ABSTRACT
Objective:
To characterize clinical features, neuroimaging, and outcomes of herpes simplex encephalitis (HSE) in immunocompromised individuals.
Methods:
We performed a retrospective case control review of patients diagnosed with HSE. Adult patients were dichotomized into immunocompromised (n = 14) and immunocompetent groups (n = 15).
Results:
Fewer immunocompromised patients presented with prodromal symptoms and focal deficits. While the majority of CSF profiles in the immunocompromised patients were mononuclear cells predominant, 3 had polymorphonuclear predominance and another 3 had normal profiles. MRI showed widespread cortical involvement, with brainstem or cerebellar involvement in some. Two immunocompromised patients had recurrent HSE. The immunosuppressed state was associated with a decrease in Karnofsky Performance Status Scale (KPSS) score of 23.1 (p = 0.018). Every 1-day delay in initiation of acyclovir was associated with a decrease in KPSS of 10.2 (p = 0.002), and every 10 cell/mm3 increase of CSF leukocytosis was associated with an increase in KPSS of 0.7 (p = 0.009). Mortality rate was 6 times higher in the immunocompromised patients.
Conclusions:
Immunocompromised states may predispose to HSE with atypical clinical and neuroradiologic features. Immunocompromised individuals with HSE have significantly worse outcomes and mortality. Early diagnosis and treatment is associated with improved outcome. The findings are particularly important in light of the increasing use of potent immunosuppressive and immunomodulatory therapies.
Herpes simplex encephalitis (HSE) is the most common cause of sporadic viral encephalitis in the Western world.1,2 It remains a rare but serious disease with an incidence of 1 in 250,000 to 500,000 population.3 The introduction of acyclovir in 1984 markedly reduced the mortality rate of HSE from about 70% previously to about 16% at 6 months follow-up.3 However, there may be renewed concern, specifically the emergence of herpes simplex virus (HSV) resistance to acyclovir, a phenomenon which may occur in immunocompromised hosts.4 Despite effective antiviral therapy, the mortality has been variably reported as 4%–28%, and only about 15%–38% of patients return to a normal level of functioning.1,2,5,6 PCR introduced in the 1990s allows the detection of DNA of HSV from CSF samples. This is more widely used compared to brain biopsy as the gold standard in the diagnosis of HSE. Although HSE is not regarded as an opportunistic infection, HSE in immunocompromised patients may have atypical presentations.7 Early recognition of the infection is critical since a delay in acyclovir administration is associated with severe morbidity and mortality.8,9 This is particularly important as newer immunotherapies are being increasingly used to treat autoimmune diseases, malignancies, and other disorders. In this study, we examined whether clinically defined immunocompromised states impacted the clinical manifestations, management, and outcome of HSE.
METHODS
We performed a retrospective review of all patients presenting with encephalitis at the Johns Hopkins Hospital (JHH), a tertiary care medical center, between January 1997 and April 2010. For the purpose of this study, encephalitis was defined as an altered mental state, change in personality or focal neurologic deficits, and ≥1 of the following: 1) fever, 2) seizure, 3) CSF pleocytosis, 4) EEG consistent with encephalopathy (focal or diffuse slow activities), 5) neuroimaging findings consistent with encephalitis (uni- or bitemporal signal hyperintensities in MRI T2/fluid-attenuated inversion recovery sequences for HSE). The exclusion criteria include delirium or encephalopathy secondary to sepsis, toxin, or metabolic causes (hypoglycemia, electrolyte disturbances). We screened the databases with the following ICD-9 coded diagnoses: encephalopathy, encephalitis, infections of the CNS, postinfectious encephalitis, and autoimmune encephalitis. From this encephalitis database, patients were included in this analysis if HSV DNA was detected in the CSF via PCR analysis. Patients with presumed HSE with negative HSV PCR or those with meningismus without encephalopathy suggestive of herpes meningitis were excluded.
We collected data on demographics, clinical characteristics, CSF analyses, MRI features, treatment, and clinical outcomes (table 1). The outcomes were graded according to Karnofsky Performance Status Scale (KPSS). HSE relapse was defined as recrudescence of symptoms within 1 month after discontinuation of acyclovir, while the term recurrent HSE was used if the interval was >1 month. Adult patients (age >18 years old) were dichotomized into immunocompromised and immunocompetent groups (table 1). The immunocompromised group included patients with chronic HIV infection, transplant recipients, patients on immunosuppressive therapies, patients with active malignancy, patients with diabetes mellitus, and patients with renal insufficiency. The immunocompetent group consisted of patients without documented immunodeficiency state.
Table 1.
Comparison of clinical characteristics of immunocompromised and immunocompetent groups

We performed a literature search with PubMed (from 1965 to current) with search items including “herpes simplex encephalitis,” “immunocompromised host,” and “immunosuppression.”
Standard protocol approvals, registrations, and patient consents
This study was approved by the Johns Hopkins University Institutional Review Board.
Statistical analysis
We assessed all potential variables for their association with outcomes in univariate models. We identified, a priori, variables that might be confounders that should be assessed in multivariate models, including age, sex, immunocompromised state, seizure at presentation, CSF pleocytosis, MRI bitemporal involvement, the delay between hospital presentation and acyclovir administration, and the completion of 21-day course of acyclovir. Continuous variables are reported as mean ± SD. All statistical tests were performed at the 2-tailed 5% level of significance. p Values of <0.05 were considered significant. Statistical analysis was performed on STATA version 11 (StataCorp, College Station, TX).
RESULTS
Of the 415 patients in the JHH encephalitis database, 35 patients had a confirmed diagnosis of HSE based on CSF PCR analysis (table 1). Six pediatric patients (age < 18 years) were excluded in the subsequent analysis. The average age was 55.1 ± 16.1 years (range 26–79 years). The male to female ratio was 1:1.1. There were 14 patients in the immunocompromised group and 15 patients in the immunocompetent group. The mean age in the immunocompromised group was 56.2 ± 12.5 years and in the immunocompetent group, 54.1± 19.3 years.
Causes of immunocompromised state
Table 2 describes immunocompromised conditions in this cohort of 14 patients. Three patients had chronic HIV infection (CD4 cells, range 6–740 cells/mm3), 4 had intracranial tumors (one received cranial radiotherapy, another had intrathecal chemotherapy), 3 had hematologic malignancies (one received a bone marrow transplant), 1 was a renal transplant recipient, and 3 patients were on immunosuppressive agents for connective tissue disorders.
Table 2.
Causes of immunocompromised state
Clinical presentations
Prodromal symptoms were present in only 28.6% of the immunocompromised group, compared to 80% of the immunocompetent group (p = 0.01). Fever was present in over two-thirds of the patients, while seizures were present in about half of the patients in both groups. Focal neurologic deficits were significantly less frequent in the immunocompromised group (28% vs 73.3%, p = 0.02).
The duration between onset of symptoms and presentation to the hospital was shorter, 3.4 ± 3.7 days, in the immunocompromised group compared to 4.9 ± 3.8 days in the immunocompetent group. The delay between hospital admission and acyclovir administration was similar between the 2 groups, averaging about 24 hours. Fewer than one-third of the immunocompromised group (28.6%) were transferred from outside hospitals compared to about two-thirds of the immunocompetent group (60.0%). Both HSV and varicella-zoster virus DNA were detected in an immunocompromised patient. Three patients in the immunocompromised group had been admitted to the hospital for treatment of unrelated conditions when they developed HSE.
CSF examination
Three patients in the immunocompromised group did not have CSF pleocytosis (patients treated for polymyositis, brain metastasis, and leptomeningeal metastasis), while only one patient in the immunocompetent group showed no CSF pleocytosis. The CSF white cell count was 132.5 ± 191.9 cells/mm3 (range: 1–621 cells/mm3) in the immunocompromised group compared to 163.0 ± 205.7 cells/mm3 (range: 1–717 cells/mm3) in the immunocompetent group. Three patients in the immunocompromised group had a polymorphonuclear predominance in the CSF (bone marrow transplantation, Hodgkin lymphoma, and cranial irradiation), and the remaining patients had predominance of mononuclear cells; all patients in the immunocompetent group had a mononuclear predominance (table 2).
Neuroimaging
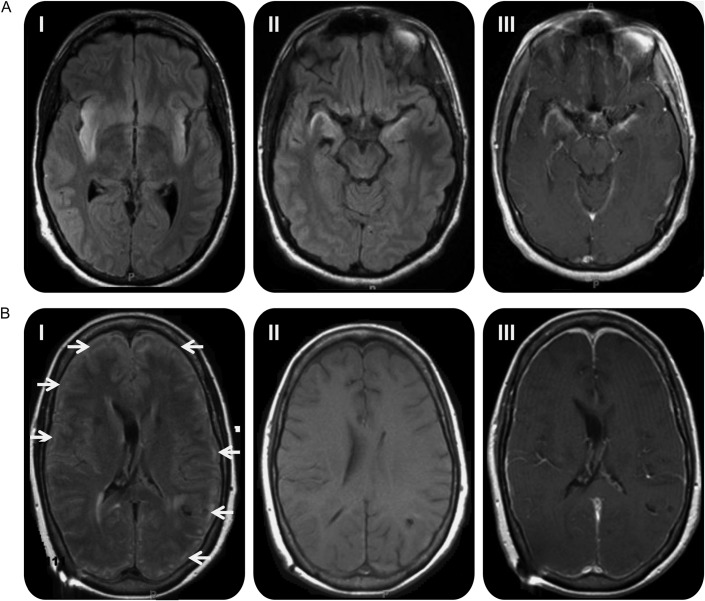
All but one patient (un-interpretable MRI due to motion artifact) in the immunocompromised group had an abnormal brain MRI. The majority (10 out of 12 patients, 78.6%) had either unilateral (8 patients) or bilateral (3 patients) temporal lobe involvement (figure, A). Three patients had additional brainstem involvement, and 2 others had additional cerebellar involvement. The remaining 2 patients had widespread cortical involvement (figure, B), and one of them also had brainstem abnormalities in addition to widespread cerebral lesions. In the immunocompetent group, temporal lobe abnormalities were observed in every case. Contrast enhancement was evident in about half of the patients in both groups.
Figure. MRI findings in patients with herpes simplex encephalitis.
(A) High signal intensity lesions are seen in both temporal lobes involving the (I) insular cortices and (II) hippocampi on T2 fluid-attenuated inversion recovery (FLAIR) sequences, with (III) gadolinium contrast enhancement in the temporal lobes and basal frontal lobes in an immunocompetent patient. (B) (I) High signal intensity lesions on T2 FLAIR sequences in the cortical gyri in an immunocompromised patient (arrows). (II) T1-weighted image without contrast and (III) with contrast does not show any evidence of contrast enhancement in the lesions.
Histopathology
Only 1 patient in our cohort (immunocompromised group) had a brain biopsy in the setting of recurrent HSE (6 episodes). Brain biopsy showed gliosis and chronic inflammation only while immunostaining for HSV was negative, a finding confirmed at autopsy despite positive HSV PCR during multiple relapses.
Acyclovir treatment
Eleven of 14 patients in the immunocompromised group, compared to 11 of 15 patients in the immunocompetent group, completed a 21-day course of acyclovir at 30 mg/kg/d. Five patients in the immunocompetent group completed a 14-day course. There were 6 deaths (16.2%): 5 (35.7%) in the immunocompromised group and 1 (6.7%) in the immunocompetent group.
Outcome
There was no significant difference in the length of hospital stay between the 2 groups, averaging about 2.5 weeks.
Two patients who had recurrent HSE were both immunocompromised: one patient had 6 episodes of recurrences, completed 3 cycles of 21-day regimen of acyclovir, and 3 cycles of a 21-day course of foscarnet; the other patient had completed 21 days of acyclovir and developed recurrence 6 months later. Two patients in the immunocompetent group relapsed within 1 month of an initial 14-day treatment. Three patients (1 immunocompromised, 2 immunocompetent) received corticosteroids in addition to acyclovir. They had a moderate outcome (KPSS between 60 and 70) despite delayed acyclovir administration with an average delay of 7 days from late presentations to the hospital.
The outcome was worse in the immunocompromised group (KPSS 39 ± 33) compared to KPSS 61 ± 26 in the immunocompetent group (p < 0.05). The mortality rate was almost 6 times as high in the immunocompromised group (35.7%) compared to the immunocompetent group (6.7%) (p = 0.05). The time from onset of symptoms to time of death was shorter, 17.6 ± 2 days, in the immunocompromised group compared to 30 days in the immunocompetent patient.
In multivariate linear regression models (table e-1 on the Neurology® Web site at www.neurology.org), every increase in 10 cells/mm3 of CSF white cell count was associated with an increase in KPSS of 0.7. Every 1-day delay in acyclovir administration from the time of hospital admission was associated with a decrease in KPSS of 10.2, after adjustment. Immunosuppressed state was significantly associated with a decrease in KPSS of 22.1. Age, gender, seizures, and bitemporal lesions on MRI and the completion of acyclovir therapy were not associated with outcome as measured by KPSS.
DISCUSSION
This is the largest series of patients reported in the literature on HSE in immunocompromised patients and the first to directly compare the presentation in immunocompromised and immunocompetent states. Despite the retrospective nature of our study, there are several important observations. 1) Immunocompromised patients may present with less prodromal symptoms, and focal deficits. 2) Immunocompromised adults have more extensive involvement of the brain and abnormalities may involve the brainstem, cerebellum, and atypical regions including scattered lesions in the cerebrum, in the absence of temporal lobe involvement. 3) Absence of CSF pleocytosis was not uncommon in immunocompromised patients. 4) The morbidity and mortality of HSE were substantially higher in the immunocompromised group. 5) Immunocompromised state, lower CSF pleocytosis, and delayed acyclovir administration were associated with poor outcomes.
The extensive and contiguous areas of brain involvement in the immunocompromised patients may reflect a wider spread of HSV due to hosts’ ineffective immune response to control the infection. This is further reflected in the frequent absence of pleocytosis in the CSF of immunocompromised patients as seen in this and other reports.10–14 However, the absence of pleocytosis may be also be seen, albeit rarely, in immunocompetent patients, particularly if CSF is evaluated early in the course of the illness.10,15 Additionally, in CSF, polymorphonuclear predominance was noted in some immunocompromised patients. These atypical features can make the diagnosis of HSE in immunocompromised patients challenging. Recurrence of herpes encephalitis may occur despite adequate antiviral therapy in the immunocompromised patients, likely because the antiviral drugs only prevent viral replication and the immune system is needed to eliminate the replicating virus or to maintain it in the latent state.
Sporadic HSE is the most common form of nonepidemic encephalitis in adults and the majority of cases (94%–96%) are caused by HSV-1.11 Latent HSV-1 infections of the trigeminal ganglia, by retrograde axonal transportation from a primary infection of the lip or buccal mucosa, were found in 65% of normal individuals.12 HSV-1 genomic sequences were also detected in the medulla, pons, olfactory bulbs, and gyrus rectus in 28%–34% of normal individuals.12,13 Although HSV-1 reactivation may occur by immunosuppression, the mechanism underlying the reactivation resulting in HSE is not well understood.
One hypothesis is that local breach of immune surveillance in the instance of cranial irradiation leads to reactivation of the latent virus in the ganglia and the brain14; and likewise, in a systemic immunocompromised state, reactivation of the virus may occur due to the breakdown of immune surveillance. Recent evidence suggests that HSV-1 may not achieve a true stage of latency and immune surveillance is critical in preventing its spread.16 The findings of a recent Swedish nationwide retrospective study examining the incidence, morbidity, and mortality did not suggest an overrepresentation of immunocompromised hosts in the cohort.2 Some, however, have argued that the atypical presentation of HSE in immunocompromised individuals might have led to an underestimation of the incidence in immunocompromised hosts.7 Immunosuppression alone is probably not enough to cause reactivation, but may enhance reactivation in conjunction with other factors.17
Our literature review yielded 28 articles reporting HSE in immunocompromised hosts (table e-2). Overall, 49 cases were reported, 27 cases predating the widespread use of MRI and HSV PCR in the diagnosis of HSE. Cases in which diagnoses were confirmed at autopsy provided histopathologic characterization. There is a broad spectrum of immunocompromised conditions associated with HSE.
Atypical clinical manifestations have been previously reported in HIV-infected patients. HSE may present as a diffuse non-necrotizing encephalitis involving the hemispheres and brainstem.18,19 In earlier series, about one-sixth of the patients (4 out of 24) had mild or atypical disease characterized by the absence of focal findings and slower progression, regardless of CD4+ T-cell counts.20 In another series of 8 patients with HIV infection/AIDS, the speed of disease progression and lack of inflammation were proportional to the degree of immunocompromised states. Patients with advanced AIDS had chronic neurologic dysfunction and diffuse leukoencephalopathy on autopsy.21
Typical neuroimaging abnormalities involve the medial temporal lobes, either unilaterally or bilaterally, and spread along limbic pathways to involve the orbital frontal lobe and insular cortex. With further spread, cingulate gyri, parietal lobes, occipital lobes, brainstem, and internal capsules may be involved.22 Atypical neuroimaging abnormalities have been reported frequently in the literature, although this may represent a reporting bias. Some of the earlier case series and reports only utilized CT brain scans. Unremarkable CT and MRI were reported in some,7,20,23,24 while widespread signal abnormalities throughout the brain, involving cortex, basal ganglia, thalamus, brainstem, and cerebellum, were reported in the others.7,25,26
The extensive and rapid spread of the infection may explain the lesser prodromal presentations and a shorter delay between onset of symptoms and presentation to the hospital in immunocompromised patients.
Clinicohistopathologic correlations in HSE differ between immunocompromised and immunocompetent patients. In typical HSE, fever and headache preceded the development of encephalopathy and personality change by 1–5 days. During the second week, inflammation and necrosis appeared predominantly in medial temporal and olfactory stria/inferior frontal lobes, cingulate gyri, and occasionally pontine nuclei. By the third week, there was extensive necrosis, inflammation, and gliosis with scant HSV-1.17,27 In contrast, in immunocompromised hosts, there was a conspicuous lack of inflammation, necrosis, and hemorrhage on histopathology, with persistence of abundant viral antigens.7,20,24,28 This may reflect the host’s inability to mount an adequate immune response to limit the course of HSE in immunocompromised hosts, paradoxically limiting tissue damage with the lack of frank necrosis. While an intact immune system may mount an intense inflammatory response leading to significant CNS injury, the lack of it severely hampers the ability to clear the infection, leading to increased morbidity and mortality. The differing mechanistic pathogenesis may help explain the atypical clinical presentations. More recently, primary immunodeficiencies involving and related to mutations in toll-like receptors (TLR-3) have been implicated in the reactivation of HSV and HSE in children.29–31 The potential role of the innate immune response in the pathogenesis of HSE in adults needs to be more fully investigated.
HSE has been reported in 3 patients with rheumatologic disorders treated with anti–tumor necrosis factor antibody therapies.32 Whether the immunosuppressive state predisposes to HSE is pertinent in this era of increasing use of potent immunomodulatory therapies. There was a fatal case of HSE during the clinical trial for FTY720 in the treatment of multiple sclerosis (MS). In the same clinical trial, the incidence of HSV infection was reportedly twice as high in the higher dose FTY720 group as compared to the placebo group.33 There was also at least one case of HSE fatality in a patient treated with natalizumab, a monoclonal antibody against α-4 integrin for treatment of relapsing-remitting MS in postmarketing surveillance (personal communication, Biogen-Idec, 2011). In patients on immunomodulatory therapies, a more systemic analysis is required to determine if there is an increased risk of HSE. Nonetheless, a high vigilance for HSE including atypical presentations must be maintained.
We analyzed patients with different degrees, spectrum, and conditions of immunocompromised states under one group. Descriptive analyses as attempted in table 2 did not demonstrate patterns of clinical presentation, or investigation or outcome across different spectrum and severity of immunosuppression. The size of the study did not allow meaningful subanalyses and interpretation of clinical characteristics of various conditions. However, this study served to raise awareness of the potential increased risk, atypical presentations, and worse outcomes among immunocompromised patients.
A limitation of our study is its retrospective nature. In addition, HSV PCR performed in the clinical laboratory at our institution did not routinely distinguish between HSV-1 and HSV-2. Only patients who had HSV detectable by PCR were included in this study, so it is possible that cases in which the viral copy numbers were below the level of detection of the PCR assay may have been excluded. This had been reported in immunocompromised patients.34 The experience of our institute as a tertiary care center may reflect a referral bias with an over-representation of immunocompromised hosts. In addition, the high proportion of immunocompetent patients transferred from outside hospitals may reflect a sicker patient cohort and may thus underestimate the difference in outcome between the 2 groups. The literature review may also be biased in selectively reporting cases of atypical presentation of HSE in immunocompromised hosts.
Thus, the immunodeficiency state may alter the extent and magnitude of HSE-associated CNS injury. HSE with atypical clinical presentations, CSF profile of low white cell count or polymorphonuclear cells predominance, and widespread MRI abnormalities were more likely in immunocompromised hosts. HSE in immunocompromised hosts had a more rapid course of progression with increased morbidity and mortality. Immunocompromised state, lower CSF white cells, and delayed administration of acyclovir were associated with worse outcomes.
Supplementary Material
GLOSSARY
- HSE
herpes simplex encephalitis
- HSV
herpes simplex virus
- JHH
Johns Hopkins Hospital
- KPSS
Karnofsky Performance Status Scale
- MS
multiple sclerosis
Footnotes
Supplemental data at www.neurology.org
AUTHOR CONTRIBUTIONS
I.L. Tan: design, analysis, and writing of the manuscript. J.C. McArthur: design, review, and writing of the manuscript. A. Venkatesan: design, review, and writing of the manuscript. A. Nath: design, analysis writing, and review of the manuscript.
DISCLOSURE
I.L. Tan reports no disclosures. J.C. McArthur has received grants from NIH and Biogen-Idec and stock options from Gliamed. A. Venkatesan and A. Nath report no disclosures. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Granerod J, Ambrose HE, Davies NW, et al. Causes of encephalitis and differences in their clinical presentations in England: a multicentre, population-based prospective study. Lancet Infect Dis 2010;10:835–844 [DOI] [PubMed] [Google Scholar]
- 2.Hjalmarsson A, Blomqvist P, Skoldenberg B. Herpes simplex encephalitis in Sweden, 1990–2001: incidence, morbidity, and mortality. Clin Infect Dis 2007;45:875–880 [DOI] [PubMed] [Google Scholar]
- 3.Whitley RJ. Herpes simplex encephalitis: adolescents and adults. Antiviral Res 2006;71:141–148 [DOI] [PubMed] [Google Scholar]
- 4.Rozenberg F, Deback C, Agut H. Herpes simplex encephalitis: from virus to therapy. Infect Disord Drug Targets 2011;11:235–250 [DOI] [PubMed] [Google Scholar]
- 5.Whitley RJ, Alford CA, Hirsch MS, et al. Vidarabine versus acyclovir therapy in herpes simplex encephalitis. N Engl J Med 1986;314:144–149 [DOI] [PubMed] [Google Scholar]
- 6.Riera-Mestre A, Gubieras L, Martinez-Yelamos S, Cabellos C, Fernandez-Viladrich P. Adult herpes simplex encephalitis: fifteen years' experience. Enferm Infecc Microbiol Clin 2009;27:143–147 [DOI] [PubMed] [Google Scholar]
- 7.Schiff D, Rosenblum MK. Herpes simplex encephalitis (HSE) and the immunocompromised: a clinical and autopsy study of HSE in the settings of cancer and human immunodeficiency virus-type 1 infection. Hum Pathol 1998;29:215–222 [DOI] [PubMed] [Google Scholar]
- 8.McGrath N, Anderson NE, Croxson MC, Powell KF. Herpes simplex encephalitis treated with acyclovir: diagnosis and long term outcome. J Neurol Neurosurg Psychiatry 1997;63:321–326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Raschilas F, Wolff M, Delatour F, et al. Outcome of and prognostic factors for herpes simplex encephalitis in adult patients: results of a multicenter study. Clin Infect Dis 2002;35:254–260 [DOI] [PubMed] [Google Scholar]
- 10.Domingues RB, Tsanaclis AM, Pannuti CS, Mayo MS, Lakeman FD. Evaluation of the range of clinical presentations of herpes simplex encephalitis by using polymerase chain reaction assay of cerebrospinal fluid samples. Clin Infect Dis 1997;25:86–91 [DOI] [PubMed] [Google Scholar]
- 11.Whitley RJ, Soong SJ, Linneman C, Jr, Liu C, Pazin G, Alford CA. Herpes simplex encephalitis: clinical assessment. JAMA 1982;247:317–320 [PubMed] [Google Scholar]
- 12.Baringer JR, Pisani P. Herpes simplex virus genomes in human nervous system tissue analyzed by polymerase chain reaction. Ann Neurol 1994;36:823–829 [DOI] [PubMed] [Google Scholar]
- 13.Sanders VJ, Waddell AE, Felisan SL, Li X, Conrad AJ, Tourtellotte WW. Herpes simplex virus in postmortem multiple sclerosis brain tissue. Arch Neurol 1996;53:125–133 [DOI] [PubMed] [Google Scholar]
- 14.Koudriavtseva T, Onesti E, Tonachella R, Pelagalli L, Vidiri A, Jandolo B. Fatal herpetic encephalitis during brain radiotherapy in a cerebral metastasized breast cancer patient. J Neurooncol 2010;100:137–140 [DOI] [PubMed] [Google Scholar]
- 15.Schlageter N, Jubelt B, Vick NA. Herpes simplex encephalitis without CSF leukocytosis. Arch Neurol 1984;41:1007–1008 [DOI] [PubMed] [Google Scholar]
- 16.Zhou L, Li J, Wang X, et al. IL-29/IL-28A suppress HSV-1 infection of human NT2-N neurons. J Neurovirol 2011;17:212–219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Johnson M, Valyi-Nagy T. Expanding the clinicopathologic spectrum of herpes simplex encephalitis. Hum Pathol 1998;29:207–210 [DOI] [PubMed] [Google Scholar]
- 18.Lach B, Atack E. Disseminated hemorrhagic leukoencephalomyelitis with localized herpes simplex brain stem infection. Acta Neuropathol 1988;75:354–361 [DOI] [PubMed] [Google Scholar]
- 19.Martin JR, Holt RK, Webster HD. Herpes-simplex-related antigen in human demyelinative disease and encephalitis. Acta Neuropathol 1988;76:325–337 [DOI] [PubMed] [Google Scholar]
- 20.Fodor PA, Levin MJ, Weinberg A, Sandberg E, Sylman J, Tyler KL. Atypical herpes simplex virus encephalitis diagnosed by PCR amplification of viral DNA from CSF. Neurology 1998;51:554–559 [DOI] [PubMed] [Google Scholar]
- 21.Levy RM, Bredesen DE, Rosenblum ML. Neurological manifestations of the acquired immunodeficiency syndrome (AIDS): experience at UCSF and review of the literature. J Neurosurg 1985;62:475–495 [DOI] [PubMed] [Google Scholar]
- 22.Wasay M, Mekan SF, Khelaeni B, et al. Extra temporal involvement in herpes simplex encephalitis. Eur J Neurol 2005;12:475–479 [DOI] [PubMed] [Google Scholar]
- 23.Auyeung P, Dunn A. Atypical case of herpes simplex encephalitis. Intern Med J 2008;38:294–295 [DOI] [PubMed] [Google Scholar]
- 24.Price R, Chernik NL, Horta-Barbosa L, Posner JB. Herpes simplex encephalitis in an anergic patient. Am J Med 1973;54:222–228 [DOI] [PubMed] [Google Scholar]
- 25.Bordes J, Kenane N, Meaudre E, et al. A case of atypical and fatal herpes simplex encephalitis in a severe burn patient. Burns 2009;35:590–593 [DOI] [PubMed] [Google Scholar]
- 26.Laskin OL, Stahl-Bayliss CM, Morgello S. Concomitant herpes simplex virus type 1 and cytomegalovirus ventriculoencephalitis in acquired immunodeficiency syndrome. Arch Neurol 1987;44:843–847 [DOI] [PubMed] [Google Scholar]
- 27.Skoldenberg B. Herpes simplex encephalitis. Scand J Infect Dis Suppl 1996;100:8–13 [PubMed] [Google Scholar]
- 28.Manz HJ, Phillips TM, McCullough DC. Herpes simplex type 2 encephalitis concurrent with known cerebral metastases. Acta Neuropathol 1979;47:237–240 [DOI] [PubMed] [Google Scholar]
- 29.Casrouge A, Zhang SY, Eidenschenk C, et al. Herpes simplex virus encephalitis in human UNC-93B deficiency. Science 2006;314:308–312 [DOI] [PubMed] [Google Scholar]
- 30.Zhang SY, Jouanguy E, Ugolini S, et al. TLR3 deficiency in patients with herpes simplex encephalitis. Science 2007;317:1522–1527 [DOI] [PubMed] [Google Scholar]
- 31.Lima GK, Zolini GP, Mansur DS, et al. Toll-like receptor (TLR) 2 and TLR9 expressed in trigeminal ganglia are critical to viral control during herpes simplex virus 1 infection. Am J Pathol 2010;177:2433–2445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bradford RD, Pettit AC, Wright PW, et al. Herpes simplex encephalitis during treatment with tumor necrosis factor-alpha inhibitors. Clin Infect Dis 2009;49:924–927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cohen JA, Barkhof F, Comi G, et al. Oral fingolimod or intramuscular interferon for relapsing multiple sclerosis. N Engl J Med 2010;362:402–415 [DOI] [PubMed] [Google Scholar]
- 34.Graber JJ, Rosenblum MK, DeAngelis LM. Herpes simplex encephalitis in patients with cancer. J Neurooncol 2011;105:415–421 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.