Abstract
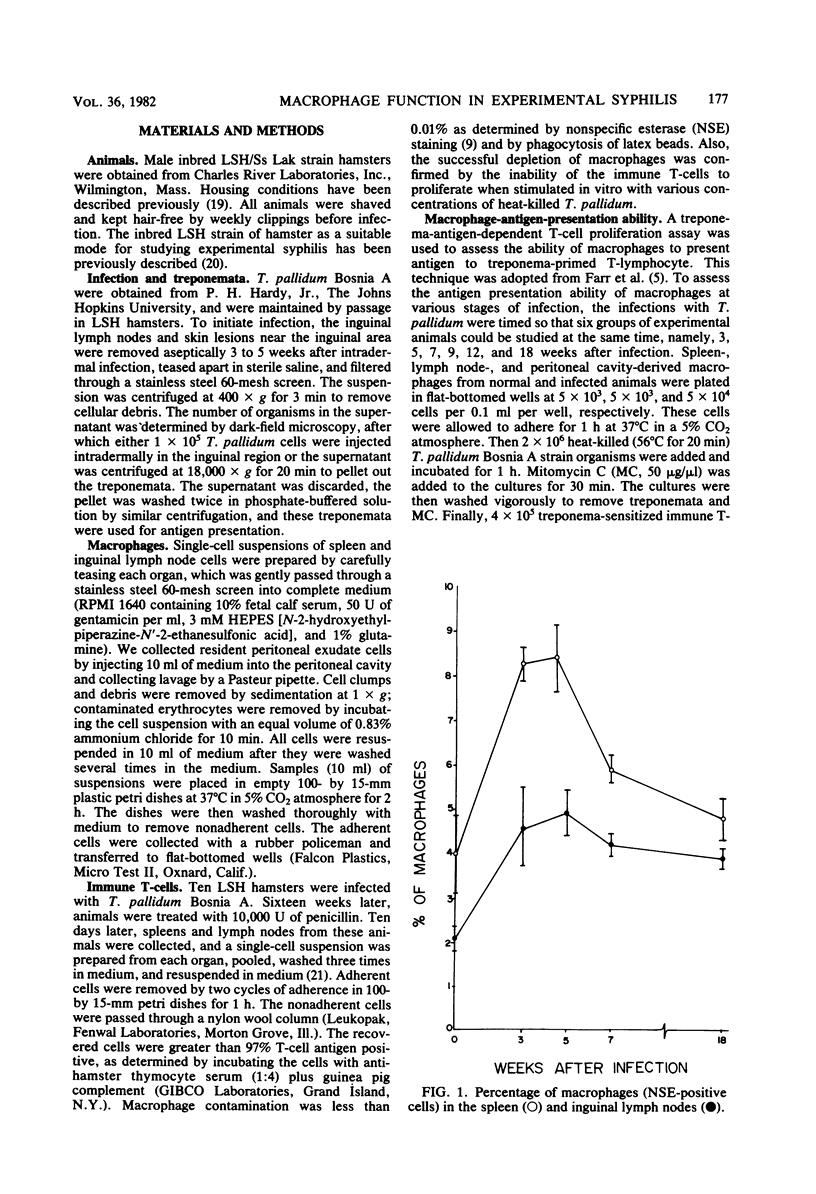
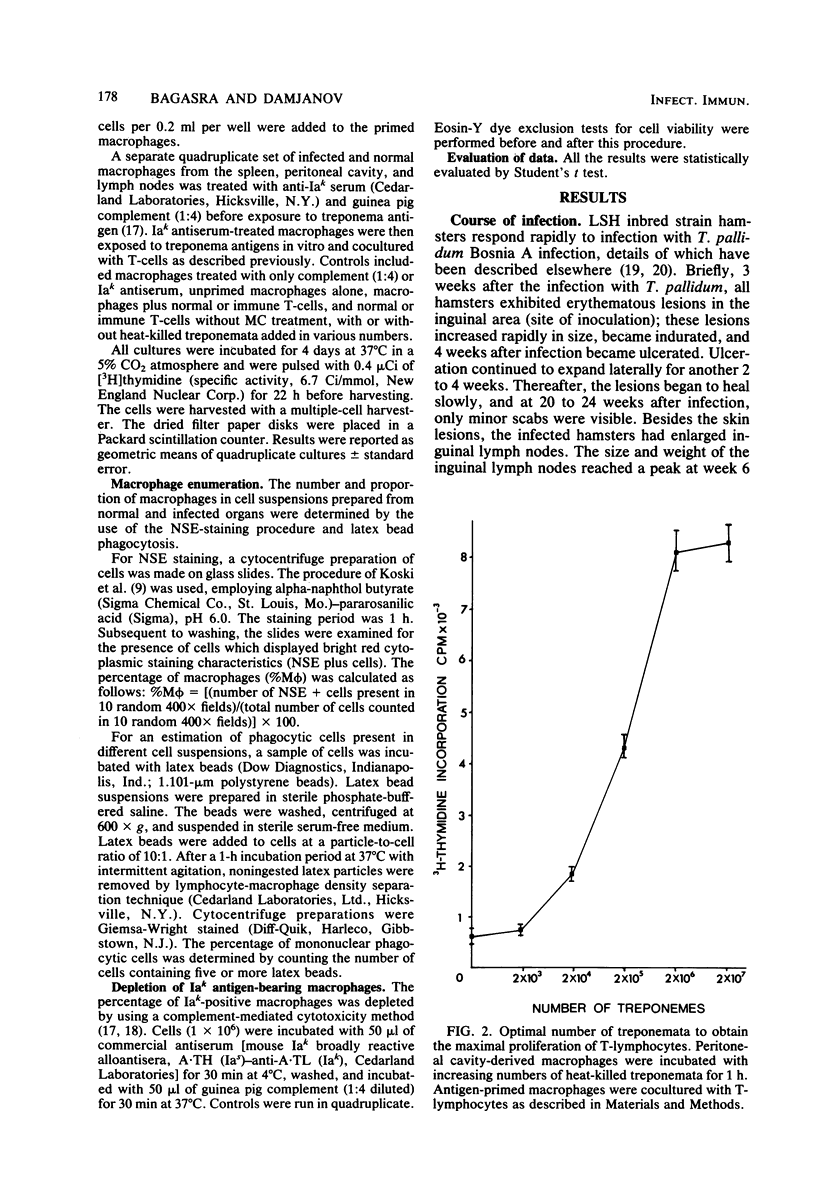
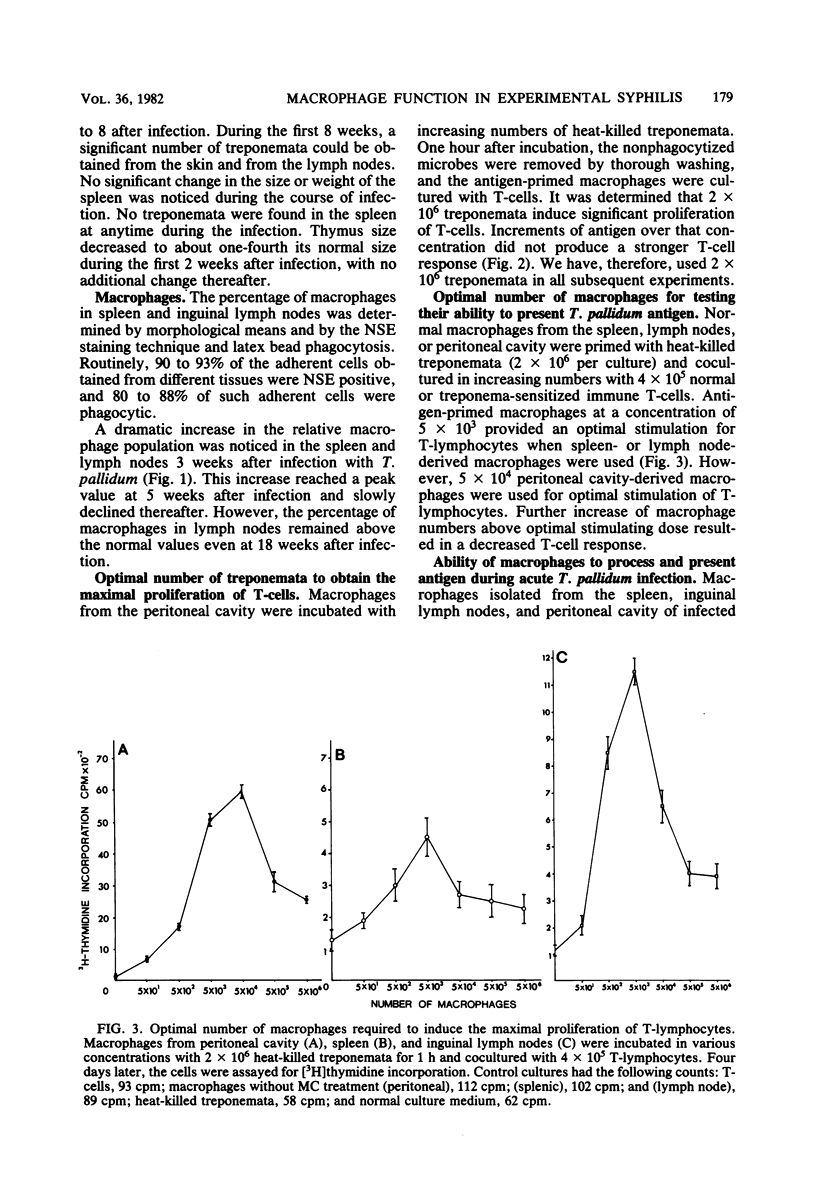
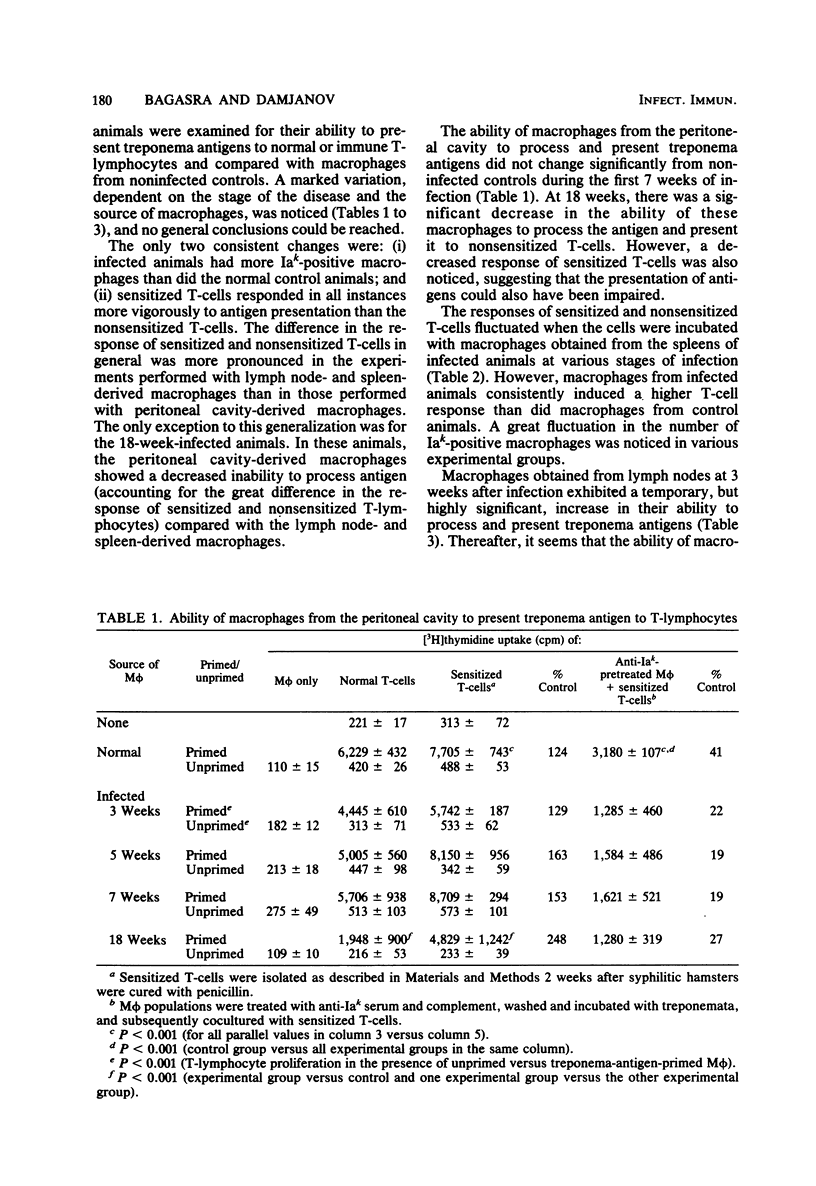
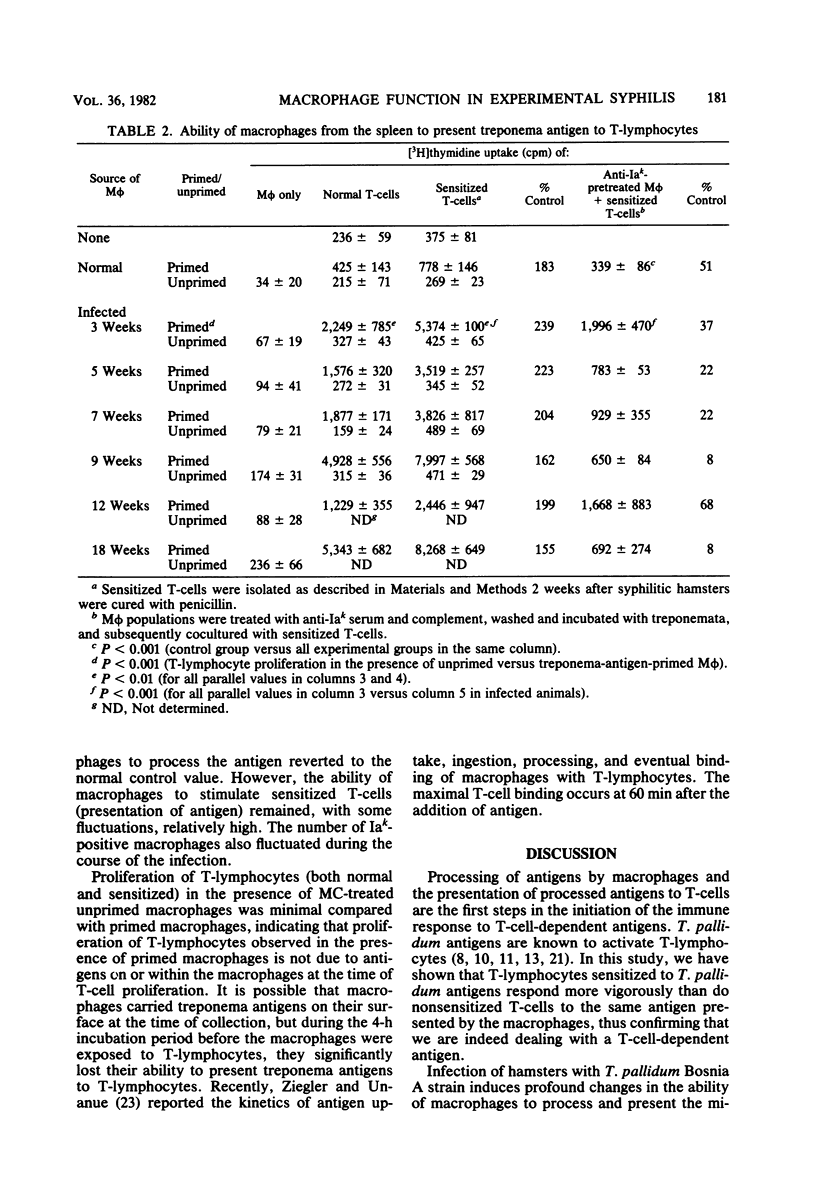
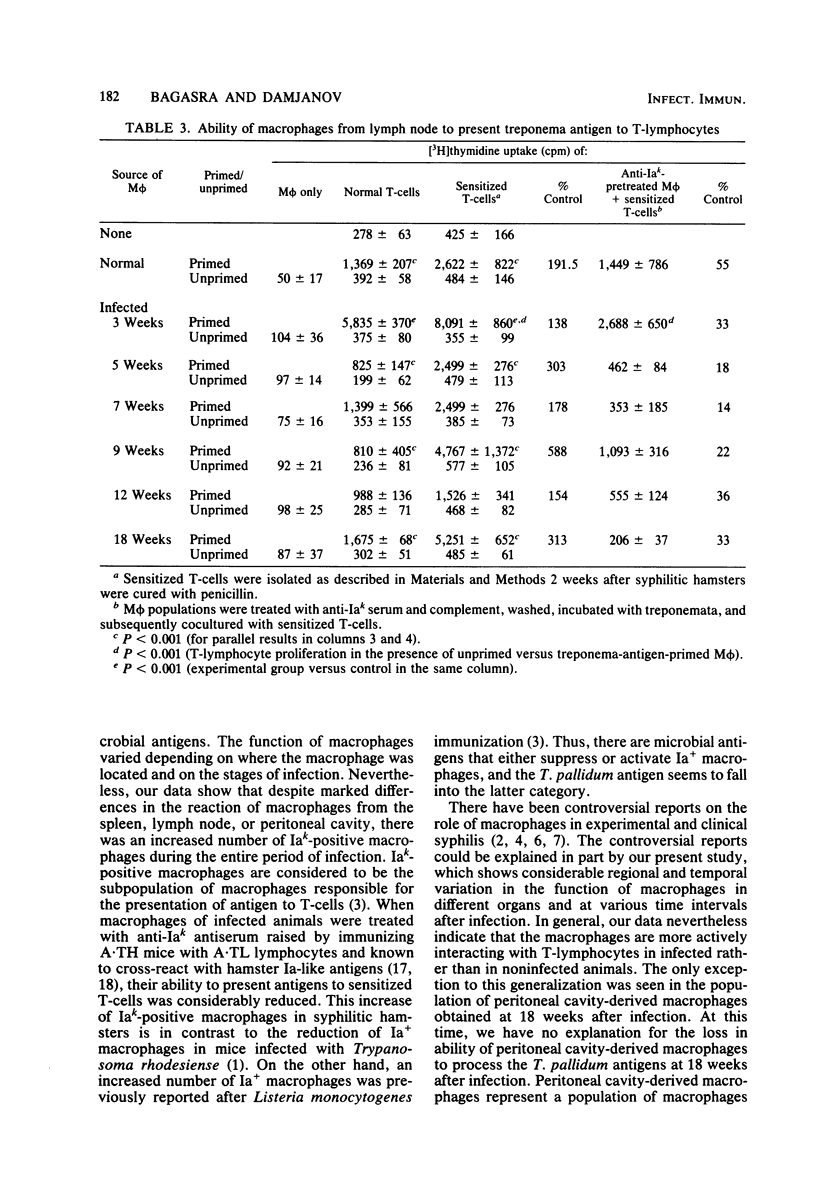
The ability of macrophages to process and present treponemal antigens to T-lymphocytes was studied in early stages of experimental syphilis produced by Treponema pallidum Bosnia A strain (the causative agent of endemic syphilis) infection of inbred Syrian hamsters (LSH/Ss Lak strain). A difference was noticed in the response of macrophages obtained from the peritoneal cavity, lymph nodes, and spleens of the infected animals. In all of these locations, a general increase in the population of Iak-positive macrophage was seen during the entire period of infection, i.e., 3 to 18 weeks after inoculation. Peritoneal cavity-derived macrophages showed no difference in antigen presentation to sensitized and nonsensitized T-lymphocytes for the first 7 weeks of infection. However, at 18 weeks after infection, peritoneal macrophages lost their ability to process treponema antigens. Spleen- and lymph node-derived macrophages did not exhibit a parallel loss in their ability to process treponema antigens. A fluctuation without a consistent pattern was noticed in the antigen processing and presentation by macrophages from the spleen and lymph nodes. In general, the sensitized T-lymphocytes responded to treponema antigen presented by macrophages more vigorously than the nonsensitized T-lymphocytes. An increased ability of spleen-derived macrophages to process and present antigens was noticed throughout the entire period of infection. The macrophages from the lymph nodes showed such an increase only temporarily at 3 weeks after infection. These data suggest that the processing and presentation of treponema antigens by macrophages in acute syphilitic infection fluctuates considerably and depends on the source of macrophages and the duration of the infection. The differences in the response of peritoneal cavity-, spleen-, and lymph node-derived macrophages probably reflect the complex interactions between the macrophage and other cells involved in the immune response to treponema infection.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bagasra O., Schell R. F., Le Frock J. L. Evidence for depletion of Ia+ macrophages and associated immunosuppression in African trypanosomiasis. Infect Immun. 1981 Apr;32(1):188–193. doi: 10.1128/iai.32.1.188-193.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baughn R. E., Musher D. M., Knox J. M. Effect of sensitization with Propionibacterium acnes on the growth of Listeria monocytogenes and Treponema pallidum in rabbits. J Immunol. 1977 Jan;118(1):109–113. [PubMed] [Google Scholar]
- Beller D. I., Kiely J. M., Unanue E. R. Regulation of macrophage populations. I. Preferential induction of Ia-rich peritoneal exudates by immunologic stimuli. J Immunol. 1980 Mar;124(3):1426–1432. [PubMed] [Google Scholar]
- Brause B. D., Roberts R. B. Attachment of virulent Treponema pallidum to human mononuclear phagocytes. Br J Vener Dis. 1978 Aug;54(4):218–224. doi: 10.1136/sti.54.4.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farr A. G., Kiely J. M., Unanue E. R. Macrophage-T cell interactions involving Listeria monocytogenes--role of the H-2 gene complex. J Immunol. 1979 Jun;122(6):2395–2404. [PubMed] [Google Scholar]
- Graves S. R., Johnson R. C. Effect of pretreatment with Mycobacterium bovis (strain BCG) and immune syphilitic serum on rabbit resistance to Treponema pallidum. Infect Immun. 1975 Nov;12(5):1029–1036. doi: 10.1128/iai.12.5.1029-1036.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy P. H., Jr, Graham D. J., Nell E. E., Dannenberg A. M., Jr Macrophages in immunity to syphilis: suppressive effect of concurrent infection with Mycobacterium bovis BCG on the development of syphilitic lesions and growth of Treponema pallidum in tuberculin-positive rabbits. Infect Immun. 1979 Nov;26(2):751–763. doi: 10.1128/iai.26.2.751-763.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein J. R., Collector M. I., Hardy P. H., Jr, Monjan A. A. In vitro antigen-specific response of spleen cells from Treponema pallidum-infected mice. J Immunol. 1980 Jul;125(1):459–460. [PubMed] [Google Scholar]
- Lukehart S. A., Baker-Zander S. A., Sell S. Characterization of lymphocyte responsiveness in early experimental syphilis. I. In vitro response to mitogens and Treponema pallidum antigens. J Immunol. 1980 Jan;124(1):454–460. [PubMed] [Google Scholar]
- Lukehart S. A., Miller J. N. Demonstration of the in vitro phagocytosis of Treponema pallidum by rabbit peritoneal macrophages. J Immunol. 1978 Nov;121(5):2014–2024. [PubMed] [Google Scholar]
- Metzger M., Podwińska J., Smogór W. The protective function of cell-mediated immunity in syphilis. Arch Immunol Ther Exp (Warsz) 1978;26(1-6):641–645. [PubMed] [Google Scholar]
- Mottram P. L., Miller J. F. Delayed-type hypersensitivity induced by antigen-pulsed, bone marrow-derived macrophages. Eur J Immunol. 1980 Mar;10(3):165–170. doi: 10.1002/eji.1830100302. [DOI] [PubMed] [Google Scholar]
- Musher D. M., Izzat N. N., Min K. W., Györkey F. In vitro phagocytosis of avirulent T. pallidum by rabbit macrophages. Acta Derm Venereol. 1972;52(5):349–352. [PubMed] [Google Scholar]
- Phillips J. T., Duncan W. R., Streilein J. W. The biochemical characterization of Syrian hamster cell-surface alloantigens. II. Immunochemical relationships between cell-surface alloantigens and class II MHC homologues. Immunogenetics. 1981 Mar 1;12(5-6):485–496. doi: 10.1007/BF01561690. [DOI] [PubMed] [Google Scholar]
- Phillips J. T., Streilein J. W., Proia D. A., Duncan W. R. Immunochemical characterization of Syrian hamster major histocompatibility complex homologues. Adv Exp Med Biol. 1981;134:69–85. doi: 10.1007/978-1-4757-0495-2_7. [DOI] [PubMed] [Google Scholar]
- Schell R. F., Chan J. K., LeFrock J. L., Bagasra O. Endemic syphilis: transfer of resistance to Treponema pallidum strain Bosnia A in hamsters with a cell suspension enriched in thymus-derived cells. J Infect Dis. 1980 Jun;141(6):752–758. doi: 10.1093/infdis/141.6.752. [DOI] [PubMed] [Google Scholar]
- Schell R. F., LeFrock J. L., Chan J. K., Bagasra O. LSH hamster model of syphilitic infection and transfer of resistance with immune T cells. Adv Exp Med Biol. 1981;134:291–300. doi: 10.1007/978-1-4757-0495-2_26. [DOI] [PubMed] [Google Scholar]
- Schell R. F., LeFrock J. L., Chan J. K., Bagasra O. LSH hamster model of syphilitic infection. Infect Immun. 1980 Jun;28(3):909–913. doi: 10.1128/iai.28.3.909-913.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler K., Unanue E. R. Identification of a macrophage antigen-processing event required for I-region-restricted antigen presentation to T lymphocytes. J Immunol. 1981 Nov;127(5):1869–1875. [PubMed] [Google Scholar]


