ABSTRACT
Objective:
Circulating endothelial cells (CECs) and microparticles (MPs) have been reported to reflect endothelial injury, cellular activation, and MP-mediated thrombin generation. We tested the hypothesis that these indices differ between children with cerebral arteriopathy and arterial ischemic stroke (AIS) recurrence, and those with a single event.
Methods:
This was a single-center cross-sectional study of 46 children with AIS and cerebral arteriopathy matched with pediatric controls. AIS recurrence was defined as new acute neurologic deficit with radiologic evidence of further cerebral infarction. CECs and MPs were identified with immunomagnetic bead extraction and flow cytometry, respectively. MP function as assessed by thrombin generation was determined using a fluorogenic assay.
Results:
Ten children had AIS recurrence while 36 had a single AIS event. CECs were raised in children with recurrent AIS, compared to those with no recurrence (p = 0.0001), and in controls (p = 0.0001). Total circulating annexin V+ MPs were significantly greater in children with recurrence than in those with no recurrence (p = 0.0020). These MPs were of endothelial or platelet origin, and a subpopulation expressed tissue factor. Finally, MP-mediated thrombin generation was enhanced in children with recurrent AIS compared to those with no recurrence (p = 0.0001), providing a link between inflammation, endothelial injury, and increased thrombotic tendency.
Conclusion:
Despite the wide spectrum of clinical and radiologic presentation of childhood AIS, indices of endothelial injury and cellular activation are different in patients with single and recurrent events. This novel approach has potential for furthering understanding of AIS pathophysiology and prognosis.
Childhood arterial ischemic stroke (AIS) results in neurologic morbidity in over two-thirds of survivors and recurrence in up to 20%.1–3 Current treatment approaches primarily focus on preventing recurrence.4 Most affected children have cerebral arteriopathies, which although radiologically similar, are likely to represent distinct pathologic entities.1,5–8 For example, both focal cerebral arteriopathy (FCA) and primary angiitis of the CNS (PACNS) manifest focal proximal occlusive disease radiologically. However, FCA is generally monophasic, while PACNS typically has a recurrent course, possibly due to persistence of an aberrant inflammatory response.9 Previous studies have proposed radiologic predictors of arteriopathy progression and AIS recurrence but neither these, nor any currently described biomarker, robustly distinguish between patients with a likely monophasic or recurrent course.7,8
Endothelial microparticles (MPs) and whole circulating endothelial cells (CECs) allow tracking of the endothelial events associated with vascular injury in patients with systemic vasculitides.10–15 MPs are membrane vesicles <1 μm in size, released during cell activation and rich in aminophospholipids.10,12–14 The procoagulant activity of MPs can be assessed by determining the MP-mediated thrombin generation in vitro.13,16,17 We have previously shown that children with systemic vasculitis and thrombosis have an enhanced MP-mediated thrombin generation thus linking inflammation, endothelial injury, and prothrombotic propensity.13
In this study we examined whether children with recurrent AIS have evidence of increased endothelial injury (increased CEC and endothelial MP) and excessive MP-mediated thrombin generation compared with those with a single AIS event.
METHODS
Study design and patient population
This was a cross-sectional study of children aged >28 days with AIS and imaging evidence of cerebral/cervical arteriopathy presenting or under follow-up at Great Ormond Street Hospital (GOSH) from September 2007 to January 2011. AIS was defined as an acute focal neurologic deficit attributable to cerebral infarction in a corresponding arterial distribution. Cerebral/cervical arteriopathy was defined as focal or segmental stenosis or occlusion, with regular or irregular abnormalities of the arterial wall7 and categorized according to current consensus definitions.18 The category of transient cerebral arteriopathy in this classification has more recently been superseded by the term FCA, which was used in this study.19 Exclusion criteria were systemic conditions that cause endothelial injury (sickle cell disease, systemic vasculitis, other autoimmune diseases); histopathologic confirmation of cerebral vasculitis; cardiac disease; and other potentially confounding syndromic diagnoses. Patients with arterial occlusion but no other arterial wall changes were also excluded as these radiologic appearances could result from cardioembolic or paradoxical embolism.7 In addition to the cross-sectional study, children with a new presentation of AIS and cervical/cerebral arteriopathy were evaluated prospectively. Blood samples were obtained from healthy controls comprising children undergoing minor surgical procedures (such as hernia repair, preoperative blood samples obtained) with no identifiable medical history of chronic illnesses or syndromic diagnosis. A disease control group was included comprising of children with cerebral arteriovenous malformations (AVM) since these patients have noninflammatory vascular pathologies.
Standard protocol approvals, registration, and patient consent
The study was approved by the Institutional Ethics Committee (COREC 07/Q0508/58). Informed consent was obtained from all parents/guardians and participants/child healthy controls.
Clinical, laboratory, and radiologic data
All children with AIS routinely underwent magnetic resonance imaging (MRI) and magnetic resonance angiography (MRA) within 48 hours of presentation for confirmation of the diagnosis and identification of possible cerebral/cervical arteriopathy. MRI and MRA were performed on a 1.5-T MR scanner using a standard imaging protocol, including T2-weighted turbo spin-echo imaging in the axial plane, fluid-attenuated inversion recovery (FLAIR) sequence in the coronal plane, T1-weighted spin-echo imaging in the sagittal plane, and 3-dimensional short-echo time-of-flight MRA of the circle of Willis. Catheter arteriography (CA) was performed at the clinician's discretion, as this may provide adjunctive yield for detection of arteriopathy.20 Blood was drawn before CA was performed to exclude procedure-related endothelial injury. Cerebral/cervical arteriopathy was identified on MRA, or CA where available, and categorized using currently recommended definitions.6,7,18,19,21 All children underwent comprehensive investigation for AIS risk factors (see supplemental material on the Neurology® Web site at www.neurology.org). Treatment was based on current international management guidelines.21–23 Patients were then re-imaged with MRI/MRA at 6 and 12 months and annually thereafter for clinical monitoring.
Patients were categorized into 2 groups according to AIS recurrence (>1 week after initial clinical event) and identified at re-presentation to our institution. Where clinical recurrence occurred, children underwent additional MRI/MRA imaging to identify new areas of arterial territory infarction or progression of arteriopathy, defined as progression of previously identified arterial disease (more extensive segment involved, more severe occlusive disease) or involvement of previously unaffected vessels or new arterial occlusion.
Circulating endothelial cells
CECs were identified with immunomagnetic bead extraction based on an international consensus protocol.11,24 CECs were defined as Ulex europaeus lectin (Sigma-Aldrich) bright cells >10 μm in size, with >5 magnetic beads attached (figure e-1A).11,15,24
Cell-derived microparticles
MPs were identified by flow cytometry as previously described (figure e-1, B–D).12,13 Blood was collected in 3.2% buffered citrate and centrifuged at 5,000 g for 5 minutes twice to obtain platelet-poor plasma (PPP). MPs were sedimented from 200 μL of PPP after centrifugation at 17,000 g for 60 minutes and resuspended in An V binding buffer (BD PharMingen, Oxford, UK) prior to incubating with fluorescent monoclonal antibodies: fluorescein isothiocyanate (FITC), phycoerythrin (PE) or PERCP-Cy5·5 labeled annexin V (BD PharMingen), mouse PE-labeled antihuman CD62e (clone 68-5H11, BD PharMingen), mouse PE-labeled antihuman CD31 (clone L133.1, BD PharMingen), mouse PE-labeled antihuman CD11b activation epitope (clone CBRM1/5, Biolegend), mouse Cy5-labeled antihuman CD14 (clone 61D3,AbD Serotec), mouse FITC-labeled antihuman TF (clone VD8, American Diagnostica), and mouse PE-labeled antihuman P-selectin glycoprotein ligand 1, PSGL-1 (clone KPL1, BD PharMingen). Additional labeling with mouse antihuman CD42a-PERCP (BD PharMingen) to exclude platelet origin was conducted. Samples were analyzed with a FACS Calibur flow cytometer (BD PharMingen).
Thrombin generation assay and von Willebrand factor antigen levels
The thrombin generation assay (TGA) measures the amount of active thrombin produced in plasma or whole blood after re-calcification by monitoring the conversion of a fluorogenic substrate cleaved by thrombin as the latter is generated.13,16,17 Direct calculation of molecular concentrations of thrombin are possible by comparison to a concurrently run calibrator.13,16,17 For the previously described MP-mediated TGA,13,17 MPs were sedimented from PPP and resuspended in 200 μL of control microparticle-free plasma (MPFP) containing 30 μg/mL of corn trypsin inhibitor (Sigma) to inhibit contact activation. The MPFP was prepared from approximately 50 mL plasma obtained from healthy volunteers. Subsequently 40 μL of MPs resuspended in control MPFP was added to the plate well, followed by 50 μL of calcium-fluorogenic substrate (0.5 mmol/L of Z-G-G-R-AMC and 7.5 mmol/L of calcium final concentrations, Pathway Diagnostics). No exogenous TF or phospholipids were added. The thrombin generated was measured by fluorogenic excitation/emission at 360/460 nm at 1-minute time intervals for 90 minutes in an Optima fluorescence plate reader (BMG). Indices recorded were 1) height of peak thrombin (nM); 2) lag time (time to onset of thrombin generation); 3) velocity index; and 4) endogenous thrombin potential (ETP), equivalent to the area under the curve (figure e-1E). Levels of von Willebrand factor (vWF) antigen were measured in PPP using a commercially available enzyme-linked immunoassay (American Diagnostica).
Statistical analysis
Numeric results were summarized as median and range. The Kruskal-Wallis test was used to examine overall differences in experimental laboratory markers between the study groups followed by the Mann-Whitney U test. Fisher exact test was used to compare categorical data between groups. Associations between biomarkers were assessed using Spearman rank correlation coefficient. The independent association of CECs, total annexin V+ MPs, and peak thrombin on the primary outcome of AIS recurrence were assessed using a multivariable logistic regression model, unadjusted and adjusted for age, gender, and time from stroke event to blood test sampling. Results were expressed as odds ratio (OR) with 95% confidence intervals (CIs) and p values. Sensitivity, specificity, and likelihood ratios were then calculated to examine the potential test characteristics of CECs for identification of AIS recurrence. p Values of less than 0.05 (2-sided) were regarded as significant. Statistical analysis was performed using SPSS version 17.
RESULTS
Characteristics of the study population
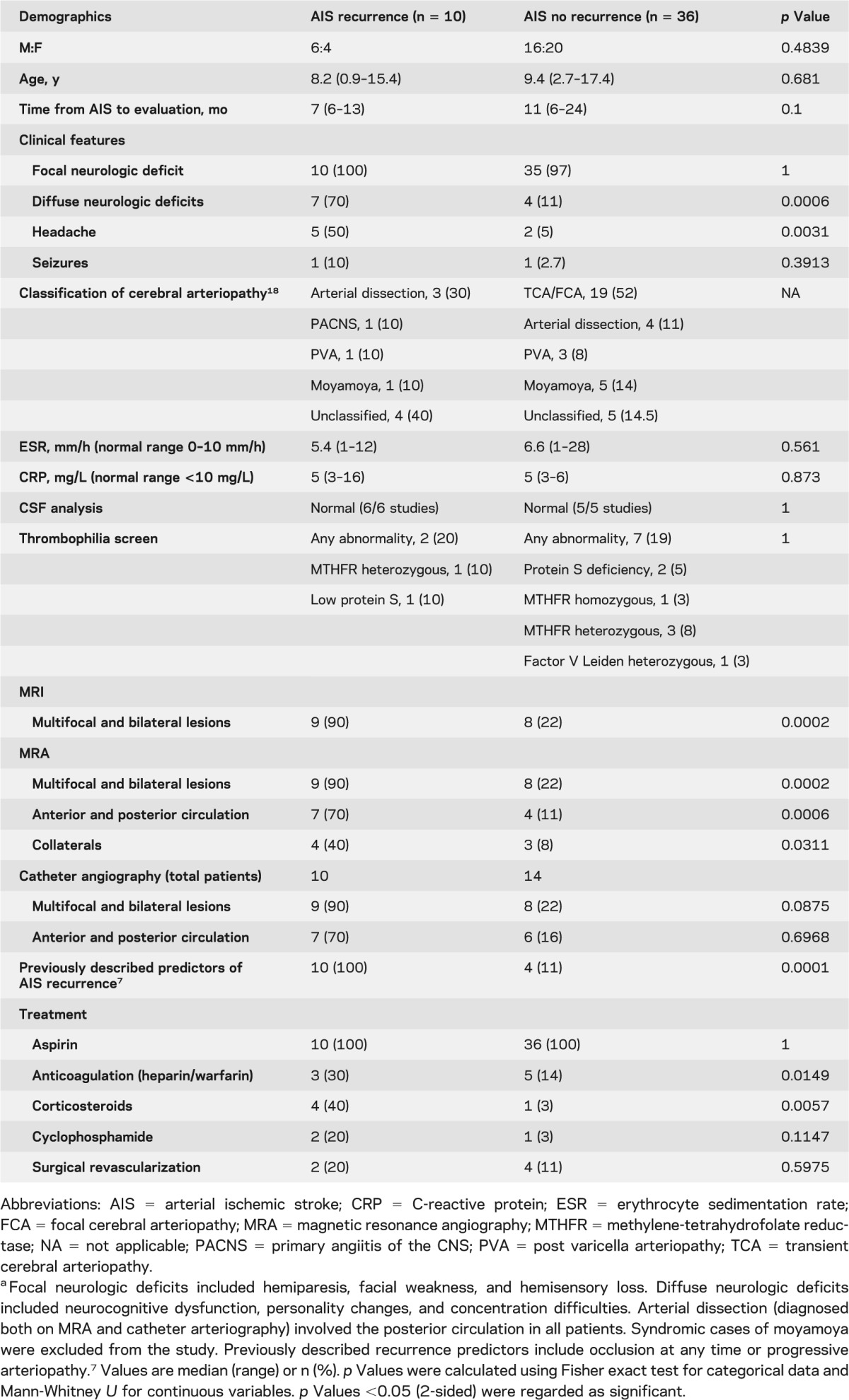
A total of 116 children with AIS were identified during the study period. Of those, 54 met the inclusion criteria; however, 8 patients/families refused participation. Of the 46 participants (age 8.8 years, range 0.9–17.4), 10 had AIS recurrence and 36 had a single event. The children who refused consent had an age of 6.2 years (4.2–8.4) and none had recurrent events. None of the children with a single AIS event had evidence of clinically silent infarction on repeat imaging at 6 and 12 months after index AIS. The disease control group included 10 children, median age 9 years (3–16), with cerebral AVM, 4 of whom had a history of intracranial hemorrhage. The demographics of the study population, the routine clinical laboratory parameters, imaging features, and arteriopathy classification are summarized in table 1. None of the children had clinical signs or symptoms of systemic inflammatory disease or evidence of a cardiac abnormality or intracardiac shunt. Evaluation took place at a median of 7 months (6–13) following recent AIS for those children with AIS recurrence compared to 11 months (6–24) for those with no recurrence (p = 0.1000). Those with recurrent AIS more commonly had diffuse neurologic deficits, headaches, multifocal and bilateral lesions, and lesions in both anterior and posterior circulation territories on MRI. They were also more likely to have been treated with steroids. Treatment with either steroids or immunosuppresion was initiated following recurrence. All children with AIS recurrence had evidence of new brain infarction with progression of previously identified arterial disease in 6 children (60%), new arterial lesions in 8 children (80%), and new arterial occlusion in the presence of other arterial wall changes in 2 patients (20%).
Table 1.
Study population characteristicsa
Eight children, aged 6.5 years (5–13), were studied prospectively at initial presentation with AIS and at latest follow up of 12 months (9–18). Six had no AIS recurrence following the initial event, while 2 children (treated with aspirin and warfarin but no immunosuppresion) re-presented with AIS recurrence during follow-up (see table e-1 for presenting features).
Circulating endothelial cells
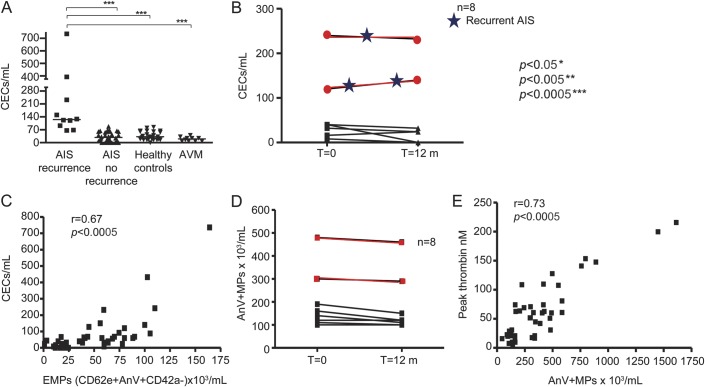
Levels of peripheral blood CEC were significantly higher in children with AIS compared with healthy child controls (p = 0.03). As depicted in figure 1A, the CEC count in children with recurrent AIS was significantly higher at 120/mL (64–732) compared to 32/mL (0–64) in the 36 children with no recurrence (p = 0.0001), 16/mL (8–40) in children with cerebral AVM (p = 0.0002), and 24/mL (0–60) in 20 child controls (p = 0.0001).
Figure 1. Circulating endothelial cells (CECs) and cellular microparticles (MP) in children with recurrent arterial ischaemic stroke (AIS).
A) CEC count in children with recurrent AIS was significantly higher compared to those children with no recurrence (p = 0.0001), children with cerebral arteriovenous malformation (AVM) (p = 0.0002), and child controls (p = 0.0001). (B) Prospective changes in CEC count in 8 children with arteriopathy and AIS. CECs at presentation for 6 children with a monophasic disease course were 36 (8–40) cells/mL and at follow-up 24 (0–32) cells/mL. For 2 children there was clinical AIS recurrence associated with cerebral arteriopathy progression (shown in red) associated with a sustained increase in CEC count at latest follow-up. (C) Circulating endothelial-derived MPs expressing CD62E correlated significantly with CECs, r = 0.67, p = 0.0001. (D) For the 6 children with a stable course with no AIS recurrence, total AnV+ MPs were at 130 (120–190) × 103/mL at initial assessment to 115 (120–150) × 103/mL at follow-up, and in 2 children with recurrence (marked in red), 390 (300–480) × 103/mL at presentation to 375 (290–440) × 103/mL at follow-up. (E) The total circulating AnV+ MPs correlated significantly with MP-mediated peak thrombin nM, r = 0.73, p = 0.0001. Spearman rank correlation was employed to examine the association between studied parameters. Kruskal-Wallis test followed by the Mann-Whitney U test was used to examine differences between the study groups. p Values of less than 0.05 (2-sided) were regarded as significant. EMP = endothelial microparticles.
Figure 1B summarizes the CEC changes for the 8 children studied prospectively. The 6 children with a monophasic disease course had no significant changes in CEC counts (p = 0.545) while the 2 children with AIS recurrence showed a sustained raised CEC count at 184 (136–232) cells/mL during follow-up.
Circulating microparticles
Children with AIS recurrence had significantly higher total AnV+ MPs compared to those with no recurrence, p = 0.0020; children with cerebral AVM, p = 0.0001; and controls, p = 0.0001 (table 2). Children with recurrent AIS had higher platelet MPs expressing P-selectin, endothelial MPs (EMPs) expressing either the endothelial activation marker CD62E (E-selectin) or CD31+, and neutrophil-derived MPs expressing the activation marker aCD11b compared to children with single AIS (table 2). Of note, circulating EMPs expressing CD62E correlated significantly with CECs, r = 0.67, p = 0.0001, suggesting a significant association between both biomarkers of endothelial injury (figure 1C).
Table 2.
Circulating microparticle profiles in children with arterial ischemic strokea
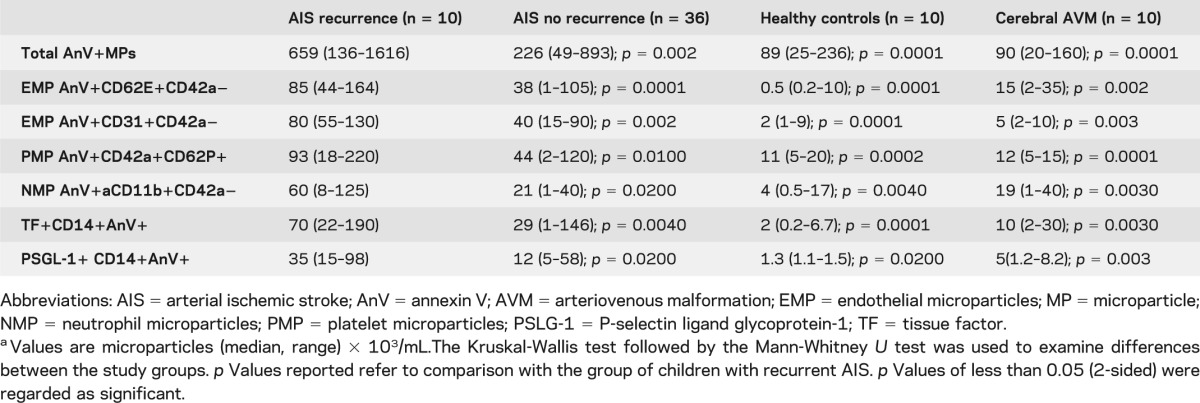
We then examined tissue factor (TF) expression on MP of monocytic (CD14) origin between the study groups since these MPs are known to be highly prothrombotic.13,25 TF+CD14+AnV+ MPs were elevated in those with AIS recurrence compared to those with no recurrence and healthy controls (table 2). As TF activity at the thrombus edge depends on interactions between platelet P-selectin and PSGL-1 on monocyte MPs,25 we determined whether there were any differences in PSGL-1+CD14+AnV+ MPs between the patient groups. Those with AIS recurrence had significantly higher levels compared to those with no recurrence.
In the 8 children studied prospectively, AnV+ MPs remained low in 6 children with no AIS recurrence; in contrast, AnV+ MPs were elevated and remained high at follow-up in 2 children with AIS recurrence (figure 1D).
TGA and vWF antigen
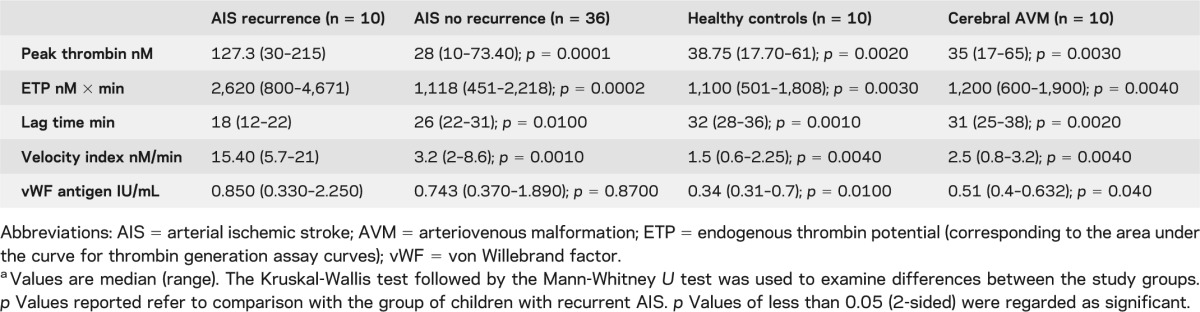
Having demonstrated higher AnV+ MPs (confirming high procoagulant phosphatidylserine content), we then investigated their capacity to generate thrombin. TGA parameters for all studied groups are summarized in table 3. Children with AIS recurrence had significantly enhanced MP-mediated peak thrombin generation, ETP, lag time, and velocity index compared to those children with nonrecurrent AIS. The total circulating AnV+ MPs correlated significantly with peak thrombin nM (figure 1E), consistent with our hypothesis that the hypercoagulability in patients with AIS was directly related to the increased total number of PS-expressing (AnV+) MPs. Finally, levels of plasma vWF antigen (previously reported to relate to cerebrovascular inflammation)26 were not significantly different between patient groups, but were significantly higher in the group of children with AIS recurrence compared to controls (table 3).
Table 3.
Thrombin-generation parameters and von Willebrand factor antigen in children with arterial ischemic strokea
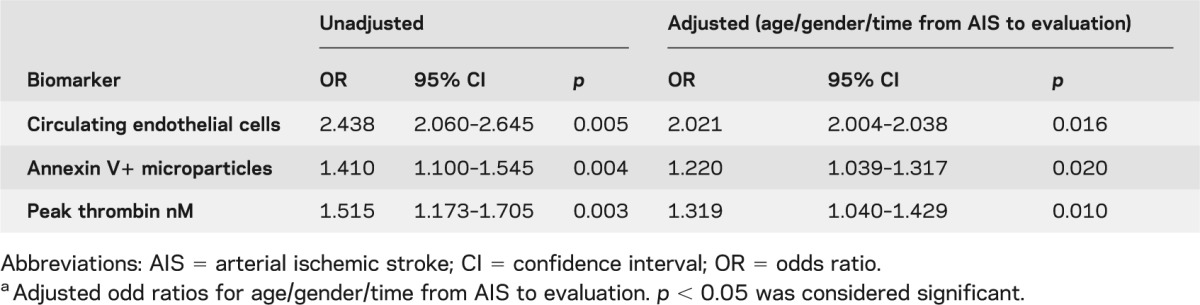
Logistic regression analysis of biomarkers and AIS recurrence
Logistic regression analysis was used to examine the relationship of CECs, total AnV+MPs, and peak thrombin to AIS recurrence (table 4). AIS recurrence was associated with high CECs, high total AnV+MPs, and elevated peak thrombin in both nonadjusted and adjusted analyses. In addition, CECs together with the previously described predictors of AIS recurrence (occlusion or progressive arteriopathy)7 resulted in an increased post-test probability of identifying children at risk of AIS recurrence from 67 (95% CI 44%–79%) to 86% (95% CI 55%–94%) (table e-2).
Table 4.
Unadjusted and adjusted odds ratios by multivariable logistic regression analysis for arterial ischemic stroke recurrencea
DISCUSSION
In this cross-sectional study of a group of children with AIS and cerebral arteriopathy, we have identified biomarkers that differ significantly between children with recurrent AIS and those with a monophasic course. Our observations suggest that a state of chronic endothelial activation and injury (increased CECs and EMPs), platelet activation (increased platelet MPs), and increased MP-mediated thrombin generation could be important determinants of AIS recurrence.
The morphology and course of cerebral/cervical arteriopathies are the most important determinants of recurrence thus far identified in childhood AIS.1,5 Current classifications are radiologic,6,7 although it is well-recognized that MRA and CA have limitations in identifying and differentiating between distinct pathologic entities. In this study we chose to classify patients based solely on AIS recurrence (corroborated both clinically and radiologically) as this is a robust, verifiable, and clinically important event.
CECs have been used as markers of endothelial damage across an ever-widening variety of diseases, including systemic vasculitis.11,12,15 Importantly, CEC levels are predominantly affected by disease activity and endothelial injury and not treatment per se.11,12,15 Our study now suggests that elevated CECs, and therefore persistence of endothelial injury, vary between children with single and recurrent AIS. CECs could therefore be an attractive candidate biomarker to detect those at risk of recurrent AIS, a finding that is consistent with the strong correlation between CECs and disease activity in systemic vasculitis.12
We also examined levels and functions of circulating MPs in our patient groups. Circulating MPs possess a plethora of potent proinflammatory effects including endothelial binding and activation by upregulation of oxidative stress.14,27 MPs also have important prothrombotic properties.13,14,25 This is largely due to PS exposure on the MP outer surface directly contributing to activation of the extrinsic coagulation pathway and indirectly enhancing the TF capability as well as delivering TF to the thrombus edge.13,14,25 Further support for this latter mechanism comes from our observation that these TF+ MP also express PSGL-1 responsible for binding of TF+MP to activated platelets expressing P-selectin.25,28
In combination, the biomarker data presented here implicate persistence of endothelial inflammation and injury and excess MP-mediated thrombin generation as potentially important mechanisms determining AIS recurrence in children. These data provide a scientific rationale for currently used strategies to prevent AIS recurrence with antiplatelet therapy (such as aspirin), but if combined with radiologic and histopathologic features could provide a rationale for corticosteroids, or more aggressive immunosuppression (cyclophosphamide or mycophenolate mofetil) to suppress vascular inflammation in select cases.9,29
Our study has limitations. It will be of considerable interest to establish the specificity of our findings by comparison with patients with AIS without arteriopathy (who may have small vessel CNS vasculitis, as suggested by some authors),30 and in relation to other well-established risk factors such as cardiac disease. We chose to initially focus on children with AIS and cerebral arteriopathy specifically in order to inform the controversy surrounding the role of persistent vascular inflammation contributing to progression of arteriopathy in this group. Secondly, we did not perform cerebrovascular biopsies to confirm persistent cerebral vasculitis, but this is reflective of current practice, where cerebroarterial or other CNS biopsy is rarely undertaken. In addition, the AIS recurrence rate in our study was higher (22%) than that reported in other cohorts,1 reflecting probable referral bias to our tertiary center. Even though several lines of evidence suggest that CEC and MP are important pathogenic mediators that can amplify endothelial injury and inflammation,27,31 the cross-sectional nature of this study precludes any causal effect to be determined. Finally, the sample size was small and the patients did not all receive the same treatments, although they were treated in line with published clinical guidelines.21–23 Ultimately the goal would be to prevent AIS recurrence by identifying the high-risk profile patients based on these biomarkers and initiate therapy after first AIS. Further prospective studies to confirm or refute our findings and form the basis for secondary stroke prevention risk-stratified approaches will require multicenter collaboration.
Our data suggest that persistent endothelial injury, platelet activation, and excessive thrombin generation could be important mechanisms underlying recurrent childhood AIS. Prospective validation of our findings is now required to firmly establish the role of these biomarkers as prognostic markers in childhood AIS and cerebral arteriopathy.
Supplementary Material
ACKNOWLEDGMENT
The authors thank the patients, their parents, and the healthy controls who participated in this study.
GLOSSARY
- AIS
arterial ischemic stroke
- AVM
arteriovenous malformation
- CA
catheter arteriography
- CEC
circulating endothelial cell
- CI
confidence interval
- EMP
endothelial microparticle
- ETP
endogenous thrombin potential
- FCA
focal cerebral arteriopathy
- FITC
fluorescein isothiocyanate
- FLAIR
fluid-attenuated inversion recovery
- GOSH
Great Ormond Street Hospital
- MP
microparticle
- MPFP
microparticle-free plasma
- MR
magnetic resonance
- MRA
magnetic resonance angiography
- OR
odds ratio
- PACNS
primary angiitis of the CNS
- PE
phycoerythrin
- PPP
platelet-poor plasma
- PSGL-1
P-selectin glycoprotein ligand 1
- TF
tissue factor
- TGA
thrombin generation assay
- vWF
von Willebrand factor
Footnotes
Editorial, page 2084
Supplemental data at www.neurology.org
AUTHOR CONTRIBUTIONS
Despina Eleftheriou: study design, data collection, laboratory measurements, statistics, writing of the manuscript. Vijeya Ganesan: study design and data acquisition, writing of the manuscript. Ying Hong: data collection, laboratory measurements and review of the manuscript. Nigel J. Klein: study design, review of the manuscript. Paul A. Brogan: study design, data collection, statistics, writing of the manuscript.
DISCLOSURE
D. Eleftheriou was supported by a clinical research fellowship awarded by Action Medical Research. V. Ganesan received research support from Action Medical Research. Y. Hong received research support by Arthritis research UK and a National Institute for Health Research/Biomedical Research Centre project grant. N. Klein is receiving research support from Action Medical research, British Heart Foundation, and the Wellcome trust. P.A. Brogan received honoraria from serving on the scientific advisory board of Novartis. He is receiving research support from the British Heart Foundation and Action Medical Research for studies for which he serves as a primary investigator. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Fullerton HJ, Wu YW, Sidney S, Johnston SC. Risk of recurrent childhood arterial ischemic stroke in a population-based cohort: the importance of cerebrovascular imaging. Pediatrics 2007;119:495. [DOI] [PubMed] [Google Scholar]
- 2.Ganesan V, Prengler M, McShane MA, Wade AM, Kirkham FJ. Investigation of risk factors in children with arterial ischemic stroke. Ann Neurol 2003;53:167–173 [DOI] [PubMed] [Google Scholar]
- 3.Ganesan V, Hogan A, Shack N, Gordon A, Isaacs E, Kirkham FJ. Outcome after ischaemic stroke in childhood. Dev Med Child Neurol 2000;42:455–461 [DOI] [PubMed] [Google Scholar]
- 4.Eleftheriou D, Ganesan V. Controversies in childhood arterial ischemic stroke and cerebral venous sinus thrombosis. Expert Rev Cardiovasc Ther 2009;7:853–861 [DOI] [PubMed] [Google Scholar]
- 5.Ganesan V, Prengler M, Wade A, Kirkham FJ. Clinical and radiological recurrence after childhood arterial ischemic stroke. Circulation 2006;114:2170. [DOI] [PubMed] [Google Scholar]
- 6.Mackay MT, Wiznitzer M, Benedict SL, Lee KJ, deVeber G, Ganesan V. Arterial ischemic stroke risk factors: the International Pediatric Stroke Study. Ann Neurol 2011;69:130–140 [DOI] [PubMed] [Google Scholar]
- 7.Braun KPJ, Bulder MMM, Chabrier S, et al. The course and outcome of unilateral intracranial arteriopathy in 79 children with ischaemic stroke. Brain 2009;132:544–557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Benseler SM, Silverman E, Aviv RI, et al. Primary central nervous system vasculitis in children. Arthritis Rheum 2006;54:1291–1297 [DOI] [PubMed] [Google Scholar]
- 9.Hutchinson C, Elbers J, Halliday W, et al. Treatment of small vessel primary CNS vasculitis in children: an open-label cohort study. Lancet Neurol 2010;9:1078–1084 [DOI] [PubMed] [Google Scholar]
- 10.Brogan PA, Shah V, Brachet C, et al. Endothelial and platelet microparticles in vasculitis of the young. Arthritis Rheum 2004;50:927–936 [DOI] [PubMed] [Google Scholar]
- 11.Clarke LA, Shah V, Arrigoni F, et al. Quantitative detection of circulating endothelial cells in vasculitis: comparison of flow cytometry and immunomagnetic bead extraction. J Thromb Haemost 2008;6:1025–1032 [DOI] [PubMed] [Google Scholar]
- 12.Clarke LA, Hong Y, Eleftheriou D, et al. Endothelial injury and repair in systemic vasculitis of the young. Arthritis Rheum 2010;62:1770–1780 [DOI] [PubMed] [Google Scholar]
- 13.Eleftheriou D, Hong Y, Klein NJ, Brogan PA. Thromboembolic disease in systemic vasculitis is associated with enhanced microparticle mediated thrombin generation. J Thromb Haemost 2011;9:1864–1867 [DOI] [PubMed] [Google Scholar]
- 14.Piccin A, Murphy WG, Smith OP. Circulating microparticles: pathophysiology and clinical implications. Blood Rev 2007;21:157–171 [DOI] [PubMed] [Google Scholar]
- 15.Woywodt A, Streiber F, de Groot K, Regelsberger H, Haller H, Haubitz M. Circulating endothelial cells as markers for ANCA-associated small-vessel vasculitis. Lancet 2003;361:206–210 [DOI] [PubMed] [Google Scholar]
- 16.Hemker HC, Wielders S, Kessels H, Beguin S. Continuous registration of thrombin generation in plasma, its use for the determination of the thrombin potential. Thromb Haemost 1993;70:617. [PubMed] [Google Scholar]
- 17.Bidot L, Jy W, Bidot C, Jr, et al. Microparticle mediated thrombin generation assay: increased activity in patients with recurrent thrombosis. J Thromb Haemost 2008;6:913–919 [DOI] [PubMed] [Google Scholar]
- 18.Sebire G, Fullerton H, Riou E, deVeber G. Toward the definition of cerebral arteriopathies of childhood. Curr Opin Pediatr 2004;16:617. [DOI] [PubMed] [Google Scholar]
- 19.Amlie-Lefond C, Bernard TJ, Sebire G, et al. Predictors of cerebral arteriopathy in children with arterial ischemic stroke: results of the International Pediatric Stroke Study. Circulation 2009;119:1417–1423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eleftheriou D, Cox T, Saynders D, Klein NJ, Brogan PA, Ganesan V. Investigation of childhood central nervous system vasculitis: magnetic resonance angiography versus catheter cerebral angiography. Dev Med Child Neurol 2010;52:863–867 [DOI] [PubMed] [Google Scholar]
- 21.Roach ES, Golomb MR, Adams R, et al. Management of stroke in infants and children: a scientific statement from a Special Writing Group of the American Heart Association Stroke Council and the Council on Cardiovascular Disease in the Young. Stroke 2008;39:2644–2691 [DOI] [PubMed] [Google Scholar]
- 22.Monagle P, Chan A, Massicotte P, Chalmers E, Michelson AD. Antithrombotic therapy in children: the Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy. Chest 2004;126:645S–687S [DOI] [PubMed] [Google Scholar]
- 23.Paediatric Stroke Working Group Stroke in childhood: clinical guidelines for diagnosis, management and rehabilitation. Available at: http://bookshop.rcplondon.ac.uk/contents/f98c6540-a541-4bed-837d-ef293ac458bf.pdf. Accessed July 14, 2011
- 24.Woywodt A, Blann AD, Kirsch T, et al. Isolation and enumeration of circulating endothelial cells by immunomagnetic isolation: proposal of a definition and a consensus protocol. J Thromb Haemost 2006;4:671–677 [DOI] [PubMed] [Google Scholar]
- 25.Falati S, Liu Q, Gross P, et al. Accumulation of tissue factor into developing thrombi in vivo is dependent upon microparticle P-selectin glycoprotein ligand 1 and platelet P-selectin. J Exp Med 2003;197:1585–1598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cellucci T, Pullenayegum E, Tyrrell P, Benseler SM. Von Willebrand factor antigen: a novel biomarker of disease activity in childhood CNS vasculitis. Arthritis Rheum 2009;60:1573. [DOI] [PubMed] [Google Scholar]
- 27.Hong Y, Eleftheriou D, Hussain A, et al. Anti-neutrophil cytoplasmic antibodies stimulate release of neutrophil microparticles causing endothelial activation and increased thrombin generation. J Am Soc Nephrol 2012;23:49–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zwicker JI, Trenor CC, III, Furie BC, Furie B. Tissue factor-bearing microparticles and thrombus formation. Arterioscler Thromb Vasc Biol 2011;31:728–733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Eleftheriou D, Dillon MJ, Brogan PA. Advances in childhood vasculitis. Curr Opin Rheumatol 2009;21:411–418 [DOI] [PubMed] [Google Scholar]
- 30.Benseler SM, deVeber G, Hawkins C, et al. Angiography negative primary central nervous system vasculitis in children: a newly recognized inflammatory central nervous system disease. Arthritis Rheum 2005;52:2159–2167 [DOI] [PubMed] [Google Scholar]
- 31.Kirsch T, Woywodt A, Beese M, et al. Engulfment of apoptotic cells by microvascular endothelial cells induces proinflammatory responses. Blood 2007;109:2854–2862 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.