Abstract
During pregnancy, fetal glucocorticoid is derived from both maternal supply and fetal secretion. We have created mice with a disruption of the Cyp11a1 gene resulting in loss of fetal steroid secretion but preserving the maternal supply. Cyp11a1null embryos have appreciable although lower amounts of circulating corticosterone, the major mouse glucocorticoid, suggesting that transplacental corticosterone is a major source of corticosterone in fetal circulation. These embryos thus provide a means to examine the effect of fetal glucocorticoids. The adrenal in Cyp11a1 null embryos was disorganized with abnormal mitochondria and oil accumulation. The adrenal medullary cells did not express phenylethanolamine N-methyltransferase and synthesized no epinephrine. Cyp11a1 null embryos had decreased diencephalon Hsd11b1, increased diencephalon Crh, and increased pituitary Pomc expression, leading to higher adrenocorticotropin level in the plasma. These data indicate blunted feedback suppression despite reasonable amounts of circulating corticosterone. Thus, the corticosterone synthesized in situ by the fetus is required for negative feedback suppression of the hypothalamus-pituitary-adrenal axis and for catecholamine synthesis in adrenal medulla.
The majority of circulating steroids are secreted from the adrenal cortex, which contains enzymes that function in steroid synthesis, such as CYP11A1, CYP11B1, and HSD3B. The levels of glucocorticoids in the body are tightly controlled in the hypothalamus-pituitary-adrenal (HPA) axis, which is activated by CRH in the hypothalamus and ACTH in the pituitary, and attenuated by glucocorticoids in a negative feedback loop (1). The major glucocorticoid in most mammalian species is cortisol, but rodents use corticosterone as their major glucocorticoid.
Glucocorticoids play important roles in glucose homeostasis, lung maturation, antiinflammation, and the development of adrenal medulla. The adrenal medulla contains enzymes for catecholamine secretion such as tyrosine hydroxylase (TH) and phenylethanolamine N-methyltransferase (PNMT). PNMT expression is induced by glucocorticoids (2–5).
Glucocorticoids are present in a large quantity during pregnancy to support the growth and development of the fetus. Prenatal exposure to glucocorticoids affects HPA development and permanently changes the HPA activity in the adulthood (1). Prenatal glucocorticoids come from both fetal secretion and maternal supply transferred through the placenta (6). Maternal glucocorticoids are necessary for the development of adrenal medulla, pancreatic β-cells, cerebral cortex, and lung maturation (7–10). The functions of glucocorticoids secreted from the fetus, however, is still unclear.
The gap in our understanding about the functions of fetal steroids comes from the lack of an animal model that differentiates the roles of maternal steroids and de novo synthesized steroids. Here, we have used the Cyp11a1 null fetus that is devoid of fetal synthesis but retains maternal steroid supply. Using this mouse model, here we show that de novo synthesized steroids affect circulating corticosterone levels, medulla function, and the negative feedback of the HPA axis during the fetal stage. In this study, we demonstrate that steroids from the fetus itself are necessary for normal fetal development.
Materials and Methods
The generation of Cyp11a1 mutant mice
The original Cyp11a1 null mice contained a neo marker in its exon 1 (11). This neo gene was removed from the genome after mating with EIIa-CRE transgenic mice, which express the CRE recombinase gene at the one-cell stage (12). The resulting mouse strain contains a loxP site in the first exon, which creates a frame-shift mutation and thus still results in a null phenotype. This mouse has been backcrossed with inbred strain (C57BL/6) female mice for 7–10 generations. Mice were housed in specific pathogen-free environment under a 14-h light, 10-h dark cycle. The use of mice complied with the guidelines set forth by the Institutional Animal Care and Utilization Committee. All experiments were performed on at least three animals for each genotype.
Histological analysis
The specimens were fixed with Bouin's solution overnight, embedded in paraffin after standard sectioning, and stained in hematoxylin/eosin (H&E). For Oil Red O staining, frozen tissue sections were stained with Oil Red O (Sigma, St. Louis, MO) as previously described (11).
Immunohistochemistry
For immunohistochemical analysis, samples were fixed in 4% paraformaldehyde/PBS in 4 C overnight and embedded in paraffin. Paraffin-embedded sections were dewaxed and rehydrated in a series of alcohol to PBS. The endogenous peroxidase activity was blocked by 3% H2O2 (in methanol) for 8 min and rinsed with PBS three times for 5 min each. Slides were pretreated in the microwave in 0.1 mm citrate acid for 1 min. After preincubating with 1.5% normal goat serum in PBS for 30 min, sections were incubated with the anti-PNMT or anti-TH antibody (diluted 1:1000 in phosphate-buffered saline with 0.1% Tween-20 containing 1.5% normal goat serum; Chemicon International, Inc., Temicula, CA) at 4 C overnight. For proliferating cell nuclear antigen (PCNA), slides were stained with Zymed's PCNA staining kit (Zymed Laboratories, Inc., CA.) according to the manufacturer's instructions. After rinsing with PBST, sections were incubated with biotinylated antibody for 30 min and avidin-biotin-peroxidase complex for 10 min (ABC kit; Vector Laboratory, Inc., Burlingame, CA). Visualization of the immune complex was achieved by incubating the sections in 3,3′-diaminobenzidine for 2 min. Slides were dehydrated and mounted in Permount.
Hormone assays
Plasma was collected from embryonic mice that were killed by decapitation at 0900 or 1800 h. Plasma was collected in ice-cold EDTA-rinsed tubes. Hormonal analyses were performed with RIA kits for corticosterone (ICN Biomedicals, Inc., Palo Alto, CA) and ACTH (Nichols Institute Diagnostics, San Juan Capistrano, CA) according to the manufacturer's instructions. Two microliters of plasma were used for corticosterone test, and 50 μl of plasma were used for ACTH test.
Real-time PCR
Total RNA was isolated from each adrenal, diencephalon, or pituitary using the RNAeasy kit from QIAGEN (Valencia, CA). The RNA pellet was dissolved in 20 μl of water, and 10 μl of the RNA solution were used for RT. RT was performed by Oligo(dT) primer and reverse transcriptase (SuperSript II; Invitrogen, Carlsbad, CA). First-strand cDNA was used as the template together with 250 nm each primer in a LightCycler quantitative PCR (Roche Diagnostics, Grenzacherstrasse, Switzerland) with QuantiTect SYBR Green PCR Master Mixture (QIAGEN) to follow the progress of DNA synthesis. RNA amounts were calculated with relative standard curves for glyceraldehyde-3-phosphate dehydrogenase and each gene. Primers used were: TGCGGGCTCACCTACCA (forward) and AAGGCAGGCAGGACGACA (reverse) for Crh, GTGTTTCCTGGCAACGGAGATG (forward) and CATGA AGCCACCGTA ACGCTTG (reverse) for Pomc, TCATGGCGTG AGTACCTC (forward) and GGGCTTGAATATCCATTAGAA (reverse) for GR, GGTGTGCCTTTCCCCATCATT (forward) and CAACATGTAGGTGATGCCCAG (reverse) for Crhr, ACCCCGACATCATATTTAAGGA (forward) and TTAACTTGTCTTTGCACCTCTGA (reverse) for Shh, TCAGCCCATATCCACAAT (forward) and CAGGGAACCAGAAAGCAG for Ucn3, and GCTGTAGCC AAATTCGTTGTC and GATGACATCAAGAAGGTGGTG for Gapdh. For Hsd11b1 and Avp, real-time quantitative PCR was performed using TaqMan Assay-on-Demand (Applied Biosystems, Foster City, CA) and using the 7000 Sequence Detector (Applied Biosystems).
Epinephrine and norepinephrine measurement
Each adrenal was homogenized in 500 μl of ice-cold 0.4 m perchloric acid containing 0.5 mm EDTA. Homogenates were centrifuged at 1000 × g at 4 C for 20 min, and the supernatants were store at −80 C until assay. Each sample was adjusted to 4 ml with 10 n HCl for standard epinephrine and norephinephrine HPLC assays.
Chromaffin reaction
Adrenal was stained with chromium salt by soaking in Muller's fluid (2.5 g of K2Cr2O7, 1 g of Na2SO4 · 10H2O in 100 ml H2O) for 48 h and washed in running water overnight. Tissues were fixed in 10% neutral formaldehyde overnight, embedded in wax, and sliced into 15-μm sections. Adrenal sections were dewaxed and mounted in Permount followed by visualization under a microscope.
Ultrastructural studies
Tissues were fixed in 2.5% glutaraldehyde in 0.1 m phosphate buffer (pH 7.2) overnight followed by washing in 0.1 m phosphate buffer (15 min, three times). After refixation in 1% osmium tetraoxide for 2 h at room temperature, they were washed in phosphate buffer, dehydrated in graded series of acetone/phosphate buffer for 15 min each (30, 50, 70, 80, 90, 95, and 100%), equilibrated, and embedded in ERL 4206 epoxy resin. For transmission electron microscopic studies, ultrathin 80-nm sections were mounted on coated 50-mesh copper grids, contrasted with aqueous solutions of uranyl acetate and lead citrate, and viewed and photographed using the FEI Tecnai TM G2 Transmission Electron Microscope.
Results
Cyp11a1 null mice suffer from mild defects of steroid secretion during the embryonic stage
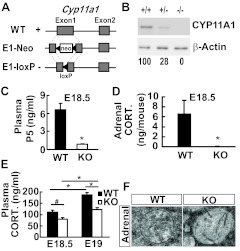
We have previously generated Cyp11a1E1-neo mice with the insertion of a neo gene into the first exon of Cyp11a1 resulting in complete loss of gene function and neonatal death (11). To eliminate the interference of the neo cassette, we have since removed the neo gene in the first exon using the Cre recombinase, which was introduced into the Cyp11a1E1-neo mice since fertilization via mating with the EIIa-cre transgenic mice. The resulting Cyp11a1E1-loxP mice had a LoxP sequence inserted into the first exon of Cyp11a1 causing a frame shift mutation (Fig. 1A). These mice expressed no CYP11A1 in their adrenals at embryonic d 18.5 (E18.5) (Fig. 1B). Their plasma pregnenolone (Fig. 1C) and adrenal corticosterone (Fig. 1D) levels were greatly reduced, indicative of the defect in de novo steroid synthesis at the embryonic stage. The resulting mice survived throughout gestation but died after birth.
Fig. 1.
Defective hormone secretion of CYP11A1 null embryos. A, Diagrams of the Cyp11a1 alleles showing the insertion of the neo gene or of the loxP site in the first exon in E1-Neo and E1-loxP, respectively. B, Western blot analysis of E18.5 adrenal CYP11A1 from the wild-type (WT) (+/+), heterozygous (+/−), or homozygous (−/−) Cyp11a1 mutant mice. C, Plasma levels of pregnenolone (P5) at E18.5 measured by RIA are shown as mean ± sem [*, P = 0.002 vs. control littermates; WT, n = 5; knockout (KO), n = 4]. D, Tissue levels of corticosterone (CORT.) in E18.5 adrenal measure by RIA are shown as mean ± sem (*, P = 0.03 vs. control littermates; WT, n = 4; KO, n = 5). E, Plasma levels of corticosterone at E18.5 (WT, n = 15; KO, n = 10) and E19 (WT, n = 11; KO, n = 10) measured by RIA are shown as mean ± sem (*, P = 0.001; #, P = 0.02). F, Transmission electron micrographs of mitochondria in adrenocortical cells at E17.5.
The survival of these knockout mice during gestation indicates that some steroids are present to sustain their life. Indeed, we detected appreciable although lower amounts of corticosterone, the major mouse glucocorticoid, in the plasma of knockout mice at both E18.5 (morning) and E19 (evening) (Fig. 1E). Because the knockout adrenal synthesized no corticosterone (Fig. 1D), the corticosterone detected in their plasma should all come from the maternal supply. Thus, the transplacental corticosterone appeared to be the major source of corticosterone in fetal circulation. The decrease of plasma corticosterone also indicates that these knockout mice suffer from mild steroid deficiency even at the embryonic stage.
Disorganized embryonic Cyp11a1 null adrenals
The mitochondria from the zona fasciculata of E17.5 adrenal were examined by electron microscopy. Wild-type mitochondria appeared tubulo-vesicular, indicating these mitochondria were functional with steroidogenic activity (Fig. 1F). The null mitochondria, on the contrary, contained only a few stalks and lacked discernable cisternae (Fig. 1F). This indicates that mitochondrial abnormality existed even in the fetal stage.
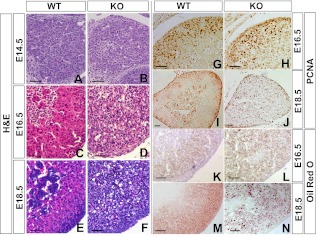
We examined histology of the fetal adrenal at different developmental stages. At E14.5, both wild-type and null adrenals were composed of cells with no distinct features, and no recognizable difference was observed (Fig. 2, A and B). In E16.5 wild-type adrenal, the medulla was easily recognized inside the cortex, and a preliminary zonation of adrenal cortex was observed (Fig. 2C). However, in null mice, the adrenals appeared disorganized, and separation of the cortex and the medulla was not recognized (Fig. 2D). At E18.5, vacuolated cytoplasm was present in the adrenocortical cells of Cyp11a1 null mice (Fig. 2, E and F).
Fig. 2.
Cyp11a1 null adrenal cells are disorganized and accumulate oil. A–F, H&E staining was performed on adrenal sections. G–J, Immunohistochemistry for PCNA was performed on adrenal sections. K–N, Oil Red O staining was performed on adrenal sections. Scale bars, 50 μm. WT, Wild type; KO, knockout.
We examined the growth of the fetal adrenal by histological staining of PCNA, a cell proliferation marker. In the wild-type adrenal, PCNA staining was located in the cortex at E16.5 and was further restricted to the outer periphery of the adrenal at E18.5 (Fig. 2, G and I). In the null adrenal, PCNA positive signals were evident but were randomly distributed at E16.5 (Fig. 2H) and E18.5 (Fig. 2J). This indicates that the defect in the Cyp11a1 null mice affects the distribution, but not the proliferation, of the cortical cells.
Lipid accumulation in null adrenal
Cyp11a1-deficient adrenals accumulate foamy vacuoles and lipid droplets at postnatal d 3 (11). We would like to follow the time course of oil accumulation in fetal adrenals. Upon staining lipid with Oil Red O, mild oil droplets started to appear in the cortex of wild-type adrenal at E16.5 (Fig. 2K) and were more evident at E18.5 (Fig. 2M). In the null adrenal, oil droplets appeared normal at E16.5 (Fig. 2L) but were much larger and randomly distributed in the adrenal at E18.5 (Fig. 2N). This severe oil accumulation parallels the appearance of foamy adrenal observed in H&E staining. Thus, the defect of lipid accumulation already occurred during the embryonic stage.
Cyp11a1 null adrenal lacks adrenergic chromaffin cells
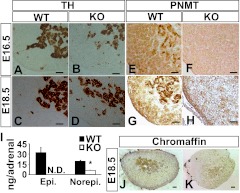
The medulla of Cyp11a1 null adrenal was examined by immunohistochemical staining of medullary markers TH and PNMT. Both E16.5 and E18.5 null adrenals stained normally for TH (Fig. 3, A–D). Because TH is the first enzyme in the synthesis of catecholamine in the medulla, this result suggests that TH-positive neural crest cells migrate into Cyp11a1 null adrenal normally.
Fig. 3.
Cyp11a1 null embryos fail to express medullary PNMT and synthesize epinephrine. Immunohistochemistry for (A–D) TH (brown staining) and (E–H) PNMT (brown staining) on adrenal sections. I, Amount of epinephrine (Epi.) and norepinephrine (Norepi.) in E18.5 adrenal measured by HPLC are shown as mean ± sd [wild type (WT), n = 5; knockout (KO), n = 6]. J and K, Chromaffin reaction was performed on E18.5 adrenals. N.D., Undetected. Scale bars, 50 μm.
Contrary to TH expression, null adrenals expressed no PNMT at E16.5 and E18.5, whereas wild-type adrenal contained plenty of it (Fig. 3, E–H). PNMT is a marker for adrenergic chromaffin cells; the lack of PNMT expression indicates that the Cyp11a1 null adrenal contains no adrenergic chromaffin cells and therefore cannot synthesize epinephrine. Indeed, epinephrine was undetected in the Cyp11a1 null adrenal (n = 5) (Fig. 3I). The level of norepinephrine was also reduced. Moreover, Cyp11a1 null adrenal also reacted poorly in chromaffin reaction (Fig. 3, J and K), indicating that the null medulla contains little catecholamine.
Activated HPA axis in Cyp11a1 null fetus
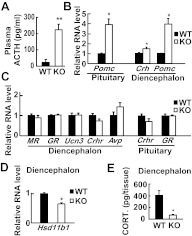
In addition to the adrenal, we checked ACTH secretion in the negative feedback suppression of glucocorticoid. In the null fetus at E18.5, the plasma ACTH level was about 10-fold higher than that in the wild-type fetus (Fig. 4A). This unsuppressed ACTH indicates that the negative feedback in null fetus is absent, even when an appreciable amount of transplacental corticosterone was in the circulation.
Fig. 4.
Unsuppressed HPA axis in Cyp11a1 null embryos. A, Plasma levels of ACTH at E18.5 measured by RIA are shown as mean ± sem. B, Relative RNA levels of Pomc and Crh at E18.5 measured by real-time RT-PCR are shown as mean ± sem [pituitary Pomc: *, P < 0.01 vs. control littermates; wild type (WT), n = 4; knockout (KO), n = 4; diencephalon Crh: *, P = 0.04 vs. control littermates; WT, n = 6; KO, n = 7; and diencephalon Pomc: **, P < 0.01 vs. control littermates; WT, n = 6; KO, n = 7]. C, RNA levels of genes related to HPA axis activity at E18.5 measured by real-time RT-PCR are shown as mean ± sem (diencephalon: WT, n = 6; KO, n = 6; pituitary: WT, n = 4; KO, n = 4). D, Relative RNA levels of Hsd11b1 in E18.5 diencephalon measured by real-time RT-PCR are shown as mean ± sem (*, P < 0.001 vs. control littermates; WT, n = 5; KO, n = 8). E, Tissue levels of corticosterone (CORT.) in E18.5 diencephalon measured by RIA are shown as mean ± sem (*, P = 0.01 vs. control littermates; WT, n = 4; KO, n = 5).
Besides plasma ACTH, the expression levels of pituitary Pomc, diencephalon Crh, and diencephalon Pomc, as measured by real-time RT-PCR, were higher than those of the wild-type control (Fig. 4B), indicating the HPA axis in the null fetus is consistently activated. The expression levels of other genes in the diencephalon, including MR, GR, Ucn3, Crhr, and Avp, however, were not altered in Cyp11a1 null mice. Pituitary Crhr and GR were also unchanged (Fig. 4C).
Lower Hsd11b1 expression in Cyp11a1 null mice brain
Besides the circulating corticosterone, the source of active corticosterone in the brain includes that converted from the inactive 11-dehydrocorticosterone by HSD11B1 (13). The brain HSD11b1 expression was measured by real-time RT-PCR (Fig. 4D) and found to be lower in the null fetus than the wild type. Consistent with lower levels of Hsd11b1, the corticosterone level in the diencephalon was also much lower than that in the wild type (Fig. 4E).
Discussion
A mouse model of Cyp11a1 deficiency
In this report, we have characterized prenatal phenotypes of mice devoid of Cyp11a1. We show here that fetal glucocorticoid is important for the development of adrenal medulla and the establishment of HPA feedback suppression. The adrenal deficiency in Cyp11a1 null mice is similar to those reported for human and rabbit Cyp11a1 deficiency (14–22). Because human and rabbit Cyp11a1 deficiencies have not been characterized in details, our mouse model may provide a means for detailed analysis of the fetal glucocorticoid functions in the suppression of the HPA axis.
Functions of fetal steroids vs. maternal steroids
Steroids in the fetal circulation are derived from two sources: the mother and the fetus; yet the functions of maternal vs. fetal steroids were never discerned. In this study, we have evidence indicating that the maternal supply constitutes a major source of circulating corticosterone that sustains gestation. Fetal secretion, on the other hand, is important for proper development of the HPA feedback loop and the differentiation of adrenal medullary adrenergic chromaffin cells. Thus, we can now distinguish the functions of maternal vs. fetal steroids during embryogenesis.
Unsuppressed HPA axis in Cyp11a1 null fetus
In this report, we show that Cyp11a1 null mice produce unsuppressed ACTH in utero. It indicates that transplacental corticosterone, even though constituting the majority of the corticosterone supply in the fetal circulation, is insufficient to suppress ACTH expression. It also proves that the steroids synthesized de novo are required to suppress the HPA axis activity even before birth. This is consistent with the presence of multidrug-resistance gene 1-type P-glycoproteins as a natural blood-brain barrier that controls the access of circulating corticosterone into the brain (23). It confirms that the negative feedback loop is already present at the fetal stage (1, 24). Our results, however, do not mean that maternal corticosterone is not important. When the circulating corticosterone is high enough to saturate the blood-brain barrier, some of it will leak into the brain exerting an effect.
In addition to those in the circulation, other sources of steroids are also involved in the regulation of the HPA axis. In the brain, neurosteroids are synthesized in situ in response to stress, and this stress-induced neurosteroid can negatively feedback the HPA axis (25). Here, we showed that the expression of Hsd11b1 was reduced in Cyp11a1 null mouse brain perhaps due to insufficient corticosterone, because Hsd11b1 can be up-regulated by glucocorticoids (26). This reduction of Hsd11b1 may lead to lower amounts of active glucocorticoids in the brain. Thus, besides the lack of neurosteroid production, decreased expression of Hsd11b1 may be the other cause for the reduction of active corticosterone and, thus, the uninhibited HPA axis.
It is interesting that Crh mRNA in Cyp11a1 null mice was mildly increased by only 1.5-fold, but Pomc mRNA and ACTH levels were greatly increased by 3- and 10-fold, respectively. This indicates that only a little CRH can induce high Pomc expression, similar to the earlier observation in stressed rats with mild increase in CRH but high Pomc induction (27). GRdim/dim mice, whose glucocorticoid receptor (GR) cannot dimerize, also have unaffected CRH but increased Pomc levels (28). This demonstrates that the effect of ACTH in the pituitary may be amplified by CRH in the hypothalamus.
The link of cortex and medulla during adrenal development
Cyp11a1 null mice have degenerating adrenal cortex and improper medulla differentiation. These defects were also observed in mice with a homozygous disruption of the StAR, Cyp21, Crhr1, and GR genes, heterozygous for Sf1, and conditionally mutated for GR (29–34). Cyp11a1 and StAR null mice are most similar in their adrenal phenotypes; both are deficient in glucocorticoid secretion and accumulate lipid droplets in their adrenals. The accumulated lipid is most likely cholesterol ester, because free cholesterol stained by Filipin was not increased in Cyp11a1 null adrenals (Supplemental Fig. 1, published on The Endocrine Society's Journals Online web site at http://endo.endojournals.org).
We find that the level of norepinephrin is reduced in Cyp11a1 null mice. This is similar to the case of GR null mice (29), which also contain reduced levels of norepinephrine and no epinephrine. The lack of epinephrine is due to the absence of adrenergic cells. Because these adrenergic cells also produce some norepinephrine, their absence leads to decreased norepinephrine levels in the GR and Cyp11a1 knockout adrenals.
Although Cyp11a1 and GR null mice have similar adrenal medullary defects, there are differences between them. Mutations of GR block both maternal and fetal corticosteroid response, but Cyp11a1 null mice can still respond to maternal corticosterone, because they have normal GR. Thus, the phenotypes in Cyp11a1 null embryos do not represent total corticosterone deficiency, but they manifest the absence of fetal corticosterone. Cyp11a1 null embryos enable the distinction between maternal and fetal steroid supply, and it proves that de novo synthesized fetal corticosterone is responsible for medulla development.
The regulation of the medulla by the glucocorticoid secreted from the cortex is likely based on the relative location of these two tissues. The adrenal is a vascular organ. In rats, adrenals typically comprise around 0.02% of the total body weight but receive approximately 0.14% of the cardiac output (35). The sinusoids are arranged as a network in the cortex continuing to the medulla. The blood supply by this sinusoid network runs from the cortex to the medulla. Thus, the medullary cells receive a steroid-rich blood supply. In the hypophysectomized rat, medullary Pnmt expression requires relatively high circulating glucocorticoid (4, 36). The Cyp11a1 null fetus further provides strong evidence that Pnmt expression during development depends on vicinal glucocorticoid directly supplied by the adrenal either through the sinusoid network or a paracrine mechanism (35, 38). Maternal glucocorticoids, on the other hand, are also required for the differentiation of adrenal medulla, because mice born from adrenalectomized mother lacking maternal corticosterone have reduced catecholamine (7).
Mitochondrial structure is indicative of the activity of a steroidogenic cell (39). The number of cristae in the mitochondria is reduced in StAR null mice (37). We detected mitochondria defect in Cyp11a1-deficient mice as early as E9.5 in placenta (data not shown), in E18.5 adrenal, and also in a mild Cyp11a1 promoter mutant as late as 18-wk-old adrenal glands (40). Thus, mitochondria appear defective as soon as cells encounter Cyp11a1 deficiency when they differentiate into steroidogenic cells.
Adrenal growth and lipid accumulation
Lipoid congenital adrenal hyperplasia patients or mice with mutated StAR or Cyp11a1 have larger adrenals that accumulate lipid aberrantly (11, 34, 41, 42). We have analyzed pathogenesis in Cyp11a1 null fetal adrenals and showed that lipid accumulation started at E16.5 and became progressively worse. About one-half of the adrenal was already smaller at E18.5 (data not shown). Thus, lipid starts to accumulate in the adrenocortical cells, leading to their degeneration soon after these cells are differentiated.
Besides lipid, ACTH may also affect adrenal growth. Disruption of the ACTH receptor gene, Mc2r, results in the atrophy of adrenal zona fasciculata and thickening of the capsule, the adrenal stem/progenitor, in the adulthood (43). The adrenal cortex secretes Sonic Hedgehog, which stimulates the growth of the capsule starting from the embryonic stages (44, 45). It is unclear whether ACTH can control the growth of adrenal capsule by modulating Sonic Hedgehog secretion from the cortex. Our preliminary data showed that Cyp11a1 null adrenals expressed less Shh than the wild type (Supplemental Fig. 2). Because Cyp11a1 null embryos have hypoplastic adrenal but excess prenatal ACTH, it will be a good tool to the study of adrenal stem/progenitor cells.
Supplementary Material
Acknowledgments
We thank Dr. Yu-Yao Huang in Chang-Gung Memorial Hospital for the epinephrine and norepinephrine HPLC assays, Shu-Jan Chou for excellent technical assistant, and Shu-Ping Lee for assistance in the use of electron microscope.
Present address for C.-C.J.H.: National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health, Bethesda, Maryland 20892.
Present address for M.-C.M.S.: Cancer and Stem Cell Biology Program, Duke-National University of Singapore Graduate Medical School, Singapore 169857, Singapore.
This work was supported by the Academia Sinica Grants AS92IMB4PP and NHRI-EX101-9710SI and the National Science Council Grant NSC100-2321-B-001-006).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- E
- Embryonic d
- GR
- glucocorticoid receptor
- H&E
- hematoxylin/eosin
- HPA
- hypothalamus-pituitary-adrenal
- PCNA
- proliferating cell nuclear antigen
- PNMT
- phenylethanolamine N-methyltransferase
- TH
- tyrosine hydroxylase.
References
- 1. Matthews SG. 2002. Early programming of the hypothalamo-pituitary-adrenal axis. Trends Endocrinol Metab 13:373–380 [DOI] [PubMed] [Google Scholar]
- 2. Kvetnanský R, Pacák K, Fukuhara K, Viskupic E, Hiremagalur B, Nankova B, Goldstein DS, Sabban EL, Kopin IJ. 1995. Sympathoadrenal system in stress. Interaction with the hypothalamic-pituitary-adrenocortical system. Ann NY Acad Sci 771:131–158 [DOI] [PubMed] [Google Scholar]
- 3. Evinger MJ, Towle AC, Park DH, Lee P, Joh TH. 1992. Glucocorticoids stimulate transcription of the rat phenylethanolamine N-methyltransferase (PNMT) gene in vivo and in vitro. Cell Mol Neurobiol 12:193–215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wong DL, Lesage A, Siddall B, Funder JW. 1992. Glucocorticoid regulation of phenylethanolamine N-methyltransferase in vivo. FASEB J 6:3310–3315 [DOI] [PubMed] [Google Scholar]
- 5. Huber K. 2006. The sympathoadrenal cell lineage: specification, diversification, and new perspectives. Dev Biol 298:335–343 [DOI] [PubMed] [Google Scholar]
- 6. Mulay S, Solomon S. 1992. Adrenal cortical function during pregnancy. In: James VT, ed. The adrenal gland. 2nd ed New York: Raven Press; 105–116 [Google Scholar]
- 7. Leret ML, Peinado V, González JC, Suárez LM, Rúa C. 2004. Maternal adrenalectomy affects development of adrenal medulla. Life Sci 74:1861–1867 [DOI] [PubMed] [Google Scholar]
- 8. Komatsu S, Yamamoto M, Arishima K, Eguchi Y. 1998. Maternal adrenocortical hormones maintain the early development of pancreatic B cells in the fetal rat. J Anat 193(Pt 4):551–557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Arahuetes RM, Carretero V, Diebold Y, Rua C. 1991. Effects of maternal bilateral adrenalectomy and betamethasone administration on fetal rat encephalic development. Biol Neonate 59:303–313 [DOI] [PubMed] [Google Scholar]
- 10. Venihaki M, Carrigan A, Dikkes P, Majzoub JA. 2000. Circadian rise in maternal glucocorticoid prevents pulmonary dysplasia in fetal mice with adrenal insufficiency. Proc Natl Acad Sci USA 97:7336–7341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hu MC, Hsu NC, El Hadj NB, Pai CI, Chu HP, Wang CK, Chung BC. 2002. Steroid deficiency syndromes in mice with targeted disruption of Cyp11a1. Mol Endocrinol 16:1943–1950 [DOI] [PubMed] [Google Scholar]
- 12. Lakso M, Pichel JG, Gorman JR, Sauer B, Okamoto Y, Lee E, Alt FW, Westphal H. 1996. Efficient in vivo manipulation of mouse genomic sequences at the zygote stage. Proc Natl Acad Sci USA 93:5860–5865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Harris HJ, Kotelevtsev Y, Mullins JJ, Seckl JR, Holmes MC. 2001. Intracellular regeneration of glucocorticoids by 11β-hydroxysteroid dehydrogenase (11β-HSD)-1 plays a key role in regulation of the hypothalamic-pituitary-adrenal axis: analysis of 11β-HSD-1-deficient mice. Endocrinology 142:114–120 [DOI] [PubMed] [Google Scholar]
- 14. Kim CJ, Lin L, Huang N, Quigley CA, AvRuskin TW, Achermann JC, Miller WL. 2008. Severe combined adrenal and gonadal deficiency caused by novel mutations in the cholesterol side chain cleavage enzyme, P450scc. J Clin Endocrinol Metab 93:696–702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hiort O, Holterhus PM, Werner R, Marschke C, Hoppe U, Partsch CJ, Riepe FG, Achermann JC, Struve D. 2005. Homozygous disruption of P450 side-chain cleavage (CYP11A1) is associated with prematurity, complete 46,XY sex reversal, and severe adrenal failure. J Clin Endocrinol Metab 90:538–541 [DOI] [PubMed] [Google Scholar]
- 16. Tajima T, Fujieda K, Kouda N, Nakae J, Miller WL. 2001. Heterozygous mutation in the cholesterol side chain cleavage enzyme (p450scc) gene in a patient with 46,XY sex reversal and adrenal insufficiency. J Clin Endocrinol Metab 86:3820–3825 [DOI] [PubMed] [Google Scholar]
- 17. Katsumata N, Ohtake M, Hojo T, Ogawa E, Hara T, Sato N, Tanaka T. 2002. Compound heterozygous mutations in the cholesterol side-chain cleavage enzyme gene (CYP11A) cause congenital adrenal insufficiency in humans. J Clin Endocrinol Metab 87:3808–3813 [DOI] [PubMed] [Google Scholar]
- 18. al Kandari H, Katsumata N, Alexander S, Rasoul MA. 2006. Homozygous mutation of P450 side-chain cleavage enzyme gene (CYP11A1) in 46, XY patient with adrenal insufficiency, complete sex reversal, and agenesis of corpus callosum. J Clin Endocrinol Metab 91:2821–2826 [DOI] [PubMed] [Google Scholar]
- 19. Rubtsov P, Karmanov M, Sverdlova P, Spirin P, Tiulpakov A. 2009. A novel homozygous mutation in CYP11A1 gene is associated with late-onset adrenal insufficiency and hypospadias in a 46,XY patient. J Clin Endocrinol Metab 94:936–939 [DOI] [PubMed] [Google Scholar]
- 20. Parajes S, Kamrath C, Rose IT, Taylor AE, Mooij CF, Dhir V, Grötzinger J, Arlt W, Krone N. 2011. A novel entity of clinically isolated adrenal insufficiency caused by a partially inactivating mutation of the gene encoding for P450 side chain cleavage enzyme (CYP11A1). J Clin Endocrinol Metab 96:E1798–E1806 [DOI] [PubMed] [Google Scholar]
- 21. Yang X, Iwamoto K, Wang M, Artwohl J, Mason JI, Pang S. 1993. Inherited congenital adrenal hyperplasia in the rabbit is caused by a deletion in the gene encoding cytochrome P450 cholesterol side-chain cleavage enzyme. Endocrinology 132:1977–1982 [DOI] [PubMed] [Google Scholar]
- 22. Pang S, Yang X, Wang M, Tissot R, Nino M, Manaligod J, Bullock LP, Mason JI. 1992. Inherited congenital adrenal hyperplasia in the rabbit: absent cholesterol side-chain cleavage cytochrome P450 gene expression. Endocrinology 131:181–186 [DOI] [PubMed] [Google Scholar]
- 23. Uhr M, Holsboer F, Müller MB. 2002. Penetration of endogenous steroid hormones corticosterone, cortisol, aldosterone and progesterone into the brain is enhanced in mice deficient for both mdr1a and mdr1b P-glycoproteins. J Neuroendocrinol 14:753–759 [DOI] [PubMed] [Google Scholar]
- 24. Henry C, Kabbaj M, Simon H, Le Moal M, Maccari S. 1994. Prenatal stress increases the hypothalamo-pituitary-adrenal axis response in young and adult rats. J Neuroendocrinol 6:341–345 [DOI] [PubMed] [Google Scholar]
- 25. Purdy RH, Morrow AL, Moore PH, Jr, Paul SM. 1991. Stress-induced elevations of γ-aminobutyric acid type A receptor-active steroids in the rat brain. Proc Natl Acad Sci USA 88:4553–4557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Low SC, Moisan MP, Noble JM, Edwards CR, Seckl JR. 1994. Glucocorticoids regulate hippocampal 11 β-hydroxysteroid dehydrogenase activity and gene expression in vivo in the rat. J Neuroendocrinol 6:285–290 [DOI] [PubMed] [Google Scholar]
- 27. Makino S, Smith MA, Gold PW. 1995. Increased expression of corticotropin-releasing hormone and vasopressin messenger ribonucleic acid (mRNA) in the hypothalamic paraventricular nucleus during repeated stress: association with reduction in glucocorticoid receptor mRNA levels. Endocrinology 136:3299–3309 [DOI] [PubMed] [Google Scholar]
- 28. Reichardt HM, Kaestner KH, Tuckermann J, Kretz O, Wessely O, Bock R, Gass P, Schmid W, Herrlich P, Angel P, Schütz G. 1998. DNA binding of the glucocorticoid receptor is not essential for survival. Cell 93:531–541 [DOI] [PubMed] [Google Scholar]
- 29. Cole TJ, Blendy JA, Monaghan AP, Krieglstein K, Schmid W, Aguzzi A, Fantuzzi G, Hummler E, Unsicker K, Schütz G. 1995. Targeted disruption of the glucocorticoid receptor gene blocks adrenergic chromaffin cell development and severely retards lung maturation. Genes Dev 9:1608–1621 [DOI] [PubMed] [Google Scholar]
- 30. Bornstein SR, Tajima T, Eisenhofer G, Haidan A, Aguilera G. 1999. Adrenomedullary function is severely impaired in 21-hydroxylase-deficient mice. FASEB J 13:1185–1194 [DOI] [PubMed] [Google Scholar]
- 31. Bland ML, Jamieson CA, Akana SF, Bornstein SR, Eisenhofer G, Dallman MF, Ingraham HA. 2000. Haploinsufficiency of steroidogenic factor-1 in mice disrupts adrenal development leading to an impaired stress response. Proc Natl Acad Sci USA 97:14488–14493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Yoshida-Hiroi M, Bradbury MJ, Eisenhofer G, Hiroi N, Vale WW, Novotny GE, Hartwig HG, Scherbaum WA, Bornstein SR. 2002. Chromaffin cell function and structure is impaired in corticotropin-releasing hormone receptor type 1-null mice. Mol Psychiatry 7:967–974 [DOI] [PubMed] [Google Scholar]
- 33. Parlato R, Otto C, Tuckermann J, Stotz S, Kaden S, Gröne HJ, Unsicker K, Schütz G. 2009. Conditional inactivation of glucocorticoid receptor gene in dopamine-β-hydroxylase cells impairs chromaffin cell survival. Endocrinology 150:1775–1781 [DOI] [PubMed] [Google Scholar]
- 34. Caron KM, Soo SC, Wetsel WC, Stocco DM, Clark BJ, Parker KL. 1997. Targeted disruption of the mouse gene encoding steroidogenic acute regulatory protein provides insights into congenital lipoid adrenal hyperplasia. Proc Natl Acad Sci USA 94:11540–11545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Vinson GP, Hinson JP. 1992. Blood flow and hormone secretion in the adrenal gland. In: James VT, ed. The adrenal gland. 2nd ed New York: Raven Press; 71–86 [Google Scholar]
- 36. Wurtman RJ. 1966. Control of epinephrine synthesis in the adrenal medulla by the adrenal cortex: hormonal specificity and dose-response characteristics. Endocrinology 79:608–614 [DOI] [PubMed] [Google Scholar]
- 37. Ishii T, Hasegawa T, Pai CI, Yvgi-Ohana N, Timberg R, Zhao L, Majdic G, Chung BC, Orly J, Parker KL. 2002. The roles of circulating high-density lipoproteins and trophic hormones in the phenotype of knockout mice lacking the steroidogenic acute regulatory protein. Mol Endocrinol 16:2297–2309 [DOI] [PubMed] [Google Scholar]
- 38. Huang CC, Liu C, Yao HH. 2012. Investigating the role of adrenal cortex in organization and differentiation of the adrenal medulla in mice. Mol Cell Endocrinol 361:165–171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Webb PD. 1980. Development of the adrenal cortex in the fetal sheep: an ultrastructural study. J Dev Physiol 2:161–181 [PubMed] [Google Scholar]
- 40. Shih MC, Hsu NC, Huang CC, Wu TS, Lai PY, Chung BC. 2008. Mutation of mouse Cyp11a1 promoter caused tissue-specific reduction of gene expression and blunted stress response without affecting reproduction. Mol Endocrinol 22:915–923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Fujieda K, Okuhara K, Abe S, Tajima T, Mukai T, Nakae J. 2003. Molecular pathogenesis of lipoid adrenal hyperplasia and adrenal hypoplasia congenita. J Steroid Biochem Mol Biol 85:483–489 [DOI] [PubMed] [Google Scholar]
- 42. Hasegawa T, Zhao L, Caron KM, Majdic G, Suzuki T, Shizawa S, Sasano H, Parker KL. 2000. Developmental roles of the steroidogenic acute regulatory protein (StAR) as revealed by StAR knockout mice. Mol Endocrinol 14:1462–1471 [DOI] [PubMed] [Google Scholar]
- 43. Chida D, Nakagawa S, Nagai S, Sagara H, Katsumata H, Imaki T, Suzuki H, Mitani F, Ogishima T, Shimizu C, Kotaki H, Kakuta S, Sudo K, Koike T, Kubo M, Iwakura Y. 2007. Melanocortin 2 receptor is required for adrenal gland development, steroidogenesis, and neonatal gluconeogenesis. Proc Natl Acad Sci USA 104:18205–18210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Huang CC, Miyagawa S, Matsumaru D, Parker KL, Yao HH. 2010. Progenitor cell expansion and organ size of mouse adrenal is regulated by sonic hedgehog. Endocrinology 151:1119–1128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. King P, Paul A, Laufer E. 2009. Shh signaling regulates adrenocortical development and identifies progenitors of steroidogenic lineages. Proc Natl Acad Sci USA 106:21185–21190 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.