Abstract
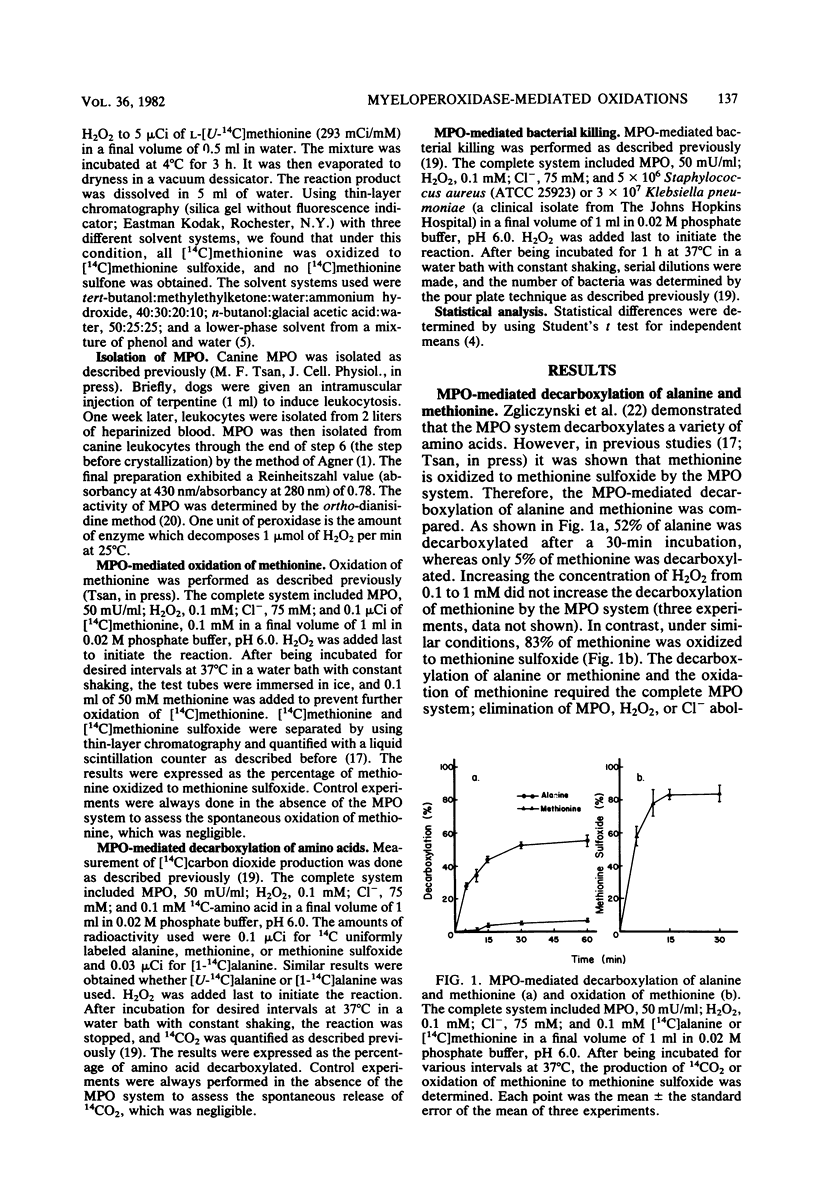
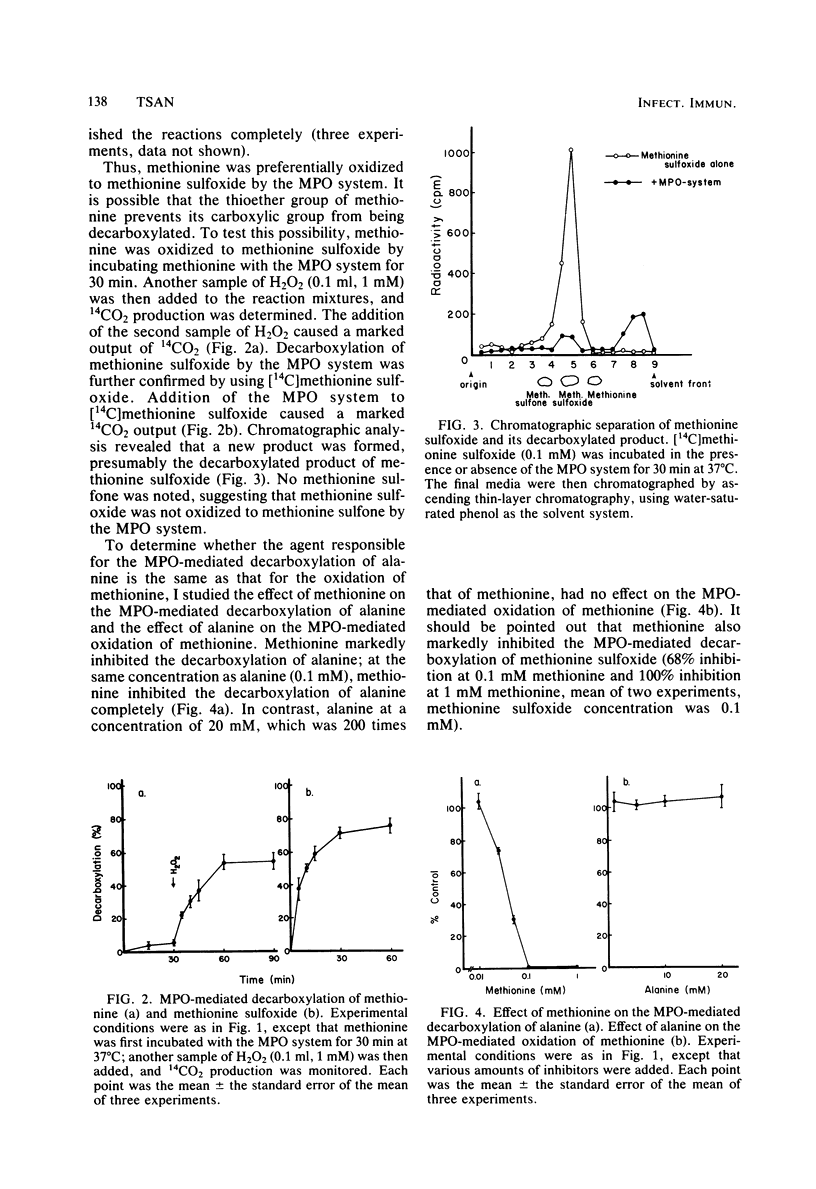
The myeloperoxidase (MPO)-mediated decarboxylation of amino acids and the MPO-mediated oxidation of methionine, two potential bactericidal mechanisms, were compared. In the presence of the MPO system (MPO, 50 mU/ml; H2O2, 0.1 mM; Cl−, 75 mM), 50% of alanine (0.1 mM) was decarboxylated, whereas only 5% of methionine (0.1 mM) was decarboxylated. In contrast, under similar conditions, 80% of methionine was oxidized to methionine sulfoxide. Once methionine was oxidized to methionine sulfoxide, it was decarboxylated (75%) by the MPO system. Methionine at 0.1 mM completely inhibited the decarboxylation of alanine, whereas alanine at a concentration 200 times that of methionine had no effect on the MPO-mediated oxidation of methionine. Sodium azide, an MPO inhibitor, inhibited the decarboxylation of alanine and the oxidation of methionine to the same extent. Tryptophan markedly inhibited the oxidation of methionine, whereas histidine stimulated it. Alanine, glycine, and taurine had no effect. In contrast, all of these amino acids and taurine markedly inhibited the MPO-mediated decarboxylation of alanine. NaN3, tryptophan, and methionine, which inhibited the MPO-mediated oxidation of methionine, also inhibited the killing of Staphylococcus aureus or Klebsiella pneumoniae by the MPO system; whereas histidine, alanine, and glycine, which did not inhibit the oxidation of methionine, had less or no effect on the killing of these two bacteria by the MPO system. Results suggest that methionine is preferentially oxidized to methionine sulfoxide by the MPO system. Once methionine is oxidized to methionine sulfoxide, it is then readily decarboxylated by the MPO system. The agent responsible for the oxidation of methionine may play an important role in the MPO-mediated killing of bacteria.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Carp H., Janoff A. Potential mediator of inflammation. Phagocyte-derived oxidants suppress the elastase-inhibitory capacity of alpha 1-proteinase inhibitor in vitro. J Clin Invest. 1980 Nov;66(5):987–995. doi: 10.1172/JCI109968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark R. A., Szot S., Venkatasubramanian K., Schiffmann E. Chemotactic factor inactivation by myeloperoxidase-mediated oxidation of methionine. J Immunol. 1980 Apr;124(4):2020–2026. [PubMed] [Google Scholar]
- Harrison J. E., Watson B. D., Schultz J. Myeloperoxidase and singlet oxygen: a reappraisal. FEBS Lett. 1978 Aug 15;92(2):327–332. doi: 10.1016/0014-5793(78)80780-7. [DOI] [PubMed] [Google Scholar]
- Held A. M., Hurst J. K. Ambiguity associated with use of singlet oxygen trapping agents in myeloperoxidase-catalyzed oxidations. Biochem Biophys Res Commun. 1978 Apr 14;81(3):878–885. doi: 10.1016/0006-291x(78)91433-x. [DOI] [PubMed] [Google Scholar]
- Klebanoff S. J. Antimicrobial mechanisms in neutrophilic polymorphonuclear leukocytes. Semin Hematol. 1975 Apr;12(2):117–142. [PubMed] [Google Scholar]
- Klebanoff S. J. Iodination of bacteria: a bactericidal mechanism. J Exp Med. 1967 Dec 1;126(6):1063–1078. doi: 10.1084/jem.126.6.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matheson N. R., Wong P. S., Travis J. Enzymatic inactivation of human alpha-1-proteinase inhibitor by neutrophil myeloperoxidase. Biochem Biophys Res Commun. 1979 May 28;88(2):402–409. doi: 10.1016/0006-291x(79)92062-x. [DOI] [PubMed] [Google Scholar]
- Paul B. B., Jacobs A. A., Strauss R. R., Sbarra A. J. Role of the Phagocyte in Host-Parasite Interactions XXIV. Aldehyde Generation by the Myeloperoxidase-H(2)O(2)-Chloride Antimicrobial System: a Possible In Vivo Mechanism of Action. Infect Immun. 1970 Oct;2(4):414–418. doi: 10.1128/iai.2.4.414-418.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen H., Klebanoff S. J. Formation of singlet oxygen by the myeloperoxidase-mediated antimicrobial system. J Biol Chem. 1977 Jul 25;252(14):4803–4810. [PubMed] [Google Scholar]
- Selvaraj R. J., Paul B. B., Strauss R. R., Jacobs A. A., Sbarra A. J. Oxidative peptide cleavage and decarboxylation by the MPO-H2O2-Cl- antimicrobial system. Infect Immun. 1974 Feb;9(2):255–260. doi: 10.1128/iai.9.2.255-260.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stelmaszyńska T., Zgliczyński J. M. Myeloperoxidase of human neutrophilic granulocytes as chlorinating enzyme. Eur J Biochem. 1974 Jun 1;45(1):305–312. doi: 10.1111/j.1432-1033.1974.tb03555.x. [DOI] [PubMed] [Google Scholar]
- Strauss R. R., Paul B. B., Jacobs A. A., Sbarra A. J. Role of the Phagocyte in Host-Parasite Interactions XXVII. Myeloperoxidase-H(2)O(2)-Cl-Mediated Aldehyde Formation and Its Relationship to Antimicrobial Activity. Infect Immun. 1971 Apr;3(4):595–602. doi: 10.1128/iai.3.4.595-602.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss R. R., Paul B. B., Jacobs A. A., Sbarra A. J. Role of the phagocyte in host-parasite interactions. XXII. H2O2-dependent decarbosylation and deamination by myeloperoxidase and its relationship to antimicrobial activity. J Reticuloendothel Soc. 1970 Jun;7(6):754–761. [PubMed] [Google Scholar]
- Tsan M. F., Chen J. W. Oxidation of methionine by human polymorphonuclear leukocytes. J Clin Invest. 1980 May;65(5):1041–1050. doi: 10.1172/JCI109756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsan M. F., Denison R. C. Oxidation of n-formyl methionyl chemotactic peptide by human neutrophils. J Immunol. 1981 Apr;126(4):1387–1389. [PubMed] [Google Scholar]
- Tsan M. F., Newman B., McIntyre P. A. Surface sulphydryl groups and phagocytosis-associated oxidative metabolic changes in human polymorphonuclear leucocytes. Br J Haematol. 1976 Jun;33(2):189–204. doi: 10.1111/j.1365-2141.1976.tb03530.x. [DOI] [PubMed] [Google Scholar]
- Zgliczyński J. M., Stelmaszyńska T., Domański J., Ostrowski W. Chloramines as intermediates of oxidation reaction of amino acids by myeloperoxidase. Biochim Biophys Acta. 1971 Jun 16;235(3):419–424. doi: 10.1016/0005-2744(71)90281-6. [DOI] [PubMed] [Google Scholar]
- Zgliczyński J. M., Stelmaszyńska T., Ostrowiski W., Naskalski J., Sznajd J. Myeloperoxidase of human leukaemic leucocytes. Oxidation of amino acids in the presence of hydrogen peroxide. Eur J Biochem. 1968 May;4(4):540–547. doi: 10.1111/j.1432-1033.1968.tb00246.x. [DOI] [PubMed] [Google Scholar]



