Abstract
Neurokinin B (NKB), encoded by Tac2 in rodents, and its receptor, NK3R, have recently emerged as important regulators of reproduction; NKB has been proposed to stimulate kisspeptin output onto GnRH neurons. Accordingly, NKB has been shown to induce gonadotropin release in several species; yet, null or even inhibitory effects of NKB have been also reported. The basis for these discrepant findings, as well as other key aspects of NKB function, remains unknown. We report here that in the rat, LH responses to the NK3R agonist, senktide, display a salient sexual dimorphism, with persistent stimulation in females, regardless of the stage of postnatal development, and lack of LH responses in males from puberty onward. Such dimorphism was independent of the predominant sex steroid after puberty, because testosterone administration to adult females failed to prevent LH responses to senktide, and LH responsiveness was not restored in adult males treated with estradiol or the nonaromatizable androgen, dihydrotestosterone. Yet, removal of sex steroids by gonadectomy switched senktide effects to inhibitory, both in adult male and female rats. Sexual dimorphism was also evident in the numbers of NKB-positive neurons in the arcuate nucleus (ARC), which were higher in adult female rats. This is likely the result of differences in sex steroid milieu during early periods of brain differentiation, because neonatal exposures to high doses of estrogen decreased ARC NKB neurons at later developmental stages. Likewise, neonatal estrogenization resulted in lower serum LH levels that were normalized by senktide administration. Finally, we document that the ability of estrogen to inhibit hypothalamic Tac2 expression seems region specific, because estrogen administration decreased Tac2 levels in the ARC but increased them in the lateral hypothalamus. Altogether, our data provide a deeper insight into relevant aspects of NKB function as major regulator of the gonadotropic axis in the rat, including maturational changes, sexual dimorphism, and differential regulation by sex steroids.
The secretion of GnRH is driven by the dynamic interplay of numerous central and peripheral factors (1). Among the central regulators, neurokinin B (NKB) has drawn special attention recently. This is mainly because of findings of human genetic studies, linking loss-of-function mutations in TAC3 and TACR3 genes (encoding NKB and its receptor, NK3R, respectively) with hypogonadotropic hypogonadism and infertility (2, 3). These observations prompted the reassessment of the physiological roles of the NKB/NK3R system in the control of the gonadotropic axis in mammals. Thus, initial studies in gonadectomized (GNX) female rodents documented inhibitory actions of NKB on gonadotropin secretion (4), which seemed contradictory to the predicted stimulatory actions of NKB suggested by human genetic studies (2, 3). However, compelling evidence has recently demonstrated a predominant stimulatory role of NKB on GnRH/gonadotropin release in different mammalian species (5–13). Admittedly, however, null responses and even inhibitory actions of the agonist of NK3R, senktide, on LH secretion have been also reported lately in rodents (7, 14, 15); the latter mainly in GNX female models without sufficient 17β-estradiol (E2) replacement. In fact, differences in the sex steroid milieu, and eventually in the prevailing gonadotropin levels, have been proposed to play a role in such a paradoxical switch between the stimulatory and inhibitory effects of NKB, with inhibitory actions being detected in conditions of null/low sex steroid replacement and enhanced gonadotropin levels (16).
Anatomically, NKB is coexpressed in the arcuate nucleus (ARC) with kisspeptin and dynorphin A (5, 6, 13, 17) in a population of neurons, also termed KNDy (kisspeptin/neurokinin B/dynorphin), which is subjected to regulation by sex steroids through estrogen receptor-α (18, 19) and androgen receptor (8, 20). Recent experimental evidence suggests that NKB from this neuronal population participates in the (auto)regulation of kisspeptin output from KNDy neurons and thereby critically contributes to two key physiological events: the timing of puberty onset and the shaping of kisspeptin (and hence GnRH) pulsatile release (16). This hypothetical model for NKB-regulatory actions was initially developed based on neuroanatomical and hormonal data from different mammalian species (7) and has been recently supported by findings showing that 1) ARC Kiss1 neurons are activated by senktide in rats and mice, as demonstrated by c-fos induction and electrophysiological recordings, respectively (7, 8); and 2) the stimulatory actions of senktide are severely attenuated, if not totally lost, in the absence of proper kisspeptin signaling in mice and monkeys (10, 12).
Despite the recent progress in the field, some aspects of NKB function as up-stream regulator of kisspeptin signaling in the reproductive brain remain partially unknown. These include not only the elucidation of the mechanisms underlying the switch between stimulatory and inhibitory actions of NKB, but also the physiological relevance of such NKB-dependent gonadotropin regulation during the lifespan and its modulation by sex steroids. Notably, NKB expression in the ARC is suppressed by sex steroids (5, 7, 21); hence, ARC NKB has been suggested to participate in the negative feedback regulation of gonadotropins. In addition, previous studies had documented a marked sexual dimorphism of ARC NKB neuronal population in the sheep, with higher number of NKB neurons in females that was reverted by prenatal androgenization (22, 23). Likewise, a sexual dimorphism in the pattern of axonal distribution of NKB neurons has been described in the rat (24, 25). This dimorphism, however, was shown to emerge progressively during puberty and seemed to be dependent on pubertal exposure to the rising levels of testosterone (T) (24, 25). To our knowledge, the functional relevance of such dimorphism has not been experimentally addressed in rodents.
In the present work, we aimed to shed further light on the role of NKB signaling in the control of the gonadotropic axis in the rat, with special attention to its interplay with developmental cues and sex steroids. To this end, LH responses to senktide were monitored in male and female rats at different stages of postnatal maturation and after manipulation of sex steroid milieu in the early (neonatal) period and adulthood. Hormonal studies were coupled with expression analyses, addressing changes in either numbers of NKB neurons or Tac2 (gene encoding NKB in rodents) mRNA expression after the above sex steroid manipulations.
Materials and Methods
Animals and drugs
Wistar rats bred in the vivarium of the University of Córdoba, Spain, were used. The day the litters were born was considered as d 1 of age. The animals were maintained under constant conditions of light (14 h of light, from 0700 h) and temperature (22 C) and were weaned at 21 d postpartum, when they were housed in groups of five rats per cage and with free access to standard rat chow and water ad libitum. For hormonal tests involving intracerebroventricular (icv) cannulation, rats were caged individually from the day before cannulae implantation until termination of experiments. Correct positioning of the cannulae was checked by visual inspection, to exclude animals showing obvious displacement or deattachment, and was confirmed at necropsy by serial coronal sectioning of randomly selected animals, a procedure that confirms that (virtually) all animals displayed proper location of the cannulae as to allow delivery into the lateral ventricle. Experimental procedures were approved by the University of Córdoba Ethical Committee for animal experimentation and were conducted in accordance with the European Union normative for care and use of experimental animals.
The NK3R agonist, senktide, and the sex steroids, testosterone (T), dihydrotestosterone (DHT), E2, and estradiol benzoate (EB), were purchased from Sigma Chemical Co. (St. Louis, MO). The dose of senktide, 600 pmol of senktide in 10 μl of physiological saline, 0.9% NaCl, was selected on the basis of previous references because it is maximally effective in inducing gonadotropin responses in the rat (4, 7). For experiments involving adult female rats, adult virgin animals were daily monitored by vaginal cytology to confirm the occurrence of regular estrous cycles; only rats with at least two consecutive regular 4-d estrous cycles were subsequently used for hormonal and molecular analyses, as previously described (26). In addition, for experiments involving ovariectomy (OVX) and steroid replacement, groups of adult female rats were subjected to bilateral gonadectomy (GNX) via abdominal incision under light ether anesthesia 2 wk before hormonal tests or tissue sampling. Likewise, groups of adult male rats were castrated (ORX) through a single abdominal incision. Immediately after GNX, capsules filled with E2, T, or DHT were implanted sc via a small midscapular incision at the base of the neck; wound clips were used to close the incision. Sham-operated animals served as GNX controls. SILASTIC tubing (Dow Corning Corp., Midland, MI; 20 mm long, 0.62 mm inner diameter, 1.25 mm outer diameter) was used for capsule preparation. Dilutions of crystalline E2 at a low dose (100 μg/ml, in olive oil) were used for capsule filling; this dose was selected to achieve moderate levels of circulating E2, as previously described (7). Similarly, in animals with androgen replacement, capsules filled with crystalline T or DHT were used, as described previously (8).
Experimental design
LH responses to the NKB agonist, senktide, at different developmental stages in male and female rats (experiment 1)
The ability of the NKB agonist, senktide, to acutely modify LH secretion in male and female rats (n = 10–12 rats per group), at different stages of postnatal development, was first explored. To this end, central administration of senktide into the lateral cerebral ventricle was conducted in both male and females during infantile (10 d), juvenile/prepubertal (25 d and 30 d), pubertal (36 d in female and 45 d in male), and adult (60 d) stages; the latter (adult female rats) were confirmed to display regular estrous cyclicity by monitoring of vaginal smears and were sampled in the morning of diestrus 1. To allow delivery of senktide or vehicle into the lateral cerebral ventricle, animals were implanted with icv cannulae lowered to a depth of 3 (prepubertal) or 4 (adult) mm beneath the surface of the skull; the insert point was placed 1 mm posterior and 1.2 mm lateral to bregma. Hormonal tests were conducted at least 1–2 d after implantation of cannulae, a time point when animals were fully recovered. Blood samples were collected 20 min after senktide injection. Animals injected with vehicle (physiological saline, 0.9% NaCl) served as controls. To note, because attenuated to null responses to the standard dose of 600 pmol were consistently detected in males, testing of the effects of a single bolus of 3 nmol senktide icv was also conducted selectively in prepubertal (25-d-old) males, following a similar experimental procedure.
LH responses to senktide in adult GNX rats, with or without sex steroid replacement (experiments 2 and 3)
Experiment 1 documented a clear-cut sexual dimorphism in LH responses to senktide from puberty onward, with no secretory responses being detected in adult males. Therefore, we aimed to analyze whether this phenomenon is a consequence of the different postpubertal steroid milieu in males vs. females. To this end, in experiment 2, the effect of icv injection of a bolus of senktide on LH secretion was assessed in intact adult male and female rats, as well as in OVX or ORX rats supplemented with T or E2, respectively (i.e. sex steroid switched). In addition, to complement this initial experiment, in experiment 3, similar tests were implemented either in GNX male and female rats without sex steroid replacement, or in OVX and ORX rats subjected to hormonal replacement with effective doses of DHT. Thus, a total of eight groups (n = 10–12 rats per group) were studied: 1) Cyclic adult female rats at diestrus-1; only females displaying at least two consecutive regular cycles and cytological features of diestrus-1 in the morning of the hormonal test were used. 2) Adult OVX rats, without sex steroid replacement (sham). 3) OVX rats supplemented with T (OVX + T); T-containing capsules were implanted sc into adult OVX rats, as described above. 4) OVX rats supplemented with DHT (OVX + DHT); DHT-containing capsules were implanted to OVX rats, as described above. 5) Adult male rats; age paired to females. 6) Adult ORX rats, without sex steroid replacement (sham). 7) ORX rats supplemented with E2 (ORX+E2); E2-containing capsules were implanted sc into adult ORX rats, as described above. 8) ORX rats supplemented with DHT (ORX+DHT); DHT-containing capsules were implanted in adult ORX rats, as described above. In each group, the animals were icv injected with senktide, and blood samples were serially obtained by jugular venipuncture before (0 min), and at 20, 60, and 120 min after icv injection. Animals injected with physiological saline served as controls.
Analyses of NKB-ir and LH responses to senktide in neonatally estrogenized male and female rats (experiments 4 and 5)
To address other aspects of the functional organization of the NKB system, in the next set of experiments we analyzed the influence of the steroid environment during the neonatal period, as critical window for brain differentiation, on key structural and functional features of this system in later (pubertal/adult) developmental stages. To this end, in experiment 4, groups (n = 5–7) of 1-d-old male and female rats were injected with a single bolus of a high dose of EB (500 μg/rat for males; 100 μg/rat for females), following standard procedures of neonatal estrogenization. Of note, EB was dissolved in olive oil and injected sc, thus allowing a depot release over several days after neonatal injection, a period that actually covers most of the postnatal window of brain sex differentiation in rats (27). Vehicle-injected animals (olive oil; 100 μl sc) served as controls. This protocol was selected on the basis of our previous studies, because it was found to be the most effective in inducing complete estrogenization. In adulthood, NKB-positive cells were counted in the ARC of adult male and female rats, subjected or not to neonatal estrogenization, using standard immunohistochemical (IHC) procedures. Demonstration of the impact of neonatal treatments with EB on the NKB content in the ARC prompted us to conduct experiment 5, where the ability of senktide to elicit LH secretion was assessed in young (>45-d-old) adult male and female rats subjected to neonatal estrogenization. Estrogenized male and female rats (n = 10–12/group) were icv injected with a single dose of senktide, and blood samples were collected after 20 min for LH determinations. Animals injected with saline served as controls.
Effect of E2 replacement on Tac2 expression in the hypothalamus of female rats (experiment 6)
The ability of sex steroids to decrease Tac2 mRNA expression in the ARC has been previously reported for both sexes in a number of species (5, 7, 18, 28–30). To note, Tac2 mRNA presents a widespread distribution in the brain (7, 31). Moreover, within the hypothalamus, it is expressed in a number of different areas, including not only the ARC but also the lateral hypothalamus (LHA), which is relevant in the control of reproductive and related functions; nevertheless, analysis of sex steroid regulation of Tac2 has been mostly restricted to the ARC. In this experiment, we aimed to compare the effects of E2 on the expression of Tac2 mRNA in the LHA, as a potentially relevant nucleus in the control of gonadotropin release, and the ARC of OVX female rats, replaced or not with effective doses of E2. To this end, animals were OVX and implanted sc with E2-containing capsules (n = 5/group), as described above. OVX animals implanted with empty capsules served as controls. Animals were euthanized 1 wk after surgery by decapitation in the morning (1000 h), and trunk blood and brains were collected, frozen on dry ice, and stored at −80 C for in situ hybridization (ISH). Plasma levels of LH and uterine weight were measured to determine the efficacy of the hormone replacement.
Tissue preparation
Blood was centrifuged to isolate the serum, which was stored at −20 C until hormone measurements. Uteri were removed and weighed to provide an indirect marker of plasma E2 levels and of their biological effect. In selected experiments (see experiment 5), brains were removed for ISH, frozen on dry ice, and then stored at −80 C until sectioned. Five sets of 20-μm sections in the coronal plane were cut on a cryostat, from the diagonal band of Broca to the mammillary bodies, thaw mounted onto SuperFrost Plus slides (VWR Scientific), and stored at −80 C. A single set was used for ISH (adjacent sections 100 μm apart).
NKB immunohistochemisty (IHC)
IHC assays were conducted in hypothalamic sections of brain samples from adult male and female rats, subjected to standard protocols of neonatal estrogenization, as described earlier in this section. The animals were perfused through the ascending aorta, under ketamine-xylazine anesthesia, with 250 ml fixative solution (4% paraformaldehyde) (32, 33). Brains were collected, immersed in fixative overnight, and cryoprotected in 30% sucrose for 2–4 d. Coronal sections (40 μm thick) were cut in parallel series of four on a freezing microtome, and one series of sections covering the entire rostro-caudal extension of the ARC was processed for detection of NKB-immunoreactivity (ir).
The sections were incubated in 1% H2O2-PBS to block endogenous peroxidase activity and in 0.01 m PBS with 0.3% Triton X-100, 5% swine serum, and 1% BSA to block nonspecific binding sites. The sections were then incubated at 4 C for 24 h with a purified rabbit antiserum against NKB (IS-39; dilution 1:5000), as described in detail elsewhere (24, 25). This antiserum has been previously used to successfully label NKB neurons and fibers in rats (24, 25). NKB-ir was detected by the avidin-biotin method using diaminobenzidine as chromagen. The sections were incubated for 60 min in biotinylated secondary antirabbit IgG (The Jackson Laboratory, Bar Harbor, ME) diluted 1:1000, and washed and transferred to the avidin-biotin complex (Vector Laboratories, Burlingame, CA) diluted 1:250. Thereafter, the sections were incubated in 0.1% diaminobenzidine (Sigma). The sections were mounted on gelatinized glass slides, dried, and coverslipped in Pertex. Positive cell bodies were counted bilaterally throughout the extent of the ARC by an observer blinded to the identity of each section. The total counts were multiplied by 4 as to represent the total number of NKB-ir neurons in the ARC of each animal.
RIA for LH
Serum LH levels were measured in 50-μl samples using a double-antibody method and RIA kits supplied by the National Institutes of Health (Dr. A. F. Parlow, National Hormone and Peptide Program, Torrance, CA). Rat LH-I-10 was labeled with 125I with the use of Iodo-gen tubes, following the instructions of the manufacturer (Pierce Chemical Co., Rockford, IL). Hormone concentrations were expressed with the reference preparation LH-RP-3 as a standard. Intraassay and interassay coefficients of variation were less than 8% and 10%, respectively. The sensitivity of the assay was 5 pg/tube.
Detection of Tac2 mRNA-probe generation
The probe used for the detection of Tac2 mRNA was as described previously (5). A sense probe for Tac2 was used as control for the specificity of the ISH procedures, which are outlined below.
Single-label ISH of Tac2 mRNA
Tac2 mRNA sense and antisense probes were transcribed with T7 or T3 polymerase (Fermentas, Inc., Glen-Burnie, MD), as described previously (5). Briefly, radiolabeled probes were synthesized in vitro by inclusion of the following ingredients in a volume of 20 μl: 250 Ci [33P]UTP (Perkin-Elmer Life and Analytical Sciences), 1 μg of PCR product, 0.5 mm each ATP, CTP, and GTP, and 40 U of polymerase. Residual DNA was digested with 4 U of deoxyribonuclease (Ambion, Austin, TX), and the deoxyribonuclease reaction was terminated by addition of 2 μl of 0.5 m EDTA, pH 8.0. The riboprobes were separated from unincorporated nucleotides with NucAway Spin Columns (Ambion). Slides with rat hypothalamic sections from the different experimental groups, covering the key nuclei/areas under analysis (namely, ARC and LHA), were processed as reported previously (7).
Quantification and analysis of Tac2 mRNA
Brain sections were divided in five sets every 20 μm (thickness of slice); thus, within each set, sections were 100 μm apart. Brain sections were analyzed unilaterally. Slides from all of the animals were assigned a random three-letter code, alphabetized, and read under dark-field illumination with custom-designed software designed to count the total number of cells and the number of silver grains (corresponding to radio-labeled Tac2 mRNA) over each cell. Data are presented as total mRNA content, depicting the number of cells within the coronal sections containing the hypothalamic nucleus studied for each set (one section every 100 μm), not the total mRNA in this specific nucleus. The starting and ending point of quantification was determined according to the atlas of Paxinos and Franklin (48).
Statistical analysis
All data are expressed as the mean ± sem for each group. In addition, when appropriate, integrated LH secretory responses expressed as the area under the curve (AUC) were calculated following the trapezoidal rule (34), over the 120-min period after the administration of senktide. ANOVA followed by Tukey's post hoc test or Student's nonparametric t test were used to assess variation among experimental groups. Significance level was set at P ≤ 0.05. All analyses were performed with GraphPad Prism Software, Inc. (San Diego, CA).
Results
LH responses to the NKB agonist, senktide, in male and female rats during postnatal maturation
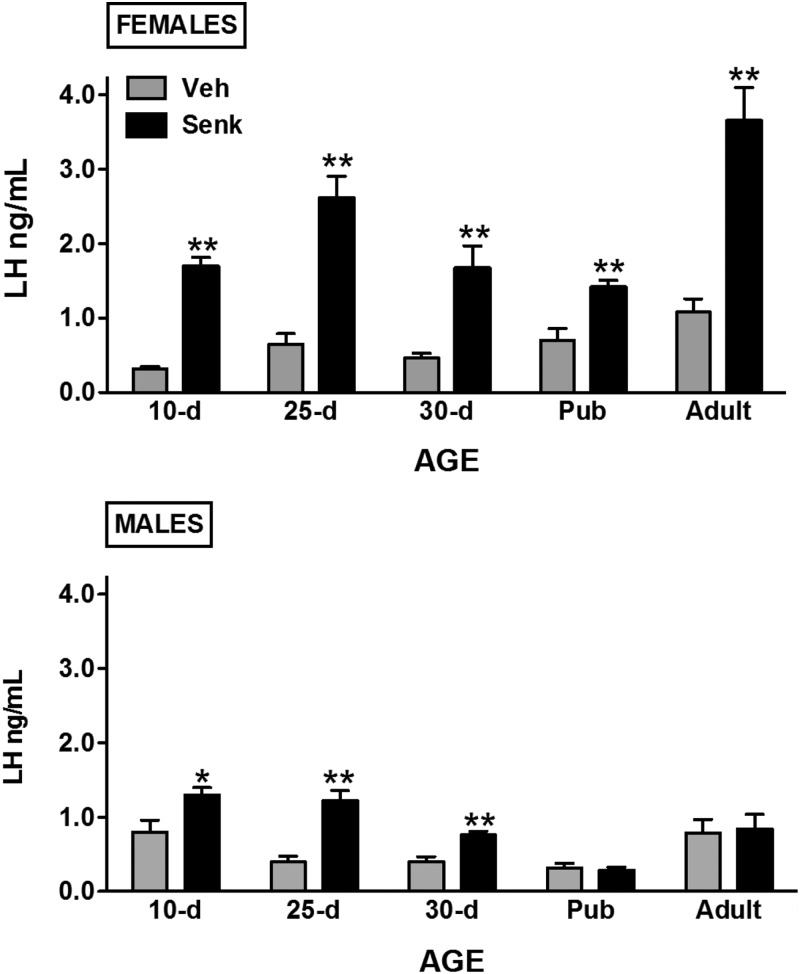
Senktide (600 pmol) was injected centrally to male and female rats at different stages of postnatal maturation; namely, at infantile (10 d), juvenile/prepubertal (25 d and 30 d), pubertal (36 d in female and 45 d in male), and adult (>60 d) ages. Of note, for adult cyclic females, hormonal tests were conducted in the morning of diestrus 1. This phase has been shown to be sensitive to the stimulatory effects of senktide on LH secretion (7) and is devoid of major changes in endogenous gonadotropin levels, as those seen in the proestrus-to-estrus transition. Nonetheless, results obtained at this phase are likely representative of other stages, because our previous work has documented clear-cut LH responses to the NKB agonist at other phases of the cycle and in E2-supplemented OVX rats (7). Conspicuous LH responses were detected 20 min after icv injection of senktide in female rats, at all ages tested (see Fig. 1, upper panels; **, P ≤ 0.01), with a relative magnitude of 2- to 4-fold increase over vehicle-injected values depending on the developmental stage. In contrast, male rats displayed markedly attenuated LH responses to senktide during postnatal maturation. Indeed, modest, albeit significant (*, P ≤ 0.05; **, P ≤ 0.01), LH responses were detected in infantile and juvenile/prepubertal males but the magnitude of such responses was lower than in corresponding pair-aged females (see Fig. 1, lower panels). Moreover, from puberty onward, male rats became irresponsive to senktide, with null LH responses to the NKB agonist in pubertal (45-d-old) and adult male rats. Of note, the attenuated LH responses to senktide in prepubertal males cannot be attributed to the submaximal dose used in our experiments, because icv injection of 3 nmol senktide evoked LH secretory bursts similar in magnitude to those elicited by the 600-pmol dose of the agonist (LH levels at 20 min after icv senktide or vehicle injection: vehicle = 0.39 ± 0.08 ng/ml; senktide 600 pmol = 1.22 ± 0.14 ng/ml; senktide 3 nmol = 1.18 ± 0.18 ng/ml).
Fig. 1.
LH responses to NKB agonist, senktide, in male and female rats during postnatal maturation. Male and female rats, at different postnatal ages (indicated on x-axis), were icv injected with an effective dose (600 pmol) of senktide or vehicle, and blood samples were obtained after 20 min for LH determinations. Pubertal animals were 36 d old for females and 45 d old for males. *, P < 0.05; **, P < 0.01 vs. corresponding vehicle-injected groups (Student's t test). Pub, Pubertal; Senk, senktide; Veh, vehicle.
Influence of actual sex steroids levels on sexually dimorphic LH responses to senktide in adult rats
Our hormonal tests in male and female rats demonstrated a marked sexual dimorphism in LH responses to central administration of senktide. To assess whether such dimorphism may be due to differences in the circulating levels of the main sex steroid (T in males; E2 in females), the effects of senktide in intact adult male and female rats were compared with those of sex steroid-switched animals (OVX+T females and ORX+E2-replaced males); LH responses were monitored at 20, 60, and 120 min after icv injection of senktide. In addition, the above studies were complemented by similar analyses in GNX animals without sex steroid replacement and in OVX and ORX rats supplemented with DHT, as a means to discriminate between androgen- vs. estrogen-specific effects.
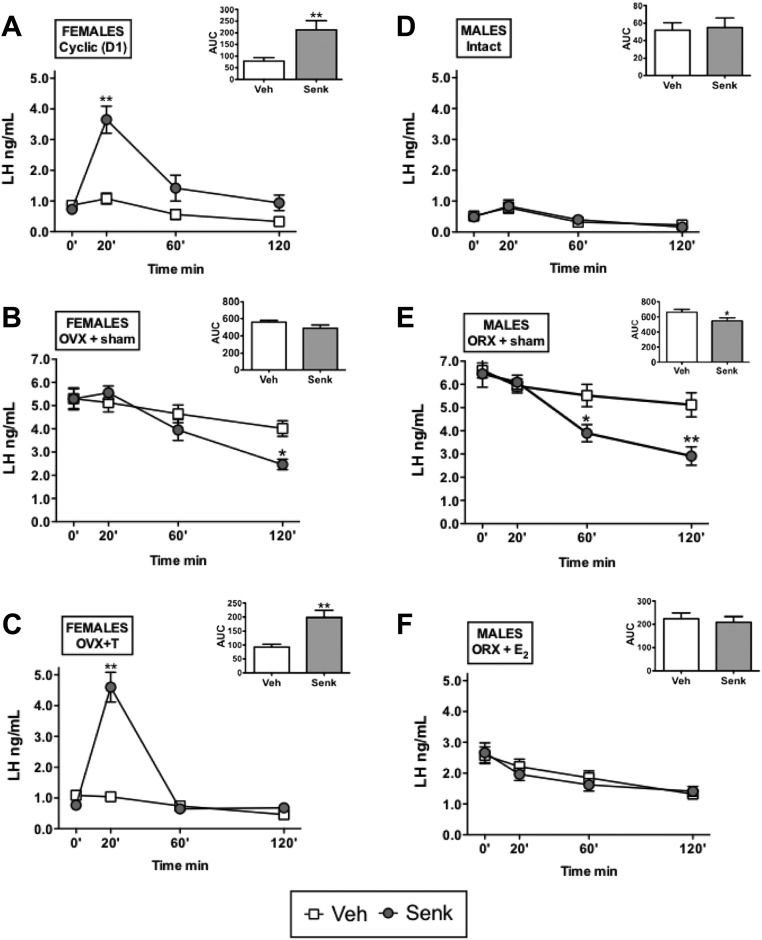
Detailed time-course analyses confirmed the patterns of LH response to central senktide injection in gonadal-intact, adult female, and male rats. Thus, in cyclic female rats in diestrus 1, activation of NK3R induced a significant increase in serum LH levels at 20 min, which was followed by a progressive decline at 60 and 120 min after senktide administration; overall, senktide injection evoked a 3-fold increase in LH secretory mass, estimated as the AUC during the 120 min after icv injection (Fig. 2-A). In contrast, in adult male rats, no LH response was detected at any time point after central administration of senktide (Fig. 2D). In clear contrast, in GNX rats (without sex steroid replacement), LH secretion was actually inhibited by central injection of senktide in both sexes. In these groups, the expected rise of basal, preinjection levels of LH due to GNX was clearly detectable. In both male and female GNX animals, icv administration of senktide lowered circulating LH levels vs. corresponding values in vehicle-injected groups. This inhibitory effect became significant at 60 min (only males) and 120 min (both males and females) after senktide injection (Fig. 2, B and E).
Fig. 2.
LH responses to senktide in adult male and female rats after manipulation of the sex steroid milieu. LH secretory responses to the NKB agonist, senktide, were studied in the following groups: adult cyclic female rats (at diestrus 1; panel A), adult male rats (panel D), OVX female rats implanted with empty capsules (OVX+sham; panel B), ORX male rats implanted with empty capsules (ORX+sham; panel E), OVX rats supplemented with T (OVX+T; panel C), and ORX rats and supplemented with E2 (ORX+E2; panel F). The animals were icv injected with an effective dose (600 pmol/rat) of senktide or vehicle, and blood samples were obtained before (0 min) or at 20, 60, and 120 min after injection. In addition to time course profiles, integral LH secretory responses (AUC) during the 120 min after icv injection of senktide or vehicle are also shown for each experimental group in the insets. *, P < 0.05; **, P < 0.01 vs. corresponding values in vehicle-injected animals (for time course data: ANOVA followed by Student-Newman-Keuls multiple range test; for AUC data: Student's t test). Senk, Senktide; Veh, vehicle.
Effectiveness of sex steroid replacement in our models was demonstrated by the lowering (to a different extent) of basal preinjection LH levels in GNX animals, compared with the circulating LH concentrations in OVX and ORX rats without sex steroid replacement. As per sex steroid-switched groups, T administration to OVX rats returned basal LH levels to the physiological range (i.e. to levels similar to those seen in adult males), whereas our protocol of E2 administration, despite being effective in inducing a marked and significant suppression of basal LH concentrations, did not bring those values to the physiological range, therefore suggesting a submaximal replacement. In this context, T supplementation of OVX female rats failed to prevent the induction of LH release by senktide in females, which displayed a significant 3- to 4-fold increase at 20 min and in the integral LH secretory (AUC) mass during 120 min after senktide injection, which was roughly similar to the profile of cyclic female rats (Fig. 2C). In turn, despite the fact that E2 treatment significantly lowered basal LH levels in ORX male rats, such replacement did not rescue LH responsiveness to senktide, so that ORX+E2 rats behaved as intact males (not as females) in terms of LH responses to the NKB agonist (Fig. 2F).
The above trends were confirmed by our DHT-replacement experiments in GNX female and male rats. Of note, despite the use of doses similar to those of T supplementation, DHT evoked a marked suppression of basal LH levels, which dropped to nearly 20% of basal values in gonadal intact female and male rats. Nonetheless, OVX rats treated with such a supraphysiological doses of DHT retained their ability to respond to senktide, which induced an approximately 2.5-fold increase in LH levels at 20 min over the corresponding values in vehicle-injected controls. In contrast, DHT-supplemented male rats did not display any detectable LH response to senktide, at any time point studied over the 120 min after its icv administration (Supplemental Fig.1 published on The Endocrine Society's Journals Online web site at http://endo.endojournals.org).
NKB-ir and LH responses to senktide in neonatally estrogenized male and female rats
Because the above results suggested that the predominant adult sex steroid milieu (androgens in males; estrogens in females) is not the major determinant for the sexual dimorphism of LH responses to senktide, we sought to determine whether these differences might be wired from early stages of brain sexual differentiation. Two complementary approaches were undertaken to address this issue. First, we assessed in adulthood the numbers of NKB immunoreactive (NKB-ir) neurons in the ARC of neonatally estrogenized male and female rats, by means of IHC. Second, we evaluated potential changes in basal LH levels and responses to senktide in young male and female rats subjected to similar protocols of neonatal estrogenization.
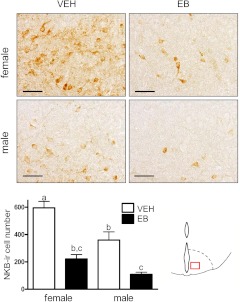
A single bolus of EB on postnatal d 1 led to a persistent, and significant, reduction of the number of NKB-ir neurons in the ARC of adult animals of both sexes, compared with vehicle-treated animals (Fig. 3). Of note, control females exhibited significantly higher amounts of NKB-ir cells than control males (P ≤ 0.5); this sexual dimorphism was obliterated by neonatal EB treatments, which markedly decrease NKB cell numbers in both sexes.
Fig. 3.
Number of NKB-ir neurons in the ARC of male and female rats after neonatal estrogenization. NKB-ir cells were counted in the ARC of adult male and female rats, treated neonatally with vehicle (controls) or an effective dose of EB. Representative photomicrographs of IHC assays are shown for the four groups, at ×40 magnification. Quantitative values of NKB-ir neurons within the ARC in the corresponding experimental groups are provided in the histogram. In addition, a schematic drawing depicting the area of counting of NKB-ir cells has been included. Groups with different superscript letters are statistically different (ANOVA followed by Student-Newman-Keuls multiple range test). Bar, 50 μm. VEH, Vehicle.
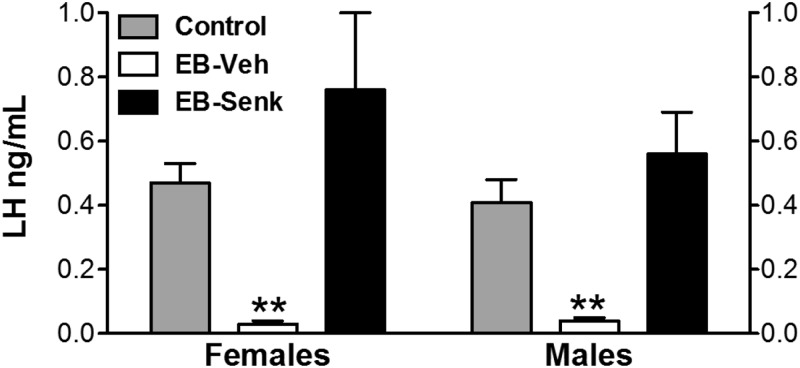
In addition to the above changes in NKB-ir neurons in the ARC, neonatal estrogenization evoked a dramatic decrease in circulating LH levels in young male and female rats, compared with their controls (**, P ≤ 0.01), suggesting a functional coupling between lower NKB expression and reduced LH secretion in these animals with perturbed hypothalamic brain differentiation. Such association was further backed up by the finding that senktide administration to neonatally estrogenized rats was able to normalize LH levels in both male and female animals (see Fig. 4).
Fig. 4.
LH levels in neonatally estrogenized rats, in basal conditions, and after senktide injection. LH levels are shown for young female and male rats, from three different experimental conditions: Controls (neonatally injected with vehicle); EB-Veh (neonatally estrogenized rats, 20 min after injection of vehicle); and EB-Senk (neonatally estrogenized rats, 20 min after injection of 600 pmol senktide). **, P < 0.01 vs. corresponding values in control animals (ANOVA followed by Student-Newman-Keuls multiple-range test). Senk, Senktide; Veh, vehicle.
Regulation of Tac2 expression by E2 in the ARC and LHA of adult female rats
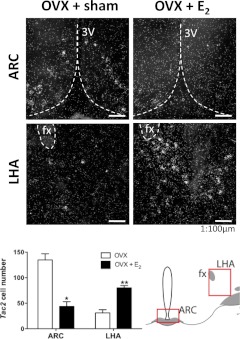
In addition to the early organizing effects of estrogen on NKB neuronal populations in the ARC, we evaluated the impact of acute E2 exposures on NKB expression in different hypothalamic areas, by ISH analyses of Tac2 mRNA levels. Of note, whereas the organizing effects of early sex steroid exposures were explored using IHC (see above), because this technique may eventually allow a better appreciation of plastic changes of the population of NKB neurons at later ages, the transient regulatory effects of E2 in adulthood were assessed by ISH, because this approach is better suited for the appreciation of (eventually, subtle) quantitative changes in expression, as extensively documented in previous literature. Our studies confirmed that E2 replacement for 1 wk to adult OVX female rats induced a significant decrease in Tac2 mRNA levels in the ARC (*, P ≤ 0.05), in keeping with previous references (5, 7, 8, 18, 28, 30) (Fig. 5). However, our studies also documented that estrogen regulation of Tac2 expression in the hypothalamus is region specific because Tac2 responses to E2 supplementation were diametrically opposite in the LHA in the same animals, in which a significant increase in Tac2 mRNA levels was detected in OVX+E2 rats (**, P ≤ 0.01; Fig. 5).
Fig. 5.
Tac2 mRNA levels in the ARC and LHA after estrogen administration to adult OVX female rats. Tac2 mRNA levels in the ARC and LHA were assessed by ISH in adult female rats, subjected to OVX and hormonal replacement with E2. For further details see Materials and Methods. In addition to representative photomicrographs, quantitative values of numbers of Tac2-expressing neurons in the indicated hypothalamic areas of the different experimental groups are provided in the histogram (lower panel). The most relevant landmarks (third ventricle, 3V; fornix, Fx), as well as the neuroanatomical areas selected for quantitative analyses, are also displayed in the representative photomicrographs and the associated cartoon, respectively. *, P < 0.05; **, P < 0.01 vs. corresponding OVX values (Student's t test).
Discussion
Recent reports have documented robust stimulatory actions of NKB (or its agonist, senktide) on gonadotropin secretion in a number of mammalian species (5–13). These findings are in keeping with the reported phenotype of hypogonadotropic hypogonadotropism in humans (2, 3), and subfertility in mice (35), with inactivating mutations of the NKB/NK3R system. However, conflicting results on the ability of NKB/senktide to regulate LH secretion can be found in the literature, with negligible or even inhibitory responses being reported in specific experimental conditions (14, 15). In fact, rodent GNX models without physiological replacement of sex steroids appear to display conspicuous inhibitory LH responses to NKB (4, 7, 15), therefore suggesting that the presence of sex steroids and/or the prevailing gonadotropin levels may be major contributing factors for the repertoire of regulatory effects (from stimulatory to null or inhibitory) of NKB of gonadotropin release. Admittedly, however, our understanding of the gonadotropic effects of NKB remains incomplete, mainly because most of the analyses on this issue have been carried out so far in adult females. Our present data provide a comparative view of the profiles of LH responses to NKB stimulation during the postnatal maturation in male and female rats. As most salient findings, our hormonal analyses disclose that, in the rat, 1) LH responsiveness to NKB is consistently higher in females than in males, with females being responsive to senktide regardless of the stage of postnatal maturation; and 2) male rats become irresponsive to NKB activation from puberty onward, therefore unveiling a marked sexual dimorphism in the actual roles of the NKB system in adulthood in this species.
In keeping with previous reports on the stimulatory effects of kisspeptins during early postnatal maturation (36), our present data demonstrate that NKB can evoke unambiguous LH secretory responses in immature male and female rats, well before puberty onset. However, although LH responses to kisspeptin-10 were equally robust in infantile and prepubertal male and female rats (36), our current results disclose that the magnitude of NKB effects is much higher in immature females, whereas pubertal and adult male rats become irresponsive to the NKB agonist in terms of LH secretion. The above observations, together with our recent findings on the up-surge of Tac2 and Tacr3 expression in the hypothalamus during prepubertal maturation (9), suggest a potential role of NKB signaling in the central activational events leading to puberty onset, especially in the female. Considering the hypothetical model for NKB modulation of kisspeptin output of KNDy neurons (16), and the fact that in the female rat the increase in hypothalamic Tac2 expression seems to slightly precede that of Kiss1 (9), our present data are compatible with a putative role of NKB as (one of) the driving signals for the increase in the kisspeptin tone that takes place during puberty. This might explain why the LH-secretory effects of senktide are of higher magnitude in prepubertal rats, when the endogenous kisspeptin release is still low but could be considerably enhanced by NKB, than in peripubertal animals, in which kisspeptin release is already high and further stimulation by NKB might only be of moderate amplitude. In addition, our present results suggest differences in the physiological role and relative importance of NKB (auto)-regulatory actions between male and female rats. Likewise, considering our recent demonstration of very potent stimulatory actions of senktide on Kiss1 neurons and LH release in adult male mice (8, 10), our current findings reveal also striking divergences regarding the ability of NKB to stimulate LH secretion between two, otherwise closely related species (rat and mouse). Admittedly, the basis for the above phenomena is yet to be elucidated and warrants further investigation.
Given the marked differences in LH responses to the NKB agonist between adult male and female rats, we sought to determine whether such divergent responses might stem from the differences in the circulating levels of the predominant sex steroids in adulthood. Our studies in sex steroid-switched animals strongly suggest that this is not the case. Thus, adult female rats exposed to T levels supposedly comparable to those of adult males (as reflected by basal LH levels) retained a feminine pattern of LH response to senktide, whereas E2-treated adult males did not acquire the capacity to respond to the NKB agonist. Moreover, supramaximal doses of the nonaromatizable androgen, DHT, failed to alter those qualitative responses: OVX+DHT females displayed detectable responses to senktide, whereas ORX-DHT males did not show such responses. These observations strongly suggest that the sexual dimorphism in LH responsiveness to NKB in the rat is not merely the result of the differences in the predominant sex steroid milieu, which maximally diverges form puberty onward, and point out to earlier maturational events as major contributing factors. Nonetheless, our studies confirm previous observations on the inhibitory effects of NKB signaling on LH release in conditions of derepressed gonadotropin secretion, as those seen in GNX animals. Although this action had been consistently demonstrated in females, to our knowledge, our findings are the first to document the same phenomenon also in GNX male rats. The mechanisms for the switch between stimulatory (in adult female rats) or null (in adult male rats) responses to the inhibitory effects of senktide (in GNX adult rats) on LH secretion might involve changes in the endogenous NKB tone among the different conditions and of its interaction with the agonist, but the ultimate basis of this phenomenon is yet to be elucidated.
Several pieces of evidence strongly suggest the relevance of early organizing events in the configuration of sexual dimorphisms affecting the NKB/NK3R system in the rat. First, as previously reported in the sheep (22, 23), the numbers of NKB-ir neurons in the ARC were moderately, but significantly, higher in female rats, and this sex difference was obliterated by neonatal estrogen treatments known to perturb the normal process of brain differentiation (27, 37). Of note, previous studies have suggested the existence of a sexual dimorphism in the patterns of axonal projection of NKB neurons in the rat that appeared to be dependent on the divergent sex steroid milieus detected from puberty onward between males and females (24, 25). Although our study did not address the time course for the observed sexual dimorphism in the NKB neuronal population, it cannot exclude the contribution of factors other than the organizing effects of sex steroids; to our knowledge, this is the first report to document the impact of early manipulations of the sex steroid levels during critical periods of brain sex differentiation in the rat on the organization of NKB neuronal networks in adulthood. This is in keeping with previous data in the sheep demonstrating that neonatal exposure to T reduced the number of NKB-ir neurons in the ARC of female sheep (22, 23). These findings strongly suggest that NKB neurons in the ARC are sensitive to the organizing effects of sex steroids during critical windows of brain maturation. Similarly, previous observations from our group have documented that neonatal estrogenization results in a significant suppression of hypothalamic Kiss1 mRNA levels in the rat hypothalamus, both at puberty and adulthood (27, 37). Taken as a whole, these findings suggest that KNDy neurons in the rat undergo a maturational program that is highly sensitive to the organizing effects of sex steroids during critical developmental windows.
Further support for the functional relevance of the above expression (ir) changes in hypothalamic NKB came from our hormonal studies in neonatally estrogenized rats. Thus, the reported suppression of NKB-ir detected in models of neonatal estrogenization was coupled to a dramatic decrease in circulating LH levels in young animals. In turn, exogenous activation of NKB signaling, by administration of senktide, was able to normalize serum LH levels in estrogenized male and female rats. The fact that young adult male rats subjected to neonatal estrogenization did respond to senktide might be indicative of their immature state, because estrogenized rats fail to undergo puberty, and prepubertal males show LH responses to NKB activation. Again, there are considerable similarities with previous findings on the effects of kisspeptin-10 on gonadotropin secretion in neonatally estrogenized rats. Admittedly, however, the magnitude of LH responses to kisspeptin-10 was higher than those elicited by senktide in our current study. Assuming the indirect mode of action of NKB, via regulation of kisspeptin output onto GnRH neurons (5, 10, 12, 38), it is tenable that such reduced LH responses (compared with kisspeptin-10) may stem from the prevailing suppression of endogenous kisspeptin tone in models of neonatal estrogenization (Ref. 27 and A.H. Bentsen, Tena-Sempere, M., and Mikkelsen, J.D., unpublished data). Nonetheless, our present findings strongly suggest that the rescue of NKB signaling in such conditions, by means of senktide administration, would be sufficient to ameliorate the defective kisspeptin and, hence, GnRH secretion, thus reinforcing the idea that NKB is an important central regulator of the gonadotropic axis.
Finally, our study addresses also the intriguing issue of potential differences in the sex steroid regulation of NKB expression in various hypothalamic nuclei. In this sense, although there is compelling evidence for the scattered expression of NKB in different hypothalamic areas (7, 31), and for the regulation of the hypothalamic NKB/NK3R system by sex steroids in a number of species (5, 7, 8, 18, 23, 25, 28–30), most of the studies on NKB regulation have been restricted to the ARC/infundibular nucleus, whereas other hypothalamic areas with prominent NKB expression, such as the LHA, remain virtually unexplored. The LHA is the source of an array of neuroendocrine factors known to participate in the central control of energy balance and reproductive function, such as orexins or melanin-concentrating hormone (MCH) (39, 40). Of note, MCH-positive neurons in the LHA have been described to express NK3R (41–45), and MCH has been shown to modulate the action of kisspeptins on GnRH neurons (46). These data indirectly support the potential interplay between NKB/MCH/kisspeptin/GnRH through networks involving the LHA. To our knowledge, our data are the first to document a striking site-specific dimorphism in the action of E2 on Tac2 expression, with consistent inhibitory effects in the ARC but unambiguous stimulatory actions in the LHA; in fact, E2 also stimulated Tacr3 mRNA levels at this hypothalamic area (our unpublished data). This phenomenon resembles the dual, opposite regulation of Kiss1 gene expression by estrogen between the ARC (inhibition) vs. the anteroventral periventricular nucleus (stimulation) (19, 47). Although the molecular mechanisms (via nonclassical and classical estrogen receptor-α pathways) and physiological relevance (e.g. for negative and positive feedback) of such differential regulation of Kiss1 expression have been characterized in recent years, the basis and putative roles of this dual regulation of Tac2 expression by E2 in the ARC and LHA remain to be determined. In any event, our present findings broaden the number (and pave the way for specific analyses) of possible modes of action of NKB in the control of the gonadotropic and, potentially, other related axes. As call of caution, however, it must be stressed that our initial attempts have failed to provide conclusive evidence for the presence of NKB-ir in the LHA in adult male and female rats, an observation that might suggest either the lack of significant translation, or the rapid turnover or release, of NKB in this hypothalamic area in normal conditions. Considering the fact that basal Tac2 mRNA levels in the LHA were low but increased with acute E2 treatment (see Fig. 5), it also remains possible that NKB-ir may become detectable only under E2-stimulated conditions. The above possibilities are currently being investigated in our laboratory.
In sum, we present herein an extensive series of experiments addressing as yet unsolved aspects of the physiology of the NKB/NK3R system regarding reproductive control in male and female rats. Our current data unveil intriguing species differences, postnatal maturational changes, sexual dimorphisms, and differential regulatory actions of sex steroids on NKB expression/actions in the context of gonadotropin regulation in the rat. These findings will help to refine our current understanding of the nature and functional relevance of NKB as master central modulator of the reproductive axis in this relevant preclinical species.
Acknowledgments
This research was supported by: Spanish research grants BFU 2008-00984 and BFU2011-25021 (Ministerio de Economía y Competitividad; cofunded by European Union FEDER Program) and P08-CVI-00603 (Junta de Andalucía); European Union research contract Developmental Effects of Environment on Reproductive Health FP7-ENV-2007-1; training grants awarded by the FP7 Marie Curie Outgoing International Fellowship of the European Union; the NOVO Nordisk Foundation; and National Institutes of Health (NIH) Grant R01 HD 049651 and the Eunice Kennedy Shriver National Institute of Child Health and Human Development/NIH through cooperative agreement U54HD12629 (to R.A.S.).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- ARC
- Arcuate nucleus
- AUC
- area under the curve
- DHT
- dihydrotestosterone
- E2
- 17β-estradiol
- EB
- estradiol benzoate
- GNX
- gonadectomized/gonadectomy
- icv
- intracerebroventricular
- IHC
- immunohistochemistry/immunohistochemical
- ISH
- in situ hybridization
- KNDy
- kisspeptin/neurokinin B/dynorphin
- LHA
- lateral hypothalamus
- MCH
- melanin-concentrating hormone
- NKB
- neurokinin B
- NK3R
- NKB receptor
- NKB-ir
- NKB immunoreactive
- ORX
- castrated/castration
- OVX
- ovariectomized/ovariectomy
- T
- testosterone.
References
- 1. Herbison A. 2006. Physiology of the GnRH neuronal network. In: Neil J, Knobil E, eds. Physiology of reproduction. San Diego, CA: Academic Press; 1415–1482 [Google Scholar]
- 2. Topaloglu AK, Reimann F, Guclu M, Yalin AS, Kotan LD, Porter KM, Serin A, Mungan NO, Cook JR, Ozbek MN, Imamoglu S, Akalin NS, Yuksel B, O'Rahilly S, Semple RK. 2009. TAC3 and TACR3 mutations in familial hypogonadotropic hypogonadism reveal a key role for Neurokinin B in the central control of reproduction. Nat Genet 41:354–358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Young J, Bouligand J, Francou B, Raffin-Sanson ML, Gaillez S, Jeanpierre M, Grynberg M, Kamenicky P, Chanson P, Brailly-Tabard S, Guiochon-Mantel A. 2010. TAC3 and TACR3 defects cause hypothalamic congenital hypogonadotropic hypogonadism in humans. J Clin Endocrinol Metab 95:2287–2295 [DOI] [PubMed] [Google Scholar]
- 4. Sandoval-Guzmán T, Rance NE. 2004. Central injection of senktide, an NK3 receptor agonist, or neuropeptide Y inhibits LH secretion and induces different patterns of Fos expression in the rat hypothalamus. Brain Res 1026:307–312 [DOI] [PubMed] [Google Scholar]
- 5. Navarro VM, Gottsch ML, Chavkin C, Okamura H, Clifton DK, Steiner RA. 2009. Regulation of gonadotropin-releasing hormone secretion by kisspeptin/dynorphin/neurokinin B neurons in the arcuate nucleus of the mouse. J Neurosci 29:11859–11866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wakabayashi Y, Nakada T, Murata K, Ohkura S, Mogi K, Navarro VM, Clifton DK, Mori Y, Tsukamura H, Maeda K, Steiner RA, Okamura H. 2010. Neurokinin B and dynorphin A in kisspeptin neurons of the arcuate nucleus participate in generation of periodic oscillation of neural activity driving pulsatile gonadotropin-releasing hormone secretion in the goat. J Neurosci 30:3124–3132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Navarro VM, Castellano JM, McConkey SM, Pineda R, Ruiz-Pino F, Pinilla L, Clifton DK, Tena-Sempere M, Steiner RA. 2011. Interactions between kisspeptin and neurokinin B in the control of GnRH secretion in the female rat. Am J Physiol Endocrinol Metab 300:E202–E210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Navarro VM, Gottsch ML, Wu M, García-Galiano D, Hobbs SJ, Bosch MA, Pinilla L, Clifton DK, Dearth A, Ronnekleiv OK, Braun RE, Palmiter RD, Tena-Sempere M, Alreja M, Steiner RA. 2011. Regulation of NKB pathways and their roles in the control of Kiss1 neurons in the arcuate nucleus of the male mouse. Endocrinology 152:4265–4275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Navarro VM, Ruiz-Pino F, Sánchez-Garrido MA, García-Galiano D, Hobbs SJ, Manfredi-Lozano M, León S, Sangiao-Alvarellos S, Castellano JM, Clifton DK, Pinilla L, Steiner RA, Tena-Sempere M. 2012. Role of neurokinin B in the control of female puberty and its modulation by metabolic status. J Neurosci 32:2388–2397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. García-Galiano D, van Ingen Schenau D, Leon S, Krajnc-Franken MA, Manfredi-Lozano M, Romero-Ruiz A, Navarro VM, Gaytan F, van Noort PI, Pinilla L, Blomenröhr M, Tena-Sempere M. 2012. Kisspeptin signaling is indispensable for neurokinin B, but not glutamate, stimulation of gonadotropin secretion in mice. Endocrinology 153:316–328 [DOI] [PubMed] [Google Scholar]
- 11. Billings HJ, Connors JM, Altman SN, Hileman SM, Holaskova I, Lehman MN, McManus CJ, Nestor CC, Jacobs BH, Goodman RL. 2010. Neurokinin B acts via the neurokinin-3 receptor in the retrochiasmatic area to stimulate luteinizing hormone secretion in sheep. Endocrinology 151:3836–3846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ramaswamy S, Seminara SB, Plant TM. 2011. Evidence from the agonadal juvenile male Rhesus monkey (Macaca mulatta) for the view that the action of Neurokinin B to trigger gonadotropin-releasing hormone release is upstream from the kisspeptin receptor. Neuroendocrinology 94:237–245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ramaswamy S, Seminara SB, Ali B, Ciofi P, Amin NA, Plant TM. 2010. Neurokinin B stimulates GnRH release in the male monkey (Macaca mulatta) and is colocalized with kisspeptin in the arcuate nucleus. Endocrinology 151:4494–4503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Corander MP, Challis BG, Thompson EL, Jovanovic Z, Loraine Tung YC, Rimmington D, Huhtaniemi IT, Murphy KG, Topaloglu AK, Yeo GS, O'Rahilly S, Dhillo WS, Semple RK, Coll AP. 2010. The effects of neurokinin B upon gonadotrophin release in male rodents. J Neuroendocrinol 22:181–187 [DOI] [PubMed] [Google Scholar]
- 15. Kinsey-Jones JS, Grachev P, Li XF, Lin YS, Milligan SR, Lightman SL, O'Byrne KT. 2012. The inhibitory effects of neurokinin B on GnRH pulse generator frequency in the female rat. Endocrinology 153:307–315 [DOI] [PubMed] [Google Scholar]
- 16. Navarro VM, Tena-Sempere M. 2012. Neuroendocrine control by kisspeptins: role in metabolic regulation of fertility. Nat Rev Endocrinol 8:40–53 [DOI] [PubMed] [Google Scholar]
- 17. Goodman RL, Lehman MN, Smith JT, Coolen LM, de Oliveira CV, Jafarzadehshirazi MR, Pereira A, Iqbal J, Caraty A, Ciofi P, Clarke IJ. 2007. Kisspeptin neurons in the arcuate nucleus of the ewe express both dynorphin A and neurokinin B. Endocrinology 148:5752–5760 [DOI] [PubMed] [Google Scholar]
- 18. Dellovade TL, Merchenthaler I. 2004. Estrogen regulation of neurokinin B gene expression in the mouse arcuate nucleus is mediated by estrogen receptor α. Endocrinology 145:736–742 [DOI] [PubMed] [Google Scholar]
- 19. Smith JT, Cunningham MJ, Rissman EF, Clifton DK, Steiner RA. 2005. Regulation of Kiss1 gene expression in the brain of the female mouse. Endocrinology 146:3686–3692 [DOI] [PubMed] [Google Scholar]
- 20. Smith JT, Dungan HM, Stoll EA, Gottsch ML, Braun RE, Eacker SM, Clifton DK, Steiner RA. 2005. Differential regulation of KiSS-1 mRNA expression by sex steroids in the brain of the male mouse. Endocrinology 146:2976–2984 [DOI] [PubMed] [Google Scholar]
- 21. Lehman MN, Coolen LM, Goodman RL. 2010. Minireview: kisspeptin/neurokinin B/dynorphin (KNDy) cells of the arcuate nucleus: a central node in the control of gonadotropin-releasing hormone secretion. Endocrinology 151:3479–3489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Cheng G, Coolen LM, Padmanabhan V, Goodman RL, Lehman MN. 2010. The kisspeptin/neurokinin B/dynorphin (KNDy) cell population of the arcuate nucleus: sex differences and effects of prenatal testosterone in sheep. Endocrinology 151:301–311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Goubillon ML, Forsdike RA, Robinson JE, Ciofi P, Caraty A, Herbison AE. 2000. Identification of neurokinin B-expressing neurons as an highly estrogen-receptive, sexually dimorphic cell group in the ovine arcuate nucleus. Endocrinology 141:4218–4225 [DOI] [PubMed] [Google Scholar]
- 24. Ciofi P, Lapirot OC, Tramu G. 2007. An androgen-dependent sexual dimorphism visible at puberty in the rat hypothalamus. Neuroscience 146:630–642 [DOI] [PubMed] [Google Scholar]
- 25. Ciofi P, Leroy D, Tramu G. 2006. Sexual dimorphism in the organization of the rat hypothalamic infundibular area. Neuroscience 141:1731–1745 [DOI] [PubMed] [Google Scholar]
- 26. Roa J, Vigo E, Castellano JM, Gaytan F, García-Galiano D, Navarro VM, Aguilar E, Dijcks FA, Ederveen AG, Pinilla L, van Noort PI, Tena-Sempere M. 2008. Follicle-stimulating hormone responses to kisspeptin in the female rat at the preovulatory period: modulation by estrogen and progesterone receptors. Endocrinology 149:5783–5790 [DOI] [PubMed] [Google Scholar]
- 27. Navarro VM, Sánchez-Garrido MA, Castellano JM, Roa J, García-Galiano D, Pineda R, Aguilar E, Pinilla L, Tena-Sempere M. 2009. Persistent impairment of hypothalamic KiSS-1 system after exposures to estrogenic compounds at critical periods of brain sex differentiation. Endocrinology 150:2359–2367 [DOI] [PubMed] [Google Scholar]
- 28. Danzer SC, Price RO, McMullen NT, Rance NE. 1999. Sex steroid modulation of neurokinin B gene expression in the arcuate nucleus of adult male rats. Brain Res Mol Brain Res 66:200–204 [DOI] [PubMed] [Google Scholar]
- 29. Pillon D, Caraty A, Fabre-Nys C, Bruneau G. 2003. Short-term effect of oestradiol on neurokinin B mRNA expression in the infundibular nucleus of ewes. J Neuroendocrinol 15:749–753 [DOI] [PubMed] [Google Scholar]
- 30. Rance NE, Bruce TR. 1994. Neurokinin B gene expression is increased in the arcuate nucleus of ovariectomized rats. Neuroendocrinology 60:337–345 [DOI] [PubMed] [Google Scholar]
- 31. Warden MK, Young WS., III 1988. Distribution of cells containing mRNAs encoding substance P and neurokinin B in the rat central nervous system. J Comp Neurol 272:90–113 [DOI] [PubMed] [Google Scholar]
- 32. Ciriza I, Carrero P, Azcoitia I, Lundeen SG, Garcia-Segura LM. 2004. Selective estrogen receptor modulators protect hippocampal neurons from kainic acid excitotoxicity: differences with the effect of estradiol. J Neurobiol 61:209–221 [DOI] [PubMed] [Google Scholar]
- 33. Hansen HH, Timmermann DB, Peters D, Walters C, Damaj MI, Mikkelsen JD. 2007. α-7 Nicotinic acetylcholine receptor agonists selectively activate limbic regions of the rat forebrain: an effect similar to antipsychotics. J Neurosci Res 85:1810–1818 [DOI] [PubMed] [Google Scholar]
- 34. Protter M, Morrey C. 1964. College calculus with analytical geometry. Reading, MA: Addison-Wesley Publishing Co [Google Scholar]
- 35. Yang JJ, Caligioni CS, Chan YM, Seminara SB. 2012. Uncovering novel reproductive defects in neurokinin B receptor null mice: closing the gap between mice and men. Endocrinology 153:1498–1508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Castellano JM, Navarro VM, Fernandez-Fernandez R, Castano JP, Malagon MM, Aguilar E, Dieguez C, Magni P, Pinilla L, Tena-Sempere M. 2006. Ontogeny and mechanisms of action for the stimulatory effect of kisspeptin on gonadotropin-releasing hormone system of the rat. Mol Cell Endocrinol 257–258:75–83 [DOI] [PubMed] [Google Scholar]
- 37. Navarro VM, Castellano JM, Fernández-Fernández R, Barreiro ML, Roa J, Sanchez-Criado JE, Aguilar E, Dieguez C, Pinilla L, Tena-Sempere M. 2004. Developmental and hormonally regulated messenger ribonucleic acid expression of KiSS-1 and its putative receptor, GPR54, in rat hypothalamus and potent luteinizing hormone-releasing activity of KiSS-1 peptide. Endocrinology 145:4565–4574 [DOI] [PubMed] [Google Scholar]
- 38. Young J, George JT, Tello JA, Francou B, Bouligand J, Guiochon-Mantel A, Brailly-Tabard S, Anderson RA, Millar RP. 24 February 2012. Kisspeptin restores pulsatile LH secretion in patients with neurokinin B signaling deficiencies: physiological, pathophysiological and therapeutic implications. Neuroendocrinology 10.1159/000336376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Nurmio M, Tena-Sempere M, Toppari J. 2010. Orexins and the regulation of the hypothalamic-pituitary-testicular axis. Acta Physiol (Oxf) 198:349–354 [DOI] [PubMed] [Google Scholar]
- 40. Williamson-Hughes PS, Grove KL, Smith MS. 2005. Melanin concentrating hormone (MCH): a novel neural pathway for regulation of GnRH neurons. Brain Res 1041:117–124 [DOI] [PubMed] [Google Scholar]
- 41. Griffond B, Ciofi P, Bayer L, Jacquemard C, Fellmann D. 1997. Immunocytochemical detection of the neurokinin B receptor (NK3) on melanin-concentrating hormone (MCH) neurons in rat brain. J Chem Neuroanat 12:183–189 [DOI] [PubMed] [Google Scholar]
- 42. Brischoux F, Fellmann D, Risold PY. 2001. Ontogenetic development of the diencephalic MCH neurons: a hypothalamic 'MCH area' hypothesis. Eur J Neurosci 13:1733–1744 [DOI] [PubMed] [Google Scholar]
- 43. Brischoux F, Cvetkovic V, Griffond B, Fellmann D, Risold PY. 2002. Time of genesis determines projection and neurokinin-3 expression patterns of diencephalic neurons containing melanin-concentrating hormone. Eur J Neurosci 16:1672–1680 [DOI] [PubMed] [Google Scholar]
- 44. Cvetkovic V, Poncet F, Fellmann D, Griffond B, Risold PY. 2003. Diencephalic neurons producing melanin-concentrating hormone are influenced by local and multiple extra-hypothalamic tachykininergic projections through the neurokinin 3 receptor. Neuroscience 119:1113–1145 [DOI] [PubMed] [Google Scholar]
- 45. Croizier S, Franchi-Bernard G, Colard C, Poncet F, La Roche A, Risold PY. 2010. A comparative analysis shows morphofunctional differences between the rat and mouse melanin-concentrating hormone systems. PLoS One 5:e15471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Wu M, Dumalska I, Morozova E, van den Pol A, Alreja M. 2009. Melanin-concentrating hormone directly inhibits GnRH neurons and blocks kisspeptin activation, linking energy balance to reproduction. Proc Natl Acad Sci USA 106:17217–17222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Gottsch ML, Navarro VM, Zhao Z, Glidewell-Kenney C, Weiss J, Jameson JL, Clifton DK, Levine JE, Steiner RA. 2009. Regulation of Kiss1 and dynorphin gene expression in the murine brain by classical and nonclassical estrogen receptor pathways. J Neurosci 29:9390–9395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Paxinos G, Watson C. 2009. The rat brain in stereotaxic coordinates, 6th ed New York: Academic; [DOI] [PubMed] [Google Scholar]