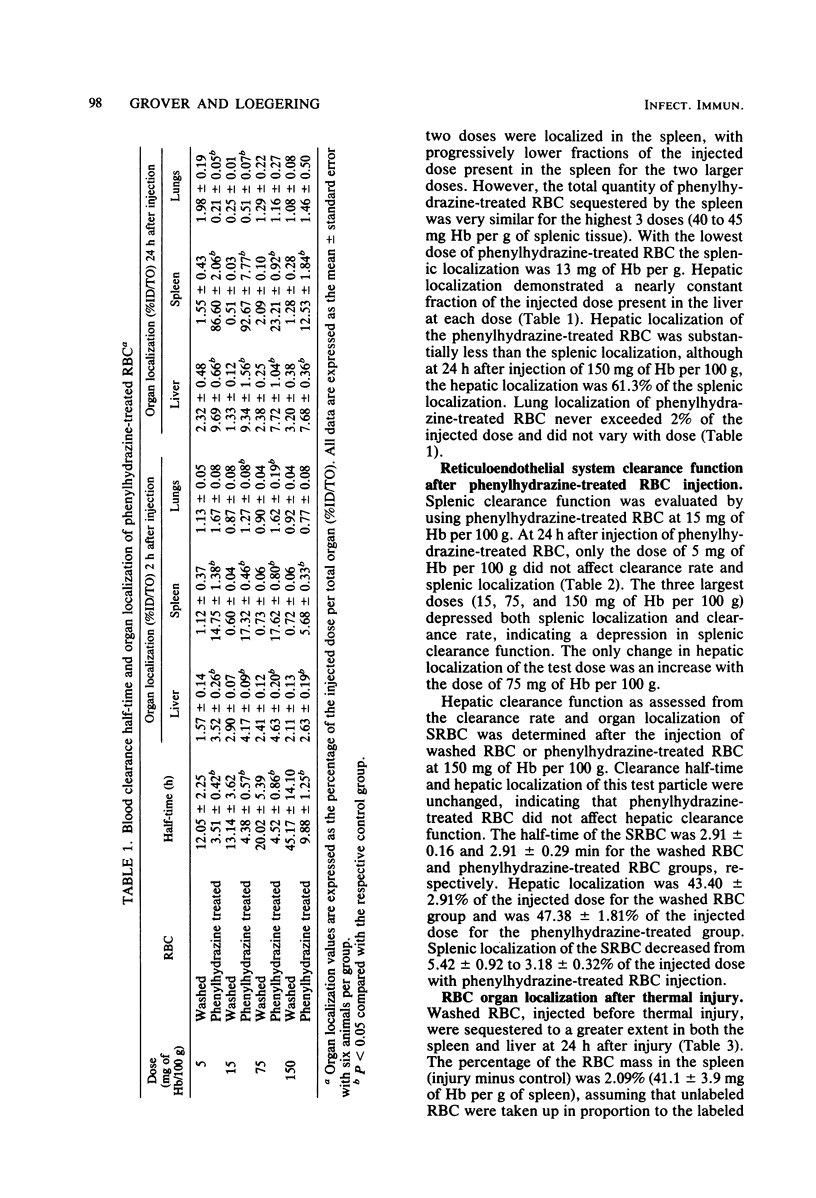
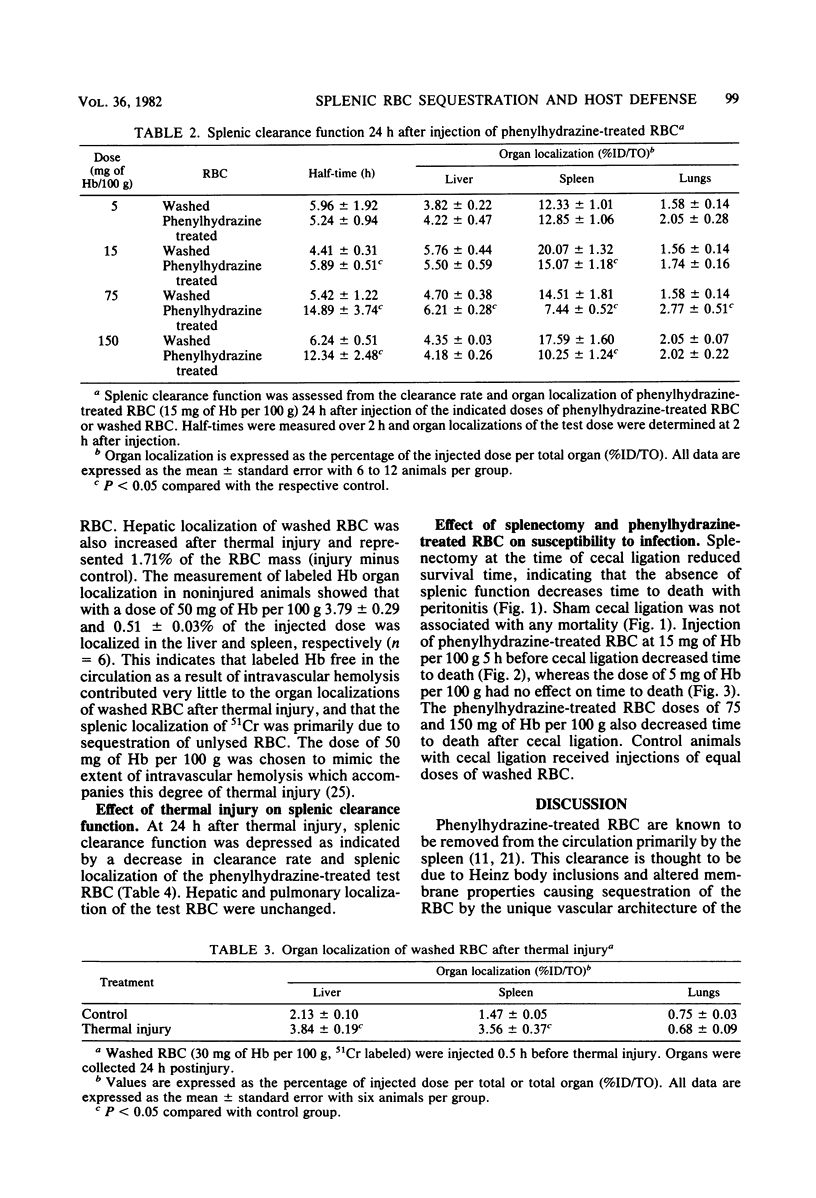
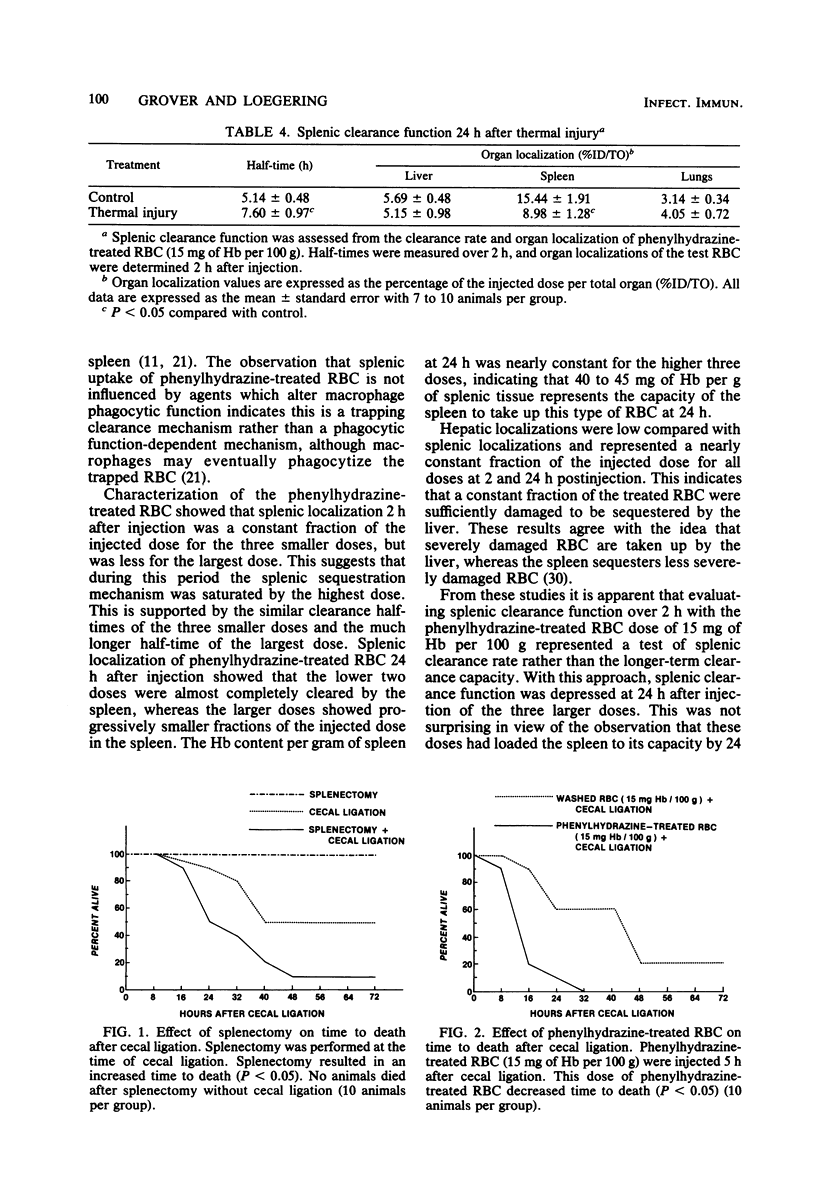
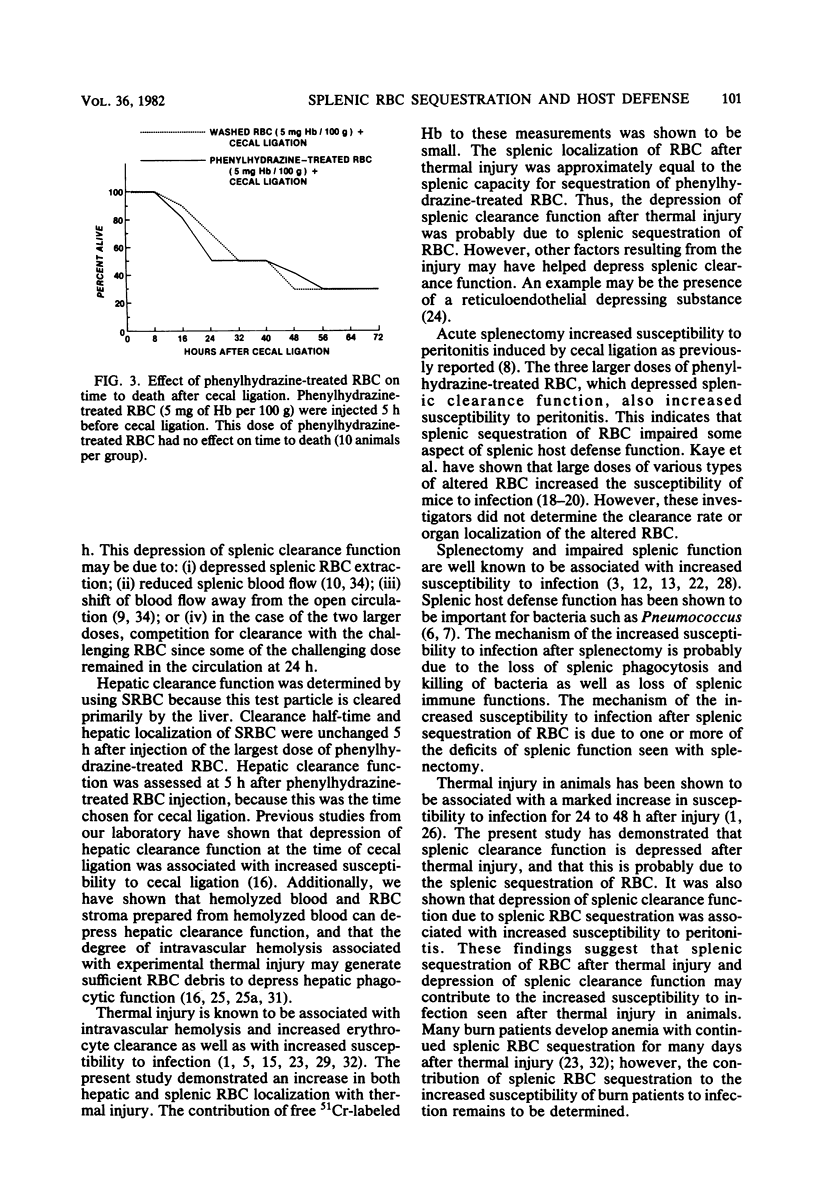
The effect of splenic sequestration of erythrocytes (RBC) on splenic clearance function and susceptibility to septic peritonitis was studied. Homologous RBC were treated with 20 mM phenylhydrazine hydrochloride in vitro and injected into rats at doses of 5, 15, 75, and 150 mg of hemoglobin per 100 g. Splenic 93, 23, and 13% of the injected dose, respectively, whereas less than 2.1% of the same doses of washed RBC were localized in the spleen. The total quantity of phenylhydrazine-treated RBC sequestered by the spleen was similar for the three larger doses (40 to 45 mg of hemoglobin per g of tissue), indicating that this is the capacity of the spleen to take up this type of RBC in 234 h. The phenylhydrazine-treated RBC in doses of 15, 75, and 150 mg of hemoglobin per 100 g depressed the clearance rate and splenic localization of a test dose of phenylhydrazine-treated RBC, whereas splenic clearance function was unchanged after a dose of 5 mg of hemoglobin per 100 g. Splenectomy or injection of doses of phenylhydrazine-treated RBC which depressed splenic clearance function increased the susceptibility to septic peritonitis induced by cecal ligation and puncture. After thermal injury, splenic clearance function was found to be depressed. Also, splenic localization of RBC 24 h after thermal injury was found to be approximately equal to that after injection of the doses of phenylhydrazine-treated RBC which depressed splenic clearance function. It is concluded that splenic sequestration of RBC can depress splenic clearance function and increase susceptibility to peritonitis, and that this may be one mechanism for the increased susceptibility to infection seen after thermal injury.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alexander J. W. Effect of thermal injury upon the early resistance to infection. J Surg Res. 1968 Mar;8(3):128–137. doi: 10.1016/0022-4804(68)90074-7. [DOI] [PubMed] [Google Scholar]
- Balfanz J. R., Nesbit M. E., Jr, Jarvis C., Krivit W. Overwhelming sepsis following splenectomy for trauma. J Pediatr. 1976 Mar;88(3):458–460. doi: 10.1016/s0022-3476(76)80267-3. [DOI] [PubMed] [Google Scholar]
- Barrett-Connor E. Bacterial infection and sickle cell anemia. An analysis of 250 infections in 166 patients and a review of the literature. Medicine (Baltimore) 1971 Mar;50(2):97–112. [PubMed] [Google Scholar]
- Bhargava M., Agarwal K. N., Kumar S. Sites of red cell destruction in the anaemia of experimental burns. Br J Haematol. 1969 Aug;17(2):179–185. doi: 10.1111/j.1365-2141.1969.tb01357.x. [DOI] [PubMed] [Google Scholar]
- Brown E. J., Hosea S. W., Frank M. M. The role of complement in the localization of pneumococci in the splanchnic reticuloendothelial system during experimental bacteremia. J Immunol. 1981 Jun;126(6):2230–2235. [PubMed] [Google Scholar]
- Brown E. J., Hosea S. W., Frank M. M. The role of the spleen in experimental pneumococcal bacteremia. J Clin Invest. 1981 Apr;67(4):975–982. doi: 10.1172/JCI110148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CSIZMAS L. Preparation of formalinized erythrocytes. Proc Soc Exp Biol Med. 1960 Jan;103:157–160. doi: 10.3181/00379727-103-25444. [DOI] [PubMed] [Google Scholar]
- Chaudry I. H., Tabata Y., Schleck S., Baue A. E. Effect of splenectomy on reticuloendothelial function and survival following sepsis. J Trauma. 1980 Aug;20(8):649–656. doi: 10.1097/00005373-198008000-00003. [DOI] [PubMed] [Google Scholar]
- Chen L. T., Scheffel U. Blood flow and phagocytic activity of the spleen of the rat with acute hemolytic anemia. J Reticuloendothel Soc. 1977 Dec;22(6):507–521. [PubMed] [Google Scholar]
- Chen L. T., Weiss L. The role of the sinus wall in the passage of erythrocytes through the spleen. Blood. 1973 Apr;41(4):529–537. [PubMed] [Google Scholar]
- Cooney D. R., Dearth J. C., Swanson S. E., Dewanjee M. K., Telander R. L. Relative merits of partial splenectomy, splenic reimplantation, and immunization in preventing postsplenectomy infection. Surgery. 1979 Oct;86(4):561–569. [PubMed] [Google Scholar]
- Cooney D. R., Swanson S. E., Dearth J. C., Dewanjee M. K., Telander R. L. Heterotopic splenic autotransplantation in prevention of overwhelming postsplenectomy infection. J Pediatr Surg. 1979 Jun;14(3):337–342. [PubMed] [Google Scholar]
- DAVIS A. K., ALPEN E. L. Mechanism of erythrocyte destruction in the burned rat. Am J Physiol. 1956 Jan;184(1):151–154. doi: 10.1152/ajplegacy.1955.184.1.151. [DOI] [PubMed] [Google Scholar]
- Grover G. J., Loegering D. J. Effect of erythrocyte debris on reticuloendothelial function and susceptibility to experimental peritonitis. Proc Soc Exp Biol Med. 1981 May;167(1):30–35. doi: 10.3181/00379727-167-41120. [DOI] [PubMed] [Google Scholar]
- HOOK E. W. Salmonellosis: certain factors influencing the interaction of Salmonella and the human host. Bull N Y Acad Med. 1961 Jul;37:499–512. [PMC free article] [PubMed] [Google Scholar]
- KAYE D., HOOK E. W. THE INFLUENCE OF HEMOLYSIS ON SUSCEPTIBILITY TO SALMONELLA INFECTION: ADDITIONAL OBSERVATIONS. J Immunol. 1963 Oct;91:518–527. [PubMed] [Google Scholar]
- KAYE D., HOOK E. W. THE INFLUENCE OF HEMOLYSIS OR BLOOD LOSS ON SUSCEPTIBILITY TO INFECTION. J Immunol. 1963 Jul;91:65–75. [PubMed] [Google Scholar]
- Kaye D., Gill F. A., Hook E. W. Factors influencing host resistance to Salmonella infections: the effects of hemolysis and erythrophagocytosis. Am J Med Sci. 1967 Aug;254(2):205–215. doi: 10.1097/00000441-196708000-00011. [DOI] [PubMed] [Google Scholar]
- Klausner M. A., Hirsch L. J., Leblond P. F., Chamberlain J. K., Klemperer M. R., Segel G. B. Contrasting splenic mechanisms in the blood clearance of red blood cells and colloidal particles. Blood. 1975 Dec;46(6):965–976. [PubMed] [Google Scholar]
- Leung L. S., Szal G. J., Drachman R. H. Increased susceptibility of splenectomized rats to infection with Diplococcus pneumoniae. J Infect Dis. 1972 Nov;126(5):507–513. doi: 10.1093/infdis/126.5.507. [DOI] [PubMed] [Google Scholar]
- Loebl E. C., Baxter C. R., Curreri P. W. The mechanism of erythrocyte destruction in the early post-burn period. Ann Surg. 1973 Dec;178(6):681–686. doi: 10.1097/00000658-197312000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loegering D. J. Circulating reticuloendothelial depressing substance following thermal injury and intestinal ischemia. Proc Soc Exp Biol Med. 1981 Apr;166(4):515–521. doi: 10.3181/00379727-166-41100. [DOI] [PubMed] [Google Scholar]
- Loegering D. J., Garrett L. J. Reticuloendothelial system depression with hemolyzed blood and susceptibility to endotoxin shock and thermal injury. Circ Shock. 1981;8(4):473–482. [PubMed] [Google Scholar]
- Loegering D. J. Hemolysis following thermal injury and depression of reticuloendothelial system phagocytic function. J Trauma. 1981 Feb;21(2):130–134. [PubMed] [Google Scholar]
- MCRIPLEY R. J., GARRISON D. W. EFFECT OF BURNS IN RATS ON DEFENSE MECHANISMS AGAINST PSEUDOMONAS AERUGINOSA. J Infect Dis. 1965 Apr;115:159–170. doi: 10.1093/infdis/115.2.159. [DOI] [PubMed] [Google Scholar]
- Pearson H. A. Sickle cell anemia and severe infections due to encapsulated bacteria. J Infect Dis. 1977 Aug;136 (Suppl):S25–S30. doi: 10.1093/infdis/136.supplement.s25. [DOI] [PubMed] [Google Scholar]
- Pearson H. A., Spencer R. P., Cornelius E. A. Functional asplenia in sickle-cell anemia. N Engl J Med. 1969 Oct 23;281(17):923–926. doi: 10.1056/NEJM196910232811703. [DOI] [PubMed] [Google Scholar]
- Rifkind R. A. Destruction of injured red cells in vivo. Am J Med. 1966 Nov;41(5):711–723. doi: 10.1016/0002-9343(66)90032-5. [DOI] [PubMed] [Google Scholar]
- Schneidkraut M. J., Loegering D. J. Effect of hemolyzed blood on reticuloendothelial function and susceptibility to hemorrhagic shock. Proc Soc Exp Biol Med. 1978 Dec;159(3):418–423. doi: 10.3181/00379727-159-40361. [DOI] [PubMed] [Google Scholar]
- Turinsky J., Patterson S. A. Proximity to a burn wound as a new factor in considerations of postburn insulin resistance. J Surg Res. 1979 Feb;26(2):171–174. doi: 10.1016/0022-4804(79)90096-9. [DOI] [PubMed] [Google Scholar]
- Vaupel P., Ruppert H., Hutten H. Splenic blood flow and intrasplenic flow distribution in rats. Pflugers Arch. 1977 Jul 19;369(3):193–201. doi: 10.1007/BF00582184. [DOI] [PubMed] [Google Scholar]
- Wichterman K. A., Baue A. E., Chaudry I. H. Sepsis and septic shock--a review of laboratory models and a proposal. J Surg Res. 1980 Aug;29(2):189–201. doi: 10.1016/0022-4804(80)90037-2. [DOI] [PubMed] [Google Scholar]



