Abstract
The analysis of several isolates of foot-and-mouth disease virus by RNase T1 fingerprinting of the 32P-labeled RNA is described. It has been shown that use of the 35S induced RNA instead of the virus particle RNA has two advantages. (i) About 40 times more radioactivity is incorporated into the induced RNA. (ii) The RNA can be prepared much more rapidly, thus increasing the value of the technique in rapid diagnosis. One-dimensional maps, in which the RNase T1 oligonucleotides are separated according to size, have been shown to provide a valuable screening method for distinguishing between viruses. Those viruses giving similar one-dimensional maps also gave similar two-dimensional maps. The value of using the length of the polycytidylic acid tract of foot-and-mouth disease virus as a diagnostic tool is also discussed.
Full text
PDF

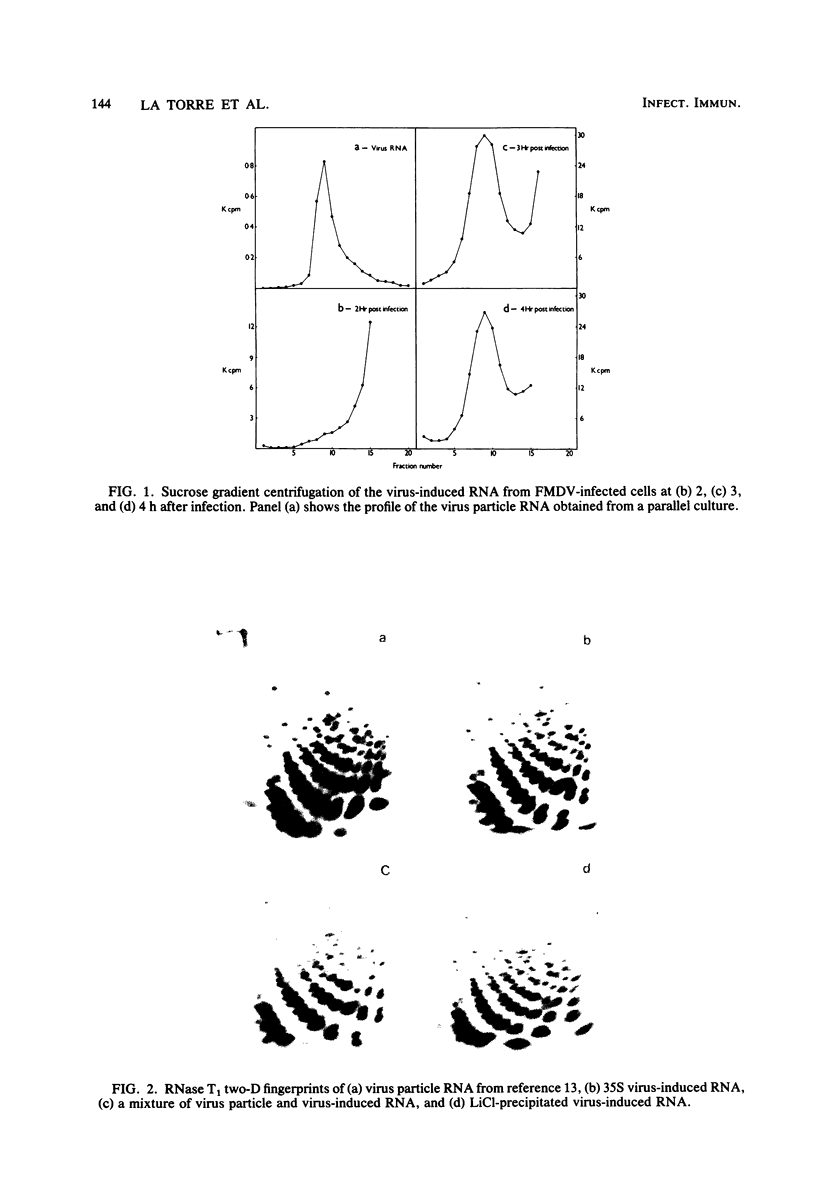
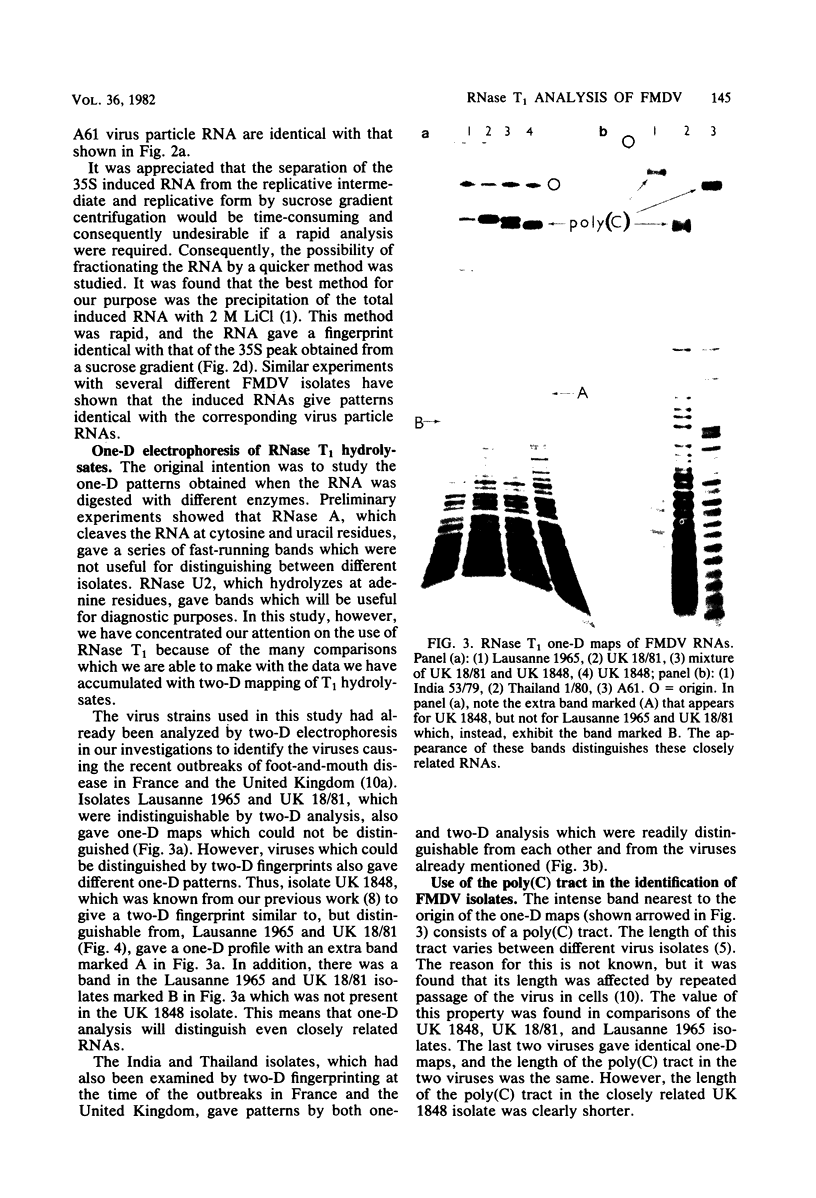


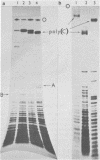
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BROWN F., CARTWRIGHT B. PURIFICATION OF RADIOACTIVE FOOT-AND-MOUTH DISEASE VIRUS. Nature. 1963 Sep 21;199:1168–1170. doi: 10.1038/1991168a0. [DOI] [PubMed] [Google Scholar]
- Baltimore D. Purification and properties of poliovirus double-stranded ribonucleic acid. J Mol Biol. 1966 Jul;18(3):421–428. doi: 10.1016/s0022-2836(66)80034-7. [DOI] [PubMed] [Google Scholar]
- Black D. N., Stephenson P., Rowlands D. J., Brown F. Sequence and location of the poly C tract in aphtho- and cardiovirus RNA. Nucleic Acids Res. 1979 Jun 11;6(7):2381–2390. doi: 10.1093/nar/6.7.2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown F. A molecular approach to the diagnosis of virus infections. Ann Neurol. 1981;9 (Suppl):39–43. doi: 10.1002/ana.410090708. [DOI] [PubMed] [Google Scholar]
- Brown F., Newman J., Stott J., Porter A., Frisby D., Newton C., Carey N., Fellner P. Poly(C) in animal viral RNAs. Nature. 1974 Sep 27;251(5473):342–344. doi: 10.1038/251342a0. [DOI] [PubMed] [Google Scholar]
- Denoya C. D., Scodeller E. A., Gimenez B. H., Vásquez C., La Torre J. L. Foot and mouth disease virus. I. Stability of its ribonucleic acid. Virology. 1978 Jan;84(1):230–235. doi: 10.1016/0042-6822(78)90241-6. [DOI] [PubMed] [Google Scholar]
- Franklin R. M. Purification and properties of the replicative intermediate of the RNA bacteriophage R17. Proc Natl Acad Sci U S A. 1966 Jun;55(6):1504–1511. doi: 10.1073/pnas.55.6.1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frisby D. P., Newton C., Carey N. H., Fellner P., Newman J. F., Harris T. J., Brown F. Oligonucleotide mapping of picornavirus RNAs by two-dimensional electrophoresis. Virology. 1976 Jun;71(2):379–388. doi: 10.1016/0042-6822(76)90365-2. [DOI] [PubMed] [Google Scholar]
- Harris T. J., Brown F. Biochemical analysis of a virulent and an avirulent strain of foot-and-mouth disease virus. J Gen Virol. 1977 Jan;34(1):87–105. doi: 10.1099/0022-1317-34-1-87. [DOI] [PubMed] [Google Scholar]
- Harris T. J., Brown F. The location of the ploy(C) tract in the RNA of foot-and-mouth disease virus. J Gen Virol. 1976 Dec;33(3):493–501. doi: 10.1099/0022-1317-33-3-493. [DOI] [PubMed] [Google Scholar]
- King A. M., Underwood B. O., McCahon D., Newman J. W., Brown F. Biochemical identification of viruses causing the 1981 outbreaks of foot and mouth disease in the UK. Nature. 1981 Oct 8;293(5832):479–480. doi: 10.1038/293479a0. [DOI] [PubMed] [Google Scholar]
- La Torre J. L., Grubman M. J., Baxt B., Bachrach H. L. The structural polypeptides of aphthovirus are phosphoproteins. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7444–7447. doi: 10.1073/pnas.77.12.7444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter A., Carey N., Fellner P. Presence of a large poly(rC) tract within the RNA of encephalomyocarditis virus. Nature. 1974 Apr 19;248(5450):675–678. doi: 10.1038/248675a0. [DOI] [PubMed] [Google Scholar]
- Robson K. J., Doel T. R., Gorman B. M., Brown F. Biochemical aspects of variation in foot-and-mouth disease virus. J Gen Virol. 1980 Jan;46(1):179–193. doi: 10.1099/0022-1317-46-1-179. [DOI] [PubMed] [Google Scholar]
- Sanger F., Coulson A. R. The use of thin acrylamide gels for DNA sequencing. FEBS Lett. 1978 Mar 1;87(1):107–110. doi: 10.1016/0014-5793(78)80145-8. [DOI] [PubMed] [Google Scholar]
- Scodeller E. A., Denoya C. D., Vasquez C., La Torre J. L. A new method for the isolation of undegraded FMDV-specific RNA from infected BHK cells. Arch Virol. 1979;62(3):253–262. doi: 10.1007/BF01317557. [DOI] [PubMed] [Google Scholar]
- Wild T. F., Martin S. J., Brown F. A study of the heterogeneous 37S ribonucleic acid induced by foot-and-mouth-disease virus. Biochem J. 1968 Apr;107(3):395–401. doi: 10.1042/bj1070395. [DOI] [PMC free article] [PubMed] [Google Scholar]