Abstract
Introduction:
Ketamine and dexmedetomidine decrease anesthetic requirement and provide analgesia to patients. We designed this study to compare the effect of dexmedetomidine and ketamine when added to lignocaine in intravenous regional anesthesia (IVRA).
Materials and Methods:
Seventy two patients undergoing hand surgery were randomly assigned to three groups to receive IVRA. They received 20 ml of 1% lignocaine and either 1 ml of isotonic saline (Group L, n = 24); or 0.5 mg/kg body weight ketamine (Group LK, n = 24) or 1 mcg/kg body weight dexmedetomidine (Group LD, n = 24). Sensory and motor block onset and recovery time were noted. After the tourniquet deflation, pain and sedation values, time to first analgesic requirement and any side effects were noted.
Results:
Shortened sensory and motor block onset times (69.17 min and 7.83 min respectively, P < 0.0001) and improved quality of anesthesia (satisfaction score = 3, P < 0.05) were found in ketamine group. Visual analog scale scores (3.21 ± 0.41) were comparable while time to first analgesic requirement (166.25 ± 25.89 min, P < 0.0001) was significantly longer in dexmedetomidine group after tourniquet release.
Conclusion:
We conclude that the addition of 1 mcg/kg of body weight dexmedetomidine or 0.5 mg/kg of body weight ketamine to lignocaine for IVRA improves quality of anesthesia and perioperative analgesia without causing side effects. We considered ketamine reduced the time for onset of block, delayed the onset of tourniquet pain, and reduced postoperative analgesic requirement and had a better patient satisfaction than placebo or dexmedetomidine.
Keywords: Biers block, dexmedetomidine, intravenous regional anesthesia, ketamine, lignocaine, regional anesthesia
Introduction
Intravenous regional anesthesia (IVRA), first described by August Bier in 1902, is technically simple and reliable, with success rates between 94% and 98%.[1] IVRA is a method of producing analgesia in the distal part of a limb by intravenous (IV) injection of a local anesthetic solution into the vein of the same limb, while circulation to the limb is occluded by the application of tourniquet.
Different agents have been used as additive to local anesthetic for IVRA including phencyclidines, non-steroidal anti inflammatory drugs, opioids, and muscle relaxants.[2] Ketamine is an effective anesthetic agent for IVRA at concentrations between 0.3% and 0.5%.[3] Dexmedetomidine, a potent alpha (α) 2-adrenoceptor agonist, has been shown to decrease anesthetic requirements by up to 90% and to induce analgesia.[4,5] Addition of dexmedetomidine to lignocaine in IVRA also improves the quality of anesthesia but has no effect on the sensory and motor block onset and regression time.[6]
No study till date has compared ketamine and dexmedetomidine as additives in IVRA. We compared the onset of anesthesia and onset of tourniquet pain during IVRA using lignocaine alone, lignocaine with ketamine, and lignocaine with dexmedetomidine and assessed postoperative analgesic requirement and satisfaction score of the patients.
Materials and Methods
This prospective randomized double blind study was conducted after obtaining clearance from Institutional Ethical Committee of the institute and written informed consent from all patients. Patients of American Society of Anesthesiologists physical status I, aged between 20 to 50 years scheduled for surgery in distal region of upper limb under IVRA were included. Those with history of allergic reaction to lignocaine, significant cardiovascular disease, sickle cell disease, history of chronic pain or regular medication with analgesics, history of opioid dependence, drug or alcohol abuse, psychiatric disorder, peripheral vascular disease, and neurological diseases were excluded from the study. At the pre-operative visit, on the evening before surgery, the visual analog scale (VAS) scoring system was explained to all the patients.
Patients were assigned randomly to one of three groups depending on the drug solution used for IVRA (n = 24 in each group). Patients in Group L received lignocaine only; Group LK received lignocaine along with ketamine (0.5 mg/ kg); and Group LD received lignocaine and dexmedetomidine (1 mcg/ kg). The lignocaine used in the study was 2% preservative free and constant (200 mg) in all groups, as was the total volume of test solution (20 ml). Normal saline (0.9 %) was added to make up the volume as required.
No premedication was given to any of the patients. Standard monitoring was used in all patients, which included noninvasive arterial blood pressure, heart rate, electrokardiogram and pulse oximetry. Prior to administration of IVRA, an infusion of 0.9 % normal saline was started in the normal limb. A 20G IV cannula was inserted into distal vein of the extremity that was to be studied; cotton pad was applied to the arm to protect the skin. Two tourniquets were placed over the cotton pad. The arm was exsanguinated by using an Esmarch bandage. The proximal tourniquet was inflated to 100 mm Hg above the systolic pressure of the patient. The absence of radial artery pulsations and failure of pulse oximetry tracing in the ipsilateral index finger was confirmed. 20 ml of test solution was injected over 10 seconds by an anesthesiologist who was blinded to the drug being administered.
After the injection of different study solutions, the onset of sensory block (defined as loss of pain sensation) was determined by the pin prick method, with a 22G hypodermic needle, distal to the tourniquet at 20 sec intervals. The motor block was assessed at one min interval till the patient was not able to produce movement of any fingers. On complaint of the tourniquet pain by patient intraoperatively, the distal cuff was inflated and the proximal cuff was deflated. If required, the timing of the second cuff inflation was noted.
Need for intraoperative analgesia was also recorded. At the end of the operation, a blinded anesthesiologist recorded the satisfaction score of the patient for the anesthetic technique according to the following numeric scale: 3 = good (no complaint from patient), 2 = moderate (minor complaint with no need for supplemental analgesics), 1 = poor (complaint which required supplemental analgesics). Recovery room pain score, time to first analgesic demand, and sedation score (Ramsay Sedation Score) were compared. We also recorded the occurrence of any unpleasant psychologic effects postoperatively.
Postoperatively patients received diclofenac 75 mg intramuscular if VAS was >4.
All values were calculated with a 95% Confidence Interval (CI). The parameters were expressed as mean ± SD and t-test was used for comparing demographic and clinical data. Analysis of variance (ANOVA) technique was used for comparison between the three groups for parametric data. Chi-square test was used for non-parametric data. For the comparisons, p< 0.05 was considered to be statistically significant. The sample size was chosen after reviewing many randomized control studies on the same subject and had a sample size ranging between 20 and 40 patients. The statistical evaluation was performed using SPSS version 11.0.
Result
A total of 72 patients were enrolled in the study (n = 24 in each group). The mean age and duration of surgery were not different between groups (P>0.05). All the patients operated during the study period were male. Among the patients, none was excluded from the study because of technical failure. The patients in Group L weighed less than those in Group LK or Group LD. Duration of surgery were similar in all the groups [Table 1]. Types of surgery performed are depicted in [Figure 1]. No patient needed treatment for hypotension or bradycardia.
Table 1.
Patient characteristics and outcome measures
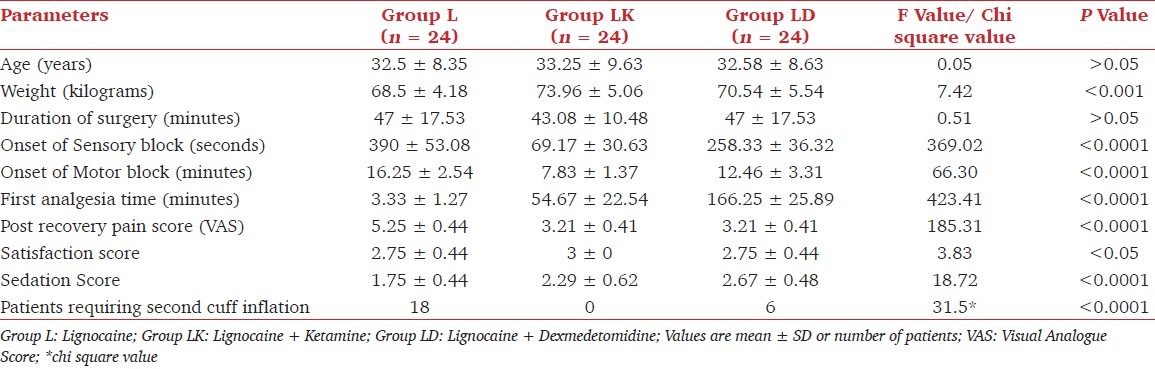
Figure 1.
Multiple bar diagram showing types of surgery in study groups
The onset of sensory and motor block was faster in Group LK as compared to other groups (P<0.0001) although the duration of analgesia, as seen by time to first analgesia demand by the patient, was more in patients in Group LD (P < 0.0001). Post recovery pain was similar in Group LK and LD but less than in Group L (P< 0.0001). Sedation score was highest in Group LD while lowest in Group L (P< 0.0001). Patients in Group LK were more satisfied than other groups in which the satisfaction scores were similar (P< 0.05). Almost two third patients in Group L complained of tourniquet pain after an average duration of 26.67 minutes requiring the second cuff to be inflated while none in Group LK and only one third in Group LD complained of tourniquet pain after an average duration of 35 min (P< 0.0001). One patient had dizziness in Group LD, two had restlessness in Group LK, and no one developed any unpleasant effect in Group L. Patients were more satisfied in Group LK as compared to Group L or LD (P< 0.05)
Discussion
IVRA technique is widely used for surgery on arms. IVRA is safe and problems are few. The advantages of IVRA are high indices of reliability, rapid onset of analgesia within 5-10 minutes and good muscular relaxation. The disadvantage of IVRA is the application of a tourniquet, which must remain inflated continuously throughout the procedure. The duration of surgery is limited by the time during which the arterial tourniquet could be kept safely inflated. Tourniquet pain, which is described as a dull and aching pain sensation, is a well-known limitation of IVRA. Skin compression, tourniquet size, and inflation pressure have been implicated as factors involved in tourniquet pain. Another drawback with this technique is the absence of postoperative analgesia. In several studies it was tried to find a local anesthesia mixture that allows relief from tourniquet pain and prolonged duration of analgesia after tourniquet release. Non-steroidal anti-inflammatory drugs, opioids, and combination of opioid and muscle relaxant have been used without demonstrating clear advantage.[2]
Ketamine, a phenyl-piperidine derivative, was first synthesized in the early 1960s as an IV anesthetic agent. At subanesthetic doses, ketamine exerts a noncompetitive blockade of N-methyl-aspartate (NMDA) receptors. NMDA receptors play a major role in synaptic plasticity and are specifically implicated in central nervous system facilitation of pain processing. NMDA receptor antagonists have been implicated in perioperative pain management. Ketamine also has local anesthetic qualities, which have been studied as a sole agent for IVRA.[7] In addition to spinal cord NMDA receptors, NMDA receptors have also been identified on peripheral unmyelinated sensory axons. This can explain why ketamine as an NMDA receptor antagonist was able to attenuate the tourniquet pain. Ketamine 0.5 and 0.3% produced adequate IVRA. Anesthesia was inadequate when a 0.2% concentration was used. Although 0.3% concentration provides complete sympathetic, sensory, and motor blockade when injected into the isolated extremity. Unpleasant psychotomimetic effects after the release of the tourniquet limit the usefulness of this use of ketamine.[8,9] When ketamine is used with lignocaine (0.5%) in a dose of 3 mg/kg of body weight, duration of analgesia after release of tourniquet is longer, and the quality of analgesia is superior. The onset of analgesia and motor blockade remains similar and all patients suffered from disorientation and hallucinations.[8] In comparison to systemic administration, there is no selective benefit to adding ketamine to the IVRA injectate.[10] Ketamine cannot be recommended as a sole agent for IVRA unless these unpleasant side effects are abolished or controlled by means of pharmacologic adjuvant.[11] When used in the doses of 0.1 to 0.5 mg/kg of body weight in IVRA no central nervous system symptoms have been observed.[12] Our study clearly demonstrated benefit of ketamine in IVRA when compared to a placebo. There was an early onset of sensory and motor block and good postoperative analgesia, although postoperative analgesia was longer in the dexmedetomidine group. No second cuff inflation was required in any patient denoting delay in onset of the tourniquet pain. Ketamine has well known hemodynamic effects (hypertension and tachycardia) but it failed to show any of these effects when given as an adjuvant in IVRA in our study. This could be due to the fact that the tourniquet was not deflated before 30 min.
Clonidine has been shown to decrease tourniquet pain and intraoperative analgesic requirement in IVRA.[13] Dexmedetomidine is approximately eight times more selective toward the α2 -adrenoceptors than clonidine.[5] Centrally active α -adrenergic agonists exert powerful analgesic action that probably is transduced at several levels. Dexmedetomidine has been shown to enhance the local anesthetic action of lignocaine via α 2A adrenoceptor.[14] Perioperative dexmedetomidine administration decreases the requirements for opioid or non-opioid analgesics both intra and postoperatively.[15] IV dexmedetomidine as a premedication has been effective before IVRA because it reduces patient anxiety, sympathoadrenal responses, and opioid analgesic requirements but it did not reduce tourniquet pain.[16,17] Addition of dexmedetomidine to prilocaine in IVRA decreases pain scores, improves anesthesia quality, decreases analgesic requirement, shortens sensory block onset time, and prolongs sensory block recovery time.[6,18] Addition of dexmedetomidine to lignocaine in IVRA also improves the quality of anesthesia and decreases the analgesic requirements but has no effect on the sensory and motor block onset and regression time.[6] Our study demonstrated that the addition of dexmedetomidine, in dose of one mcg/kg of body weight, to lignocaine for IVRA not only improved quality of anesthesia and postoperative analgesia without causing significant side effects but also shortened the onset of sensory and motor block as compared to placebo. Dexmedetomidine administration produces abrupt hypertension and bradycardia until the central sympatholytic effect dominates, resulting in moderate decrease in both mean arterial pressure and heart rate from baseline.[19] In our study, no such hemodynamic changes were observed with use of dexmedetomidine in IVRA. IV dexmedetomidine is also known to exert a sedative effect, which was significantly higher than other groups in our study.
This is the first clinical study comparing the addition of dexmedetomidine and ketamine to lignocaine for IVRA. Superiority of one over the other could not be established as ketamine produced early onset of block and delayed onset of tourniquet pain, whereas dexmedetomidine when added in IVRA provided better postoperative analgesia. Ketamine use was associated with better patient satisfaction than placebo or dexmedetomidine.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Holmes CM. Intravenous regional anesthesia. A useful method of producing analgesia of the limbs. Lancet. 1963;1:245–7. doi: 10.1016/s0140-6736(63)90954-1. [DOI] [PubMed] [Google Scholar]
- 2.Choyce A, Peng P. A systematic review of adjuncts for intravenous regional anesthesia for surgical procedures. Can J Anaesth. 2002;49:32–45. doi: 10.1007/BF03020416. [DOI] [PubMed] [Google Scholar]
- 3.Durrani Z, Winnie AP, Zsigmond EK, Burnett ML. Ketamine for Intravenous Regional Anesthesia. Anesth Analg. 1989;68:628–32. [PubMed] [Google Scholar]
- 4.Kamibayashi T, Maze M. Clinical uses of alpha-2-adrenergic agonists. Anesthesiology. 2000;93:1345–9. doi: 10.1097/00000542-200011000-00030. [DOI] [PubMed] [Google Scholar]
- 5.Kalso EA, Pøyhia R, Rosenberg PH. Spinal antinociception by dexmedetomidine, a highly selective α2-adrenergic agonist. Pharmacol Toxicol. 1991;68:140–3. doi: 10.1111/j.1600-0773.1991.tb02052.x. [DOI] [PubMed] [Google Scholar]
- 6.Esmaoglu A, Mizrak A, Akin A, Turk Y, Boyaci A. Addition of dexmedetomidine to lignocaine for intravenous regional anesthesia. Eur J Anesth. 2005;22:447–51. doi: 10.1017/s0265021505000761. [DOI] [PubMed] [Google Scholar]
- 7.Hegazy NA, Elmetwaly KF, Aboelseoud AA, Alshaer AA. Does the use of ketamine or nitroglycerin as an adjuvant to lignocaine improve the quality of intravenous regional anesthesia? Saudi J Anaesth. 2010;4:55–62. doi: 10.4103/1658-354X.65122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Durrani Z, Winnie AP, Zsigmond EK, Burnett ML. Ketamine for intravenous regional anesthesia. Anesth Analg. 1989;68:328–32. [PubMed] [Google Scholar]
- 9.Roy DD, Deshpande RA. IVRA with ketamine: A study of 30 cases. Indian J Anesth. 1987;35:355–8. [Google Scholar]
- 10.Viscomi CM, Friend A, Parker C, Murphy T, Yarnell M. Ketamine as an Adjuvant in Lignocaine Intravenous Regional Anesthesia: A Randomized, Double-blind, Systemic Control Trial. Reg Anesth Pain Med. 2009;34:130–3. doi: 10.1097/AAP.0b013e31819bb006. [DOI] [PubMed] [Google Scholar]
- 11.Kaul TK, Mittal BR, Gupta S. Comparison of lignocaine and ketamine as intravenous regional anesthetics. Ind J Anaesth. 1993;41:234–9. [Google Scholar]
- 12.Gorgias NK, Maidatsi PG, Kyriakidis AM, Karakoulas KA, Alvanos DN, Giala MM. Clonidine versus ketamine to prevent tourniquet pain during intravenous regional anesthesia with lignocaine. Reg Anesth Pain Med. 2001;26:512–7. doi: 10.1053/rapm.2001.27857. [DOI] [PubMed] [Google Scholar]
- 13.Lurie SD, Reuben SS, Gibson CS, DeLuca PA, Maciolek HA. Effect of clonidine on upper extremity tourniquet pain in healthy volunteers. Reg Anesth Pain Med. 2000;25:502–5. doi: 10.1053/rapm.2000.8460. [DOI] [PubMed] [Google Scholar]
- 14.Yoshitomi T, Kohjitani A. Dexmedetomidine enhances the Local Anesthetic action of Lignocaine via an α2A adrenoceptor. Anesth Analg. 2008;107:96–101. doi: 10.1213/ane.0b013e318176be73. [DOI] [PubMed] [Google Scholar]
- 15.Mizrak A, Gul R, Erkutlu I, Alptekin M, Oner U. Premedication with Dexmedetomidine alone or together with 0.5% Lignocaine for IVRA. J Surg Res. 2010;164:242–7. doi: 10.1016/j.jss.2009.03.005. [DOI] [PubMed] [Google Scholar]
- 16.Scheinin H, Jaakola ML, Sjövall S, Ali-Melkkilä T, Kaukinen S, Turunen J, et al. Intramuscular dexmedetomidine as premedication for general anesthesia. Anesthesiology. 1993;78:1065–75. doi: 10.1097/00000542-199306000-00008. [DOI] [PubMed] [Google Scholar]
- 17.Jaakola ML. Dexmedetomidine premedication before intravenous regional anesthesia in minor outpatient hand surgery. J Clin Anesth. 1994;6:204–11. doi: 10.1016/0952-8180(94)90060-4. [DOI] [PubMed] [Google Scholar]
- 18.Kol IO, Ozturk H, Kaygusuz K, Gursoy S, Comert B, Mimaroglu C. Addition of Dexmedetomidine or Lornoxicam to Prilocaine in Intravenous Regional Anesthesia for Hand or Forearm Surgery: A Randomized Controlled Study. Clin Drug Investig. 2009;29:121–9. doi: 10.2165/0044011-200929020-00006. [DOI] [PubMed] [Google Scholar]
- 19.Dyck JB, Shafer SL. Dexmedetomidine pharmacokinetics and pharmacodynamics. Anesth Pharmacol Rev. 1993;1:238–45. [Google Scholar]


