Abstract
Vitamin D is a potent stimulator of monocyte innate immunity, with this effect being mediated via intracrine conversion of 25-hydroxyvitamin D (25OHD) to 1,25-dihydroxyvitamin D (1,25(OH)2D). In the kidney synthesis of 1,25(OH)2D is suppressed by fibroblast growth factor 23 (FGF23), via transcriptional suppression of the vitamin D-activating enzyme 1α-hydroxylase (CYP27B1). We hypothesized that FGF23 also suppresses CYP27B1 in monocytes, with concomitant effects on intracrine responses to 1,25(OH)2D. Monocytes from healthy donor peripheral blood mononuclear cells (PBMCm) and from peritoneal dialysate effluent from kidney disease patients (PDm) were assessed at baseline to confirm the presence of mRNA for FGF23 receptors (FGFRs), with Klotho and FGFR1 being more strongly expressed than FGFR2/3/4 in both cell types. Immunohistochemistry showed co-expression of Klotho and FGFR1 in PBMCm and PDm, with this effect being enhanced following treatment with FGF23 in PBMCm but not PDm. Treatment with FGF23 activated MAP kinase (MAPK) and Akt pathways in PBMCm, demonstrating functional FGFR signaling in these cells. FGF23 treatment of PBMCm and PDm decreased expression of mRNA for CYP27B1. In PBMCm this was associated with downregulation of 25OHD to 1,25(OH)2D metabolism, and concomitant suppression of intracrine induced 24-hydroxylase (CYP24A1) and antibacterial cathelicidin (LL37). FGF23 suppression of CYP27B1 was particularly pronounced in PBMCm treated with interleukin-15 to stimulate synthesis of 1,25(OH)2D. These data indicate that FGF23 can inhibit extra-renal expression of CYP27B1 and subsequent intracrine responses to 1,25(OH)2D in two different human monocyte models. Elevated expression of FGF23 may therefore play a crucial role in defining immune responses to vitamin D and this, in turn, may be a key determinant of infection in patients with CKD.
Keywords: monocytes, vitamin D, innate immunity, FGF23, peritoneal dialysis
Introduction
Fibroblast Growth Factor 23 (FGF23) is a protein synthesized by osteocytes and osteoblasts that plays a key role in the ‘bone-parathyroid-kidney’ axis and the regulation of phosphate/calcium/vitamin D metabolism (1–3). FGF23 acts mainly as a phosphaturic factor, inhibiting the expression of type IIa sodium-phosphate co-transporters on the apical membrane of proximal tubular cells, thus leading to inhibition of phosphate reabsorption (4). However, FGF23 also suppresses renal synthesis of the active form of vitamin D, 1,25-dihydroxyvitamin D (1,25(OH)2D) by inhibiting expression of the enzyme 25-hydroxyvitamin D-1α-hydroxylase (CYP27B1) whilst stimulating the catabolic enzyme vitamin D-24-hydroxylase (CYP24A1)(4). FGF23 null mice present with a premature aging phenotype as well as severe growth retardation, abnormal skeletogenesis, vascular and soft tissue calcification, hyperphosphatemia, and increased renal expression of CYP27B1 (5). The importance of CYP27B1 as a target for FGF23 is further illustrated by the abrogation of FGF23-knockout skeletal and biochemical abnormalities in FGF23/CYP27B1 double knockout mice (6,7).
In addition to its effects on mineral and skeletal homeostasis, vitamin D can also act as a potent modulator of immune function (8,9). In particular, active 1,25(OH)2D can enhance innate immune handling of bacteria via the induction of antibacterial proteins such as cathelicidin (LL37) and β-defensin 2 (10,11). Cells such as monocytes expressing both CYP27B1 and the nuclear vitamin D receptor (VDR) are able to achieve this by localized conversion of 25OHD to 1,25(OH)2D and subsequent VDR-mediated transactivation of antibacterial proteins (12,13). This intracrine mode of action is enhanced by a variety of immunomodulatory factors, notably following the recognition of pathogen-associated molecular patterns by pattern-recognition receptors (12,13). Monocyte synthesis of 1,25(OH)2D and associated induction of antibacterial activity can also be enhanced by cytokines such as interleukin-15 (IL-15) (14), and interferon γ (IFNγ) (15). Conversely, other cytokines such as interleukin-4 (IL-4) suppress monocyte accumulation of 1,25(OH)2D by enhancing its catabolism via the enzyme 24-hydroxylase (CYP24A1), and thereby inhibit intracrine induction of antibacterial activity in these cells (15). These observations highlight the pivotal importance of vitamin D metabolism in mediating innate immune responses to infection, and underline the potential immune impact of factors that are able to regulate this metabolism. In the current study, we sought to determine whether FGF23 can contribute to this immune regulation of vitamin D metabolism.
In humans, aberrant expression of FGF23 was initially linked to hypophosphatemic rickets (16), but current data suggest that this is far more complex, and includes a role for FGF23 in oncogenic osteomalacia (17). In chronic kidney disease (CKD), elevated serum levels of FGF23 are detectable at early stages of the disease, prior to dysregulation of serum phosphate and parathyroid hormone (PTH) (18,19). In this setting, FGF23 levels have a prognostic value with higher levels of serum FGF23 being associated with poorer health outcomes (20–23). Although this appears to be due, at least in part, to vascular and atherogenic effects of FGF23 (24,25), infection is also a key cause of mortality in CKD patients. Aside from the increased risk of infection due to immunosuppressive therapies in CKD patients undergoing kidney transplantation (26), CKD itself is a state of acquired immune deficiency (27). The incidence of bacterial infections in CKD dialysis patients is higher than in the general population (28,29) and infections are the second leading cause of death in such patients. The immune dysfunction observed in CKD patients, such as impaired polymorphonuclear cell apoptosis (30), has been attributed to many different factors including iron overload, uremic toxins, and dialysis (27). To date, there have been no published studies of FGF23 and immune function, although some reports of FGF23-null mice have described atrophy of the thymus, spleen and lymph nodes, and decreased capacity for T cell proliferation (6). In the current study we have explored a possible role for FGF23 as a mediator of immune dysfunction via its effects on vitamin D metabolism in monocytes.
Materials and Methods
Isolation and initial treatment of peripheral blood mononuclear cells from healthy donors
Ficoll-isolated peripheral blood mononuclear cells (PBMCs) derived from anonymous healthy donors (screened in accordance with standard transfusion medicine protocols) were obtained from the Center for AIDS Research Virology Core/BSL3 Facility (supported by National Institutes of Health award AI-28697 and by the UCLA AIDS Institute and the UCLA Council of Bioscience Resources). PBMC monocytes (PBMCm) were enriched by adherence by incubating 5×106 PBMCs per well in 12-well plates for 2 hours in RPMI (Invitrogen, Carlsbad, CA) with 1% human serum (HS, Human AB serum, Omega Scientific, Tarzana, CA, USA).
Isolation of monocytes from peritoneal dialysate effluents by iterative centrifugation
Samples of overnight dwell dialysates were collected from patients undergoing peritoneal dialysis as approved by the UCLA Human Subject Protection Committee, with consent forms being obtained from all parents/patients. Dialysate samples were decanted into 500 ml sterile centrifuge tubes, and then centrifuged at 1200 G for 10 mins at room temperature. Supernatants were discarded and cell pellets were combined and recentrifuged using the same parameters. After again discarding supernatants, viable cells were counted by haemocytometer following staining with Trypan blue. After a third centrifugation the remaining cells were used in vitro for isolation of peritoneal dialysate monocytes (PDm). PDm were selected by adherence by incubating 5×106 PD cells per well in 12-well plates for 12 hrs in RPMI (Invitrogen, Invitrogen, Carlsbad, CA) with 1% HS. All samples were obtained from patients with no evidence of peritonitis.
Culture and treatment of PBMCm and PDm
PBMCm and PDm were cultured at 37oC in 5% CO2 in 12-well plastic cell culture plates using medium containing RPMI 1640, 10% HS and granulocyte-macrophage colony stimulating factor (GM-CSF) 10 IU/ml (PeproTechInc, Rocky Hood, NJ, USA) for various time periods (6 or 24 hours). Treatments were carried out using recombinant human FGF23 (100 ng/ml, 2604-FG, R&D systems, Minneapolis, MN, USA), recombinant human IL-15 (200 ng/ml)(247-IL, R&D systems) or both in comparison to vehicle-treated cells (PBS 1x). The pan fibroblast growth factor receptor (FGFR) inhibitor PD173074 (250 nM) (Sigma Aldrich, St Louis, MO, USA) was used 1hr before treating cells with FGF23, with control cells receiving DMSO as vehicle. At the end of incubation periods cells were either lysed with RNAzol and frozen at −80 °C or processed for Western blot analyses. All culture conditions were carried out in duplicate for RT-PCR analyses.
Extraction of RNA and reverse transcription
After lysing cells PBMCm or PDm with RNAzol, RNA was extracted using choloroform, isopropanol, ethanol and glycogen, as described previously (13). After resuspending the resulting RNA in RNase-free water, aliquots (300ng) were reverse-transcribed as recommended by the manufacturer (SuperScript III Reverse Transcriptase, Invitrogen, Carlsbad, CA) as previously described (13).
Quantitative real time RT-PCR amplification of cDNAs
Expression of mRNAs for VDR, CYP27B1, CYP24A1, LL37, fibroblast growth factor receptor (FGFR)1, FGFR2, FGFR3, FGFR4, Klotho and tumor necrosis factor α (TNF-α) was quantified using a Stratagene MX3005P device using PBMCm or PDm, as recommended by the manufacturer and as previously described (13). Approximately 7.5 ng of cDNA was used per reaction. All reactions were normalized by multiplex analysis with the housekeeping 18S rRNA gene (Applied Biosystems, Foster City, CA, USA). Data were obtained as Ct values, corresponding to the cycle number at which logarithmic PCR plots cross a calculated threshold line, and were further used to determine ΔCt values, corresponding to the difference between the Ct of the target gene and the Ct of the housekeeping 18S rRNA gene. PCR amplification of target gene cDNA was conducted using Taqman human gene expression assays, as previously described (13). The probes and primer kits used for each gene were as follows: Hs00172113-m1 for VDR, Hs00168017-m1 for CYP27B1, Hs00167999-m1 for CYP24A1, Hs00189038-m1 for LL37, Hs00915134-g1 for FGFR1, Hs01552926-m1 for FGFR2, Hs00179829-m1 for FGFR3, Hs01106908-m1 for FGFR4, Hs00183100-m1 for Klotho and Hs00174128-m1 for TNFα (Applied Biosystems). All reactions were amplified under the following conditions: 95°C for 10 mins followed by 40 cycles of 95°C for 30 seconds, 55°C for 1 min and 72°C for 1 min. Reactions were initially expressed as mean ± SD ΔCt values and values for fold-change relative to vehicle-treated cells were determined using the equation 2−ΔCt.
Western Blot analyses
For Western blot analysis of FGFR-mediated MAPK and Akt phosphorylation pathways in PBMCm, preliminary experiments showed that optimal expression of phosphorylated MAPK (pMAPK) and Akt (pAkt) was achieved after serum-starvation (0.1% HS) of these cells for 1 and 2 hrs respectively. During this serum starvation period, cells were treated with vehicle or an FGFR inhibitor (FGFRi, 250 nM), and then treated for a further 1 hr with either vehicle, FGF23 (100 ng/ml), FGFRi (250 nM) or FGF23 in combination with FGFRi. At the end of this treatment period, cells were treated with protein lysis buffer (NP40 Cell Lysis Buffer, Invitrogen, Camarillo, CA, USA), the proteinase inhibitor phenylmethylsulfonyl fluoride (PMSF), a proteinase inhibitor cocktail (P8340, 10 μl per ml of NP40, Sigma Aldrich, St Louis, MO, USA) and phosphatase inhibitor cocktail (P5726, 10 μl per ml of NP40, Sigma Aldrich, St Louis, MO, USA), and centrifuged at 4°C at 5000 G for 20 mins. Supernatants were then collected and stored at −20°C. Equal amounts of lysate protein were separated by electrophoresis using 10% SDS-PAGE gels with a biotinylated protein ladder (7727, Cell Signaling Technology, Danvers, MA, USA). Resulting blots were incubated for 24 hrs with primary antibodies directed against total MAPK (p44/42 Erk1/2) (dilution 1/1000), phosphoMAPK (p44/42 Erk1/2) (dilution 1/800), total Akt (1/1000) and phospho-Akt (1/400) (all Cell Signaling Technology, Danvers, MA, USA - catalog numbers 9102, 9101, 4691 and 4060, respectively). A horse-radish peroxidase (HRP)-system with an ECL system was then used for the incubation of secondary antibodies (1hr, dilution 1/2000) and image development (Phototype-HRP Western Blot detection system anti-rabbit 7074, anti-mouse 7076, anti-biotin 7075 and 20X LumiGlo Reagent/20X Peroxide 7003, Cell Signaling Technology, Danvers, MA, USA). Western blot analyses were quantified using ImageJ software (National Institutes of Health, Bethesda, MD).
Immunofluorescence analyses
PBMCm or PDm were seeded at 2 × 106 per well in a 4-well glass slide (Lab Tek II Chamber Slide System, NalgeNuncInc, Naperville, IL, USA), and cultured in the same medium and with the same reagents described above. After an initial fixation in 500 μl per well of paraformaldehyde (4%) for 10 mins, slides were subject to the following: 3 washes with PBS 1x; incubation with 500 μl of a mixture of PBS 1x and Tween 0.2% for 10 mins; 3 washes with PBS 1x; incubation with 500 μl of a mixture of PBS 1x and dry milk 5% for 30 mins; 3 washes with PBS 1x; incubation for 1 hr in 300 μl of a mixture of PBS 1x, bovine serum albumin and the following primary antibodies: FGFR1 (Flg-C-15-sc-121, 1/100, Santa Cruz Biotechnologies Inc, Santa Cruz, CA, USA) and Klotho (246, 1/100, a kind gift from Dr. Jeffrey Lavigne, Immutopics Inc, San Clemente, CA, USA). After 3 washes with PBS 1x, slides were then incubated with secondary antibodies labeled with Alexa 488 and 594 (anti-goat and anti-rabbit, Invitrogen, Camarillo, CA, USA) for 1 hr, washed 3 times with PBS 1x. Nuclear staining was carried out using 100 μl of 4′,6-diamidino-2-phenylindole dihydrochloride (DAPI, Sigma-Aldrich) (1/10 000 for 5 mins). Slides were then fixed with mounting media (Prolong Gold Antifade Reagent, P36930, Invitrogen) and stored at 4°C in the dark. Imaging was then carried out using a 60x lens, with the Nikon NIS Elements software.
Analysis of 25OHD metabolism by PBMCm
The effects of FGF23 on synthesis of 1,25(OH)2D and 24,25-dihydroxyvitamin D (24,25(OH)2D) by PBMCm were assessed by quantifying the metabolism of radiolabeled 25OHD as described previously (31). Briefly, aliquots of cells were incubated with radiolabeled 3H-25OHD substrate (300,000 cpm, 155Ci/mmol; Perkin Elmer, Waltham, Massachusetts) for 6 hrs in serum-free culture medium. The resulting mix of 3H-vitamin D metabolites was then extracted from the total cell lipids using initial C18 Sep-pak purification (Waters, Milford, Massachusetts) and subsequent HPLC (ZorbaxSil column; Agilent, Santa Clara, California) separation of 1α-hydroxylated and 24-hydroxylated vitamin D metabolites. 3H-25OHD, 3H-24,25(OH)2D and 3H-1,25(OH)2D was quantified by Beta-Ram in-line scintillation counting (Lablogic, Brandon, Florida). Data were reported as fmoles of vitamin D metabolites produced/hr/106 cells from n=5 separate cell preparations.
Statistics
Data are presented as mean ± standard error (SEM) for fold-changes in mRNA expression and HPLC metabolism data, and as mean ± standard deviation (SD) for raw RT-PCR data and Western blot analyses. Experimental means were compared statistically using an unpaired Student’s t-test. Where indicated, multifactorial data involving FGF23 and co-treatments were compared using one way analysis of variance (ANOVA) with the Holm-Sidak method used as a post hoc multiple comparison procedure. Statistical analyses were carried out using raw ΔCt values and fold-changes. Spearman correlation test was used for bivariate analyses.
Results
Expression and regulation of FGFRs in PBMCm
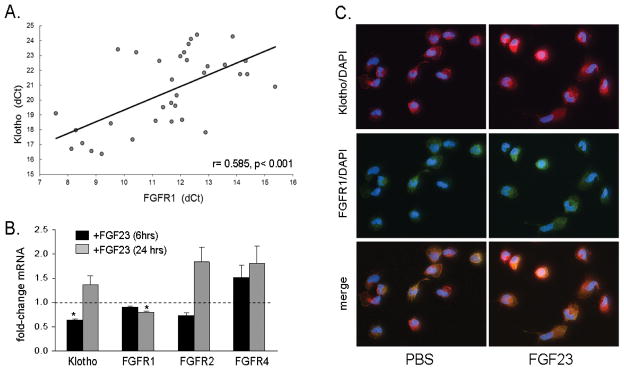
To assess the ability of monocytes to respond to FGF23, PBMCm were analysed for expression of different FGFRs and the FGFR co-receptor Klotho. As shown in Table 1, basal, untreated cultures of PBMCm expressed mRNA for FGFR1, -2 and -4 as well as Klotho but did not express FGFR3. Although expression of FGFR1 was much higher than other FGFRs or Klotho, analysis of PBMCm from multiple donors showed a strong positive correlation between mRNA for FGFR1 and Klotho (Figure 1A), CYP27B1 (supplemental Figure 1A), and the antibacterial factor LL37 (supplemental Figure 1B), but not CYP24A1 (supplemental Figure 1C). Treatment of PBMCm with FGF23 (100 ng/ml) suppressed expression of mRNA for Klotho at 6 hrs, and FGFR1 at 24 hrs (Figure 1B). Immunofluorescence confirmed that PBMCm express protein for Klotho and FGFR1, with coincident expression of the two proteins in some but not all untreated PBMCm cells (Figure 1C). Treatment with FGF23 (24 hrs) enhanced expression of protein for Klotho and FGFR1 in some but not all cells (Figure 1C).
Table 1. Expression of mRNA for FGFRs and Klotho in PBMC-derived monocytes (PBMCm).
Expression of mRNA for FGFR1, -2, -3, -4, and Klotho in baseline (untreated) PBMCm.
| ΔCt (mean ± SD) | Fold change to relative to FGFR1 | |
|---|---|---|
| FGFR1 | 11.32 ± 1.56 | 1 |
| FGFR2 | 22.15 ± 2.07 | 0.00055 |
| FGFR3 | Undetectable | 0 |
| FGFR4 | 24.34 ± 1.90 | 0.00012 |
| Klotho | 21.12 ± 2.04 | 0.00112 |
Data are shown as mean ΔCt PCR amplification values ± SD, normalized to expression of the housekeeping gene 18S rRNA (central column), and fold-change in mRNA expression relative to the most highly expressed mRNA (FGFR1) (right column), both n = 35 donor batches of PBMCm.
Figure 1. Expression and regulation of fibroblast growth factor receptors (FGFR) and Klotho in PBMCm.
1A. Baseline expression of FGFR1 mRNA in PBMCm correlates with Klotho mRNA (Spearman correlation coefficient of 0.585, p<0.001, 35 different batches of PBMCs). 1B. Effect of FGF23 (100 ng/ml) on expression of mRNA Klotho, FGFR1, FGFR2 and FGFR4 in PBMCm in vitro was assessed after 6 hr (black bars) and 24 hr (grey bars) treatments. Data show combined results using PBMCm from healthy donors at 6 hrs (8 donors) and 24 hrs (5 donors). 1C. Effect of FGF23 (100 ng/ml, 24 hrs) on expression of Klotho (red), FGFR-1 (green), and nuclear DAPI (blue) protein in PBMCm, as determined by immunofluoresence microscopy. Merged immunofluoresence shows co-expression of FGFR1 and Klotho in PBMCm.
Effects of FGF23 on FGFR signaling in PBMCm
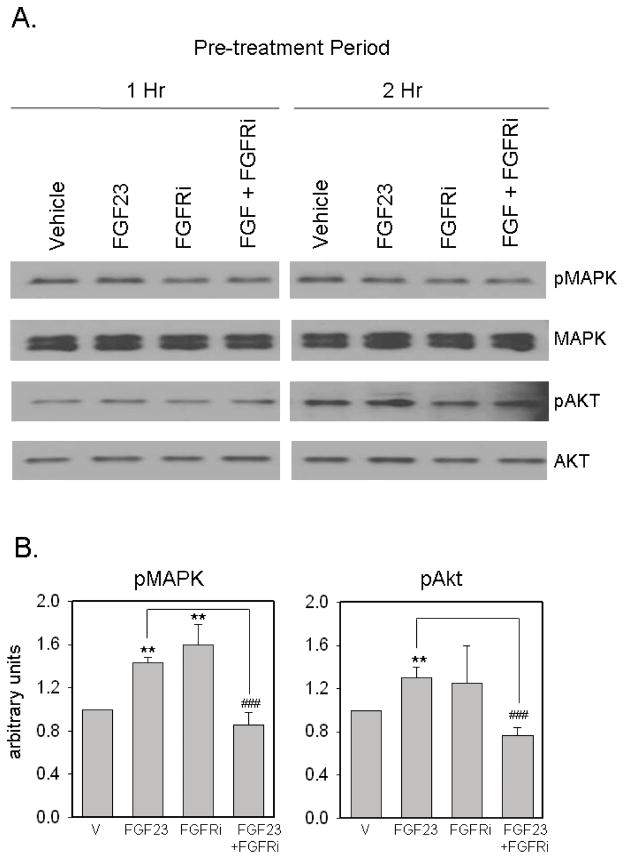
Having demonstrated expression of FGFR1 and Klotho in PBMCm, we next sought to characterize the signaling response of these receptors following exposure to FGF23. Initial analyses showed that optimal expression of phosphorylated MAPK (pMAPK) and Akt (pAkt) was achieved after serum-starvation (1% HS) pre-treatment for 1 and 2 hrs respectively (Figure 2A). Expression of pMAPK and pAkt was induced after treatment with FGF23 (100ng/ml) for 30 mins (Figure 2A and 2B), with no further induction of pMAPK or pAkt after 60 or 120 mins (data not shown). Pre-treatment of PBMCm with the FGFR inhibitor PD173074 (FGFRi, 250 nM) for 1 hr suppressed the activation of these two phosphorylation pathways following treatment with FGF23 (Figure 2A and 2B).
Figure 2. FGF23 modulates FGFR signaling in PBMCm.
2A. Effect of FGF23 (100 ng/ml) on MAPK and Aktsignaling in PBMCm. Data are shown as representative Western blots showing expression of protein for: total MAPK; phosphoMAPK (pMAPK); total Akt; phosphoAkt (pAkt). PBMC were pre-treated under conditions of serum deprivation (0.1% human serum) for either 1 hr or 2 hrs with or without an FGFR inhibitor (FGFRi, 250 nM), and then treated with or without vehicle or FGF23 (100 ng/ml) for a further 1 hr. 2B. Quantification of changes in expression of pMAPK (1 hr pre-treatment, left panel) and pAkt (2 hr pre-treatment, right panel) expression normalized to total MAPK and Akt respectively. Data were determined using ImageJ software and represent mean ± SD values for n = 3 separate donor PBMCm cultures. ** = statistically different from vehicle-treated PBMCm, p < 0.01. ## = statistically different from FGF23-treated PBMCm, p < 0.01.
FGF23 decreases expression of CYP27B1 and intracrine synthesis of 1,25(OH)2D in PBMCm
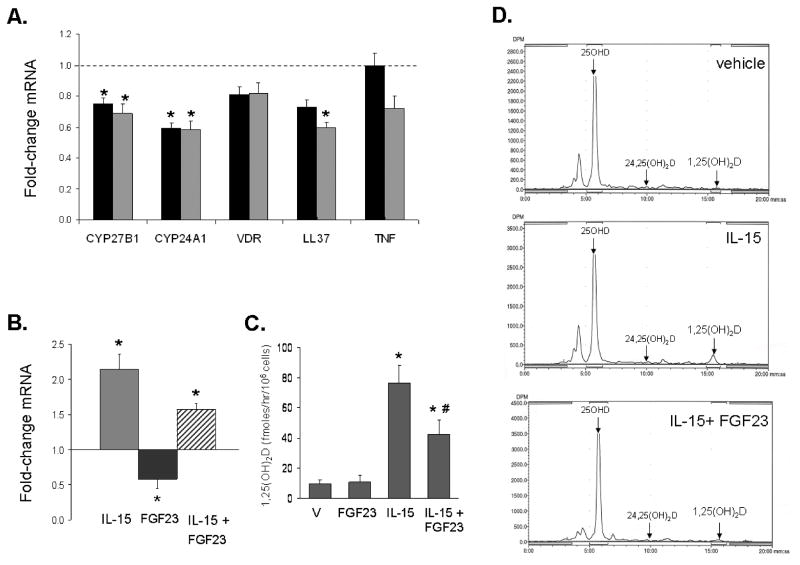
Treatment with FGF23 (100 ng/mL) decreased expression of mRNA for CYP27B1 in PBMCm after 6 and 24 hrs (Figure 3A). This effect was paralleled by decreased expression of the 1,25(OH)2D-target genes CYP24A1 (6 and 24 hrs) and LL37 (24 hrs) (Figure 3A). PBMCm pre-treated with IL-15 (48 hrs) showed a 2-fold increase in CYP27B1 mRNA relative to vehicle-treated cells, but this effect was inhibited by subsequent exposure to FGF23 (100 ng/ml, 24 hrs)(Figure 3B). HPLC analysis of vitamin D metabolism in 5 different batches of PBMCm showed that treatment with FGF23 inhibited monocyte synthesis of active 1,25(OH)2D (Figure 3C and 3D). Vehicle-treated cells (9.9 ± 2.9 fmoles/hr/106 cells), and cells treated with FGF23 alone (10.9 ± 4.2 fmoles/hr/106 cells) showed similar levels of 1,25(OH)2D production. However, cells treated with IL-15 showed a significant increase in synthesis of 1,25(OH)2D (76.3 ± 12.1 fmoles/hr/106 cells), with this effect being significantly inhibited by co-treatment with FGF23 (42.5 ± 9.5 fmol/hr/106 cells). Synthesis of 24,25(OH)2D was undetectable under each culture condition (Figure 3D).
Figure 3. FGF23 suppresses expression and activity of CYP27B1 in PBMCm.
3A. Effect of FGF23 (100 ng/ml) at 6hrs (black bars) and 24 hrs (grey bars) on expression of mRNA for CYP27B1, CYP24A1, VDR, LL37 and tumor necrosis factor α (TNFα) in PBMCm. Data shown are mean ± SEM fold-changes in mRNA expression relative to vehicle-treated cells for PBMCm from 8 different healthy donors. 3B. Effect of FGF23 (100 ng/ml, 24 hrs) alone or following pre-activation of cells with interleukin-15 (IL-15, 200 ng/ml, 48 hrs) on CYP27B1 mRNA expression in PBMCm. Data are shown as fold-change in CYP27B1 mRNA relative to vehicle-treated cells for PBMCm from 3 different healthy donors. 3C. Effect of vehicle (V) or FGF23 (100 ng/ml, 24 hrs) alone or following pre-activation of cells with IL-15 (200 ng/ml, 48 hrs) on conversion of 25OHD to 1,25(OH)2D in PBMCm. Data are shown as fmoles 1,25(OH)2D synthesized/hr/106 cells for PBMCm from n = 5 different healthy donors. 3D. Effect of FGF23 (100 ng/ml, 24 hrs) alone or after pre-activation of cells with IL-15 (200 ng/ml, 48 hrs) on conversion of 25OHD to 1,25(OH)2D in PBMCm. Data are shown as representative HLPC analyses for each treatment. * = statistically different compared to vehicle p< 0.05; # = statistically different from IL-15-treated cells, p < 0.05.
FGF23-mediated suppression of intracrine vitamin D activity in monocytes isolated from peritoneal dialysate effluent (PDm)
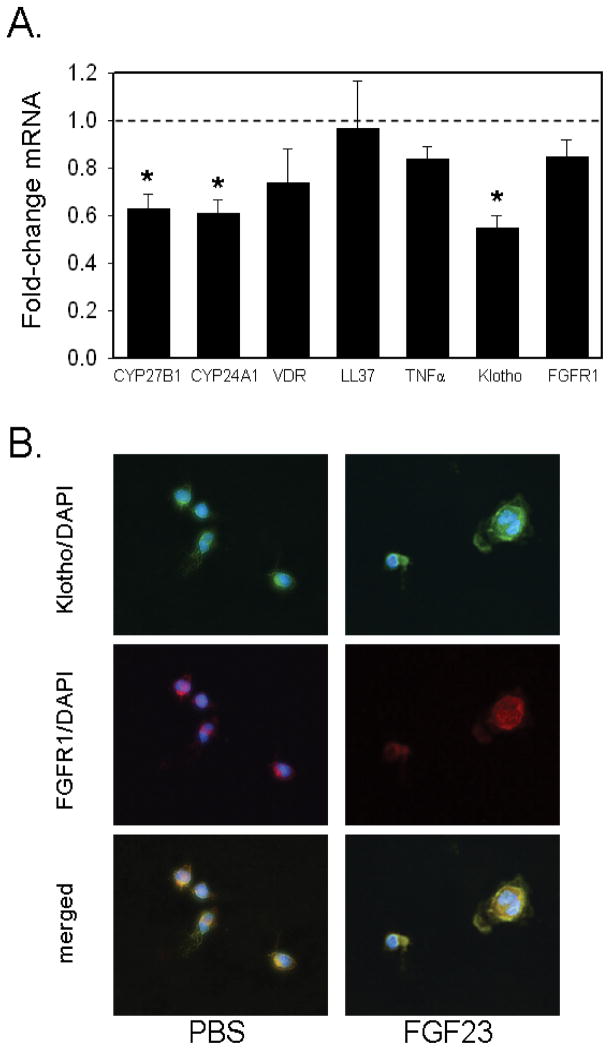
Monocytes isolated from peritoneal effluents of patients undergoing maintenance continuous peritoneal dialysis (PDm), showed a 5-fold higher basal expression of FGFR1 and 8-fold higher levels of CYP24A1 (Table 2). Treatment of PDm in vitro with FGF23 (100 ng/ml) resulted in similar responses to those observed with PBMCm: expression of mRNA for CYP27B1, CYP24A1 and Klotho was decreased after 6 hrs of treatment with FGF23 (Figure 4A); by contrast, expression of LL37 was unaffected by treatment with FGF23, although baseline expression of LL37 in PDm cells was 0.12 relative to that observed in PBMCm (Table 2). At the protein level, co-expression of Klotho and FGFR1 was observed in basal and FGF23-treated cultures of PDm (Figure 4B).
Table 2. Relative expression of vitamin D- and FGF23-related genes in PDm versus PBMCm.
Baseline expression of mRNAs for CYP27B1, CYP24A1, VDR, cathelicidin (LL37), Klotho and FGFR1 in PDm was compared to the expression of these genes in PBMCm.
| ΔCtPDm (mean ± SD) | ΔCtPBMCm (mean ± SD) | Fold-change relative to PBMCm | |
|---|---|---|---|
| CYP27B1 | 12.09 ± 2.21 | 11.90 ± 2.94 | 0.88 |
| CYP24A1 | 17.59 ± 2.90 | 20.58 ± 3.11 | 7.96* |
| VDR | 12.47 ± 3.60 | 14.14 ± 4.04 | 3.17 |
| LL37 | 22.38 ± 3.58 | 19.38 ± 3.05 | 0.12* |
| Klotho | 19.23 ± 2.03 | 20.57 ± 2.42 | 2.53 |
| FGFR1 | 9.57 ± 3.06 | 11.78 ± 2.05 | 4.63* |
Data in left-hand and central columns are shown as raw mRNA expression (ΔCt) data (mean ± SD) for the gene of interest normalized to expression of the housekeeping 18S rRNA gene. Data in the right-hand column are shown as fold-change mRNA expression for PDm relative to the equivalent gene in PBMCm. Combined results from 14 batches of PD cells and 45 batches of PBMCm.
statistically different from PBMCm, p< 0.05
Figure 4. FGF23 suppresses expression of CYP27B1 in monocytes from peritoneal dialysates.
4A. Effect of FGF23 (100 ng/ml, 6hrs) on expression of mRNA for CYP27B1, CYP24A1, Klotho and FGFR1 in peritoneal dialysate monocytes (PDm). Data are shown as mean ± SEM fold-change in mRNA expression relative to vehicle-treated cells for PDm from n = 7 different donors. 4B. Immunofluorescence analysis of protein for Klotho (green), FGFR-1 (red) and nuclear DAPI (blue) in PDm. Merged immunofluoresence shows co-expression of FGFR1 and Klotho in PDm. * = statistically different from vehicle-treated PDm, p< 0.05.
Discussion
Vitamin D is a potent regulator of innate (32) and adaptive immunity (8), with these effects being highly dependent on local conversion of 25OHD to 1,25(OH)2D by monocytes (12,13) and/or dendritic cells (DCs) (33,34). This intracrine activation of vitamin D within the immune system is sensitively induced by immunogenic stimuli (12,13), or exposure to cytokines such as IL-15 (14) or IFNγ (15). Although induction of CYP27B1 appears to be important for normal immune function, over-activity of the enzyme has been linked to pathological conditions such as granulomatous disease and associated hypercalcemia(35). Specific mechanisms that may help to prevent CYP27B1 over-activity following a pathogenic challenge include induction of the vitamin D catabolic enzyme CYP24A1 (36). In monocytes, as classically observed in the kidney, 1,25(OH)2D itself potently induces expression of CYP24A1 with this effect being enhanced following TLR activation (12). However, induction of monocyte vitamin D catabolism is not restricted to 1,25(OH)2D-mediated self-regulation. Recent studies have shown that the cytokine IL-4 suppresses intracrine-induced antibacterial responses to 25OHD through enhanced monocyte 24-hydroxylase activity (15). This mechanism suggests that T cells from the adaptive immune system are able to attenuate synthesis of 1,25(OH)2D by monocytes and/or DCs indirectly via induction of 24-hydroxylase vitamin D catabolism. What is less clear is whether CYP27B1 itself is a target for suppression of monocyte 1,25(OH)2D production. In classical vitamin D endocrinology, renal expression of CYP27B1 is inhibited by FGF23 as a counterpoint to the stimulatory actions of PTH (5). The aim of the current study was to determine whether similar FGF23-mediated regulation of CYP27B1 occurs in an extra-renal setting.
The significant decrease of CYP27B1 expression and activity in FGF23-treated monocytes is consistent with the classical actions of FGF23 on renal synthesis of 1,25(OH)2D. However, our data contrast previous studies describing FGF23-mediated upregulation of CYP27B1 in another extra-renal tissue, namely parathyroid cells (37). The precise physiological basis for these different extra-renal activities of FGF23 is unclear, and effects on parathyroid cells may simply represent an alternative mechanism to enhance vitamin D-mediated control of PTH secretion. In classical vitamin D endocrinology, FGF23-mediated suppression of CYP27B1 is accompanied by increased expression of the catabolic enzyme CYP24A1, with the latter acting to further attenuate renal production of 1,25(OH)2D (38–40). It was therefore interesting to note that monocyte expression of CYP24A1 is suppressed by FGF23 in parallel with its effects on CYP27B1. The most likely explanation is that, in the absence of cytokines such as IL-4, monocyte expression of CYP24A1 is primarily dependent on local synthesis of 1,25(OH)2D. Because synthesis of 1,25(OH)2D is decreased in the presence of FGF23, expression of CYP24A1 will also decline. Renal expression of CYP24A1 is also stimulated by 1,25(OH)2D but it is known to be sensitive to other endocrine factors such as PTH, which has been reported to regulate both transcription of CYP24A1 (41) and stability of the resulting mRNA (42). Thus, FGF23-mediated regulation of CYP24A1 in the kidney may be entirely different to its effects in monocytes.
Previous studies have reported expression of FGFR1 in interstitial inflammatory renal monocytes (43) but, to the best of our knowledge, our data provide the first evidence for combined expression of FGFR1 and Klotho in monocytes outside the kidney. In other cell types FGF23 has been shown to bind to multiple FGFRs (38,44–46). For example, recent studies have shown that FGFR3 and FGFR4 are the most likely receptors for FGF23-mediated regulation of serum 1,25(OH)2D levels (45). Nevertheless the relatively high levels of FGFR1 in PBMCm and PDm suggest that this is the likely principal target for FGF23 in these cells, with Klotho acting as a co-receptor. Irrespective of the precise receptor isoform involved in mediating inhibition of monocyte CYP27B1, the MAPK and Akt phosphorylation pathways that appear to be activated by FGF23 in monocytes have also been linked to FGF23 responses in other cell types (38,44,47,48). This contrasts recent studies of FGF23 responses in cardiomyocytes, which were Klotho-and MAPK/Akt-independent (46). Functional interplay between FGF23 and vitamin D in monocytes is supported by other observations from our study. First, both PBMC and PD monocytes show decreased expression of Klotho and FGFR1 after treatment with FGF23. Second, at baseline, there is a strong positive relationship between mRNA expression for Klotho and FGFR1, but also between FGFR1 and CYP27B1 and between FGFR1 and LL37. Finally, the PD cells, from patients chronically exposed to high circulating levels of FGF23 (49), have a lower baseline expression of LL37 relative to PBMCm. This provides a potential explanation for the lack of significant suppression of LL37 expression in PDm treated with FGF23 in vitro.
Decreased monocyte function following exposure to FGF23 may influence several disease scenarios. The key role of FGF23 in phosphate physiology was first highlighted in pediatric autosomal dominant hypophosphatemic rickets (ADHR)(16). Unlike CKD and end-stage renal disease (49,50), ADHR involves only moderately increased circulating levels of FGF23, and is not known to present with the same infections observed in CKD, except for a tendency to dental abscesses (51), and some reported pulmonary infections (52). The latter may be due to effects of increased FGF23, but may also involve thoracic deformations induced by rickets or direct effects of vitamin D deficiency (53,54). However, elevated FGF23 may influence other aspects of vitamin D physiology. Increased levels of vitamin D have been associated with improved survival and cardiovascular status in CKD patients (55), while vitamin D treatments have also been linked to decreased risk of cardiovascular deaths among dialysis patients (56,57). Conversely, high circulating levels of FGF23 are strongly associated with risk of mortality and cardiovascular disease in patients with CKD (23), and we propose that this may be due, at least in part, to FGF23-mediated dysregulation of extra-renal vitamin D function. The precise mechanisms linking FGF23, vitamin D and cardiovascular disease are as yet unclear but could involve suppression of the intracrine immunomodulatory actions of 25OHD in disease affected cells. This may include monocytes and DCs, but endothelial cells are also known to express CYP27B1, with the latter being induced by inflammatory stimuli (58).
The most immediate clinical implication of the data presented in this study is the potential link between elevated FGF23 and impaired innate immunity in CKD. As detailed above, CKD is a state of acquired immune deficiency involving both cellular and humoral immunity (27), and circulating levels LL37 in this population appear to be an independent risk factor of mortality by infections (59). In a similar fashion, higher quartiles of serum FGF23 are associated with an increased risk of mortality across the spectrum of CKD (23,60), and non-CKD populations (61), mainly in terms of cardiovascular mortality; therapy with vitamin D sterols is associated with reduced mortality in dialysis patients (56,57). Prospective randomized trials will be required to define the role of FGF23 on overall patient survival as well as the impact of therapy with vitamin D. Although therapeutic targeting of FGF23 may be a strategy to delay the onset of secondary hyperparathyroidism and bone and mineral disorders associated with CKD, the effects of such an approach on global and cardiovascular morbi-mortality have yet to be studied. However, based on data presented here we propose that therapies aimed at lowering circulating levels of FGF23 may have immediate benefits for the morbi-mortality induced by infections in the CKD population.
To date, studies of vitamin D-induced innate immunity have focused exclusively on monocytes derived from PBMCs (12,13). Stubbs et al. demonstrated modulation of vitamin D-dependent biomarkers in PBMC following native oral vitamin D supplementation in hemodialysis patients, but without any evaluation of a potential interplay between vitamin D and FGF23 pathways (62). However, in CKD it seems more likely that antibacterial activity will arise from tissue-localized monocytes and macrophages rather than from the circulating populations of these cells. With this in mind, a key objective of the current study was to characterize the vitamin D system and vitamin D-induced immunity in a population of cells more closely associated with infection in CKD patients, namely PDm. Previous studies have shown that peritoneal macrophages from dialysis patients are able to synthesize 1,25(OH)2D (63), but the relevance of this finding to innate immunity remains unclear. PDm and PBMCm express similar levels of CYP27B1, but the relatively high levels of FGFR1/Klotho in PDm may facilitate FGF23 suppression of CYP27B1 in these cells. Despite this, FGF23 treatment did not significantly suppress LL37 expression in PDm. One explanation for this is that baseline levels of LL37 are lower in PDm relative to PBMC, and may thus be resistant to further suppression. Conversely, baseline expression of CYP24A1 is significantly higher in PDm relative to PBMCm and this is also likely to influence intracrine responses to 25D in these cells.
In future studies, it will be interesting to characterize further the difference between PDm and PBMCm with respect to vitamin D-mediate immune function. For the current study it was not possible to obtain sufficient blood from the pediatric CKD donors of the PD cells to enable parallel isolation of PBMCm. However, direct comparison between these two populations of monocytes may provide important new insights on the immunomodulatory effects of vitamin D in vivo. Future studies will also need to further clarify the wider impact of FGF23 on immune function. The current study focuses on the intracrine effects of vitamin D on monocyte function but it is also possible that FGF23 will act to suppress CYP27B1 in other immune cells that express this enzyme such as DCs (33,34). In this way, FGF23 may not only influence innate antibacterial responses to infection but it may also affect antigen presentation by DCs and concomitant adaptive T cell/B cell immune activity. This may be particularly important in CKD patients where inflammation is a key feature of disease pathophysiology, with reported links to patient vitamin D status (64). Effects of FGF23 on DC vitamin D metabolism and antigen presentation may also impact on adaptive immune response to kidney transplantation. Recent studies from our group have highlighted association between FGF23 levels, deteriorating kidney function and host-graft rejection in pediatric CKD transplantation patients (65). The role of immune vitamin D metabolism in this setting has yet to be studied but may provide an important mechanism for future therapeutic intervention to improve transplantation success.
Supplementary Material
As well as correlating with Klotho expression, in 35 different batches of PBMCs at baseline, FGFR-1 levels also correlated with the expression of vitamin D dependent genes in PBMCm: CYP27B1 (Spearman correlation, r= 0.716, p< 0.001, 1S-A) and LL37(Spearman correlation, r= 0.583, p< 0.001, 1S-B). Of note, there was no relationship between baseline FGFR-1 expression and CYP24A1 (1S-C), nor between CYP27B1 and CYP24A1 (1S-D); Klotho expression was associated with no other genes apart from FGFR-1 (Figure 1A). By contrast, the baseline expression of CYP27B1 and LL37 correlated (r= 0.571, p<0.001, 1S-E).
Effect of the toll-like receptor (TLR)2 ligand 19 kDa lipoprotein (100 ng/ml, 6hrs) on expression of mRNA for CYP27B1, CYP24A1, VDR, LL37 and tumor necrosis factor α (TNFα), FGFR1 and Klotho in PBMCm. Data shown are mean ± SEM fold-changes in mRNA expression relative to vehicle-treated cells for PBMCm from 5 different healthy donors. * = statistically different from vehicle-treated PBMCm, p< 0.05.
Acknowledgments
This work was supported in part by educational grants (AcadémieFrançaise/Jean Walter Zellidja, RéunionPédiatrique de la Région Rhône Alpes, SociétéFrançaise de Pédiatrie/Evian, Fondation pour la RechercheMédicale, Philippe Foundation, JB), by a grant from the Center for D-receptor Activation Research (CeDAR, MH), by NIH grant DK0911672 (MH), by USPHS grants DK 67563, DK 35423, DK 80984 and funds from the Casey Lee Ball Foundation (IBS and KWP). We would like to thank Deborah Krakow MD, Anna Sarukhanov, and Margarita Ivanova (UCLA) for their help with the Western blot analyses.
Justine Bacchetta (MD, PhD) performed the experiments as well as the statistical analyses, and wrote the manuscript; Jessica Sea, Rene Chun (PhD) and Thomas Lisse (PhD) did some of the experimental work; Katherine Wesseling-Perry (MD), Isidro Salusky (MD) helped to design the study and to analyze the results; John Adams (MD) helped to analyze data; Barbara Gales (RN) was involved in patient recruitment; Martin Hewison (PhD) initially designed the study, helped to analyze the results and edited the manuscript.
Footnotes
Disclosure
All authors state that they have no conflicts of interest
References
- 1.Liu S, Quarles LD. How fibroblast growth factor 23 works. J Am Soc Nephrol. 2007;18(6):1637–47. doi: 10.1681/ASN.2007010068. [DOI] [PubMed] [Google Scholar]
- 2.Yamazaki Y, Tamada T, Kasai N, Urakawa I, Aono Y, Hasegawa H, Fujita T, Kuroki R, Yamashita T, Fukumoto S, Shimada T. Anti-FGF23 neutralizing antibodies show the physiological role and structural features of FGF23. J Bone Miner Res. 2008;23(9):1509–18. doi: 10.1359/jbmr.080417. [DOI] [PubMed] [Google Scholar]
- 3.Yoshiko Y, Wang H, Minamizaki T, Ijuin C, Yamamoto R, Suemune S, Kozai K, Tanne K, Aubin JE, Maeda N. Mineralized tissue cells are a principal source of FGF23. Bone. 2007;40(6):1565–73. doi: 10.1016/j.bone.2007.01.017. [DOI] [PubMed] [Google Scholar]
- 4.Shimada T, Hasegawa H, Yamazaki Y, Muto T, Hino R, Takeuchi Y, Fujita T, Nakahara K, Fukumoto S, Yamashita T. FGF-23 is a potent regulator of vitamin D metabolism and phosphate homeostasis. Journal of bone and mineral research: the official journal of the American Society for Bone and Mineral Research. 2004;19(3):429–35. doi: 10.1359/JBMR.0301264. [DOI] [PubMed] [Google Scholar]
- 5.Shimada T, Kakitani M, Yamazaki Y, Hasegawa H, Takeuchi Y, Fujita T, Fukumoto S, Tomizuka K, Yamashita T. Targeted ablation of Fgf23 demonstrates an essential physiological role of FGF23 in phosphate and vitamin D metabolism. J Clin Invest. 2004;113(4):561–8. doi: 10.1172/JCI19081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Razzaque MS, Sitara D, Taguchi T, St-Arnaud R, Lanske B. Premature aging-like phenotype in fibroblast growth factor 23 null mice is a vitamin D-mediated process. The FASEB journal: official publication of the Federation of American Societies for Experimental Biology. 2006;20(6):720–2. doi: 10.1096/fj.05-5432fje. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sitara D, Razzaque MS, St-Arnaud R, Huang W, Taguchi T, Erben RG, Lanske B. Genetic ablation of vitamin D activation pathway reverses biochemical and skeletal anomalies in Fgf-23-null animals. The American journal of pathology. 2006;169(6):2161–70. doi: 10.2353/ajpath.2006.060329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hewison M. Vitamin D and innate and adaptive immunity. Vitam Horm. 2011;86:23–62. doi: 10.1016/B978-0-12-386960-9.00002-2. [DOI] [PubMed] [Google Scholar]
- 9.Adams JS, Hewison M. Unexpected actions of vitamin D: new perspectives on the regulation of innate and adaptive immunity. Nat Clin Pract Endocrinol Metab. 2008;4(2):80–90. doi: 10.1038/ncpendmet0716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gombart AF, Borregaard N, Koeffler HP. Human cathelicidin antimicrobial peptide (CAMP) gene is a direct target of the vitamin D receptor and is strongly up-regulated in myeloid cells by 1,25-dihydroxyvitamin D3. Faseb J. 2005;19(9):1067–77. doi: 10.1096/fj.04-3284com. [DOI] [PubMed] [Google Scholar]
- 11.Wang TT, Nestel FP, Bourdeau V, Nagai Y, Wang Q, Liao J, Tavera-Mendoza L, Lin R, Hanrahan JW, Mader S, White JH. Cutting edge: 1,25-dihydroxyvitamin D3 is a direct inducer of antimicrobial peptide gene expression. J Immunol. 2004;173(5):2909–12. doi: 10.4049/jimmunol.173.5.2909. [DOI] [PubMed] [Google Scholar]
- 12.Liu PT, Stenger S, Li H, Wenzel L, Tan BH, Krutzik SR, Ochoa MT, Schauber J, Wu K, Meinken C, Kamen DL, Wagner M, Bals R, Steinmeyer A, Zugel U, Gallo RL, Eisenberg D, Hewison M, Hollis BW, Adams JS, Bloom BR, Modlin RL. Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science. 2006;311(5768):1770–3. doi: 10.1126/science.1123933. [DOI] [PubMed] [Google Scholar]
- 13.Adams JS, Ren S, Liu PT, Chun RF, Lagishetty V, Gombart AF, Borregaard N, Modlin RL, Hewison M. Vitamin d-directed rheostatic regulation of monocyte antibacterial responses. J Immunol. 2009;182(7):4289–95. doi: 10.4049/jimmunol.0803736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Krutzik SR, Hewison M, Liu PT, Robles JA, Stenger S, Adams JS, Modlin RL. IL-15 links TLR2/1-induced macrophage differentiation to the vitamin D-dependent antimicrobial pathway. J Immunol. 2008;181(10):7115–20. doi: 10.4049/jimmunol.181.10.7115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Edfeldt K, Liu PT, Chun R, Fabri M, Schenk M, Wheelwright M, Keegan C, Krutzik SR, Adams JS, Hewison M, Modlin RL. T-cell cytokines differentially control human monocyte antimicrobial responses by regulating vitamin D metabolism. Proc Natl Acad Sci U S A. 2010;107(52):22593–8. doi: 10.1073/pnas.1011624108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Consortium A. Autosomal dominant hypophosphataemic rickets is associated with mutations in FGF23. Nat Genet. 2000;26(3):345–8. doi: 10.1038/81664. [DOI] [PubMed] [Google Scholar]
- 17.Jonsson KB, Zahradnik R, Larsson T, White KE, Sugimoto T, Imanishi Y, Yamamoto T, Hampson G, Koshiyama H, Ljunggren O, Oba K, Yang IM, Miyauchi A, Econs MJ, Lavigne J, Juppner H. Fibroblast growth factor 23 in oncogenic osteomalacia and X-linked hypophosphatemia. N Engl J Med. 2003;348(17):1656–63. doi: 10.1056/NEJMoa020881. [DOI] [PubMed] [Google Scholar]
- 18.Danziger J. The bone-renal axis in early chronic kidney disease: an emerging paradigm. Nephrol Dial Transplant. 2008;23(9):2733–7. doi: 10.1093/ndt/gfn260. [DOI] [PubMed] [Google Scholar]
- 19.Isakova T, Wahl P, Vargas GS, Gutierrez OM, Scialla J, Xie H, Appleby D, Nessel L, Bellovich K, Chen J, Hamm L, Gadegbeku C, Horwitz E, Townsend RR, Anderson CA, Lash JP, Hsu CY, Leonard MB, Wolf M. Fibroblast growth factor 23 is elevated before parathyroid hormone and phosphate in chronic kidney disease. Kidney Int. 2011;79(12):1370–8. doi: 10.1038/ki.2011.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fliser D, Kollerits B, Neyer U, Ankerst DP, Lhotta K, Lingenhel A, Ritz E, Kronenberg F, Kuen E, Konig P, Kraatz G, Mann JF, Muller GA, Kohler H, Riegler P. Fibroblast growth factor 23 (FGF23) predicts progression of chronic kidney disease: the Mild to Moderate Kidney Disease (MMKD) Study. J Am Soc Nephrol. 2007;18(9):2600–8. doi: 10.1681/ASN.2006080936. [DOI] [PubMed] [Google Scholar]
- 21.Kazama JJ, Gejyo F, Shigematsu T, Fukagawa M. Role of circulating fibroblast growth factor 23 in the development of secondary hyperparathyroidism. Ther Apher Dial. 2005;9(4):328–30. doi: 10.1111/j.1744-9987.2005.00291.x. [DOI] [PubMed] [Google Scholar]
- 22.Nakanishi S, Kazama JJ, Nii-Kono T, Omori K, Yamashita T, Fukumoto S, Gejyo F, Shigematsu T, Fukagawa M. Serum fibroblast growth factor-23 levels predict the future refractory hyperparathyroidism in dialysis patients. Kidney Int. 2005;67(3):1171–8. doi: 10.1111/j.1523-1755.2005.00184.x. [DOI] [PubMed] [Google Scholar]
- 23.Gutierrez OM, Mannstadt M, Isakova T, Rauh-Hain JA, Tamez H, Shah A, Smith K, Lee H, Thadhani R, Juppner H, Wolf M. Fibroblast growth factor 23 and mortality among patients undergoing hemodialysis. N Engl J Med. 2008;359(6):584–92. doi: 10.1056/NEJMoa0706130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mirza MA, Hansen T, Johansson L, Ahlstrom H, Larsson A, Lind L, Larsson TE. Relationship between circulating FGF23 and total body atherosclerosis in the community. Nephrol Dial Transplant. 2009;24(10):3125–31. doi: 10.1093/ndt/gfp205. [DOI] [PubMed] [Google Scholar]
- 25.Mirza MA, Larsson A, Lind L, Larsson TE. Circulating fibroblast growth factor-23 is associated with vascular dysfunction in the community. Atherosclerosis. 2009;205(2):385–90. doi: 10.1016/j.atherosclerosis.2009.01.001. [DOI] [PubMed] [Google Scholar]
- 26.Parasuraman R, Samarapungavan D, Venkat KK. Updated principles and clinical caveats in the management of infection in renal transplant recipients. Transplantation reviews. 2010;24(2):43–51. doi: 10.1016/j.trre.2009.09.001. [DOI] [PubMed] [Google Scholar]
- 27.Cohen G, Haag-Weber M, Horl WH. Immune dysfunction in uremia. Kidney Int Suppl. 1997;62:S79–82. [PubMed] [Google Scholar]
- 28.Sarnak MJ, Jaber BL. Mortality caused by sepsis in patients with end-stage renal disease compared with the general population. Kidney Int. 2000;58(4):1758–64. doi: 10.1111/j.1523-1755.2000.00337.x. [DOI] [PubMed] [Google Scholar]
- 29.Collins AJ, Foley RN, Gilbertson DT, Chen SC. The state of chronic kidney disease, ESRD, and morbidity and mortality in the first year of dialysis. Clinical journal of the American Society of Nephrology: CJASN. 2009;4(Suppl 1):S5–11. doi: 10.2215/CJN.05980809. [DOI] [PubMed] [Google Scholar]
- 30.Sardenberg C, Suassuna P, Andreoli MC, Watanabe R, Dalboni MA, Manfredi SR, dos Santos OP, Kallas EG, Draibe SA, Cendoroglo M. Effects of uraemia and dialysis modality on polymorphonuclear cell apoptosis and function. Nephrol Dial Transplant. 2006;21(1):160–5. doi: 10.1093/ndt/gfi095. [DOI] [PubMed] [Google Scholar]
- 31.Wu S, Ren S, Nguyen L, Adams JS, Hewison M. Splice variants of the CYP27b1 gene and the regulation of 1,25-dihydroxyvitamin D3 production. Endocrinology. 2007;148(7):3410–8. doi: 10.1210/en.2006-1388. [DOI] [PubMed] [Google Scholar]
- 32.Hewison M. Antibacterial effects of vitamin D. Nat Rev Endocrinol. 2011;7(6):337–45. doi: 10.1038/nrendo.2010.226. [DOI] [PubMed] [Google Scholar]
- 33.Hewison M, Freeman L, Hughes SV, Evans KN, Bland R, Eliopoulos AG, Kilby MD, Moss PA, Chakraverty R. Differential regulation of vitamin D receptor and its ligand in human monocyte-derived dendritic cells. J Immunol. 2003;170(11):5382–90. doi: 10.4049/jimmunol.170.11.5382. [DOI] [PubMed] [Google Scholar]
- 34.Fritsche J, Mondal K, Ehrnsperger A, Andreesen R, Kreutz M. Regulation of 25-hydroxyvitamin D3-1 alpha-hydroxylase and production of 1 alpha,25-dihydroxyvitamin D3 by human dendritic cells. Blood. 2003;102(9):3314–6. doi: 10.1182/blood-2002-11-3521. [DOI] [PubMed] [Google Scholar]
- 35.Kallas M, Green F, Hewison M, White C, Kline G. Rare causes of calcitriol-mediated hypercalcemia: a case report and literature review. J Clin Endocrinol Metab. 2010;95(7):3111–7. doi: 10.1210/jc.2009-2673. [DOI] [PubMed] [Google Scholar]
- 36.Sakaki T, Kagawa N, Yamamoto K, Inouye K. Metabolism of vitamin D3 by cytochromes P450. Front Biosci. 2005;10:119–34. doi: 10.2741/1514. [DOI] [PubMed] [Google Scholar]
- 37.Krajisnik T, Bjorklund P, Marsell R, Ljunggren O, Akerstrom G, Jonsson KB, Westin G, Larsson TE. Fibroblast growth factor-23 regulates parathyroid hormone and 1alpha-hydroxylase expression in cultured bovine parathyroid cells. J Endocrinol. 2007;195(1):125–31. doi: 10.1677/JOE-07-0267. [DOI] [PubMed] [Google Scholar]
- 38.Razzaque MS. FGF23-mediated regulation of systemic phosphate homeostasis: is Klotho an essential player? Am J Physiol Renal Physiol. 2009;296(3):F470–6. doi: 10.1152/ajprenal.90538.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Juppner H, Wolf M, Salusky IB. FGF-23: More than a regulator of renal phosphate handling? J Bone Miner Res. 2010;25(10):2091–7. doi: 10.1002/jbmr.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shimada T, Hasegawa H, Yamazaki Y, Muto T, Hino R, Takeuchi Y, Fujita T, Nakahara K, Fukumoto S, Yamashita T. FGF-23 is a potent regulator of vitamin D metabolism and phosphate homeostasis. J Bone Miner Res. 2004;19(3):429–35. doi: 10.1359/JBMR.0301264. [DOI] [PubMed] [Google Scholar]
- 41.Yang W, Friedman PA, Kumar R, Omdahl JL, May BK, Siu-Caldera ML, Reddy GS, Christakos S. Expression of 25(OH)D3 24-hydroxylase in distal nephron: coordinate regulation by 1,25(OH)2D3 and cAMP or PTH. Am J Physiol. 1999;276(4 Pt 1):E793–805. doi: 10.1152/ajpendo.1999.276.4.E793. [DOI] [PubMed] [Google Scholar]
- 42.Zierold C, Mings JA, DeLuca HF. Regulation of 25-hydroxyvitamin D3-24-hydroxylase mRNA by 1,25-dihydroxyvitamin D3 and parathyroid hormone. J Cell Biochem. 2003;88(2):234–7. doi: 10.1002/jcb.10341. [DOI] [PubMed] [Google Scholar]
- 43.Rossini M, Cheunsuchon B, Donnert E, Ma LJ, Thomas JW, Neilson EG, Fogo AB. Immunolocalization of fibroblast growth factor-1 (FGF-1), its receptor (FGFR-1), and fibroblast-specific protein-1 (FSP-1) in inflammatory renal disease. Kidney Int. 2005;68(6):2621–8. doi: 10.1111/j.1523-1755.2005.00734.x. [DOI] [PubMed] [Google Scholar]
- 44.Shimada T, Urakawa I, Isakova T, Yamazaki Y, Epstein M, Wesseling-Perry K, Wolf M, Salusky IB, Juppner H. Circulating fibroblast growth factor 23 in patients with end-stage renal disease treated by peritoneal dialysis is intact and biologically active. J Clin Endocrinol Metab. 2010;95(2):578–85. doi: 10.1210/jc.2009-1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gattineni J, Twombley K, Goetz R, Mohammadi M, Baum M. Regulation of serum 1,25(OH)2 vitamin D3 levels by fibroblast growth factor 23 is mediated by FGF receptors 3 and 4. Am J Physiol Renal Physiol. 2011;301(2):F371–7. doi: 10.1152/ajprenal.00740.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Faul C, Amaral AP, Oskouei B, Hu MC, Sloan A, Isakova T, Gutierrez OM, Aguillon-Prada R, Lincoln J, Hare JM, Mundel P, Morales A, Scialla J, Fischer M, Soliman EZ, Chen J, Go AS, Rosas SE, Nessel L, Townsend RR, Feldman HI, St John Sutton M, Ojo A, Gadegbeku C, Di Marco GS, Reuter S, Kentrup D, Tiemann K, Brand M, Hill JA, Moe OW, Kuro OM, Kusek JW, Keane MG, Wolf M. FGF23 induces left ventricular hypertrophy. J Clin Invest. 2011;121(11):4393–408. doi: 10.1172/JCI46122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Goetz R, Beenken A, Ibrahimi OA, Kalinina J, Olsen SK, Eliseenkova AV, Xu C, Neubert TA, Zhang F, Linhardt RJ, Yu X, White KE, Inagaki T, Kliewer SA, Yamamoto M, Kurosu H, Ogawa Y, Kuro-o M, Lanske B, Razzaque MS, Mohammadi M. Molecular insights into the klotho-dependent, endocrine mode of action of fibroblast growth factor 19 subfamily members. Mol Cell Biol. 2007;27(9):3417–28. doi: 10.1128/MCB.02249-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ranch D, Zhang MY, Portale AA, Perwad F. Fibroblast growth factor 23 regulates renal 1,25-dihydroxyvitamin D and phosphate metabolism via the MAP kinase signaling pathway in Hyp mice. J Bone Miner Res. 2011;26(8):1883–90. doi: 10.1002/jbmr.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wesseling-Perry K, Pereira RC, Wang H, Elashoff RM, Sahney S, Gales B, Juppner H, Salusky IB. Relationship between plasma fibroblast growth factor-23 concentration and bone mineralization in children with renal failure on peritoneal dialysis. J Clin Endocrinol Metab. 2009;94(2):511–7. doi: 10.1210/jc.2008-0326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bacchetta J, Dubourg L, Harambat J, Ranchin B, Abou-Jaoude P, Arnaud S, Carlier MC, Richard M, Cochat P. The influence of glomerular filtration rate and age on fibroblast growth factor 23 serum levels in pediatric chronic kidney disease. J Clin Endocrinol Metab. 2010;95(4):1741–8. doi: 10.1210/jc.2009-1576. [DOI] [PubMed] [Google Scholar]
- 51.Bacchetta J, Salusky IB. Evaluation of hypophosphatemia: lessons from patients with genetic disorders. Am J Kidney Dis. 2012;59(1):152–9. doi: 10.1053/j.ajkd.2011.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kitajima I, Maruyama I, Matsubara H, Osame M, Igata A. Immune dysfunction in hypophosphatemic vitamin D-resistant rickets: immunoregulatory reaction of 1 alpha(OH) vitamin D3. Clin Immunol Immunopathol. 1989;53(1):24–31. doi: 10.1016/0090-1229(89)90097-4. [DOI] [PubMed] [Google Scholar]
- 53.Koeffler HP, Bishop JE, Reichel H, Singer F, Nagler A, Tobler A, Walka M, Norman AW. Lymphocyte cell lines from vitamin D-dependent rickets type II show functional defects in the 1 alpha,25-dihydroxyvitamin D3 receptor. Mol Cell Endocrinol. 1990;70(1):1–11. doi: 10.1016/0303-7207(90)90053-b. [DOI] [PubMed] [Google Scholar]
- 54.Muhe L, Lulseged S, Mason KE, Simoes EA. Case-control study of the role of nutritional rickets in the risk of developing pneumonia in Ethiopian children. Lancet. 1997;349(9068):1801–4. doi: 10.1016/S0140-6736(96)12098-5. [DOI] [PubMed] [Google Scholar]
- 55.Souberbielle JC, Body JJ, Lappe JM, Plebani M, Shoenfeld Y, Wang TJ, Bischoff-Ferrari HA, Cavalier E, Ebeling PR, Fardellone P, Gandini S, Gruson D, Guerin AP, Heickendorff L, Hollis BW, Ish-Shalom S, Jean G, von Landenberg P, Largura A, Olsson T, Pierrot-Deseilligny C, Pilz S, Tincani A, Valcour A, Zittermann A. Vitamin D and musculoskeletal health, cardiovascular disease, autoimmunity and cancer: Recommendations for clinical practice. Autoimmun Rev. 2010;9(11):709–15. doi: 10.1016/j.autrev.2010.06.009. [DOI] [PubMed] [Google Scholar]
- 56.Shoji T, Shinohara K, Kimoto E, Emoto M, Tahara H, Koyama H, Inaba M, Fukumoto S, Ishimura E, Miki T, Tabata T, Nishizawa Y. Lower risk for cardiovascular mortality in oral 1alpha-hydroxy vitamin D3 users in a haemodialysis population. Nephrology, dialysis, transplantation: official publication of the European Dialysis and Transplant Association - European Renal Association. 2004;19(1):179–84. doi: 10.1093/ndt/gfg513. [DOI] [PubMed] [Google Scholar]
- 57.Negri AL. Association of oral calcitriol with improved survival in non-dialysed and dialysed patients with CKD. Nephrology, dialysis, transplantation: official publication of the European Dialysis and Transplant Association - European Renal Association. 2009;24(2):341–4. doi: 10.1093/ndt/gfn624. [DOI] [PubMed] [Google Scholar]
- 58.Zehnder D, Bland R, Chana RS, Wheeler DC, Howie AJ, Williams MC, Stewart PM, Hewison M. Synthesis of 1,25-dihydroxyvitamin D(3) by human endothelial cells is regulated by inflammatory cytokines: a novel autocrine determinant of vascular cell adhesion. J Am Soc Nephrol. 2002;13(3):621–9. doi: 10.1681/ASN.V133621. [DOI] [PubMed] [Google Scholar]
- 59.Gombart AF, Bhan I, Borregaard N, Tamez H, Camargo CA, Jr, Koeffler HP, Thadhani R. Low plasma level of cathelicidin antimicrobial peptide (hCAP18) predicts increased infectious disease mortality in patients undergoing hemodialysis. Clin Infect Dis. 2009;48(4):418–24. doi: 10.1086/596314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jean G, Terrat JC, Vanel T, Hurot JM, Lorriaux C, Mayor B, Chazot C. High levels of serum fibroblast growth factor (FGF)-23 are associated with increased mortality in long haemodialysis patients. Nephrol Dial Transplant. 2009;24(9):2792–6. doi: 10.1093/ndt/gfp191. [DOI] [PubMed] [Google Scholar]
- 61.Parker BD, Schurgers LJ, Brandenburg VM, Christenson RH, Vermeer C, Ketteler M, Shlipak MG, Whooley MA, Ix JH. The associations of fibroblast growth factor 23 and uncarboxylated matrix Gla protein with mortality in coronary artery disease: the Heart and Soul Study. Ann Intern Med. 2010;152(10):640–8. doi: 10.1059/0003-4819-152-10-201005180-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Stubbs JR, Idiculla A, Slusser J, Menard R, Quarles LD. Cholecalciferol supplementation alters calcitriol-responsive monocyte proteins and decreases inflammatory cytokines in ESRD. J Am Soc Nephrol. 2010;21(2):353–61. doi: 10.1681/ASN.2009040451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hayes ME, O’Donoghue DJ, Ballardie FW, Mawer EB. Peritonitis induces the synthesis of 1 alpha,25-dihydroxyvitamin D3 in macrophages from CAPD patients. FEBS Lett. 1987;220(2):307–10. doi: 10.1016/0014-5793(87)80836-0. [DOI] [PubMed] [Google Scholar]
- 64.Zehnder D, Quinkler M, Eardley KS, Bland R, Lepenies J, Hughes SV, Raymond NT, Howie AJ, Cockwell P, Stewart PM, Hewison M. Reduction of the vitamin D hormonal system in kidney disease is associated with increased renal inflammation. Kidney Int. 2008;74(10):1343–53. doi: 10.1038/ki.2008.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wesseling-Perry K, Tsai EW, Ettenger RB, Juppner H, Salusky IB. Mineral abnormalities and long-term graft function in pediatric renal transplant recipients: a role for FGF-23? Nephrol Dial Transplant. 2011;26(11):3779–84. doi: 10.1093/ndt/gfr126. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
As well as correlating with Klotho expression, in 35 different batches of PBMCs at baseline, FGFR-1 levels also correlated with the expression of vitamin D dependent genes in PBMCm: CYP27B1 (Spearman correlation, r= 0.716, p< 0.001, 1S-A) and LL37(Spearman correlation, r= 0.583, p< 0.001, 1S-B). Of note, there was no relationship between baseline FGFR-1 expression and CYP24A1 (1S-C), nor between CYP27B1 and CYP24A1 (1S-D); Klotho expression was associated with no other genes apart from FGFR-1 (Figure 1A). By contrast, the baseline expression of CYP27B1 and LL37 correlated (r= 0.571, p<0.001, 1S-E).
Effect of the toll-like receptor (TLR)2 ligand 19 kDa lipoprotein (100 ng/ml, 6hrs) on expression of mRNA for CYP27B1, CYP24A1, VDR, LL37 and tumor necrosis factor α (TNFα), FGFR1 and Klotho in PBMCm. Data shown are mean ± SEM fold-changes in mRNA expression relative to vehicle-treated cells for PBMCm from 5 different healthy donors. * = statistically different from vehicle-treated PBMCm, p< 0.05.