Abstract
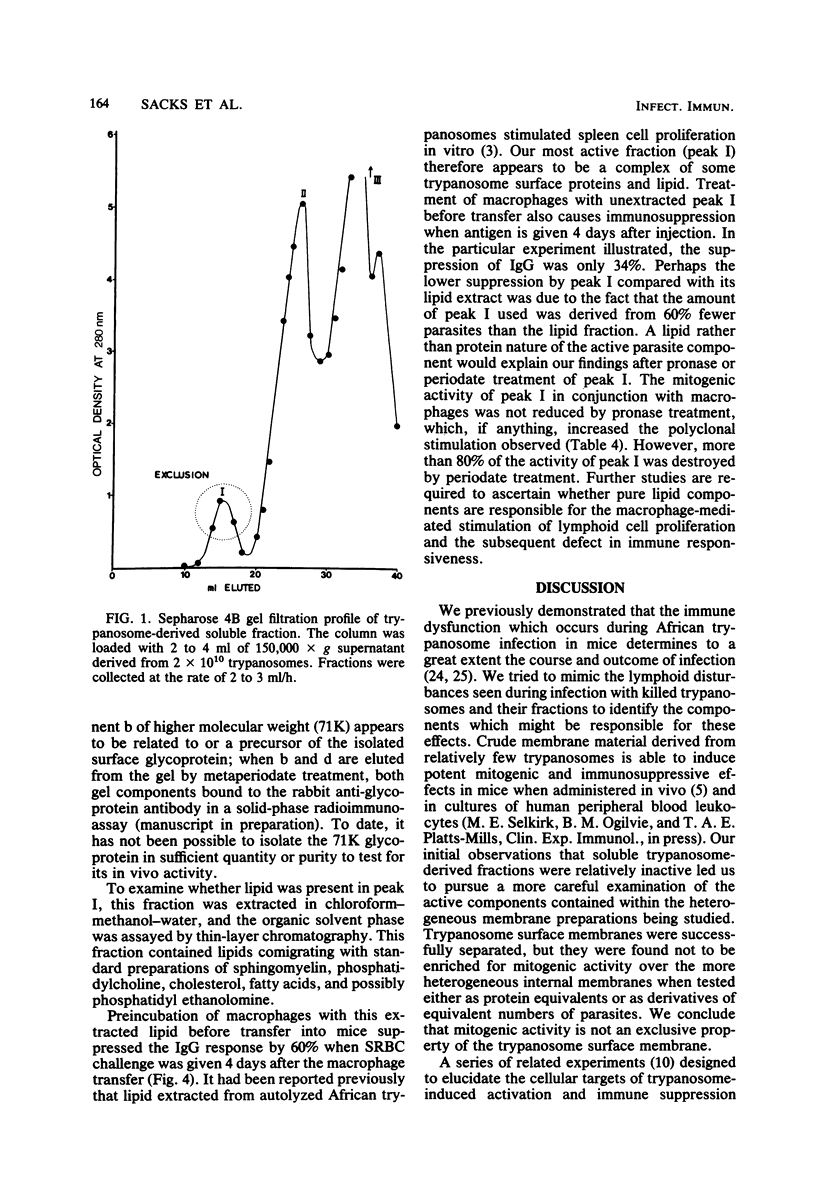
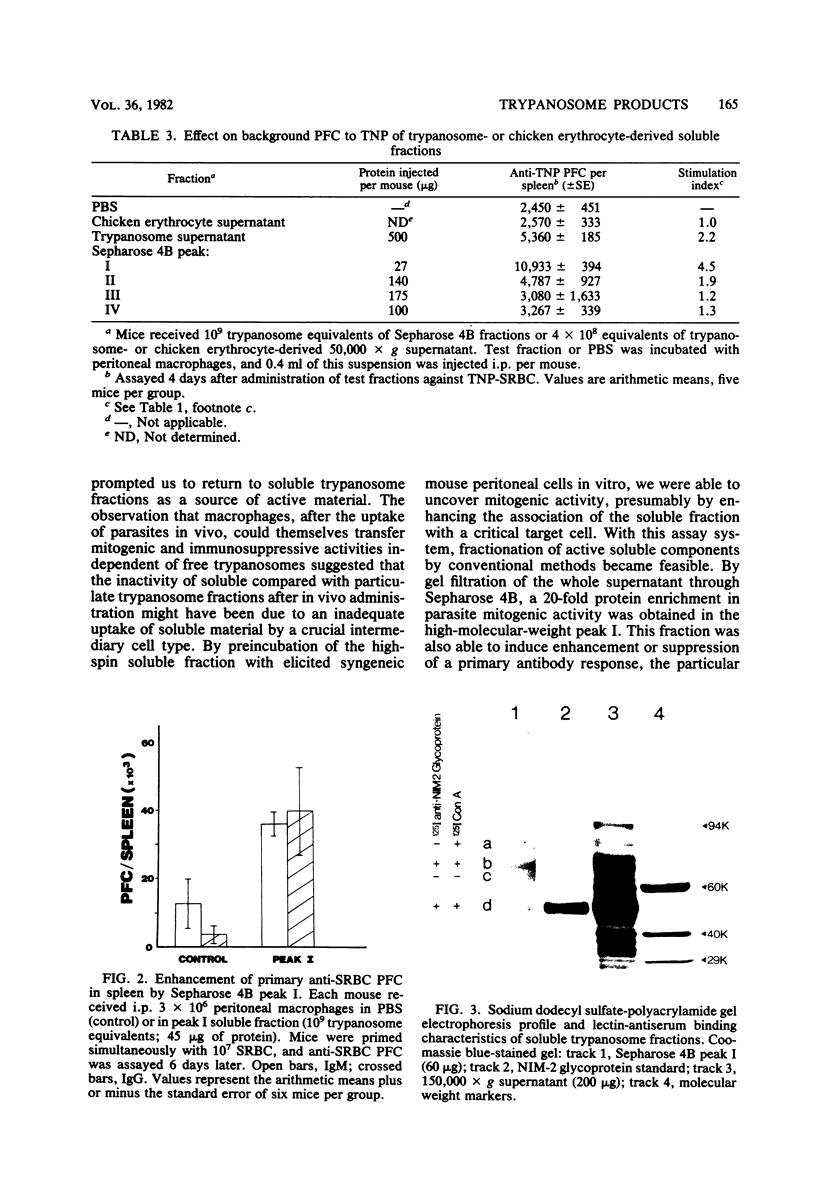
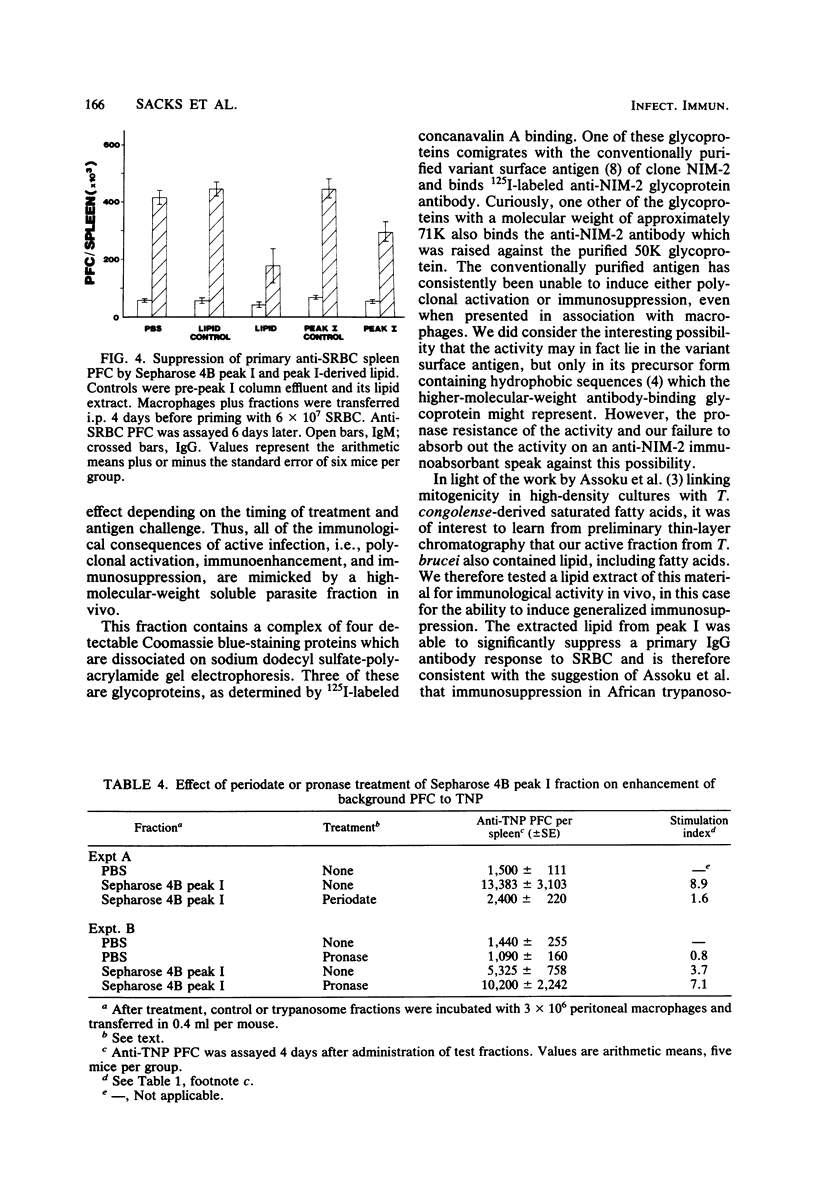
This report describes further attempts to define the nature of the parasite product(s) responsible for the extensive changes in lymphoid tissue in mice during infection with Trypanosoma brucei. As previously described, potent mitogenic and immunosuppressive effects are induced by a trypanosome-derived crude membrane fraction in vivo. There was no enrichment in these activities when purified parasite surface membranes were used. Mitogenic activity can be recovered from soluble trypanosome material only when it is incubated with peritoneal macrophages before transfer into syngeneic recipients. Thus, by encouraging association with a critical target cell, soluble parasite products can be studied, and their active components can be separated by conventional methods. Preliminary fractionation of high-spin trypanosome supernatant over Sepharose 4B confined the mitogenic activity to the high-molecular-weight fraction, which is a macromolecular complex of proteins, glycoproteins, and lipid. Extracted lipid from this material was able to significantly suppress a primary immunoglobulin G anti-sheep erythrocyte response. The activity was periodate sensitive and pronase resistant. The use of macrophages in vitro may be a general method whereby important biological activities lost as a result of fractionation procedures can be recovered and the active components studied in greater detail.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albright J. W., Albright J. F. Inhibition of murine humoral immune responses by substances derived from trypanosomes. J Immunol. 1981 Jan;126(1):300–303. [PubMed] [Google Scholar]
- Askonas B. A., Corsini A. C., Clayton C. E., Ogilvie B. M. Functional depletion of T- and B-memory cells and other lymphoid cell subpopulations-during trypanosomiasis. Immunology. 1979 Feb;36(2):313–321. [PMC free article] [PubMed] [Google Scholar]
- Assoku R. K., Hazlett C. A., Tizard I. Immunosuppression in experimental African trypanosomiasis. Polyclonal B-cell activation and mitogenicity of trypanosome-derived saturated fatty acids. Int Arch Allergy Appl Immunol. 1979;59(3):298–307. [PubMed] [Google Scholar]
- Boothroyd J. C., Cross G. A., Hoeijmakers J. H., Borst P. A variant surface glycoprotein of Trypanosoma brucei synthesized with a C-terminal hydrophobic 'tail' absent from purified glycoprotein. Nature. 1980 Dec 11;288(5791):624–626. doi: 10.1038/288624a0. [DOI] [PubMed] [Google Scholar]
- Clayton C. E., Sacks D. L., Ogilvie B. M., Askonas B. A. Membrane fractions of trypanosomes mimic the immunosuppressive and mitogenic effects of living parasites on the host. Parasite Immunol. 1979 Autumn;1(3):241–249. doi: 10.1111/j.1365-3024.1979.tb00709.x. [DOI] [PubMed] [Google Scholar]
- Clayton C. E., Selkirk M. E., Corsini C. A., Ogilvie B. M., Askonas B. A. Murine trypanosomiasis: cellular proliferation and functional depletion in the blood, peritoneum, and spleen related to changes in bone marrow stem cells. Infect Immun. 1980 Jun;28(3):824–831. doi: 10.1128/iai.28.3.824-831.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corsini A. C., Clayton C., Askonas B. A., Ogilvie B. M. Suppressor cells and loss of B-cell potential in mice infected with Trypanosoma brucei. Clin Exp Immunol. 1977 Jul;29(1):122–131. [PMC free article] [PubMed] [Google Scholar]
- Cross G. A. Identification, purification and properties of clone-specific glycoprotein antigens constituting the surface coat of Trypanosoma brucei. Parasitology. 1975 Dec;71(3):393–417. doi: 10.1017/s003118200004717x. [DOI] [PubMed] [Google Scholar]
- Grosskinsky C. M., Askonas B. A. Macrophages as primary target cells and mediators of immune dysfunction in African trypanosomiasis. Infect Immun. 1981 Jul;33(1):149–155. doi: 10.1128/iai.33.1.149-155.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurd J. W., Evans W. H. Identification of liver plasma membrane glycoproteins which bind to 125I-labelled concanavalin A following electrophoresis in sodium dodecyl sulfate. Can J Biochem. 1976 May;54(5):477–480. doi: 10.1139/o76-068. [DOI] [PubMed] [Google Scholar]
- Hudson K. M., Byner C., Freeman J., Terry R. J. Immunodepression, high IgM levels and evasion of the immune response in murine trypanosomiasis. Nature. 1976 Nov 18;264(5583):256–258. doi: 10.1038/264256a0. [DOI] [PubMed] [Google Scholar]
- Jayawardena A. N., Waksman B. H., Eardley D. D. Activation of distinct helper and suppressor T cells in experimental trypanosomiasis. J Immunol. 1978 Aug;121(2):622–628. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lanham S. M., Godfrey D. G. Isolation of salivarian trypanosomes from man and other mammals using DEAE-cellulose. Exp Parasitol. 1970 Dec;28(3):521–534. doi: 10.1016/0014-4894(70)90120-7. [DOI] [PubMed] [Google Scholar]
- Mansfield J. M., Bagasra O. Lymphocyte function in experimental African trypanosomiasis. I. B cell responses to helper T cell-independent and -dependent antigens. J Immunol. 1978 Mar;120(3):759–765. [PubMed] [Google Scholar]
- Mayor-Withey K. S., Clayton C. E., Roelants G. E., Askonas B. A. Trypanosomiasis leads to extensive proliferation of B, T and null cells in spleen and bone marrow. Clin Exp Immunol. 1978 Dec;34(3):359–363. [PMC free article] [PubMed] [Google Scholar]
- Miller H. C., Esselman W. J. Modulation of the immune response by antigen-reactive lymphocytes after cultivation with gangliosides. J Immunol. 1975 Sep;115(3):839–843. [PubMed] [Google Scholar]
- Morrison W. I., Roelants G. E., Mayor-Withey K. S., Murray M. Susceptibility of inbred strains of mice to Trypanosoma congolense: correlation with changes in spleen lymphocyte populations. Clin Exp Immunol. 1978 Apr;32(1):25–40. [PMC free article] [PubMed] [Google Scholar]
- Murray P. K., Jennings F. W., Murray M., Urquhart G. M. The nature of immunosuppression in Trypanosoma brucei infections in mice. II. The role of the T and B lymphocytes. Immunology. 1974 Nov;27(5):825–840. [PMC free article] [PubMed] [Google Scholar]
- Rittenberg M. B., Pratt K. L. Antitrinitrophenyl (TNP) plaque assay. Primary response of Balb/c mice to soluble and particulate immunogen. Proc Soc Exp Biol Med. 1969 Nov;132(2):575–581. doi: 10.3181/00379727-132-34264. [DOI] [PubMed] [Google Scholar]
- Rovis L., Baekkeskov S. Sub-cellular fractionation of Trypanosoma brucei. Isolation and characterization of plasma membranes. Parasitology. 1980 Jun;80(3):507–524. doi: 10.1017/s0031182000000974. [DOI] [PubMed] [Google Scholar]
- Ryan J. L., Shinitzky M. Possible role for glycosphingolipids in the control of immune responses. Eur J Immunol. 1979 Feb;9(2):171–175. doi: 10.1002/eji.1830090215. [DOI] [PubMed] [Google Scholar]
- Sacks D. L., Askonas B. A. Trypanosome-induced suppression of anti-parasite responses during experimental African trypanosomiasis. Eur J Immunol. 1980 Dec;10(12):971–974. doi: 10.1002/eji.1830101216. [DOI] [PubMed] [Google Scholar]
- Sacks D. L., Selkirk M., Ogilvie B. M., Askonas B. A. Intrinsic immunosuppressive activity of different trypanosome strains varies with parasite virulence. Nature. 1980 Jan 31;283(5746):476–478. doi: 10.1038/283476a0. [DOI] [PubMed] [Google Scholar]
- Wellhausen S. R., Mansfield J. M. Lymphocyte function in experimental African trypanosomiasis. II. Splenic suppressor cell activity. J Immunol. 1979 Mar;122(3):818–824. [PubMed] [Google Scholar]