Abstract
In higher plants formate dehydrogenase (FDH, EC 1.2.1.2.) is a mitochondrial, NAD-dependent enzyme. We previously reported that in potato (Solanum tuberosum L.) FDH expression is high in tubers but low in green leaves. Here we show that in isolated tuber mitochondria FDH is involved in formate-dependent O2 uptake coupled to ATP synthesis. The effects of various environmental and chemical factors on FDH expression in leaves were tested using the mitochondrial serine hydroxymethyltransferase as a control. The abundance of FDH transcripts is strongly increased under various stresses, whereas serine hydroxymethyltransferase transcripts decline. The application of formate to leaves strongly enhances FDH expression, suggesting that it might be the signal for FDH induction. Our experiments using glycolytic products suggest that glycolysis may play an important role in formate synthesis in leaves in the dark and during hypoxia, and in tubers. Of particular interest is the dramatic accumulation of FDH transcripts after spraying methanol on leaves, as this compound is known to increase the yields of C3 plants. In addition, although the steady-state levels of FDH transcript increase very quickly in response to stress, protein accumulation is much slower, but can eventually reach the same levels in leaves as in tubers.
FDH catalyzes the oxidation of formate into CO2, and is a widespread enzyme in bacteria, yeasts, fungi, mammals, and plants. Several types of FDHs have been reported with differing cofactors (NAD or FAD), electron acceptors, substrates, and cellular locations. A wide diversity of FDH types is found in the bacteria, where they are involved in respiration (for review, see Sawers, 1994) and possibly in the maintenance of a reducing environment (Haynes et al., 1995). The NAD-dependent FDHs (EC 1.2.1.2) have been extensively studied in yeast and bacteria over the past decade because the favorable thermodynamics of the reaction, the easy removal of the product (CO2), and the low cost of the substrate (formate) have made this enzyme a good candidate for industrial NADH regeneration (Allen and Holbrook, 1995). In methanol-utilizing yeasts such as Hansenula polymorpha, Candida boidinii, and Pichia pinus, NAD-dependent FDH plays a crucial role, together with formaldehyde dehydrogenase, in providing NADH to the respiratory chain (Van Dijken et al., 1976). Aside from formate, it is possible that S-formylglutathione might also be a substrate for FDH, as was suggested for yeasts (Van Dijken et al., 1976), pea (Uotila and Koivusalo, 1979), and Pseudomonas sp. 101 (Popov and Lamzin, 1994).
In higher plants only NAD-dependent FDHs have been found, and these are localized in the mitochondrial matrix (Halliwell, 1974; Oliver, 1981; Colas des Francs-Small et al., 1993). Oliver (1981) showed that isolated leaf mitochondria from spinach (and, to a lesser extent, that from beet and tobacco) can oxidize formate with a tight coupling to oxidative phosphorylation. The first plant FDH cDNA to be published encodes a potato (Solanum tuberosum L.) enzyme (Colas des Francs-Small et al., 1993) that is 8 to 10 times more abundant in mitochondria isolated from tubers and dark-grown shoots than in leaf mitochondria (Colas des Francs-Small et al., 1992). Similar variations were found in many other plant species, suggesting drastic changes in formate metabolism between tissues. For example, there is a variable abundance of a polypeptide designated W in pea (Pisum sativum L.) tissues (Humpherey-Smith et al., 1992), which was subsequently identified in our laboratory as FDH.
In this paper we describe the expression of potato mitochondrial FDH in various tissues and under several stresses. The mRNA levels of the mitochondrial SHMT isoform were studied in parallel because this enzyme was previously described to be abundant in pea photosynthetic tissues and scarce in green tissues kept in the dark (Turner et al., 1992, 1993). Like FDH, SHMT is involved in C1 metabolism, which is important for the biosynthesis and degradation of some amino acids (Ser, Gly, and Met), purines, sugars, and organic acids (Cossins, 1980). Our results show that FDH expression is increased by various stresses and that SHMT is either decreased or unchanged. These changes in expression were relatively slow and therefore probably result from metabolite changes rather than direct induction or repression by the environmental alterations. As formate would be a good candidate for the final signal in transduction pathways leading to FDH responses, we tested the effects of spraying leaves with metabolites that could be directly or indirectly involved in formate biosynthesis at the level of FDH and SHMT mRNAs.
MATERIALS AND METHODS
Plant Material and Growth Conditions
Leaves, stems, roots, flowers, shoots, and tubers were obtained from potato (Solanum tuberosum L. cv BF15 or Désirée) plants (2n = 4× = 48) grown in 15-cm pots in a greenhouse at 20°C under a 16-h photoperiod. Hypoxia (2–3% O2 [v/v]) was created for 8 to 48 h in an airtight Perspex chamber (made in the lab) flushed with N2 for 2 out of 12 h. Cold stress was induced at 4°C for 24 or 48 h. To induce drought stress, plants were dug up carefully and placed for 24 to 48 h on a sheet of Parafilm. These stresses were performed under the same photoperiod as the control plants. Dark treatment was performed at 20°C in a dark chamber for 8 h to 5 d, and dark-to-light shift treatment was performed by transferring plants from the dark chamber (24- or 48-h treatment) back to the greenhouse for an extra 24 h. In the wounding experiment, leaves were lacerated and left for 20 min to 3 d on the plants prior to extraction. Chemical treatments were performed by spraying the plants with various solutions, such as 100 μm ABA, 10 mm formate, acetate, pyruvate, Ser, or sarcosine, 10% ethanol, or 20% methanol. Twenty-four to 48 h after treatment, the leaves were harvested and immediately frozen in liquid N2 for mRNA isolation.
RNA Isolation and Northern Analysis
Total RNAs were isolated from various potato tissues according to either the procedure of Logemann et al. (1987) or a modified method of Sambrook et al. (1989). Plant tissues were ground in liquid N2, and the powder was homogenized in extraction buffer (8 m guanidium hydrochloride, 20 mm Mes, pH 7, 20 mm Na4EDTA, and 50 mm 2-mercaptoethanol) and centrifuged for 20 min at 17,000 rpm (rotor SS-34, model RC5C, Sorvall) at 4°C. RNAs were then purified by an overnight centrifugation through a 4-mL 5.7 m CsCl cushion at 20°C at 32,000 rpm (rotor SW41, model L7–55, Beckman). For each sample, 10 μg of RNA was run on a 3% formaldehyde, 1.5% agarose gel, transferred by blotting onto nylon membranes (Hybond-N+, Amersham), and probed with either an 850-bp potato FDH cDNA (Colas des Francs-Small et al., 1993) or a 1.5-kb pea (Pisum sativum L.) SHMT cDNA (Turner et al., 1992). RNA loading on gels was controlled by EtBr staining under UV light, and transfer efficiency by hybridization with a 2.42-kb wheat mitochondrial 18S rRNA gene fragment (Bonen and Gray, 1980).
Measurement of Formate Oxidation by Isolated Mitochondria
Potato tuber mitochondria were isolated and subsequently purified on Percoll gradients as previously described (Diolez and Moreau, 1985). O2 uptake and membrane potential were measured simultaneously with an O2 electrode (Hansatech, Kings Lynn, UK) and a TPP-sensitive electrode (Diolez and Moreau, 1985). Experiments were carried out at 25°C in a medium containing 1 mL (0.5 mg) of mitochondrial protein in 400 mm mannitol, 10 mm K2PO4 buffer, pH 7.2, 100 mm KCl, 5 mm MgCl2, 1 mg/mL BSA, and 4 μm TPP+ as final concentrations. Respiration was initiated with 15 mm formate, and a limiting amount of ADP (100 μm) was then added to ensure a state 3-to-state 4 transition. Membrane potential was calculated using the equation:
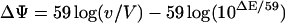 |
where v is the mitochondrial matrix volume taken as 1 μL/mg protein, V is the volume of the incubation medium, and ΔE is the electrode potential in mV (Kamo et al., 1979). Membrane potential was collapsed at the end of each experiment run by the addition of 1 μm carbonylcyanide m-chlorophenylhydrazone. The calculation of ΔΨ was carried out without correction for the probe binding. The mitochondrial protein concentration was determined according to the method of Lowry et al. (1951).
Mitochondrial Protein Analysis
Two-dimensional SDS-PAGE of mitochondrial proteins was performed on a Mini Protean II apparatus (Bio-Rad), as described previously (Colas des Francs-Small et al., 1992). The gels were Coomassie blue stained and the polypeptides were quantified (Scanalytics MasterScan I, Computer Signal Processing, Billerica, MA) equipped with two-dimensional gel-analysis software. The polypeptides of interest were identified by immunodetection with various antibodies after transfer onto nitrocellulose sheets (Hybond C, Amersham) using the Mini Trans-Blot cell (Bio-Rad).
RESULTS
FDH and SHMT mRNA Expression in Various Potato Tissues
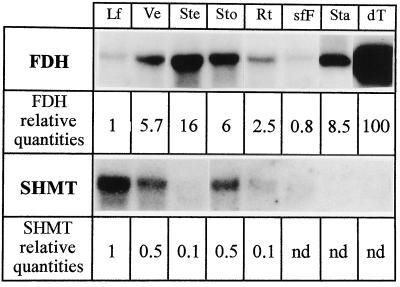
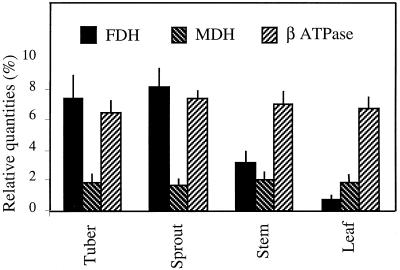
The levels of FDH and SHMT mRNAs were studied in parallel in various tissues from greenhouse-grown potato plants and are presented in Figure 1. The lowest FDH transcript levels were found in leaves and stamen-free flowers, whereas developing tubers exhibited a very high amount of FDH mRNAs (about 100-fold higher than in leaves). Veins, stems, stolons, stamens, and roots showed intermediate amounts of FDH transcripts: about 16-fold higher in stems than in leaves (used as the reference for quantitations), 6-fold higher in veins, 8-fold higher in stamens, 6-fold higher in stolons, and 2.5-fold higher in roots. Compared with FDH, SHMT transcript levels were high in leaves, one-half as abundant in veins and stolons, and very scarce or even undetectable in other tissues. FDH protein amounts followed mRNA levels in the various tissues tested. The histogram of the quantities of FDH, MDH (matrix protein), and β-ATPase (membrane protein) illustrates the variations in FDH and the relatively constant amounts of MDH and β-ATPase in these tissues (Fig. 2).
Figure 1.
Northern analysis of RNA isolated from various potato tissues and probed with either potato FDH cDNA or pea SHMT cDNA. Transcript sizes are, respectively, 1.4 and 1.5 kb. Ten micrograms of RNA was loaded onto each lane, except for developing tubers, the lanes of which were loaded with only 5 μg of RNA. Lf, Leaves; Ve, veins; Ste, stems; Sto, stolons; Rt, roots; sfF, stamen-free flowers; Sta, stamens; and dT, developing tubers. The autoradiographs were scanned using the leaf sample as the reference for both probes, and the figures were corrected according to the RNA loadings.
Figure 2.
Relative contents of FDH, MDH, and β-ATPase proteins in potato mitochondria isolated from different tissues, as estimated by quantitation of the corresponding spots on Coomassie blue-stained two-dimensional gels, as described in Methods. The relative abundance of each spot was expressed as a percentage of the total number of protein spots detected on the gels. The figures are an average of five quantitations performed on five gels resulting from five distinct mitochondrial preparations.
Formate Oxidation in Potato Tuber Mitochondria
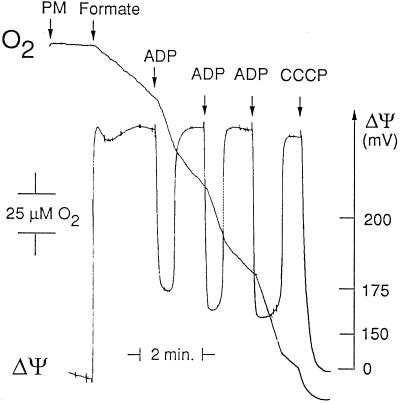
From simultaneous measurements of O2 uptake and membrane potential in mitochondria isolated from potato tubers, it could be demonstrated that NAD-linked FDH produced NADH, which was reoxidized by the respiratory chain (Fig. 3). Formate oxidation in state 4 (10.5 ± 0.5 nmol O2 min−1 mg−1 protein) was associated with high values of membrane potential (228 ± 2 mV), whereas the addition of ADP induced a large depolarization (55 ± 5 mV) (Fig. 3). The rates of formate oxidation (32 ± 2 nmol O2 min−1 mg−1 protein) were one-half that of those obtained with malate (64 ± 14 nmol O2 min−1 mg−1 protein). By contrast, the ADP-to-O2 ratio found with either formate or malate was identical (2.15 ± 0.35), indicating that formate oxidation was coupled to the three proton translocation sites in plant mitochondria (see also Oliver, 1981).
Figure 3.
Simultaneous recording of respiratory rate and membrane potential in purified mitochondria from potato tuber oxidizing formate. Experimental conditions are described in Methods. Mitochondria (0.5 mg protein/mL) were incubated in the presence of 4 μm TPP+ and 1 mm NAD+. Concentrations used were: formate, 15 mm; ADP, 100 μm; and carbonylcyanide m-chlorophenylhydrazone, 1 μm. PM, Purified mitochondria.
The Expression of FDH in Leaves Is Enhanced under Various Environmental Stresses
Hypoxia
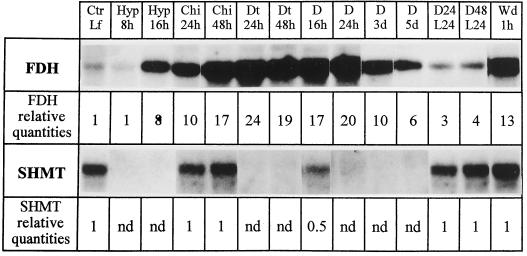
Hypoxia led to a strong FDH mRNA induction. Although no effect was observed after 8 h of hypoxia, the FDH mRNA level increased 8-fold after 16 h (Fig. 4), and remained high after 24 and 48 h of hypoxia (data not shown).
Figure 4.
RNA expression of FDH and SHMT in potato leaves under various stress treatments. Each lane was loaded with 10 μg of RNA. Ctr Lf, Control leaves; Hyp 8h and Hyp 16h, 8 and 16 h of hypoxia, respectively; Chi 24h and Chi 48h, 24 and 48 h of chilling, respectively; Dt 24h and Dt 48h, 24 and 48 h of drought treatment, respectively; D 16h, D 24h, D 3d, and D 5d, 16 h, 24 h, 3 d, and 5 d of dark treatment, respectively; D24 and L24, 24 h of dark treatment followed by 24 h under a 16-h photoperiod; D48 and L24, 48 h of dark treatment followed by 24 h under a 16-h photoperiod; Wd 1h, 1 h after wounding. The autoradiographs were scanned using the control leaf sample as the reference for both probes, and the figures were corrected according to the RNA loadings.
Chilling and Drought
Chilling and drought also considerably increased FDH mRNA (respectively, 10- and 24-fold higher than the control) after 24 h of treatment (Fig. 4). Chilling, however, induced a slower response than drought, as the transcript abundance continued to increase for another 24 h.
Dark and Dark-to-Light Transition Effects
A dramatic increase of FDH mRNA was observed after 16 h of dark treatment (Fig. 4), which continued to increase up to 24 h (17- and 20-fold higher than the control, respectively), remained high after 48 h (data not shown), and started to decrease after 3 d (down to 10-fold higher than the control). Dark induction of FDH mRNAs was almost completely reversible by placing the plants under a 16-h photoperiod for another 24 h (Fig. 4, lanes D24-L24 and D48-L24).
Wounding
Unlike the previous stress treatments, wounding led to a very rapid FDH mRNA response, as the transcripts were very abundant only 20 min after wounding. They accumulated (13-fold higher than the control) for up to 1 h (Fig. 4), remained high for 24 h, and then decreased between 24 h and 3 d (data not shown).
SHMT and FDH Leaf mRNAs Show Opposite Responses to Various Environmental Stresses
SHMT mRNA levels were unchanged after chilling or wounding (Fig. 4). On the contrary, they were undetectable after 8 h of hypoxia, 24 h of drought, or 24 h of dark treatment. SHMT mRNAs reappeared when plants kept for 24 to 48 h in the dark were placed back under a 16-h photoperiod for another 24 h. In summary, SHMT and FDH mRNAs showed opposite responses (except for chilling and wounding), demonstrating that we are dealing with specific regulations. In regard to dark and hypoxia stresses, however, the kinetics of the increase or decrease were different, i.e. more rapid and drastic for SHMT transcripts.
The Expression of FDH and SHMT mRNAs in Leaves Are Altered Differently by External Addition of Various Metabolites
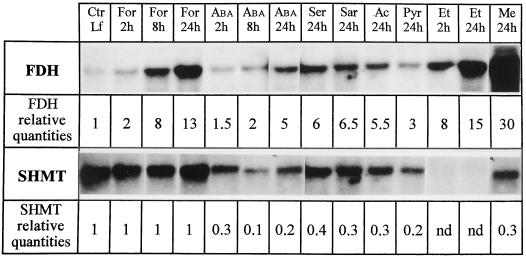
Formate
Spraying plants with 10 mm formate (the substrate for FDH) increased FDH mRNAs 13-fold after 24 h, whereas the amount of SHMT mRNAs was unchanged (Fig. 5).
Figure 5.
RNA expression of FDH and SHMT after spraying leaves with various metabolites. Each lane was loaded with 10 μg of RNA. Ctr Lf, Control leaves (water); For 2h, For 8h, and For 24h, 2, 8, and 24 h after 10 mm formate application, respectively; ABA 2h, ABA 8h, and ABA 24h, 2, 8, and 24 h after spraying 100 μm ABA, respectively; Ser 24h, 24 h after 10 mm Ser application; Sar 24h, 24 h after spraying 10 mm sarcosine; Ac 24h, 24 h after spraying 10 mm acetate; Pyr 24h, 24 h after spraying 10 mm pyruvic acid; Et 2h and Et 24h, 2 and 24 h after spraying 10% ethanol, respectively; and Me 24h, 24 h after spraying 20% methanol. The autoradiographs were scanned using the control leaf sample as the reference for both probes, and the figures were corrected according to the RNA loadings.
ABA
ABA, a compound involved in the response to several stresses such as cold, hypoxia, drought, and wounding, was tested for its ability to regulate FDH or SHMT mRNA levels. Twenty-four hours after spraying 100 μm ABA, the amount of FDH mRNAs was multiplied by five (Fig. 5), and the amount of SHMT mRNAs decreased.
Ser and Sarcosine
Ser and sarcosine, two products derived from Gly and methylene THF by the action of SHMT and sarcosine dehydrogenase, respectively, induced a 2.5- to 3-fold decrease of SHMT mRNA levels in leaves, whereas the amount of FDH mRNA was increased (about 6-fold higher in both cases) (Fig. 5).
Pyruvate, Acetate, and Ethanol
Because glycolysis is known to be induced by dark and hypoxia, some products of glycolysis (pyruvate, acetate, and ethanol) were tested. Northern analyses showed an increase of FDH expression level 24 h after spraying 10 mm acetate (5.5-fold) or 10 mm pyruvate (3-fold), and a greater increase (15-fold) 24 h after 10% ethanol treatment (Fig. 5). On the contrary, SHMT mRNAs decreased after spraying acetate or pyruvate (3- and 5-fold, respectively), and disappeared after ethanol treatment (Fig. 5).
Methanol
Methanol led to a dramatic increase (30-fold higher than the control) of FDH mRNAs (Fig. 5) and a 3-fold decrease of SHMT mRNAs (Fig. 5).
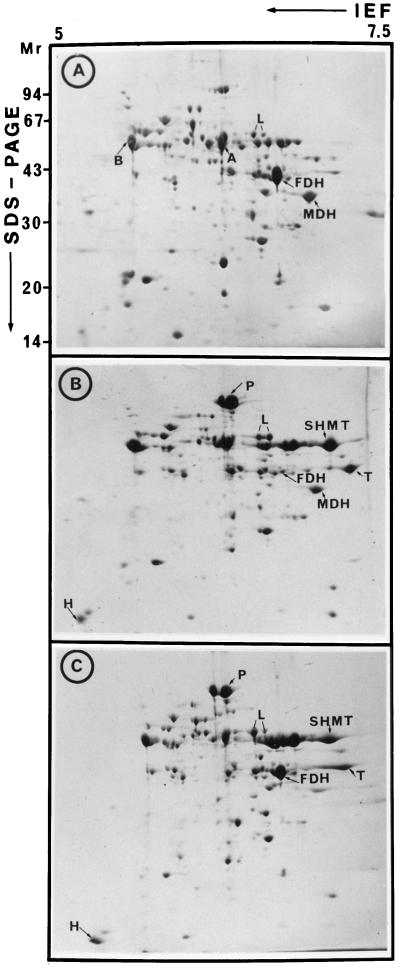
Two-Dimensional Analysis of Mitochondrial Polypeptides
Figure 6, A and B, shows the polypeptide patterns obtained with mitochondria isolated from tubers and leaves from young, actively growing plants (summer plants). The relative FDH content was 10-fold higher in tubers than in leaves, whereas MDH and α- and β-ATPase were roughly constant in both tissues (Fig. 2). However, it was evident that in mitochondria isolated from the leaves of old, slowly growing plants (winter plants, mostly grown under artificial light), the relative FDH content observed was similar to that found in tubers (Fig. 6C). The two-dimensional mitochondrial protein patterns of tubers from summer and winter plants were similar (not shown).
Figure 6.
Two-dimensional protein patterns of mitochondria isolated from various potato tissues. A, Tubers; B, green leaves from a young, rapidly growing plant (spring); and C, green leaves from an old plant grown under poor conditions (winter). Two-dimensional PAGE was performed according to the method of Colas des Francs-Small et al. (1992), and the gels were Coomassie blue stained. Input was 150 μg. Some polypeptides of interest identified by immunoblotting are indicated: A and B, α- and β-ATPase, respectively; P, L, T, and H, subunits of the Gly decarboxylase complex.
DISCUSSION
Differential Expression of FDH in Various Potato Tissues
Our results show that under normal growth conditions, FDH transcript and protein contents are high in nonphotosynthetic tissues (stems, stolons, stamens, tubers, and dark-grown sprouts) and scarce in leaf mitochondria. An exception to this rule is the roots, where FDH content is only slightly higher than in leaves. The fact that formate can be excreted by roots, as reported for oats and maize under poorly oxygenated conditions (Davison, 1951), might explain this discrepancy.
The highest FDH expression levels were found in developing tubers, as opposed to dormant tubers, where no FDH transcripts were detected (Colas des Francs-Small et al., 1993). FDH activity, however, remains high in dormant tubers, sprouting tubers, and sprouts. Why do nonphotosynthetic tissues need more FDH than green tissues? What does this particularly high FDH activity in potato tubers mean? In some plant tissues, such as tubers and sprouts, FDH might be involved in cell respiration when the TCA cycle is not fully operative. Norton (1963) suggested that in fresh potato tuber slices, the bulk of respiration is mediated by systems other than the TCA cycle, because only 10% of the O2 uptake is inhibited by malonate, a potent inhibitor of succinate dehydrogenase. In addition, it was shown that during the aging of tuber slices, malonate-sensitive respiration increases greatly, whereas malonate-insensitive respiration remains roughly constant.
In isolated mitochondria a formate-dependent O2 uptake coupled with ATP synthesis was described by Oliver (1981) and found to vary greatly between tissues and plant species (it was very high in spinach leaves). Our results clearly demonstrate that formate is a good respiratory substrate for isolated potato tuber mitochondria and is consistent with the high level of FDH protein found in tuber (Fig. 2) and other nongreen tissues (Colas des Francs-Small et al., 1993). Furthermore, formate is able to strongly inhibit the oxidation of single substrates such as malate or succinate (not shown).
FDH Response to Stress
Some environmental signals to which tubers are naturally submitted (e.g. dark and hypoxia) and a few other stresses were investigated for their ability to enhance FDH expression in potato leaves, where it is low under normal conditions. The mRNA level of the mitochondrial isoform of SHMT, a key enzyme of C1 metabolism, was studied in parallel as a control. Our results show that FDH mRNA expression is strongly increased by dark, hypoxia, wounding, cold, and drought. We performed western analyses and activity tests for most of these stresses and observed that FDH quantity and activity follow the mRNA increase 1 to 3 d after the stress treatment. In all cases, the final activity was 1.5- to 1.7-fold higher than the control (not shown).
Under all of these stresses, SHMT was regulated in an opposite manner, except during chilling, when the mRNA level remains as high as in control leaves. The disappearance of SHMT mRNAs is faster than the increase of FDH mRNAs. Only wounding led to a very rapid response of FDH mRNAs (20 min). The relatively slow responses to all the other stresses tested here (8 h on average) probably reflect regulation of FDH expression by changes in concentration of certain intracellular metabolites due to the activation of specific metabolic pathways; for example, dark, hypoxia, and chilling all induce glycolysis. The fact that the response of FDH transcripts to ABA is minor compared with their induction by most stresses suggests that ABA-independent transduction pathways are probably involved in the FDH response to stress.
An important observation reported here is that FDH can accumulate to high levels in leaves of continuously stressed plants, e.g. after 2 to 3 months of poor growth conditions in small pots. We hypothesize that in such stressed tissues, formate respiration might complement classic respiration.
FDH Response to Metabolites
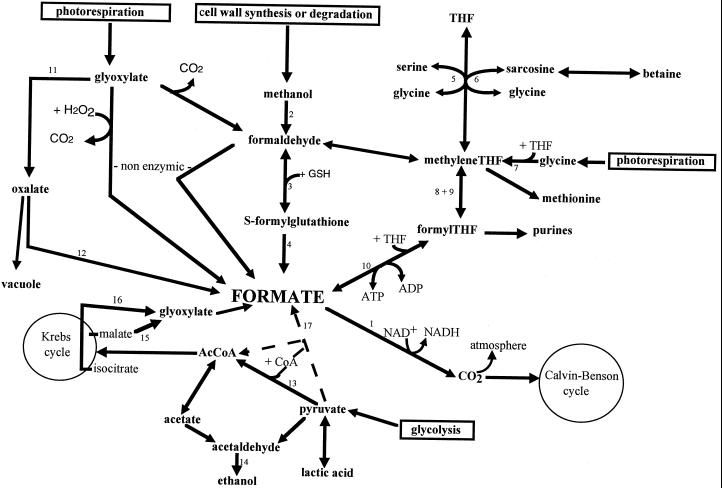
To elucidate the transduction pathways involved in the FDH plant response to stress, the effects of spraying leaves with various metabolites were studied. The effects of the metabolites should be considered to be qualitative rather than quantitative because of the uncertainties concerning the amount of metabolite actually entering the plant cells. The observation that formate application greatly enhances FDH expression in potato leaves led us to focus on formate biosynthesis. Under normal growth conditions, formate can arise from various pathways (Fig. 7). The photorespiratory origin of formate in leaves was the first to be described (Tolbert et al., 1949), and in a recent review, Fall and Benson (1996) suggested rapid formate biosynthesis via methanol metabolism (reactions 2, 3, and 4), with the methanol arising from cell wall degradation or synthesis. Finally, glycolytic products may generate glyoxylate from malate (via malate synthase, reaction 15) or from isocitrate (via isocitrate lyase, reaction 16). This glyoxylate will give rise to formate in peroxisomes. In stressed plants formate biosynthesis could result from the enhancement of any of these pathways or from newly induced ones. Three metabolic pathways thought to be involved in response to stress were studied here.
Figure 7.
Possible pathways of formate production in higher plants. Reactions shown are: 1, FDH; 2, methanol oxidase; 3, formaldehyde dehydrogenase; 4, S-formyl hydrolase; 5, SHMT; 6, sarcosine dehydrogenase; 7, Gly decarboxylase; 8, 5,10-methylene-THF dehydrogenase; 9, 5,10-methenyl-THF cyclohydrolase; 10, 10-formyl-THF synthetase; 11, glycollate oxidase; 12, oxalate decarboxylase; 13, pyruvate dehydrogenase; 14, alcohol dehydrogenase; 15, malate synthase; 16, isocitrate lyase; 17, pyruvate formate lyase. Reactions 2, 3, 4, 12, and 14 are cytosolic; reactions 1 and 7 are mitochondrial; reactions 11, 15, and 16 are peroxisomal; reactions 6, 8, and 9 have been described in both cytosol and mitochondria; reactions 5 and 13 are both plastidial and mitochondrial; and reaction 17 has only been described in microorganisms and mitochondria from unicellular algae.
The Ser and Sarcosine Degradation Pathway
Our results show that spraying Ser and sarcosine increases FDH transcription in potato leaves. The catabolism of sarcosine and Ser to formate was first described in yeast and mammals (Barlow and Appling, 1988), but whether plants use sarcosine is not clear. When photorespiration occurs, the mitochondrial SHMT isoform catalyzes the synthesis of Ser, whereas the thermodynamic equilibrium of this reaction is normally in favor of Ser degradation (Rebeillé et al., 1994). In nongreen tissues Ser is thought to be cleaved by other isoforms of SHMT to yield Gly, 5,10-methylene THF, and, subsequently, formate (Schirch, 1984). Furthermore, Ser catabolism was shown to occur in grapevine leaves under water stress (Morchiladze, 1969). We suggest that under drought, Ser might be partly metabolized into formate (reactions 5, 6, 7, 8, 9, and 10) and possibly account for a part of the FDH induction in potato leaves. The advantages of such a degradation pathway under stress would be to produce ATP (reaction 10) and to provide NADH to the respiratory chain (reaction 1). It would be interesting to study this Ser catabolism in more detail in tissues in which FDH expression is high.
Glycolysis
Dark and hypoxia are known to induce glycolysis, which leads to the production of pyruvate, acetate, and, in the case of hypoxia, ethanol. Spraying acetate and pyruvate increased FDH transcript levels, but less than dark or hypoxia. This suggests that these compounds could be involved in the FDH response to dark or hypoxia, but the concentrations used might not be sufficient to reflect these stresses. On the other hand, 10% ethanol (2 m) led to a higher FDH increase and to total disappearance of SHMT transcripts, close to what was observed under dark and hypoxic stresses. Acetate, pyruvate, and ethanol could act either directly as formate precursors, or indirectly by induction of the enzymes involved in formate biosynthesis. These results suggest that glycolysis may play an important role in the FDH response to dark and hypoxia. The important glycolytic flux that occurs in nonphotosynthetic tissues (germinating tubers, sprouts, germinating pollen, stems, and leaves in the dark) might to a certain extent account for the high FDH levels in such tissues. However, the pathway from glycolytic products to formate remains obscure. We suggest two possibilities: (a) via malate and isocitrate or (b) directly from pyruvate to formate via a pyruvate formate lyase (reaction 17), which has been described in mitochondria from unicellular algae (Kreutzberg, 1984; Kreutzberg et al., 1987) but remains to be found in higher plants.
The Metabolism of Methanol
Both cell wall growth and degradation lead to methanol production in plants (Fall and Benson, 1996). Methanol catabolism to formate is particularly interesting, because methanol-treated plants exhibited very high levels of FDH mRNA. The lack of response of SHMT after methanol treatment indicates that FDH induction is specific. Moreover, recent papers demonstrate that the application of methanol to leaves enhances the yields in a wide range of C3 plants (unlike C4 plants) (Nonomura and Benson, 1992; Devlin and al., 1994; Rowe et al., 1994). These authors suggest that an increase in available CO2 might contribute to this response. Cossins (1964) showed that methanol is rapidly metabolized into CO2 in leaves, but although formaldehyde dehydrogenase and FDH exist in plants, methanol oxidase has so far only been found in microorganisms. Furthermore, their role in this metabolic pathway is not yet established (Fall and Benson, 1996). Formaldehyde dehydrogenase, in particular, could be partly or entirely overshadowed by other pathways, as formaldehyde can also give rise to formate directly or via methylene THF (Doman and Romanova 1962; Cossins, 1964). The dramatic induction of FDH after methanol treatment strongly favors the hypothesis of its participation in methanol oxidation to CO2. Mutants deficient in one or more enzymes of this latter pathway would be very helpful for studying the role of these enzymes in C3 plant growth.
ACKNOWLEDGMENTS
The authors wish to thank Dr. Steve Rawsthorne for providing the pea SHMT probe and antibody. We are grateful to R. Boyer for taking the photographs and to Drs. Ian Small and Steve Rawsthorne for critical reading of the manuscript. We also thank F. Vedel for his continuous interest in this work.
Abbreviations:
- α- and β-ATPase
α- and β-subunits of the mitochondrial ATP synthase, respectively
- FDH
formate dehydrogenase
- MDH
malate dehydrogenase
- SHMT
Ser hydroxymethyltransferase
- THF
tetrahydrofolate
- TPP
tetraphenylphosphonium
Footnotes
This work was supported by the Centre National de la Recherche Scientifique (Strasbourg, France).
LITERATURE CITED
- Allen SJ, Holbrook JJ. Gene. 1995;162:99–104. doi: 10.1016/0378-1119(95)00347-9. [DOI] [PubMed] [Google Scholar]
- Barlow CK, Appling DR. In vitro evidence for the involvement of mitochondrial folate metabolism in the supply of cytoplasmic one-carbon units. Biofactors. 1988;1:171–176. [PubMed] [Google Scholar]
- Bonen L, Gray MW. Organization and expression of mitochondrial genomes of plants. The genes for wheat mitochondrial ribosomal and transfer RNA: evidence for an unusual arrangement. Nucleic Acid Res. 1980;8:319–335. doi: 10.1093/nar/8.2.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colas des Francs-Small C, Ambard-Bretteville F, Darpas A, Sallantin M, Huet JC, Pernollet JC, Rémy R. Variation of the polypeptide composition of mitochondria isolated from different potato tissues. Plant Physiol. 1992;98:273–278. doi: 10.1104/pp.98.1.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colas des Francs-Small C, Ambard-Bretteville F, Small ID, Rémy R. Identification of a major soluble protein in mitochondria from nonphotosynthetic tissues as NAD-dependent formate dehydrogenase. Plant Physiol. 1993;102:1171–1177. doi: 10.1104/pp.102.4.1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cossins EA. The utilization of carbon-1 compounds by plants. I. The metabolism of methanol-C14 and its role in amino-acid biosynthesis. Can J Bot. 1964;42:1793–1802. [Google Scholar]
- Cossins EA (1980) One-carbon metabolism. In DD Davies, eds, The Biochemistry of Plants, Vol II. Academic Press, New York, pp 365–418
- Davison DC. Studies on formate dehydrogenases. Biochem J. 1951;49:520–526. doi: 10.1042/bj0490520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devlin RM, Bhowmik PC, Karczmarczyk SJ. Influence of methanol on plant growth. Plant Growth Regul Soc Am Q. 1994;22:102–108. [Google Scholar]
- Diolez P, Moreau F. Correlations between ATP synthesis, membrane potential and oxidation rate in plant mitochondria. Biochem Biophys Acta. 1985;806:56–63. [Google Scholar]
- Doman NG, Romanova AK. Transformations of labeled formic acid, formaldehyde, methanol and CO2 absorbed by bean and barley leaves from air. Plant Physiol. 1962;37:833–840. doi: 10.1104/pp.37.6.833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fall R, Benson AA. Leaf methanol: the simpliest natural product from plants. Trends Plant Sci. 1996;1:296–301. [Google Scholar]
- Halliwell B. Oxidation of formate by peroxisomes and mitochondria from spinach leaves. Biochem J. 1974;138:77–85. doi: 10.1042/bj1380077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haynes TS, Klemm DJ, Ruocco JJ, Barton LL. Formate dehydrogenase activity in cells and outer membrane blebs of Desulfovibrio gigas. Anaerobe. 1995;1:175–182. doi: 10.1006/anae.1995.1016. [DOI] [PubMed] [Google Scholar]
- Humphery-Smith I, Colas des Francs-Small C, Ambard-Bretteville F, Rémy R. Tissue-specific variations of pea mitochondrial polypeptides detected by computerized image analysis of two-dimensional electrophoresis gels. Electrophoresis. 1992;13:168–172. doi: 10.1002/elps.1150130134. [DOI] [PubMed] [Google Scholar]
- Kamo N, Muratsugu M, Hongoh R, Kobakate Y. Membrane potential of mitochondria measured with an electrode sensitive to tetraphenylphosphonium and relationship between proton electrochemical potential and phosphorylation potential in steady state. J Membr Biol. 1979;49:105–121. doi: 10.1007/BF01868720. [DOI] [PubMed] [Google Scholar]
- Kreutzberg K. Starch fermentation via formate producing pathway in Chlamydomonas reinhardtii, Chlorogonium elongatum, and Chlorella fusca. Physiol Plant. 1984;61:87–94. [Google Scholar]
- Kreutzberg K, Klöck G, Grobheiser D. Subcellular distribution of pyruvate-degrading enzymes in Chlamydomonas reinhardtii studied by an improved protoplast fractionation procedure. Physiol Plant. 1987;69:481–488. [Google Scholar]
- Logemann J, Schell J, Willmitzer L. Improved method for the isolation of RNA from plant tissues. Anal Biochem. 1987;163:16–20. doi: 10.1016/0003-2697(87)90086-8. [DOI] [PubMed] [Google Scholar]
- Lowry OH, Rosenbrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- Morchiladze ZN. Transformation of 3 C14 series in the grapevine. Soobshch Akad Nauk Gruz SSR. 1969;54:705–708. [Google Scholar]
- Nonomura AM, Benson AA. The path of carbon in photosynthesis: improved crop yields with methanol. Proc Natl Acad Sci USA. 1992;89:9794–9798. doi: 10.1073/pnas.89.20.9794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norton G. The respiratory pathways in potato tubers. In: Irvins JD, Milthorpe FL, editors. The Growth of the Potato. Proceedings of the Tenth Easter School in Agricultural Science. University of Nottingham, London: Butterworths; 1963. pp. 148–169. [Google Scholar]
- Oliver DJ. Formate oxidation and oxygen reduction by leaf mitochondria. Plant Physiol. 1981;68:703–705. doi: 10.1104/pp.68.3.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popov VO, Lamzin VS. NAD(+)-dependent formate dehydrogenase. Biochem J. 1994;302:967. doi: 10.1042/bj3010625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebeillé F, Neuburger M, Douce R. Interaction between glycine decarboxylase, serine hydroxymethyltransferase and tetrahydrofolate polyglutamates in pea leaf mitochondria. Biochem J. 1994;302:223–228. doi: 10.1042/bj3020223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe RN, Farr DJ, Richards BAJ. Effects of foliar and root applications of methanol or ethanol on the growth of tomato plants (Lycopersicon esculentum Mill.) N Z J Crop Hort Sci. 1994;22:335–337. [Google Scholar]
- Sambrook J, Fritsch EF, Manniatis T. Molecular Cloning: A Laboratory Manual. Cold Spring Harbor, New York: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- Sawers G. The hydrogenases and formate dehydrogenases of Escherichia coli. Antonie Van Leeuwenhoek. 1994;66:57–88. doi: 10.1007/BF00871633. [DOI] [PubMed] [Google Scholar]
- Schirch L (1984) Folates in serine and glycine metabolism. In RL Blakely, SJ Benkovic, eds, Folates and Pterins. Wiley, New York, pp 399–431
- Tolbert NE, Clagett CO, Burris RH. Products of the oxidation of glycolic acid and lactic acid by enzymes from tobacco leaves. J Biol Chem. 1949;181:905–914. [PubMed] [Google Scholar]
- Turner SR, Hellens R, Ireland R, Ellis N, Rawsthorne S. The organisation and expression of the genes encoding the mitochondrial glycine decarboxylase complex and serine hydroxymethyltransferase in pea (Pisum sativum) Mol Gen Genet. 1993;236:402–408. doi: 10.1007/BF00277140. [DOI] [PubMed] [Google Scholar]
- Turner SR, Ireland R, Morgan C, Rawsthorne S. Identification and localization of multiple forms of serine hydroxymethyltransferase in pea (Pisum sativum) and characterization of a cDNA encoding a mitochondrial isoform. J Biol Chem. 1992;267:13528–13534. [PubMed] [Google Scholar]
- Uotila L, Koivusalo M. Purification of formaldehyde and formate dehydrogenases from pea seeds by affinity chromatography and S-formylglutathione as the intermediate of formaldehyde metabolism. Arch Biochem Biophys. 1979;196:33–45. doi: 10.1016/0003-9861(79)90548-4. [DOI] [PubMed] [Google Scholar]
- Van Dijken JP, Oostra-Demkes GT, Otto R, Harder W. S-Formylglutathione: the substrate for formate dehydrogenase in methanol-utilizing yeasts. Arch Microbiol. 1976;111:77–83. doi: 10.1007/BF00446552. [DOI] [PubMed] [Google Scholar]