Abstract
We report the case of a 7-year-old girl operated for craniopharyngioma who developed hyperkalemic cardiac arrest in the post-operative period. She was diagnosed as Neuroleptic malignant syndrome (NMS) and the causative drug was carbamazepine. It was essentially a diagnosis of exclusion, and treatment was mainly supportive in form of withdrawal of the neuroleptic medication (carbamazepine) and administration of dantrolene and bromocriptine. Although, relatively uncommon, NMS can be fatal. NMS presents a clinical challenge as the patient outcome depends on its prompt recognition and treatment.
Keywords: Carbamazepine, craniopharyngioma, neuroleptic malignant syndrome
Introduction
Neuroleptic malignant syndrome (NMS) is an uncommon, but serious, adverse reaction to neuroleptics drugs. Dehydration and malnutrition are considered risk factors for NMS, and hence cancer patients represent a high risk group for NMS. NMS can be difficult to diagnose due to multiple complicating and confusing factors associated with its presentation. Given the widespread prescription of neuroleptics, clinicians need to be aware and be able to recognize and appropriately manage NMS.
Case Report
A 7-year-old girl, presented with headache, diplopia and imbalance. Magnetic resonance imaging (MRI) revealed a solid-cystic suprasellar mass, suggestive of a craniopharyngioma. All routine investigations were normal except hormonal studies [Table 1]. She was scheduled for surgery on a semi-emergency basis as she was clinically euthyroid. A right pterional craniotomy with radical resection of the tumor was performed.
Table 1.
Hormonal studies

Postoperatively patient developed a left-sided spastic hemiplegia and phenytoin, mannitol, dexamethasone and thyroxine therapy was started. Patient also fluctuated between diabetes insipidus (DI) and syndrome of inappropriate antidiuretic hormone (SIADH) requiring free water and desmopressin. Since, phenytoin is known to cause DI by decreasing the ADH release, it was substituted by carbamazepine on post-operative day (POD)-2, which was stopped after two days. Computerized tomography scan showed routine post-operative changes and thyroid function tests showed a significant improvement [Table 1].
On the POD-4, she developed wide QRS-complex with tall T waves and suffered a hyperkalemic cardiac arrest. Investigations revealed a K+ value of 9.4 meq/l (previous K+-3.1meq/l), Creatine phosphokinase (CPK) level 506 IU/L and a normal pH. She was successfully resuscitated with calcium gluconate, sodium bicarbonate and glucose-insulin infusion. Patient was mechanically ventilated.
The following morning, as all vital parameters were stable, the patient was weaned off mechanical ventilation. However, in a couple of hours her neurological status worsened (Glasgow coma scale - E3M4V1) and patient developed 101 to 103° F fever, for which active cooling was instituted. Blood, urine, stool and CSF cultures were negative. Patient developed increased spasticity of limbs with involuntary movements. Electroencephalogram (EEG) was suggestive of NMS with no evidence of seizure activity. Repeat CPK levels showed an increasing trend [Figure 1]. Fever lasted for 3 days but there was no hemodynamic instability.
Figure 1.
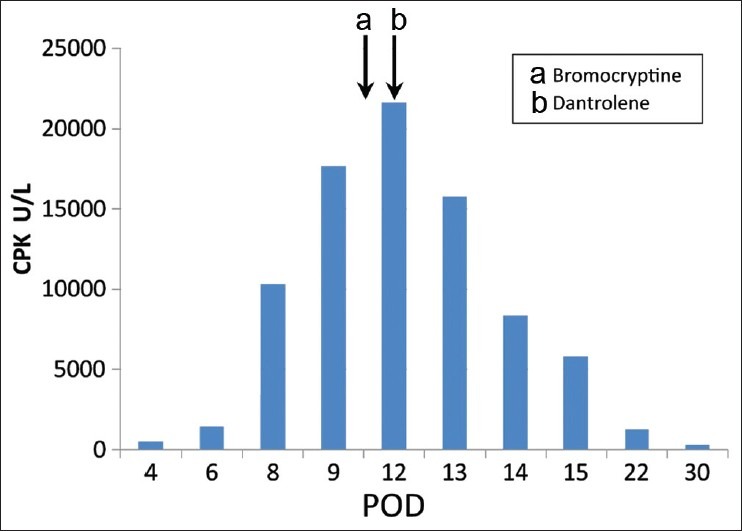
CPK levels (a) Drug Bromocriptine first administered (b) Drug Dantrolene first administered POD: Post-operative day
Rising CPK levels, fluctuating serum sodium and wavering neurological status persisted. The spasticity of the limbs improved after baclofen was started on POD-7. She was started on bromocriptine on POD-10 and dantrolene on POD-12 after which her CPK levels started falling. Once CPK levels were normalized dantrolene and bromocriptine were stopped on POD-16. A rigorous regimen for limb physiotherapy and ambulation was instituted. She was discharged on POD-33 with left hemiparesis and on desmopressin, hydrocortisone, thyroxin and baclofen. At 3 months follow-up, she had significantly improved and had only minimal residual left hemiparesis. Histopathology revealed a malignant germ cell tumor and she was referred for further adjuvant therapy.
Discussion
NMS is an uncommon idiosyncratic disorder characterized by severe rigidity, tremor, fever, altered mental status, autonomic dysfunction, leukocytosis and elevated serum CPK.[1,2] It is caused by a variety of neuroleptic and non-neuroleptic drugs that produce massive and sudden reduction in dopamine activity following dopamine blockade. NMS is known for its diverse clinical presentation, where clinical features may be easily missed causing delay in diagnosis. Features like muscle rigidity, hyperthermia and mental changes are quite similar to those of malignant hyperthermia (MH), central cholinergic syndrome and serotonin syndrome. Severe central nervous system infection, tetanus, and drug interactions with monoamine oxidase inhibitors can also mimic this picture. The usual clinical course of NMS runs from 2 to 14 days after recognition and stoppage of offending drug, however it may be prolonged.[1]
MH was the first differential diagnosis because our patient had received general anesthesia. MH was however ruled out as symptoms of MH after sevoflurane anesthesia occurs within 24 h and are unlikely after 72 h.[3] Serotonin syndrome was ruled out because patient was not on any selective serotonin reuptake inhibitors (SSRIs) or selective serotonin-norepinephrine reuptake inhibitors (SSNRIs)
It was possible that the patient suffered from severe hypothyroidism causing rhabdomyolysis.[4] Sellar-suprasellar tumors are known to present with multiple endocrine abnormalities but the possibility of an autoimmune hypothyroidism was excluded as antithyroglobulin antibodies were negative and patient became biochemically euthyroid post-operative, despite which CPK levels continued to rise.
The diagnosis was further complicated by the possibility of inadvertent damage to basal ganglia during surgery, which could result in rigidity, bradykinesia and tremors. We attributed the electrolyte disturbances to craniopharyngioma surgery, however, they are generally in the form of disturbances in sodium homeostasis and not fluctuations in potassium. In our patient, the sequence of events strongly suggested a hyperkalemic cardiac arrest but the cause leading to it was uncertain.
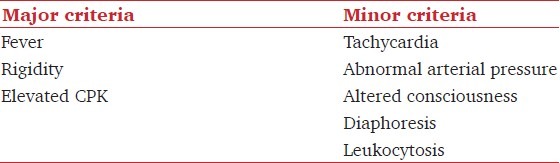
Levenson in 1985 had proposed a set of diagnostic criteria, incorporating physical signs and routine laboratory tests which are being used routinely to diagnose NMS [Table 2].[5] Our patient presented with all the three Levenson's major criteria along with tachycardia, altered consciousness and diarrhea (autonomic instability).
Table 2.
Levenson's criteria for the diagnosis of NMS (Presence of 3 major or two major and four minor signs indicate a high probability of NMS)
Although not commonly implicated, carbamazepine has been associated with NMS.[2,6] The onset of NMS following administration of neurolept drug is not related with the duration or dose of drug but rather on the physiological state at the time of administration of the drug. It typically develops over a period of 24-72 h but the risk may continue up to 10-20 days after neuroleptics have been discontinued.[5] Additional risk factors for NMS include high ambient temperatures, humidity, dehydration, concomitant illness, head trauma, and organic brain disease.[1,2,7] Our patient suffered from hyperkalemia cardiac arrest 48 h after administration of first dose of carbamazepine. She was volume depleted due to DI and had organic brain lesion.
Treatment of NMS is mainly in form of early recognition and prompt withdrawal of the offending agent along with supportive care.[5] Supportive treatment includes temperature control, rehydration and restoration of electrolyte balance. Though, there is no strong data supporting use of specific drugs, bromocriptine and dantrolene sodium have been recommended. Bromocriptine in dose of 2.5mg-10mg six-hourly activates postsynaptic receptors and offsets the central inhibition of dopamine. It also stimulates production of dopamine from the pituitary gland to reverse the hyperthermic responses. Dantrolene is drug of choice in MH but its role in NMS is less well defined.[5] It is a direct muscle relaxant that acts by blocking the release of calcium from the sarcoplasmic reticulum, and works in tandem with the effects of central dopamine agonists to counteract the peripheral pyrexic mechanisms of NMS.[1,2] After stoppage of carbamazepine, our patient received only symptomatic care for six days before bromocriptine was added but her CPK levels started declining only after the start of dantrolene.
To conclude, NMS is a potentially lethal disorder where early recognition and prompt intervention is necessary. Treatment is largely supportive but the spectrum of clinical features may cause confusion and delay diagnosis as occurred in our patient. High level of suspicion is therefore the key to early recognition.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Pelonero AL, Levenson JL, Pandurangi AK. Neuroleptic malignant syndrome: A review. Psychiatr Serv. 1998;49:1163–72. doi: 10.1176/ps.49.9.1163. [DOI] [PubMed] [Google Scholar]
- 2.Bottoni TN. Neuroleptic malignant syndrome: A brief review. Hosp Physician. 2002;15:58–63. [Google Scholar]
- 3.Litman RS, Flood CD, Kaplan RF, Kim YL, Tobin JR. Postoperative malignant hyperthermia: An analysis of cases from the North American Malignant Hyperthermia Registry. Anesthesiology. 2008;109:825–9. doi: 10.1097/ALN.0b013e31818958e5. [DOI] [PubMed] [Google Scholar]
- 4.Hekimsoy Z, Oktem IK. Serum creatine kinase levels in overt and subclinical hypothyroidism. Endocr Res. 2005;31:171–5. doi: 10.1080/07435800500371706. [DOI] [PubMed] [Google Scholar]
- 5.Adnet P, Lestavel P, Krivosic-Horber R. Neuroleptic malignant syndrome. Br J Anaesth. 2000;85:129–35. doi: 10.1093/bja/85.1.129. [DOI] [PubMed] [Google Scholar]
- 6.O’Griofa FM, Voris JC. Neuroleptic malignant syndrome associated with carbamazepine. South Med J. 1991;84:1378–80. doi: 10.1097/00007611-199111000-00023. [DOI] [PubMed] [Google Scholar]
- 7.Harsch HH. Neuroleptic malignant syndrome: Physiological and laboratory findings in a series of nine cases. J Clin Psychiatry. 1987;48:328–33. [PubMed] [Google Scholar]


