Abstract
New approaches consisting of ‘multistage' vaccines against (TB) are emerging that combine early antigenic proteins with latency-associated antigens. In this study, HspX was tested for its potential to elicit both short- and long-term protective immune responses. HspX is a logical component in vaccine strategies targeting protective immune responses against primary infection, as well as against reactivation of latent infection, because as previously shown, it is produced during latency, and as our studies show, it elicits protection within 30 days of infection. Recent studies have shown that the current TB vaccine, bacilli Calmette-Guerin (BCG), does not induce strong interferon-γ T-cell responses to latency-associated antigens like HspX, which may be in part why BCG fails to protect against reactivation disease. We therefore tested HspX protein alone as a prophylactic vaccine and as a boost to BCG vaccination, and found that HspX purified from M. tuberculosis cell lysates protected mice against aerosol challenge and improved the protective efficacy of BCG when used as a booster vaccine. Native HspX was highly immunogenic and protective, in a dose-dependent manner, in both short- and long-term infection models. Based on these promising findings, HspX was produced as a recombinant protein in E. coli, as this would enable facile purification; however, recombinant HspX (rHspX) alone consistently failed to protect against aerosol challenge. Incubation of rHspX with mycobacterial cell lysate and re-purification following incubation restored the capacity of the protein to confer protection. These data suggest the possibility that the native form may chaperone an immunogenic and protective antigen that is mycobacteria-specific.
Keywords: α-crystallin, chaperone, heat shock protein, immunity, subunit, tuberculosis, vaccine
Declared a global health emergency in the early 1990s, tuberculosis (TB) remains a major health problem today, with over two million deaths because of this disease each year, and one-third of the world's population infected with Mycobacterium tuberculosis.1 Most infected people initially control the infection by generating cell-mediated immunity; however, residual latent bacilli remain viable in healthy hosts for many years and can reactivate into contagious TB disease. In fact, latent infections that have reactivated into active disease may be the predominate source of contagious TB.2
The only vaccine currently available against TB is live attenuated M. bovis, bacilli Calmette-Guerin (BCG). BCG is effective in preventing severe forms of childhood TB, but its protective effect wanes in adulthood,3 and it does not protect against reactivation of latent TB, which partially explains why this vaccine has had little impact on the global TB epidemic.4 Therefore, concerted research efforts have focused on developing a TB vaccine that confers consistent, long-term protection against adult pulmonary TB, and ideally one that can protect against initial infection, as well as reactivation of latent infection.
It is well-established that T helper cell type 1 immunity is required for protection against M. tuberculosis infection,5 but mounting evidence suggests that interferon-γ (IFN-γ) induction alone does not guarantee long-term protection. Therefore, it is necessary to assess additional cytokines beyond IFN-γ, such as tumor necrosis factor (TNF)-α and interleukin (IL)-2 to generate a more complete functional assessment of effector and memory T cells.6, 7 Forbes et al.8 showed that the induction of multifunctional T cells (that is, T cells that produced IFN-γ, TNF-α and IL-2) was associated with markedly reduced mycobacterial burden in the lungs of mice given a prime-boost vaccine with BCG followed by virally encoded M. tuberculosis antigen Ag85A compared with BCG vaccination alone.
Several conventional subunit protein vaccines have been shown to induce multifunctional T cells,9 and many of these subunit vaccines have been shown to confer protection rivaling that of BCG in several animal models.10, 11 Until recently, the majority of subunit vaccines were based on antigens expressed during the early stages of M. tuberculosis infection; however, to target protection during latency, several current studies have incorporated the latency-associated antigen, 16-kDa α-crystallin protein (also called HspX, acr and Rv2031c) in heterologous prime-boost strategies using protein HspX subunit vaccines, DNA vaccines expressing HspX and recombinant BCG strains overexpressing HspX.12, 13, 14, 15
The HspX protein is associated with latency because it is the dominant antigen produced by M. tuberculosis during the latent stage of infection, accounting for up to 25% of the total protein expressed during static growth or under oxygen deprivation, as has been demonstrated in models aiming to mimic the granulomatous environment in vitro.16, 17 The proposed role of HspX is to enhance long-term stability of proteins16 and cell structures,18 which in turn helps the bacilli maintain long-term survival. In addition, rapid and robust expression of HspX has been demonstrated upon entry into macrophages and under oxygen-deprivation in vitro,19 and during log-phase growth, low levels of HspX protein are produced as early as day four of incubation.20 As this antigen is associated with latent M. tuberculosis infection and it is also reported to be produced during early log-phase growth, HspX is a rational vaccine target antigen as it should stimulate an immune response during early infection, as well as during latency. Interestingly, even though both M. tuberculosis and M. bovis BCG express functional HspX,18 BCG-vaccinated individuals not exposed to M. tuberculosis fail to recognize HspX,4, 21 whereas both B-cell and T-cell responses to HspX were detected in patients with active TB or in healthy people with latent TB.4, 22, 23
In light of the epidemic status of TB and the ability of BCG to confer protection in children, boosting BCG with a latency-associated antigen like HspX, rather than replacing BCG completely, may prove the most realistic and effective way to control this highly infectious disease. In this study, we first set out to determine if HspX alone could induce protective immune responses in mice both in short-term and long-term models of aerosol infection with M. tuberculosis. Our results indicate that not only does the native form of HspX (nHspX) confer short- and long-term protective immunity in mice, but it also significantly boosted the protective efficacy of BCG. Overall, the results of this study show that a latency-associated antigen can confer prophylactic protection, as well as improve upon the protective efficacy of BCG alone, and that the concentration dose of the subunit vaccination affects the quality of the immune response generated. In contrast, recombinant HspX (rHspX) did not confer protection, even though the native protein is not post-translationally modified.24 Thus, this study also demonstrates the importance of the construct chosen to produce a subunit vaccine, and implies that the chaperone function of HspX may contribute to its antigenicity in our model.
RESULTS
Native HspX, when used as a vaccine, confers short- and long-term protection that is concentration-dependent
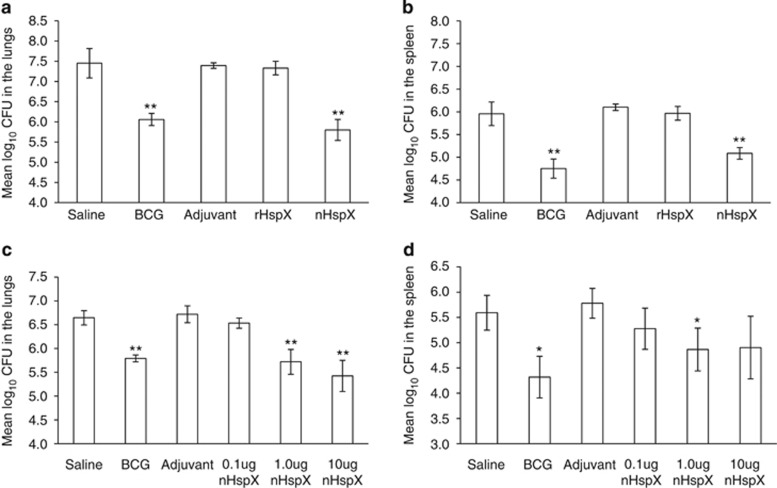
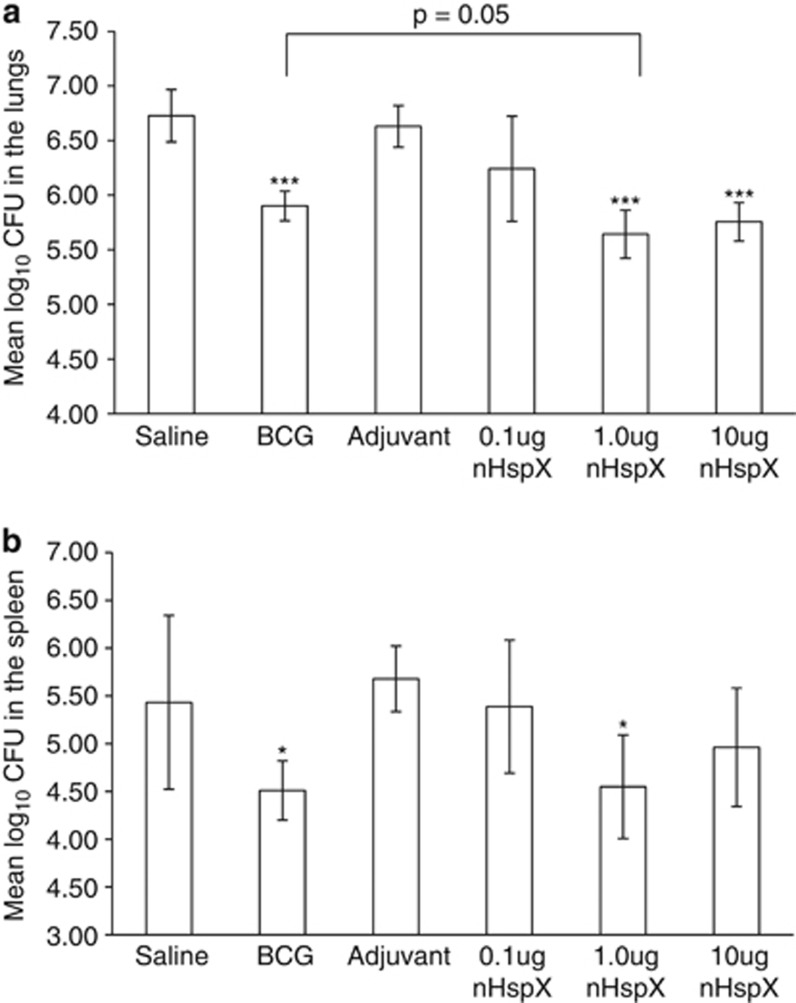
To determine whether native or rHspX subunit vaccines could protect against infection, mice were vaccinated with protein in adjuvant and challenged with M. tuberculosis 30 days later. The bacterial burden in the lungs and spleens was assessed 30 days following the aerosol infection. Native HspX conferred statistically significant protection in both the lungs and spleens (Figures 1a and b, respectively), while rHspX protein failed to protect in either organ. We next wished to determine what concentration per dose of nHspX provided optimal protection. The protocol currently accepted for subunit vaccines consists of three sequential injections of protein at 10 μg per dose; however, this may not elicit the ideal immune response necessary for long-term protection against M. tuberculosis. Therefore, we tested the ability of nHspX to protect at varied concentrations per dose. As shown in Figure 1c, the 1.0 μg and 10 μg concentrations of nHspX generated protection in the lungs while the lower concentration, 0.1 μg, did not protect. In the spleen, the 1.0 μg concentration conferred some protection (Figure 1d). These results show that as little as 1.0 μg of nHspX protected mice against challenge in a short-term infection model. Next, we wished to determine if nHspX elicited long-term protection and whether this protection was also concentration-dependent. Mice were immunized as described above, rested for 6 months and then challenged by aerosol with M. tuberculosis. The bacterial load was assessed 30 days following infection. Again, both the 1.0 μg and 10 μg concentrations of nHspX elicited protection in the lungs that was significantly different (P<0.001) from the saline control group, and in fact, mice vaccinated with 1.0 μg of nHspX had fewer bacilli in the lungs than mice vaccinated with BCG (Figure 2a). As in the short-term model, 1.0 μg of nHspX significantly decreased dissemination of the bacilli to the spleen in the long-term model (Figure 2b).
Figure 1.
Native HspX, but not rHspX, confers short-term protection against M. tuberculosis infection that is concentration-dependent. Mice were immunized s.c. with 10 μg native HspX (nHspX), 10 μg rHspX (rHpsX) or adjuvant three times, 2 weeks apart and then infected with a low-dose aerosol of H37Rv M. tuberculosis 30 days after the last immunization. BCG-vaccinated mice were given a single 105 CFU s.c. dose at the time of the third immunization. Mice were killed 30 days post infection and the bacterial burden was assessed in the (a) lungs and the (b) spleen. In a separate experiment, mice were immunized with 0.1–10 μg nHspX three times, 2 weeks apart, and then infected with a low-dose aerosol of H37Rv M. tuberculosis 30 days after the last immunization. The bacterial burden was assessed in the (c) lungs and the (d) spleen. Data are expressed as the mean (n=5 mice) s.e.m. *P<0.05 and **P<0.01 when compared with the saline-vaccinated group using a 2-tailed Student's t-test.
Figure 2.
Native HspX vaccine confers long-term protection that is concentration-dependent against aerosol infection with M. tuberculosis. Mice were immunized with native HspX (nHspX) at 0.1 μg, 1.0 μg or 10 μg per dose, three times, 2 weeks apart, and then infected with a low-dose aerosol of H37Rv M. tuberculosis 6 months after the last immunization. BCG-vaccinated mice were given a single dose at the time of the third immunization. Mice were killed 30 days post infection and the bacterial burden was assessed in the (a) lungs and the (b) spleen. Data are expressed as the mean (n=5 mice) s.d. ***P<0.001 when compared with the saline-vaccinated group or BCG group (brackets) using a 2-tailed Student's t-test. *P<0.05 when compared with the saline-vaccinated group using a 1-tailed Student's t-test.
Native HspX vaccine generates short-term and long-term acquired IFN-γ responses specifically against HspX
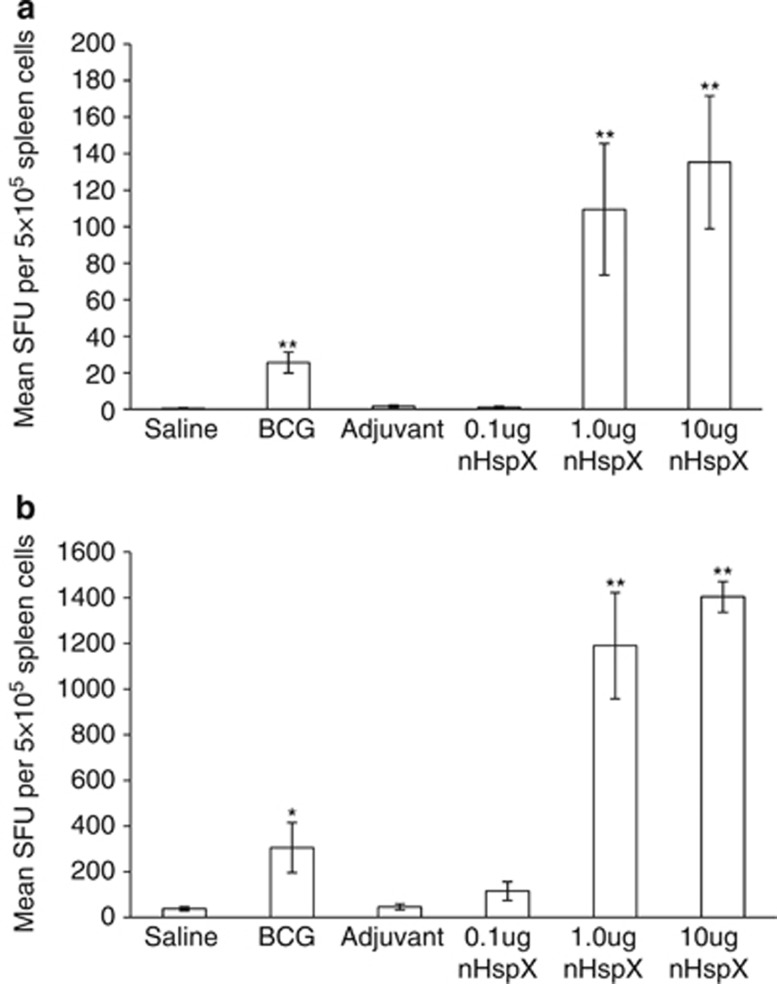
To investigate whether the concentration of HspX used to vaccinate mice affected the generation and the quality of long-term immunity, splenocytes from vaccinated mice were harvested at either 30 days or 6 months after the last vaccination, stimulated with nHspX in vitro, and assessed by ELISpot assay for IFN-γ production. Cells from mice vaccinated with 1.0 μg and 10 μg HspX produced significantly more IFN-γ-producing cells than BCG-vaccinated mice in response to HspX both 30 days and 6 months after the vaccination (Figures 3a and b, respectively), with over a 10-fold increase in the number of IFN-γ-producing cells after 6 months. Interestingly and in line with previous reports,4, 21 splenocytes from BCG-vaccinated mice recognized HspX but produced very little IFN-γ in comparison with mice vaccinated with the 1.0 μg and 10 μg concentrations of HspX.
Figure 3.
Native HspX vaccine induces short-term and long-term immunity. The mean number of IFN-γ-producing cells per 5 × 105 spleen cells after in vitro stimulation was determined by ELISpot assay. Splenocytes were obtained from vaccinated mice at (a) 30 days and (b) 6 months post vaccination and were stimulated in vitro with native HspX protein. Data are expressed as the mean (n=5 mice) s.e.m. and are representative of those from two experiments. *P<0.05 and **P<0.01 when compared with the saline-vaccinated group using a 2-tailed Student's t-test.
Native HspX subunit vaccination induces multifunctional CD4+ T cells
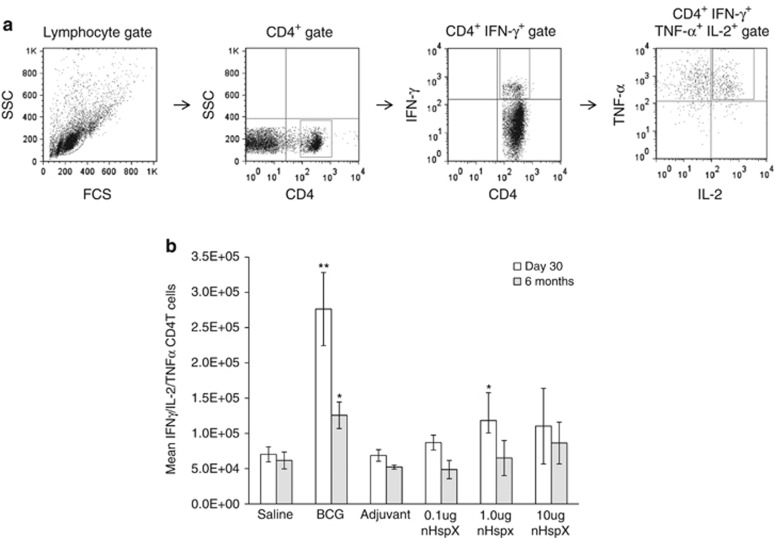
We next looked at the ability of the nHspX subunit vaccine to stimulate multifunctional CD4+ T cells both in the short- and long-term models. Spleen cells, harvested from mice at 30 days and 6 months after the last vaccination, were stained for surface CD4 and intracellular IFN-γ, IL-2 and TNF-α cytokines. Before staining, the cells were incubated and stimulated with brefeldin A and anti-CD3/anti-CD28 antibodies as a means to assess what the cells were capable of producing ex vivo. As Figure 4 shows, the number of multifunctional T cells capable of producing IFN-γ, IL-2 and TNF-α was increased in the BCG- and 1.0 μg nHspX-vaccinated mice at day 30. At 6 months after the last vaccination, however, only the BCG-vaccinated group had increased numbers of multifunctional T cells compared with the saline control group. While not statistically significant, there was an increase in the number of multifunctional T cells in the HspX-vaccinated groups that was concentration-dependent, with the highest concentration of nHspX (10 μg) eliciting the greatest number of multifunctional T cells at the 6-month time point. Interestingly, the 1.0 μg concentration of nHspX was able to induce multifunctional CD4+ T cells within the first 30 days after immunization.
Figure 4.
Native HspX vaccine induces multifunctional CD4+ T cells. Splenocytes were obtained from vaccinated mice at 30 days (white bars) and 6 months (gray bars) post vaccination. Single cell suspensions were treated with brefeldin A, anti-CD28 and anti-CD3 for 6 h and then stained for surface CD4 and for intracellular IFN-γ, IL-2 and TNF-α. (a) Gating strategy: lymphocytes were gated according to their scatter profile and then further gated upon CD4-positive, IFN-γ-positive cells that were double positive for TNF-α and IL-2. (b) The data are expressed as the percentage of multifunctional CD4+ T cells within the lymphocyte gate. Data are expressed as the mean (n=5 mice) s.e.m. **P<0.01 and *P<0.05 when compared with the saline-vaccinated group using a 2-tailed Student's t-test.
Native HspX vaccine boosts the immunogenicity and protective efficacy of BCG
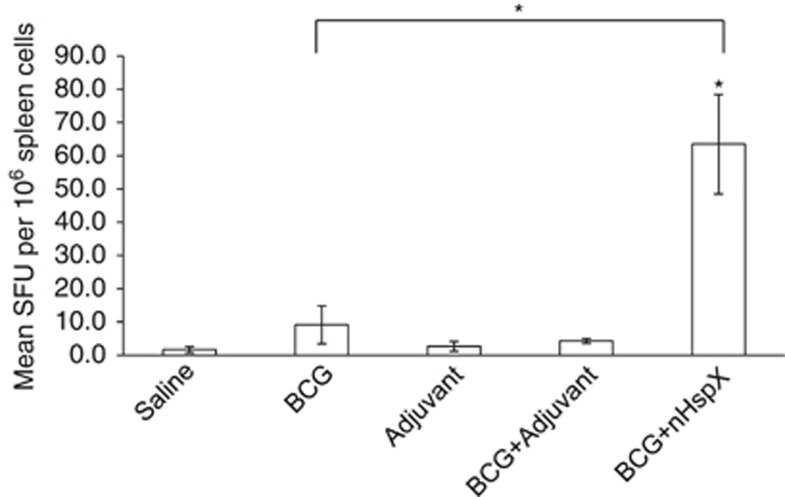
One shortcoming of the BCG vaccine is that it fails to induce protective T-cell responses against latency-associated antigens. As the nHspX subunit vaccine protected mice in both the short-term and long-term studies, we next wished to know whether it could boost the protective immune response of BCG vaccination, and potentially compensate for the lack of T-cell responses to dormancy antigens like HspX in BCG-vaccinated individuals. Mice were vaccinated once with BCG followed by one dose of nHspX 6 weeks later. The mice were exposed to a low-dose aerosol challenge, and their lungs assayed for viable M. tuberculosis bacilli 30 days post infection. Table 1 shows that BCG boosted with nHspX was highly effective (P<0.0001 compared with the saline control mice), decreasing the bacterial load in both the lungs and the spleen better than BCG vaccination alone (P=0.05). Accordingly, the number of IFN-γ-secreting spleen cells was significantly greater in the mice vaccinated with BCG and boosted with nHspX compared with the BCG vaccine alone (Figure 5).
Table 1. Native HspX vaccine boosts the protective efficacy of BCG vaccination.
| Mean log10 CFU of M. tuberculosis±s.d. (n=5)a | Mean log10 CFU reductionb | |||
|---|---|---|---|---|
|
Vaccine groupc |
Lung |
Spleen |
Lung |
Spleen |
| Saline | 7.27±0.20 | 5.82±0.08 | ||
| BCG | 6.43±0.21** | 4.98±0.22** | 0.85** | 0.84** |
| Adjuvant | 7.28±0.23 | 5.80±0.09 | 0.00 | 0.02 |
| BCG+Adjuvant | 6.57±0.11** | 5.27±0.22* | 0.71** | 0.55* |
| BCG+rHspX | 6.57±0.30** | 5.15±0.27* | 0.70** | 0.66* |
| BCG+nHspX | 6.17±0.13***,ψ | 4.59±0.26** | 1.10 *** | 1.23** |
Abbreviations: BCG, bacilli Calmette-Guerin; CFU: colony forming units. ***P<0.0001, **P<0.01 and *P<0.05 compared with the saline-vaccinated group, and ψP=0.05 compared with BCG alone using a 2-tailed Student's t-test.
Number of bacilli isolated from the lung and spleen 30 days after aerosol challenge.
Calculated by subtracting the mean Log10 bacilli in each group from that in of the saline group.
Mice were given one boosting dose of rHspX or native HspX (nHspX) 6 weeks following s.c. immunization with 105 BCG. A low-dose aerosol of M. tuberculosis H37Rv was administered 30 days after the boost.
Figure 5.
Native HspX improves protective immunity of BCG vaccination. The mean number of IFN-γ-producing cells per 5 × 105 spleen cells after in vitro stimulation was determined by ELISpot assay and expressed as spot forming units. Splenocytes were obtained from C57BL/six mice 14 days post vaccination and were stimulated in vitro with native HspX (nHspX) protein. Data are expressed as the mean (n=5 mice) s.d. *P<0.05 when compared with the saline-vaccinated group or BCG group (bracket) using a 2-tailed Student's t-test.
Granulomatous response in the lungs varies depending on native HspX vaccine concentration
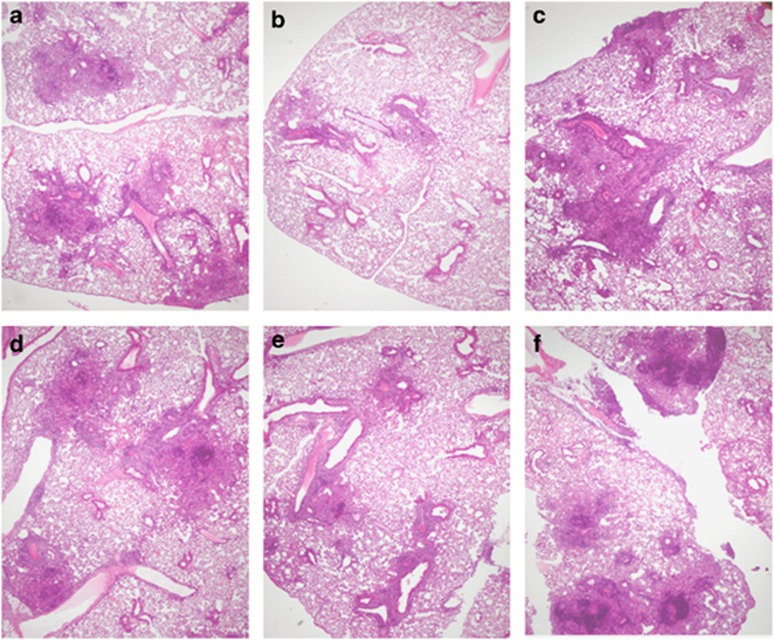
To examine if the concentration of native HspX subunit affected granuloma formation and lung architecture, mice were immunized with 0.1 μg, 1.0 μg, or 10 μg nHspX three times over a 6-week period, and then infected with M. tuberculosis by the aerosol route 30 days after the last vaccine dose. Lungs were harvested 30 days post infection and processed for microscopy. The inflammation in each group of nHspX-vaccinated animals (Figure 6, panels d: 0.1 μg; e: 1.0 μg; f: 10 μg) was quantitatively similar to that observed in the saline- and adjuvant-control animals (Figures 6a and c, respectively). The lesions in all of the groups were markedly dominated by lymphocytes bordered by activated macrophages, and no neutrophils were observed. BCG-vaccinated mice (Figure 6b) had quantitatively milder lung inflammation, but the composition of the infiltrates was similar to that of nHspX-vaccinated animals. Interestingly, the number and size of the granulomatous lesions in the 1.0 μg nHspX-vaccinated mice (Figure 6e) was most similar to those in the BCG-vaccinated mice. These results are not surprising considering the number of live bacilli was most reduced in the lungs of the groups vaccinated with either BCG or the 1.0 μg concentration of native HspX.
Figure 6.
Native HspX induces anti-mycobacterial inflammation dominated by lymphocytes and activated macrophages. Mice were immunized with 0.1–10 μg native HspX (nHspX) three times, 2 weeks apart, and then infected with a low-dose aerosol of H37Rv M. tuberculosis 30 days after the last immunization. Mice were killed 30 days post infection and the lungs were processed for microscopy. While inflammation in the nHspX-vaccinated animals (d: 0.1 μg; e: 1.0 μg; f: 10 μg) did not differ quantitatively from that seen in the saline- and adjuvant-control animals (a, c, respectively), the lesions were notably dominated by lymphocytes and a rim of activated macrophages. Neutrophils were absent. BCG-vaccinated animals (b) had quantitatively milder lung inflammation, but the composition of the infiltrates were similar to that of nHspX-vaccinated animals. Hematoxylin and eosin; 40 × .
rHspX protein incubated with whole-cell lysate protects against M. tuberculosis
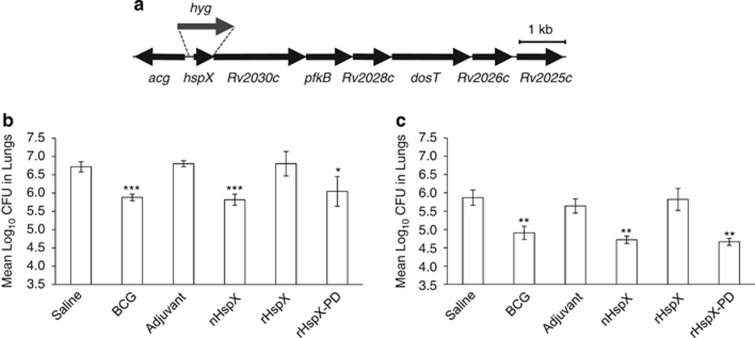
Our findings suggest that native HspX, not rHspX, confers protection in our model. This finding is unexpected, given that native HspX does not appear to be post-translationally modified.24 We further investigated the ineffectiveness of rHspX compared with native HspX to explore other rationale for this difference in protection. Given that one of the major roles of heat shock proteins is to chaperone other proteins, particularly during cell stress as is the case with α-crystallins,25 we hypothesized that native HspX protein conferred protection because of either its ability to chaperone another antigen or other unique attribute present only when the protein is produced by M. tuberculosis. To test this possibility, we treated rHspX with whole-cell lysate from a M. tuberculosis hspX deletion mutant (ΔHspX) and then used it as a vaccine to determine if it could protect mice from infection with either wild type or ΔHspX. Whole-cell lysate from the ΔHspX strain was used because of binding of the rHspX to nHspX protein in the wild-type strain cell lysate (data not shown). The resultant protein, now termed rHspX-pulldown, was used to immunize mice. Mice were challenged 30 days later with either wild type or ΔHspX strains of M. tuberculosis. Lungs were assessed 30 days post infection for the number of viable bacilli. Consistent with that shown previously, rHspX did not protect against either strain of M. tuberculosis, but rHspX-pulldown protein significantly reduced the bacterial load in mice infected with either the wild type or ΔHspX strain of M. tuberculosis (Figure 7).
Figure 7.
rHspX protein incubated with whole-cell lysate protects against M. tuberculosis. (a) Diagrammatic representation of the hspX deletion/hyg insertion presents in the ΔHspX M. tuberculosis mutant strain. Open reading frames and relative orientations are indicated (arrows), and the hspX region replaced with a hygromycin resistance gene (hyg) is indicated (dashed lines). Mice were immunized with 5.0 μg native HspX (nHspX), rHspX or rHspX-pulldown three times, 2 weeks apart, and then infected with a low-dose aerosol of (b) H37Rv or (c) ΔHspX strains of M. tuberculosis 30 days after the last immunization. BCG-vaccinated mice were given a single dose at the time of the third immunization. Mice were killed 30 days post infection and the bacterial burden was assessed in the lungs. Data are expressed as the mean (n=5 mice) s.d. ***P<0.001, **P<0.01 and *P<0.05 when compared with the saline-vaccinated group using a 2-tailed Student's t-test.
DISCUSSION
While most TB vaccines currently in clinical trials target early expressed antigens, targeting latency-associated antigens, like HspX, has recently gained momentum in the field of TB vaccine development.12, 13, 14, 15, 26 In the present study, we tested the ability of HspX protein to induce short- and long-term protective immunity both as a prophylactic vaccine and as a booster to BCG vaccination. We found that native HspX conferred both short- and long-term protection, with long-term protection superior to that conferred by BCG. In fact, native HspX boosted BCG both in terms of protective efficacy and immunogenicity. The protective effect of the native HspX subunit vaccine was concentration-dependent, which also affected the quality of the immune response and extent of immunopathology in the lungs. Interestingly, rHspX was ineffective against infection, possibly due to chaperoning and/or folding differences between the native and recombinant forms of the protein.
Owing to the nature of TB infection, an effective TB vaccine should protect prophylactically, as well as against reactivation of latent TB. HspX is a logical vaccine target antigen because it is produced both during latency and, as our results support, during the early phase of infection in vivo. Here, the subunit form of the vaccine induced protective immunity within the first 30 days of infection (Figures 1a and b), suggesting that HspX protein is produced by the bacilli and is detected by the immune response during the early stage of infection, thereby rendering HspX an effective prophylactic vaccine candidate. Futhermore, the concentration of native HspX protein used per dose to vaccinate the animals affected the ability of the vaccine to protect. These data suggest that the concentration of a vaccine antigen may determine its ability to protect and that higher concentrations do not provide better protection. As in the lungs, the 0.1 μg concentration was ineffective in the spleen, indicating that the concentration of the vaccine affected the dissemination of bacilli, and that the optimal concentration for preventing dissemination was 1.0 μg. Compellingly, the native HspX vaccine was able to generate long-term protective immunity that rivaled that of BCG (Figure 2a, P=0.05).
The vaccinating dose of native HspX also affected the quality of the immune response. Induction of cell-mediated immunity is essential for protection against TB and CD4+ T cells are thought to impart protection by producing T helper cell type 1 cytokines.27 The native HspX vaccine used in the present study, therefore, is a promising vaccine candidate because it induced IFNγ-producing cells both in the short- and long-term models (Figure 3). The marked increase in the number of HspX-specific spleen cells producing IFNγ 30 days post vaccination compared with almost 10-times as many cells observed after 6 months suggests that the native HspX vaccine induced T helper cell type 1 memory immunity that correlated with a significant reduction in the bacterial load following immunization with the 1.0 μg dose. Expansion of fewer HspX-specific cells in the BCG-vaccinated animals may be explained by the fact that BCG, as a live replicating vaccine, induces a very diverse T-cell pool, and therefore the number of HspX-specific T cells in these mice may be diluted compared with vaccination with a single protein. Moreover, it has recently been shown that multifunctional T cells, that is, T cells capable of simultaneously producing IFNγ, IL-2 and TNFα, are functionally superior to single cytokine-producing T cells,28, 29 and are correlated with protection against TB.30, 31 As shown in Figure 4, multifunctional CD4+ T cells could be detected in the spleens of BCG- and HspX-vaccinated mice after 30 days and their occurence was concentration-dependent. Only the BCG group maintained these cells over time, which were not necessarily HspX-specific as the cells were stimulated ex vivo with antibodies against CD3 and CD28. As such, it is not unexpected that BCG would induce a greater pool of multifunctional T cells specific to a wide variety of different antigens.
Besides affecting the quality of the immune response, the concentration of native HspX protein used to vaccinate mice also affected the pulmonary pathology following infection with M. tuberculosis. Immunization with the 1.0 μg concentration of native HspX resulted in the least amount of pathology (Figure 6e). Although the composition of the infiltrates was similar in all the groups, the inflammatory response in the lungs of the mice vaccinated with the 0.1 μg and 10 μg concentrations (Figures 6d and e, respectively) was more severe than in the BCG- or 1.0 μg HspX-vaccinated mice, with larger lesions resembling those observed in the saline- and adjuvant-control groups (Figures 6a and c, respectively). These histological data may lend insight into the observation that, while both the 1.0 μg and 10 μg concentrations of native HspX conferred protection, only the 1.0 μg concentration decreased dissemination of the bacilli to the spleen.
Here, we tested the ability of the native HspX vaccine to boost the protective efficacy of BCG and found that this approach significantly increased the protective efficacy of BCG vaccination alone. Boosting BCG with the native HspX vaccine significantly increased the number of HspX-specific, IFNγ-producing cells in mice, which may have been, in part, the reason the prime-boost approach worked better than BCG alone. Overall, our results confirm data in the literature indicating that HspX, particularly when used in conjunction with BCG, is a promising target for developing vaccine regimens effective against the multiple stages of M. tuberculosis infection. It has been proposed that the failure of BCG to sterilize the lungs of M. tuberculosis may be due to its inability to induce long-lived T-cell responses against latency-associated antigens like HspX.32 That said, replacing BCG seems unlikely at this point as it is the most widely distributed vaccine in the world,33 and it confers protection in children. Several investigators have recently tested different vaccine strategies aimed at inducing both prophylactic and therapeutic protection against M. tuberculosis. Aagaard et al.26 recently tested a ‘multistage' vaccine regimen directed against both early-secreted and dormancy-associated antigens and found it to be protective prophylactically, as well as against reactivation TB disease. Others have employed HspX-based vaccines as a means to improve upon the protective effect of BCG in mice.
Based on these data combined with those from other laboratories, incorporating HspX into vaccine regimens may prove a powerful strategy. Our finding, however, that the rHspX was ineffective against pulmonary TB (Figure 1a and Figure 7), indicated that the engineering of this protein (and possibly others) must be considered in vaccine strategy design. The HspX protein is a member of the α-crystallin-like heat shock protein family34 and functions as a molecular chaperone,25 in part by preventing heat-induced aggregation of proteins in M. tuberculosis.16, 35 Although it is unclear how this chaperoning function may contribute to the hypoxic response of the bacilli, it has been proposed that HspX may help to stabilize cell structures in the thickening cell wall, thereby allowing the bacilli to survive low oxygen tension within the granuloma.18 In this regard, we hypothesized that protective immunity is generated against a molecule the native HspX protein chaperones, and that this chaperoned molecule is produced by M. tuberculosis but not by E. coli. If this hypothesis is correct, the reason rHspX protein failed to protect was because E. coli did not produce the substrate molecule to which HspX binds, thereby preventing epitope exposure and subsequent generation of a protective immune response when rHspX alone is used as the vaccine. As shown in Figure 7, the fact that the recombinant protein, when incubated with whole-cell lysate from ΔHspX M. tuberculosis, was able to confer statistically significant protection against infection, suggested that this hypothesis could be true. Further investigation of potential molecular and functional differences among the native, recombinant and recombinant pulldown proteins is currently in progress.
In summary, the results of this study showed that native HspX subunit vaccination protected mice when used on its own and that protective immunity was long-lived. Perhaps more clinically relevant, though, is that native HspX boosted the protective effect of BCG vaccination, maintaining the positive aspects of BCG while improving upon its shortcomings, namely BCG's inability to stimulate strong T-cell responses against latency-associated antigens. Another important finding of this study was that the native HspX subunit vaccine was protective, while the recombinant form was not, most likely because it chaperoned an immunogenic, mycobacterial molecule produced by M. tuberculosis. Based on the results presented here, the native HspX proves to be a promising multistage vaccine for use in both prophylactic and therapeutic approaches and to boost BCG vaccination.
Methods
Mice
Specific pathogen-free female, 6–8-week-old C57BL/six mice were purchased from Charles River Laboratories (Wilmington, MA, USA). All animals were maintained under barrier conditions with sterile mouse chow and water ad libitum. The specific pathogen-free nature of the mouse colonies was demonstrated by testing sentinel animals, which were shown to be negative for 12 known mouse pathogens. All experimental procedures were approved by the Colorado State University Animal Care and Use Committee.
Bacterial strains and growth
M. tuberculosis H37Rv (wild type) and HspX gene deleted (ΔHspX) were used. ΔHspX was made by allelic exchange using a modified protocol of Pelicic et al.36 Briefly, hspX-flanking regions from 825 bp to 107 bp upstream of hspX and from the second last codon of hspX to 644 bp downstream were amplified from genomic DNA of M. tuberculosis strain H37Rv with primer pairs hspXupF1[SpeI]/hspXupR1[PacI] and hspXdownF1[PacI]/hspXdownR1[BamHI], respectively, and inserted into SpeI/BamHI-digested plasmid pPR27.36 The cloned regions in the resulting plasmid (pPR27ΔhspX) were determined to be free of mutations by DNA sequencing. Plasmid was digested with PacI, blunted and ligated with a ∼1300 bp blunted BspHI/SmaI hygromycin resistance gene from plasmid p16R1.37 Resulting plasmid, pPR27ΔhspXhyg1, which contains the hygromycin resistance gene in the same orientation as the original hspX gene, was electroporated into M. tuberculosis strain H37Rv and the cells were plated on Middlebrook 7H11 agar supplemented with OADC (oleic acid, albumin, dextrose and catalase), 0.05% Tween-80 and 50 μg ml−1 hygromycin (7H11OADCTH) and incubated at 30 °C. Three transformants were cultured in 5 ml Dubos broth supplemented with OADC, 0.05% Tween-80, and 50 μg ml−1 hygromycin for 6 weeks at 32 °C. To obtain candidates that integrated the plasmid into the chromosome and lost the plasmid-encoded sacB gene, 100 μl of each culture was spread onto 7H11OADCTH supplemented with 2% sucrose and the plates incubated at 32 °C for 6 weeks. To confirm plasmid loss by screening for loss of the plasmid-encoded gentamycin resistance gene, twenty colonies from each plate were patched onto 7H11OADCTH+2% sucrose plates containing or lacking 5 μg ml−1 gentamycin and incubated for 6 weeks at 32 °C. Two of the 60 candidates were sensitive to gentamycin. Genomic DNA from both candidates was purified and screened by PCR with primers hspXoutF1 and hspXoutR1. Results demonstrated that only one of the strains (subsequently named ΔHspX) contained the single band corresponding to replacement of hspX with the hygromycin resistance gene. The mutant was confirmed by Southern analysis (data not shown).
Primers used:
hspXupF1[SpeI]: GGACTAGTTCGGCATGATACGACGA
hspXupR1[PacI]: ACCTTAATTAACCATTGACCCTGTTGTCTG
hspXdownF1[PacI]: ACCTTAATTAACTGACCACTGGGTCCGT
hspXdownR1[BamHI]: CGGGATCCTCCTCGTCGGTGACCT
hspXoutF1: GCACCCGATCCTTGTCGAG
hspXoutR1: CACGACGTCGTCATTGACC
One milliliter each of wild type and ΔHspX was transferred into a glass tube containing 9 ml of Proskauer-Beck (PB) medium38 and static cultures incubated at 37 °C for 3 weeks, or until pellicle formation was visible on top of the media. Pellicles were harvested and used for sequential inoculation into 25 ml and 100 ml of PB media and incubated as before. Pellicles of M. tuberculosis from the final passage were transferred to 20 ml of PB media containing 20% glycerol (v/v); cells were mixed by agitation, and suspensions bath sonicated at 4 °C for 10 min. One milliliter seed stock vials were prepared and stored at −80 °C. One vial of each strain of seed stock was added to 9 ml of 7H9+OADC medium containing 0.1% Tween-80 and incubated at 37 °C with shaking for 14 days. Cultures were inoculated into 45 ml of 7H9+OADC+0.1% Tween-80 and incubated at 37 °C with shaking, harvested, and 1 ml working stock vials were prepared and stored at −80 °C. Three vials of each strain were randomly recovered, thawed, serially diluted in 7H9+OADC medium and plated in triplicate for the determination of CFU.
Protein purification
Native HspX (nHspX) protein was purified from whole γ-irradiated Mtb cells following cell disruption using a French Press as described previously.39 The whole-cell lysate was centrifuged at 40 000 g, the supernatant discarded, and the pellet suspended in phosphate-buffered saline with 1% (v/v) n-octyl-β-𝒟-thioglucopyranoside, and centrifuged at 27 000 g. The supernatant was collected, exchanged into 10 mℳ ammonium bicarbonate and lyophilized. The dried material was suspended in 7.25 ℳ urea, 0.4% pH 3–10 ampholytes, 1.6% pH 4–7 ampholytes, 1% n-octyl-β-𝒟-thioglucopyranoside and 2 mℳ dithiothreitol (DTT). The protein concentration was determined by bicinchoninic acid assay (Pierce Chemical Company, Rockford, IL, USA). A sample of 100 mg of soluble protein was applied to a preparative Rotorfor apparatus (BioRad, Hercules, CA, USA), and proteins separated as per the manufacturer's instructions. Individual fractions (representing a pH range of 3–10) were harvested and resolved by SDS–PAGE using 4–12% gradient gels and transferred to nitrocellulose for analysis by western blot using CS-49 (anti-HspX antibody). Membranes were developed with 4-bromo-3-chloro-2-indoyl-1-phospate following incubation with secondary antibody. Fractions containing the HspX were pooled, exchanged into 3ℳ urea, 20 mℳ tris–HCl, 0.15 ℳ NaCl and 0.1% n-Octyl-β-𝒟-thioglucopyranoside, and the protein was purified by size exclusion chromatography using Sephadex S-75 resin (GE Life Sciences, Piscataway, NJ, USA) with isocratic elution. Fractions were resolved by SDS–PAGE and analyzed by western blot as described above. Fractions containing purified HspX were pooled, exchanged into 10 mℳ ammonium bicarbonate, protein quantified by bicinchoninic acid assay, and the purity was confirmed by SDS–PAGE and staining with silver nitrate. Endotoxin levels were assessed with a Limulus amebocyte lysate assay as described previously40 and samples were found to contain less than 10 ng EU mg−1 protein.
rHspX was produced in and purified from E. coli. The gene fragment encoding HspX was amplified by PCR using the primers 5′-CATATGGCCACCACCCTTCC-3′ (forward) and 5′-CTCGAGTCAGTTGGTGGACCGGATCT-3′ (reverse). The amplified gene product was ligated into the multiple cloning site for pET15B (EMD Biosciences, Gibbstown, NJ, USA) following digestion with NdeI and XhoI and the recombinant plasmid was transformed into E. coli BL21(DE3)pLysS (Invitrogen, Carlsbad, CA, USA). The transformed E. coli was plated onto LB media containing ampicillin and chloramphenicol, followed by sequential inoculation of 30 ml, then 2 l of LB broth and cultures grown to 1.0 OD600 at 37 °C with shaking. The cultures were induced with 0.5 mℳ isopropylthio-β-galactoside (IPTG) and harvested after 16 h of growth at 37 °C. The cells were broken by probe sonication and soluble rHspX was purified by Ni2+ chromatography.40 Endotoxin levels were measured as described above and found to contain <10 ng EU mg−1 of protein.
rHspX-pulldown protein was produced by incubation of purified, rHspX with ΔHspX WCL (10:1; lysate:recHspX ratio) in 10 mℳ ammonium bicarbonate at 4 °C overnight with gentle agitation. The mixture was applied to Ni2+ resin and rHspX-pulldown purified with any binding partners as described above.
Vaccination procedure
In the studies comparing rHspX and nHspX, mice were injected subcutaneously (s.c.) three times at 2-week intervals with either protein. For the dosing studies, mice were injected three times at 2-week intervals with nHspX protein at 0.1 μg, 1.0 μg or to 10 μg. As a positive control, one group of mice was vaccinated with a single dose of BCG Pasteur (1 × 105 CFU) at the time of the third vaccination dose. Negative controls included one group injected s.c. with saline, and one group injected s.c. with the adjuvant formulation alone. For the BCG prime-boost study, mice were vaccinated s.c. with BCG as above, followed by one dose of recombinant or native HspX 6 weeks later. In all studies, the HspX was emulsified in an adjuvant formulation containing dioctadecylammonium bromide (250 μg per dose) and monophosphoryl lipid A (25 μg per dose) as previously described,41 and all mice were injected s.c. with 100 μl of the vaccine formulation in the scruff of the neck.
Aerosol infection
Mice were infected by using procedures described previously.42 Briefly, bacterial stocks were diluted in 5 ml sterile distilled water to 2 × 106CFU ml−1 and placed in a nebulizer attached to an airborne infection system (Glass-Col, Terre Haute, IN, USA). At 30 days post vaccination or at 6 months post vaccination, mice were exposed to 40 min of aerosol, during which time approximately 50–100 bacteria were deposited in the lungs of each animal. Bacterial load was determined by plating whole-organ homogenates onto nutrient 7H11 agar supplemented with OADC. Colonies were enumerated after 21-day incubation at 37 °C. For the BCG prime-boost study, mice were infected by the aerosol route as described above 30 days after the last vaccination.
Spleen cell isolation
At 30 days or 6 months after the last vaccination, mice were euthanized by CO2 asphyxiation and the body cavity opened. The spleen was removed and passed through a cell strainer (BD Biosciences, Mountain View, CA, USA). Remaining red blood cells were then lysed with Gey's solution (0.15 ℳ NH4Cl and 10 mℳ KHCO3), and the cells washed with RPMI (Invitrogen) containing 5% heat-inactivated fetal bovine serum (Atlas Biologicals, Fort Collins, CO, USA). Total viable cell numbers were determined using a Neubauer chamber (IMV International, Minneapolis, MN, USA) and 2% trypan blue solution.
IFNγ ELISpot
MultiScreen 96-well high throughput screening (HTS) IP sterile plates (Millipore, Bedford, MA, USA) were coated with anti-IFNγ antibody at 4 °C overnight. The plates were blocked according to the manufacturer (eBioscience, San Diego, CA, USA), and spleen cells (5 × 105 cells per well) were cultured with 10 μg ml−1 nHspX or rHspX protein in complete RPMI 1640 (10% fetal bovine serum, penicillin–streptomycin, non-essential amino acids, 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES) buffer and ℒ-glutamine). ConA (5 μg ml−1; Sigma, St Louis, MO, USA) treatment was used as a positive control and media without a stimulant as a negative control. Cells were incubated for 48 h at 37 °C in a 5% CO2 incubator and then stained with an anti-IFNγ-horseradish peroxidase conjugate and developed for horseradish peroxidasereactivity with 30% H2O2 and 3-amino-9-ethylcarbazole substrate (Sigma). IFNγ-positive spots were counted with an Immunospot reader (Cellular Technology Limited, Cleveland, OH, USA).
Flow cytometric analysis
Levels of intracellular IFN-γ, IL-2 and TNF-α produced by stimulated T lymphocytes was measured by pre-incubation of 106 cells with Monensin (3 μℳ) and anti-CD3 and anti-CD28 (both at 0.2 μg per 106 cells) for 4 h at 37 °C, 5% CO2. The cells were then stained with fluorescent-labeled antibodies against the surface markers CD4, washed, fixed and permeabilized with PermFix/Perm Wash (BD Pharmingen, San Diego, CA, USA), and stained for intracellular IFN-γ, IL-2 and TNF-α using fluorescent-labeled antibodies and appropriate isotype controls. Cells were gated on lymphocytes by forward and side scatter according to their characteristic scatter profile. Antibodies were used at 0.2 μg per 106 cells, and all analyses were performed with an acquisition of at least 100 000 events on a Becton-Dickinson FACSCalibur (BD Biosciences). The data were analyzed using FlowJo software (Tree Star Inc., Ashland, OR, USA).
Histological analysis
Whole lungs from euthanized mice (n=3) were inflated with 10% formalin, embedded in paraffin and five, 4 μm serial sections were cut. Sections 1 and 5 were then stained with hematoxylin and eosin and examined by a veterinary pathologist with no prior knowledge of the experimental design.
Statistical analysis
Residuals from a one-way analysis of variance were tested for normality using a Wilk-Shapiro test with P-values ranging from 0.09 to 0.98 across all of the data presented in this manuscript. As these P-values are consistent with the assumption of normality (that is, P>0.05), the Student t-test was used to determine statistical significance.
Acknowledgments
Funding for this research was provided by NIH, NIAID NO1-AI-40091 and the CVMBS College Research Council at Colorado State University.
References
- Blanc L, Martinez L. Reaching the targets for TB control: call for papers. Bull World Health Organ. 2006;84:688. doi: 10.2471/blt.06.035709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arend SM, van Dissel JT. Evidence of endogenous reactivation of tuberculosis after a long period of latency. J Infect Dis. 2002;186:876–877. doi: 10.1086/342604. [DOI] [PubMed] [Google Scholar]
- Rodrigues LC, Diwan VK, Wheeler JG. Protective effect of BCG against tuberculosis meningitis and miliary tuberculosis: a meta-analysis. Int J Epidemiol. 1993;22:1154–1158. doi: 10.1093/ije/22.6.1154. [DOI] [PubMed] [Google Scholar]
- Geluk A, Lin MY, van Meijgaarden KE, Leyten EM, Franken KL, Ottenhof TH, et al. T-cell recognition of the HspX protein in Mycobacterium tuberculosis correlates with latent M. tuberculosis infection but not with M. bovis BCG vaccination. Infect Immun. 2007;75:2914–2921. doi: 10.1128/IAI.01990-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caruso A, Serbina NV, Klein E, Triebold KJ, Bloom BR, Flynn J. Mice deficient in CD4 T cells have only transiently diminished levels of IFN-g, yet succumb to tuberculosis. J Immunol. 1999;162:5407–5416. [PubMed] [Google Scholar]
- Foulds KE, Wu C, Seder RA. Th1 memory; implications for vaccine development. Immunol Rev. 2006;211:58–66. doi: 10.1111/j.0105-2896.2006.00400.x. [DOI] [PubMed] [Google Scholar]
- Goldsack L, Kirman JR. Half-truths and selective memory: interferon gamma CD4(+) T cells and protective memory against tuberculosis. Tuberculosis. 2007;87:465–473. doi: 10.1016/j.tube.2007.07.001. [DOI] [PubMed] [Google Scholar]
- Forbes EK, Sander C, Ronan EO, McShane H, Hill AV, Beverley PC, et al. Multifunctional, high-level cytokine-producing Th1 cells in the lung, but not spleen, correlate with protection against Mycobacterium tuberculosis aerosol challenge in mice. J Immunol. 2008;181:4955–4964. doi: 10.4049/jimmunol.181.7.4955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindenstrøm T, Agger EM, Korsholm KS, Darrah PA, Aagaard C, Seder RA, et al. Tuberculosis subunit vaccination provides long-term protective immunity characterized by multifunctional CD4 memory T cells. J Immunol. 2009;182:8047–8055. doi: 10.4049/jimmunol.0801592. [DOI] [PubMed] [Google Scholar]
- Brandt L, Elhay MJ, Rosenkrands I, Lindblad EB, Andersen P. ESAT-6 subunit vaccination against Mycobacterium tuberculosis. Infect Immun. 2000;68:791–795. doi: 10.1128/iai.68.2.791-795.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langermans JA, Doherty TM, Vervenne RA, van der Laan T, Lyashchenko K, Greenwald R, et al. Protection of macaques against Mycobacterium tuberculosis infection by a subunit vaccine based on a fusion protein of antigen 85B and ESAT-6. Vaccine. 2005;23:2740–2750. doi: 10.1016/j.vaccine.2004.11.051. [DOI] [PubMed] [Google Scholar]
- Dey B, Jain R, Gupta UD, Katoch VM, Ramanathan VD, Tyagi AK. A booster vaccine expressing a latency-associated antigen augments BCG induced immunity and confers enhanced protection against Tuberculosis. PLoS One. 2011;6:e23360. doi: 10.1371/journal.pone.0023360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Xu M, Wang ZY, Chen BW, Du WX, Su C, et al. The development and preliminary evaluation of a new Mycobacterium tuberculosis vaccine comprising Ag85b, HspX and CFP-10:ESAT-6 fusion protein with CpG DNA and aluminum hydroxide adjuvants. FEMS Immunol Med Microbiol. 2010;59:42–52. doi: 10.1111/j.1574-695X.2010.00660.x. [DOI] [PubMed] [Google Scholar]
- Spratt JM, Britton WJ, Triccas JA. In vivo persistence and protective efficacy of the bacille Calmette Guerin vaccine overexpressing the HspX latency antigen. Bioeng Bugs. 2010;1:61–65. doi: 10.4161/bbug.1.1.10027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Yu H, Zhang Y, Wang B, Jiang W, Da Z, et al. Immunogenicity and protective efficacy of a fusion protein vaccine consisting of antigen Ag85B and HspX against Mycobacterium tuberculosis infection in mice. Scand J Immunol. 2011;73:568–576. doi: 10.1111/j.1365-3083.2011.02531.x. [DOI] [PubMed] [Google Scholar]
- Yuan Y, Crane DD, Barry CEr. Stationary phase-associated protein expression in Mycobacterium tuberculosis: function of the mycobacterial alpha-crystallin homolog. J Bacteriol. 1996;178:4484–4492. doi: 10.1128/jb.178.15.4484-4492.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan Y, Crane DD, Simpson RM, Zhu YQ, Hickey MJ, Sherman DR, et al. The 16-kDa alpha-crystallin (Acr) protein of Mycobacterium tuberculosis is required for growth in macrophages. Proc Natl Acad Sci. 1998;95:9578–9583. doi: 10.1073/pnas.95.16.9578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham AF, Spreadbury CL. Mycobacterial stationary phase induced by low oxygen tension: cell wall thickening and localization of the 16-kilodalton alpha-crystallin homolog. J Bacteriol. 1998;180:801–808. doi: 10.1128/jb.180.4.801-808.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan Y, Crane DD, Simpson RM, Zhu Y, Hickey MJ, Sherman DR, et al. The 16-kDa α-crystallin (Acr) protein of Mycobacterium tuberculosis is required for growth in macrophages. Proc Natl Acad Sci. 1998;95:9578–9583. doi: 10.1073/pnas.95.16.9578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Y, Coates AR. Transcription of the stationary-phase-associated hspX gene of Mycobacterium tuberculosis is inversely related to synthesis of the 16-kilodalton protein. J Bacteriol. 1999;181:1380–1387. doi: 10.1128/jb.181.5.1380-1387.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin MY, Geluk A, Smith SG, Stewart AL, Friggen AH, Franken KL, et al. Lack of immune responses to Mycobacterium tuberculosis DosR regulon proteins following Mycobacterium bovis BCG vaccination. Infect Immun. 2007;75:3523–3530. doi: 10.1128/IAI.01999-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidow A, Kanaujia GV, Shi L, Kaviar J, Guo X, Sung N, et al. Antibody profiles characteristic of Mycobacterium tuberculosis infection state. Infect Immun. 2005;73:6846–6851. doi: 10.1128/IAI.73.10.6846-6851.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demissie A, Leyten EM, Abebe M, Wassie L, Aseffa A, Abate G, et al. Recognition of stage-specific mycobacterial antigens differentiates between acute and latent infections with Mycobacterium tuberculosis. Clin Vaccine Immunol. 2006;13:179–186. doi: 10.1128/CVI.13.2.179-186.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee BY, Hefta SA, Brennan PJ. Characterization of the major membrane protein of virulent Mycobacterium tuberculosis. Infect Immun. 1992;60:2066–2074. doi: 10.1128/iai.60.5.2066-2074.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwitz J. Alpha-crystallin can function as a molecular chaperone. Proc Natl Acad Sci. 1992;89:10449–10453. doi: 10.1073/pnas.89.21.10449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aagaard C, Hoang T, Dietrich J, Cardona PJ, Izzo A, Dolganov G, et al. A multistage tuberculosis vaccine that confers efficient protection before and after exposure. Nat Med. 2011;17:189–194. doi: 10.1038/nm.2285. [DOI] [PubMed] [Google Scholar]
- Stenger S, Modlin RL. T cell mediated immunity to Mycobacterium tuberculosis. Curr Opin Microbiol. 1999;2:89–93. doi: 10.1016/s1369-5274(99)80015-0. [DOI] [PubMed] [Google Scholar]
- Beveridge NE, Price DA, Casazza JP, Pathan AA, Sander CR, Asher TE, et al. Immunisation with BCG and recombinant MVA85A induces long-lasting, polyfunctional Mycobacterium tuberculosis-specific CD4+ memory T lymphocyte populations. Eur J Immunol. 2007;37:3089–3100. doi: 10.1002/eji.200737504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wille-Reece U, Flynn BJ, Loré K, Koup RA, Miles AP, Saul A, et al. Toll-like receptor agonists influence the magnitude and quality of memory T cell responses after prime-boost immunization in nonhuman primates. J Exp Med. 2006;203:1249–1258. doi: 10.1084/jem.20052433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aagaard CS, Hoang TT, Vingsbo-Lundberg C, Dietrich J, Andersen P. Quality and vaccine efficacy of CD4+ T cell responses directed to dominant and subdominant epitopes in ESAT-6 from Mycobacterium tuberculosis. J Immunol. 2009;183:2659–2668. doi: 10.4049/jimmunol.0900947. [DOI] [PubMed] [Google Scholar]
- Hoang TT, Nansen A, Roy S, Billeskov R, Aagaard C, Elvang T, et al. Distinct differences in the expansion and phenotype of TB10.4 specific CD8 and CD4 T cells after infection with Mycobacterium tuberculosis. PLoS One. 2009;4:e5928. doi: 10.1371/journal.pone.0005928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vekemans J, Ota MO, Sillah J, Fielding K, Alderson MR, Skeiky YA, et al. Immune responses to mycobacterial antigens in the Gambian population: implications for vaccines and immunodiagnostic test design. Infect Immun. 2004;72:381–388. doi: 10.1128/IAI.72.1.381-388.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefanova T, Chouchkova M, Hinds J, Butcher PD, Inwald J, Dale J, et al. Genetic composition of Mycobacterium bovis BCG substrain Sofia. J Clin Microbiol. 2003;41:5349. doi: 10.1128/JCM.41.11.5349.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Jong WW, Caspers GJ, Leunissen JA. Genealogy of the alpha-crystallin--small heat-shock protein superfamily. Int J Biol Macromol. 1998;22:151–162. doi: 10.1016/s0141-8130(98)00013-0. [DOI] [PubMed] [Google Scholar]
- Chang Z, Primm TP, Jakana J, Lee IH, Serysheva I, Chiu W, et al. Mycobacterium tuberculosis 16-kDa antigen (Hsp16.3) functions as an oligomeric structure in vitro to suppress thermal aggregation. J Biol Chem. 1996;271:7218–7223. [PubMed] [Google Scholar]
- Pelicic V, Jackson M, Reyrat JM, Jacobs WR, Gicquel B, Guilhot C. Efficient allelic exchange and transposon mutagenesis in Mycobacterium tuberculosis. Proc Natl Acad Sci. 1997;94:10955–10960. doi: 10.1073/pnas.94.20.10955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garbe TR, Barathi J, Barnini S, Zhang Y, Abou-Zeid C, Tang D, et al. Transformation of mycobacterial species using hygromycin resistance as selectable marker. Microbiol. 1994;140:133–138. doi: 10.1099/13500872-140-1-133. [DOI] [PubMed] [Google Scholar]
- Kruh NA, Troudt J, Izzo A, Prenni J, Dobos KM. Portrait of a pathogen: the Mycobacterium tuberculosis proteome in vivo. PLoS One. 2010;5:e13938. doi: 10.1371/journal.pone.0013938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirschfield GR, McNeil M, Brennan PJ. Peptidoglycan-associated polypeptides of Mycobacterium tuberculosis. J Bacteriol. 1990;172:1005–1013. doi: 10.1128/jb.172.2.1005-1013.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H, Troudt J, Grover A, Arnett K, Lucas M, Cho YS, et al. Three protein cocktails mediate delayed-type hypersensitivity responses indistinguishable from that elicited by purified protein derivative in the guinea pig model of Mycobacterium tuberculosis infection. Infect Immun. 2011;79:716–723. doi: 10.1128/IAI.00486-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldridge JR, Crane RT. Monophosphoryl lipid A (MPL) formulations for the next generation of vaccines. Methods. 1999;19:103–107. doi: 10.1006/meth.1999.0834. [DOI] [PubMed] [Google Scholar]
- Cooper AM, Dalton DK, Stewart TA, Griffin JP, Russell DG, Orme IM. Disseminated tuberculosis in interferon gamma gene-disrupted mice. J Exp Med. 1993;178:2243–2247. doi: 10.1084/jem.178.6.2243. [DOI] [PMC free article] [PubMed] [Google Scholar]