ABSTRACT
Objective:
To evaluate the relationship between antihypertensive (AH) drug adherence and cardiovascular (CV) outcomes among patients with a recent ischemic stroke and assess the validity of our approach.
Methods:
A cohort of 14,227 patients diagnosed with an ischemic stroke was assembled from individuals 65 years and older who were treated with AH agents from 1999 to 2007 in Quebec, Canada. A nested case-control design was used to evaluate the occurrence of nonfatal major CV outcomes and mortality. Each case was matched to 15 controls by age and cohort entry time. Medication possession ratio was used for AH agent adherence level. Adjusted conditional logistic regression models were used to estimate the rate ratio of CV events. The validity of the approach was assessed by evaluating the adherence level of CV-protective and non–CV-protective drugs.
Results:
Mean age was 75 years, 54% were male, 38% had coronary artery disease, 23% had diabetes, 47% dyslipidemia, and 14% atrial fibrillation or flutter. High adherence to AH therapy was mirrored by similar adherence to statins and antiplatelet agents and was associated with a lower risk of nonfatal vascular events compared with lower adherence (rate ratio 0.77 [0.70–0.86]). We observed a paradoxic link between adherence to several drugs and all-cause mortality.
Conclusion:
Adherence to AH agents is associated with adherence to other secondary preventive therapies and a risk reduction for nonfatal vascular events after an ischemic stroke. Overestimation of all-cause mortality reduction may be related to frailty and comorbidities, which may confound the apparent benefit of different drugs.
Stroke represents one of the most common and devastating disorders worldwide.1,2 Approximately 795,000 strokes occur each year, resulting in 134,000 deaths, making it a leading cause of mortality and health care costs in the United States, and also in Canada.3,4
Hypertension is the strongest modifiable risk factor for all types of strokes5 and its prevalence is increasing.6 The available evidence shows that a 10-mm Hg reduction in systolic blood pressure is associated with a relative risk reduction for stroke of approximately one-third.7 This is also true for individuals who have previously had a cerebrovascular event.8 Current knowledge suggests that the variability and instability of blood pressure could have an important role in the progression of organ damage and occurrence of vascular events. Clinicians should be aware of the implications of blood pressure variability and the benefit of drug class effects.9–11
A large discrepancy exists between recommended guidelines for treatment of hypertension and blood pressure control within the community.12–14 Nonadherence to antihypertensive (AH) medication is recognized as a major contributor to the lack of adequate control of blood pressure.15,16 However, there are no large-scale effectiveness studies assessing the link between adherence to AH medications and major cardiovascular (CV) outcomes in high-risk individuals who have recently had an ischemic stroke. Our aim was to evaluate this relationship in a cohort of older patients hospitalized for an ischemic stroke and returning to the community. We also assessed the potential influence of a healthy user or frailty bias on our findings.17
METHODS
Data sources
We present a nested case-control study of hypertensive patients with a recent ischemic stroke in the province of Quebec, Canada. Data were obtained from a linked administrative health database. The RAMQ (Régie Assurance Maladie Québec) covers all Quebec residents for the cost of physician visits, hospitalizations, procedures, and 94% of citizens aged 65 and older for drugs. The databases are composed of linkage data files that capture the following information: 1) demographics data; 2) data on delivered medication in community pharmacies such as the date of filling, name of the drug, dose, quantity, dosage form, and duration of therapy; 3) all hospitalizations with dates of admission and discharge for the primary and up to 15 secondary diagnoses that are coded using the International Classification of Diseases, 9th and 10th revisions (ICD-9 and ICD-10); 4) all surgical procedure codes follow the Canadian classification of diagnostic, therapeutic, and surgical procedures; and 5) physician visits with the date of service, the diagnosis using ICD-9 and ICD-10 coding, and medical procedures. The databases have been previously validated.18,19
Ethical approval
Approval for access to study data was provided by the ethic board of the province (Commission d’Accés l’Information du Québec) and of the ethic committee of the University of Montreal.
Cohort definition
We selected patients aged 65 years and older hospitalized for an ischemic stroke (ICD-9 codes: 433, 434, 436; ICD-10 codes: I67.2, I63, I64) from all individuals who initiated AH agents with diuretics, β-blockers, calcium channel blockers, angiotensin-converting enzyme inhibitors, or angiotensin II receptor blockers between January 1, 1999, and December 31, 2007. The stroke cohort entry date was defined as the date of the first prescription of an AH agent in the first month after hospital discharge. Patients had to be insured by the RAMQ for at least 1 year before entering the cohort and were followed up from the date of issuance of the first prescription of AH agent until stroke recurrence, myocardial infarction, death, or the end of the study period (June 30, 2008).
Nested case-control study
Nonfatal vascular events were defined as a composite outcome of stroke recurrence and classified as hemorrhagic (ICD-9 431 or ICD-10 I61), ischemic, or myocardial infarction (ICD-9 410 or ICD-10 I21). Those codes have been validated and shown to have high sensitivity and specificity.20,21 Secondary outcomes were vascular death (death within 30 days after a CV event) and all-cause mortality.
All cases of primary and secondary outcomes were identified and up to 15 controls were randomly selected from the cohort based on the risk set of each case using density sampling. Sampling for each control was selected in proportion to time contribution to the person-time at risk in the source population (i.e., the whole cohort), which gives an unbiased estimate of the rate ratio.22 Cases and controls were matched for age at entry into the cohort and for the duration of follow-up.
Exposure assessment
Treatment adherence was estimated by calculating the medication possession ratio (MPR). The MPR reflects the total number of days’ supply of medication dispensed divided by the length of follow-up.23 For cases, adherence was calculated from the start of follow-up to time of the vascular event (index date). For controls, the adherence was calculated from the start of follow-up to time of selection (index date). The MPR was dichotomized, setting a threshold of MPR (<80%) to identify patients who were nonadherent.24
Covariates
We used gender and diagnoses in the hospital discharge databases, vascular procedures, and drug markers to obtain comorbidity data, defined as 1) coronary artery disease: ICD-9 codes 411–414 or ICD-10 I24, I20, I25, vascular medical procedure (coronary artery bypass graft, angiography, angioplasty, or stent), or use of oral nitrate; 2) atrial fibrillation or flutter: ICD-9 427.3 or ICD-10 I48 or prescription of drug markers; 3) chronic heart failure: ICD-9 398.91, 402.01, 402.11, 402.91, 428.0, 428.1, and 428.9 or ICD-10 I09.81, I11, I50.9, and I50.1 or the use of furosemide alone or with digoxin, angiotensin-converting enzyme inhibitors, spironolactone, or β-blockers; and 4) peripheral arterial disease: ICD-9 440–447 or ICD-10 I70.0–I74.9, medical procedure for noncoronary angioplasty, or use of pentoxifylline. In addition, diabetes and dyslipidemia were identified at entry and during follow-up using ICD-9 or ICD-10 codes or drug markers.
Statistical analysis
Conditional logistic regression models were developed to evaluate crude and adjusted rate ratios for primary and secondary outcomes. Validation of our approach was done with an analysis of 4 drug variables, which included statins, antidiabetics, proton pump inhibitors, and antiresorptive agents for osteoporosis, and determining different patterns of use (nonusers, adherence level <80%, and adherence level ≥80%). The goal was to assess whether adherence to CV-protective and non–CV-protective drugs was also associated with a risk reduction for nonfatal vascular events, vascular mortality, and all-cause mortality in order to examine the potential presence of a bias.25,26
Multivariate models were constructed to maximally adjust for confounders. Rate ratios and 95% confidence intervals were calculated. All analyses were performed using SAS version 9.1 (SAS Institute, Cary, NC).
RESULTS
Patient characteristics
Among 34,163 patients hospitalized with a diagnosis of ischemic stroke from January 1999 to December 2007, 9,467 patients were excluded because they died during hospitalization, were admitted to long-term institutions, or did not have a confirmed diagnosis of hypertension. The cohort consisted of 14,227 patients with an ischemic stroke who were users of AH agents in the first month after hospital discharge. The mean age of the cohort was 75 years, 54% were male, 38% had coronary artery disease, 23% were diabetic, and 47% had dyslipidemia; other CV comorbidities are listed in table 1. Demographics and clinical characteristics regarding AH drug adherence in the nested case-control study are quite similar (table e-1 on the Neurology® Web site at www.neurology.org). No major clinically significant difference (>5%) was observed between groups, except for dyslipidemia.
Table 1.
Baseline characteristics and initial antihypertensive medication of patients with stroke
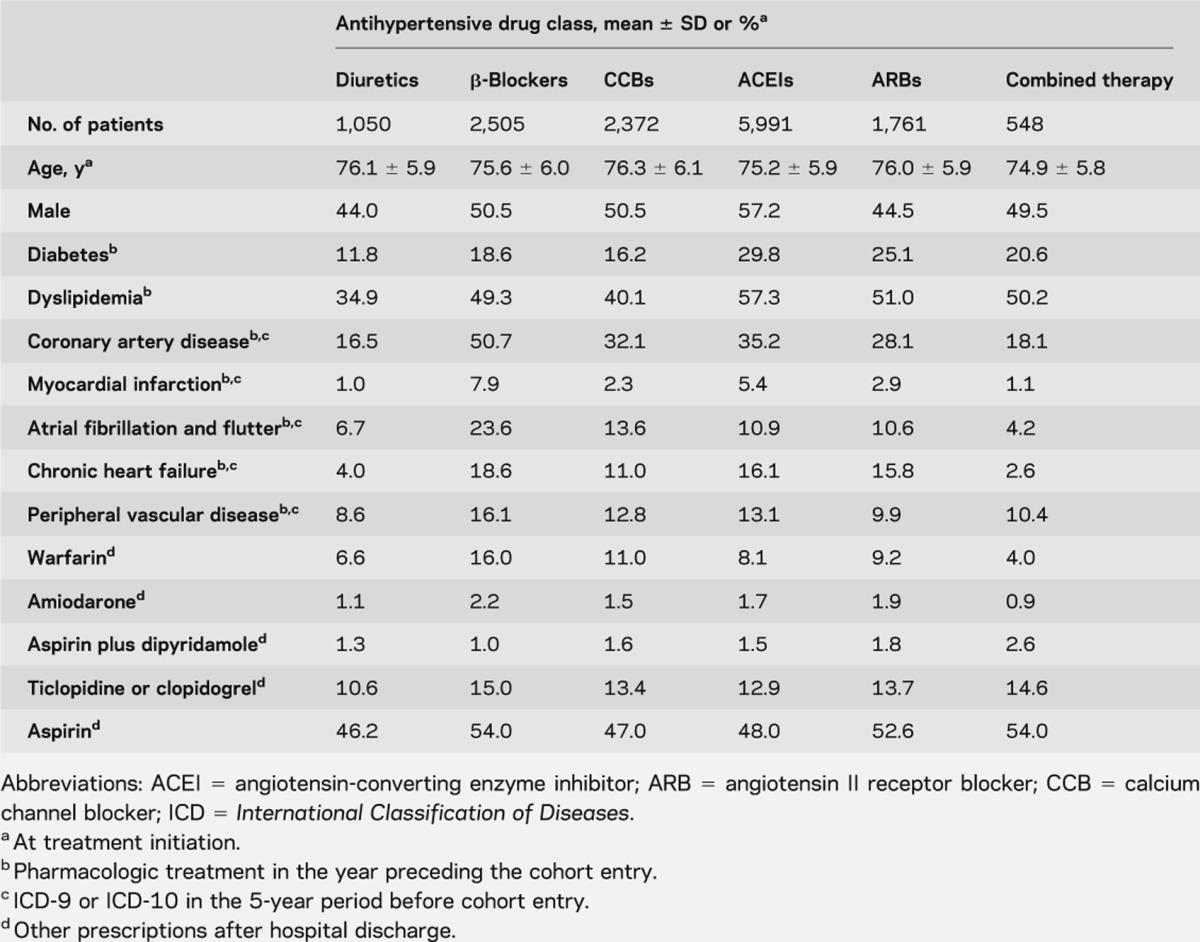
During a mean follow-up of 3.3 years, there were 11.7% stroke recurrences (1,670 events including 147 hemorrhagic and 1523 ischemic strokes [3.9 per 100 person-years]), 7.0% myocardial infarctions (990 events, 2.3 per 100 person-years), 2.8% CV deaths (399 events, 1.0 per 100 person-years), and 11.4% all-cause mortality (1,628 events, 3.8 per 100 person-years). Male gender and all CV comorbidities were more prevalent among cases than controls (table e-2).
Impact of adherence to AH drugs on nonfatal vascular events
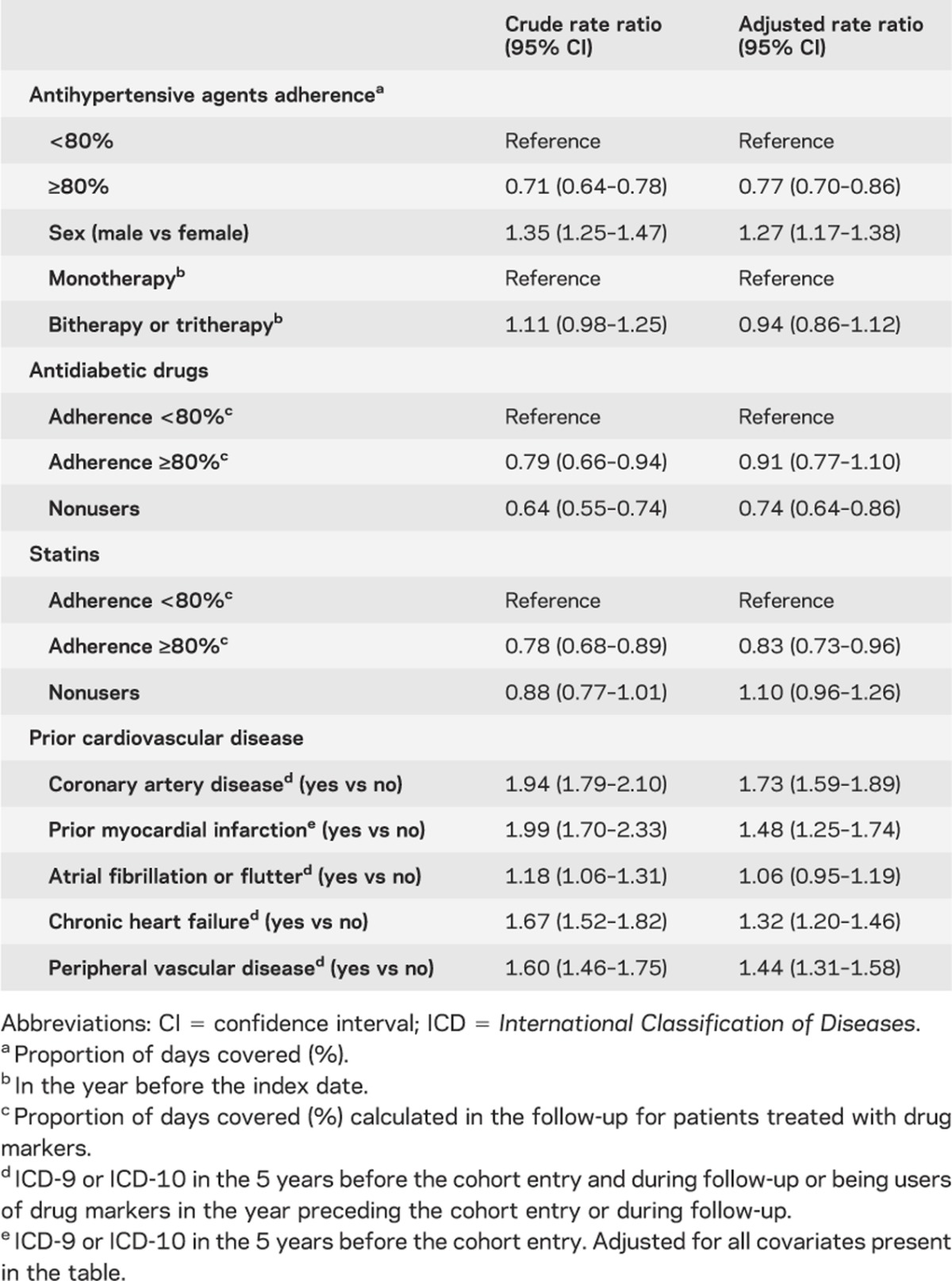
Mean adherence to AH agents was approximately 98% for high-adherence and 45% for low-adherence groups. Patients with high adherence had a risk reduction of 23% of nonfatal vascular events compared with patients at low-adherence levels (table 2). As shown in table e-3, the proportions of patients at each level of adherence among cases and controls for AH agents, dyslipidemia agents, and antiplatelets are quite similar.
Table 2.
Nonfatal vascular events rate ratios
Risk factors for nonfatal vascular events
Male gender (table 2) was associated with more nonfatal vascular events (rate ratio 1.27; 1.17–1.38). Coronary artery disease, myocardial infarction, chronic heart failure, and peripheral vascular disease were also associated with increased nonfatal vascular events. High adherence to antidiabetic drugs did not have a significant impact on nonfatal vascular events, whereas patients with high adherence to statins had a significant risk reduction for nonfatal vascular events (rate ratio 0.83; 0.73–0.96).
Other outcomes and subgroup analyses
Rate ratios for nonfatal stroke (0.70; 0.61–0.81) and all-cause mortality (0.52; 0.47–0.59) were significantly lower with high adherence to AH medications, and a trend was observed for nonfatal myocardial infarction (0.86; 0.72–1.01) and vascular death (0.81; 0.60–1.05).
We observed a risk reduction for nonfatal ischemic stroke (rate ratio: 0.70; 0.61–0.82); however, the level of risk for nonfatal vascular events remained similar in patients below or above age 75 (rate ratio: 0.73; 0.62–0.86 vs 0.81; 0.70–0.93).
Validity of the methodologic approach
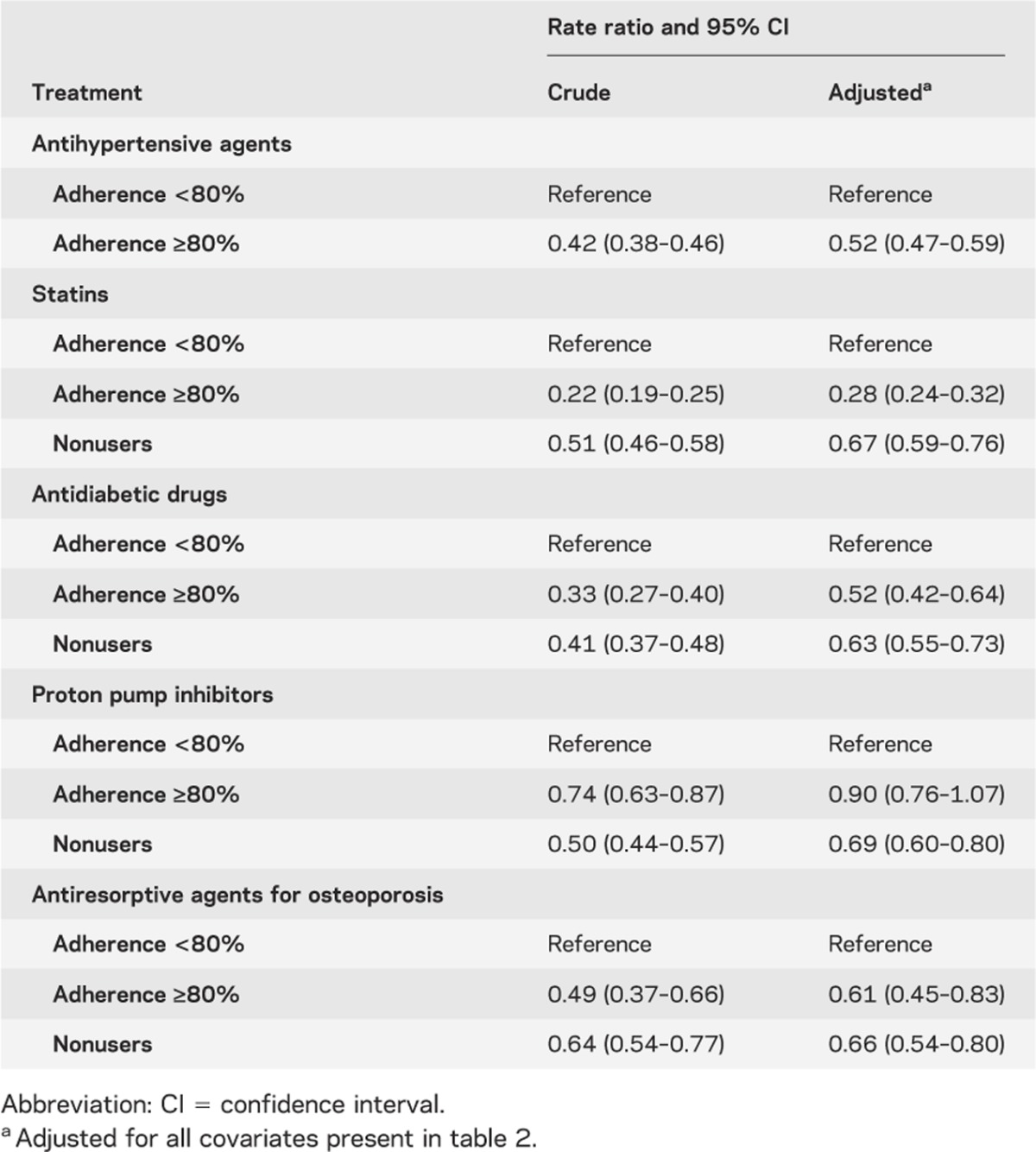
Table 3 shows that adherence to different drug classes has a different impact on nonfatal vascular events. High adherence to CV-protective medications was associated with a reduction of nonfatal vascular events, but this effect was not present with non–CV-protective drugs. However, as shown in table 4, a trend in the risk reduction of vascular mortality was associated with better drug adherence to AH agents. Compared with nonusers and nonadherers, a high level of adherence to statins showed a very high risk reduction for vascular mortality. Conversely, non–CV-protective drugs had no effect on vascular mortality. Finally, as shown in table 5, we observed a paradoxic relation between several drugs and all-cause mortality, giving an important risk reduction for all-cause mortality. Adjustment for patient characteristics, comorbidity, and polypharmacy had no effect on these results.
Table 3.
Effect of prescription pattern for different drugs on all nonfatal vascular events
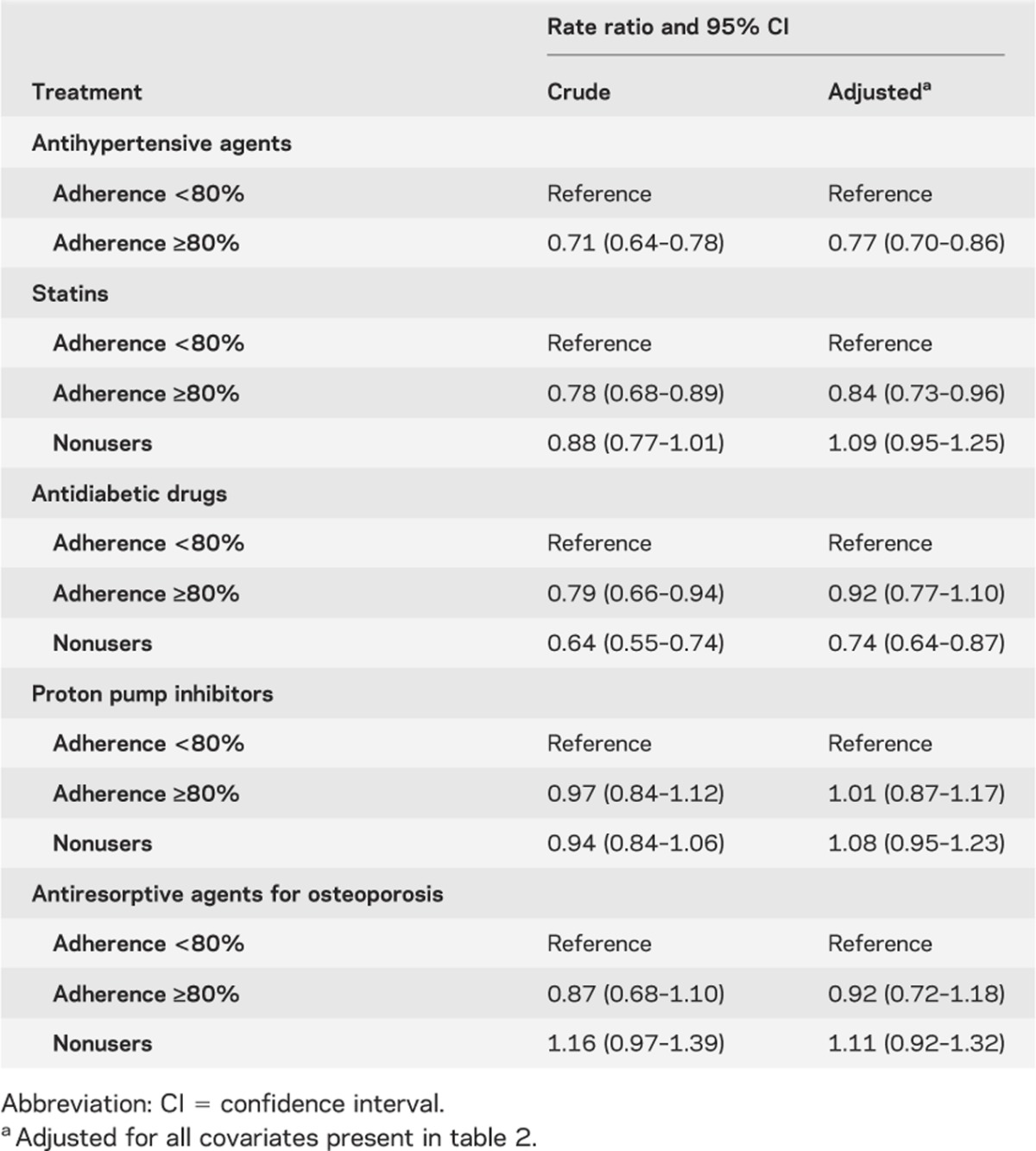
Table 4.
Effect of prescription pattern for different drugs on CV mortality
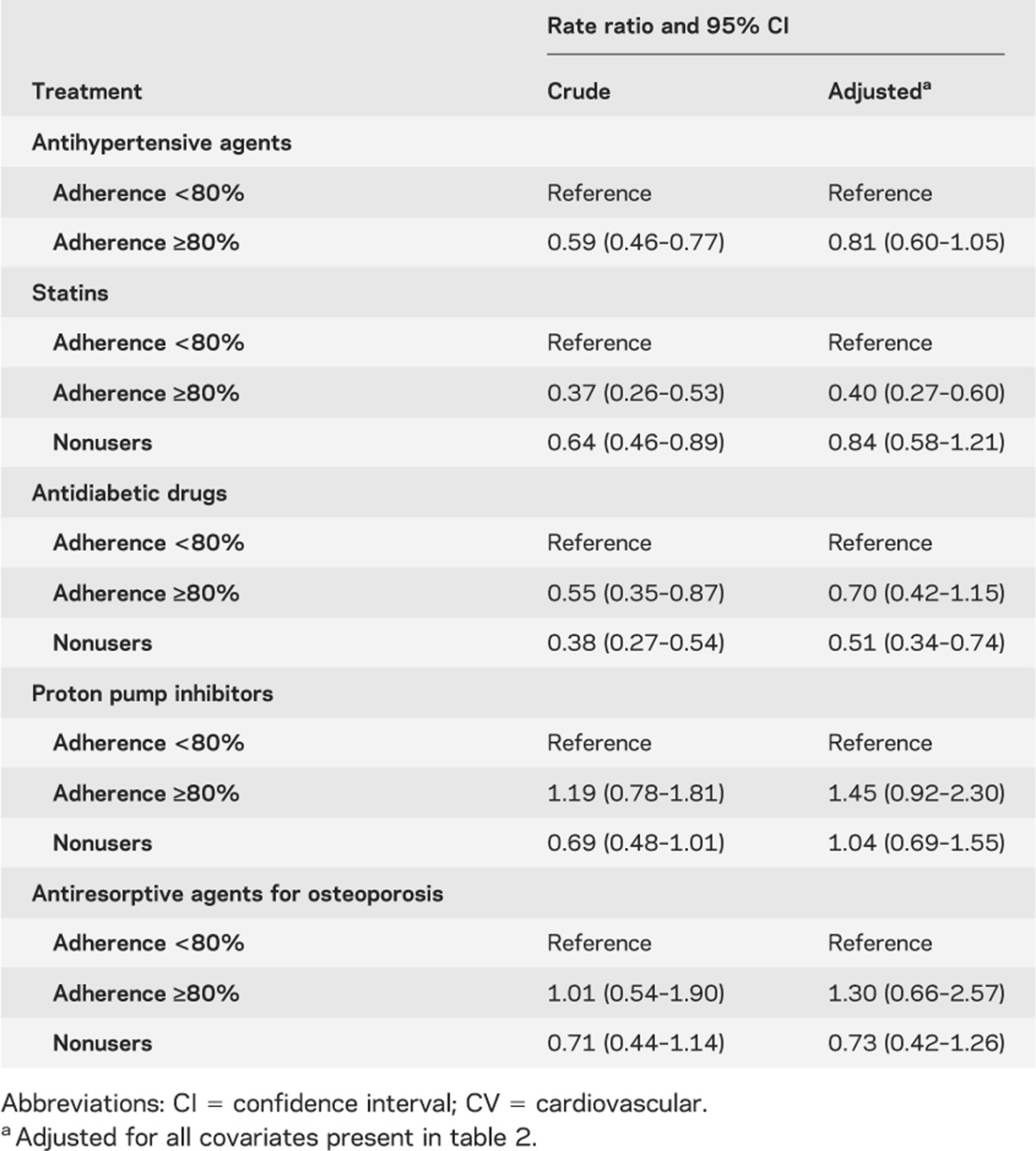
Table 5.
Effect of prescription pattern for different drugs on all-cause mortality
DISCUSSION
Our study reveals that adherence to AH treatment of ≥80% after a recent ischemic stroke is associated with adherence to other secondary preventive therapies and a significant 23% decreased risk of nonfatal vascular event. In addition, results indicate that male gender, presence of cardiac disease, and peripheral vascular disease are associated with increased nonfatal vascular events. Consistent with previously published studies, our data also show that high adherence to dyslipidemia therapy is associated with lower vascular events.27–29 Also in keeping with the current literature, high adherence to antidiabetic medications did not significantly affect major vascular outcomes. Indeed, although tight glycemic control has been shown to improve diabetic microvascular complications, this is still uncertain for macrovascular disease.30 Because the profile of each specific level of adherence among cases and controls for AH agents, dyslipidemia agents, and antiplatelets is quite similar, this suggests that at least part of the AH effect may be explained by better overall medical compliance and compliance with other drugs such as statins and antiplatelets.
The association between AH drug adherence and risk of major vascular events had not been evaluated previously in a real-life clinical setting focusing on secondary prevention after an ischemic stroke. A systematic review of higher-risk individuals who previously had a cerebrovascular event reported a stroke risk reduction of approximately 25% with different AH agents. Our results are also comparable to the results of the PROGRESS trial,31 in which active treatment with perindopril was compared with placebo in individuals who had a previous stroke or transient ischemic attack over a 4-year follow-up period. The study reported a nonfatal stroke risk reduction of 29% and a 24% risk reduction for ischemic stroke; there was no significant reduction for vascular death and all-cause mortality.31 Although the impact of AH therapy adherence on the actual blood pressure level is beyond the scope of our study, recent publications have shown that imperfect adherence can lead to significant blood pressure fluctuations resulting in increased CV risk.32
The risk coefficients reported in our study associated with known vascular risk factors such as male gender, presence of CV disease, or dyslipidemia are in agreement with the literature.3,33 Previous studies have shown that men are at higher risk for cerebrovascular disease than women.33 In addition, patients with evidence of coronary artery disease, chronic heart failure, or peripheral artery disease have an increased risk of cerebrovascular disease compared with those without such conditions.3,34 Finally, high total cholesterol levels are also associated with a higher risk of ischemic stroke.3
We also explored the presence of a healthy-user effect by analyzing observational data to create causal models to assess the associations between high adherence to CV-protective and non–CV-protective therapies and their relation to certain outcomes such as vascular mortality and all-cause mortality.25 A trend in risk reduction for vascular mortality was associated with better drug adherence to AH agents. However, we suspect the effect of a healthy-user bias specifically for individuals taking statin therapies. Compared with nonusers, a high level of adherence to statins had a very high risk reduction for vascular mortality that is beyond the results reported from randomized trials.35,36 As well, we also suspect a healthy-adherer effect, because compared with nonadherers, statin-adherent patients had a much lower rate of vascular mortality with little effect after adjustment.35
In addition, users of CV-protective and non–CV-protective therapies presented an important risk reduction for all-cause mortality. Adjustment had little effect on these results. Drug-use patterns are often used to provide surrogate measures of disease, but selective underuse of certain drugs by elderly patients with potentially unmeasured comorbidities may lead to false-protective associations between the use of specific drugs and mortality. We suspect that controlling for the propensity to use drugs will not totally eliminate this risk.26 The presence of frailty and comorbidities that influence the use of preventive therapies can act as confounders and lead to an apparent benefit of certain drugs on all-cause mortality. These observations raise concerns about using observational studies with high-risk populations to evaluate associations between drug use and mortality.26,37 This is supported by some results from the Vitamins in Stroke Prevention study.38
Our design took into account the potential for some methodologic limitations. To avoid selection bias, we included only incident AH users. The potential for confounding by indication should be cautiously assessed, but given that the global study population was receiving AH agents, the likelihood of such a bias is reduced. However, other potential limitations remain. First, we could not control for all characteristics that may have influenced physicians’ choice of medication, including unmeasured variables and missing data for blood pressure control, which could lead to residual confounding effects. However, there is no particular reason to believe that the choice of AH agents would be strongly influenced by the hypertension level. Second, because patients with more comorbidities are more likely to have CV events, we adjusted for several risk factors. Nevertheless, residual confounding caused by incomplete or inaccurate measurement of covariates or unmeasured confounders cannot be excluded. For instance, patients who do not adhere to their therapeutic regimen may have other traits that contribute to worse outcomes, including factors such as depression, lower socioeconomic status, and adverse health behaviors.24 We were able to adjust in part for these factors. Third, we could not adjust directly for blood glucose and cholesterol levels, but we determined the adherence level of patients receiving drugs for diabetes or dyslipidemia. In particular, our results revealed that high adherence to lipid-lowering drugs decreased the risk for nonfatal vascular events by 17%. These results are consistent with previous studies,36 and this finding remains true even compared to patients without dyslipidemia, which is in keeping with previous publications on the pleiotropic effect of statins on the CNS.39 Fourth, although our follow-up period was shorter compared with some randomized clinical trials, our median time of follow-up of 3.3 years represented a sufficient length of time to detect differences in major vascular outcomes. Fifth, some individuals with a history of CV disease may not have been identified because of errors in diagnostic coding. The probability of this occurring would be low because we had access to relevant medical and drug information for all individuals over a period of several years before their entry into the cohort. Sixth, we utilized prescription refill patterns to assess exposure and therefore we cannot ascertain whether the dispensed medication was actually taken by the patient. However, some available evidence suggests a good correlation between pharmacy dispensing records and cumulative drug exposure and gaps in medication supply.40 Finally, the relationships between initiation, adherence, and health-seeking tendencies may vary by drug class and outcome; this association is likely stronger for medications used to treat asymptomatic disease, and for outcomes with a potential behavioral component.
Our study suggests that adherence to AH medication for at least 80% of the time is associated with adherence to other secondary preventive therapies and a significantly decreased risk of nonfatal vascular events after a recent ischemic stroke. However, users of specific classes of CV-protective and non–CV-protective therapies present an important unrealistic risk reduction on all-cause mortality, demonstrating that other unmeasured confounders must explain part of the association. These findings also raise concerns about using observational studies in high-risk populations to infer associations between drug use and certain outcomes. Finally, clinicians may consider the level of adherence as a potential marker of healthy behaviors that may be useful in targeting stroke patients with unhealthy practices.38
Supplementary Material
Glossary
- AH
antihypertensive
- CV
cardiovascular
- ICD
International Classification of Diseases
- MPR
medication possession ratio
- RAMQ
Régie Assurance Maladie Québec
Footnotes
Supplemental data at www.neurology.org
AUTHOR CONTRIBUTIONS
S. Perreault: drafting/revising the manuscript, study concept or design, analysis or interpretation of data, acquisition of data, statistical analysis, study supervision, obtaining funding. A.Y.X. Yu: drafting/revising the manuscript. R. Côté: drafting/revising the manuscript, study concept or design, analysis or interpretation of data. A. Dragomir: drafting/revising the manuscript, study concept or design, analysis or interpretation of data, statistical analysis. B. White-Guay: drafting/revising the manuscript, analysis or interpretation of data. S. Dumas: analysis or interpretation of data, statistical analysis, literature review.
DISCLOSURE
S. Perreault and A.Y.X. Yu report no disclosures. R. Côté received honoraria for serving on scientific advisory boards of Boehringer-Ingelheim, Pfizer, Bristol-Myers Squibb, and Bayer. A. Dragomir, B. White-Guay, and S. Dumas report no disclosures. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Beaglehole R. Global cardiovascular disease prevention: time to get serious. Lancet 2001;358:661–663 [DOI] [PubMed] [Google Scholar]
- 2.Johnston SC, Mendis S, Mathers CD. Global variation in stroke burden and mortality: estimates from monitoring, surveillance, and modelling. Lancet Neurol 2009;8:345–354 [DOI] [PubMed] [Google Scholar]
- 3.Goldstein LB, Bushnell CD, Adams RJ, et al. Guidelines for the primary prevention of stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2011;42:517–584 [DOI] [PubMed] [Google Scholar]
- 4.Public Health Agency of Canada Tracking Heart Disease and Stroke in Canada. Ottawa: Government of Canada; 2009:132 [Google Scholar]
- 5.Ohira T, Shahar E, Chambless LE, Rosamond WD, Mosley TH, Jr, Folsom AR. Risk factors for ischemic stroke subtypes: the Atherosclerosis Risk in Communities Study. Stroke 2006;37:2493–2498 [DOI] [PubMed] [Google Scholar]
- 6.Fields LE, Burt VL, Cutler JA, Hughes J, Roccella EJ, Sorlie P. The burden of adult hypertension in the United States 1999 to 2000: a rising tide. Hypertension 2004;44:398–404 [DOI] [PubMed] [Google Scholar]
- 7.Lawes CM, Bennett DA, Feigin VL, Rodgers A. Blood pressure and stroke: an overview of published reviews. Stroke 2004;35:1024. [PubMed] [Google Scholar]
- 8.Rashid P, Leonardi-Bee J, Bath P. Blood pressure reduction and secondary prevention of stroke and other vascular events: a systematic review. Stroke 2003;34:2741–2748 [DOI] [PubMed] [Google Scholar]
- 9.Rothwell PM. Limitations of the usual blood-pressure hypothesis and importance of variability, instability, and episodic hypertension. Lancet 2010;375:938–948 [DOI] [PubMed] [Google Scholar]
- 10.Rothwell PM, Howard SC, Dolan E, et al. Effects of beta blockers and calcium-channel blockers on within-individual variability in blood pressure and risk of stroke. Lancet Neurol 2010;9:469–480 [DOI] [PubMed] [Google Scholar]
- 11.Webb AJ, Fischer U, Mehta Z, Rothwell PM. Effects of antihypertensive-drug class on interindividual variation in blood pressure and risk of stroke: a systematic review and meta-analysis. Lancet 2010;375:906–915 [DOI] [PubMed] [Google Scholar]
- 12.Lloyd-Jones DM, Evans JC, Levy D. Hypertension in adults across the age spectrum: current outcomes and control in the community. JAMA 2005;294:466–472 [DOI] [PubMed] [Google Scholar]
- 13.Mazzaglia G, Ambrosioni E, Alacqua M, et al. Adherence to antihypertensive medications and cardiovascular morbidity among newly diagnosed hypertensive patients. Circulation 2009;120:1598–1605 [DOI] [PubMed] [Google Scholar]
- 14.Paul SL, Thrift AG. Control of hypertension 5 years after stroke in the North East Melbourne Stroke Incidence Study. Hypertension 2006;48:260–265 [DOI] [PubMed] [Google Scholar]
- 15.Bramley TJ, Gerbino PP, Nightengale BS, Frech-Tamas F. Relationship of blood pressure control to adherence with antihypertensive monotherapy in 13 managed care organizations. J Manag Care Pharm 2006;12:239–245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Burnier M. Medication adherence and persistence as the cornerstone of effective antihypertensive therapy. Am J Hypertens 2006;19:1190–1196 [DOI] [PubMed] [Google Scholar]
- 17.Simpson SH, Eurich DT, Majumdar SR, et al. A meta-analysis of the association between adherence to drug therapy and mortality. BMJ 2006;333:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tamblyn R, Lavoie G, Petrella L, Monette J. The use of prescription claims databases in pharmacoepidemiological research: the accuracy and comprehensiveness of the prescription claims database in Quebec. J Clin Epidemiol 1995;48:999–1009 [DOI] [PubMed] [Google Scholar]
- 19.Wilchesky M, Tamblyn RM, Huang A. Validation of diagnostic codes within medical services claims. J Clin Epidemiol 2004;57:131–141 [DOI] [PubMed] [Google Scholar]
- 20.Tirschwell DL, Longstreth WT., Jr Validating administrative data in stroke research. Stroke 2002;33:2465–2470 [DOI] [PubMed] [Google Scholar]
- 21.Monfared AA, Rahme E, LeLorier J. Accuracy of ICD-9 diagnosis code ‘410’ to identify episodes of hospitalizations for acute myocardial infarction in RAMQ. Can J Clin Pharmacol 2004;11:e42 [Google Scholar]
- 22.Essebag V, Genest J, Jr, Suissa S, Pilote L. The nested case-control study in cardiology. Am Heart J 2003;146:581–590 [DOI] [PubMed] [Google Scholar]
- 23.Hess LM, Raebel MA, Conner DA, Malone DC. Measurement of adherence in pharmacy administrative databases: a proposal for standard definitions and preferred measures. Ann Pharmacother 2006;40:1280–1288 [DOI] [PubMed] [Google Scholar]
- 24.Osterberg L, Blaschke T. Adherence to medication. N Engl J Med 2005;353:487–497 [DOI] [PubMed] [Google Scholar]
- 25.LaFleur J, Nelson RE, Sauer BC, Nebeker JR. Overestimation of the effects of adherence on outcomes: a case study in healthy user bias and hypertension. Heart 2011;97:1862–1869 [DOI] [PubMed] [Google Scholar]
- 26.Glynn RJ, Knight EL, Levin R, Avorn J. Paradoxical relations of drug treatment with mortality in older persons. Epidemiology 2001;12:682–689 [DOI] [PubMed] [Google Scholar]
- 27.Kettani FZ, Dragomir A, Cote R, et al. Impact of a better adherence to antihypertensive agents on cerebrovascular disease for primary prevention. Stroke 2009;40:213–220 [DOI] [PubMed] [Google Scholar]
- 28.Perreault S, Ellia L, Dragomir A, et al. Effect of statin adherence on cerebrovascular disease in primary prevention. Am J Med 2009;122:647–655 [DOI] [PubMed] [Google Scholar]
- 29.Simpson RJ, Jr, Mendys P. The effects of adherence and persistence on clinical outcomes in patients treated with statins: a systematic review. J Clin Lipidol 2010;4:462–471 [DOI] [PubMed] [Google Scholar]
- 30.Stolar M. Glycemic control and complications in type 2 diabetes mellitus. Am J Med 2010;123:S3–S11 [DOI] [PubMed] [Google Scholar]
- 31.PROGRESS Collaborative Group Randomised trial of a perindopril-based blood-pressure-lowering regimen among 6,105 individuals with previous stroke or transient ischaemic attack. Lancet 2001;358:1033–1041 [DOI] [PubMed] [Google Scholar]
- 32.Lowy A, Munk VC, Ong SH, et al. Effects on blood pressure and cardiovascular risk of variations in patients’ adherence to prescribed antihypertensive drugs: role of duration of drug action. Int J Clin Pract 2011;65:41–53 [DOI] [PubMed] [Google Scholar]
- 33.Li C, Engstrom G, Hedblad B, Janzon L. Sex-specific cardiovascular morbidity and mortality in a cohort treated for hypertension. J Hypertens 2006;24:1523–1529 [DOI] [PubMed] [Google Scholar]
- 34.Amarenco P, Steg PG. Stroke is a coronary heart disease risk equivalent: implications for future clinical trials in secondary stroke prevention. Eur Heart J 2008;29:1605–1607 [DOI] [PubMed] [Google Scholar]
- 35.Patrick AR, Shrank WH, Glynn RJ, et al. The association between statin use and outcomes potentially attributable to an unhealthy lifestyle in older adults. Value Health 2011;14:513–520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Amarenco P, Bogousslavsky J, Callahan A, 3rd, et al. High-dose atorvastatin after stroke or transient ischemic attack. N Engl J Med 2006;355:549–559 [DOI] [PubMed] [Google Scholar]
- 37.Glynn RJ, Schneeweiss S, Wang PS, Levin R, Avorn J. Selective prescribing led to overestimation of the benefits of lipid-lowering drugs. J Clin Epidemiol 2006;59:819–828 [DOI] [PubMed] [Google Scholar]
- 38.Ovbiagele B, Campbell S, Faiz A, Chambless LE. Relationship between non-specific prescription pill adherence and ischemic stroke outcomes. Cerebrovasc Dis 2010;29:146–153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen J, Zhang ZG, Li Y, et al. Statins induce angiogenesis, neurogenesis, and synaptogenesis after stroke. Ann Neurol 2003;53:743–751 [DOI] [PubMed] [Google Scholar]
- 40.Choo PW, Rand CS, Inui TS, et al. Validation of patient reports, automated pharmacy records, and pill counts with electronic monitoring of adherence to antihypertensive therapy. Med Care 1999;37:846–857 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


