Abstract
Sorafenib and sunitinib cause hypothyroidism in a subset of patients. This retrospective study examined the incidence of hypothyroidism and its relationship to progression-free survival in renal cell carcinoma. We found that hypothyroidism occurs more frequently in patients treated with sunitinib and correlates with a longer progression-free survival, which could be useful as a biomarker for response to therapy.
Introduction
Sunitinib and sorafenib are tyrosine kinase inhibitors used in metastatic renal cell carcinoma and are known to cause hypothyroidism in a subset of patients. The goal of this study was to better characterize the development of hypothyroidism in patients and to examine its relationship to progression-free survival.
Patients and Methods
A retrospective chart review was performed on patients treated with sunitinib or sorafenib from January 1, 2005, to January 1, 2011. Data pertaining to the treatment course and development of hypothyroidism were extracted. Patients with hypothyroidism at the beginning of treatment were analyzed separately.
Results
A total of 73 treatment periods had sufficient data to analyze. Among patients with normal baseline thyroid function, 15 (44%) of 34 patients treated with sunitinib and 6 (27%) of 22 patients treated with sorafenib developed hypothyroidism. The hazard ratio for the development of hypothyroidism with sorafenib vs. sunitinib treatment was significant, at 0.38 (95% CI, 0.14–0.97). There was a statistically significant difference in the progression-free survival between patients who developed hypothyroidism while receiving treatment compared with those who did not, 18.2 vs. 10.1 months (P = .01).
Conclusions
This study demonstrated a significant difference in the incidence of hypothyroidism during treatment with sunitinib and sorafenib, with a higher incidence of hypothyroidism in patients treated with sunitinib. The development of hypothyroidism was associated with a longer progression-free survival.
Keywords: Biologic markers, Enzyme inhibitors, Hypothyroidism, Kidney neoplasms, Protein kinase inhibitors, Sorafenib, Sunitinib
Introduction
Sunitinib malate (Sutent [SU11248]; Pfizer, New York, NY) and sorafenib tosylate (Nexavar [BAY 43-9006]; Bayer, West Haven, CT) are oral multitargeted tyrosine kinase inhibitors (TKI). They have antiangiogenic properties through inhibition of several tyrosine kinases, including vascular endothelial growth factor receptors 1 to 3, platelet-derived growth factor receptor α/β, c-kit receptor, fms-like tyrosine kinase 3 receptor, and RET kinase.1–3 Sorafenib also inhibits Raf kinase.1,3 Sunitinib and sorafenib were the first 2 drugs of their class to be approved for advanced renal cell carcinoma (RCC) on the basis of phase III trials that demonstrated efficacy.4,5 In addition, sunitinib is also US Food and Drug Administration approved for use in gastrointestinal stromal tumor and pancreatic neuroendocrine tumors, whereas sorafenib is approved for use in hepatocellular carcinoma.6,7
Common adverse effects observed with both drugs include fatigue, diarrhea, nausea, mucositis and/or stomatitis, rash, and hand-foot syndrome.4,5 Hypothyroidism was not reported in the initial phase III trials. However, the high incidence of fatigue prompted clinicians to check thyroid-stimulating hormone (TSH) levels, and it was found that hypothyroidism was associated with sunitinib.8–10 Since then, sunitinib-induced hypothyroidism has been well documented in both prospective and retrospective studies, with reported rates of thyroid function test abnormalities in 20% to 85% of treated patients.8,9,11–18 Hypothyroidism has also been reported in patients treated with sorafenib, although there are fewer studies that examined thyroid function in this population.16,17,19–23 In most case series, the incidence has been lower with sorafenib (ranging from 18–38%)17,19,21 than that with sunitinib, but this finding has not been universally. One recent study in a Japanese population reported sorafenib-associated hypothyroidism in 67.7% of patients,20 and another study reported an incidence of hypothyroidism of 42.1% in patients treated with sorafenib compared with 20.5% for those treated with sunitinib.16
Although many cases of sunitinib- and sorafenib-induced hypothyroidism are mild, multiple case reports of patients with severe symptoms that required hospitalization for problems such as pericardial effusion, symptomatic bradycardia, and myxedematous coma have been published.11,24–26 Although sunitinib-induced hypothyroidism may present over time, it can also develop suddenly during treatment.27,28
It is not clear if the development of hypothyroidism is a prognostic or a predictive marker.23 Recently, a few studies have been designed to look at the relationship between the development of hypothyroidism and clinical outcome to answer this question. The results have been mixed to date, with 1 study reporting a significant difference in overall survival in patients who developed hypothyroidism but not in progression-free survival (PFS), whereas another study found a significant difference in PFS, and a third study found no difference in PFS.16–18
Given the mixed results of these studies, more data are needed. The goal of our study was to examine the incidence of hypothyroidism in patients with metastatic RCC who were treated with sunitinib or sorafenib, and to examine a possible correlation between the development of hypothyroidism and PFS. Another objective of this work was to expand on our understanding of sorafenib-associated hypothyroidism and the effect of both drugs on the thyroid function of patients who were hypothyroid at baseline, a topic on which there is little published information.
Patients and Methods
Patients
A retrospective chart review was performed on all patients with histologically confirmed metastatic RCC treated with sunitinib or sorafenib who were seen at the University of Colorado Hospital urological oncology clinic. They were identified from a list of clinic patients with RCC treated from January 1, 2005, to January 1, 2011. Patients were excluded if there was missing or insufficient follow-up information, including a lack of a baseline TSH value or TSH values during treatment. The study population consisted of 78 patients. Some patients had separate therapeutic trials of sunitinib and sorafenib during the study period; consequently, there were a total of 108 treatment periods examined. For patients on 2 separate drug trials, the washout periods were adequate, and there was no evidence of a cross-over effect.
Treatment
Patients were treated with either sunitinib, 50 mg daily, on a 6-week cycle (4 weeks on treatment, 2 weeks off) or sorafenib, 400 mg twice daily, continuously. Ten patients were started at a reduced dose based on the treating physician’s judgment. Dose adjustments for toxicity or intolerance were performed according to the manufacturers’ recommendations. PFS was defined as the time from the start of treatment until therapy was discontinued due to disease progression or drug intolerance. Computed tomography scans to assess for response were generally performed every 2 to 3 cycles, although formal RECIST (Response Evaluation Criteria in Solid Tumors) criteria for radiographic progression were not used to define PFS in this retrospective study, with progression based on the treating physician’s assessment of radiographic and clinical changes.
Outcomes
Patient charts were examined for demographic data; treatment details; time from the start of treatment to disease progression; history of hypothyroidism; development of hypothyroidism during treatment, including laboratory data and symptoms; whether or not thyroid replacement was started and dose of replacement required; and when or if the hypothyroidism resolved. The time from the start of treatment to development of hypothyroidism also was examined. TSH values were not routinely obtained until late 2006, when reports of hypothyroidism were beginning to be published. Patients were excluded if they did not have a baseline TSH level and at least 1 TSH level while on treatment. Hypothyroidism was defined as any TSH increase above the upper limit of normal (5.0 mIU/L). Thyroid replacement was started at the treating physician’s discretion, generally if the TSH level was >10 mIU/L or if the patient had clinically significant symptoms thought to be due to hypothyroidism.
Patients with a history of hypothyroidism who were already receiving thyroid replacement at the start of TKI treatment were analyzed separately from the larger study population. To examine thyroid dysfunction in these patients, we followed the dose of thyroid replacement required. A dose increase of ≥25 µg was considered a marker of worsening thyroid function.
Statistical Analysis
Patient demographics were described and the differences between patients treated with sunitinib and the treated with sorafenib were analyzed by using a χ2 test or Fisher exact test, if needed, for categorical variables, and a 2-group t test for continuous variables. A Kaplan-Meier plot was used to describe the probability of time to incidence of sunitinib-induced and of sorafenib-induced hypothyroidism, and P values were determined by using a log-rank test. A Kaplan-Meier plot was also used to display the PFS stratified by the thyroid functional status. The Cox proportional hazard regression model was used to estimate the hazard ratio for hypothyroidism between the 2 drugs. A P value ≤.05 was considered statistically significant throughout this study.
Results
Of the 78 patients and 108 treatment periods identified, 61 patients, with a total of 73 treatment periods, had sufficient data to be analyzed, with most excluded due to a lack of TSH values at baseline or during treatment. Patient characteristics are described in Table 1. Patients with a history of hypothyroidism on thyroid replacement were separated from patients with normal thyroid function at baseline. In the group with normal baseline thyroid function, there were 56 treatment periods, 22 with sorafenib, 34 with sunitinib; their thyroid function during treatment is described in Table 2. Free T4 values were not reported here because this value was not checked consistently. Six (27%) of 22 patients treated with sorafenib developed hypothyroidism during treatment, of which 3 (13.6%) were given thyroid replacement; the median dose required was 50 µg. The median peak TSH level was 12.3 mIU/L (range, 5.5–28.13 mIU/L). Fifteen of 34 (44%) patients developed hypothyroidism while being treated with sunitinib. Twelve (35%) of the 34 were treated with thyroid replacement, with a median dose of 75 µg. The average peak TSH level was 34.8 mIU/L (range, 5.87–162.64 mIU/L). There was a significant difference in the probability of time to incidence of hypothyroidism while on therapy with sorafenib vs. sunitinib (P = .038, log-rank test). The median time to develop hypothyroidism was 11 and 20 months for patients on sunitinib and sorafenib, respectively, with this finding. Cox proportional hazard regression models were used to examine the association between the type of therapy (sorafenib vs. sunitinib) and the time to develop hypothyroidism when adjusting for therapy time. Analysis of the results indicated that the hazard of hypothyroidism for patients on sorafenib is 38% of the hazard for patients on sunitinib (95% CI, 14%–97%) (P = .04). There also was a trend toward a significant difference between the percentage of patients who required replacement, 13.6% of patients who received sorafenib vs. 35.3% of patients who received sunitinib, P = .07.
Table 1.
Patient Characteristics
| Characteristics | Sunitinib | Sorafenib | P Value |
|---|---|---|---|
| Total no. Patient | 58 | 50 | |
| No. Patients With Sufficient Data to Analyze | 45 | 28 | .3 |
| Mean (SD) Age, y | 59 ± 9.7 | 60 ± 8.1 | .73 |
| Men, no. (%) | 40 (69) | 41 (82) | .12 |
| Prior Sunitinib Therapy, The Number of Patients | — | 5 | |
| Prior Sorafenib Therapy, The Number of Patients | 6 | — | |
| No. (%) Patients With a History of Hypothyroidism at Baseline | 11 (19) | 6 (12) | .77 |
Table 2.
Thyroid Function During Treatment
| Sorafenib | Sunitinib | P Value | |
|---|---|---|---|
| Patients Without a History of Hypothyroidism | |||
| No. (%) patients developing hypothyroidism | 6/22 (27) | 15/34 (44) | .16 |
| Hazard ratio for hypothyroidism | 0.38 (sorafenib/sunitinib) (95% CI, 0.14–0.97 | .04 | |
| Patients with hypothyroidism, median peak TSH level (range), mIU/L | 12.3 (5.5–28.13) | 34.8 (5.87–162.64) | .10 |
| No. (%) patients required thyroid replacement | 3/22 (13.6) | 12/34 (35.3) | .07 |
| Median thyroid replacement dose (range), µg | 50 (25–150) | 75 (50–175) | .56 |
| Survival function with estimated median time to development of hypothyroidism, mo | 20 | 11 | .038a |
| Patients With a History of Hypothyroidism | |||
| No. (%) patients with a history of hypothyroidism develop thyroid dysfunction | 2/6 (33) | 8/11 (73) | .16 |
| Patients with hypothyroidism, median peak TSH (range), mIU/L | 25.8 (7.9–43.8) | 28.9 (7.5–72.43) | .99 |
| Survival function with estimated median time to development, mo | 8 | 6 | .3a |
| Median thyroid replacement dose increase required (range), µg | 43.8 (50–37.5) | 37.5 (25–50) | .69 |
| All Patients | |||
| Average baseline TSH level | 2.10 | 2.12 | .97 |
| Average peak TSH level | 6.23 | 18.12 | .04 |
| No. (%) patients with peak TSH between 5 and 10 mIU/L | 4 (14.3) | 4 (8.9) | .52 |
| No. (%) patients with peak TSH level >10 mIU/L | 4 (14.3) | 19 (42.2) | .06 |
| No. (%) patients with hypothyroidism or worsening thyroid dysfunction | 8/28 (28.6) | 23/45 (51.1) | .058 |
| Survival function with estimated median time to development, mo | 20 | 10 | .01a |
| Hazard ratio for hypothyroidism | 0.38 (sorafenib as compared to sunitinib) (95% CI, 0.17–0.86) | .03 | |
Abbreviation: TSH = thyroid-stimulating hormone.
Log-rank test for the difference in survival function between the 2 drugs.
A total of 17 patients had a history of hypothyroidism at the beginning of treatment and were already on a stable dose of thyroid replacement, with TSH values in the normal range at the start of treatment (Table 2). Eight (73%) of the 11 patients treated with sunitinib developed worsening thyroid dysfunction as identified by the use of higher doses of thyroid replacement. The median dose increase was 37.5 µg (range, 25–50 µg) and the average peak TSH level during treatment was 28.9 mIU/L (range, 7.5–72.43 mIU/L). During treatment with sorafenib, 2 (33%) of 6 patients developed worsening thyroid dysfunction, which required dose increases of 50 and 37.5 µg, and their peak TSH values during treatment were 43.8 and 7.9 mIU/L. No patients had a low TSH level during treatment or required their thyroid replacement dose to be decreased.
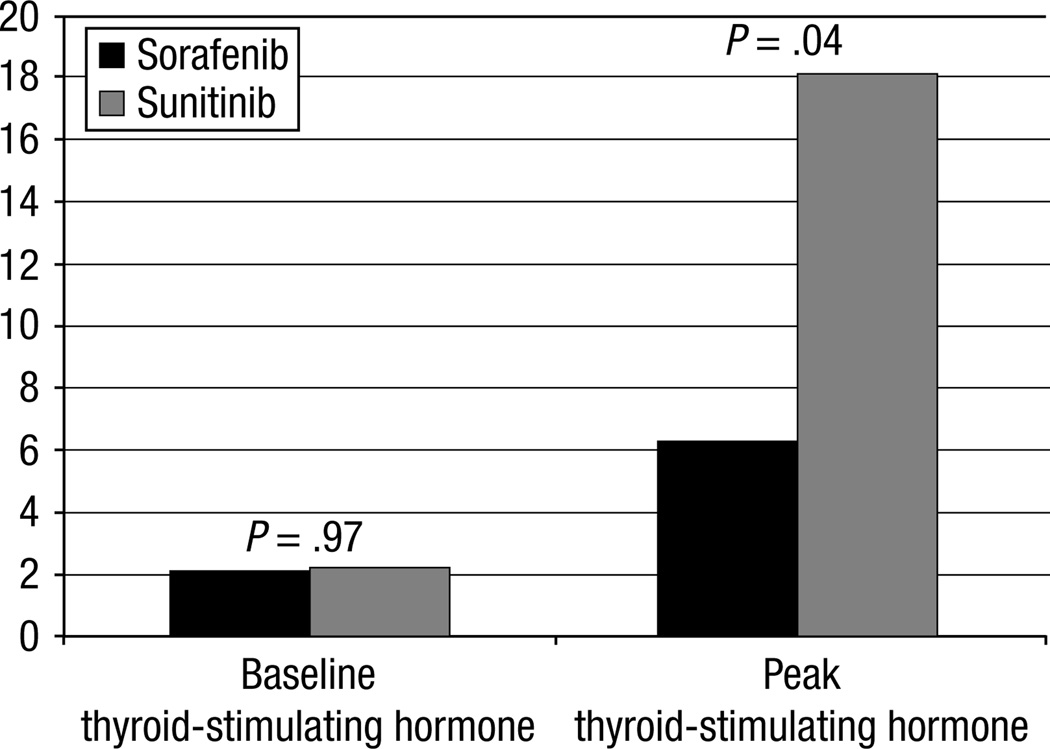
When combining both the normal thyroid and baseline hypothyroid groups, 8 (28.6%) of 28 patients developed hypothyroidism or worsening thyroid dysfunction during sorafenib treatment and 23 (51.1%) of 45 with sunitinib. With this larger cohort, the survival analyses indicate that the difference in the development of hypothyroidism on sorafenib vs. sunitinib is more statistically significant (P = .01, log-rank test). Cox proportional regression modeling revealed that the hazard ratio of hypothyroidism for patients on sorafenib is only 38% of the hazard for patients on sunitinib, which is the same result reported earlier for the group patients with normal thyroid function at baseline but with a more narrow 95% CI (17%–86%). Moreover, the severity of thyroid dysfunction was greater in sunitinib compared with sorafenib-treated subjects (Table 2), as evaluated by the difference in the percentage of patients with a peak TSH level >10 mIU/L while on sorafenib vs. sunitinib, 14.3% vs. 42.2%, which is significant (P = .01). The average peak TSH level of all patients (including those who did not develop hypothyroidism) is also significantly higher in patients treated with sunitinib, 18.1 vs. 6.2 (P = .04), as shown in Figure 1.
Figure 1.
Baseline Thyroid-Stimulating Hormone (TSH) and Peak TSH. Average Baseline and Peak TSH for All Patients Treated With Sorafenib as Compared to Sunitinib
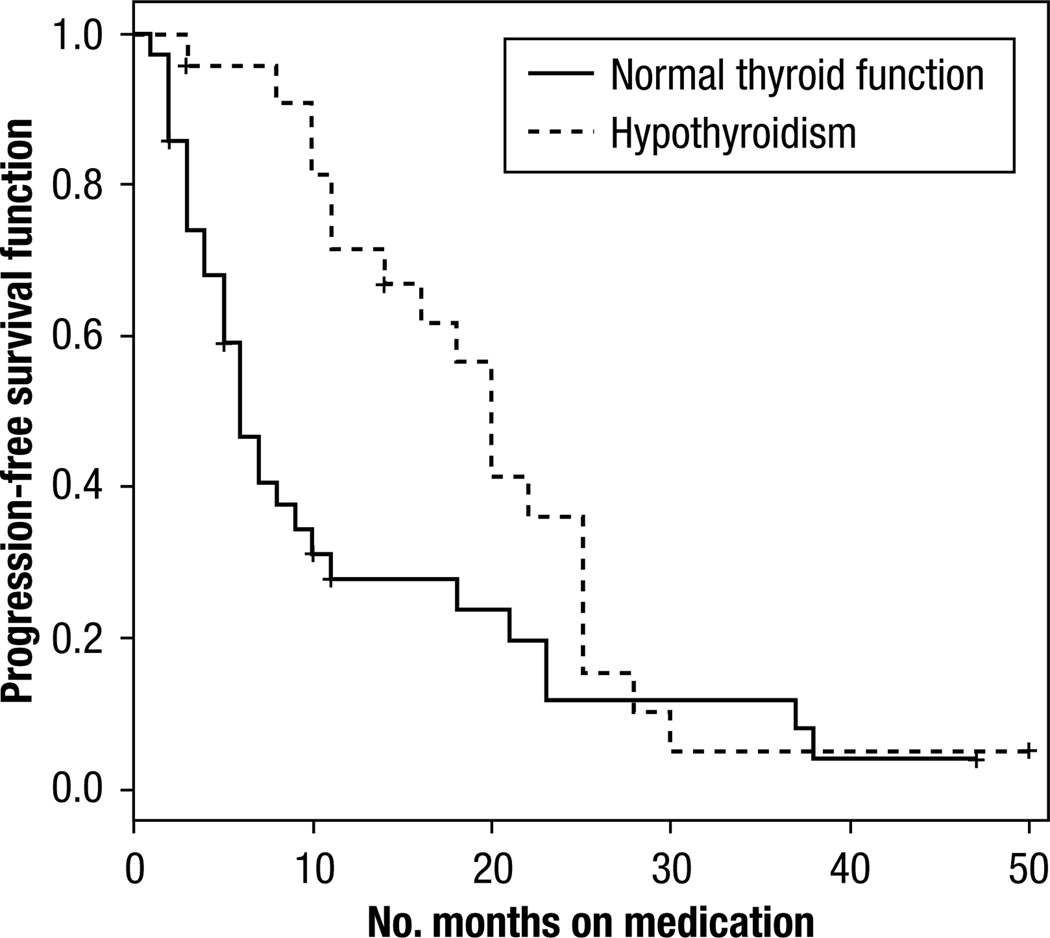
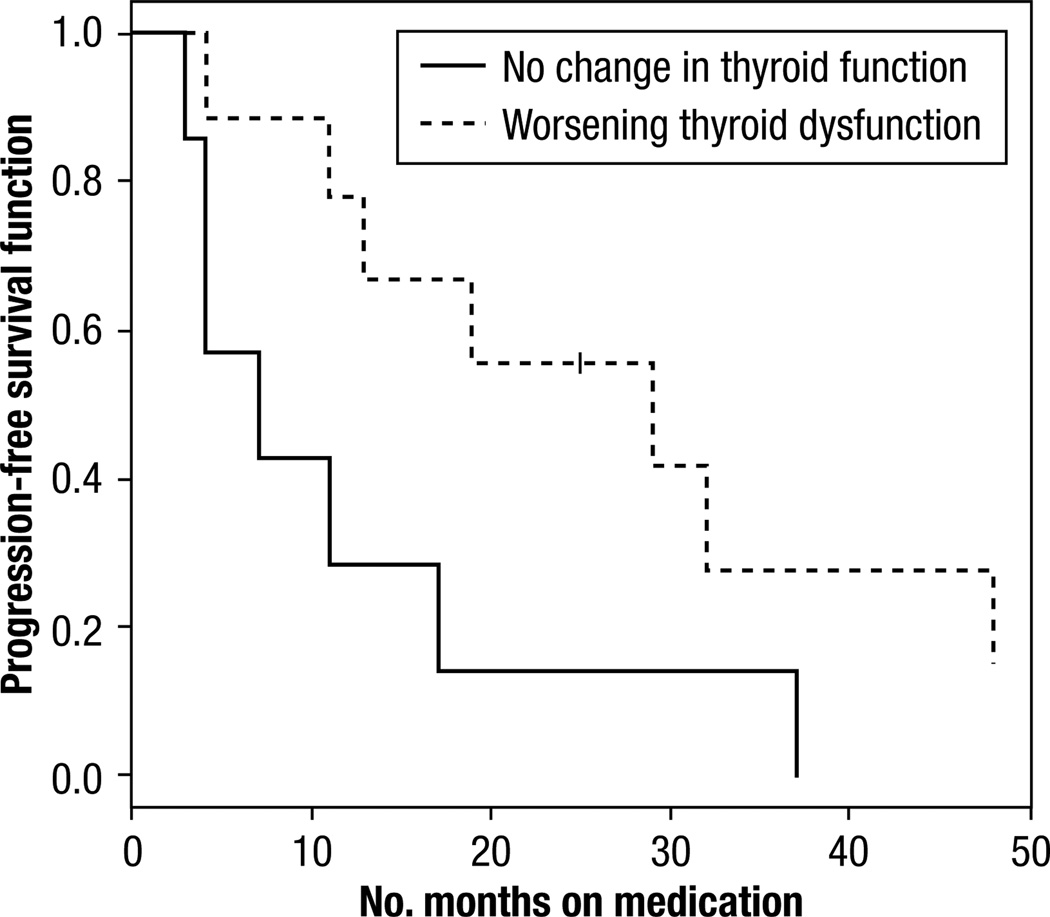
There was a statistically significant difference in PFS between patients with normal thyroid function at baseline who developed hypothyroidism and patients who remained euthyroid (Table 3) (P = .02, log-rank test), as revealed in the Kaplan-Meier plot (Figure 2). The median time to progression was 20 months (95% CI, 11–25 months) in hypothyroid vs. 6 months (95% CI, 4–10 months) in patients who were euthyroid. This trend was also seen in patients who were hypothyroid at baseline and developed worsening thyroid dysfunction during treatment (Figure 3), a median PFS of 29 months (95% CI, 4–48 months) vs. 7 months (95% CI, 3–17 months) (P = .05, log-rank test). Overall survival data were not included because we could not obtain complete survival data in this retrospective study.
Table 3.
Difference in Progression-free Survival in Patients Who Develop Hypothyroidism
| Group of Patients | No. Patients |
Median Progression-Free Survival (95% CI), mo |
P Value |
|---|---|---|---|
| Develop hypothyroidism while on treatment with sunitinib or sorafenib | 21 | 20 (11–25) | .02 |
| Remain euthyroid while on treatment with sunitinib or sorafenib | 35 | 6 (4–10) | |
| History of hypothyroidism, develop worsening thyroid dysfunction | 10 | 29 (4–48) | .05 |
| History of hypothyroidism, no change in thyroid function | 7 | 7 (3–17) |
Figure 2.
Kaplan Meier Curve, Progression-Free Survival for Patients With Baseline Euthyroidism Who Developed Hypothyroidism While on Sunitinib or Sorafenib Treatment Versus Those Who Remained Euthyroid (P = 0.02).
Figure 3.
Kaplan Meier Curve of Progression-Free Survival for Development of Worsening Hypothyroidism in Those Patient With Baseline Thyroid Dysfunction While on Sunitinib or Sorafenib Treatment (P = 0.05)
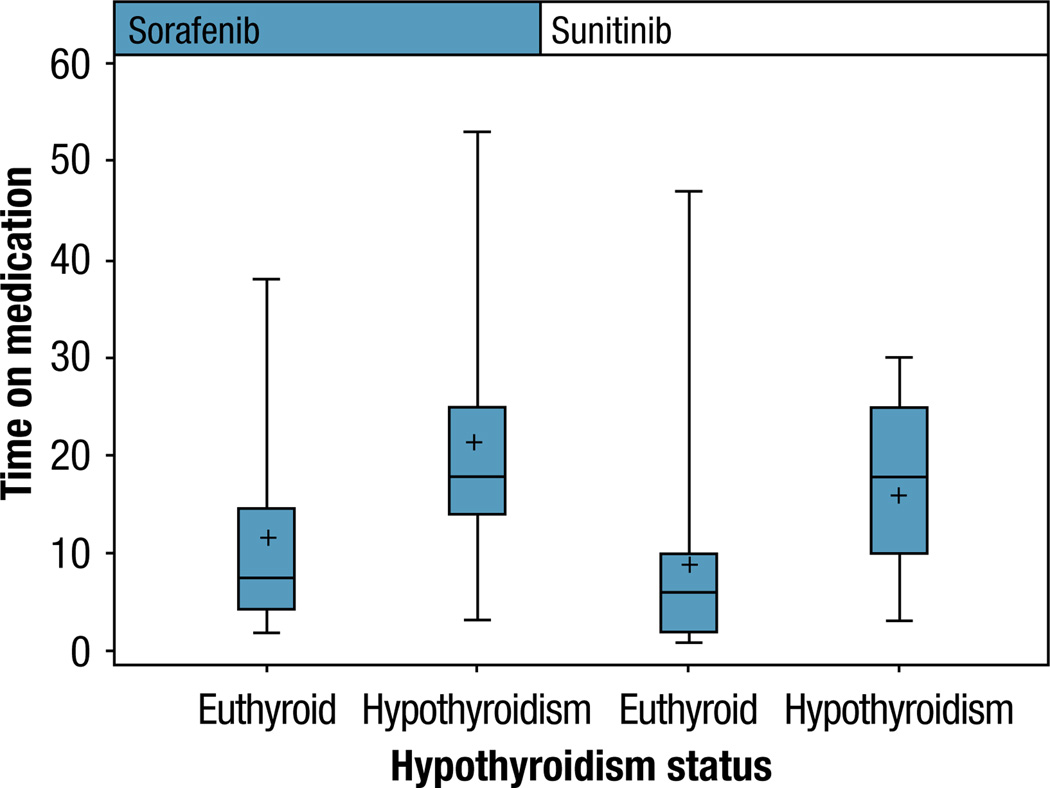
When patients on sunitinib were analyzed separately from the patients on sorafenib, there was still a difference in PFS. Patients who developed hypothyroidism while on sunitinib had a significantly longer PFS than those who did not, with a median PFS of 20 months (95% CI, 10–25 months) vs. 6 months (95% CI, 3–7 months), although the survival function did not reach significance (P = .2, log-rank test). Patients who developed hypothyroidism while on sorafenib also had a longer PFS than those who did not, with a median PFS of 18 vs. 8 months (P = .07). The difference in PFS when sunitinib and sorafenib-treated patients are analyzed separately is displayed in Figure 4.
Figure 4.
Box Plot, Difference in Progression-Free Survival Between Euthyroid and Hypothyroid Patients When Separated by Treatment Drug
Finally, after therapy was discontinued, hypothyroidism resolved in 11 patients (median time, 1.5 months; range, 0–12 months), and they were tapered off their thyroid replacement. It did not resolve in 7 patients with normal baseline thyroid function who remained on thyroid replacement at the end of this study period, with the last visit for each of these patients at a median of 20 months after the end of treatment (range, 6–36 months).
Discussion
There are 3 main findings in this study: the difference in the natural history of the development of hypothyroidism during treatment with sunitinib and sorafenib, the description of a subset of patients with baseline hypothyroidism treated with sunitinib or sorafenib, and the relationship between the development of hypothyroidism and PFS. First, there is a significant difference in the incidence of thyroid dysfunction between patients treated with sunitinib and the treated with sorafenib. Few studies have directly compared patients treated with sunitinib and those treated with sorafenib in the same institution for treatment-associated thyroid dysfunction. The overall incidence of sorafenib-induced hypothyroidism was lower than sunitinib-induced hypothyroidism, with a hazard ratio of 0.38 (95% CI, 0.17–0.86), which is consistent with the results in a recent study done by Riesenbeck et al17 and what would be expected from clinical experience with both drugs, which is contrary to another recent trial in which the incidence of hypothyroidism in patients treated with sorafenib was 42.1%, twice that found in patients treated with sunitinib (20.5%).16 Furthermore, our observed incidence of hypothyroidism during treatment with sorafenib (27%) is similar to that reported in a recent phase I trial of patients treated with sorafenib and everolimus (20%) and by other groups.17,19,21
It also appears that sorafenib-induced hypothyroidism may be milder and slower to develop. Fewer patients who developed hypothyroidism on sorafenib required thyroid replacement (P = .07), the peak TSH level of all patients treated with sorafenib was lower (P = .04), the percentage of patients with a peak TSH level >10 mIU/L on sorafenib was lower (P = .06), and the median time to detection was longer (P = .01). These findings are in line with other reported values; in a Japanese study of sorafenib, the incidence of hypothyroidism was 67.7%, but nearly all the patients had a peak TSH level <10.0 mIU/L, and only 5% of the patients were given thyroid replacement.20 In another study, in which 18% of patients treated with sorafenib developed hypothyroidism, the average peak TSH level was 6.32, with a range of 2.4 to 9.21.19 Our study had the advantage of reporting on the outcomes of thyroid dysfunction from patients treated with sorafenib and those treated with sunitinib from a single institution and time period.
Second, we report on subsets of patients with existing thyroid dysfunction who have largely been excluded in previous reports. There were 17 patients with a history of hypothyroidism who were already on thyroid replacement, of whom, 10 required an increase of ≥25 µg during treatment. These results support the findings of Schmidinger et al,16 who reported that 7 of 14 patients in their study population who had a history of hypothyroidism at baseline required an increase of their thyroid replacement dose. A second patient population included in this present study consisted of the patients previously treated with sorafenib or sunitinib who progressed and were then switched to the other drug. In the final analysis, we had 12 patients who were treated with both sorafenib and sunitinib in this study period and were included in both groups. Seven of these patients did not develop hypothyroidism during either treatment, one became hypothyroid with both treatments, 2 did not have hypothyroidism while on sorafenib but consequently developed hypothyroidism while on sunitinib, and 2 had hypothyroidism on sunitinib that resolved, and they did not develop hypothyroidism while on sorafenib. Overall, the development of hypothyroidism was not clearly related to previous treatment, consistent with a recent study that found that thyroid dysfunction did not occur more frequently in patients who had previous treatment compared with those who did not (no significant difference between those patient populations, P = .62).18
Third, we describe the relationship between the development of thyroid dysfunction and prolonged PFS. The median PFS of patients who developed hypothyroidism during treatment with sorafenib or sunitinib was 20 months (95% CI, 11–25 months) compared with 6 months (95% CI, 4–10 months) in patients who remained euthyroid (P = .02). This is similar to the results found in the study by Riesenbeck et al,17 who reported that the development of hypothyroidism in patients treated with sorafenib and the treated with sunitinib was associated with a prolonged PFS, 16.0 ± 0.8 months vs. 6.0 ± 0.8 months; P = .032.
These results point to a relationship between the development of hypothyroidism or worsening thyroid dysfunction and a prolonged PFS. The difference in PFS is independent of the treatment drug and cannot be attributed to a PFS difference between the 2 drugs and a higher prevalence of hypothyroidism with sunitinib. When analyzing patients treated with sunitinib and patients treated with sorafenib separately, there was still a difference in PFS that related to thyroid dysfunction.
However, what is not clear is if hypothyroidism could be used as a biomarker for treatment efficacy or if it is just identified more frequently in patients with a longer treatment duration. At least 1 study reported that the risk of developing hypothyroidism increases with treatment duration.8 To answer this question, it would be helpful to know when hypothyroidism develops, and the data that has been reported is variable. Among the studies that describe the median time to detection, 5 studies report a time of 1 to 3 months.12,13,17,19,20 However, even in studies with a median time to detection of 1 month, the range extends to 7 months, and there were 3 other studies that reported a time of 4 to 5 months, with a range up to the 36th month of treatment.8,14,18
The studies that looked at the relationship between hypothyroidism and PFS have used different approaches to deal with the issue of timing and may explain why there have been mixed results. Schmidinger et al16 found the overall survival, but not the PFS, to be statistically significantly longer in patients with hypothyroidism, but thyroid function was only assessed at 2 months. A second study, by Sabatier et al,18 used a landmark method to assess a possible correlation and their landmark time was 6 months; therefore, patients who developed hypothyroidism or progressed before this time period were not included. This study did not find a difference in mean PFS, possibly due to excluding too many patients who progressed and did not develop hypothyroidism: the landmark population had 2 patients with progressive disease as the best response as opposed to 19 in the overall study population.
One of the major limitations of this retrospective review is that thyroid function tests were not checked at standard intervals, so the median time to detection reported here is likely to be much less accurate than the time reported in prospective trials with proscribed testing intervals. In addition to the other limitations inherent in a retrospective review, another potential bias is that TSH values were not checked routinely before 2006 when the hypothyroidism association was first reported in the literature. The median time to first TSH level check from the start of treatment was 6 months in this series, which is slightly skewed by the beginning of the study period when it was checked less frequently. The treating physician only monitored thyroid function when there was a clinical suspicion of hypothyroidism; consequently, the incidence reported here may underestimate the true rate of hypothyroidism. However, there were only 7 patients included who were treated before 2006 and the overall incidence rates in both sunitinib and sorafenib treatment groups are similar to the rates reported in other prospective studies.
The mechanism of sunitinib- and sorafenib-induced hypothyroidism is not fully understood. There have been many studies that looked at this question, with conflicting results. It does not appear to be immune related; most studies have documented negative thyroid antibodies,8,11,12,22,24 and only two reported a positive thyroglobulin antibody.9,29 A number of groups have described transient mild hyperthyroidism preceding development of hypothyroidism in 17% to 44% of patients, which indicates that TKI-associated thyroid dysfunction may develop secondary to a destructive thyroiditis after an initial thyrotoxic phase.8,12,13,15,20,22 Supporting this hypothesis, thyroid atrophy has been documented on ultrasound and computed tomography.8,13,22,30 A possible mechanism is vascular endothelial growth factor receptor tyrosine kinase inhibition on thyroid cells leading to capillary regression,31,32 although 1 group found no change in thyroid volume or vasculature.11 Other investigators have suggested that altered thyroid hormone production and metabolism may play a role by causing an increase in deiodinase 3 activity33 or by inhibiting peroxidase activity.14 Finally, TKIs have been shown to inhibit growth of FRTL-5 thyroid cells,34 to regulate growth and function of normal and neoplastic thyroid cell kinases,35 and to treat advanced thyroid cancer.36
Finally, 2 clinical issues regarding thyroid hormone replacement for patients who become hypothyroid are when to initiate it and if it can be stopped after treatment. There are some data to suggest that hypothyroidism may actually be protective in cancer,37 which implies that one should wait to start thyroid replacement until patients become overtly hypothyroid with clear symptoms secondary to hypothyroidism and low free thyroxine levels. The effect of thyroid hormone replacement on outcomes and the optimal time to begin replacement will be an important question to address in more depth in future studies. There have been a few algorithms suggested by previous groups.38 It was our practice to generally follow the guidelines put forth by the American Thyroid Association and withhold thyroid hormone replacement in patients who were asymptomatic with a TSH level <10 µM/L.39 However, the majority of patients treated with TKI have fatigue, which complicates the assessment of hypothyroid-associated symptoms. Finally, sunitinib- and sorafenib-induced hypothyroidism can be transient, resolving 2 to 6 months after the end of therapy, in some patients even at the end of the 2-week off period of each cycle.11,12,22 But there are data that suggest that, in some patients, thyroid dysfunction may be irreversible and that patients will continue to need hormone replacement therapy indefinitely.13,30,40 Our experience supports these findings. In patients with adequate data to analyze, hypothyroidism resolved in 11 of 18 patients, with 7 patients remaining hypothyroid and requiring ongoing replacement. We would suggest continuing to monitor TSH values and to taper the thyroid replacement dose as indicated but to counsel patients that TKI hypothyroidism may be persistent, even permanent.
Conclusion
This study demonstrated a significant difference in the incidence of TKI-associated hypothyroidism during treatment with sunitinib vs. sorafenib, with a higher incidence of thyroid dysfunction in patients treated with sunitinib. The development of hypothyroidism with sunitinib and sorafenib was associated with a longer PFS, which was statistically significant. Hypothyroidism is a potential predictive biomarker for treatment response with sunitinib and sorafenib, but future studies need to confirm this relationship and address the best manner to manage patients who do develop hypothyroidism on TKI therapy.
Clinical Practice Points.
Hypothyroidism is a known adverse effect of sunitinib and sorafenib treatment. In this single-institution study, hypothyroidism occurs more frequently with sunitinib than with sorafenib and appears to be more clinically significant with sunitinib.
In a subset of patients with hypothyroidism at baseline, treatment with sunitinib and with sorafenib caused worsening thyroid dysfunction as measured by increased thyroid supplementation requirements.
The development of hypothyroidism is correlated with prolonged PFS, which suggests that it could be used as a biomarker for treatment efficacy.
Acknowledgments
We thank Dr. Aleck Hercbergs for his helpful review of the manuscript and advice on this project. This work was supported in part by a Paul Calabresi K12 clinical scholar grant (T.W.F.) awarded to the University of Colorado Denver (K12CA086913).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure
T. W. Flaig has participated in Pfizer and Bayer sponsored clinical trials of these agents and has also receive honorarium from Amgen and consulted for Sanofi-Aventis. This work was supported by the National Institutes of Health. The other authors have stated that they have no conflicts of interest
References
- 1.Wilhelm SM, Carter C, Tang L, et al. BAY 43-9006 exhibits broad spectrum oral antitumor activity and targets the RAF/MEK/ERK pathway and receptor tyrosine kinases involved in tumor progression and angiogenesis. Cancer Res. 2004;64:7099–7109. doi: 10.1158/0008-5472.CAN-04-1443. [DOI] [PubMed] [Google Scholar]
- 2.Mendel DB, Laird AD, Xin X, et al. In vivo antitumor activity of SU11248, a novel tyrosine kinase inhibitor targeting vascular endothelial growth factor and platelet-derived growth factor receptors: determination of a pharmacokinetic/pharmacodynamic relationship. Clin Cancer Res. 2003;9:327–337. [PubMed] [Google Scholar]
- 3.Wilhelm S, Carter C, Lynch M, et al. Discovery and development of sorafenib: a multikinase inhibitor for treating cancer. Nat Rev Drug Discov. 2006;5:835–844. doi: 10.1038/nrd2130. [DOI] [PubMed] [Google Scholar]
- 4.Motzer RJ, Hutson TE, Tomczak P, et al. Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. N Engl J Med. 2007;356:115–124. doi: 10.1056/NEJMoa065044. [DOI] [PubMed] [Google Scholar]
- 5.Escudier B, Eisen T, Stadler WM, et al. Sorafenib in advanced clear-cell renal-cell carcinoma. N Engl J Med. 2007;356:125–134. doi: 10.1056/NEJMoa060655. [DOI] [PubMed] [Google Scholar]
- 6.Demetri GD, van Oosterom AT, Garrett CR, et al. Efficacy and safety of sunitinib in patients with advanced gastrointestinal stromal tumour after failure of imatinib: a randomised controlled trial. Lancet. 2006;368:1329–1338. doi: 10.1016/S0140-6736(06)69446-4. [DOI] [PubMed] [Google Scholar]
- 7.Abou-Alfa GK, Schwartz L, Ricci S, et al. Phase II study of sorafenib in patients with advanced hepatocellular carcinoma. J Clin Oncol. 2006;24:4293–4300. doi: 10.1200/JCO.2005.01.3441. [DOI] [PubMed] [Google Scholar]
- 8.Desai J, Yassa L, Marqusee E, et al. Hypothyroidism after sunitinib treatment for patients with gastrointestinal stromal tumors. Ann Intern Med. 2006;145:660–664. doi: 10.7326/0003-4819-145-9-200611070-00008. [DOI] [PubMed] [Google Scholar]
- 9.Rini BI, Tamaskar I, Shaheen P, et al. Hypothyroidism in patients with metastatic renal cell carcinoma treated with sunitinib. J Natl Cancer Inst. 2007;99:81–83. doi: 10.1093/jnci/djk008. [DOI] [PubMed] [Google Scholar]
- 10.Wolter P, Dumez H, Schöffski P. Sunitinib and hypothyroidism. N Engl J Med. 2007;356:1580. doi: 10.1056/NEJMc070327. [DOI] [PubMed] [Google Scholar]
- 11.Mannavola D, Coco P, Vannucchi G, et al. A novel tyrosine-kinase selective inhibitor, sunitinib, induces transient hypothyroidism by blocking iodine uptake. J Clin Endocrinol Metab. 2007;92:3531–3534. doi: 10.1210/jc.2007-0586. [DOI] [PubMed] [Google Scholar]
- 12.Wolter P, Stefan C, Decallonne B, et al. The clinical implications of sunitinib-induced hypothyroidism: a prospective evaluation. Br J Cancer. 2008;99:448–454. doi: 10.1038/sj.bjc.6604497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shinohara N, Takahashi M, Kamishima T, et al. The incidence and mechanism of sunitinib-induced thyroid atrophy in patients with metastatic renal cell carcinoma. Br J Cancer. 2011;104:241–247. doi: 10.1038/sj.bjc.6606029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wong E, Rosen LS, Mulay M, et al. Sunitinib induces hypothyroidism in advanced cancer patients and may inhibit thyroid peroxidase activity. Thyroid. 2007;17:351–355. doi: 10.1089/thy.2006.0308. [DOI] [PubMed] [Google Scholar]
- 15.Grossmann M, Premaratne E, Desai J, et al. Thyrotoxicosis during sunitinib treatment for renal cell carcinoma. Clin Endocrinol (Oxf) 2008;69:669–672. doi: 10.1111/j.1365-2265.2008.03253.x. [DOI] [PubMed] [Google Scholar]
- 16.Schmidinger M, Vogl UM, Bojic M, et al. Hypothyroidism in patients with renal cell carcinoma: blessing or curse? Cancer. 2011;117:534–544. doi: 10.1002/cncr.25422. [DOI] [PubMed] [Google Scholar]
- 17.Riesenbeck LM, Bierer S, Hoffmeister I, et al. Hypothyroidism correlates with a better prognosis in metastatic renal cancer patients treated with sorafenib or sunitinib. World J Urol. 2011;29:807–813. doi: 10.1007/s00345-010-0627-2. [DOI] [PubMed] [Google Scholar]
- 18.Sabatier R, Eymard JC, Walz J, et al. Could thyroid dysfunction influence outcome in sunitinib-treated metastatic renal cell carcinoma? Ann Oncol. 2012;23:714–721. doi: 10.1093/annonc/mdr275. [DOI] [PubMed] [Google Scholar]
- 19.Tamaskar I, Bukowski R, Elson P, et al. Thyroid function test abnormalities in patients with metastatic renal cell carcinoma treated with sorafenib. Ann Oncol. 2008;19:265–268. doi: 10.1093/annonc/mdm483. [DOI] [PubMed] [Google Scholar]
- 20.Miyake H, Kurahashi T, Yamanaka K, et al. Abnormalities of thyroid function in Japanese patients with metastatic renal cell carcinoma treated with sorafenib: a prospective evaluation. Urol Oncol. 2010;28:515–519. doi: 10.1016/j.urolonc.2009.08.011. [DOI] [PubMed] [Google Scholar]
- 21.Harzstark AL, Small EJ, Weinberg VK, et al. A phase I study of everolimus and sorafenib for metastatic clear cell renal cell carcinoma. Cancer. 2011;117:4194–4200. doi: 10.1002/cncr.25931. [DOI] [PubMed] [Google Scholar]
- 22.van Doorn L, Eskens FA, Visser TJ, et al. Sorafenib induced thyroiditis in two patients with hepatocellular carcinoma. Thyroid. 2011;21:197–202. doi: 10.1089/thy.2010.0234. [DOI] [PubMed] [Google Scholar]
- 23.Rini B. Does hypothyroidism predict clinical outcome? Nat Rev Urol. 2010 doi: 10.1038/nrurol.2010.191. Published online. [DOI] [PubMed] [Google Scholar]
- 24.Prat A, Serrano C, Valverde C, et al. Acute severe hypothyroidism induced by sunitinib. Radiother Oncol. 2008;89:124–125. doi: 10.1016/j.radonc.2008.05.007. [DOI] [PubMed] [Google Scholar]
- 25.Collinson FJ, Vasudev NS, Berkin L, et al. Sunitinib-induced severe hypothyroidism with cardiac compromise. Med Oncol. 2011;28(suppl 1):S699–S701. doi: 10.1007/s12032-010-9757-z. [DOI] [PubMed] [Google Scholar]
- 26.Block MS, Kohli M. Clinical hypothyroidism in a renal cell carcinoma patient treated with sorafenib. Clin Adv Hematol Oncol. 2011;9:335–338. [PubMed] [Google Scholar]
- 27.de Groot JWB, Links TP, van der Graaf WTA. Tyrosine kinase inhibitors causing hypothyroidism in a patient on levothyroxine. Ann Oncol. 2006;17:1719–1720. doi: 10.1093/annonc/mdl112. [DOI] [PubMed] [Google Scholar]
- 28.Hutson TE, Figlin RA, Kuhn JG, et al. Targeted therapies for metastatic renal cell carcinoma: an overview of toxicity and dosing strategies. Oncologist. 2008;13:1084–1096. doi: 10.1634/theoncologist.2008-0120. [DOI] [PubMed] [Google Scholar]
- 29.Faris JE, Moore AF, Daniels GH. Sunitinib (Sutent)-induced thyrotoxicosis due to destructive thyroiditis: a case report. Thyroid. 2007;17:1147–1149. doi: 10.1089/thy.2007.0104. [DOI] [PubMed] [Google Scholar]
- 30.Rogiers A, Wolter P, Op de Beeck K, et al. Shrinkage of thyroid volume in sunitinib-treated patients with renal cell carcinoma: a potential marker of irreversible thyroid dysfunction? Thyroid. 2010;20:317–322. doi: 10.1089/thy.2009.0125. [DOI] [PubMed] [Google Scholar]
- 31.Kamba T, Tam BY, Hashizume H, et al. VEGF-dependent plasticity of fenestrated capillaries in the normal adult microvasculature. Am J Physiol Heart Circ Physiol. 2006;290:H560–H576. doi: 10.1152/ajpheart.00133.2005. [DOI] [PubMed] [Google Scholar]
- 32.Makita N, Miyakawa M, Fujita T, et al. Sunitinib induces hypothyroidism with a markedly reduced vascularity. Thyroid. 2010;20:323–326. doi: 10.1089/thy.2009.0414. [DOI] [PubMed] [Google Scholar]
- 33.Abdulrahman RM, Verloop H, Hoftijzer H, et al. Sorafenib-induced hypothyroidism is associated with increased type 3 deiodination. J Clin Endocrinol Metab. 2010;95:3758–3762. doi: 10.1210/jc.2009-2507. [DOI] [PubMed] [Google Scholar]
- 34.Salem AK, Fenton MS, Marion KM, et al. Effect of sunitinib on growth and function of FRTL-5 thyroid cells. Thyroid. 2008;18:631–635. doi: 10.1089/thy.2007.0336. [DOI] [PubMed] [Google Scholar]
- 35.Fagin JA. How thyroid tumors start and why it matters: kinase mutants as targets for solid cancer pharmacotherapy. J Endocrinol. 2004;183:249–256. doi: 10.1677/joe.1.05895. [DOI] [PubMed] [Google Scholar]
- 36.Gupta-Abramson V, Troxel AB, Nellore A, et al. Phase II trial of sorafenib in advanced thyroid cancer. J Clin Oncol. 2008;26:4714–4719. doi: 10.1200/JCO.2008.16.3279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hercbergs AH, Ashur-Fabian O, Garfield D. Thyroid hormones and cancer: clinical studies of hypothyroidism in oncology. Curr Opin Endocrinol Diabetes Obes. 2010;17:432–436. doi: 10.1097/MED.0b013e32833d9710. [DOI] [PubMed] [Google Scholar]
- 38.Torino F, Corsello SM, Longo R, et al. Hypothyroidism related to tyrosine kinase inhibitors: an emerging toxic effect of targeted therapy. Nat Rev Clin Oncol. 2009;6:219–228. doi: 10.1038/nrclinonc.2009.4. [DOI] [PubMed] [Google Scholar]
- 39.Gharib H, Tuttle RM, Baskin HJ, et al. Subclinical thyroid dysfunction: a joint statement on management from the American Association of Clinical Endocrinologists, the American Thyroid Association, and the Endocrine Society. J Clin Endocrinol Metab. 2005;90:581–585. doi: 10.1210/jc.2004-1231. discussion 586-7. [DOI] [PubMed] [Google Scholar]
- 40.Sakurai K, Fukazawa H, Arihara Z, et al. Sunitinib-induced thyrotoxicosis followed by persistent hypothyroidism with shrinkage of thyroid volume. Tohoku J Exp Med. 2010;222:39–44. doi: 10.1620/tjem.222.39. [DOI] [PubMed] [Google Scholar]