Abstract
Quinine is a vital anti-malarial drug used in the management of resistant Falciparum malaria. There are previous reports of quinine-induced pulmonary edema and infiltrates. We report the first case of biopsy-proven bronchiolitis obliterans organizing pneumonia (BOOP), confirmed by the Naranjo Adverse Drug Reaction Probability Scale (NADRS) of 5 and a severity scale of 5, secondary to intravenous quinine, in a 15-year-old girl with Plasmodium falciparum infection after a visit to Kenya. Clinical course of the patient followed by review of the literature and appropriate medical interventions for quinine-induced BOOP are suggested.
Keywords: Bronchiolitis obliterans and organizing pneumonia, malaria, quinine
Introduction
Quinine is a naturally occurring cinchona alkaloid that possesses anti-malarial activity. Quinine is a preferred parentral drug for the treatment of severe malaria because of the emergence of chloroquine resistance.[1] Although highly effective against the malarial parasite, quinine is associated with side-effects, mainly a triad of cinchonism, hypotension and hypoglycemia. There are reports of Acute Respiratory Distress Syndrome (ARDS) with toxic ingestion of quinine.[2] In addition, pulmonary edema and transient infiltrates have recently been reported in patients taking routine quinine for leg cramps.[3,4] The mechanism is believed to be an immunologic reaction that can occur after a single or few doses.[4]
ARDS is also reported with severe malaria. ARDS is ascribed primarily to severe malaria. However, there is an opinion among physicians that some cases of non-cardiogenic pulmonary edema or ARDS in patients with malaria are caused by an adverse reaction to quinine,[4] which is a common drug used in treating severe resistant malaria. We describe a case of bronchiolitis obliterans organizing pneumonia (BOOP) caused by an adverse reaction to quinine in a case of Falciparum malaria.
Case Report
A 15-year-old Kenyan girl was referred to our hospital from a peripheral hospital as a case of complicated Falciparum malaria, where she initially presented with fever, lethargy and body aches. She had visited Kenya where she stayed for 5 weeks. She received malaria prophylaxis with a sulfa agent only after arrival in Kenya. Two weeks prior to admission, she had fever, which was treated by self-medication without response. On admission, the diagnosis of Falciparum malaria was confirmed by peripheral smear with a parasite count of 4%. Treatment was begun with mefloquine but, due to lack of clinical improvement, intravenous quinine was started the following day. No loading dose of quinine was given. On Day 3, the patient developed respiratory distress for which she was transferred to our hospital. On admission her vital signs were blood pressure 110/60 mmHg, pulse 80 beats/min, respiratory rate 40 breaths/min, temperature 38.2°C and oxygen saturation 92% on 2 L of oxygen supplementation by nasal cannula. Physical examination revealed moderate respiratory distress and coarse crackles at the bases. Chest radiograph showed basilar alveolar infiltrate . Her initial leukocyte count was 6.7 × 103/mL, hemoglobin 9.9 g/dL, platelet count 109 × 103/mL and International Normalized Ratio for prothrombin time 1.2. Serum electrolyte panel and liver function were within normal limits. Parasite count had dropped to less than 0.1%. Intravenous doxycycline and lumefantrine plus arthemether were added to her anti-malarial regimen. Piperacillin–tazobactam was started to cover possible bacterial infection. The patient's respiratory function deteriorated and she was mechanically ventilated on the second day of admission. Chest radiograph showed bilateral alveolar infiltrates. Echocardiography showed an ejection fraction of 60% and right ventricular systolic pressure of 32 mmHg. Whole blood exchange transfusion was carried out on Day 2. Alveolar recruitment maneuvers and nitric oxide were used to improve oxygenation. On Day 10, she developed bilateral pneumothorax, for which bilateral chest tubes were inserted. Quinine was discontinued on Day 10. On Day 12, bronchosopy with bronchoalveolar lavage was done, which failed to grow any organism. On Day 13, due to persistent hypoxemia, a chest computerized tomography scan was done, which showed bilateral ground glass infiltrates [Figure 1]. Intravenous Methylprednisone 60 mg every 8 h was started and an open lung biopsy was done [Figure 2]. Biopsy findings showed bronchopulmonary tissue with extensive intra-alveolar loose fibromyxoid tissue formation extending into the terminal bronchioles that was consistent with BOOP. In addition, scattered lymphocytes and plasma cells were seen together with foamy macrophages. No microorganisms or reactive pneumocytes were visualized and there was no evidence of malignancy. The patient was weaned off mechanical ventilation on Day 19 and transferred to the ward on Day 23. Despite a prolonged Intensive Care Unit stay, the patient did not develop any additional organ dysfunction.
Figure 1.
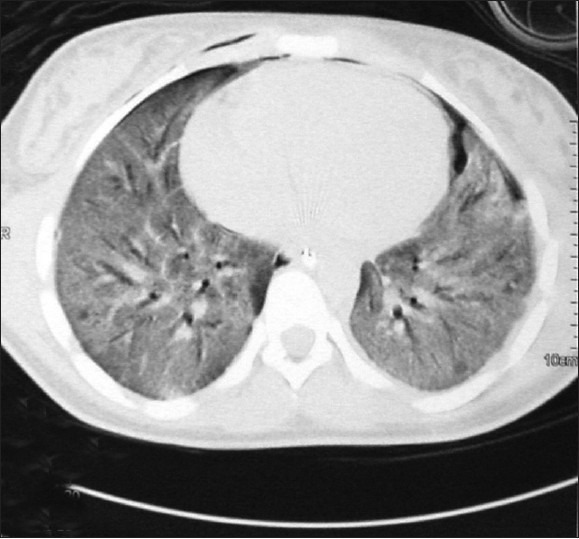
Chest computed tomography scan
Figure 2.
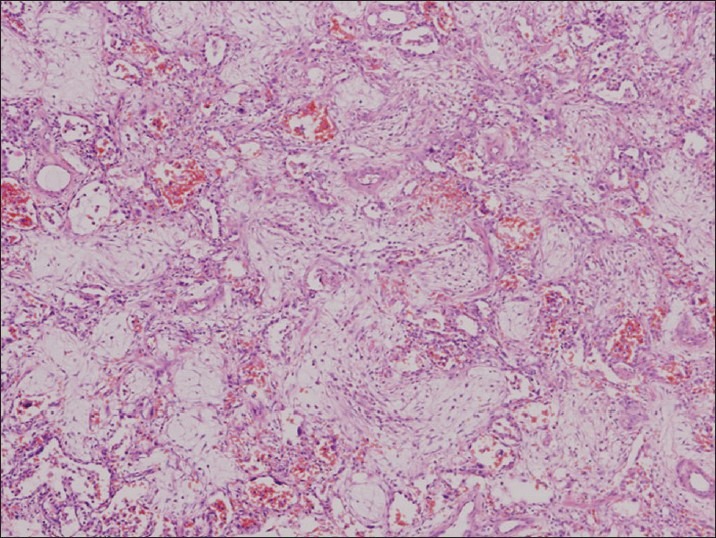
Open lung biopsy. The alveolar spaces are filled with myxoid fibrous tissue consisted of fibroblasts and collagen fibers. H&E, ×400
Discussion
Quinine is associated with a variety of hypersensitivity reactions, including cinchonism, fever, angioedema, flushed skin, pruritis, urticaria, asthma, dyspnea, acute renal failure and thrombocytopenia. Quinine has been reported to cause ARDS when used in toxic doses.[2] There are reports of acute pulmonary edema and transient pulmonary infiltrates caused by quinine.[3,4] However, to the best of our knowledge, there is no reported case of quinine-induced BOOP in the medical literature.
BOOP is a non-specific entity caused by damage to the small conducting airways, alveolar ducts and peri-alveolar space that defines this histological condition. Histologically, it is characterized by polypoid endobronchial masses composed of myxoid fibroblastic tissue resembling granulation tissue filling the lumens of terminal and respiratory bronchioles and extending in an uninterrupted manner into the alveolar ducts and alveoli. This represents an organizing pneumonia.[5]
Pulmonary involvement is known to occur in Falciparum malaria, and has rarely been described in other forms.[6] This is thought to be due to cytoadherence of parasitized erythrocytes to the vascular endothelium causing vascular blockage, particularly at high parasitic loads. Unlike BOOP, histopathological findings in Falciparum-induced ARDS include pulmonary edema, disseminated intravascular coagulation, intra-alveolar hemorrhage, congestion, hyaline membrane formation, malarial pigment, parasitized erythrocytes and giant cells in the septa.[7]
It is possible that BOOP in our patient was caused by Falciparum malaria. However, we believe this was a quinine-induced BOOP for the following reasons. First, the Naranjo Adverse Drug Reaction Probability Scale (NADRS) was 5, which indicates a probable adverse drug reaction (ADR) due to the administration of quinine.[8] This score was based on the ADR after quinine was administered, improved with discontinuing the drug and administering steroids as specific treatment for BOOP, and lastly confirmed by the histology slides.[8] Second, ARDS with Falciparum malaria is part of multisystem involvement, generally associated with intravascular coagulation, renal failure, cerebral malaria, shock and a high parasite count.[9] Our patient had isolated respiratory failure, which is very unusual in patients with respiratory failure due to malaria.[10] Third, there was a clear temporal relationship between infusion of quinine and development of bilateral infiltrates, which favors its association with the drug. Fourth, to the best of our knowledge there is no reported case of BOOP reported secondary to Falciparum malaria. Although there is a report of Plasmodium vivax-induced BOOP,[11] unlike our patient, the case reported had multi-organ dysfunction.
There are two cases of quinine-induced pulmonary edema in the literature.[3,4] None of these cases underwent biopsy to delineate the underlying histology. Thus, this is the first case where a biopsy was obtained.
This case report supports the theory that ARDS in patients with malaria can be caused by an adverse reaction to quinine, especially if there is isolated respiratory failure without multiorgan involvement. This may also explain reports of pulmonary edema in patients with a relatively low level of parasitemia[12] or even with the successful elimination of malaria parasites.[13] In a case series of 12 patients with Plasmodium falciparum complicated by acute lung injury, the damage occurred within 24 h after administration of IV quinine in seven patients and 48–96 h after such therapy was begun in five patients.[8] While in patients with ARDS complicating Plasmodium vivax, the pulmonary edema resolved only after quinine was stopped.[13]
In conclusion, quinine is yet another addition to the vast etiologies of BOOP. As BOOP is highly steroid responsive, we suggest the immediate discontinuation of quinine (followed by early open lung biopsy), if there is no clinical improvement, followed by steroids in patients with malaria who develop isolated respiratory failure with pulmonary infiltrates while on quinine.
Acknowledgment
The authors thank Professor Fikri Abu Zidan, Department of Surgery, Faculty of Medicine and Health Sciences, UAE University, Al-Ain, UAE for his review of this case. They also thank Dr. Amer Al Jundi, Clinical Pharmacist, Department of Pharmacy, Tawam Hospital, Al-Ain, UAE for the technical help.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.2nd ed. Geneva: World Health Organization; 2010. Guidelines for the treatment of malaria. [PubMed] [Google Scholar]
- 2.Wenstone R, Bell M, Mostafa SM. Fatal adult respiratory distress syndrome after quinine overdose. Lancet. 1989;1:1143–4. doi: 10.1016/s0140-6736(89)92425-2. [DOI] [PubMed] [Google Scholar]
- 3.Krantz MJ, Dart RC, Mehler PS. Transient pulmonary infiltrates possibly induced by quinine sulfate. Pharmacotherapy. 2002;22:775–8. doi: 10.1592/phco.22.9.775.34070. [DOI] [PubMed] [Google Scholar]
- 4.Everts RJ, Hayhurst MD, Nona BP. Acute pulmonary edema caused by quinine. Pharmacotherapy. 2004;24:1221–4. doi: 10.1592/phco.24.13.1221.38087. [DOI] [PubMed] [Google Scholar]
- 5.Epler GR. Bronchiolitis obliterans organizing pneumonia. Arch Intern Med. 2001;161:158–64. doi: 10.1001/archinte.161.2.158. [DOI] [PubMed] [Google Scholar]
- 6.Carlini ME, White AC, Jr, Atmar RL. Vivax malaria complicated by adult respiratory distress syndrome. Clin Infect Dis. 1999;28:1182–3. doi: 10.1086/517779. [DOI] [PubMed] [Google Scholar]
- 7.Duarte MI, Corbett CE, Boulos M, Amato Neto V. Ultrastructure of the lung in falciparum malaria. Am J Trop Med Hyg. 1985;34:31–5. doi: 10.4269/ajtmh.1985.34.31. [DOI] [PubMed] [Google Scholar]
- 8.Naranjo CA, Busto J, Sellers EM, Sandor P, Ruiz I, Roberts EA, et al. A method for estimating the probability of adverse drug reactions. ClinPharmacolTher. 1981;30:239–45. doi: 10.1038/clpt.1981.154. [DOI] [PubMed] [Google Scholar]
- 9.Gachot B, Wolff M, Nissack G, Veber B, Vachon F. Acute lung injury complicating imported plasmodium falciparum malaria. Chest. 1995;108:746–9. doi: 10.1378/chest.108.3.746. [DOI] [PubMed] [Google Scholar]
- 10.Sheehy TW, Reba RC. Complications of falciparum malaria and their treatment. Ann Intern Med. 1967;66:807–9. doi: 10.7326/0003-4819-66-4-807. [DOI] [PubMed] [Google Scholar]
- 11.Yale SH, Adlakha A, Sebo TJ, Ryu JH. Bronchiolitis obliterans organizing pneumonia caused by plasmodium vivax malaria. Chest. 1993;104:1294–6. doi: 10.1378/chest.104.4.1294. [DOI] [PubMed] [Google Scholar]
- 12.Curlin ME, Barat LM, Walsh DK, Granger DL. Noncardiogenic pulmonary edema during vivax malaria. Clin Infect Dis. 1999;28:1166–7. doi: 10.1086/517767. [DOI] [PubMed] [Google Scholar]
- 13.Tanios MA, Kogelman L, McGovern B, Hassoun PM. Acute respiratory distress syndrome complicating plasmodium vivax malaria. Crit Care Med. 2001;29:665–7. doi: 10.1097/00003246-200103000-00037. [DOI] [PubMed] [Google Scholar]


