Abstract
The interaction of Matrix metalloproteinases (MMPs), its tissue inhibitors (TIMPs) and pro-inflammatory cytokines in response to Mycobacterium tuberculosis (MTB) infection is important to understand the immune response at the site of infection. We compared the levels of MMPs, TIMPs and cytokines in plasma (BL) and pleural fluid (PF) of tuberculosis (TB) and non tuberculosis (NTB) patients. Comparison between BL and PF showed significantly higher levels of MMP-1, TIMP-1 and -3 in TB PF; of MMP-7, -8, -9 in BL of both groups. Also, levels of MMP-1,-8,-9 and TIMP-3 were significantly higher in TB PF compared to NTB. Cytokines INF-γ, TNF-α, and IL-6 significantly increased in PF of both groups. A positive correlation of MMPs with TIMPs in TB, MMP-1 and -9 with IL-6 in TB PF and MMP-9 with IFN-γ in NTB PF was observed. This study implicates the possible usage of MMPs as bio-markers aiding diagnosis in TB pleuritis.
1. Introduction
Tuberculosis pleurisy (TP) denotes a form of inflammatory pleural disease due to Mycobacterium tuberculosis (MTB) infection, involving pleura [1]. Rupture of sub pleural caseous focus in the lung into the pleural space triggers hypersensitivity reaction due to mycobacterial antigens resulting in the possible clinical manifestations [1, 2]. In a normal course, phagocytosis of MTB by alveolar macrophages results in influx of lymphocytes and activated macrophages into the lesion resulting in granuloma formation, a protective mechanism to keep the infection under check and latent. But during reactivation, granuloma breaks down; the lung fibers are degraded due to proteolytic activity and dissemination of MTB is achieved resulting in caseation [3]. These proteolytic processes are carried out by Matrix metalloproteinases (MMPs) capable of degrading almost all components of extra cellular matrix [4]. The proteolytic processes also increase the capillary permeability resulting in the accumulation of pro-inflammatory cytokines and inflammatory cells in the pleural cavity [2, 5, 6].
Cytokines like Tumor necrosis factor (TNF-α) and Interferon (IFN)-γ upregulate the recruited monocytes and macrophages to produce MMPs, in turn increase inflammation by activating the pro-inflammatory cytokines [7]. This alters the integrity of underlying basement membrane and facilitates fluid influx into the pleural space [4, 8]. MMPs are inhibited by specific tissue inhibitors of metalloproteinases (TIMPs) with which they form 1:1 complexes. The MMP:TIMP ratio is critical in regulating the proteolysis of connective tissues and in controlling tissue damage [9]. High concentrations of MMP-1 (Collagenase-1), MMP-8 (Collagenase-2), MMP-9 (Gelatinase-B), TIMP-1 and -2 and pro-inflammatory cytokines in pleural fluids has been reported in earlier studies [5, 10, 11]. However, their levels have not been studied and/or compared with systemic plasma levels or with other disease conditions. The secretion of these molecules at the site of infection and their systemic spread is of much importance because they possess all possible characters to act as valuable biomarker for various disease conditions. Similarly, the action of proteinases and pro-inflammatory cytokines in response to bacterial infection is also important in identifying the immunopathology of tuberculosis (TB) and possible remedial measures to overcome it. Hence, in the present study, we compared the levels of various MMPs, TIMPS and pro-inflammatory cytokines in pleural fluid (PF) and plasma (BL) of TB and non TB (NTB) patients to identify possible correlations with the aim of aiding diagnosis of TB pleuritis.
2. Materials and methods
2.1. Study subjects
Blood and pleural fluid samples were collected from 44 patients of Government General Hospital (GGH). The patients were between the age group of 18–60 years with a mean of 39. Out of them, 28 had exudative pleural effusions with lymphocytic predominance (TB). The rest 16 patients had non-tuberculosis etiology [malignancy (n = 5), liver failure (n = 5), parapneumonic (n = 1), cardiac failure (n = 2) and renal failure (n = 2), secondary infection (n = 1)] and hence were grouped as non-tuberculosis control group (NTB). The study patients were sero negative for HIV. A written and informed consent was obtained from each patient. The collection of the samples and the study followed the ethical guidelines of GGH, Chennai. The blood and the PF samples collected for diagnostics and therapeutic purpose were utilized for the study.
The diagnosis for tuberculosis was based on the smear, culture and polymerase chain reaction (PCR) positivity of the sputum or the chest X-ray. These patients showed positivity in at least any two of the above criteria and hence were categorized as TB groups. All the patients were first time diagnosed as TB and were not relapsed cases. They responded well to anti tuberculous treatment (ATT) and were followed for three months. In NTB group, patients with clinical evidence of heart failure, liver cirrhosis and renal failure had transudative effusions whereas malignant patients had exudative effusions. The samples were collected before the start of the treatment.
2.2. Collection of blood and pleural fluid
The blood samples were collected by venipuncture and the pleural fluids samples obtained via thoracentesis. The collected pleural fluid and blood were immediately processed to separate the cell free plasma and pleural fluids which were subsequently stored at −70 °C until used for other assays.
2.3. Multiplex assay system
An automated fluorescent microsphere-based multiplex immunoassay to detect multiple proteins in a 96-well microplate format was used. Matrix metalloproteinases (MMP-1, MMP-7, MMP-8 and MMP-9), tissue inhibitors of metalloproteinases (TIMP-1, TIMP-2, TIMP-3, and TIMP-4) were estimated in plasma and pleural fluids by commercially available Multiplex ELISA kits (R&D systems, Minneapolis, MN, USA). Cytokines IFN-γ, TNF-α, IL-4 and IL-6 were estimated using commercially available Bioplex multiplex cytokine assay system (Biorad, Hercules, CA).
All assays were performed according to protocols specified by the manufacturers. The lowest detection limit for the various analytes were MMP-1: 9.33 pg/ml, MMP-7: 125.51 pg/ml, MMP-8: 109 pg/ml, MMP-9: 65.71 pg/ml, TIMP-1: 13.31 pg/ml, TIMP-2: 43.90 pg/ml, TIMP-3: 137.71 pg/ml, TIMP-4: 6.58 pg/ml, TNF-α: 4.89 pg/ml, IFN-γ: 2.14 pg/ml, IL-4: 0.3 pg/ml and IL-6: 2.31 pg/ml.
2.4. Statistical analysis
All statistical analyses were performed using Graph pad prism software (Version 5.0 for Windows; Graphpad software, Inc., San Diego, CA, USA). Significance between paired samples was compared using Wilcoxon matched pair test; between TB and NTB groups using Mann–Whitney test. P values, less than 0.05 were considered significant.
3. Results
We looked into the levels of different MMPs, TIMPs and cytokines in plasma (BL) and pleural fluids (PF) of TB and NTB patients. A comparative analysis of BL versus PF and TB versus NTB was done. The data are represented in ng/ml for MMPs and TIMPs; in pg/ml and ng/ml for cytokines concentration.
3.1. MMP levels in plasma and pleural fluids of tuberculosis and nontuberculosis patients
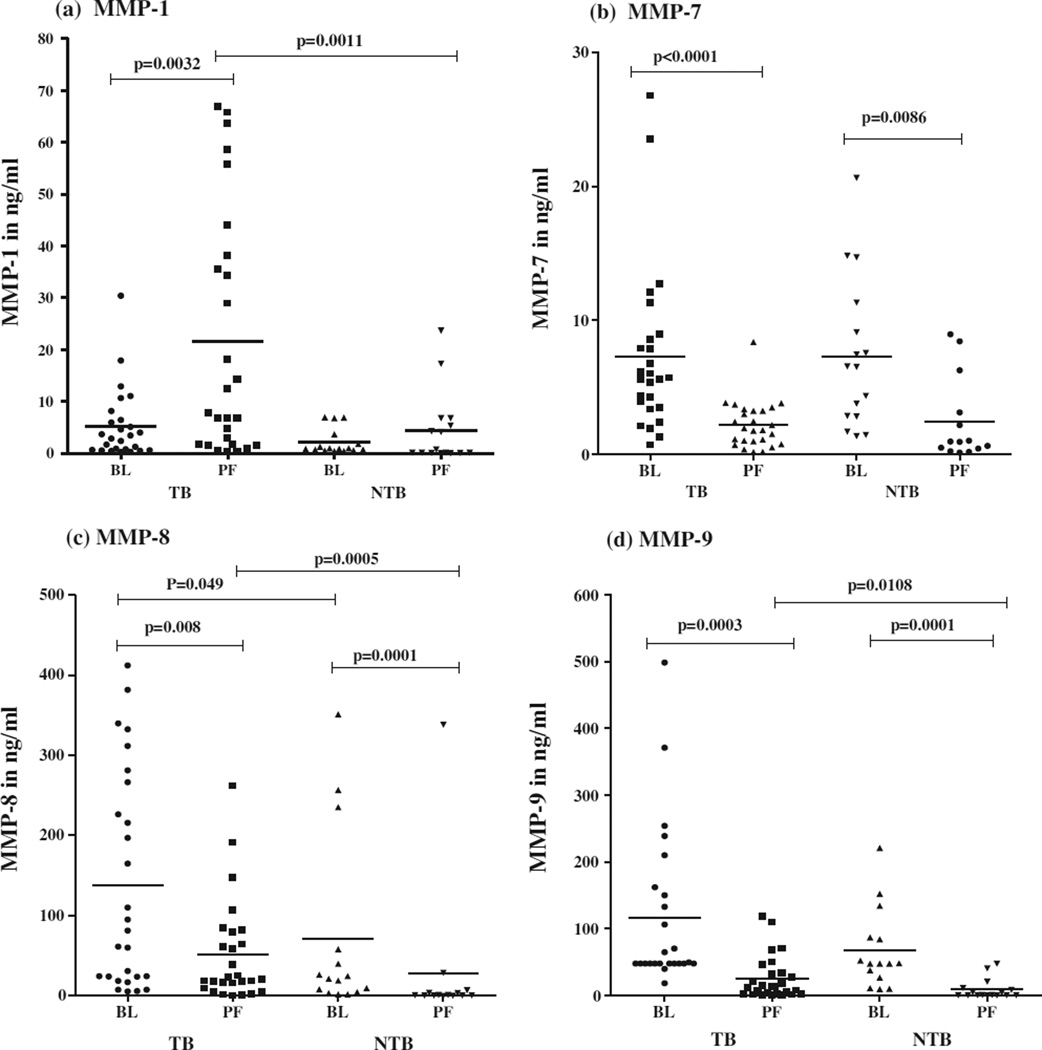
As represented in Fig 1(a), the levels of MMP-1 were significantly higher (p = 0.0032) in PF compared to BL in TB group. No significant difference was observed between BL and PF of NTB group. A comparison of MMP-1 in PF between two groups again showed significantly higher level (p = 0.0011) in TB PF, such difference was not observed in MMP-1 levels in BL between these two groups.
Fig. 1.
In vivo levels of MMPs in plasma (BL) and pleural fluid (PF) of 28 tuberculous pleuritis patients and 16 non-tuberculous pleuritis patients. Horizontal line represents mean value. P values are represented.
As shown in Fig 1(b), MMP-7 levels were significantly higher in BL compared to PF in both TB (p = 0.0001) and NTB (p = 0.0086) groups. No significance was observed in MMP-7 levels of either BL or PF when compared between two groups.
Figure 1(c) represents MMP-8 levels in BL and PF of two groups. The MMP-8 levels were significantly higher in BL of both TB (p = 0.008) and NTB (p = 0.0001) compared to their respective PF levels. The comparison of MMP-8 levels in BL and PF between two groups showed significantly higher levels (p = 0.049, 0.0005) in TB compared to NTB.
The comparison of MMP-9 levels in TB and NTB groups is represented in Fig. 1(d). The results showed a significantly higher MMP-9 levels in BL compared to PF of both TB (p = 0.0003) and NTB (p = 0.0001) groups. The comparison of MMP-9 levels of PF between two groups showed significantly higher levels only in TB PF (p = 0.0108). There was no such difference in the levels of MMP-9 in BL between these two groups.
3.2. TIMP levels in plasma and pleural fluids of tuberculosis and non-tuberculosis patients
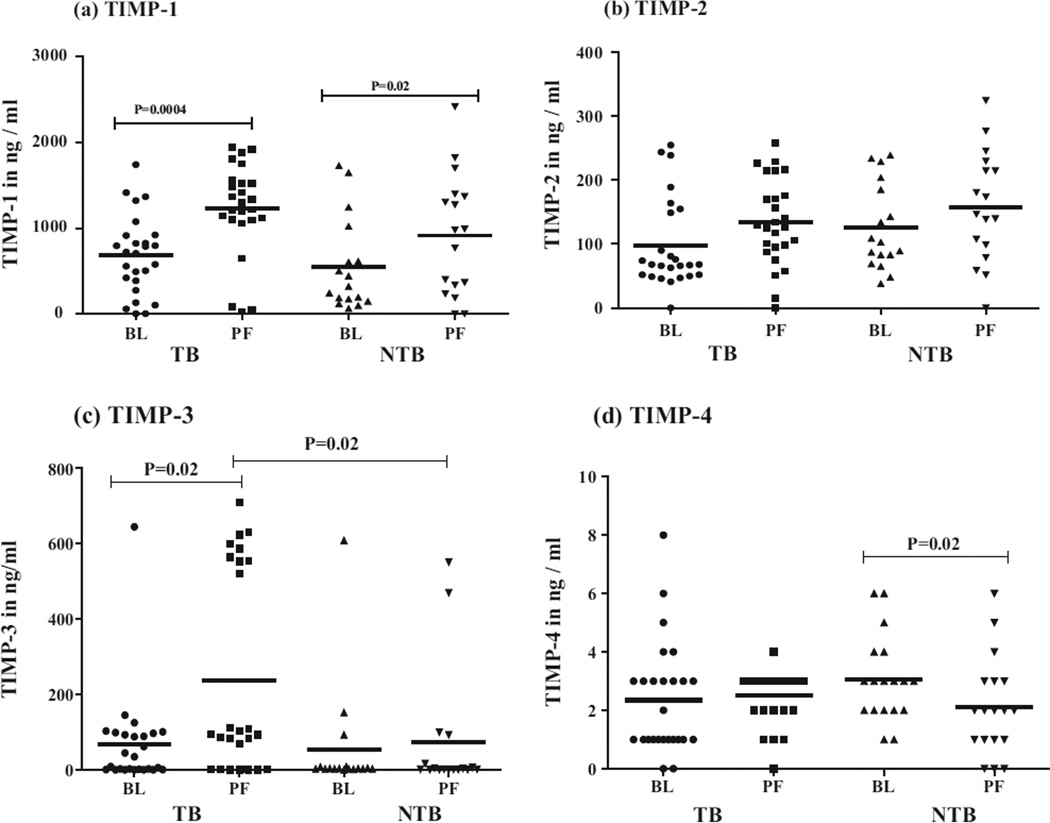
The levels of TIMP-1 are represented in Fig. 2(a). The TIMP-1 levels were significantly higher in PF compared to BL in both TB (P = 0.0004) and NTB (P = 0.02) groups. No significance was observed in TIMP-1 levels of either BL or PF between the two groups.
Fig. 2.
In vivo levels of TIMPs in plasma (BL) and pleural fluid (PF) of 28 tuberculous pleuritis patients and 16 non-tuberculous pleuritis patients. Horizontal line represents mean value. P values are represented.
Fig. 2(b) represents the TIMP-2 levels in BL and PF of two groups. The TIMP-2 levels were higher in PF than BL in both TB and NTB groups but were not statistically significant. Similarly, no significance was observed in TIMP-2 levels either in BL or PF when compared between TB and NTB groups.
As shown in Fig. 2(c), the TIMP-3 level in PF was significantly higher (P = 0.02) compared to BL in TB group. When TIMP-3 levels of PF between two groups were compared, significantly higher levels (P = 0.02) were observed in TB group. The levels of TIMP-4 were significantly higher (P = 0.02) in BL than PF only in NTB group, such significance was not observed in TB group (Fig. 2(d)).
3.3. In vivo levels of pro-inflammatory cytokines in tuberculosis and non-tuberculosis patients
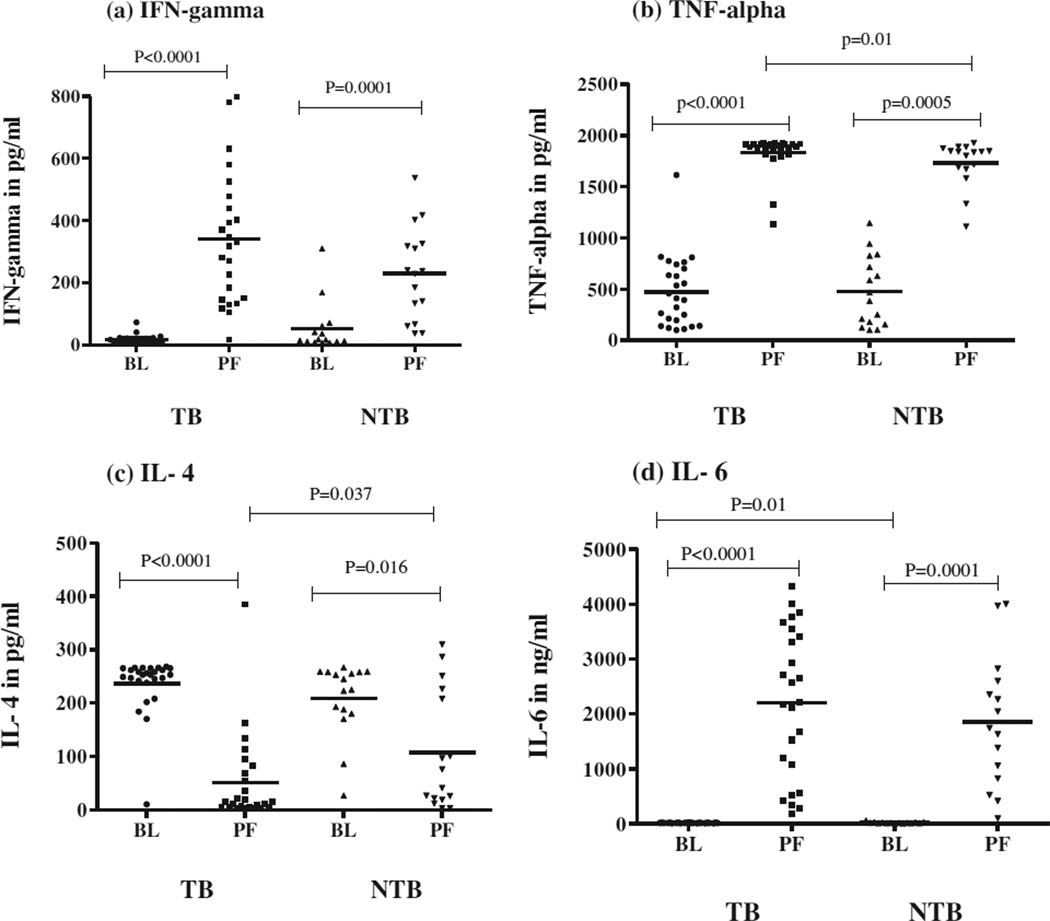
As shown in Fig 3, pro-inflammatory cytokines, IFN-γ (a), TNF-α (b) and IL-6 (d) were significantly higher in PF compared to BL in both TB and NTB groups. However, IL-4 levels were significantly lower in PF of both the groups, more so in TB PF (c).
Fig. 3.
In vivo levels of pro-inflammatory cytokines in plasma (BL) and pleural fluid (PF) of 28 tuberculous pleuritis (TB) patients and 16 non-tuberculous pleuritis (NTB) patients. Horizontal line represents mean value. P values are represented.
3.4. Correlation of MMPs with pro-inflammatory cytokines in tuberculous and non tuberculous pleuritis patients
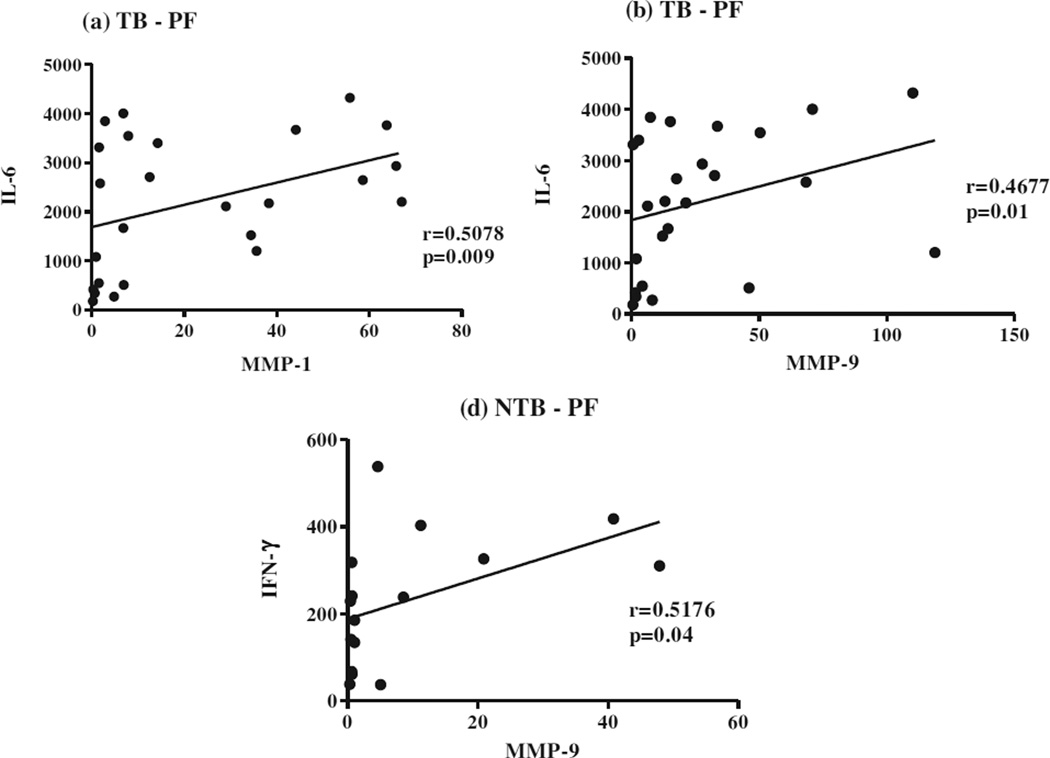
As shown in Fig 4, MMP-1 (a) and MMP-9 (b) showed significant positive correlation with IL-6 in TB PF (r = 0.5078, p = 0.009 and r = 0.4677, p = 0.01, respectively). Similarly, MMP-9 (c) had significant positive correlation with IFN-γ (r = 0.5176, p = 0.04) in NTB PF. MMP-7 and -8 did not show any correlation with any cytokines in PF of either TB or NTB group. No correlation was observed between MMPs and cytokines in BL of either group (data not shown).
Fig. 4.
Correlation of in vivo levels of MMP-1, -7 and -9 with pro-inflammatory cytokines (IFN-γ and IL-6) in pleural fluid (PF) from 28 tuberculous pleuritis patients (a and b) and 16 non-tuberculous pleuritis patients (c).
3.5. Correlation of MMPs with TIMPs in tuberculous pleuritis patients
As shown in Table 1a significant positive correlation of all MMPs with TIMP-4 in PF while MMP-1, -7 and -8 with TIMP-1 in BL was observed. A positive correlation was also observed in TB PF between MMP-7, -8, -9 and TIMP-3. Only MMP-8 and -9 showed positive correlation with TIMP-2 in PF and BL. However, no significant correlation of these two parameters was observed in BL or PF of NTB group (data not shown).
Table 1.
Correlation (r values) of MMPs with TIMPs in plasma (BL) and pleural fluid (PF) of 28 tuberculous pleuritis (TB) patients.
| Parameters | TIMP-1 | TIMP-2 | TIMP-3 | TIMP-4 | |
|---|---|---|---|---|---|
| MMP-1 | BL | 0.4144* | ns | 0.4553* | ns |
| PF | ns | ns | ns | 0.5439* | |
| MMP-7 | BL | 0.4094* | ns | ns | ns |
| PF | ns | ns | 0.5700* | 0.6603** | |
| MMP-8 | BL | 0.4274* | ns | ns | ns |
| PF | ns | 0.5386* | 0.4411* | 0.5340* | |
| MMP-9 | BL | ns | 0.5045* | ns | ns |
| PF | ns | ns | 0.6852** | 0.6838** |
BL, plasma; PF, pleural fluid; ns, not significant.
P < 0.05.
P < 0.001.
4. Discussion
The presence of metalloproteinases, their inhibitors and proinflammatory cytokines play an important role in integrity and remodeling of extra cellular matrix components in inflammatory conditions. In the present study, we measured the levels of MMPs -1, -7, -8, -9; TIMPs-1, -2, -3, -4 and pro-inflammatory cytokines (TNF-α, INF-γ, IL-4 and IL-6) in the plasma and pleural fluid samples of TB and NTB patients. Our aim was to compare these proteins under two conditions, one systemic versus localized and second TB versus non TB etiologies; hence we segregated the patients in two broader/polar groups of TB and NTB.
It is known that cleavage of matrix is original function of MMP-1 and it can drive lung matrix destruction without caseous necrosis. It is also reported that MTB infection alone can cause up regulation of MMP-1 at gene level followed by its excessive secretion [3]. Besides this, cytokines like TNF-α and IL-1β activate stromal cells to synthesis MMP-1. Moreover, activated macrophages being a good source of MMP-1 [12] when infected with MTB secrete large amount of MMP-1 in lungs of TB patients [13]. Thus MTB induced upregulation of MMP-1 and cytokines result in higher levels of MMP-1 in PF of TB compared to NTB patients as observed in this study. Our observation also correlated well with the previous work where high concentrations of MMP-1, -2, and TIMP-1, -2 in the PF of TB compared to coronary heart failure (CHF) were reported [10]. The increased levels of MMPs in PF compared to plasma also indicated their compartmentalization at the site of infection.
A soluble metalloproteinase MMP-7 (matrilysin) is profoundly secreted and found in airway epithelial cells. MMP-7 knock out mouse showed impaired wound healing and tissue remodeling [14]. TNF-α secreted by inflamed or wounded tissue may also affect the secretion of MMP-7 [15]. We observed significant increase in MMP-7 levels in BL compared to PF in both TB and NTB groups. The mechanism behind the increased plasma levels of MMP-7 under MTB infection needs further study.
Neutrophil collagenase or MMP-8 is predominantly secreted by neutrophilic granules at the inflammatory loci. Similar to our results, higher levels of MMP-8 have been reported in serum and plasma samples compared to fluids from the disease site in patients with periodontitis, acute coronary syndrome (ACS) and sepsis. Extracellular matrix degradation leading to the vulnerability of lesions is said to be the reason for this [16–18]. Similarly, in TB pleuritis, one can argue in favor of degrading action of MMP-8 causing instability of pleura which may further lead to the leakage of MMP-8 into the systemic circulation.
MMP-9 is the most widely studied of all metalloproteinases in almost all disease conditions. It degrades type 4 collagen which is one of the main constituents of basement membrane. MMP-9 is a double-edged sword. In TB, it plays an important role in granuloma formation and containing the infection with recruitment of macrophages into the granuloma [4, 7]. But when secreted in excess it leads to severity of the disease helping in the dissemination of MTB [19]. An increased infiltration of the immune cells like monocytes/macrophages [4, 20], mesothelial cells [8] and neutrophils [20] into the site of infection is said to increase the production of MMP-9. Earlier studies have reported compartmentalization of MMP-8 and -9 at the site of active TB disease [10]. Many researchers have also studied the expression of MMP-8 and -9 in pleural effusions of different origin [8] and have reported its increased levels in PF of emphysema [11]. In another study, increased expression of MMP-9 was found in TB PF compared to lung cancer [5]. In this study also, significantly increased levels of MMP-8 and -9 were observed in PF of TB compared to NTB patients. In our previous work, we have assessed functional activity of Proand active forms of MMPs by zymography and showed increase in MMP-9 activity in both BL and PF of TB compared to NTB (data unpublished). This further extends support to our current observation.
We also observed significantly higher levels of MMP-9 in BL of both the groups. Not many studies have reported MMP-9 levels in circulation. Only study, where authors have reported an increased serum MMP-9 in TB patients compared to normal subjects and attributed the cause as augmentation of synthesis and/or secretion of this enzyme by inflammatory cells in response to MTB infection [20]. In another study, the levels of MMP-9 were compared in serum and bronchial lavage fluid (BLF) obtained from patients with lung cancer and nonmalignant lung disease. A significantly increased serum level of MMP-9 was found only in the lung cancer patients and no such significance was observed when MMP-9 levels were compared in BLF of two groups [21]. This increase in serum MMP-9 levels was attributed to disease condition in above mentioned studies. Contrary to this, it is also reported that MMP-9 measurements in serum may reflect release of proteases from leucocytes during clotting in the blood tube and may not be reflective of the disease process at all [22]. However, in our study, we have measured MMP-9 in blood plasma and not in serum and hence the increase in MMP-9 levels is truly related to disease condition and not to clotting or sample processing effect.
All the four isoforms of tissue inhibitor proteins (TIMP-1, -2, -3 and -4) inhibit active forms of all metalloproteinases [23]. Apart from inhibition function, TIMPs also have other actions in tumor progression [24], tumor regression [25] and varied action in programmed cell death [26]. It has been suggested in earlier studies that constitutive expression of TIMP-1 and -2 in the infectious pleural fluid may be due to their expression by resident mesothelial cells triggered by inflammatory mediators [11]. In this study, the increased levels of TIMP-1 and -3 in TB PF indicated its increased secretion at the site of active disease in response to increased pro-inflammatory cytokines and MMP production. These results indicate that TIMPs along with MMPs are compartmentalized at the site of infection due to their increased production and secretion by local cells or their passive diffusion from blood into the pleural space [10].
Further, correlation analysis of these parameters in TB pleuritis showed that TIMP-1 positively correlated with MMP-1, -7, -8 in BL but not in PF. On the contrary, TIMP-4 correlated with all MMPs in PF but not in BL. A positive correlation was also observed between TIMP-3 and MMP-7, -8, -9 in PF. However, TIMP-2 showed positive correlation only with MMP-8 in PF. These results suggest that TIMP-3 and -4 may play major role in inhibiting the degrading action of metalloproteinases at the site of infection.
In immune cells, pro-inflammatory cytokines like TNF-α and IFN-γ influence the secretion of MMPs [27]. In our earlier reports and this study, we have shown compartmentalization of proinflammatory cytokines TNF-α and IFN-γ in the pleural space of TB pleuritis patients [28–30] indicating their role in activating local cells for the production of MMPs and TIMPs. In inflammation, IL-6 is an important regulatory factor often produced by inflammatory cells present in the pleural effusion and down regulates the production of TNF-α [31].
We observed a significant increase in IL-6 in PF of both the groups but its positive correlation with MMP-1 and -9 only in TB group suggest its protective nature against the excessive damage due to catabolic effects of TNF-α [32]. On the other hand, positive correlation of IFN-γ with MMP-9 in NTB PF may indicate its active role in pathology of various non tuberculous diseases.
As observed earlier, decreased levels of IL-4, an anti-inflammatory cytokine in the PF of both the groups indicated its antagonistic activity against other pro-inflammatory cytokines. Together, these results confirm that cytokines and cellular components of mycobacteria induce the production of MMPs and TIMPs in the pleural effusion.
Extensive studies on the levels of MMPs in the systemic circulation have been done in various cancers [21, 33] and sepsis conditions [34, 35] but not in TB pleuritis. Moreover, the systemic levels of MMPs have been seldom compared with the localized levels in infectious diseases like TB. In this study, we compared the in vivo levels of these proteins in plasma and pleural fluid. The striking observation was increased plasma levels of MMP-7, -8 and -9 compared to PF in both the groups, more pronounced in TB. It is known that MTB as a whole or its cell wall components lipoarabinomannan (LAM) act as a potent activator of MMPs. Also it causes tissue destruction by stimulating macrophages to release cytokines like TNF-α and IFN-γ which in turn induces the fibroblast to release MMPs [19, 36]. In vitro studies have shown that macrophages stimulated with BCG and MTB expressed certain MMPs [19]. Thus MTB specific components or general inflammatory response might be the contributing factors for the observed increase in the systemic levels of these MMPs.
The collection of PF samples from the site of infection requires invasive procedures which are sometimes difficult and risky with respect to the location. Under such circumstances, blood acts as a good and potential indicator for various maladies. Hence studying MMPs along with TIMPs in blood plasma may prove useful in predicting the disease and will help in avoiding invasive procedures. Further, comparing their plasma levels with the other fluids, if available from the site of infection will help in understanding pathophysiology of the disease. In different disease conditions like periodontitis, acute coronary syndrome and sepsis, MMPs and TIMPs were identified as biomarkers with practical implications in both diagnostics and therapeutics [16–18]. In conclusion, increased levels of MMP-1, -8 and -9 in TB PF may help to confirm the diagnosis of TB pleuritis where the gold standard of microscopy and culture fails. In addition, increased MMP-7, -8 and -9 in TB plasma may act as an alarm in conjunction with other markers like TIMPs and proinflammatory cytokines for the confirmed diagnosis of TB pleuritis.
Acknowledgments
This work received partial support from the National Institutes of Health through NIAID/TRC/ICER program. Swetha Sundararajan thanks DBT for providing financial support to carry out this research. We extend our gratitude to all the patients whose samples formed base for this study. The technical help and useful tips from Ph.D. student (ICER) C. Anuradha is greatly acknowledged.
Abbreviations
- TB
tuberculosis
- MTB
Mycobacterium tuberculosis
- MMP
Matrix metalloproteinases
- TNF-α
tumor necrosis factor alpha
- IFN-γ
interferon gamma
- TIMP
tissue inhibitor of metalloproteinases
- NTB
non tuberculosis
- BL
blood plasma
- PF
pleural fluid
- BLF
bronchial lavage fluid
- ATT
anti tuberculous treatment
- BCG
Bacillus Calmette–Guerin
References
- 1.Berger HW, Mejia E. Tuberculous pleurisy. Chest. 1973;63:88–92. doi: 10.1378/chest.63.1.88. [DOI] [PubMed] [Google Scholar]
- 2.Gopi A, Madhavan SM, Sharma SK, Sahn SA. Diagnosis of treatment of tuberculous pleural effusion. Chest. 2007;131:880–889. doi: 10.1378/chest.06-2063. [DOI] [PubMed] [Google Scholar]
- 3.Elkington P, Shiomi T, Breen R, Nuttal R, Ugarte-Gil CA, Walker N, et al. MMP-1 drives immunopathology in human tuberculosis and transgenic mice. J Clin Invest. 2011;121:1827–1833. doi: 10.1172/JCI45666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sheen P, O’Kane CM, Chaudhary K, Tovar M, Santillan C, Sosa J, et al. High MMP-9 activity characterizes pleural tuberculosis correlating with granuloma formation. Eur Respir J. 2009;33:134–141. doi: 10.1183/09031936.00127807. [DOI] [PubMed] [Google Scholar]
- 5.Park KJ, Hwang SC, Sheen SS, Oh YJ, Han JH, Lee KB. Expression of matrix metalloproteinase-9 in pleural effusions of tuberculosis and lung cancer. Respiration. 2005;72:166–175. doi: 10.1159/000084048. [DOI] [PubMed] [Google Scholar]
- 6.Lin FC, Chen YC, Chen FJ, Chang SC. Cytokines and fibrinolytic enzymes in tuberculous and parapneumonic effusions. Clin Immunol. 2005;116:166–173. doi: 10.1016/j.clim.2005.03.015. [DOI] [PubMed] [Google Scholar]
- 7.Taylor JL, Hattle JM, Dreitz SA, Troudt JM, Izzo LS, Basaraba RJ, et al. Role for matrix metalloproteinase 9 in granuloma formation during pulmonary Mycobacterium tuberculosis infection. Infect Immun. 2006;74:6135–6144. doi: 10.1128/IAI.02048-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eickelberg O, Sommerfeld CO, Wyser C, Tamm M, Reichenberger F, Bardin PG, et al. MMP and TIMP expression pattern in pleural effusions of different origins. Am J Respir Crit Care Med. 1997;156:1987–1992. doi: 10.1164/ajrccm.156.6.9704112. [DOI] [PubMed] [Google Scholar]
- 9.Anand SP, Selvaraj P. Effect of 1, 25 dihydroxyvitamin D(3) on matrix metalloproteinases MMP-7, MMP-9 and the inhibitor TIMP-1 in pulmonary tuberculosis. Clin Immunol. 2009;133:126–131. doi: 10.1016/j.clim.2009.06.009. [DOI] [PubMed] [Google Scholar]
- 10.Hoheisel G, Sack U, Hui DS, Huse K, Chan KS, Chan KK, et al. Occurrence of matrix metalloproteinases and tissue inhibitors of metalloproteinases in tuberculous pleuritis. Tuberculosis. 2001;81:203–209. doi: 10.1054/tube.2000.0276. [DOI] [PubMed] [Google Scholar]
- 11.Iglesias D, Alegre J, Alemán C, Ruíz E, Soriano T, Armadans LI, et al. Metalloproteinases and tissue inhibitors of metalloproteinases in exudative pleural effusions. Eur Respir J. 2005;25:104–109. doi: 10.1183/09031936.04.00010504. [DOI] [PubMed] [Google Scholar]
- 12.Ma C, Tarnuzzer RW, Chengini N. Expression of matrix metalloproteinases and tissue inhibitor of matrix metalloproteinases in mesothelial cells and their regulation by transforming growth factor-β1. Wound Repair Regen. 1999;7:477–485. doi: 10.1046/j.1524-475x.1999.00477.x. [DOI] [PubMed] [Google Scholar]
- 13.Elkington PTG, Nuttall RK, Boyle JJ, O’Kane CM, Horncastle DE, Edwards DR, et al. Mycobacterium tuberculosis, but not vaccine bcg, specifically upregulates Matrix metalloproteinase-1. Am J Respir Crit Care Med. 2005;172:1596–1604. doi: 10.1164/rccm.200505-753OC. [DOI] [PubMed] [Google Scholar]
- 14.Dunsmore SE, Saarialho-Kere UK, Roby JD, Wilson CL, Matrisian LM, Welgus HG, Parks WC. Matrilysin expression and function in airway epithelium. J Clin Invest. 1998;102:1321–1331. doi: 10.1172/JCI1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lopez- Boado YS, Wilson CL, Parks WC. Regulation of Matrilysin expression in airway epithelial cells byPseudomonas aeruginosaflagellin. J Biol Chem. 2001;276:41417–41423. doi: 10.1074/jbc.M107121200. [DOI] [PubMed] [Google Scholar]
- 16.Sorsa T, Tervahartiala T, Leppilahti J, Hernandez M, Gamonal J, Tuomainen AM, et al. Collagenase-2 (MMP-8) as a point-of-care biomarker in periodontitis and cardiovascular diseases. Therapeutic response to non-antimicrobial properties of tetracyclines. Pharmacol Res. 2011;63:108–113. doi: 10.1016/j.phrs.2010.10.005. [DOI] [PubMed] [Google Scholar]
- 17.Pussinen PJ, Sarna S, Puolakkainen M, Ohlin H, Sorsa T, Pesonen E. The balance of serum matrix metalloproteinase-8 and its tissue inhibitor in acute coronary syndrome and its recurrence. Int J Cardiol. 2012 doi: 10.1016/j.ijcard.2011.12.095. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 18.Lauhio A, Hästbacka J, Pettilä V, Tervahartiala T, Karlsson S, Varpula T, et al. Serum MMP-8, -9 and TIMP-1 in sepsis: high serum levels of MMP-8 and TIMP-1 are associated with fatal outcome in a multicentre, prospective cohort study. Hypothetical impact of tetracyclines. Pharmacol Res. 2011;64:590–594. doi: 10.1016/j.phrs.2011.06.019. [DOI] [PubMed] [Google Scholar]
- 19.Quiding-Järbrink M, Smith DA, Bancroft GJ. Production of matrix metalloproteinases in response to mycobacterial infection. Infect Immun. 2001;69:5661–5670. doi: 10.1128/IAI.69.9.5661-5670.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hrabec E, Strek M, Zieba M, Kwiatkowska S, Hrabec Z. Circulation level of matrix metalloproteinase-9 is correlated with disease severity in tuberculosis patients. Int J Tuberc Lung Dis. 2002;6:713–719. [PubMed] [Google Scholar]
- 21.Koç M, Ediger D, Budak F, Karadağ M, Oral HB, Uzaslan E, et al. Matrix metalloproteinase-9 (MMP-9) elevated in serum but not in bronchial lavage fluid in patients with lung cancer. Tumori. 2006;92:149–154. doi: 10.1177/030089160609200211. [DOI] [PubMed] [Google Scholar]
- 22.Zucker S, Cao J. Measurement of Matrix metalloproteinases in serumof patients with melanoma: snarled intechnical pitfalls. Commentary on Nikkola et al., p. 5158. Clin Cancer Res. 2005;11:5069–5070. doi: 10.1158/1078-0432.CCR-05-0774. [DOI] [PubMed] [Google Scholar]
- 23.Baker AH, Edwards DR, Murphy G. Metalloproteinase inhibitors: biological actions and therapeutic opportunities. J Cell Sci. 2002;115:3719–3727. doi: 10.1242/jcs.00063. [DOI] [PubMed] [Google Scholar]
- 24.Jiang Y, Wang M, Celiker M, Liu Y, Sang Q, Goldberg I, et al. Stimulation of mammary tumorigenesis by systemic inhibitor of matrix metalloproteinase 4 gene delivery. Cancer Res. 2001;61:2365–2370. [PubMed] [Google Scholar]
- 25.Baker AH, George SJ, Zaltsman AB, Murphy G, Newby AC. Inhibition of invasion and induction of apoptotic cell death of cancer cell lines by overexpression of TIMP-3. Br J Cancer. 1999;79:1347–1355. doi: 10.1038/sj.bjc.6690217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Smith MR, Kung HF, Durum SK, Colburn NH, Sun Y. TIMP-3 induces cell death by stabilizing TNF-alpha receptors on the surface of human colon carcinoma cells. Cytokine. 1997;9:770–778. doi: 10.1006/cyto.1997.0233. [DOI] [PubMed] [Google Scholar]
- 27.Sarén P, Welgus HG, Kovanen PT. TNF-alpha and IL-1beta selectively induce expression of 92-kDa gelatinase by human macrophages. J Immunol. 1996;157:4159–4165. [PubMed] [Google Scholar]
- 28.Jalapathy KV, Prabha C, Das SD. Correlates of protective immune response in tuberculous pleuritis. FEMS Immunol Med Microbiol. 2004;40:139–145. doi: 10.1016/S0928-8244(03)00303-1. [DOI] [PubMed] [Google Scholar]
- 29.Rajavelu P, Pokkali S, Bhatt K, Narayanan PR, Salgame P, Das SD. Comparative evaluation of cytokines, T-cell apoptosis, and costimulatory molecule expression in tuberculous and nontuberculous pleurisy. Clin Transl Sci. 2008;1:209–214. doi: 10.1111/j.1752-8062.2008.00057.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Prabha C, Jalapathy KV, Matsa RP, Das SD. Differential T helper cell response in tuberculous pleuritis. Indian J Med Microbiol. 2007;25:3–18. doi: 10.4103/0255-0857.31056. [DOI] [PubMed] [Google Scholar]
- 31.Koshimoto T, Akira S, Taga T. Interleukin-6 and its receptor: a paradigm for cytokines. Science. 1992;258:22–26. doi: 10.1126/science.1411569. [DOI] [PubMed] [Google Scholar]
- 32.Lotz M, Guerne PA. Interleukin-6 induces the synthesis of tissue inhibitor metalloproteinases-1/erythroid potentiating activity (TIMP-1/EPA) J Biol Chem. 1991;266:2017–2019. [PubMed] [Google Scholar]
- 33.Łukaszewicz-Zajac M, Mroczko B, Kozłowski M, Nikliński J, Laudański J, Szmitkowski M. Elevated levels of serum metalloproteinase 9 in patients with esophageal squamous cell carcinoma. Pol Arch Med Wewn. 2009;119:558–563. [PubMed] [Google Scholar]
- 34.Hoffmann U, Bertsch T, Dvortsak E, Liebetrau C, Lang S, Liebe V, Huhle G, Borggrefe M, Brueckmann M. Matrix-metalloproteinases and their inhibitors are elevated in severe sepsis: prognostic value of TIMP-1 in severe sepsis. Scand J Infect Dis. 2006;38:867–872. doi: 10.1080/00365540600702058. [DOI] [PubMed] [Google Scholar]
- 35.Gäddnäs FP, Sutinen MM, Koskela M, Tervahartiala T, Sorsa T, Salo TA, Laurila JJ, Koivukangas V, Ala-Kokko TI, Oikarinen A. Matrix-metalloproteinase-2, -8 and -9 in serum and skin blister fluid in patients with severe sepsis. Crit Care. 2010;14:R49. doi: 10.1186/cc8938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chang C, Wysocki A, Tchou-Wong KM, Moskowitz N, Zhang Y, Rom WN. Effect of Mycobacterium tuberculosis and its components on macrophages and the release of matrix metalloproteinases. Thorax. 1996;51:306–311. doi: 10.1136/thx.51.3.306. [DOI] [PMC free article] [PubMed] [Google Scholar]