Abstract
Menopausal women often initiate hormone treatment to alleviate the symptoms of menopause. Research suggests that these treatments also affect cognition, and studies in young animals indicate that hormone treatment can alter several neuroanatomical measures. However, very little is known about the effects of long-term hormone treatment on the aging female brain. This study investigated the effects of hormone treatment on neuron number and tyrosine hydroxylase (TH) in the rat medial prefrontal cortex (mPFC). Female Long Evans rats were ovariectomized at middle age (12–13 months) and placed in one of four groups: no replacement (NR) (n = 12), 17β-estradiol (E2) (n = 12), E2 and progesterone (n = 7), or E2 and medroxyprogesterone acetate (MPA) (n = 10). Animals were euthanized at 20 months, and the brains were Nissl stained; a subset was immunostained for TH [NR (n = 5); E2 (n = 6); E2 + MPA (n = 4); E2 + progesterone (n = 6)]. E2 was administered through the drinking water, and progestagens were administered via pellets inserted at the nape of the neck. Neuron number and TH fiber density were quantified in the mPFC. Hormone treatment did not alter neuron number. Treatment with E2 and MPA resulted in greater TH densities than NR in layer 1 (P < 0.05). In layers 2/3, animals receiving E2 had greater TH densities than NR animals (P < 0.01). These results indicate that long-term hormone treatments alter dopaminergic fibers and potentially the functioning of the aging mPFC.
Aging females experience a dramatic decrease in ovarian hormones at the onset of menopause, and many women initiate hormone treatments consisting of estrogen [e.g. 17β-estradiol (E2) or conjugated equine estrogens] or estrogen combined with a progestin [e.g. medroxyprogesterone acetate (MPA)] to alleviate the symptoms associated with menopause. The presence of these hormones may alter the course of aging. Indeed, women using estrogen therapy during menopause have a greater gray matter density in the superior frontal gyrus than nonusers (1), and nonusers have lower gray matter concentration in orbitofrontal cortices than both estrogen users and young women (2). However, the Women's Health Initiative found that hormone replacement results in an increased risk of stroke and dementia (3–5), but the timing of hormone replacement initiation may explain these negative findings (6–8).
The effects of hormone treatment on the prefrontal cortex are especially important given the changes that are occurring in this brain region during human aging. The prefrontal cortex has been identified as a region that has greater decline in gray matter volume during aging than other brain areas (9, 10). Decreases in synaptic density, spine density, and dendritic arborization have also been found in the aged human frontal cortex (11–14) and the medial prefrontal cortex (mPFC) of aged rats (15–17). In addition, a loss of neurons has been observed in the aging human cortex overall (18) and in the prefrontal cortex (PFC) during aging in nonhuman primates and rodents (19, 20). However, there is evidence from our laboratory that this loss is sexually dimorphic with males, but not females, losing neurons during aging in the mPFC (20). The presence of low levels of ovarian hormones in rats after the cessation of the estrous cycle (21) may protect females from this age-related neuron loss, thus providing a possible mechanism by which hormone treatment decreases shrinkage associated with aging.
Ovarian hormones are known to alter several neuroanatomical measures including synapse number and spine density in the prefrontal cortex of young (22, 23) and aged rhesus monkeys (24). In addition, a recent study from our laboratory found that long-term treatment with E2 in combination with MPA during aging resulted in a greater number of synapses than no replacement (NR) animals in the mPFC of female rats (25). This alteration in synapse number could result from several different cellular changes including preservation of neurotransmitter functioning. Dopamine is of particular interest because changes occur in this system during aging in the PFC. For example, dopamine receptors decrease during aging in humans, nonhuman primates, and rodents (26–29), with the fastest rate of decline commonly found in the frontal cortex (30–32). Dopaminergic functioning in the PFC is also altered by ovarian hormones. In intact animals, levels of dopamine fluctuate across the estrous cycle in the prefrontal cortex (33). Furthermore, acute E2 and an estrogen receptor β agonist increased dopamine metabolites in the PFC of young adult rats (34, 35), and tyrosine hydroxylase (TH) immunoreactivity in the PFC of adult female monkeys is decreased after ovariectomy and restored with treatment of E2 administered with progesterone (P) (36, 37). However the effects of ovarian hormones during aging on this neurotransmitter system have not been thoroughly investigated, and it is possible that effects of hormone treatment may be different in young and aged animals. In the present study, different combinations of long-term hormone treatments were administered to aging female rats to determine the effects on both neuron number and dopaminergic fibers.
Materials and Methods
Subjects
Subjects were female Long Evans hooded rats purchased from Charles River Laboratories (Wilmington, MA) as retired breeders at the age of 11–12 months. Due to limited availability from the supplier, animals were run in three experimental cohorts. Animals from all cohorts were included in the analysis of neuron number (n = 41), and animals from cohorts 1 and 2 were included in the analysis of TH density (n = 21). Animals from the same group were pair- or triple housed, in clear cages on a 12-h light, 12-h dark cycle. Standard rodent chow (8604; Harlan Teklad, Placentia, CA) and water were available ad libitum to all animals, except during behavioral procedures (38) when the animals were maintained at 85–90% of their normal body weight. Subsets of these animals were tested in two behavioral studies (38, 39), and tissue from some of these animals was used for another neuroanatomical study (25). Rats were killed at least 2 wk after behavioral testing so that weight would be normalized and to minimize potential neural effects of training. All rats were handled, checked for health problems (tumors), and weighed weekly. Only healthy animals were retained for the study. Both body and uterine weight were measured at time of death. Animal care and experimental procedures were in accordance with National Institutes of Health guidelines and were approved by the Institutional Animal Care and Use Committee.
Hormone treatment
Subjects were ovariectomized at 12–13 months under isoflurane anesthesia. Hormone administration was initiated the day of surgery and continued until euthanasia. Animals were randomly placed in one of the following four groups: NR (n = 12), E2 (n = 12), E2 + P, (n = 7), or E2 and MPA (E2 + MPA) (n = 10).
E2 administration
As in the study of Chisholm and Juraska (38) all groups receiving E2 were given E2 in their drinking water. E2 was first dissolved in 95% ethanol (2 mg/ml) and then dissolved in water as described by Gordon et al. (40). A pilot study found that an E2 dose of 47 μg/kg/d resulted in estrogen levels in the physiological range for this age group (25–30 pg/ml) (16, 41). Water consumption was measured weekly for each cage and remained between 60–80 ml/kg/d throughout the experiment for all groups. This resulted in E2 doses between 40–55 μg/kg/d. The dose of E2 was calculated by taking the amount of water consumed by a cage and dividing by the sum of the weights in that cage. This value was then multiplied by the E2 concentration in the water.
Progestogen treatment
At the time of ovariectomy, one hormone pellet of either P or MPA was inserted in the nape of the neck in the appropriate groups. P capsules were 40 mm in length and made from SILASTIC tubing (Dow Corning, Midland, MI) packed with crystalline hormone. Studies have shown that 40-mm implants produce hormone levels between those found in aging female rats in persistent estrus and persistent diestrus (42). MPA pellets (1.5 mg) were purchased from Innovative Research of America (Sarasota, FL). The 1.5-mg 90-d release pellets result in a dose similar to that in women taking 2.5 mg/d when expected daily release and average body weight are factored in. P and MPA pellets were replaced every 90 d and all other groups received sham surgeries at the time of pellet replacement.
Histology
At approximately 20 months, after 8 months of hormone treatment, rats in cohorts 1 and 2 (n = 21) were deeply anesthetized with sodium pentobarbital (2 mg/kg of a 50 mg/ml solution) and perfused intracardially with PBS followed with a solution of 4% paraformaldehyde, 4% sucrose, and 1.4% sodium cacodylate in dH2O. The brains were removed and stored in the same solution for 24 h. Brains were then transferred to a sodium cacodylate buffer solution and shipped at room temperature to Neuroscience Associates (Knoxville, TN) for sectioning. Unstained 30-μm sections were returned to our laboratory where they were stained with methylene blue/azure II, a cell body stain, and for TH.
The third cohort of animals (n = 20) were only used for neuron counts and were processed according to the following methods. All rats were deeply anesthetized with sodium pentobarbital (2 mg/kg of a 50 mg/ml solution) and intracardially perfused with Ringer's wash (2 min) followed by a solution of 4% paraformaldehyde in 0.1 m phosphate buffer. Brains were removed and stored in a solution of 4% paraformaldehyde in 0.1 m phosphate buffer for 21 d, followed by cryoprotection in 30% sucrose for 3 d. Brains were coronally sectioned, and 60-μm thick sections were mounted and then stained with methylene blue/azure II.
Immunohistochemistry
Sections from cohorts 1 and 2 [NR (n = 5); E2 (n = 6); E2 + MPA (n = 4); E2 + P (n = 6)] were immunoreacted according to procedures found in Adler et al. (43) with a few modifications. Sections were rinsed in 0.1 m PBS, pH 7.4 (3 × 15 min), incubated in 1% H2O2 in PBS for 30 min. Sections were then rinsed in Tris-buffered saline (TBS 3 × 15 min), pH 7.4, placed in blocking solution (TBS containing 10% normal swine serum) for 2 h, and incubated in primary antiserum (diluted in TBS containing 1% normal swine serum, 2 d, 4 C). Anti-TH antibodies (Chemicon International, Temecula, CA) were used at a working dilution of 1:1000. After incubation in primary antibody, sections were rinsed in TBS, placed in biotinylated secondary antibodies (Vector Laboratories, Burlingame, CA; 2 h room temperature; 1:100), rinsed in TBS, and then incubated in avidin-biotin-complexed horseradish peroxidase for 2 h at room temperature (Vector Laboratories). Sections were then rinsed in TBS, pH 7.4, and reacted by using Sigma Fast Tabs (Sigma, Saint Louis, MO). Sections were immediately mounted and allowed to dry overnight. The following day, slides were dehydrated and coverslipped.
Volume estimation
Using cytoarchitectonic criteria (44, 45) the ventral mPFC [prelimbic (PL) and infralimbic (IL)] regions were parcellated at 31.25× using a camera lucida on coded slides stained with methylene blue/azure II. The ventral mPFC was parcellated starting with the first section containing white matter continuing through the first section in which the genu of the corpus callosum appeared. This resulted in parcellation of both hemispheres in four to five sections per subject. Parcellation criteria used for the ventral mPFC have been described by Markham et al. (46). Layers 2/3 and 5/6 were measured separately (rat mPFC lacks layer 4). Camera lucida tracings were scanned into a computer, and Image J (version 1.44, 2010) was used to measure the area of each parcellation. The volume was then calculated by multiplying this area by the mounted tissue thickness between sections. Mounted tissue thickness was measured by determining the difference between the focal depth of the top and bottom of the tissue using the StereoInvestigator software program (MicroBrightField, Chicago, IL). An average section thickness was calculated per animal and used in the calculations for that animal.
Neuron number estimation
Neurons were quantified in the PL and IL of the mPFC using the StereoInvestigator software program (MicroBrightField). The optical disector was used to obtain stereologically unbiased counts of cell density in each layer of the mPFC. Using this program, contours were drawn of layers 2/3 and layers 5/6 in the ventral mPFC, and at least 200 neurons were counted within each layer (2/3, 5/6) for each subject. Section thickness was measured at every fourth site on counted sections. Only neurons fully inside the counting frame or those that contacted the inclusion line without contacting the exclusion line were included in counts. Average counts for each layer were divided by the volume of the counting frame to calculate the cell density. This number was then multiplied by the volume to obtain cell number.
Fiber density and volume
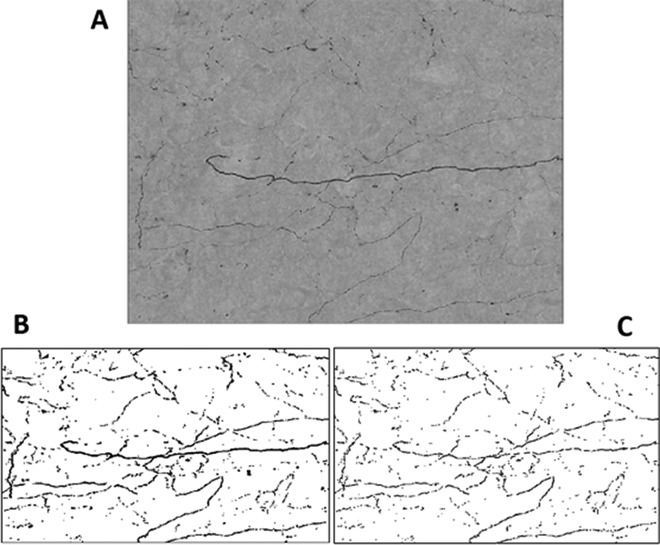
Examination of TH immunoreactive fibers was carried out in two PFC-containing sections (300 μm apart) of each brain. Z-stacked images were acquired using a Zeiss Axiovert 200M fluorescence microscope (Carl Zeiss, Thornwood, NY) and compressed using Axiovision software (Fig. 1A). Within each layer of the PFC (1, 2/3, and 5/6), three pictures were taken (two in the PL one in the IL). A total of 18 pictures were taken per animal (nine per section).
Fig. 1.
A, TH immunostained fibers in a Z-stacked image compressed with Axiovision software. Image pixel density (the percent of the image in black) was measured in two ways: first t as a binary image (B), taking thickness into account, and as a skeletonized image (C), reducing the thickness to 1 pixel wide.
Image J was used to measure image pixel density (the percent of the image in black) in two ways: first as a binary image, taking thickness into account (Fig. 1B), and then as a skeletonized image, reducing the thickness to 1 pixel wide (Fig. 1C). Skletonized images were analyzed because previous studies that have quantified TH fibers used this type of image (36); binary images were quantified to determine whether fiber thickness might be altered. In addition, the binary images were mulitplied by the volume of the mPFC for each animal to test whether the total volume of TH fiber innervation in the mPFC differed between groups.
Statistical analysis
All data analysis was performed on arithmetic means, and they were normally distributed. Body and uterine weights were analyzed using a one-way ANOVA with cohort as a covariate. Neuron number was analyzed using a one-way ANOVA with cohort as a covariate for each layer separately and then with all layers combined for total neuron number in the mPFC. The total volume of TH immunoreactive fibers was analyzed for layer 1 and layers 2/3 separately, as well as combined using a one-way ANOVA with cohort as a covariate. The total pixel density percentage in both binary and skeletonized images was analyzed in layers 1 and layers 2/3 separately using a one-way ANOVA. TH fibers were not analyzed in layers 5/6 due to the dark background in the images acquired. To correct for multiple comparisons, Tukey's Highly Significant Difference tests were used for all post hoc comparisons. A post hoc power analysis (R software) was conducted by using the values from the present study.
Results
Body and uterine weights
The analysis of body weight from all animals resulted in a significant effect of hormone treatment [F (3, 36) = 9.897; P < 0.01]. Post hoc tests revealed that the NR group weighed significantly more than all groups that received hormone treatment (E2: P < 0.01; E2 + P: P < 0.01; E2 + MPA: P < 0.01). No other comparisons reached significance (Table 1). Analysis of animals that were used only for TH analysis also resulted in a significant effect of treatment on body weight [F (3, 16) = 4.421; P < 0.02] (E2: P < 0.05; E2 + P: P < 0.05; E2 + MPA: P < 0.05).
Table 1.
Mean body and uterine weight for all subjects
| Hormone group | Mean body weight (g) | Mean uterine weight (g) |
|---|---|---|
| NR | 585.2 ± 21.9 | 0.09 ± 0.01 |
| E2 | 454.1 ± 27.6a | .16 ± 0.02a |
| E2 and P | 458.4 ± 28.3a | .12 ± 0.01a |
| E2 and MPA | 404.7 ± 28.3a | .16 ± 0.02a |
Body and uterine weights were taken at time of death for all groups. NR animals weighed significantly more and had lower uterine weights that all hormone-treated groups.
P < 0.01
The analysis of uterine weight from all animals revealed a significant effect of hormone treatment [F (3, 36) = 11.197; P < 0.01]. Post hoc revealed that uterine weight in the NR group was significantly lower than all groups that received hormone treatment, indicating that hormone treatment was physiologically effective (E2: P < 0.01; E2 + P: P < 0.01; E2 + MPA: P < 0.01) (Table 1). There was also a significant effect of treatment on uterine weight in animals that were used only for TH [F (3, 16) = 5.623, P < 0.01] (E2: P < 0.03; E2 + P: P < 0.01; E2 + MPA: P < 0.01).
Neuron number and volume
Hormone treatment did not significantly alter volume or neuron number in any layer or in all layers combined of the mPFC (Fig. 2).
Fig. 2.
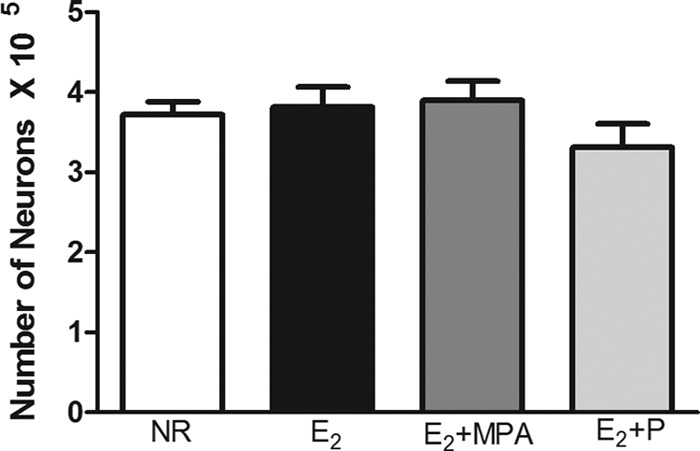
The total number of neurons in the mPFC (mean ± sem). There was not a significant effect of hormone treatment on neuron number.
Tyrosine hydroxylase
Total volume in the upper layers of the mPFC
There was an effect of hormone treatment on the total volume of TH fibers [F (3, 16) = 3.524; P < 0.04]. Post hoc tests found that animals receiving E2+MPA had a higher volume of TH immunoreactive fibers than those receiving NR (P < 0.04) (Fig. 3A).
Fig. 3.
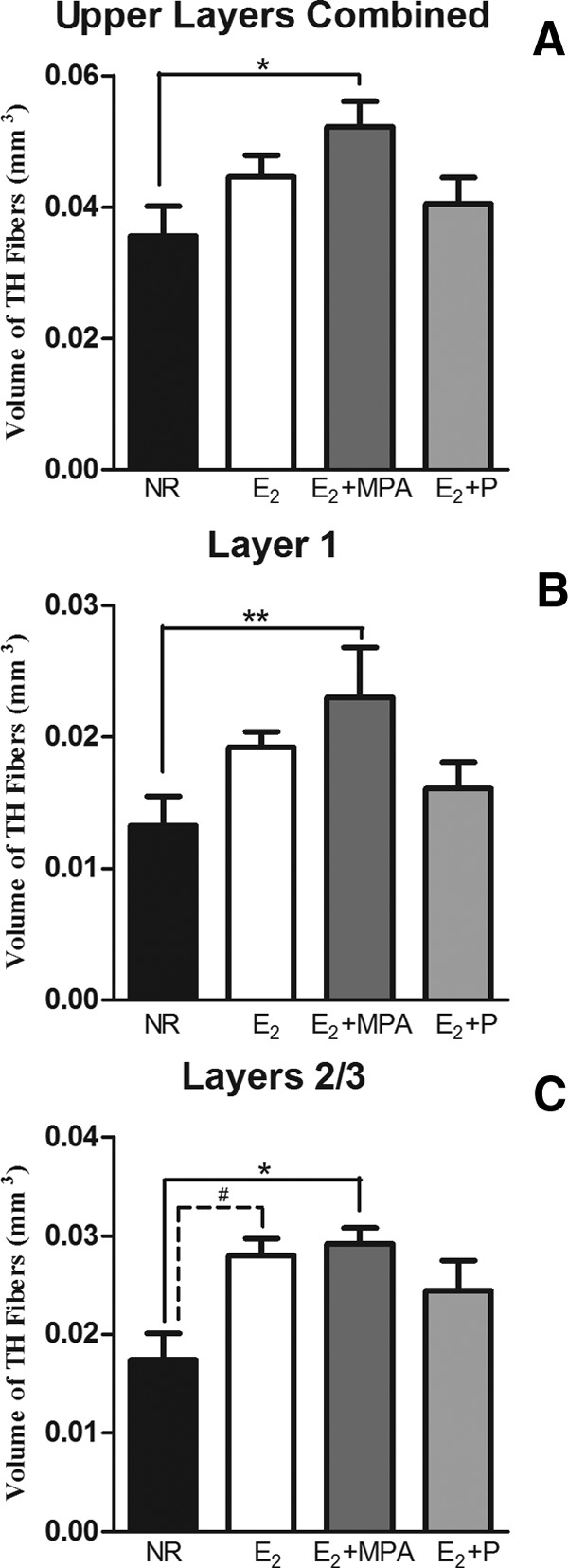
The volume of TH fibers (mean ± sem) within the mPFC. In the upper layers combined (A), layer 1 (B), and layers 2/3 (C) there was a main effect of hormone treatment (P < 0.04 for each analysis). **, P < 0.02; *, P < 0.05; #, P < 0.06.
Layer 1
There was an effect of hormone treatment on the volume of TH fibers in layer 1 [F (3, 16) = 4.286; P < 0.03]. Post hoc tests showed that animals receiving E2+MPA had a higher volume of TH immunoreactive fibers than those receiving NR (P < 0.02) (Fig. 3B). Analysis of binary images in layer 1 found that hormone treatment significantly altered the density of TH immunoreactive fibers [F (3, 17) = 3.903; P < 0.03] (Fig. 4A). Post hoc tests showed that animals receiving E2+MPA had a higher density of TH immunoreactive fibers than those receiving NR (P < 0.04). Analysis of the skeletonized images revealed a weak trend toward a similar pattern within layer 1 [F (3, 17) = 2.648; P < 0.09] (Fig. 4B).
Fig. 4.
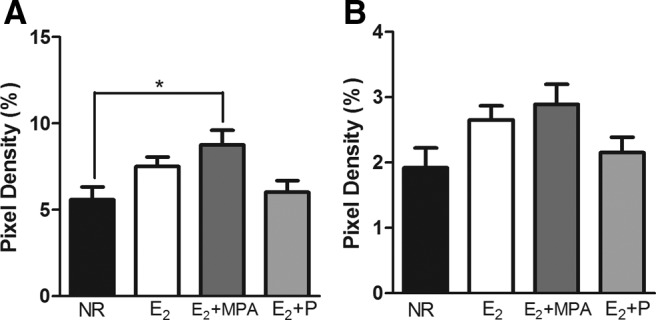
The density of TH fibers in layer 1 (mean ± sem). A, In binary images, there was an effect of hormone treatment (P < 0.03). B, In skeletonized images, there were no significant differences of hormone treatment. *, P < 0.05.
Layers 2/3
There was also a main effect of hormone treatment on the volume of TH fibers in layers 2/3 [F (3, 14) = 3.721; P < 0.04]. Post hoc tests found that animals receiving E2+MPA had significantly higher volumes of TH than NR animals (P < 0.05). There was a trend for animals receiving E2 to have a greater volume of TH than NR animals (P < 0.06) (Fig. 3C). Analysis of the binary images found that hormone treatment significantly altered the TH immunoreactive fiber density within layers 2/3 [F (3, 15) = 3.387; P < 0.05] (Fig. 5A). Post hoc tests demonstrated that animals receiving E2 had significantly higher TH fiber densities than NR animals (P < 0.04) (Fig. 5A). Analysis of skeletonized images revealed a significant effect of hormone treatment on pixel density percentage of TH immunoreactive fibers in layers 2/3 [F (3, 15) = 6.496; P < 0.01] (Fig. 5B). Post hoc tests demonstrated that animals receiving E2 had significantly higher TH fiber densities than NR animals (P < 0.01). In addition, there was a weak trend for the E2 +MPA group to have a higher pixel density than NR animals (P = 0.08).
Fig. 5.
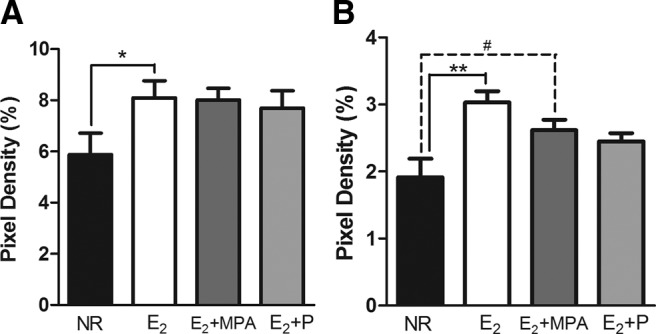
The density of TH fibers in layers 2/3 (mean ± sem). In binary images (A) there was an effect of hormone treatment (P < 0.05). In skeletonized images (B), there was an effect of hormone treatment (P < 0.01). **, P < 0.02; *, P < 0.05; #, P = 0.08.
Discussion
Long-term treatment of middle aged female rats with E2 in combination with MPA resulted in a greater volume of TH fibers in the mPFC compared with ovariectomized controls. There are also indications that the density of TH fibers was higher in the group given E2 alone. This is the first study to evaluate the effects of long-term estrogen treatment, with or without MPA, on the prefrontal dopaminergic system during aging in females. However, these results are congruent with several studies in young animals that find that the prefrontal dopaminergic system is influenced by ovarian hormones (33, 34, 36, 37). Although no studies have looked at the prefrontal cortex during aging, the effects of acute estrogen treatment on the aged striatum have been examined. Acute estradiol benzoate increased basal dopamine levels in the striatum of both young and aged rats, whereas a similar treatment increased dopamine receptors in the striatum of young females, but not middle aged females (47, 48). The changes in the dopaminergic system found after long-term treatment with E2 and MPA in the current study are also consistent with the greater number of synapses found in a previous study from our laboratory after the same treatment (25), which suggests that at least some of those preserved synapses were dopaminergic. Because dopamine fibers are known to decrease with normal aging in the male PFC (49), it is most likely that this hormone treatment is preventing the normal loss associated with aging, rather than causing new fibers to innervate the prefrontal cortex.
Interestingly, E2 in combination with P did not result in a greater volume or density of TH fibers. This was not simply due to a lack of statistical power because a power analysis indicates that even at 20 subjects per group, the E2 with P group is not likely to differ from controls (it would require at least 65 animals per group). The finding of no effect from treatment with E2 with P is in agreement with our previous study, which found that long-term E2 in combination with MPA resulted in a greater number of synapses, whereas E2 with P failed to alter synapse number (25). MPA is a synthetic analog of P; however, these two progestagens do not share identical biological properties. For example, P is metabolized to allopregnanolone (50) whereas MPA inhibits the enzymes required for this conversion (51–53), and MPA has a higher affinity for androgen and glucocorticoid receptors than P (54). These two progestagens often result in differential neural outcomes although most of these studies indicate a beneficial effect of P on the measures evaluated (55). For example, P alone and in combination with estrogen protected against glutamate toxicity whereas MPA was not protective and prevented E2's influence on neuroprotection (56, 57). In addition, P protected against kainic acid-induced neuronal loss in vitro whereas MPA did not (58). Treatment with E2 and P, but not MPA, increased proliferation of neuroprogenitor cells in culture (59). Furthermore, MPA decreased levels of brain-derived neurotrophic factor whereas P increased this measure (60). MPA, but not progesterone, significantly decreased levels of glutamic acid decarboxylase in the hippocampus (61) and suppressed cytokine production after an inflammatory stimulus in vitro (62). Most of these studies have examined the effects of acute progestagen treatment, and the current study administered hormones for approximately 7 months to evaluate the long-term effects of hormone treatment. It has been found that chronic treatment of ovarian hormones results in different outcomes than more acute treatments (63–65). The long-term hormone treatment used in the current study may result in receptors that are less sensitive, and the two progestagens may result in differential receptor sensitivities after long-term exposure.
The lack of effect on the volume of TH fibers found after E2 treatment alone may be due to low statistical power, in contrast to the lack of effects from E2 with progesterone. Treatment with E2 resulted in a trend for a significant effect on the volume of TH fibers in layers 2/3, and a post hoc power analysis indicates that in order to achieve an 80% probability of detecting a statically significant difference, 16 subjects per group were needed. Thus, a relatively high number of subjects would probably reveal an effect of E2 treatment on TH. Still the potential effects of E2 are less robust than those of E2 in combination with MPA, which was significant with a comparatively low number of subjects.
It should be noted that the current results may not generalize to other doses of hormones. Both the E2 and the P dose used in the current study were based on levels found in aging female rats in persistent estrus and persistent diestrus (16, 21). However, because MPA is a synthetic compound, the same method for calculating dose could not be used. Rather, the dose for MPA was determined by using pellets that would results in levels of MPA similar to those in humans when expected daily release and average body weight were factored in. Thus nonphysiological doses of E2 and P as well as different doses of MPA could result in different effects.
The mPFC in rodents plays a role in higher-order cognitive behaviors such as working memory and behavioral flexibility (66–68). Many of these behaviors are impaired during aging. For example, aged rats showed decreased performance, compared with young rats, on object recognition (17) and delayed spatial alternation tasks (69, 70). There is evidence that performance on tasks mediated in part by the PFC is correlated with dopamine levels in the PFC of aged female rats (71) and TH fibers in the male mPFC (49). Therefore, the greater amount of TH found in the mPFC in the current study would be expected to result in improved performance on tasks mediated by the PFC. Indeed, our laboratory has shown previously that long-term treatment with E2 and MPA improved acquisition of the t-maze in aged female rats (38). However, when a subset of these animals was tested on the water maze, this same treatment impaired performance compared with all other hormone-treated groups (39). Interestingly, E2 treatment affects monoamine levels differently in the PFC and the hippocampus. E2 increased levels of monoamines in the PFC but decreased them in the hippocampus of young adult animals (34). The water maze is thought to be heavily mediated by the hippocampus, whereas several brain regions are important for t-maze performance including the PFC and striatum (72, 73). Although the current study did not examine TH densities in the hippocampus, it is possible that E2 in combination with MPA does not alter dopamine in the hippocampus, providing an explanation for the differential behavioral outcomes observed in our previous studies.
TH is the rate-limiting enzyme in the biosynthesis of catecholamines and therefore identifies both dopaminergic and noradrenergic fibers. However, there is evidence that suggests the changes found in the current study are primarily dopaminergic. Studies using dopamine β-hydroxylase, a marker for adrenergic and noradrenergic fibers, in the primate prefrontal cortex have found a low density of fibers that were large in diameter whereas TH staining was dense and labeled slender highly varicose axons (74, 75). There also appears to be distinct morphologies between DBH and TH fibers in rodents. When noradrenergic fibers were selectively depleted in the anterior cingulate of the rat, the remaining fibers were characterized as thin delicate fibers with many varicosities and presumed to be dopaminergic (76). The fibers that were observed in the current study were thin and highly varicose, more closely resembling those previously described as dopaminergic. Importantly, ovariectomy has been shown to increase dopamine β-hydroxylase-labeled fibers in the prefrontal cortex, and this was reversed after estrogen or estrogen with P treatment (36). Based on this, any staining of noradrenergic fibers in the current study would be expected to increase the number of fibers in our ovariectomized animals and therefore underestimate the effects of hormone treatment observed on TH fibers.
Long-term hormone treatment did not alter neuron number in the mPFC. Thus the preservation of the number of mPFC neurons in intact aging females compared with males observed by Yates et al. (20) is not accounted for by the hormone replacement regimen given here. These results may seem surprising due to the numerous studies that have demonstrated that estrogens are neuroprotective in young animals and in vitro (77). However, there is a growing body of research indicating that the adult brain and aged brain respond differently to hormone treatments. For example, estrogen treatment reduced cortical infarct volume in young adult animals but resulted in greater infarct volume middle-aged animals (78). A follow-up study replicated in these findings and found that estrogen treatment increases levels of IGF-1 in young animals, while decreasing levels in middle-aged animals (79). Therefore it is important that studies examining the effects of hormone treatments on problems associated with aging do so in an appropriate model. In addition, it is unknown how neuroprotective properties seen after extreme insult relate to normal aging.
This is the first study to find that long-term treatment with E2 alone or in combination with MPA alters the prefrontal dopaminergic system during aging. Because the deterioration of the dopaminergic system during normal aging is correlated with decreased performance on cognitive tasks, the results provide a possible mechanism by which hormone treatments could benefit cognition during aging.
Acknowledgments
We thank the Microscopy Suite at the Beckman Institute (University of Illinois) for their assistance with the stereology workstation.
This work was supported by National Institutes of Health Grant AG 022499.
Nioka C. Chisholm previously published as Nioka C. Lowry
Disclosure Summary: There is no conflict of interest for either author.
Footnotes
- E2
- 17β-Estradiol
- IL region
- infralimbic region
- MPA
- medroxyprogesterone acetate
- mPFC
- medial prefrontal cortex
- NR
- no replacement
- P
- progesterone
- PFC
- prefrontal cortex
- PL region
- prelimbic region
- TBS
- Tris-buffered saline
- TH
- tyrosine hydroxylase.
References
- 1. Lord C, Engert V, Lupien SJ, Pruessner JC. 2010. Effect of sex and estrogen therapy on the aging brain: a voxel-based morphometry study. Menopause 17:846–851 [DOI] [PubMed] [Google Scholar]
- 2. Robertson D, Craig M, van Amelsvoort T, Daly E, Moore C, Simmons A, Whitehead M, Morris R, Murphy D. 2009. Effects of estrogen therapy on age-related differences in gray matter concentration. Climacteric 12:301–309 [DOI] [PubMed] [Google Scholar]
- 3. Wassertheil-Smoller S, Hendrix SL, Limacher M, Heiss G, Kooperberg C, Baird A, Kotchen T, Curb JD, Black H, Rossouw JE, Aragaki A, Safford M, Stein E, Laowattana S, Mysiw WJ, WHI Investigators 2003. Effect of estrogen plus progestin on stroke in postmenopausal women: the Women's Health Initiative: a randomized trial. JAMA 289:2673–2684 [DOI] [PubMed] [Google Scholar]
- 4. Anderson GL, Limacher M, Assaf AR, Bassford T, Beresford SA, Black H, Bonds D, Brunner R, Brzyski R, Caan B, Chlebowski R, Curb D, Gass M, Hays J, Heiss G, Hendrix S, Howard BV, Hsia J, Hubbell A, Jackson R, Johnson KC, Judd H, Kotchen JM, Kuller L, LaCroix AZ, et al. 2004. Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: the Women's Health Initiative randomized controlled trial. JAMA 291:1701–1712 [DOI] [PubMed] [Google Scholar]
- 5. Shumaker SA, Legault C, Rapp SR, Thal L, Wallace RB, Ockene JK, Hendrix SL, Jones BN, III, Assaf AR, Jackson RD, Kotchen JM, Wassertheil-Smoller S, Wactawski-Wende J, WHIMS Investigators 2003. Estrogen plus progestin and the incidence of dementia and mild cognitive impairment in postmenopausal women: the Women's Health Initiative Memory Study: a randomized controlled trial. JAMA 289:2651–2662 [DOI] [PubMed] [Google Scholar]
- 6. Daniel JM, Bohacek J. 2010. The critical period hypothesis of estrogen effects on cognition: Insights from basic research. Biochim Biophys Acta 1800:1068–1076 [DOI] [PubMed] [Google Scholar]
- 7. Sherwin BB. 2009. Estrogen therapy: is time of initiation critical for neuroprotection? Nat Rev Endocrinol 5:620–627 [DOI] [PubMed] [Google Scholar]
- 8. Gibbs RB. 2000. Long-term treatment with estrogen and progesterone enhances acquisition of a spatial memory task by ovariectomized aged rats. Neurobiol Aging 21:107–116 [DOI] [PubMed] [Google Scholar]
- 9. Raz N, Lindenberger U, Rodrigue KM, Kennedy KM, Head D, Williamson A, Dahle C, Gerstorf D, Acker JD. 2005. Regional brain changes in aging healthy adults: general trends, individual differences and modifiers. Cereb Cortex 15:1676–1689 [DOI] [PubMed] [Google Scholar]
- 10. Resnick SM, Pham DL, Kraut MA, Zonderman AB, Davatzikos C. 2003. Longitudinal magnetic resonance imaging studies of older adults: a shrinking brain. J Neurosci 23:3295–3301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. de Brabander JM, Kramers RJ, Uylings HB. 1998. Layer-specific dendritic regression of pyramidal cells with ageing in the human prefrontal cortex. Eur J Neurosci 10:1261–1269 [DOI] [PubMed] [Google Scholar]
- 12. Huttenlocher PR. 1979. Synaptic density in human frontal cortex—developmental changes and effects of aging. Brain Res 163:195–205 [DOI] [PubMed] [Google Scholar]
- 13. Jacobs B, Driscoll L, Schall M. 1997. Life-span dendritic and spine changes in areas 10 and 18 of human cortex: a quantitative Golgi study. J Comp Neurol 386:661–680 [PubMed] [Google Scholar]
- 14. Masliah E, Mallory M, Hansen L, DeTeresa R, Terry RD. 1993. Quantitative synaptic alterations in the human neocortex during normal aging. Neurology 43:192–197 [DOI] [PubMed] [Google Scholar]
- 15. Grill JD, Riddle DR. 2002. Age-related and laminar-specific dendritic changes in the medial frontal cortex of the rat. Brain Res 937:8–21 [DOI] [PubMed] [Google Scholar]
- 16. Markham JA, Juraska JM. 2002. Aging and sex influence the anatomy of the rat anterior cingulate cortex. Neurobiol Aging 23:579–588 [DOI] [PubMed] [Google Scholar]
- 17. Wallace M, Frankfurt M, Arellanos A, Inagaki T, Luine V. 2007. Impaired recognition memory and decreased prefrontal cortex spine density in aged female rats. Ann NY Acad Sci 1097:54–57 [DOI] [PubMed] [Google Scholar]
- 18. Pakkenberg B, Gundersen HJ. 1997. Neocortical neuron number in humans: effect of sex and age. J Comp Neurol 384:312–320 [PubMed] [Google Scholar]
- 19. Smith DE, Rapp PR, McKay HM, Roberts JA, Tuszynski MH. 2004. Memory impairment in aged primates is associated with focal death of cortical neurons and atrophy of subcortical neurons. J Neurosci 24:4373–4381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Yates MA, Markham JA, Anderson SE, Morris JR, Juraska JM. 2008. Regional variability in age-related loss of neurons from the primary visual cortex and medial prefrontal cortex of male and female rats. Brain Res 1218:1–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Dudley SD. 1982. Responsiveness to estradiol in central nervous system of aging female rats. Neurosci Biobehav Rev 6:39–45 [DOI] [PubMed] [Google Scholar]
- 22. Leranth C, Hajszan T, Szigeti-Buck K, Bober J, MacLusky NJ. 2008. Bisphenol A prevents the synaptogenic response to estradiol in hippocampus and prefrontal cortex of ovariectomized nonhuman primates. Proc Natl Acad Sci USA 105:14187–14191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Tang Y, Janssen WG, Hao J, Roberts JA, McKay H, Lasley B, Allen PB, Greengard P, Rapp PR, Kordower JH, Hof PR, Morrison JH. 2004. Estrogen replacement increases spinophilin-immunoreactive spine number in the prefrontal cortex of female rhesus monkeys. Cereb Cortex 14:215–223 [DOI] [PubMed] [Google Scholar]
- 24. Hao J, Rapp PR, Leffler AE, Leffler SR, Janssen WG, Lou W, McKay H, Roberts JA, Wearne SL, Hof PR, Morrison JH. 2006. Estrogen alters spine number and morphology in prefrontal cortex of aged female rhesus monkeys. J Neurosci 26:2571–2578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Chisholm NC, Juraska JM. 2012. Effects of long-term treatment with estrogen and medroxyprogesterone acetate on synapse number in the medial prefrontal cortex of aged female rats. Menopause 19:804–811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. de Keyser J, De Backer JP, Vauquelin G, Ebinger G. 1990. The effect of aging on the D1 dopamine receptors in human frontal cortex. Brain Res 528:308–310 [DOI] [PubMed] [Google Scholar]
- 27. Seeman P, Bzowej NH, Guan HC, Bergeron C, Becker LE, Reynolds GP, Bird ED, Riederer P, Jellinger K, Watanabe S. 1987. Human brain dopamine receptors in children and aging adults. Synapse 1:399–404 [DOI] [PubMed] [Google Scholar]
- 28. Gozlan H, Daval G, Verge D, Spampinato U, Fattaccini CM, Gallissot MC, el Mestikawy S, Hamon M. 1990. Aging associated changes in serotoninergic and dopaminergic pre- and postsynaptic neurochemical markers in the rat brain. Neurobiol Aging 11:437–449 [DOI] [PubMed] [Google Scholar]
- 29. Lai H, Bowden DM, Horita A. 1987. Age-related decreases in dopamine receptors in the caudate nucleus and putamen of the rhesus monkey (Macaca mulatta). Neurobiol Aging 8:45–49 [DOI] [PubMed] [Google Scholar]
- 30. Kaasinen V, Kemppainen N, Någren K, Helenius H, Kurki T, Rinne JO. 2002. Age-related loss of extrastriatal dopamine D(2) -like receptors in women. J Neurochem 81:1005–1010 [DOI] [PubMed] [Google Scholar]
- 31. Kaasinen V, Vilkman H, Hietala J, Någren K, Helenius H, Olsson H, Farde L, Rinne J. 2000. Age-related dopamine D2/D3 receptor loss in extrastriatal regions of the human brain. Neurobiol Aging 21:683–688 [DOI] [PubMed] [Google Scholar]
- 32. Inoue M, Suhara T, Sudo Y, Okubo Y, Yasuno F, Kishimoto T, Yoshikawa K, Tanada S. 2001. Age-related reduction of extrastriatal dopamine D2 receptor measured by PET. Life Sci 69:1079–1084 [DOI] [PubMed] [Google Scholar]
- 33. Dazzi L, Seu E, Cherchi G, Barbieri PP, Matzeu A, Biggio G. 2007. Estrous cycle-dependent changes in basal and ethanol-induced activity of cortical dopaminergic neurons in the rat. Neuropsychopharmacology 32:892–901 [DOI] [PubMed] [Google Scholar]
- 34. Inagaki T, Gautreaux C, Luine V. 2010. Acute estrogen treatment facilitates recognition memory consolidation and alters monoamine levels in memory-related brain areas. Horm Behav 58:415–426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Jacome LF, Gautreaux C, Inagaki T, Mohan G, Alves S, Lubbers LS, Luine V. 2010. Estradiol and ERβ agonists enhance recognition memory, and DPN, an ERβ agonist, alters brain monoamines. Neurobiol Learn Mem 94:488–498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kritzer MF, Kohama SG. 1999. Ovarian hormones differentially influence immunoreactivity for dopamine β-hydroxylase, choline acetyltransferase, and serotonin in the dorsolateral prefrontal cortex of adult rhesus monkeys. J Comp Neurol 409:438–451 [DOI] [PubMed] [Google Scholar]
- 37. Kritzer MF, Kohama SG. 1998. Ovarian hormones influence the morphology, distribution, and density of tyrosine hydroxylase immunoreactive axons in the dorsolateral prefrontal cortex of adult rhesus monkeys. J Comp Neurol 395:1–17 [PubMed] [Google Scholar]
- 38. Chisholm NC, Juraska JM. 2012. Long-term replacement of estrogen in combination with medroxyprogesterone acetate improves acquisition of an alternation task in middle-aged female rats. Behav Neurosci 126:128–136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Lowry NC, Pardon LP, Yates MA, Juraska JM. 2010. Effects of long-term treatment with 17β-estradiol and medroxyprogesterone acetate on water maze performance in middle aged female rats. Horm Behav 58:200–207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Gordon MN, Osterburg HH, May PC, Finch CE. 1986. Effective oral administration of 17β-estradiol to female C57BL/6J mice through the drinking water. Biol Reprod 35:1088–1095 [DOI] [PubMed] [Google Scholar]
- 41. Warren SG, Juraska JM. 2000. Sex differences and estropausal phase effects on water maze performance in aged rats. Neurobiol Learn Mem 74:229–240 [DOI] [PubMed] [Google Scholar]
- 42. Liu JW, Dawson DD, Peters CE, Baker MA, Walker AM. 1997. Estrogen replacement in ovariectomized rats results in physiologically significant levels of circulating progesterone, and co-administration of progesterone markedly reduces the circulating estrogen. Endocrine 6:125–131 [DOI] [PubMed] [Google Scholar]
- 43. Adler A, Vescovo P, Robinson JK, Kritzer MF. 1999. Gonadectomy in adult life increases tyrosine hydroxylase immunoreactivity in the prefrontal cortex and decreases open field activity in male rats. Neuroscience 89:939–954 [DOI] [PubMed] [Google Scholar]
- 44. Krettek JE, Price JL. 1977. The cortical projections of the mediodorsal nucleus and adjacent thalamic nuclei in the rat. J Comp Neurol 171:157–191 [DOI] [PubMed] [Google Scholar]
- 45. Van Eden CG, Uylings HB. 1985. Cytoarchitectonic development of the prefrontal cortex in the rat. J Comp Neurol 241:253–267 [DOI] [PubMed] [Google Scholar]
- 46. Markham JA, Morris JR, Juraska JM. 2007. Neuron number decreases in the rat ventral, but not dorsal, medial prefrontal cortex between adolescence and adulthood. Neuroscience 144:961–968 [DOI] [PubMed] [Google Scholar]
- 47. McDermott JL. 1993. Effects of estrogen upon dopamine release from the corpus striatum of young and aged female rats. Brain Res 606:118–125 [DOI] [PubMed] [Google Scholar]
- 48. Roy EJ, Sheinkop S, Wilson MA. 1982. Age alters dopaminergic responses to estradiol. Eur J Pharmacol 82:73–75 [DOI] [PubMed] [Google Scholar]
- 49. Mizoguchi K, Shoji H, Tanaka Y, Maruyama W, Tabira T. 2009. Age-related spatial working memory impairment is caused by prefrontal cortical dopaminergic dysfunction in rats. Neuroscience 162:1192–1201 [DOI] [PubMed] [Google Scholar]
- 50. Majewska MD, Harrison NL, Schwartz RD, Barker JL, Paul SM. 1986. Steroid hormone metabolites are barbiturate-like modulators of the GABA receptor. Science 232:1004–1007 [DOI] [PubMed] [Google Scholar]
- 51. Jarrell J. 1984. Studies on the developmental pattern of rat ovarian 3 α-hydroxysteroid dehydrogenase: inhibition of the postpubertal activity with medroxyprogesterone acetate in vivo. J Steroid Biochem 21:151–156 [DOI] [PubMed] [Google Scholar]
- 52. Lee TC, Miller WL, Auchus RJ. 1999. Medroxyprogesterone acetate and dexamethasone are competitive inhibitors of different human steroidogenic enzymes. J Clin Endocrinol Metab 84:2104–2110 [DOI] [PubMed] [Google Scholar]
- 53. Penning TM, Sharp RB, Krieger NR. 1985. Purification and properties of 3α-hydroxysteroid dehydrogenase from rat brain cytosol. Inhibition by nonsteroidal anti-inflammatory drugs and progestins. J Biol Chem 260:15266–15272 [PubMed] [Google Scholar]
- 54. Bamberger CM, Schulte HM. 2000. Molecular mechanisms of dissociative glucocorticoid activity. Eur J Clin Invest 30(Suppl 3):6–9 [DOI] [PubMed] [Google Scholar]
- 55. Singh M. 2006. Progesterone-induced neuroprotection. Endocrine 29:271–274 [DOI] [PubMed] [Google Scholar]
- 56. Nilsen J, Brinton RD. 2003. Divergent impact of progesterone and medroxyprogesterone acetate (Provera) on nuclear mitogen-activated protein kinase signaling. Proc Natl Acad Sci USA 100:10506–10511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Nilsen J, Brinton RD. 2002. Impact of progestins on estradiol potentiation of the glutamate calcium response. Neuroreport 13:825–830 [DOI] [PubMed] [Google Scholar]
- 58. Ciriza I, Carrero P, Frye CA, Garcia-Segura LM. 2006. Reduced metabolites mediate neuroprotective effects of progesterone in the adult rat hippocampus. The synthetic progestin medroxyprogesterone acetate (Provera) is not neuroprotective. J Neurobiol 66:916–928 [DOI] [PubMed] [Google Scholar]
- 59. Liu L, Zhao L, She H, Chen S, Wang JM, Wong C, McClure K, Sitruk-Ware R, Brinton RD. 2010. Clinically relevant progestins regulate neurogenic and neuroprotective responses in vitro and in vivo. Endocrinology 151:5782–5794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Jodhka PK, Kaur P, Underwood W, Lydon JP, Singh M. 2009. The differences in neuroprotective efficacy of progesterone and medroxyprogesterone acetate correlate with their effects on brain-derived neurotrophic factor expression. Endocrinology 150:3162–3168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Braden BB, Talboom JS, Crain ID, Simard AR, Lukas RJ, Prokai L, Scheldrup MR, Bowman BL, Bimonte-Nelson HA. 2010. Medroxyprogesterone acetate impairs memory and alters the GABAergic system in aged surgically menopausal rats. Neurobiol Learn Mem 93:444–453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Bamberger CM, Else T, Bamberger AM, Beil FU, Schulte HM. 1999. Dissociative glucocorticoid activity of medroxyprogesterone acetate in normal human lymphocytes. J Clin Endocrinol Metab 84:4055–4061 [DOI] [PubMed] [Google Scholar]
- 63. Pazol K, Northcutt KV, Patisaul HB, Wallen K, Wilson ME. 2009. Progesterone and medroxyprogesterone acetate differentially regulate α4 subunit expression of GABA(A) receptors in the CA1 hippocampus of female rats. Physiol Behav 97:58–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Gibbs RB. 1997. Effects of estrogen on basal forebrain cholinergic neurons vary as a function of dose and duration of treatment. Brain Res 757:10–16 [DOI] [PubMed] [Google Scholar]
- 65. Morissette M, Di Paolo T. 1993. Effect of chronic estradiol and progesterone treatments of ovariectomized rats on brain dopamine uptake sites. J Neurochem 60:1876–1883 [DOI] [PubMed] [Google Scholar]
- 66. Kolb B. 1990. Animal models for human PFC-related disorders. Prog Brain Res 85:501–519 [DOI] [PubMed] [Google Scholar]
- 67. Birrell JM, Brown VJ. 2000. Medial frontal cortex mediates perceptual attentional set shifting in the rat. J Neurosci 20:4320–4324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. De Bruin JP, Feenstra MG, Broersen LM, Van Leeuwen M, Arens C, De Vries S, Joosten RN. 2000. Role of the prefrontal cortex of the rat in learning and decision making: effects of transient inactivation. Prog Brain Res 126:103–113 [DOI] [PubMed] [Google Scholar]
- 69. Ando S, Ohashi Y. 1991. Longitudinal study on age-related changes of working and reference memory in the rat. Neurosci Lett 128:17–20 [DOI] [PubMed] [Google Scholar]
- 70. Tanila H, Taira T, Piepponen TP, Honkanen A. 1994. Effect of sex and age on brain monoamines and spatial learning in rats. Neurobiol Aging 15:733–741 [DOI] [PubMed] [Google Scholar]
- 71. Luine V, Bowling D, Hearns M. 1990. Spatial memory deficits in aged rats: contributions of monoaminergic systems. Brain Res 537:271–278 [DOI] [PubMed] [Google Scholar]
- 72. Redish AD, Touretzky DS. 1998. The role of the hippocampus in solving the Morris water maze. Neural Comput 10:73–111 [DOI] [PubMed] [Google Scholar]
- 73. Lalonde R. 2002. The neurobiological basis of spontaneous alternation. Neurosci Biobehav Rev 26:91–104 [DOI] [PubMed] [Google Scholar]
- 74. Lewis DA, Foote SL, Goldstein M, Morrison JH. 1988. The dopaminergic innervation of monkey prefrontal cortex: a tyrosine hydroxylase immunohistochemical study. Brain Res 449:225–243 [DOI] [PubMed] [Google Scholar]
- 75. Lewis DA, Morrison JH. 1989. Noradrenergic innervation of monkey prefrontal cortex: a dopamine-β-hydroxylase immunohistochemical study. J Comp Neurol 282:317–330 [DOI] [PubMed] [Google Scholar]
- 76. Berger B, Tassin JP, Blanc G, Moyne MA, Thierry AM. 1974. Histochemical confirmation for dopaminergic innervation of the rat cerebral cortex after destruction of the noradrenergic ascending pathways. Brain Res 81:332–337 [DOI] [PubMed] [Google Scholar]
- 77. Garcia-Segura LM, Azcoitia I, DonCarlos LL. 2001. Neuroprotection by estradiol. Prog Neurobiol 63:29–60 [DOI] [PubMed] [Google Scholar]
- 78. Selvamani A, Sohrabji F. 2010. Reproductive age modulates the impact of focal ischemia on the forebrain as well as the effects of estrogen treatment in female rats. Neurobiol Aging 31:1618–1628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Selvamani A, Sohrabji F. 2010. The neurotoxic effects of estrogen on ischemic stroke in older female rats is associated with age-dependent loss of insulin-like growth factor-1. J Neurosci 30:6852–6861 [DOI] [PMC free article] [PubMed] [Google Scholar]