Abstract
During spermatogenesis, preleptotene spermatocytes residing near the basement membrane of the seminiferous tubule must traverse the blood-testis barrier (BTB) at stage VIII–IX of the epithelial cycle to continue their development in the adluminal compartment. Unlike other blood-tissue barriers (e.g. the blood-brain barrier) that are created by the endothelial tight junction (TJ) barrier of capillaries, the BTB is created by specialized junctions between Sertoli cells in which TJ coexists with basal ectoplasmic specialization (basal ES, a testis-specific adherens junction). The basal ES is typified by the presence of tightly packed actin filament bundles sandwiched between cisternae of endoplasmic reticulum and the apposing plasma membranes of Sertoli cells. These actin filament bundles also confer unusual adhesive strength to the BTB. Yet the mechanisms by which these filamentous actin (F-actin) networks are regulated from the bundled to the debundled state to facilitate the transit of spermatocytes remain elusive. Herein, we provide evidence that ribosomal protein S6 (rpS6), the downstream signaling molecule of the mammalian target of rapamycin complex 1 (mTORC1) pathway, is a major regulator of F-actin organization and adhesion protein recruitment at the BTB. rpS6 is restrictively and spatiotemporally activated at the BTB during the epithelial cycle. An activation of rpS6 led to a disruption of the Sertoli cell TJ barrier and BTB integrity. Its silencing in vitro or in vivo by using small interfering RNA duplexes or short hairpin RNA was found to promote the Sertoli cell TJ permeability barrier by the recruitment of adhesion proteins (e.g. claudin-11 and occludin) to the BTB. Thus, rpS6 in the mTORC1 pathway regulates BTB restructuring via its effects on the F-actin organization and protein recruitment at the BTB.
The blood-testis barrier (BTB) is an important ultrastructure created by coexisting tight junction (TJ), basal ectoplasmic specialization (ES) and gap junction between adjacent Sertoli cells in the seminiferous epithelium near the basement membrane (1). The BTB segregates the events of meiosis I and II, and postmeiotic germ cell development (i.e. spermiogenesis) behind the host immune system, so that these cellular events all take place in a specialized microenvironment, namely the adluminal compartment (1, 2). This is to avoid the production of antisperm antibodies against germ-cell-specific antigens that are expressed transiently in developing spermatids, many of which are proto- and/or oncogenes (3). The BTB is also one of the tightest blood-tissue barriers in the mammalian body (1). This is contributed almost exclusively by the unique basal ES (a testis-specific adherens junction type) (2) that coexists with TJ in which tightly packed actin filament bundles are sandwiched between cisternae of endoplasmic reticulum and the apposing Sertoli cell plasma membranes (1, 2). In fact, this extensive actin filament bundle at the basal ES is the hallmark ultrastructure of the BTB (1). Yet, the BTB undergoes extensive restructuring at stage VIII–IX of the epithelial cycle to accommodate the transit of preleptotene spermatocytes at the site, many of which are connected in clones via intercellular bridges residing in the basal compartment, to enter the adluminal compartment to differentiate to late spermatocytes to prepare for meiosis I and II (2). Thus, it is conceivable that the extensive actin filament network at the BTB require extensive remodeling at stage VIII–IX of the epithelial cycle. Although recent studies have shown that the highly restricted temporal and spatial expression of epidermal growth factor receptor pathway substrate 8 (Eps8), an actin-barbed end capping and -bundling protein (4, 5), and actin-related protein 3 (Arp3), a component of the Arp2/3 complex that induces barnched actin polymerization (6, 7), play a crucial role to induce changes in the conformation of the actin network from its bundled to debundled state to facilitate TJ and basal ES remodeling, the underlying molecular mechanisms remain unexplored.
The mammalian target of rapamycin (mTOR) signaling pathway is well known for its role in regulating cell growth and proliferation via its effects on modulating protein synthesis (8–10). Its key signaling molecule is mTOR, which associates with raptor (regulatory-associated protein of mTOR) and other subunits, thereby creating a multiprotein complex called mTOR complex 1 (mTORC1) (8–10). Other important signaling molecules of the mTORC1 pathway included ribosomal protein S6 kinase [S6K, also known as p70 S6K (p70S6K)] and ribosomal protein S6 (rpS6) (Fig. 1A). However, recent studies have shown that besides regulating protein synthesis pertinent for cell growth and proliferation, the mTORC1 signaling pathway regulates many different cellular events (10, 11). For instance, SK6, the downstream signaling molecule of the mTORC1 but upstream of rpS6, was recently shown to be directly involved in controlling actin dynamics in metastatic cancer cells (12). Although recent studies have also shown that the mTORC1 signaling pathway regulates barrier function of podocytes in the kidney (13) and the urinary bladder epithelium (14), as well as actin cytoskeletal organization in yeast and mammalian cancer cells (15, 16), the effector of this signaling pathway has yet to be identified. Because the BTB is under extensive restructuring at stage VIII–IX and is facilitated by the remodeling of filamentous actin (F-actin), it serves as an ideal model to study how mTORC1 signaling regulates barrier function via F-actin. Herein, we sought to examine whether rpS6 is the effector and its role in actin re-organization at the BTB during the seminiferous epithelial cycle of spermatogenesis.
Fig. 1.
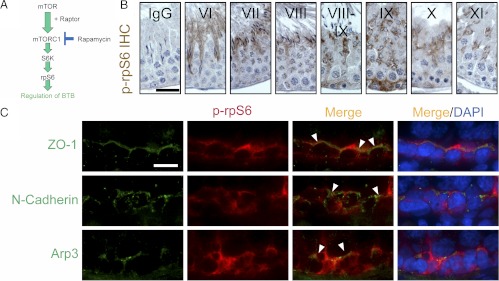
Stage-specific localization of p-rpS6 at the BTB of adult rat testes. A, Schematic diagram of the mTOR/mTORC1 signaling pathway mediated via the downstream phosphorylated (activated) rpS6 (p-rpS6) downstream that regulates multiple cellular events including BTB dynamics. B, Immunohistochemical (IHC) localization of p-rpS6 in the seminiferous epithelium of adult rat testes illustrating stage-specific expression of p-rpS6 consistent with its localization at the BTB with p-rpS6 highly expressed at late-stage VIII to stage IX at the time of BTB restructuring, and p-rpS6 was also detected at the apical ES during the transit of elongating spermatids during spermiogenesis and during apical ES degeneration at spermiation at stage VIII. The panel of micrographs shown here is an abridged version of the IHC data shown in Supplemental Fig. 1. IgG illustrates the control panel in which the anti-p-rpS6 antibody (Table 1) was substituted with normal rabbit IgG. Roman numerals illustrate stages of the epithelial cycle. Scale bar, 50 μm, which applies to all micrographs in this panel. C, Colocalization of p-rpS6 (red) with three putative BTB-associated proteins: ZO-1 (green, a TJ-adaptor protein), N-cadherin (green, a basal ES-protein), and Arp3 (green, a BTB-associated actin regulatory protein known to induce F-actin nucleation and branching) in adult rat testes. Cell nuclei were stained with DAPI, and p-rpS6 was found to colocalize with ZO-1, N-cadherin, and Arp3 as annotated by white arrowheads, at the BTB. Scale bar, 25 μm, which applies to all micrographs in this panel.
Materials and Methods
Animals
Sprague Dawley rats were obtained from Charles River Laboratories (Kingston, NY) and housed at the Rockefeller University Comparative Bioscience Center. The use of rats for studies reported herein was approved by the Rockefeller University Institutional Animal Care and Use Committee with Protocol Numbers 09-016 and 12-506.
Treatment of rats with adjudin, 1-(2,4-dichlorobenzyl)-1H-indazole-3-carbohydrazide
Adult rats [∼300 g body weight (BW)] received a single acute dose of adjudin at 250 mg/kg BW by gavage, which was recently shown to induce BTB restructuring that led to its disruption (17). Rats (n = 3 rats per time point in both treatment and control groups) were terminated at specified time points, and testes were collected, snap-frozen in liquid nitrogen, stored at −80 C until used for either lysate preparation for immunoblot analysis or to obtain frozen cross-sections in a cryostat. In some experiments, rats were terminated at specified time points for BTB integrity assay, including controls. All samples within an experimental group vs. controls were processed simultaneously to eliminate interexperimental variations.
Isolation of germ cells, primary Sertoli cell cultures, and rapamycin treatment
Total germ cells were isolated from adult rats of approximately 300 g BW using a mechanically based protocol without involving enzymatic digestion as described (18), the glass-wool filtration step was omitted so that elongating/elongated spermatids and spermatozoa were retained, and the ratio of different kinds of germ cells in our preparations was similar to in vivo when cell preparations were subjected to flow cytometry analysis as described (18). Sertoli cells were isolated from testes of 20-d-old rats as described (7, 19). At this age, Sertoli cells cease to divide and are differentiated (20), and they are capable of establishing a functional TJ permeability barrier that mimics the BTB in vivo (21–23). Furthermore, ultrastructures of TJ, basal ES, gap junction, and desmosome that mimicked cell junctions at the BTB in vivo were detected in these cultures within approximately 2–3 d by electron microscopy as described (24–26). Sertoli cells were seeded on Matrigel-coated (BD Biosciences, Billerica, MA) coverslips, culture plates, or Millicell HA culture plate inserts (Millipore, Billerica, MA) at 0.05, 0.5, and 1.0 × 106 cells/cm2, respectively, for the corresponding dual-labeled immunofluorescence analysis, lysate preparation for immunoblotting, and assessing the Sertoli cell TJ permeability barrier function by quantifying transepithelial electrical resistance (TER) across the cell epithelium as described earlier (7, 27). Sertoli cells were cultured in serum-free F12/DMEM (Sigma-Aldrich, St. Louis, MO) supplemented with growth factors, bacitracin, and gentamicin as described (19) in a humidified atmosphere of 95% air/5% CO2 (vol/vol) in a CO2 incubator at 35 C. Rapamycin (Sigma-Aldrich) was dissolved in dimethylsulfoxide and used to treat cells on d 3 at a final concentration of 100 ng/ml. The final dimethylsulfoxide concentration in these cultures was at 0.01% (vol/vol). Cells were then fixed for dual-labeled immunofluorescence analysis or terminated for lysate preparation at specified time points as described (7, 28).
Transfection of small interfering RNA (siRNA) duplexes in Sertoli cells in vitro
For in vitro rpS6 silencing, rpS6-specific siRNA duplexes vs. nontargeting control siRNA duplexes at 100 nm were transfected to Sertoli cells on d 3 using RiboJuice siRNA transfection reagent (Novagen, EMD4Biosciences, Billerica, CA) for 24 h as described (24). However, for studies to assess the effects of RNA interference (RNAi) on the Sertoli cell TJ permeability barrier function, 200 nm siRNA duplexes for both rpS6 knockdown vs. controls were used. The sequence of siRNA duplex that specifically targeted rpS6 was 5′-GCAGAAUAUGCUAAACUUUtt-3′ (s131129; Ambion, Austin, TX; s131127 and s131128 were found to be less effective in silencing rpS6 based on preliminary pilot experiments, see Table 2), and the nontargeting siRNA duplex (Silencer Select Negative Control 1 siRNA; Ambion) served as the negative control.
Table 2.
Primers and rpS6-specific siRNA duplexes used for PCR and RNAi experiments
| Primers Target gene | Genbank accession number | Primer sequence (5′-3′) | Nucleotide position | Expected size (b.p.) |
|---|---|---|---|---|
| rpS6 | NM 017160.1 | Sense: GATGATGTCCGCCAGTATGT | 466–485 | 176 |
| Antisense: TATTCTGCAGCCTCCTCC | 624–641 | |||
| S-16 | XM 001078234 | Sense: TCCGCTGCAGTCCGTTCAAGTCTT | 177–200 | 385 |
| Antisense: GCCAAACTTCTTGGATTCGCAGCG | 538–561 |
| siRNA duplexes Target gene | Gene ID | siRNA sequence (5′-3′) |
|---|---|---|
| rpS6 | s131127 | Sense: GAGCUAGUAGAAUCCGAAAtt |
| Antisense: UUUCGGAUUCUACUAGCUCtt | ||
| rpS6 | s131128 | Sense: GAAAGCCCUUAAACAAAGAtt |
| Antisense: UCUUUGUUUAAGGGCUUUCta |
Preparation of short hairpin RNA (shRNA) constructs and transfection of testes in vivo with shRNA
For in vivo silencing of rpS6, shRNA was designed based on the same sequence of siRNA duplex targeting rpS6 shown to knock down rpS6 (see findings in in vitro RNAi studies) using BLOCK-iT RNAi Designer (Invitrogen, Carlsbad, CA). Random nontargeting shRNA sequence was used for negative control. The shRNA, 50 nucleotides in length, including both rpS6 and nontargeting control, was cloned into BLOCK-iT U6 RNAi Entry Vector (Invitrogen). Plasmid DNA was treated with the MiraCLEAN Endotoxin Removal Kit (Mirus, Madison, WI) before its use for in vivo experiments. Vector containing shRNA was mixed with TransIT EE hydrodynamic delivery solution (Mirus) for in vivo transfection into rat testes via intratesticular injection using 28-gauge needles at a final volume of approximately 125 μl per testis. Four adult rats of approximately 300 g BW were used, and 8 μg of vector DNA containing shRNA targeting rpS6 or nontargeting shRNA was administered to each testis per day for 2 consecutive days (i.e. two doses) so that one testis of the same rat served as the negative control. Rats were euthanized by CO2 asphyxiation 3–5 d after the first administration, and testes were collected, snap-frozen in liquid nitrogen, and stored at −80 C until used to obtain cross-sections in a cryostat. All samples in treatment vs. control groups were processed simultaneously to eliminate interexperimental variations.
RNA extraction and RT-PCR
RNA was extracted from Sertoli cell cultures with TRIzol reagent (Invitrogen) and was reverse transcribed to cDNA using Moloney murine leukemia virus reverse transcriptase (Promega, Madison, WI). PCR was performed with GoTaq (Promega) using specific primers (see Table 2) to amplify the corresponding target genes as described (24).
Lysate preparation and immunoblotting
Lysates of testes were prepared in immunoprecipitation (IP) lysis buffer [50 mm Tris (pH 7.4) containing 0.15 m NaCl, 1% Nonidet P-40 (vol/vol), 1 mm EGTA, 2 mm N-ethylmaleimide, and 10% glycerol (vol/vol)] supplemented with protease inhibitor mixture (Sigma-Aldrich) and phosphatase inhibitor mixture I and II (Sigma-Aldrich) at a dilution of 1:100 as specified by the manufacturer. Protein concentration was estimated using a Bio-Rad Dc protein assay kit in 96-well plates with a Bio-Rad model 680 spectrophotometry reader using BSA as a standard. Approximately 100 μg of protein from testis lysates or 25 μg of protein from Sertoli cell lysates was used for immunoblotting as described (7). Antibodies used for immunoblot analysis, dual-labeled immunofluorescence, and immunohistochemistry (IHC) at appropriate dilutions are listed in Table 1.
Table 1.
Antibodies used for different experiments in this study
| Antibody | Vendor | Catalog no. | Application | Working dilution for IB or (IF) or [IP] |
|---|---|---|---|---|
| Rabbit anti-rpS6 | Cell Signaling Technology (Danvers, MA) | 2217 | IB, (IF) | 1:1000, (1:100) |
| Rabbit anti-p-rpS6 | Cell Signaling Technology | 4858 | IB, (IF), [co-IP] | 1:2000, (1:100), [1;30] |
| Rabbit anti-mTOR | Cell Signaling Technology | 2983 | IB | 1:2000 |
| Rabbit anti-S6K | Abcam (Cambridge, MA) | ab32529 | IB | 1:2500 |
| Rabbit anti-p-S6K | Abcam | ab32359 | IB | 1:3000 |
| Rabbit anti-claudin-11 | Invitrogen (Carlsbad, CA) | 36–4500 | IB, (IF) | 1:125, (1:100) |
| Rabbit anti-occludin | Invitrogen | 71–1500 | IB, (IF) | 1:125, (1:100) |
| Rabbit anti-JAMA-A | Invitrogen | 36–1700 | IB | 1:125 |
| Rabbit anti-N-cadherin | Santa Cruz Biotechnology (Santa Cruz, CA) | sc-7939 | IB | 1:200 |
| Mouse anti-ZO-1 | Invitrogen | 33–9188 | IB, (IF) | 1:125, (1:100) |
| Rabbit anti-β-catenin | Invitrogen | 71–2700 | IB | 1:200 |
| Rabbit anti-CAR | Santa Cruz Biotechnology | sc-15405 | IB | 1:200 |
| Mouse anti-c-Src | Santa Cruz Biotechnology | sc-8056 | IB | 1:200 |
| Rabbit anti-FAK | Santa Cruz Biotechnology | sc-558 | IB | 1:200 |
| Mouse anti-Arp3 | Sigma-Aldrich (St. Louis, MO) | A5979 | IB, (IF) | 1:300, (1:100) |
| Mouse anti-Eps8 | BD Biosciences (Billerica, MA) | 610143 | IB, (IF) | 1:500, (1:100) |
| Goat anti-actin | Santa Cruz Biotechnology | sc-1616 | IB | 1:300 |
Antibodies used in this study cross-reacted with the corresponding rat proteins as indicated by the manufacturers. IB, Immunoblotting; IF, immunofluorescence microscopy; co-IP, coimmunoprecipitation; CAR, coxsackievirus and adenovirus receptor.
IHC and dual-labeled immunofluorescence analysis
For IHC, Bouin's-fixed testes were embedded in paraffin and sectioned to 5 μm with a microtome and mounted on positively charged glass microscopic slides. Antigen retrieval was performed by boiling paraffin sections on slides in Tris-EDTA buffer [10 mm Tris (pH 9.0) at 22 C, containing 1 mm EDTA, 0.05% Tween (vol/vol)] for 20 min. Sections were then stained with an anti-phospho-rpS6 (p-rpS6) antibody (Table 1) with an IHC kit (Invitrogen) as described (29) using 3,3-diaminobenzidine for color development. For dual-labeled immunofluorescence analysis, frozen sections of testes (7 μm) or Sertoli cells cultured on coverslips at a density 0.05 × 106 cells/cm2 were fixed in 4% paraformaldehyde in PBS [10 mm sodium phosphate, 0.15 m NaCl (pH 7.4) at 22 C (wt/vol)] for 10 min. Sections or cells were then permeabilized with 0.1% Triton X-100 in PBS (vol/vol) for 4 min and blocked in 1% BSA in PBS (wt/vol) (blocking solution) for 30 min, followed by an overnight incubation of primary antibodies diluted in blocking solution at room temperature. Sections or cells were then incubated at room temperature with Alexa Fluor-conjugated secondary antibodies (Invitrogen; red fluorescence, Alexa Fluor 555; green fluorescence, Alexa Fluor 488) at 1:250 dilution with blocking solution. To visualize F-actin, sections or cells were incubated with fluorescein isothiocyanate (FITC)-conjugated phalloidin (Sigma-Aldrich) at 1:50 dilution together with secondary antibodies. Sections or cells were then mounted with Prolong gold antifade mounting medium with 4′,6-diamidino-2-phenylindole (DAPI) (Invitrogen) for fluorescence microscopy. Images were captured using a Nikon Eclipse 90i microscope and acquired using Nikon NIS Elements Imaging software (version 3.2) (Nikon Instruments Inc., Melville, NY). Images were then analyzed in PhotoShop using Adobe Creative Suite (version 3.0), such as to obtain merged images that stained with difference fluorescence tags for analysis of co-localization. All IHC and immunofluorescence microscopy studies were repeated three times with testes from different rats or different preparations of Sertoli cell cultures. All samples (sections of testes or Sertoli cells) within an experimental group were processed simultaneously in a single experimental session by placing three to four cross-sections of testes on a single microscopic slide such that multiple slides could be processed by a single investigator to eliminate interexperimental variations. Negative controls included the use of normal IgG of the corresponding host animals (e.g. mouse and rabbit) diluted in blocking solution to substitute the primary antibody.
Semiquantitative analysis of fluorescence images
For the frozen sections of rpS6 shRNA- vs. nontargeting shRNA-transfected testes, the intensity of fluorescence signals from phospho-rpS6 in tubules at late stage VIII–IX, claudin-11 in tubules at stage IX, or occludin in tubules at stage VIII (note that at these stages, their expression was shown to be considerably lowered based on pilot experiments and as reported) (30) was measured using ImageJ software package (version 1.45; http://rsbweb.nih.gov/ij). Intensity of the corresponding fluorescence signals from control shRNA-transfected testes was arbitrarily set at 1, against which statistical analysis was performed. At least 80 tubules were randomly selected and measured from each treatment vs. control group, with four rats in each group.
BTB integrity assay
The BTB integrity in vivo was assessed by an assay established earlier in our laboratory as detailed elsewhere (30, 31). In short, rats were under anesthesia with ketamine HCl (60 mg/kg BW) and xylazine (10 mg/kg BW) (Sigma-Aldrich) administered im. Thereafter, a small incision was made over the jugular vein to expose the blood vessel, and 1.5 mg FITC-conjugated inulin (molecular weight 4.6 kDa) (Sigma-Aldrich) in 300 μl PBS was administered to the jugular vein using a 28-gauge needle. Thereafter, the wound was stitched with one or two Clay Adams 9-mm wound clips (Becton Dickinson, Sparks, MD). Rats were allowed to recover, and approximately 45 min later, rats were euthanized by CO2 asphyxiation, and frozen cross-sections of testes were obtained in a cryostat at −20 C and examined by fluorescence microscopy. Rats treated with CdCl2 at 5 mg/kg BW ip for 3 d served as positive control because the BTB was reported to be irreversibly damaged by this treatment (32, 33). To obtain semiquantitative data, the distance traveled by the fluorescence signal from the basement membrane of a tubule (DSignal) was divided by its radius (DRadius). For tubules that were obliquely sectioned, DRadius was obtained by averaging the shortest and the longest distance from the basement membrane. A total of 180 tubules were randomly scored from three rats (i.e. ∼60 tubules from each rat).
Coimmunoprecipitation
To assess whether p-rpS6 was structurally interacting with Arp3, Eps8, or actin, 500 μg protein of testis lysates from normal rats was first incubated with 1.5 μg normal rabbit IgG for 2 h, to be followed by a 2-h incubation with 15 μl Protein A/G Plus (Santa Cruz Biotechnology, Santa Cruz, CA), and the supernatant was obtained after centrifugation. This preclearing step was crucial to remove nonspecific interaction proteins. The clear supernatant was then incubated with the corresponding primary antibody listed in Table 1 overnight at room temperature. Primary antibody was substituted with normal rabbit IgG for negative control. Thereafter, lysates was incubated with 20 μl Protein A/G Plus for 6 h, and the supernatant was collected for immunoblotting using corresponding antibodies to identify the interacting proteins.
Statistical analysis
Statistical analysis of data derived from immunoblotting, Sertoli cell TJ permeability barrier function assay based on TER measurement, and fluorescence signal intensity and BTB integrity assays was performed with GB-STAT software package (version 7.0; Dynamic Microsystems, Inc., Silver Spring, MD) using two-way ANOVA followed by Newman-Keuls test or Student's t test.
Results
rpS6, the downstream signaling molecule of the mTORC1 and an integrated component of the BTB, displays stage-specific expression during the seminiferous epithelial cycle of spermatogenesis
Figure 1A illustrates the mTORC1 pathway and its key signaling molecules known to regulate protein synthesis and actin dynamics pertinent to the BTB. rpS6, the downstream critical signaling molecule of the mTORC1 pathway, was found to be expressed by Sertoli and germ cells in the testis (Supplemental Fig. 1A published on The Endocrine Society's Journals Online web site at http://endo.endojournals.org, Fig. 1B, and Tables 1 and 2). Using antibodies specific to p-rpS6, the activated (phosphorylated) form of rpS6 (Supplemental Fig. 1B), and rpS6 (Table 1), both p-rpS6 (Fig. 1B and Supplemental Fig. 1C) and rpS6 (Supplemental Fig. 2) were found to be localized in the seminiferous epithelium, consistent with their localization at the BTB. However, the expression of p-rpS6 is stage specific, with its localization at the BTB restricted to late stage VIII and IX (Fig. 1B and Supplemental Fig. 1C). The p-rpS6 also colocalized with several putative BTB proteins: zonula occludens-1 (ZO-1) (a TJ-associated adaptor), N-cadherin (a basal ES protein), and Arp3 (a component of the Arp2/3 complex at the BTB) (1) (Fig. 1C), illustrating it is a likely BTB component protein. Consistent with the above findings, rpS6 was found to colocalize, at least in part, with F-actin at the BTB (Supplemental Fig. 2); however, p-rpS6 was expressed and colocalized with F-actin only at late-stage VIII and IX at the BTB (Supplemental Fig. 3), at the time of BTB restructuring, apparently being used to facilitate the transit of preleptotene spermatocytes at the BTB. It is of interest to note that rpS6 was confined to the basal compartment near the BTB (Supplemental Fig. 2); however, p-rpS6, besides being stage-specifically expressed at the BTB at late-stage VIII–IX, was also expressed at the apical ES, predominantly at stage VI–VIII (Fig. 1B and Supplemental Figs. 1C and 3), colocalizing with two putative apical ES proteins: nectin 3 and Arp3 (Supplemental Fig. 4). This illustrates that p-rpS6 may also be involved in apical ES dynamics during the epithelial cycle.
BTB disruption induced by adjudin associates with a significant induction and an alteration in distribution of p-rpS6 in the seminiferous epithelium
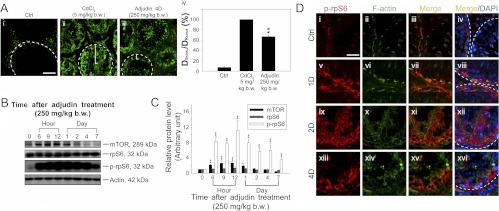
To further confirm the enhanced and stage-specific expression of p-rpS6 at the BTB detected in late-stage VIII–IX tubules indeed correlates with BTB restructuring that facilitates the transit of preleptotene spermatocytes, a recently established in vivo model was used (17). Using this model, adult rats were treated with a single acute dose of adjudin [1-(2,4-dichlorobenzyl)-1H-indazole-3-carbohydrazide, a potential male contraceptive under investigation (34)] at 250 mg/kg BW to induce BTB disruption (17). Within 4 d, BTB disruption induced by an acute dose of adjudin was confirmed (Fig. 2A, i–iv). Indeed, a significant surge in p-rpS6 (but not total rpS6) and a transient increase in mTOR were detected within hours after adjudin treatment (Fig. 2, B and C), supporting the notion that p-rpS6 is likely involved in the BTB-restructuring event. Furthermore, the organization of F-actin was also compromised at both the basal and the adluminal compartments due to BTB disruption and anchoring junction disruption, respectively (Fig. 2D, i–xvi). The disorganized F-actin also partially colocalized with the induced p-rpS6 (Fig. 2D, v–xvi vs. i–iv), suggesting the disruptive role of p-rpS6 in BTB function may be mediated by changes in the actin filament organization.
Fig. 2.
A study of using an animal model by disrupting the BTB with an acute dose of adjudin (250 mg/kg BW) to assess changes in the level and localization of p-rpS6. A, Adult rats [∼300 g BW; n = 3 rats per time point in each treatment vs. control (Ctrl) groups] were treated with adjudin (250 mg/kg BW) by gavage as described (34) at d 0, and the BTB integrity was accessed on d 4 by its ability to block inulin-FITC from entering the adluminal compartment in the seminiferous epithelium behind the BTB. Normal rats served as negative controls, whereas rats treated with CdCl2 (5 mg/kg BW, ip) for 3 d, which is known to induce BTB disruption (32), served as positive controls. In normal rat testes with intact BTB, fluorescence signals were retained at the basal compartment (Ai), but for CdCl2-treated rats in which the BTB was disrupted, signals were found in the adluminal compartment (Aii). For testes from rats treated with an acute dose of adjudin, fluorescence signal was found behind the BTB, and in some tubules, signals were even detected in the lumen of tubules (see Aiii), illustrating the BTB had been compromised. White broken lines annotate the relative location of the basement membrane in a seminiferous tubule, which is adjacent to the BTB. White brackets in tubules indicate the relative distance traveled by inulin-FITC from the BTB with the BTB location annotated by the white broken line. Scale bar in Ai, 50 μm, which applies to i–iii. The findings in Ai–iii were summarized and semiquantitatively shown in a histogram shown in Aiv by comparing the ratio of the distance traveled by inulin-FITC from the BTB near the basement membrane (DSignal) to the tubule radius (DRadius) between the three groups. For tubules that were obliquely sectioned, DRadius was obtained by averaging the shortest and the longest radius from the basement membrane. Each bar represents mean ± sd of 60 tubules that were randomly selected and scored from testes of three rats for each time point. **, P < 0.01, compared with control group. B, Immunoblots of mTORC1 signaling molecules including mTOR, rpS6, and p-rpS6 (see Fig. 1A) using testis lysates at specified time points from rats after adjudin treatment. Actin served as a protein loading control. This is a representative set of data from three independent experiments. C, Histogram summarizing immunoblotting data of B, with each data point normalized against actin. Protein levels at 0 h were arbitrarily set as 1 against which statistical comparison was performed. Each bar represents a mean ± sd of n = 3. *, P < 0.05; **, P < 0.01. D, p-rpS6 (red) colocalized with F-actin (green) in frozen sections of rat testes after adjudin treatment; cell nuclei were stained with DAPI. In control (Ctrl), p-rpS6 was expressed at the BTB near the basement membrane indicated by white broken lines and was restricted only to tubules at late-stage VIII–IX (i–iv) (see Fig. 1A and Supplemental Fig. 1). But after adjudin treatment, p-rpS6 was found in tubules regardless of their stages (v–viii). In addition, the organization of F-actin was disrupted after adjudin treatment (ii, vi, x, and xiv), and the disorganized F-actin also partially colocalized with the up-regulated p-rpS6 (v–xvi vs. i–iv), supporting the notion that p-rpS6 takes part in actin disorganization induced by adjudin. Scale bar in Di, 50 μm, which applies to i–xvi.
Disruption of p-rpS6 production by Sertoli cells promotes the Sertoli cell TJ permeability barrier in vitro
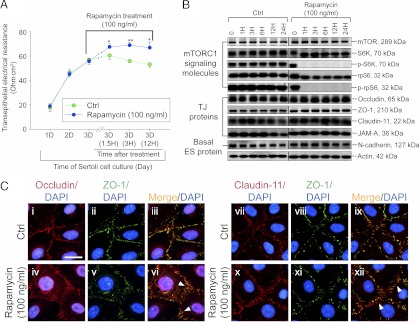
Collectively, data reported in Figs. 1 and 2 and Supplemental Figs. 1–4 have illustrated that an up-regulation of p-rpS6 is associated with BTB restructuring. We next examined the phenotype of the Sertoli cell BTB using an in vitro model. It was reported that Sertoli cells cultured in vitro established a functional TJ barrier that mimicked the BTB in vivo (23, 35, 36), containing ultrastructures of TJ, basal ES, and gap junction (24, 25), and this in vitro model is widely used by investigators to study BTB function (1). Rapamycin, a specific inhibitor of mTORC1 (Fig. 1A), by causing raptor to dissociate from mTORC1 and thus preventing mTORC1 from activating its downstream effectors, such as phosphorylation of SK6 and rpS6 (37), was used to treat the Sertoli cell epithelium as shown in Fig. 3A. After rapamycin treatment, the Sertoli cell TJ barrier was strengthened as indicated by a significant increase in TER across the cell epithelium (Fig. 3A). The inhibition of mTORC1 by rapamycin was confirmed by a virtual loss of p-S6K and p-rpS6 detected by immunoblotting (Fig. 3B), which are the two crucial downstream signaling molecules in the mTORC1 signaling pathway (Fig. 1A). The steady-state levels of several TJ proteins [e.g. occludin, ZO-1, claudin-11, and junctional adhesion molecule-A (JAM-A)] were mildly up-regulated, whereas basal ES proteins (e.g. N-cadherin) were found to be unaffected after rapamycin treatment (Fig. 3B). Furthermore, changes in protein distribution of several TJ proteins were detected with considerably more occludin and claudin-11, but not ZO-1, localized at the Sertoli cell-cell interface (Fig. 3C), which were likely being used to support the Sertoli cell TJ permeability barrier, thereby tightening the TJ barrier.
Fig. 3.
The functional role of rpS6 at the Sertoli BTB in vitro. Rapamycin is an effective inhibitor of mTORC1 that suppresses its substrate S6K in the mTOR signaling pathway; this, in turn, blocks the phosphorylation and activation of rpS6 to form p-rpS6 (8, 16), modulating the TJ permeability barrier (see Fig. 1A). A, The establishment of a functional Sertoli cell TJ permeability barrier was assessed by quantifying TER across the cell epithelium. By d 3, when the TJ barrier was established, cells were treated with rapamycin (100 ng/ml), and a blockade of rpS6 activation (p-rpS6 production was abolished, as shown in B) was found to promote Sertoli TJ barrier function. Each data point is a mean ± sd of n = 4 replicates from a representative experiment, which was repeated three times using different batches of Sertoli cells and yielded similar results. *, P < 0.05; **, P < 0.01 compared with corresponding controls (Ctrl). B, Representative immunoblots showing the steady-state levels of signaling molecules of the mTORC1 pathway vs. TJ and basal ES proteins using Sertoli cell lysates terminated at specified time points after rapamycin treatment with n = 4 independent experiments (see Supplemental Fig. 5 which summarizes results of these immunoblots). These data thus confirmed that rapamycin virtually blocked the formation of p-rpS6 (i.e. the activated form of rpS6) and p-S6K (i.e. the activated form of S6K) and inhibited the steady-state level of rpS6 but not S6K, which was accompanied by a mild increase in claudin-11, occludin, ZO-1, and JAM-A by 24-h after rapamycin treatment, but not N-cadherin. Actin served as a protein loading control. C, Changes in the localization of TJ proteins, such as occludin (red), ZO-1 (green), and clauidn-11(red), after treatment with rapamycin for 20 h. ZO-1 was found to colocalize with occludin or claudin-11 and appeared as orange fluorescence with Sertoli cell nuclei visualized by DAPI (blue) staining. After rapamycin treatment, occludin and claudin-11, but not ZO-1, were found to localize considerably more at the Sertoli cell-cell interface (see white arrowheads) but less in the cell cytosol, supporting data in A. Scale bar, 50 μm in the first micrograph, which applies to all other micrographs.
The silencing of p-rpS6 by RNAi promotes the Sertoli cell TJ permeability barrier in vitro
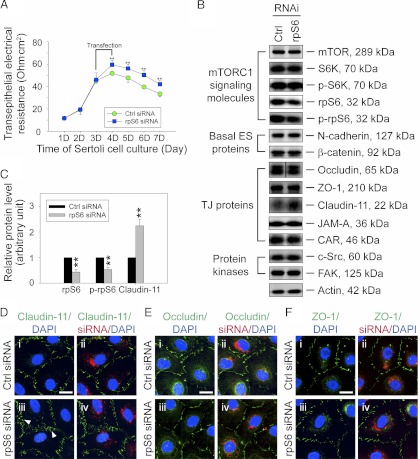
To confirm that rpS6 is a crucial effector in promoting Sertoli cell TJ barrier function, we next investigate whether the knockdown of rpS6 by RNAi would also promote the Sertoli cell TJ barrier function and modulate protein distribution at the Sertoli cell-cell interface, analogous to the rapamycin treatment. Indeed, the knockdown of rpS6 by approximately 60% (and also p-rpS6 by ∼50%) in Sertoli cells using rpS6-specific siRNA duplexes vs. nontargeting control siRNA duplexes was found to strengthen the Sertoli cell TJ barrier (Fig. 4, A–C). Although there was no detectable off-target effect of this rpS6 knockdown, the steady-state level of claudin-11, but not occludin or ZO-1, was found to be induced by more than 2-fold (Fig. 4, B and C). Furthermore, there was a change in the cellular distribution of claudin-11, but not occludin and ZO-1, in the Sertoli epithelium with more claudin-11 detected at the cell-cell interface (Fig. 4, D vs. E and F). These findings thus illustrate that rpS6 is the effector that modulates BTB dynamics. In this context, it is noted that after rapamycin treatment, the protein levels of occludin and JAM-A, besides claudin-11, were all induced; yet a knockdown of rpS6 induced only claudin-11 (Fig. 4, B and C). This could likely be the result of RNAi efficacy because the knockdown of rpS6 using specific siRNA duplexes only suppressed rpS6/p-rpS6 by approximately 60%, but rapamycin treatment suppressed rpS6 and p-rpS6 by approximately 30% and approximately 100%, respectively (Fig. 3 and Supplemental Fig. 5).
Fig. 4.
A knockdown of rpS6 by RNAi promotes the Sertoli cell TJ permeability barrier function in vitro. A, Sertoli cells cultured in vitro established a functional TJ barrier that mimicked the BTB in vivo when its barrier function was assessed by TER across the cell epithelium. On d 3, Sertoli cells were transfected with rpS6-specific siRNA duplexes vs. nontargeting control siRNA duplexes for 24 h. A knockdown of rpS6 (and also p-rpS6) by approximately 60% (B and C) was found to promote the TJ-barrier function significantly vs. controls (Ctrl). Each data point is a mean ± sd of n = 5 replicates from a representative experiment, which was repeated three times using different batches of Sertoli cells and yielded similar results. **, P < 0.01. B, Immunoblots showing the steady-state levels of signaling molecules and their activated (phosphorylated) forms in the mTORC1 pathway, basal ES proteins, TJ proteins, and protein kinases using lysates of Sertoli cells terminated 2 d after transfection. Actin served as a protein loading control. This is a representative set of data from four experiments. It was noted that the knockdown of rpS6 did not induce any off-target effects except an induction in claudin-11. C, Histogram summarizing selected immunoblotting results such as those shown in B and normalized against actin. Protein levels of the nontargeting control group (Ctrl) were arbitrarily set at 1 against which statistical comparison was performed. Each bar is a mean ± sd of n = 4. **, P < 0.01. D–F, Changes in localization of TJ proteins, such as claudin-11 (green) (D), occludin (green) (E), and ZO-1 (green) (F) at the Sertoli cell-cell interface after rpS6 knockdown were assessed 2 d after transfection. Sertoli cell nuclei were stained with DAPI, and red siGLO was used to illustrate successful transfection. Note that considerably more claudin-11 was detected at the Sertoli cell-cell interface (see white arrowheads in Diii) after rpS6 knockdown (D, iii and iv vs. i and ii), whereas there were no observable changes detected in the localization of occludin (E) and ZO-1 (F). Bar in i (D–F), 25 μm, which applies to i–iv.
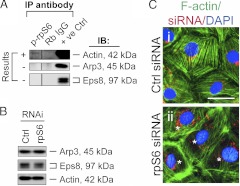
A knockdown of rpS6 induces reorganization of actin filament network in Sertoli cells
We next examined whether changes in the distribution of proteins, such as claudin-11, at the Sertoli cell-cell interface was the result of an alteration of the underlying actin filament network at the BTB. Co-IP was used to evaluate the association of p-rpS6 with actin and two actin-regulatory proteins, Arp3 and Eps8, recently shown to regulate actin filaments at the Sertoli cell BTB (5, 7). Although p-rpS6 was found to interact with actin, it did not interact with either Arp3 or Eps8 based on coimmunoprecipitation experiments (Fig. 5A). Interestingly, the knockdown of rpS6 by approximately 60% (Fig. 4, B and C) was found to induce a persistent minor decline in the steady-state level of Eps8 but had no apparent effect on Arp3 expression (Fig. 5B). Furthermore, the localization of Arp3 and Eps8 in the rpS6-silenced Sertoli cells remained unaltered vs. control cells (Supplemental Fig. 6). However, after knockdown of rpS6, F-actin was found to be aligned more extensively at the cell-cell interface (Fig. 5C, ii vs. i). This increase in the proportion of F-actin being organized at the cortical side of Sertoli cells might be responsible for tightening the Sertoli cell TJ barrier. In short, these findings illustrate that p-rpS6 regulates BTB dynamics via its effects on the F-actin organization.
Fig. 5.
rpS6 regulates actin organization in Sertoli cells. A, A co-IP study using lysates of normal adult rat testes to access any structural protein-protein interactions between p-rpS6 and actin, Arp3 (a component of the Arp2/3 complex that regulates actin filament nucleation and branching), or Eps8 (an actin filament-barbed end capping and -bundling protein). Testis lysates incubated with normal rabbit IgG instead of an anti-rpS6 antibody were used as a negative control, and lysates from normal rat testes without being subjected to co-IP served as a positive control (+ve Ctrl); p-rpS6 was found to associate with actin but not the actin-regulating proteins Arp3 and Eps8. This is a representative set of data from three independent experiments. B, Immunoblots (IB) showing the steady-state levels of actin-regulating proteins Arp3 and Eps8 using Sertoli cell lysates 2 d after transfection of siRNA duplexes for rpS6 knockdown by approximately 60% (see Fig. 4), illustrating rpS6 knockdown caused a mild reduction of actin-bundling protein Eps8. Actin served as a protein loading control. This is a representative set of data from four experiments. C, Changes in Sertoli cell F-actin organization after knockdown of rpS6. Sertoli cell nuclei were visualized by DAPI, and red siGLO was used to illustrate successful transfection. It is noted that after transfection of Sertoli cells with rpS6-specific siRNA duplexes for RNAi, F-actin was localized more intensely at the cortical side of cells (these cells are denoted by asterisks), appearing to strengthen adhesion at the Sertoli cell-cell interface (ii vs. i), supporting the data shown in Fig. 4A. Bar in Ci, 20 μm, which applies to i and ii.
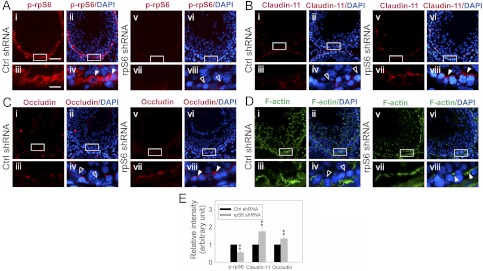
A knockdown of rpS6 in the testis by shRNA in vivo modulates actin reorganization and blocks stage-specific down-regulation of TJ proteins in the seminiferous epithelium to promote BTB integrity
It is known that the expression of many BTB constituent proteins (e.g. occludin) in the seminiferous epithelium are stage specific (1). For instance, the expression of occludin at the BTB is considerably reduced in tubules at stage VIII–XI (30). This stage-specific expression of occludin, coupled with F-actin reorganization at these stages (5, 7), is probably being used to coordinate BTB restructuring at these stages to facilitate the transit of preleptotene spermatocytes to enter the adluminal compartment to prepare for meiosis I and II. Because p-rpS6 was found to be considerably induced at late-stage VIII–IX and the knockdown of rpS6 was shown to tighten the Sertoli cell TJ barrier, we sought to examine whether a knockdown of rpS6 in vivo would abolish the down-regulation of TJ proteins and affect F-actin reorganization at the BTB in tubules at these stages. To test this possibility, an in vivo knockdown of rpS6 was performed by intratesticular injection of shRNA specifically targeting rpS6 vs. nontargeting control shRNA (Fig. 6, A–D). After the knockdown of rpS6, the intensity of p-rpS6 staining at the BTB in tubules at late-stage VIII–IX vs. the corresponding controls was indeed considerably diminished (Fig. 6A, v–viii vs. i–iv), illustrating a considerable knockdown of rpS6 in these tubules (Fig. 6A). As shown in Fig. 6, B and C, the expression of claudin-11 and occludin at the BTB in control testes at stage IX and at late-stage VIII tubule, respectively, were considerably lowered, consistent with an earlier report showing a reduced expression of occludin at the BTB in stage VIII tubules (30). However, after the knockdown of rpS6 by shRNA, the expression of either claudin-11 or occludin in corresponding tubules at these stages was considerably induced (Fig. 6, B, C, and E). Furthermore, the organization of F-actin at BTB was also weakened in late-stage VIII–IX tubules (Fig. 6D, i–iv), and these changes together with the reduced expression of claudin-11 and occludin are likely being used to induce BTB restructuring at these stages under normal physiological conditions. However, the knockdown of rpS6 by RNAi was found to induce F-actin reorganization so that distinctive F-actin networks are detected at the BTB in rats treated with rpS6-specific shRNA for silencing in these tubules (Fig. 6D). These results thus support the notion that p-rpS6 is a critical regulator of BTB dynamics at stage VIII-IX of the seminiferous epithelial cycle of spermatogenesis.
Fig. 6.
An in vivo study assessing the role of p-rpS6 in BTB function by RNAi using rpS6-specific shRNA. A–D, Eight micrograms of vector containing shRNA targeting rpS6 vs. nontargeting shRNA was administered to each testis in adult rats [∼300 g BW; n = 4 rats per time point in both treatment and control (Ctrl) groups] per day for 2 consecutive days, and testes were collected on d 3–5 after the first injection. Frozen sections of testes were used to study changes in the localization of p-rpS6 (red) (A) in late-stage VIII–IX tubules, claudin-11 (red) (B) in stage IX tubules, occludin (red) (C) in stage VIII tubules, and actin (green) (D) in late-stage VIII–IX tubules in the rpS6 knockdown group vs. control group. Tubules at these stages were selected for better illustration of changes in protein localization and/or recruitment after rpS6 knockdown by shRNA because at these stages in normal rat testes, the expression of claudin-11 and occludin at the BTB was considerably weakened vs. other stages [see i–iv in B and C, which are consistent with an earlier report (30)], and the distinctive F-actin network at the BTB was also considerably weakened (see D, i-iv). These stage-specific changes thus facilitate BTB restructuring to accommodate the transit of preleptotene spermatocytes at the site at stage VIII–IX. Nuclei were stained with DAPI. Magnified views of the boxed area shown in i, ii, v, and vi of A–D are shown in iii, iv, vii, and viii of A–D, respectively. A, Considerably more intense staining (see white arrowheads) of p-rpS6 was detected near the basement membrane of late-stage stage VIII–IX tubules at the BTB site from rat testes transfected with nontargeting control (Ctrl) shRNA (i–iv) vs. corresponding tubules from testes transfected with rpS6-specific shRNA (see open arrowheads) (v–viii), illustrating successful knockdown of rpS6 by using rpS6 shRNA. B–D, Considerably more claudin-11, occludin-11, and F-actin was also found at the BTB near the basement membrane from tubules at stage IX, VIII, and late VIII–IX, respectively, in rat testes after rpS6 knockdown (see white arrowheads) vs. the corresponding control tubules (see open arrowheads) (B, v–viii vs. i–iv; C, v–viii vs. i–iv; D, v–viii vs. i–iv). Scale bar in Ai, 100 μm, which applies to i, ii, v, and vi of A–D; scale bar in Aiii, 25 μm, which applies to iii, iv, vii, and viii of A–D. E, Changes in the intensity of claudin-11 (in stage IX tubules) and occludin (in stage VIII tubules) in the rpS6 knockdown testes by shRNA vs. the corresponding nontargeting controls shown in A–C were quantified by using ImageJ software by scoring about 40 tubules at specified stages. Each bar represents mean ± sd (n = 4 rats with approximately 10 randomly selected tubules per testis and a total of four testes were scored). The staining intensity in control testes was arbitrarily set at 1, against which statistical analysis was performed. **, P < 0.01.
Discussion
rpS6, a component of the 40S ribosomal subunit, is the substrate of S6K; the activation of rpS6 is mediated via its phosphorylation by S6K to form p-rpS6 (Fig. 1A) (38). It is a crucial signaling molecule of the mTORC1 signaling pathway known to be involved in protein synthesis, cell growth, cell cycle progression, cell proliferation and survival (10, 37), and virtually every aspect of cellular events in mammalian cells (10). For example, S6K can activate small GTPases Rac1 and Cdc42, but not RhoA, to affect actin-reorganization and act as an actin filament cross-linking protein, which in turn stabilizes actin filaments in ovarian cancer cells (12), thereby serving as a crucial regulator of actin dynamics in tumor progression (12). Furthermore, the knockout of an upstream negative regulator of mTORC1 called tuberous sclerosis complex 1 (TSC1) led to mTORC1 activation in podocytes, which in turn resulted in glomerular basement membrane thickening and proteinuria in nondiabetic young and adult mice, mimicking diabetic nephropathy (13). It is noted that podocytes are visceral epithelial cells that wrap around the capillaries of the glomerulus in the kidney to retain proteins during blood filtration at the site, and their defects lead to proteinuria and glomerulosclerosis in kidney diseases, including diabetic nephropathy, one of the most lethal complications that occur in type 1 and type 2 diabetics (39). More important, activation of mTORC1 led to redistribution of ZO-1, a TJ adaptor protein, from cell membrane to cell cytosol at the podocyte barrier, disrupting the barrier function (13). Another study performed in mice showed that, upon an up-regulation of the mTORC1 pathway caused by deletion of two mTOR upstream molecules, a loss of epithelial marker E-cadherin and TJ protein ZO-1 in bladder epithelial cells was resulted (14). In short, these findings illustrate that the mTORC1 signaling pathway is involved in TJ-barrier function; however, the effector remains unidentified.
Herein, we have demonstrated that p-rpS6 is the likely effector of the mTORC1 pathway for modulating the BTB function in the testis. The activated form of rpS6, p-rpS6, displays a stage-specific expression at the BTB, and its expression during the epithelial cycle closely associates with BTB restructuring events at late-stage VIII–IX, illustrating its expression may be necessary to induce BTB restructuring. This postulate is further supported by findings using an in vivo model in which BTB restructuring induced by an acute dose of adjudin (17) was shown to be associated with a significant induction of p-rpS6 at the BTB as reported herein. In short, these findings illustrate that an enhanced p-rpS6 expression would perturb the Sertoli cell BTB function. In other words, a reduced p-rpS6 expression would promote the BTB. Indeed, the use of either rapamycin to block mTORC1, the upstream signaling regulator of rpS6, or a knockdown of rpS6 by RNAi was found to promote the Sertoli cell TJ permeability barrier function, which was also associated with a concomitant and significant decline of p-rpS6 and also p-S6K (the upstream regulator of p-rpS6) in the Sertoli cell epithelium. Although these observations can be attributed to changes in protein synthesis, such as an increase in de novo synthesis of adhesive TJ-protein complexes at the BTB (e.g. occludin-ZO-1 and claudin-11-ZO-1) to promote the TJ-barrier function after the knockdown of rpS6 or a blockade of mTORC1. Because it is well established that the mTOR signaling pathway is crucial to regulate protein synthesis (9, 10), and a study using knock-in mice (rpS6p−/−) without p-rpS6 in these mice has shown that p-rpS6 can down-regulate protein synthesis (40). However, a tightening of the Sertoli cell TJ permeability was detected within 1.5 h after rapamycin, whereas the increase in the production of claudin-11 and several other TJ proteins was not visible until 24-h later. Instead, there were changes in protein recruitment at the Sertoli cell-cell interface with more claudin-11 and/or occludin being localized to the cell junction. These changes also correlated with a reorganization of actin filaments in the Sertoli cell epithelium, the hallmark ultrastructure of the BTB, with more actin filaments being found localized to the cell-cell interface. These findings are also consistent with data obtained by knockdown of rpS6 in testes in vivo using shRNA in which tubules at late-stage VIII–IX were found to have significantly more occludin and claudin-11 being recruited to the BTB site vs. nontargeting control shRNA. Additionally, a considerably distinctive actin filament network was also detected at the BTB in the seminiferous epithelium of the testes at late-stage VIII–IX in rpS6-knockdown rats treated with rpS6-specific shRNA. Collectively, these findings illustrate p-rpS6 in the mTOR signaling pathway regulates BTB dynamics via changes in protein localization and/or recruitment to the TJ and basal ES at the BTB via changes in reorganization of the underlying F-actin network during spermatogenesis, in addition to an alteration of protein synthesis, consistent with recent findings reporting that an activation of mTORC1 activity/function can disrupt barrier function in the kidney glomerulus (13) and the TJ barrier in mouse bladder (14).
In short, we have demonstrated that rpS6 regulates BTB dynamics via changes in the recruitment of BTB proteins at the Sertoli cell-cell interface, which is mediated by alterations of actin filament network organization. Although the molecular mechanisms underlying these changes remain to be elucidated, it is likely that the knockdown of rpS6/p-rpS6 modulates the kinetics of endocytic vesicle-mediated protein internalization, recycling, and/or endosome/ubiquitin-mediated protein degradation, so that occludin and claudin-11 can be recruited to the Sertoli cell-cell interface at the BTB in stage VIII–IX tubules as demonstrated herein based on studies in vivo after rpS6/ knockdown in the testis by shRNA. This possibility is further supported by experiments using genetic analysis, illustrating a functional link between TORC1 and actin/endocytosis-related genes, and an inhibition of TORC1 by rapamycin disrupted actin polarization in Saccharomyces cerevisiae (15). Furthermore, there is accumulating evidence that changes in the organization of the actin filament network at the BTB, such as induced by the intricate interactions of Arp3 (7), Eps8 (5), and drebrin E (41), regulate endocytic vesicle-mediated protein trafficking (42). Nonetheless, findings reported herein have demonstrated for the first time that the mTORC1 signaling pathway is crucial to maintain BTB integrity during spermatogenesis in the testis, which is mediated via changes in protein recruitment at the site, illustrating yet another function of the mTORC1 signaling pathway in regulating cellular homeostasis.
Supplementary Material
Acknowledgments
This work was supported by grants from the National Institutes of Health (R01 HD056034 to C.Y.C. and U54 HD029990 Project 5 to C.Y.C.).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- Arp3
- Actin-related protein 3
- BTB
- blood-testis barrier
- BW
- body weight
- DAPI
- 4′,6-diamidino-2-phenylindole
- Eps8
- epidermal growth factor receptor pathway substrate 8
- ES
- ectoplasmic specialization
- F-actin
- filamentous actin
- FITC
- fluorescein isothiocyanate
- IHC
- immunohistochemistry
- IP
- immunoprecipitation
- mTOR
- mammalian target of rapamycin
- JAM-A
- junctional adhesion molecule-A
- mTORC1
- mTOR complex 1
- p-rpS6
- phospho-rpS6
- RNAi
- RNA interference
- rpS6
- ribosomal protein S6
- shRNA
- short hairpin RNA
- siRNA
- small interfering RNA
- S6K
- S6 kinase
- TER
- transepithelial electrical resistance
- TJ
- tight junction
- ZO-1
- zonula occludens-1.
References
- 1. Cheng CY, Mruk DD. 2012. The blood-testis barrier and its implication in male contraception. Pharmacol Rev 64:16–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Cheng CY, Mruk DD. 2010. A local autocrine axis in the testes that regulates spermatogenesis. Nat Rev Endocrinol 6:380–395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cheng YH, Wong EW, Cheng CY. 2011. Cancer/testis (CT) antigens, carcinogenesis and spermatogenesis. Spermatogenesis 1:209–220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hertzog M, Milanesi F, Hazelwood L, Disanza A, Liu H, Perlade E, Malabarba MG, Pasqualato S, Maiolica A, Confalonieri S, Le Clainche C, Offenhauser N, Block J, Rottner K, Di Fiore PP, Carlier MF, Volkmann N, Hanein D, Scita G. 2010. Molecular basis for the dual function of Eps8 on actin dynamics: bundling and capping. PLoS Biol 8:e1000387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lie PP, Mruk DD, Lee WM, Cheng CY. 2009. Epidermal growth factor receptor pathway substrate 8 (Eps8) is a novel regulator of cell adhesion and the blood-testis barrier integrity in the seminiferous epithelium. FASEB J 23:2555–2567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Rottner K, Hänisch J, Campellone KG. 2010. WASH, WHAMM and JMY: regulation of Arp2/3 complex and beyond. Trends Cell Biol 20:650–661 [DOI] [PubMed] [Google Scholar]
- 7. Lie PP, Chan AY, Mruk DD, Lee WM, Cheng CY. 2010. Restricted Arp3 expression in the testis prevents blood-testis barrier disruption during junction restructuring at spermatogenesis. Proc Natl Acad Sci USA 107:11411–11416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zhou H, Huang S. 2010. The complexes of mammalian target of rapamycin. Curr Protein Pept Sci 11:409–424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Conn CS, Qian SB. 2011. mTOR signaling in protein homeostasis: less is more? Cell Cycle 10:1940–1947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Weichhart T. 2012. Mammalian target of rapamycin: a signaling kinase for every aspect of cellular life. Methods Mol Biol 821:1–14 [DOI] [PubMed] [Google Scholar]
- 11. Magnuson B, Ekim B, Fingar DC. 2012. Regulation and function of ribosomal protein S6 kinase (S6K) within mTOR singalling networks. Biochem J 441:1–21 [DOI] [PubMed] [Google Scholar]
- 12. Ip CK, Cheung AN, Ngan HY, Wong AS. 2011. p70 S6 kinase in the control of actin cytoskeleton dynamics and directed migration of ovarian cancer cells. Oncogene 30:2420–2432 [DOI] [PubMed] [Google Scholar]
- 13. Inoki K, Mori H, Wang J, Suzuki T, Hong S, Yoshida S, Blattner SM, Ikenoue T, Rüegg MA, Hall MN, Kwiatkowski DJ, Rastaldi MP, Huber TB, Kretzler M, Holzman LB, Wiggins RC, Guan KL. 2011. mTORC1 activation in podocytes is a critical step in the development of diabetic nephropathy in mice. J Clin Invest 121:2181–2196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Shorning BY, Griffiths D, Clarke AR. 2011. Lkb1 and Pten synergise to suppress mTOR-mediated tumorigenesis and epithelial-mesenchymal transition in the mouse bladder. PLoS One 6:e16209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Aronova S, Wedaman K, Anderson S, Yates J, 3rd, Powers T. 2007. Probing the membrane environment of the TOR kinases reveals functional interactons between TORC1, actin, and membrane trafficking in Saccharomyces cerevisiae. Mol Biol Cell 18:2779–2794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Liu L, Chen L, Chung J, Huang S. 2008. Rapamycin inhibits F-actin reorganization and phosphorylation of focal adhesion proteins. Oncogene 27:4998–5010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mok KW, Mruk DD, Lee WM, Cheng CY. 2012. Spermatogonial stem cells alone are not sufficient to re-initiate spermatogenesis in the rat testis following adjudin-induced infertility. Int J Androl 35:86–101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Aravindan GR, Pineau CP, Bardin CW, Cheng CY. 1996. Ability of trypsin in mimicking germ cell factors that affect Sertoli cell secretory function. J Cell Physiol 168:123–133 [DOI] [PubMed] [Google Scholar]
- 19. Mruk DD, Cheng CY. 2011. An in vitro system to study Sertoli cell blood-testis barrier dynamics. Methods Mol Biol 763:237–252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Orth JM. 1982. Proliferation of Sertoli cells in fetal and postnatal rats: a quantitative autoradiographic study. Anat Rec 203:485–492 [DOI] [PubMed] [Google Scholar]
- 21. Janecki A, Jakubowiak A, Steinberger A. 1992. Effect of cadmium chloride on transepithelial electrical resistance of Sertoli cell monolayers in two-compartment cultures: a new model for toxicological investigations of the “blood-testis” barrier in vitro. Toxicol Appl Pharmacol 112:51–57 [DOI] [PubMed] [Google Scholar]
- 22. Janecki A, Jakubowiak A, Steinberger A. 1991. Effects of cyclic AMP and phorbol ester on transepithelial electrical resistance of Sertoli cell monolayers in two-compartment culture. Mol Cell Endocrinol 82:61–69 [DOI] [PubMed] [Google Scholar]
- 23. Grima J, Pineau C, Bardin CW, Cheng CY. 1992. Rat Sertoli cell clusterin, α2-macroglobulin, and testins: biosynthesis and differential regulation by germ cells. Mol Cell Endocrinol 89:127–140 [DOI] [PubMed] [Google Scholar]
- 24. Lie PP, Cheng CY, Mruk DD. 2010. Crosstalk between desmoglein-2/desmocollin-2/Src kinase and coxsackie and adenovirus receptor/ZO-1 protein complexes, regulates blood-testis barrier dynamics. Int J Biochem Cell Biol 42:975–986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Siu MK, Wong CH, Lee WM, Cheng CY. 2005. Sertoli-germ cell anchoring junction dynamics in the testis are regulated by an interplay of lipid and protein kinases. J Biol Chem 280:25029–25047 [DOI] [PubMed] [Google Scholar]
- 26. Li MW, Mruk DD, Lee WM, Cheng CY. 2009. Disruption of the blood-testis barrier integrity by bisphenol A in vitro: is this a suitable model for studying blood-testis barrier dynamics? Int J Biochem Cell Biol 41:2302–2314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lui WY, Lee WM, Cheng CY. 2001. Transforming growth factor-β3 perturbs the inter-Sertoli tight junction permeability barrier in vitro possibly mediated via its effects on occludin, zonula occludens-1, and claudin-11. Endocrinology 142:1865–1877 [DOI] [PubMed] [Google Scholar]
- 28. Lie PP, Cheng CY, Mruk DD. 2011. Interleukin-1α is a regulator of the blood-testis barrier. FASEB J 25:1244–1253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Li MW, Mruk DD, Lee WM, Cheng CY. 2009. Connexin 43 and plakophilin-2 as a protein complex that regulates blood-testis barrier dynamics. Proc Natl Acad Sci USA 106:10213–10218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Li MW, Xia W, Mruk DD, Wang CQF, Yan HH, Siu MK, Lui WY, Lee WM, Cheng CY. 2006. TNFα reversibly disrupts the blood-testis barrier and impairs Sertoli-germ cell adhesion in the seminiferous epithelium of adult rat testes. J Endocrinol 190:313–329 [DOI] [PubMed] [Google Scholar]
- 31. Xia W, Wong EW, Mruk DD, Cheng CY. 2009. TGF-β3 and TNFα perturb blood-testis barrier (BTB) dynamics by accelerating the clathrin-mediated endocytosis of integral membrane proteins: A new concept of BTB regulation during spermatogenesis. Dev Biol 327:48–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wong CH, Mruk DD, Lui WY, Cheng CY. 2004. Regulation of blood-testis barrier dynamics: an in vivo study. J Cell Sci 117:783–798 [DOI] [PubMed] [Google Scholar]
- 33. Setchell BP, Waites GMH. 1970. Changes in the permeability of the testicular capillaries and of the “blood-testis barrier” after injection of cadmium chloride in the rat. J Endocrinol 47:81–86 [DOI] [PubMed] [Google Scholar]
- 34. Cheng CY, Mruk DD, Silvestrini B, Bonanomi M, Wong CH, Siu MK, Lee NP, Mo MY. 2005. AF-2364 [1-(2,4-dichlorobenzyl)-1H-indazole-3-carbohydrazide] is a potential male contraceptive: A review of recent data. Contraception 72:251–261 [DOI] [PubMed] [Google Scholar]
- 35. Janecki A, Steinberger A. 1986. Polarized Sertoli cell functions in a new two-compartment culture system. J Androl 7:69–71 [DOI] [PubMed] [Google Scholar]
- 36. Byers SW, Hadley MA, Djakiew D, Dym M. 1986. Growth and characterization of epididymal epithelial cells and Sertoli cells in dual environment culture chambers. J Androl 7:59–68 [DOI] [PubMed] [Google Scholar]
- 37. Zoncu R, Efeyan A, Sabatini DM. 2011. mTOR: from growth signal integration to cancer, diabetes and ageing. Nat Rev Mol Cell Biol 12:21–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Meyuhas O. 2008. Physiological roles of ribosomal protein S6: one of its kind. Int Rev Cell Mol Biol 268:1–37 [DOI] [PubMed] [Google Scholar]
- 39. Löwik MM, Groenen PJ, Levtchenko EN, Monnens LA, van den Heuvel LP. 2009. Molecular genetic analysis of podocyte genes in focal segmental glomerulosclerosis: a review. Eur J Pediatr 168:1291–1304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ruvinsky I, Sharon N, Lerer T, Cohen H, Stolovich-Rain M, Nir T, Dor Y, Zisman P, Meyuhas O. 2005. Ribosomal protein S6 phosphorylation is a determinant of cell size and glucose homeostasis. Genes Dev 19:2199–2211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Li MW, Xiao X, Mruk DD, Lam YL, Lee WM, Lui WY, Bonanomi M, Silvestrini B, Cheng CY. 2011. Actin binding protein drebrin E is involved in junction dynamics during spermatogenesis. Spermatogenesis 1:123–136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Cheng CY, Wong EW, Lie PP, Li MW, Mruk DD, Yan HH, Mok KW, Mannu J, Mathur PP, Lui WY, Lee WM, Bonanomi M, Silvestrini B. 2011. Regulation of blood-testis barrier dynamics by desmosome, gap junction, hemidesmosome and polarity proteins: an unexpected turn of events. Spermatogenesis 1:105–115 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.