Abstract
Four critical stages of embryogenesis, including callus induction, cellular acquisition of morphogenetic competence, expression of embryogenic program, and development and maturation of somatic embryos during somatic embryogenesis from leaf discs of eggplant (Solanum melongena L.), were identified by scanning electron microscopy. Temporal changes in arginine decarboxylase (ADC) activity and polyamines (PAs) during critical stages of embryogenesis revealed that high levels of PAs (especially putrescine [PUT]), due to higher ADC activity in discs from the apical region (with high embryogenic capacity) than from the basal region of the leaf (with poor embryogenic capacity), were correlated with differential embryogenesis response. Kinetic studies of the up- and down-regulation of embryogenesis revealed that PUT and difluoromethylarginine pretreatments were most effective before the onset of embryogenesis. Basal discs pretreated with PUT for 4 to 7 d showed improved embryogenesis that was comparable to apical discs. PA content at various critical steps in embryogenesis from basal discs were found to be comparable to that of apical discs following adjustments of cellular PA content by PUT. In contrast, pretreatment of apical discs with difluoromethylarginine for 3 d significantly reduced ADC activity, cellular PA content, and embryogenesis to levels that were comparable to basal discs. Discs from the basal region of leaves treated with PUT for 3 d during the identified stages of embryogenesis improved their embryogenic potential.
PAs, SPD, SPM, and their diamine obligate precursor PUT, are small, aliphatic amines that are ubiquitous in all plant cells. Although the precise modes of action of PAs are yet to be understood (Walden et al., 1997), extensive studies support their role in modulation of a variety of physiological processes that range from cell growth and differentiation to stress responses (Evans and Malmberg, 1989; Galston and Kaur-Sawhney, 1990; Bajaj and Rajam, 1996; Kumar et al., 1997; Rajam, 1997). They have also been labeled as a new class of growth substances (Galston and Kaur-Sawhney, 1990; Bagni and Torrigiani, 1992). In recent years the interest in PA research has increased tremendously and is now being applied to improve plant developmental processes, including SE, in a variety of agronomically and horticulturally important crops (Chi et al., 1994; Bajaj and Rajam, 1996; Rajam, 1997). PAs have been studied in relation to SE in many plant systems (Minocha and Minocha, 1995), because SE is an important pathway for plant regeneration and a potential model system for studying regulatory events of plant morphogenesis in vitro. Several reports show the involvement of PAs, particularly in their free forms, and ADC activity in SE. Furthermore, the reduction of the endogenous free PAs and ADC activity on treatment with inhibitors of PA biosynthesis such as dl-DFMA concomitantly inhibited SE. The inhibitory effects of PA-biosynthesis inhibitors could be partially or fully restored by exogenous PAs and their precursors (Helleboid et al., 1995; Minocha and Minocha, 1995), indicating the direct role of PAs in SE.
However, most of the earlier studies included free PAs (Galston and Flores, 1991; Minocha and Minocha, 1995), and omitted the conjugated and bound forms. In fact, conjugated and bound PAs are important for plant developmental processes such as flower development (Tiburcio et al., 1988; Evans and Malmberg, 1989; Kaur-Sawhney and Applewhite, 1993), because there may be interconversion between free and conjugated forms to maintain appropriate levels of free PAs during developmental processes (Torrigiani et al., 1989). The effect of exogenous PAs and their biosynthesis inhibitors have been studied usually by applying them for the entire culture period, irrespective of the developmental stage at which they may be critical for morphogenesis. Moreover, to our knowledge, no single study has monitored endogenous PA pools temporally after application of exogenous PAs/PA-biosynthesis inhibitors. In this study we monitored the changes in PA levels and ADC activity in tissues with differential embryogenic capacity during critical stages of SE to identify the regulatory role of specific PA(s) and their individual forms in SE. Furthermore, experiments were conducted to examine whether PA/PA-biosynthesis inhibitors can regulate SE by adjustment of cellular PA pools and ADC activity during the critical stages of embryogenesis.
MATERIALS AND METHODS
Plant Material and Culture Conditions
Seeds of eggplant (Solanum melongena L., cv Pusa Purple Long [PPL]), obtained from National Seeds Corp. (Indian Agricultural Research Institute, New Delhi), were used throughout the study. Seeds were surface sterilized with 0.1% HgCl2 for 3 min and rinsed three times with sterile, distilled water. Disinfected seeds were sown in plastic pots containing a sterilized mixture of garden soil and vermiculite (1:1, v/v). Seeds were germinated and raised at 26 ± 1°C with a 16-/8-h photoperiod for 6 weeks.
Leaves were excised from pot-grown 6-week-old seedlings, disinfected with 0.2% HgCl2 for 1 min, and rinsed three times with sterile, distilled water. Discs from the apical and basal regions of leaves with veinlets, but lacking the mid-vein, were cut using a 6-mm-sterilized cork borer and cultured separately on agar-solidified Murashige-Skoog medium (Murashige and Skoog, 1962) supplemented with 10.73 μm NAA (referred to as embryogenic medium), pH 5.8, for the induction of SE via the callus phase (Sharma and Rajam, 1995a; Yadav and Rajam, 1997). The cultures were incubated at 26 ± 1°C with a 16-/8-h photoperiod in cool-white fluorescent light (40 μmol m−2 s−1). PUT was added to the medium before autoclaving and DFMA was filter sterilized (using 0.2-μm filters, Millipore) and added to autoclaved medium cooled to 46 to 48°C.
SEM
Leaf discs cultured on embryogenic medium were removed at 1-d intervals for up to 21 d of culture and fixed in glutaraldehyde (2.5%, v/v, pH 7.2) for 3 h for SEM. The fixative was washed off by three gentle rinses with phosphate buffer (0.1 m, pH 7.2) followed by tissue dehydration using a graded ethanol series. The dehydrated tissue was critical point dried, mounted on an SEM specimen stub with silver-conducting cement, gold coated, and scanned under a Philips 501B scanning electron microscope (O'Brien and McCully, 1981).
PA Analysis
Free (as free cations), conjugated (acid-soluble forms conjugated with phenolics, hydroxycinnamic acids, and other low-molecular-weight compounds), and bound (acid-insoluble forms bound covalently to macromolecules and cell walls) PAs (PUT, SPD, and SPM) were extracted in prechilled perchloric acid. PAs were analyzed during the critical stages of SE from three samples randomly selected from apical and basal discs of eggplant leaves cultured on embryogenic medium (up to 21 d) and pretreated with PUT- or DFMA-amended embryogenic medium for various time intervals or during critical steps in SE, and then transferred to PA- or DFMA-free embryogenic medium, as described previously (Sharma and Rajam, 1995b). Dansylated PAs were extracted in 250 μL of benzene, and a clear benzene layer was used for loading the PAs onto a silica gel TLC plate (250 μm in thickness, Silica gel 60, E. Merck, Darmstadt, Germany). After developing in cyclohexane:ethyl acetate (5:4, v/v), dansylated PA bands were detected, scraped from plates, eluted in 3 mL of ethyl acetate, and quantified using a spectrophotofluorometer (model RF-540, Shimadzu, Tokyo, Japan) with excitation and emission wavelengths of 350 and 495 nm, respectively.
ADC Assay
The ADC activity was assayed according to the procedure described by Birecka et al. (1985) with some modifications, as detailed earlier (Bajaj and Rajam, 1996). Leaf discs (300 mg) were homogenized with a pestle in a prechilled mortar on ice in 1 mL of the extraction buffer containing 200 mm Tris-HCl (pH 8.5), 10 mm DTT, 0.1 mm pyridoxal phosphate, and 0.1 mm EDTA and kept at 4°C for at least 45 min. The homogenate was centrifuged at 20,000g at 4°C for 20 min, and the supernatant was collected and precipitated with 30% (NH4)2SO4 and again centrifuged under similar conditions for 10 min. The resulting supernatant was dialyzed overnight with at least two changes against dialysis buffer containing 10 mm Tris-HCl (pH 8.5), 2 mm DTT, 0.05 mm pyridoxal phosphate, and 0.05 mm EDTA.
The ADC activity was determined in 200 μL of reaction mixture containing 160 μL of the enzyme extract, 20 μL of the assay buffer (containing 80 mm Tris-HCl, pH 8.5, 16 mm DTT, 0.4 mm pyridoxal phosphate, and 0.4 mm EDTA), 17 μL of 100 mm l-Arg, and 3 μL of [U-14C]Arg (specific activity 300 mCi/mmol, 100 μCi/mL). The reaction mixture was incubated at 37°C for 45 min in small vials, with the cap pierced by a needle on which a piece of filter disc that had been dipped earlier in 2 m KOH was kept. The reaction was stopped by adding 10% perchloric acid and incubated again for 45 min at 37°C. Following incubation, the filter papers were carefully removed and placed in scintillation vials containing 2 mL of scintillation fluid and radioactivity was measured using a liquid-scintillation counter (LKB-1209 Rackbeta, Pharmacia). The enzyme activity was measured by the amount of 14CO2 released from [U-14C]Arg, and specific activity was measured as nanomoles of CO2 per hour per milligram of protein. Protein content was determined according to the method of Bradford (1976) using BSA as the standard.
Data Analysis
Discs from the apical and basal regions of leaves were evaluated for SE after 35 d of culture. Embryos were scored under a dissecting microscope. Each experiment with about 20 replicates (except for PA analysis, for which 3 replicates were maintained) was repeated at least twice with similar results, and the data represented are from a single experiment. Statistical analysis was done for all of the data (mean ± se) obtained using Student's t test to check differences between the treatments. Fisher's lsd procedure was used to determine the differences among the means.
RESULTS
Critical Stages during SE from Leaf Discs
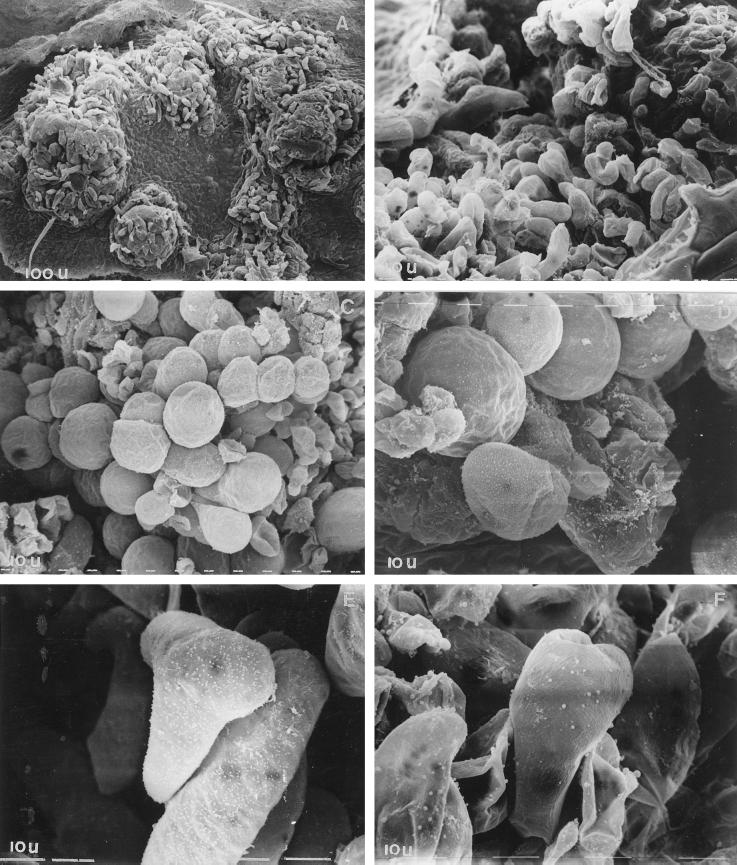
Previously, we tested the discs from the apical and basal regions of eggplant leaves for SE and showed that discs from the apical region of leaves produced about 75 embryos per culture, as compared with discs from the basal region, which yielded approximately 35 embryos per culture. In the present study four critical stages of SE were identified by scanning the leaf discs cultured on embryogenic medium during different time intervals. They included: stage I, the callus-induction phase, between 1 and 6 d of culture during which the leaf disc swelled and then developed into friable callus, initially from the edges and subsequently from other regions of the discs (Fig. 1A); stage II, the cellular acquisition of morphogenetic competence, between 6 and 9 d of culture during which the proembryogenic sectors were formed (Fig. 1B); stage III, the expression of embryogenic program, between 9 and 12 d of culture during which the proembryogenic clusters developed into the first visible globular embryos as green dots (Fig. 1, C and D); and stage IV, the development and maturation of somatic embryos between 12 and 21 d of culture, during which the globular embryos developed into the heart stage (Fig. 1E) and then the torpedo stage (Fig. 1F). Although asynchronous, most of the embryos (approximately 63%) attained maturity by 35 d of culture.
Figure 1.
Scanning electron micrographs of critical stages in SE. Induction of callus during 0 to 6 d of culture (A), enlarged view of callus cells (B), appearance of globular embryos during 9 to 12 d of culture (C), enlarged view of globular embryos (D), and development of globular embryos into the heart-shaped (E) and torpedo stage (F) during 12 to 21 d of culture. Scanning was done after 6 (A and B), 9 (C and D), 12 (E), and 21 (F) d of culture. Magnification: A, ×160; B and C, ×320; and D through F, ×1250.
Endogenous Levels of PAs, ADC Activity, and Their Temporal Changes during Critical Stages of SE
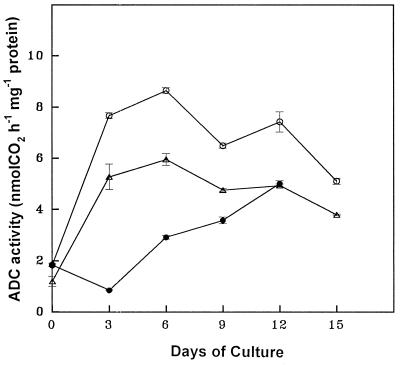
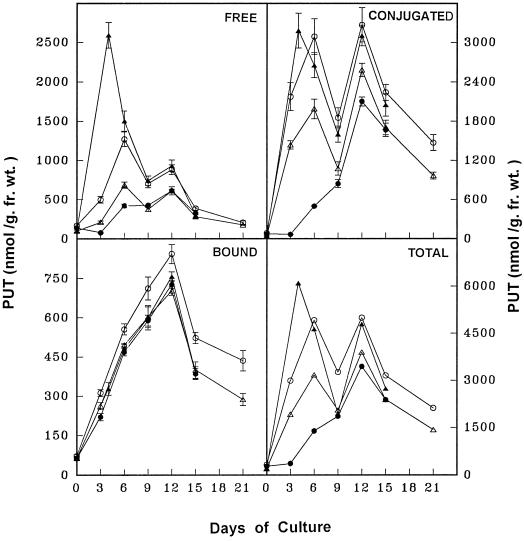
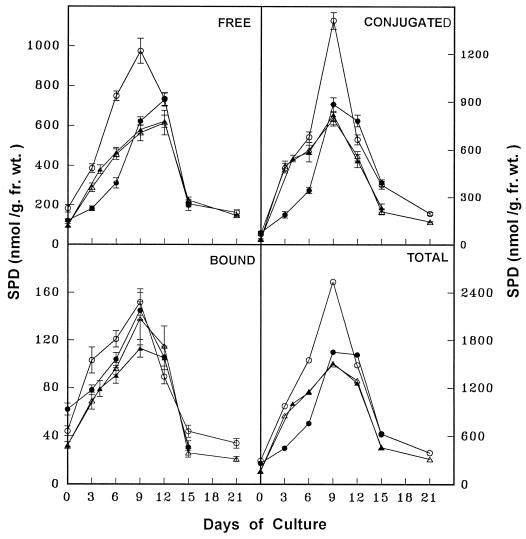
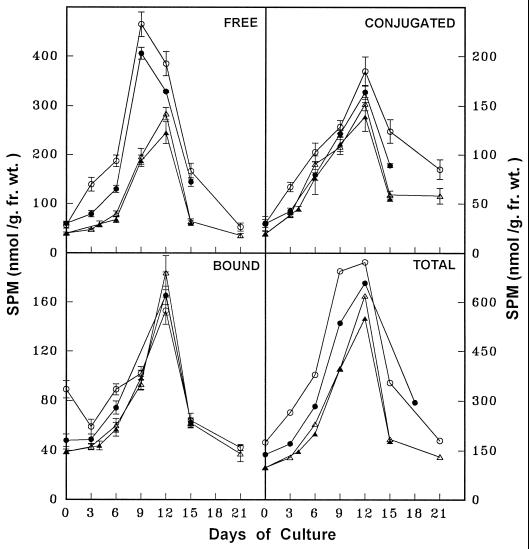
The temporal changes in free, conjugated, and bound forms of PAs and the ADC activity were monitored in discs from the apical and basal regions of leaves during critical stages of SE as described above. The PA analysis in the parent tissue (leaf discs) revealed that PUT was the predominant amine, followed by SPD and SPM. The free form of PAs was more abundant, followed by the conjugated and bound forms. The concentration of all three PAs (Figs. 2-4) and ADC activity (Fig. 5) were higher in apical discs than in basal ones (Figs. 2–5).
Figure 5.
Temporal changes in ADC activity during various stages of SE from apical leaf discs (○), basal leaf discs (▵), and apical leaf discs pretreated with DFMA for 3 d (•) of eggplant on embryogenic medium.
Figure 2.
Temporal changes in PUT concentration during various stages of SE from apical leaf discs (○), basal leaf discs (▵), apical-pretreated leaf discs with DFMA for 3 d (•), and basal pretreated leaf discs with PUT for 4 d (▴) of eggplant on embryogenic medium. fr. wt., Fresh weight.
Temporal PA analysis during stage I revealed that the PUT content (free, conjugated, and total) was highest (Fig. 2) because of high ADC activity (Fig. 5), although there was a gradual increase in all forms of SPD and SPM (Figs. 3 and 4). During stage II the cellular levels of free, conjugated, and total PUT declined sharply, along with a decline in ADC activity, although the different forms of SPD and SPM maintained their gradual increase (Figs. 3 and 4). Stage III coincided with an elevation in all forms of PUT, SPD, SPM (Figs. 2–4), and ADC activity (Fig. 5). Finally, during stage IV the content of all PAs and ADC activity declined (Figs. 2–5).
Figure 3.
Temporal changes in SPD concentration during various stages of SE from apical leaf discs (○), basal leaf discs (▵), apical pretreated leaf discs with DFMA for 3 d (•), and basal pretreated leaf discs with PUT for 4 d (▴) of eggplant on embryogenic medium. fr. wt., Fresh weight.
Figure 4.
Temporal changes in SPM concentration during various stages of SE from apical leaf discs (○), basal leaf discs (▵), apical leaf discs pretreated with DFMA for 3 d (•), and basal leaf discs pretreated with PUT for 4 d (▴) of eggplant on embryogenic medium. fr. wt., Fresh weight.
Up- and Down-Regulation of SE by Adjusting Cellular PA Pools and ADC Activity
In an earlier study we showed that the exogenous PUT (0.5 mm) could promote SE by 5- to 6- fold in eggplant, which was associated with increased cellular PUT levels; embryogenesis was completely blocked by 2 mm DFMA as a result of depletion of cellular PA levels. Exogenous SPD and SPM were shown to inhibit SE from leaf discs of eggplant. These observations prompted us to examine the regulation of SE by modulating and adjusting cellular PA pools by either exogenous PUT or DFMA treatments for different time intervals during critical stages of SE.
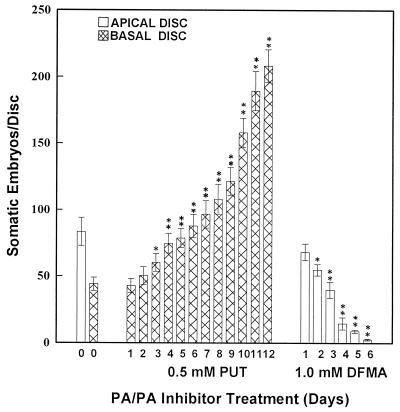
The discs from the apical and basal regions of leaves were cultured on PUT- or DFMA-amended embryogenic medium for different times (in days) or for only 3 d during the identified critical stages of SE (i.e. 0–3, 3–6, 6–9, 9–12, or 12–15 d of culture) and then transferred to PUT-/DFMA- free embryogenic medium. The activity of ADC and PA content were analyzed after pretreatments and the subsequent embryogenic response was recorded. PUT pretreatment (in days) promoted SE from basal discs in a time-dependent fashion, and pretreatment for 12 d (until the expression of embryogenic program) caused an approximately 5-fold increase in the SE response (Fig. 6). Discs from the basal region (with poor embryogenic capacity of leaves) at 4 to 7 d of PUT pretreatment showed improved SE, which was comparable to the SE response observed in apical discs (with good embryogenic capacity; Fig. 6). PA content, especially free and conjugated PUT, was elevated markedly during stage I (Fig. 2); during later stages of SE, PA content in discs from the basal region became comparable to the levels observed in apical discs following adjustment of the cellular PA pools (Figs. 2–4).
Figure 6.
SE response in leaf discs of eggplant pretreated for different durations with PUT or DFMA. * and ** denote significant differences between treated and untreated controls at 5 and 1% levels, respectively.
Increased duration of pretreatment with 1 mm DFMA reduced SE in the discs from the apical region of leaves. Three days of pretreatment of apical discs with 1 mm DFMA reduced the number of somatic embryos/disc (without affecting the callus growth and morphology), which was comparable to the embryogenic response from basal discs (Fig. 6). These observations could be well correlated with the decrease in ADC activity (Fig. 5) and free, conjugated, and total PUT contents (Fig. 2) in apical discs during the early period of stage I. However, DFMA- treated apical discs upon transfer to DFMA-free embryogenic medium showed a gradual increase in PA titers and ADC activity prior to the onset of SE (stage III) and became comparable to basal discs during the expression of embryogenesis (stage III; Figs. 2–4).
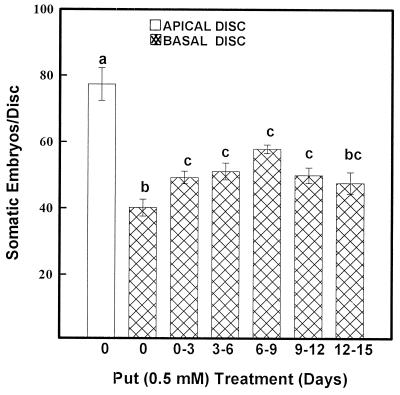
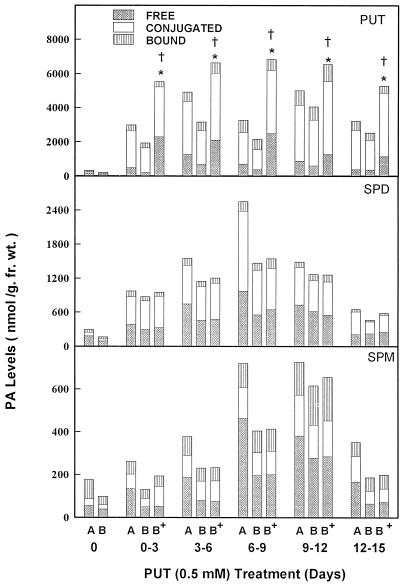
In a separate study PUT treatment for 3 d during critical stages improved SE from basal discs over basal controls (Fig. 7). This coincided with about a 1.5- to 3-fold increase in free and conjugated PUT at each stage of treatment (Fig. 8). The PUT treatment during 6 to 9 d of culture (the stage at which cells acquire morphogenetic competence) was most effective in increasing the number of embryos/disc (Fig. 7), because there was a maximum increase of cellular PUT (Fig. 8).
Figure 7.
SE response in leaf discs of eggplant treated with PUT during critical stages of SE. Bars with different letters represent significantly different means (P < 0.05) using Fischer's lsd method.
Figure 8.
PA concentrations in discs from apical (A) and basal (B) plant leaves and basal discs pretreated with PUT (B+) during critical stages of SE. * and † represent significant differences between basal discs pretreated with PUT and control, untreated basal discs for free and conjugated PAs, respectively, at 5% levels. fr. wt., Fresh weight.
DISCUSSION
Several economically important crop plants are either poorly responding or recalcitrant for in vitro morphogenesis. Very little is known about the underlying mechanisms and the use of additional means that may regulate morphogenesis in addition to plant hormone effects. Although PAs have been shown to be important for cellular differentiation to SE (Feirer et al., 1984; Fienberg et al., 1984; Galston and Flores 1991; Sharma and Rajam, 1995b; Bajaj and Rajam, 1996) and have been suggested as regulators of SE (Montague et al., 1979), their time- and duration-dependent effects (Kaur-Sawhney et al., 1990) and the precise role of PAs in the regulation of critical steps in SE (Bradley et al., 1984) still remain to be examined.
Eggplant was selected for this study because it offers a potentially good system to investigate plant growth and development in vitro (Gleddie et al., 1986; Sharma and Rajam, 1995a) and has not been fully exploited to study the role of PAs in in vitro plant morphogenesis (Sharma and Rajam, 1995b; Yadav and Rajam, 1997). Previously, we showed differences in embryogenic potential among different regions of hypocotyl (Sharma and Rajam, 1995b) and in discs from the apical and basal regions of eggplant leaves (Yadav and Rajam, 1997). Our results showed that spatial endogenous PA levels were associated with differential embryogenic ability (Sharma and Rajam, 1995b; Yadav and Rajam, 1997). Furthermore, we demonstrated the association of elevated PUT levels and the importance of the ADC pathway in SE from eggplant leaves (Yadav and Rajam, 1997). But the question of whether the increase in cellular PUT is a crucial prerequisite for cellular acquisition of embryogenic ability or merely a consequence of embryo growth and development still needs to be answered.
To gain further insight into the causal relationship between PAs and SE, temporal changes in PA metabolism were monitored during the four critical stages of SE as identified by SEM (Fig. 1). During induction of embryogenic callus (Fig. 1A), there were high titers of free, conjugated, and total PUT as compared with SPD and SPM (Figs. 2–4), because of the high activity of ADC (Fig. 5) that is a prerequisite for cell division (Fracassini et al., 1980; Maki et al., 1991) leading to callus formation. High levels of free PAs (PUT) and ADC activity have been reported in growing and dividing tissues (Kaur-Sawhney et al., 1985, 1989). Exogenous PUT has also been shown to induce mitotic divisions in dormant tubers of Helianthus (Bagni, 1966) and almond protoplasts (Wu and Kuniyuki et al., 1985). During cellular acquisition of morphogenic competence (Fig. 1B), levels of free and conjugated PUT declined (Fig. 2), and this may be due to the direct involvement of PUT in acquiring morphogenic competence (Santanen and Simola, 1994) or to the rapid conversion of PUT into SPD and SPM as their levels increased (Figs. 3 and 4). PUT contents were elevated (Fig. 2) because of high ADC activity (Fig. 5) during expression of the embryogenic program (Fig. 1, C and D), probably due to the direct involvement of PUT in SE (Helleboid et al., 1995) and/or due to indirect involvement of elevated SPD and SPM levels (Figs. 3 and 4) as a result of increased PUT synthesis. SPD has been implicated in SE in many plants (Minocha and Minocha, 1995). In stage IV (Figs. 1, E and F), the PA levels declined (Figs. 2–4), probably due to their utilization during embryo development. It is interesting that apical discs with high endogenous PA levels and good embryogenic ability attained higher levels of free and conjugated forms of PUT, SPD, and SPM than the discs from the basal region with low endogenous PA levels and a poor embryogenic response (Figs. 2–4). However, irrespective of the embryogenic potential, the pattern of overall changes of endogenous PAs was similar in discs from two regions of leaves (Figs. 2–4), suggesting that changes in levels of endogenous PAs are related to SE processes in a critical way. However, in an earlier study of SE from eggplant cotyledon, only free PAs were analyzed, which did not correlate with SE, and the exogenous PAs generally had no effect on SE (Fobert and Webb, 1988).
To examine whether the adjustment of cellular PA pools and ADC activity during critical stages of SE can regulate SE from eggplant leaves, the changes in cellular PA content and ADC activity were recorded in untreated control cultures and in cultures pretreated with PUT or DFMA for a short duration in embryogenic medium during critical stages of SE. This approach is more specific to embryogenesis because short duration pretreatment with DFMA significantly affected SE without affecting callus growth.
It is apparent from the results (Figs. 6–8) that short exposure to PUT pretreatment in discs from the basal region of leaves enhanced their SE ability due to the increased cellular PUT and not due to the increased SPD and SPM (Fig. 8), and this was compounded over time with increased duration of PUT treatment (Fig. 6). This may be because the exogenously supplied PUT is not converted into SPD and SPM (Kumar and Thorpe, 1989), probably because of the limitation of enzymes (S-adenosylmethionine decarboxylase and SPD and SPM synthases) involved in the conversion of PUT into SPD and SPM (Bastola and Minocha, 1995). It is interesting that transgenic tobacco plants (DeScenzo and Minocha, 1993) and carrot cell lines (Bastola and Minocha, 1995) expressing mouse Orn decarboxylase (which is involved in PUT synthesis) cDNA showed increased PUT but not SPD and SPM, which coincided with increased SE (in the case of carrot cell lines).
The elongated pretreatment with DFMA of discs from the apical region of leaves reduced the cellular PUT content by blocking the ADC pathway and thus reduced their SE response without causing significant changes in SPD and SPM content. The up- and down-regulation of embryogenesis in discs from the apical and basal regions of leaves following short pretreatment with PUT or DFMA in embryogenic medium may be due to the initial enhanced or reduced cellular PUT content, respectively, but during the later stages of SE, PA levels became comparable to the untreated controls.
In conclusion, PUT had an enrichment effect during the early stages of SE from eggplant, and by judicious time and dosage of PA/PA biosynthesis inhibitor the PA metabolism can be modulated for regulation of SE. These findings may be helpful in induction and promotion of plant regeneration via SE in morphogenically poor and recalcitrant species.
ACKNOWLEDGMENTS
J.S.Y. is grateful to the Council of Scientific and Industrial Research (New Delhi, India) for the award of a Senior Research Fellowship. We also thank the Merrel Dow Research Institute (Cincinnati, OH) for the generous gift of DFMA, the Department of Biotechnology-Bioinformatics SubCentre (University of Delhi South Campus) for providing the computational facility, and Mr. Rajiv Chawla for excellent word processing.
Abbreviations:
- ADC
Arg decarboxylase
- DFMA
α-difluoromethyl-Arg
- PA(s)
polyamine(s)
- PUT
putrescine
- SE
somatic embryogenesis
- SEM
scanning electron microscopy
- SPD
spermidine
- SPM
spermine
Footnotes
This research was generously supported by the Department of Science and Technology (grant no. SP/SO/A-23/92), New Delhi, India.
LITERATURE CITED
- Bagni N. Aliphatic amines and growth factor of coconut milk a stimulating cellular proliferation in Helianthus tuberosus (Jerusalem artichoke) in vitro. Experientia. 1996;22:732. [Google Scholar]
- Bagni N, Torrigiani P. Polyamines: a new class of growth substances. In: Karssen CM, Van Loon LC, Vreugdenhil D, editors. Progress in Plant Growth Regulation. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1992. pp. 264–275. [Google Scholar]
- Bajaj S, Rajam MV. Polyamine accumulation and near loss of morphogenesis in long-term callus cultures of rice. Restoration of plant regeneration by manipulation of cellular polyamine levels. Plant Physiol. 1996;112:1343–1348. doi: 10.1104/pp.112.3.1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastola DR, Minocha SC. Increased putrescine biosynthesis through transfer of mouse ornithine decarboxylase cDNA in carrot promotes somatic embryogenesis. Plant Physiol. 1995;109:63–74. doi: 10.1104/pp.109.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birecka H, Bitonti AJ, McCann PP. Assaying ornithine and arginine decarboxylases in some plant species. Plant Physiol. 1985;79:509–514. doi: 10.1104/pp.79.2.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Bradley PM, El Fikki F, Giles KL. Polyamine and arginine affect somatic embryogenesis of Daucus carota. Plant Sci Lett. 1984;34:397–401. [Google Scholar]
- Chi G-L, Lin W-S, Lee JEE, Pua E-C. Role of polyamines on de novo shoot morphogenesis from cotyledons of Brassica campestris spp. pekinensis (Lour.) Olsson in vitro. Plant Cell Rep. 1994;13:323–329. doi: 10.1007/BF00232630. [DOI] [PubMed] [Google Scholar]
- DeScenzo RA, Minocha SC. Modulation of cellular polyamines in tobacco by transfer and expression of mouse ornithine decarboxylase cDNA. Plant Mol Biol. 1993;22:113–127. doi: 10.1007/BF00039000. [DOI] [PubMed] [Google Scholar]
- Evans PT, Malmberg RL. Do polyamines have a role in plant development? Annu Rev Plant Physiol Plant Mol Biol. 1989;40:235–269. [Google Scholar]
- Feirer RP, Mignon G, Litvay JD. Arginine decarboxylase and polyamines required for embryogenesis in wild carrot. Science. 1984;223:1433–1435. doi: 10.1126/science.223.4643.1433. [DOI] [PubMed] [Google Scholar]
- Fienberg AA, Choi JH, Lubich WP, Sung ZR. Developmental regulation of polyamine metabolism in growth and differentiation of carrot cultures. Planta. 1984;162:532–539. doi: 10.1007/BF00399919. [DOI] [PubMed] [Google Scholar]
- Fobert PR, Webb DT. Effects of polyamines, polyamine precursors and polyamine biosynthetic inhibitors on somatic embryogenesis from eggplant (Solanum melongena) cotyledons. Can J Bot. 1988;66:1734–1742. [Google Scholar]
- Fracassini DS, Bagni N, Cionini PG, Bennici A. Polyamines and nucleic acids during the first cell cycle of Helianthus tuberosus tissue after the dormancy break. Planta. 1980;148:332–337. doi: 10.1007/BF00388120. [DOI] [PubMed] [Google Scholar]
- Galston AW, Flores HE. Polyamines and plant morphogenesis. In: Slocum RD, Flores HE, editors. Biochemistry and Physiology of Polyamines in Plants. Boca Raton, FL: CRC Press; 1991. pp. 175–186. [Google Scholar]
- Galston AW, Kaur-Sawhney R. Polyamines in plant physiology. Plant Physiol. 1990;94:406–410. doi: 10.1104/pp.94.2.406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gleddie S, Keller WA, Setterfield G. Somatic embryogenesis and plant regeneration from cell suspension derived protoplasts of Solanum melongena (eggplant) Can J Bot. 1986;64:355–361. [Google Scholar]
- Helleboid S, Couillerot JP, Hilbert JH, Vasseur J. Inhibition of direct somatic embryogenesis by α-difluoromethylarginine in a Cichorium hybrid: effects on polyamine content and protein patterns. Planta. 1995;196:571–576. [Google Scholar]
- Kaur-Sawhney R, Applewhite PB. Endogenous protein bound polyamines: correlation with regions of cell division in tobacco leaves, internodes and ovaries. Plant Growth Regul. 1993;12:223–227. [Google Scholar]
- Kaur-Sawhney R, Chackalamannil A, Galston AW. Effects of inhibitors of polyamine biosynthesis on meristematic centres in Petunia protoplast cultures. Plant Sci. 1989;62:123–128. [Google Scholar]
- Kaur-Sawhney R, Kandpal G, Mcgonigle B, Galston AW. Further experiments on spermidine-mediated floral bud formation in thin layer explants of Wisconsin 38 tobacco. Planta. 1990;181:212–215. doi: 10.1007/BF02411540. [DOI] [PubMed] [Google Scholar]
- Kaur-Sawhney R, Shekhawat NS, Galston AW. Polyamine levels as related to growth, differentiation and senescence in protoplast-derived cultures of Vigna aconitifolia and Avena sativa. Plant Growth Regul. 1985;3:329–337. doi: 10.1007/BF00117590. [DOI] [PubMed] [Google Scholar]
- Kumar A, Altabella T, Taylor MA, Tiburcio AF. Recent advances in polyamines research. Trends Plant Sci. 1997;2:124–130. [Google Scholar]
- Kumar PP, Thorpe TA. Putrescine metabolism in excised cotyledons of Pinus radiata cultured in vitro. Physiol Plant. 1989;76:521–526. [Google Scholar]
- Maki H, Ando S, Kodama H, Komamine A. Polyamines and the cell cycle of Catharanthus roseus cells in culture. Plant Physiol. 1991;96:1008–1013. doi: 10.1104/pp.96.4.1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minocha SC, Minocha R. Role of polyamines in somatic embryogenesis. In: Bajaj YPS, editor. Biotechnology in Agriculture and Forestry, Vol 30: Somatic Embryogenesis and Synthetic Seeds I. Berlin: Springer-Verlag; 1995. pp. 53–70. [Google Scholar]
- Montague MJ, Armstrong TA, Jaworski EG. Polyamine metabolism in embryogenic cells of Daucus carota. II. Changes in arginine decarboxylase activity. Plant Physiol. 1979;63:341–345. doi: 10.1104/pp.63.2.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murashige T, Skoog F. A revised medium for rapid growth and bioassays of tobacco tissue cultures. Physiol Plant. 1962;15:473–497. [Google Scholar]
- O'Brien TP, McCully ME (1981) The Study of Plant Structure: Principles and Selected Methods. Tharmacarpi, Melbourne, Australia
- Rajam MV. Polyamines. In: Prasad MNV, editor. Plant Ecophysiology. New York: John Wiley & Sons; 1997. pp. 343–374. [Google Scholar]
- Santanen A, Simola LK. Catabolism of putrescine and spermidine in embryogenic and non-embryogenic callus lines of Picea abies. Physiol Plant. 1994;90:125–129. [Google Scholar]
- Sharma P, Rajam MV. Genotype, explant and position effects on organogenesis and somatic embryogenesis in eggplant (Solanum melongena L.) J Exp Bot. 1995a;46:135–141. [Google Scholar]
- Sharma P, Rajam MV. Spatial and temporal changes in endogenous polyamine levels associated with somatic embryogenesis from different regions of hypocotyl of eggplant (Solanum melongena L.) J Plant Physiol. 1995b;146:658. 664. [Google Scholar]
- Tiburcio AF, Kaur-Sawhney R, Galston AW. Polyamine biosynthesis during vegetative and floral bud differentiation in thin layer cultures. Plant Cell Physiol. 1988;29:1241–1249. [Google Scholar]
- Torrigiani P, Altamura MM, Capitani F, Serafinni-Fracasini D, Bagni N. De novo root formation in thin layer cultures of tobacco: changes in free and bound polyamines. Physiol Plant. 1989;77:294–301. [Google Scholar]
- Walden R, Cordeiro A, Tiburcio AF. Polyamines: small molecules triggering pathways in plant growth and development. Plant Physiol. 1997;113:1009–1013. doi: 10.1104/pp.113.4.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu SC, Kuniyuki AH. Isolation and culture of almond protoplasts. Plant Sci. 1985;41:55–60. [Google Scholar]
- Yadav JS, Rajam MV. Spatial distribution of free and conjugated polyamines in leaves of Solanum melongena L. associated with differential morphogenetic potential: efficient somatic embryogenesis with putrescine. J Exp Bot. 1997;48:1537–1545. [Google Scholar]