Abstract
Mammalian circadian organization is governed by pacemaker neurons in the brain that communicate with oscillators in peripheral tissues. Adrenal glucocorticoids are important time-giving signals to peripheral circadian oscillators. We investigated the rhythm of Per1-luc expression in pineal, pituitary, salivary glands, liver, lung, kidney, cornea as well as suprachiasmatic nucleus from adrenalectomized and sham-operated rats kept under light-dark cycles, or exposed to single 6-h phase delays or advances of their light cycles. Adrenalectomy shifted the phases of Per1-luc in liver, kidney, and cornea and caused phase desynchrony and significant dampening in the rhythmicity of cornea. Treatment with hydrocortisone shifted the phases of Per1-luc in most of the tissues examined, even those that were not affected by adrenalectomy. The rhythm in cornea recovered in animals given hydrocortisone in vivo or when corneas were treated with dexamethasone in vitro. Adrenalectomy increased the rate of reentrainment after phase shifts in liver, kidney, cornea, pineal, lung, and suprachiasmatic nucleus but not in pituitary and salivary glands. Our data show that glucocorticoids act as strong entraining signals for peripheral circadian oscillators and may feed back on central oscillators as well.
In mammals, circadian rhythmicity is regulated by a central clock in the suprachiasmatic nucleus (SCN) of the hypothalamus. Ablation of the SCN results in complete arrhythmicity in behavior as well as loss of other circadian rhythms, such as endocrine and body temperature rhythms (1–3). The molecular clock that generates circadian rhythmicity relies on autoregulatory feedback loops in an array of clock genes and proteins. Clock and Bmal1 are primary activators of downstream elements in the pathway. Heterodimers of CLOCK and BMAL1 drive the expression of Period (Per) and Cryptochrome (Cry). PER and CRY proteins translocate back into nucleus acting as negative regulators of CLOCK:BMAL1-induced transactivation (4).
In addition to the SCN, most peripheral tissues also express circadian oscillations driven by the clock genes. The oscillators in these peripheral tissues are less robust than those in the SCN. When cultured in vitro, SCN explants show rhythmic oscillations in gene expression for many cycles whereas those in cultured peripheral oscillators tend to dampen more quickly. Peripheral tissues also appear to rely upon SCN-driven cues to align their oscillations to the light-dark (LD) cycle and to each other. When animals are exposed to delays or advances of their LD cycles, peripheral oscillators reentrain more slowly than the SCN, with the rate of reentrainment varying among oscillators (5). In the absence of the SCN, peripheral tissues continue to oscillate, but the normal relationship among their phases is disrupted (6).
Maintaining synchrony between the SCN and peripheral oscillators is important for normal function; its loss is correlated with several severe pathologies (7–10). Multiple signaling pathways (calcium, cAMP, protein kinase A and C, vasoactive intestinal peptide, glucocorticoids, ligands of nuclear receptors retinoic acid receptor α and retinoid X receptor α) have been implicated in the regulation of phases of peripheral oscillators (11–13). It is likely that SCN-driven signals provide internal phase synchrony by acting as timing cues to peripheral oscillators. Both neuronal and humoral signals from the SCN (directly or indirectly) may act this way (12, 14–16). SCN-lesioned mice parabiosed to intact partners (allowing vascular exchange but not neural communication) showed rhythmic clock gene expression in some tissues but not others (16, 17). Gonadotropins, LH, and FSH but not sympathetic nerve input, regulate the circadian phase in the clock in the ovary (18). The ovarian clock is not only entrained to gonadotropins but also governs a gating mechanism for ovulation in response to these hormones, emphasizing the importance of endocrine signals in the physiology of peripheral circadian oscillators (19).
Glucocorticoids are important timing signals for peripheral oscillators. They are rhythmically secreted by the adrenal gland peaking with the onset of activity (the beginning of dark period in nocturnal animals). Rhythmic secretion of corticosterone is tightly controlled by the SCN; SCN lesions abolish the rhythm and lead to high basal corticosterone levels (1). Glucocorticoid receptors are abundant in many peripheral tissues, but not in adult SCN neurons, which makes these hormones suitable candidates as entraining signals for circadian oscillators in peripheral organs (14, 20).
In this study, we tested the hypothesis that glucocorticoids act as entraining signals for peripheral circadian oscillators. We investigated the effects of bilateral adrenalectomy and hydrocortisone treatment on phases of Per1-luc expression rhythms in peripheral oscillators and the SCN. We tested the reentrainment kinetics of the SCN and peripheral oscillators in adrenalectomized animals subjected to phase shifts. Finally, we examined in vitro effects of dexamethasone, a synthetic glucocorticoid, on the circadian rhythm of cornea dissected from adrenalectomized rats.
Materials and Methods
Animals
Adult male Period1-luciferase (Per1-luc) transgenic rats were obtained from our breeding colony. In this transgenic rat line, the Period1 gene promoter is linked to a luciferase reporter (5). Animals were maintained on 12-h light, 12-h dark cycles and ad libitum feeding throughout all experiments. All procedures were approved by the University of Virginia Animal Care and Use Committee.
Effects of adrenalectomy and glucocorticoid replacement on phase of peripheral oscillators and SCN
Per1-luc rats were randomly assigned to one of three groups: Group 1 rats were adrenalectomized (ADX) and provided saline (0.9% sodium chloride) as drinking water ad libitum. Bilateral adrenalectomy was performed through dorsal incisions under 2.5% isoflurane anesthesia. Group 2 rats received sham surgeries (Sham ADX) in which they were exposed to bilateral laparotomy as in group 1 except their adrenal glands were left untouched. Group 3 rats were ADX and received 50 μg/ml hydrocortisone (Sigma Aldrich, St. Louis, MO) dissolved in 0.4% ethanol as glucocorticoid treatment in their drinking water (saline) ad libitum (ADX+CORT). The animals were maintained in a 12-h light, 12-h dark (LD) cycle for 6 wk after the surgeries and decapitated at approximately ZT11 (Zeitgeber time 11; 1 h before lights are off). Blood was collected for corticosterone measurement, and tissues were harvested for culture.
Effects of adrenalectomy on reentrainment
Per1-luc rats had either adrenalectomy or sham surgeries and then were maintained in 12:12 LD cycle for 6 wk. The animals were then exposed to a 6-h phase delay or advance of their light cycles. They were decapitated at approximately ZT11 of their new LD cycle either 1 d or 5 d after the phase shifts. Blood was collected for corticosterone measurement and tissues were harvested for culture.
Effects of in vitro dexamethasone treatment of cornea of ADX animals
Per1-luc rats were ADX and then maintained in a 12:12 LD cycle for 6 wk. The animals were decapitated at ZT 11, blood was collected for corticosterone measurement and corneas were isolated for culture. Corneas were cultured in a medium containing either dexamethasone (10 μm DEX) or vehicle (VEH; 0.1% ethanol).
Tissue culture
Tissue culture and preparation were performed as described elsewhere (21). After decapitation and blood collection, the tissues were harvested and placed in chilled Hank's balanced salt solution. We collected SCN, cornea, pituitary gland, liver, lung, pineal, kidney, and salivary gland. The SCN was cut from a 300-μm coronal brain section. Pineal gland was flattened and cultured whole. The anterior pituitary gland, salivary gland, liver, lung, and kidney were hand sliced into thin sections. The whole cornea was removed from one of the eyes. Explanted tissues were placed on Millicell culture inserts in 35-mm culture dishes in medium containing luciferin. Cultures were incubated for 5 d under constant conditions (constant darkness, constant temperature of 35 C, and no medium change). Light emitted from each culture was measured with photomultiplier detectors (Hamamatsu, Bridgewater, NJ).
RIA
Plasma was analyzed for corticosterone with a RIA kit (MP Biomedicals, Solon, OH). In adrenalectomy groups, only the animals that showed no detectable levels of corticosterone were included in the analyses.
Analysis of Per1-luc bioluminescence data
Bioluminescence data were detrended by subtracting the 24-h running average from the raw data. Detrended data sets were smoothed by taking 2-h running averages. The time corresponding to the highest level of bioluminescence that occurred between 24 and 48 h in culture was considered the peak phase. Average peak phases of Per1-luc expression were reported as arithmetic means ± sem in ZT (Supplemental Table 1 published on The Endocrine Society's Journals online web site at http://endo.endojournals.org). Peak phases were converted into phase angles relative to the LD cycle of the animal. Mean vectors of circular distributions based on the phase of individual tissues were calculated as in Yoshikawa et al. (2009). Rayleigh uniformity test was applied to determine whether there was significant clustering of the peak phases for each tissue. Watson-Williams F-test was applied to evaluate the differences among treatments. Reentrainment data were analyzed by two-way ANOVA; Bonferroni post hoc tests analyzed the differences between phase shift-d 1 and control groups or between phase shift-d 5 and target groups in ADX or Sham ADX tissues.
Results
Effects of adrenalectomy and glucocorticoid treatment on phase of peripheral oscillators and SCN
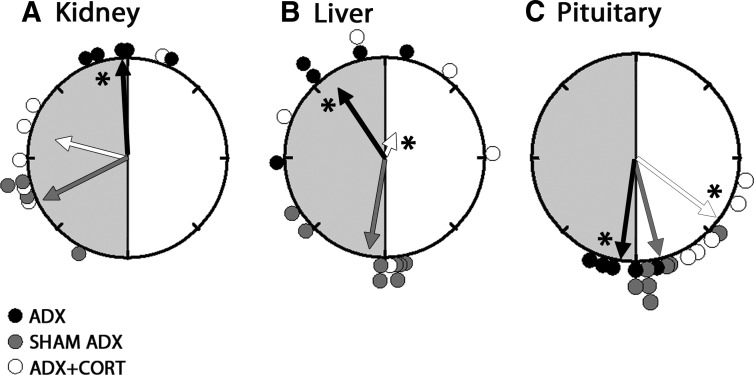
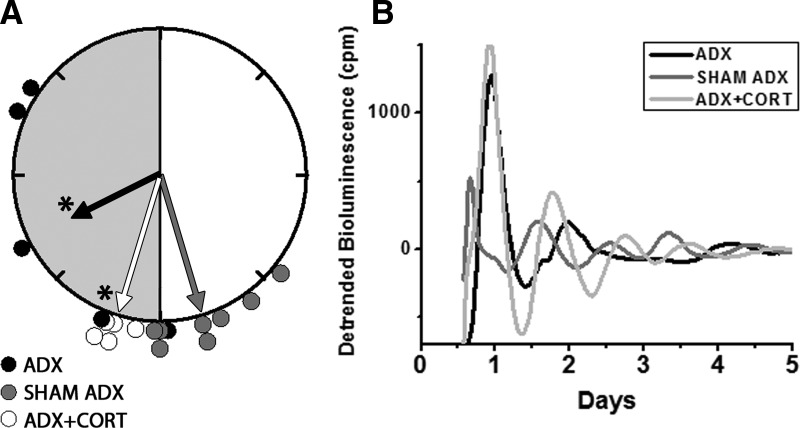
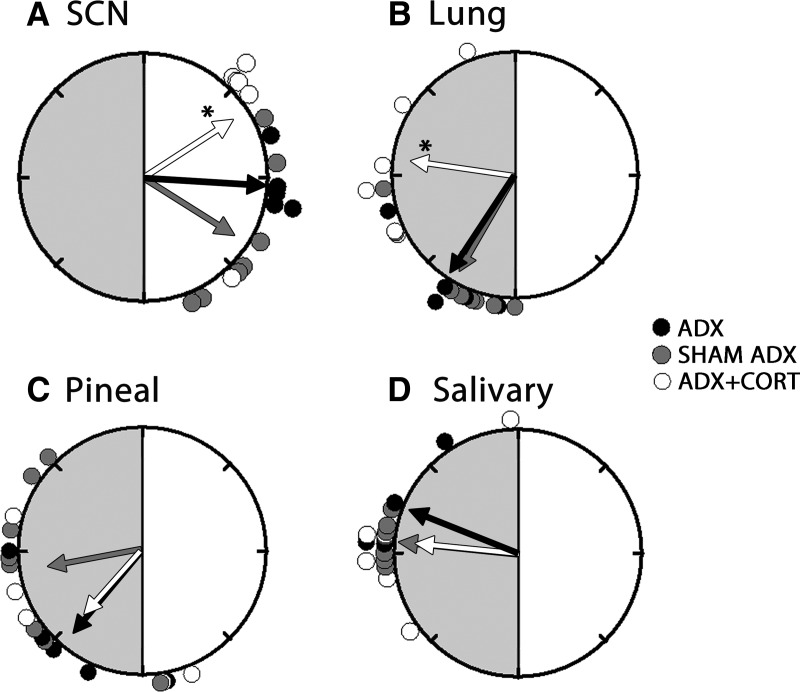
Adrenalectomy delayed the phase of Per1-luc expression in several tissues. Peak Per1-luc expression was significantly delayed in kidney, liver, cornea, and pituitary gland from ADX rats (Figs. 1 and 2) (F test, P < 0.05). The effect of adrenalectomy was particularly robust in cornea; not only was there a significant delay of peak phase, but the rhythm also was severely damped after the first peak in vitro (Fig. 2B). Moreover, the phases of corneas from ADX animals were not significantly clustered around the mean, indicating phase desynchrony (Fig. 2A) (Rayleigh uniformity test, P > 0.05). Adrenalectomy had no effect on the phase of SCN, lung, pineal, or salivary gland (Fig. 3) (Watson-Williams F test, P > 0.05).
Fig. 1.
Rayleigh plots of the peak phases of Per1-luc in kidney (A), liver (B), and pituitary (C) from ADX (black circles), sham-operated (gray circles), and ADX animals receiving hydrocortisone treatment (white circles). The light and dark portions of the animals' day before euthanasia are indicated with white and gray shaded halves of the circles, respectively. The arrows represent the mean vectors for circular distributions. The length of the vector represents the strength of the phase clustering, and the angle of the vector represents the mean vector phase. Asterisk represents the significance at P < 0.05 level for the F-test compared with sham controls. Adrenalectomy altered the mean phase in liver, kidney, and pituitary, compared with sham controls. Hydrocortisone treatment to ADX animals advanced the phase in pituitary (P < 0.05) whereas it restored the phase in kidney compared with sham controls (P > 0.05). Hydrocortisone treatment caused desynchrony of Per1-luc expression in liver (Rayleigh test, P > 0.05). CORT, Hydrocortisone.
Fig. 2.
Rayleigh plot of the peak phases of Per1-luc expression in cornea from ADX (black circles), sham-operated (gray circles), or ADX with hydrocortisone replaced (white circles) animals (A). Individual bioluminescence traces of Per1-luc recorded from cornea of ADX (black trace), sham-operated (dark gray trace), and ADX with hydrocortisone replaced (light gray trace) animals (B). Details as in Fig. 1. Adrenalectomy caused phase desynchrony and damping of the rhythmicity in cornea. Despite a robust first day peak, four of five ADX corneas did not reveal any significant period as assessed by periodogram (P > 0.05; Lumicycle, Actimetrics, Wilmetter, IL). All corneas from the hydrocortisone-treated group (n = 5) regained robust oscillations and phase synchronization (Rayleigh test, P < 0.01). CORT, Hydrocortisone.
Fig. 3.
Rayleigh plots of peak phases of Per1-luc in SCN (A), lung (B), pineal (C), and salivary (D) from ADX (black circles), sham-operated (gray circles), and ADX animals receiving hydrocortisone treatment (white circles). Details as in Fig. 1. Compared with sham controls, adrenalectomy did not have any effects on phase of SCN, lung, pineal, and salivary glands. Hydrocortisone treatment of ADX animals significantly delayed the phase in lung and advanced the phase in SCN compared with sham controls. Hydrocortisone treatment did not have any effect on the mean phases of salivary or pineal glands of ADX animals. CORT, Hydrocortisone.
Hydrocortisone treatment of ADX animals restored the robustness of the rhythm and phase synchrony in cornea and delayed the phase of Per1-luc expression (Fig. 2). It advanced the phase in pituitary (Fig. 1C) and SCN (Fig. 3A) but delayed it in lung (Fig. 3B) (F test, P < 0.05). Hydrocortisone treatment caused phase desynchrony in liver (Rayleigh uniformity test, P > 0.05). The average peak phases of Per1-luc expression in ZT are reported as arithmetic means ± sem along with the number of rhythmic tissues used in analysis in Supplemental Table 1.
Effects of adrenalectomy on reentrainment
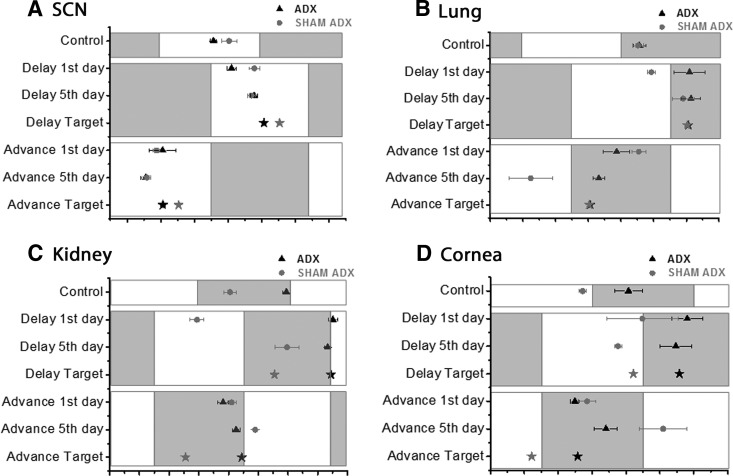
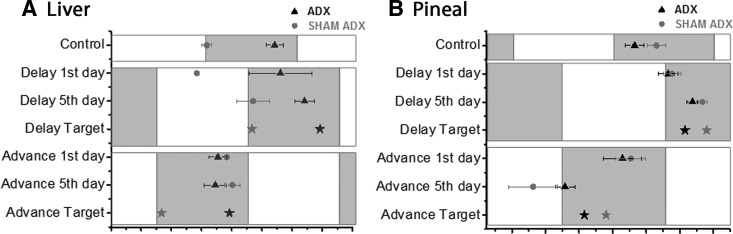
Adrenalectomy accelerated reentrainment in several tissues. After a 6-h phase delay or advance of the LD cycle, the phases of Per1-luc expression in SCN, lung, kidney, and cornea of ADX animals shifted to their target phase more rapidly than sham controls (Fig. 4). The phases of lung, kidney, and cornea from ADX rats shifted to their target phase within a single day following a phase delay or advance of the LD cycle; tissues from sham control animals did not (Fig. 4, B–D). ADX-SCN shifted to its target phase 5 d after either delay or advance of the LD cycle whereas sham controls were still not fully shifted after 5 d (Fig. 4A).
Fig. 4.
Phase maps indicating rates of reentrainment in SCN (A), lung (B), kidney (C), and cornea (D) of ADX (black symbols) or sham-operated animals (gray symbols), 1 d and 5 d after 6-h phase delays and advances. Black triangle and gray circles represent the mean phase ± sem (ZT) of the groups listed on the y-axis. The control groups are the preshift phases of the tissues. The stars represent the target phases at steady state after 6 h delay or advance. The white and gray blocks represent the LD cycles of the animals before euthanasia. Adrenalectomy increased readjustment rate of Per1-luc in SCN, lung, kidney, and cornea after either phase delay or advance.
Adrenalectomy also accelerated reentrainment in liver and pineal gland after phase advance of the LD cycle, but not after a phase delay (Fig. 5). ADX-liver and pineal shifted to their target phases 1 d and 5 d after phase advance (P > 0.05), respectively, whereas the phases of tissues from sham controls were still significantly different from their targets (P < 0.05). Adrenalectomy did not affect reentrainment in pituitary or salivary glands (Supplemental Fig. 1).
Fig. 5.
Phase maps demonstrating rates of reentrainment in liver (A) and pineal (B) of ADX (black symbols) or sham-operated animals (gray symbols), 1 d and 5 d after 6-h phase delays and advances. Details as in Fig. 4. Liver and pineal gland of ADX rats reentrained faster after phase advances relative to sham controls whereas reentrainment to phase delays did not differ.
Effects of in vitro dexamethasone treatment of cornea of ADX animals
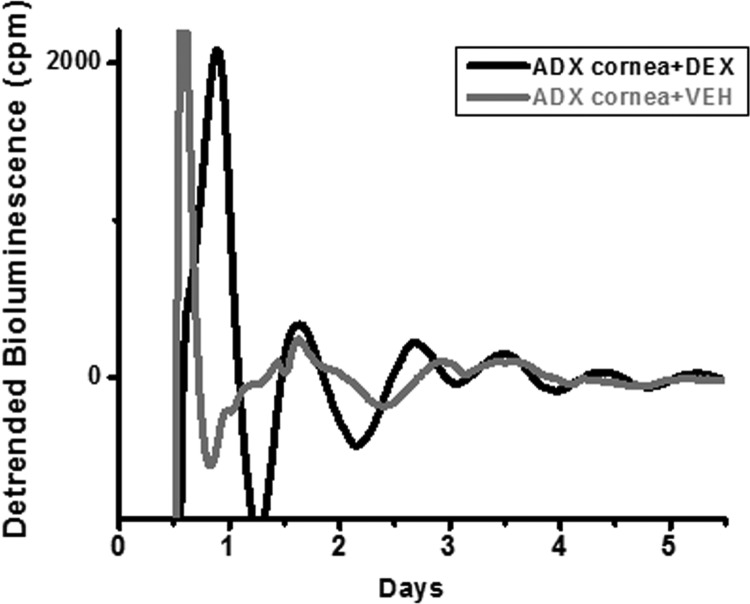
In vivo, adrenalectomy had the most robust effect on cornea, not only causing a shift in phase, but also decreasing the robustness of the rhythm of Per1-luc expression. To determine whether this effect was due to a direct action of glucocorticoids on the cornea, we tested the effect of in vitro glucocorticoid treatment on the cornea oscillator. Corneas from ADX animals were weakly rhythmic, with only two of six vehicle-treated ADX-corneas exhibiting a robust circadian period (P < 0.05) (Fig. 6). Dexamethasone treatment improved rhythmicity, with a robust circadian oscillation (as assessed by periodogram) observed in five of seven dexamethasone-treated ADX corneas.
Fig. 6.
Individual bioluminescence traces of Per1-luc recorded from single corneas of ADX animals. Corneas were either treated with dexamethasone (DEX, black trace) or vehicle (VEH, gray trace). Corneas treated with dexamethasone in vitro regained their robust oscillations.
Discussion
Maintaining order within the circadian system requires entrainment not only of behavior but also of the many oscillators at cellular and tissue levels. Our data as well as those of others (14, 22) demonstrate that glucocorticoids are critical timing signals for many peripheral oscillators, enabling their proper phase alignment and entrainment to the LD cycle. In our hands, adrenalectomy and hydrocortisone treatment altered the phase and robustness of a number of peripheral oscillators, with particularly strong effects observed in cornea. Adrenalectomy accelerated the reentrainment of peripheral oscillators after a shift in the LD cycle, further supporting a role for glucocorticoids in the maintenance of circadian entrainment.
Among mammalian peripheral tissues, liver and kidney have been found to be very sensitive to phase resetting in response to manipulation of glucocorticoid signaling. Glucocorticoid receptor agonists prednisolone and dexamethasone induce Per1 gene expression in liver, kidney, and rat primary hepatocytes whereas dexamethasone down-regulates Reverbα in liver (14, 22, 23). Moreover, for both of these tissues, neural input is not required for phase resetting of clock gene rhythms (16). Adrenalectomy abolishes circadian expression of many genes involved in liver metabolic function (24). Adrenal-specific Bmal1 mutants (with abolished corticosterone rhythms) have dampened Per1 levels in liver and kidney (25). In the present study, we have found that adrenalectomy delayed the peak phase of the steady-state rhythm of Per1-luc in kidney by 7.5 h and in liver by 9 h even though there was no change in the LD cycle (Fig. 1, A and B). In the absence of glucocorticoid signaling, kidney and liver lose their normal phase relationship to the LD cycle; perhaps these peripheral clocks are using some other internal entraining signal. Hydrocortisone treatment in ADX animals restored the phase of Per1-luc in kidney, but in liver it caused the loss of phase synchrony (Fig. 1, A and B). Mice continuously infused with prednisolone have been reported to have blunted Per1 rhythms in liver and skeletal muscle (22). Perhaps, the exogenous glucocorticoids provided by hydrocortisone treatment may overstimulate the receptors leading to desynchrony in liver.
Adrenalectomy disrupted phase synchrony and shifted the mean phase of Per1-luc expression in cornea whereas hydrocortisone treatment restored both the mean phase and phase synchrony (Fig. 2A). We reported a similar phase desynchrony in cornea when intact animals were treated with methamphetamine in their drinking water (26). In the same study, temporally restricted feeding of intact and SCN-lesioned animals caused phase shifts of PER2::LUC expression in cornea. Both methamphetamine and restricted feeding alter rhythms of circulating glucocorticoids (27–29). Methamphetamine and food entrainment may influence the oscillators in cornea (and perhaps other oscillators as well) through effects on glucocorticoids.
Adrenalectomy caused damping of the rhythm of Per1-luc expression in cornea. The normally robust rhythm in cornea that is lost in ADX animals was restored when the animals were given hydrocortisone in their drinking water (Fig. 2B) or when corneas removed from ADX animals were treated with dexamethasone in vitro (Fig. 6). Although this is the first study to show direct effects of adrenalectomy and glucocorticoids on the circadian oscillations in cornea, an early report by Cardoso and Ferreira (30) suggested that the diurnal variation in mitosis in corneal epithelial cells depended on glucocorticoid signaling.
Among the tissues examined, adrenalectomy had no (or only a very minor) effect on the SCN (Fig. 3A), pituitary gland (Fig. 1C), or lung (Fig. 3B); somewhat paradoxically, Per1-luc phases of all three tissues were affected significantly when the ADX animals were treated with hydrocortisone in their drinking water. This is a particularly surprising result in the SCN, because glucocorticoid receptors are not detectable in adult rat SCN (20). Despite the lack of glucocorticoid receptors, there have been previous reports that the SCN can respond to glucocorticoid signaling. Cheng et al. (31) have suggested that dexamethasone acts on the mammalian circadian clock through Dexras1 (dexamethasone-inducible Ras-like G protein). The immediate early genes c-fos and c-jun are induced in the SCN in response to dexamethasone injection (32). It has been reported that corticosterone is able to feedback on the SCN indirectly by affecting the serotonergic input that the SCN receives from raphe nuclei (33). Furthermore, Buijs and Escobar (34) have suggested that a feed-forward circuit promoting locomotor activity is formed by a loop involving the effects of corticosterone and serotonin on the SCN. It is possible that glucocorticoid treatment in ADX animals may dysregulate this circuit and thus produce a change in the phase of Per1-luc expression in the SCN.
A recent study has reported that adrenalectomy has no effect on Per2 and Bmal1 expression in pituitary gland (35). The unresponsiveness of lung or the weak response of pituitary gland to adrenalectomy coupled with phase shifts in response to hydrocortisone treatment may be explained by the ability of tissues to respond to high levels of glucocorticoids as in the stress response (36). Dexamethasone has been shown to reset PER2 in lung slices when applied in vitro, and when inhaled it can advance the phases of Per1, Per2, and Bmal1 in lungs (37).
Pineal (Fig. 3C) and salivary glands (Fig. 3D) are the only two tissues that are not affected by either adrenalectomy or hydrocortisone treatment. Both of these glands receive intense sympathetic innervation from the superior cervical ganglion, which itself receives input from the SCN through the paraventricular nucleus of the hypothalamus and the intermediolateral column of the spinal cord (38, 39). Vujovic et al. (40) showed that for the salivary gland, rapid phase adjustment to an inverted feeding regimen depended primarily on sympathetic innervation. This suggests that endocrine signals may not be the primary entraining signals for salivary and perhaps pineal gland as well.
Restricted feeding during the subjective day eventually uncouples the phases of peripheral oscillators from those of the SCN (41, 26). This uncoupling may be blocked by glucocorticoids, because the peripheral oscillators (specifically liver and kidney) of ADX or glucocorticoid receptor knockout animals phase reset to daytime feeding more rapidly than in intact animals (42). Here, we show that glucocorticoids act as inhibitors of uncoupling after photic phase shifts; after either phase delays or advances, most tissues readjusted their phases to the new LD cycle more rapidly in the ADX animals. The SCN has been reported to adjust its phase in 1 d after a phase shift (5). In our study, however, the phase of the Sham ADX-SCN was still not on target even 5 d after the shifts (Fig. 4A). The sham operation itself may retard reentrainment, possibly through inducing systemic inflammation. We have previously shown impaired responses to pacemaking cues (methamphetamine and restricted feeding) in sham-operated compared with intact animals (26). Adrenalectomy, on the other hand, accelerated the rate of reentrainment in SCN; in contrast to the SCN of Sham ADX animals, ADX-SCN was on target 5 d after both phase delays and advances. This interpretation is supported by behavioral studies. Decreasing cortisol levels by injection of metyrapone (an inhibitor of 11-β hydroxylase enzyme) leads to rapid behavioral reentrainment to a new light schedule, whereas increasing cortisol levels, through cortisol pellet implants or restraint stress treatment, retards reentrainment of running-wheel activity after phase advances (43). Moreover, ADX (44) or adrenal clock-deficient animals (45) have faster reentrainment kinetics in locomotor activity rhythms after phase shifts.
For liver and pineal gland, adrenalectomy did not affect reentrainment after phase delays; however, it did increase the rate of reentrainment for phase advances (Fig. 5, A and B). For pineal gland and lung, adrenalectomy did not affect phase under stable LD cycle conditions but when the animals were phase shifted, glucocorticoids retarded reentrainment in these tissues (Fig. 4B). This result for pineal gland is in agreement with the studies indicating altered pineal physiology in ADX animals under challenging situations such as saline injection (46), insulin stress (47), or immune challenge (48). The rates of reentrainment were robustly increased in kidney and cornea as these tissues adjusted their phases within 1 d of phase advance or delay in ADX animals (Fig. 4, C and D). In these cases reentrainment is so rapid that it is not possible to distinguish it from possible direct masking.
Adrenalectomy accelerated rate of reentrainment in most of the tissues examined. Even the tissues (such as SCN, lung, and pineal) that were not affected by adrenalectomy when the animals were entrained to stable LD cycles had rapid reentrainment when the animals were exposed to phase shifts. This strongly suggests that the differences in reentrainment kinetics between groups are not solely due to phase differences under stable environmental conditions.
The only exceptions to increased rate of reentrainment in ADX animals were the pituitary and salivary glands (Supplemental Fig. 1). For salivary gland, this result, together with the lack of effects of adrenalectomy under stable LD conditions (Fig. 3D), indicates that this gland uses cues other than glucocorticoids, most likely sympathetic neural signals, to entrain (40). Pituitary gland, on the other hand, may be using other neuroendocrine factors such as CRH or arginine vasopressin as entraining signals.
Our results and those of others clearly show that glucocorticoids act as entraining signals and stabilize circadian organization in the face of changes in environmental conditions. The rhythm of the corticosterone itself is relatively slow to reentrain to shifted LD cycles. After an 8-h phase delay, it takes 7 d for the corticosterone rhythm to recover its normal phase relationship to the new LD cycle (49), and 5 d are required after an inversion of the LD cycle (44). In response to a phase advance, the corticosterone peak is delayed whereas the nadir is phase advanced (50, 51). Perhaps the inability of the corticosterone rhythm to adjust rapidly to changes in the light schedule is what retards the reentrainment of peripheral oscillators in intact animals, whereas removal of corticosterone relieves the inhibition and accelerates resynchronization.
It has been shown that the phase of Per1-luc rhythm is affected by the time of animal euthanasia (52). However, the same study also showed that as long as samples are collected around ZT11 (1 h before the light offset of animals), ex vivo period-luciferase expression measurement reliably approximates phase in vivo. To minimize the timing-of-culture effect, we also chose to perform procedures at ZT11. In future studies, it might be interesting to investigate whether the phases of tissues from ADX animals would be more resilient to resetting by the time of animal euthanasia.
Glucocorticoids are implicated in regulation of many physiological functions (reviewed in Refs. 53 and 54). Their role in stress response is well established. Studies have shown that stress exposure can affect circadian rhythms of body temperature and hormone secretion (55, 56) but the effects are not large. Perhaps there are protective mechanisms at work in the intact animal. In addition to their roles in regulation of stress, immune and inflammatory responses, development, metabolism, and cognition, here we show that glucocorticoids are also involved in conveying timing information to circadian oscillators.
Supplementary Material
Acknowledgments
We thank Katie Lee, Nish Trivedi, Andaleeb Rahman, and Dr. Michael Sellix (University of Virginia, Charlottesville, VA) for technical assistance in the experiments.
This work was supported by National Institutes of Health Conte Center grant 133711.101.GO 10938.31680 (to M.M.) and Double Hoo Research grant of University of Virginia Center for Undergraduate Excellence (to P.P. and L.W.). P.P. was supported by University of Virginia Graduate School of Arts and Sciences dissertation year fellowship.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- ADX
- Adrenalectomized
- LD cycle
- light-dark cycle
- SCN
- suprachiasmatic nucleus
- ZT
- Zeitgeber time.
References
- 1. Moore RY, Eichler VB. 1972. Loss of circadian adrenal corticosterone rhythm following suprachiasmatic lesion in the rat. Brain Res 42:201–206 [DOI] [PubMed] [Google Scholar]
- 2. Stephan FK, Zucker I. 1972. Circadian rhythms in drinking behavior and locomotor activity of rats are eliminated by hypothalamic lesions. Proc Natl Acad Sci USA 69:1583–1586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Refinetti R. 1995. Effects of suprachiasmatic lesion on temperature regulation in the golden hamster. Brain Res Bull 36:81–84 [DOI] [PubMed] [Google Scholar]
- 4. Reppert SM, Weaver DR. 2002. Coordination of circadian timing in mammals. Nature 418:935–941 [DOI] [PubMed] [Google Scholar]
- 5. Yamazaki S, Numano R, Abe M, Hida A, Takahashi R, Ueda M, Block GD, Sakaki Y, Menaker M, Tei H. 2000. Resetting central and peripheral circadian oscillators in transgenic rats. Science 288:682–685 [DOI] [PubMed] [Google Scholar]
- 6. Yoo SH, Yamazaki S, Lowrey PL, Shimomura K, Ko CH, Buhr ED, Siepka SM, Hong HK, Oh WJ, Yoo OJ, Menaker M, Takahashi JS. 2004. PERIOD2::LUCIFERASE real-time reporting of circadian dynamics reveals persistent circadian oscillations in mouse peripheral tissues. Proc Natl Acad Sci USA 101:5339–5346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Schernhammer ES, Laden F, Speizer FE, Willett WC, Hunter DJ, Kawachi I, Colditz GA. 2001. Rotating night shifts and risk of breast cancer in women participating in the nurses' health study. J Natl Cancer Inst 93:1563–1568 [DOI] [PubMed] [Google Scholar]
- 8. Filipski E, Delaunay F, King VM, Wu MW, Claustrat B, Gréchez-Cassiau A, Guettier C, Hastings MH, Francis L. 2004. Effects of chronic jet lag on tumor progression in mice. Cancer Res 64:7879–7885 [DOI] [PubMed] [Google Scholar]
- 9. Mahoney MM. 2010. Shiftwork, jetlag, and female reproduction. Int J Endocrinol 2010:813764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Marcheva B, Ramsey KM, Buhr ED, Kobayashi Y, Su H, Ko CH, Ivanova G, Omura C, Mo S, Vitaterna MH, Lopez JP, Philipson LH, Bradfield CA, Crosby SD, JeBailey L, Wang X, Takahashi JS, Bass J. 2010. Disruption of the clock components CLOCK and BMAL1 leads to hypoinsulinemia and diabetes. Nature 466:627–631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Balsalobre A, Marcacci L, Schibler U. 2000. Multiple signaling pathways elicit circadian gene expression in cultured Rat-1 fibroblasts. Curr Biol 10:1291–1294 [DOI] [PubMed] [Google Scholar]
- 12. McNamara P, Seo SP, Rudic RD, Sehgal A, Chakravarti D, FitzGerald GA. 2001. Regulation of CLOCK and MOP4 by nuclear hormone receptors in the vasculature: a humoral mechanism to reset a peripheral clock. Cell 105:877–889 [DOI] [PubMed] [Google Scholar]
- 13. Loh DH, Dragich JM, Kudo T, Schroeder AM, Nakamura TJ, Waschek JA, Block GD, Colwell CS. 2011. Effects of vasoactive intestinal peptide genotype on circadian gene expression in the suprachiasmatic nucleus and peripheral organs. J Biol Rhythms 26:200–209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Balsalobre A, Brown SA, Marcacci L, Tronche F, Kellendonk C, Reichardt HM, Schütz G, Schibler U. 2000. Resetting of circadian time in peripheral tissues by glucocorticoid signaling. Science 289:2344–2347 [DOI] [PubMed] [Google Scholar]
- 15. Terazono H, Mutoh T, Yamaguchi S, Kobayashi M, Akiyama M, Udo R, Ohdo S, Okamura H, Shibata S. 2003. Adrenergic regulation of clock gene expression in mouse liver. Proc Natl Acad Sci USA 100:6795–6800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Guo H, Brewer JM, Champhekar A, Harris RB, Bittman EL. 2005. Differential control of peripheral circadian rhythms by suprachiasmatic-dependent neural signals. Proc Natl Acad Sci USA 102:3111–3116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Guo H, Guo H, Brewer JM, Lehman MN, Bittman EL. 2006. Suprachiasmatic regulation of circadian rhythms of gene expression in hamster peripheral organs: effects of transplanting the pacemaker. J Neurosci 26:6406–6412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Yoshikawa T, Sellix M, Pezuk P, Menaker M. 2009. Timing of the ovarian clock is regulated by gonadotropins. Endocrinology 150:4338–4347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sellix MT, Yoshikawa T, Menaker M. 2010. A circadian egg timer gates ovulation. Curr Biol 20:R266–R267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Rosenfeld P, Van Eekelen JA, Levine S, De Kloet ER. 1988. Ontogeny of the type 2 glucocorticoid receptor in discrete rat brain regions: an immunocytochemical study. Dev Brain Res 42:119–127 [DOI] [PubMed] [Google Scholar]
- 21. Yamazaki S, Takahashi JS. 2005. Real-time bioluminescence reporting of circadian gene expression in mammals. Methods Enzymol 393:288–301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Koyanagi S, Okazawa S, Kuramoto Y, Ushijima K, Shimeno H, Soeda S, Okamura H, Ohdo S. 2006. Chronic treatment with prednisolone represses the circadian oscillation of clock gene expression in mouse peripheral tissues. Mol Endocrinol 20:573–583 [DOI] [PubMed] [Google Scholar]
- 23. Torra IP, Tsibulsky V, Delaunay F, Saladin R, Laudet V, Fruchart JC, Kosykh V, Staels B. 2000. Circadian and glucocorticoid regulation of Rev-erbα expression in liver. Endocrinology 141:3799–3806 [DOI] [PubMed] [Google Scholar]
- 24. Oishi K, Amagai N, Shirai H, Kadota K, Ohkura N, Ishida N. 2005. Genome-wide expression analysis reveals 100 adrenal gland-dependent circadian genes in the mouse liver. DNA Res 12:191–202 [DOI] [PubMed] [Google Scholar]
- 25. Son GH, Chung S, Choe HK, Kim HD, Baik SM, Lee H, Lee HW, Choi S, Sun W, Kim H, Cho S, Lee KH, Kim K. 2008. Adrenal peripheral clock controls the autonomous circadian rhythm of glucocorticoid by causing rhythmic steroid production. Proc Natl Acad Sci USA 105:20970–20975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Pezuk P, Mohawk JA, Yoshikawa T, Sellix MT, Menaker M. 2010. Circadian organization is governed by extra-SCN pacemakers. J Biol Rhythms 25:432–441 [DOI] [PubMed] [Google Scholar]
- 27. Angeles-Castellanos M, Salgado-Delgado R, Rodriguez K, Buijs RM, Escobar C. 2010. The suprachiasmatic nucleus participates in food entrainment: a lesion study. Neuroscience 165:1115–1126 [DOI] [PubMed] [Google Scholar]
- 28. Honma S, Honma K, Shirakawa T, Hiroshige T. 1988. Rhythms in behaviors, body temperature and plasma corticosterone in SCN lesioned rats given methamphetamine. Physiol Behav 44:247–255 [DOI] [PubMed] [Google Scholar]
- 29. Honma S, Kanematsu N, Honma K. 1992. Entrainment of methamphetamine-induced locomotor activity rhythm to feeding cycles in SCN-lesioned rats. Physiol Behav 52:834–850 [DOI] [PubMed] [Google Scholar]
- 30. Cardoso SS, Ferreira AL. 1967. Effect of adrenalectomy and of dexamethasone upon circadian distribution of mitosis in the cornea of rats. I. Proc Soc Exp Biol Med 125:1254–1259 [DOI] [PubMed] [Google Scholar]
- 31. Cheng HY, Obrietan K, Cain SW, Lee BY, Agostino PV, Joza NA, Harrington ME, Ralph MR, Penninger JM. 2004. Dexras1 potentiates photic and suppresses nonphotic responses of the circadian clock. Neuron 43:715–728 [DOI] [PubMed] [Google Scholar]
- 32. Briski KP, DiPasquale BM, Gillen E. 1997. Induction of immediate-early gene expression in preoptic and hypothalamic neurons by the glucocorticoid receptor agonist, dexamethasone. Brain Res 768:185–196 [DOI] [PubMed] [Google Scholar]
- 33. Malek ZS, Sage D, Pévet P, Raison S. 2007. Daily rhythm of tryptophan hydroxylase-2 messenger ribonucleic acid within raphe neurons is induced by corticoid daily surge and modulated by enhanced locomotor activity. Endocrinology 148:5165–5172 [DOI] [PubMed] [Google Scholar]
- 34. Buijs RM, Escobar C. 2007. Corticosterone and activity: the long arms of the clock talk back. Endocrinology 148:5162–5164 [DOI] [PubMed] [Google Scholar]
- 35. Bur IM, Zouanoui S, Fontanaud P, Coutry N, Molino F, Martin AO, Mollard P, Bonnefont X. 2010. The comparison between circadian oscillators in mouse liver and pituitary gland reveals different integration of feeding and light schedules. PloS ONE 5(12):e 15316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Yamamoto T, Nakahata Y, Tanaka M, Yoshida M, Soma H, Shinohara K, Yasuda A, Mamine T, Takumi T. 2005. Acute physical stress elevates mouse period1 mRNA expression in mouse peripheral tissues via glucocorticoid-responsive element. J Biol Chem 280:42036–42043 [DOI] [PubMed] [Google Scholar]
- 37. Gibbs JE, Beesley S, Plumb J, Singh D, Farrow S, Ray DW, Loudon AS. 2009. Circadian timing in the lung; a specific role for bronchiolar epithelial cells. Endocrinology 150:268–276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Teclemariam-Mesbah R, Kalsbeek A, Pevet P, Buijs RM. 1997. Direct vasoactive intestinal polypeptide-containing projection from the suprachiasmatic nucleus to spinal projecting hypothalamic paraventricular neurons. Brain Res 748:71–76 [DOI] [PubMed] [Google Scholar]
- 39. Ueyama T, Krout KE, Nguyen XV, Karpitskiy V, Kollert A, Mettenleiter TC, Loewy AD. 1999. Suprachiasmatic nucleus: a central autonomic clock. Nat Neurosci 2:1051–1053 [DOI] [PubMed] [Google Scholar]
- 40. Vujovic N, Davidson AJ, Menaker M. 2008. Sympathetic input modulates, but does not determine, phase of peripheral circadian oscillators. Am J Physiol Regul Integr Comp Physiol 295:R355–R360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Damiola F, Le Minh N, Preitner N, Kornmann B, Fleury-Olela F, Schibler U. 2000. Restricted feeding uncouples circadian oscillators in peripheral tissues from the central pacemaker in the suprachiasmatic nucleus. Genes Dev 14:2950–2961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Le Minh N, Damiola F, Tronche F, Schütz G, Schibler U. 2001. Glucocorticoid hormones inhibit food-induced phase-shifting of peripheral circadian oscillators. Embo J 20:7128–7136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Mohawk JA, Cashen K, Lee TM. 2005. Inhibiting cortisol response accelerates recovery from a photic phase shift. Am J Physiol Regul Integr Comp Physiol 288:R221–R228 [DOI] [PubMed] [Google Scholar]
- 44. Sage D, Ganem J, Guillaumond F, Laforge-Anglade G, François-Bellan AM, Bosler O, Becquet D. 2004. Influence of the corticosterone rhythm on photic entrainment of locomotor activity in rats. J Biol Rhythms 19:144–156 [DOI] [PubMed] [Google Scholar]
- 45. Kiessling S, Eichele G, Oster H. 2010. Adrenal glucocorticoids have a key role in circadian resynchronization in a mouse model of jet lag. J Clin Invest 120:2600–2609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Toriani ME, Reiter RJ, Vaughan MK, Gonzales-Brito A, Herbert DC. 1988. The depression in rat pineal melatonin production after saline injection at night may be elicited by corticosterone. Brain Res 450(1–2):18–24 [DOI] [PubMed] [Google Scholar]
- 47. Tannenbaum MG, Reiter RJ, Vaughan MK, Troiani ME, Gonzalez-Brito A. 1987. Adrenalectomy prevents changes in rat pineal melatonin content and N-acetyltransferase activity induced by acute insulin stress. J Pineal Res 4:395–402 [DOI] [PubMed] [Google Scholar]
- 48. Lopes C, Mariano M, Markus RP. 2001. Interaction between adrenal and the pineal gland in chronic experimental inflammation induced by BCG in mice. Inflamm Res 50:6–11 [DOI] [PubMed] [Google Scholar]
- 49. Weinert D, Eimert H, Erkert HG, Schneyer U. 1994. Resynchronization of the circadian corticosterone rhythm after a light/dark shift in juvenile and adult mice. Chronobiol Int 11:222–231 [DOI] [PubMed] [Google Scholar]
- 50. Sei H, Fujihara H, Ueta Y, Morita K, Kitahama K, Morita Y. 2003. Single eight-hour shift of light-dark cycle increases brain-derived neurotrophic factor protein levels in the rat hippocampus. Life Sci 73:53–59 [DOI] [PubMed] [Google Scholar]
- 51. Mohawk JA, Pargament JM, Lee TM. 2007. Circadian dependence of corticosterone release to light exposure in the rat. Physiol Behav 92:800–806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Yoshikawa T, Yamazaki S, Menaker M. 2005. Effects of preparation time on phase of cultured tissues reveal complexity of circadian organization. J Biol Rhythms 20:500–512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Chung S, Son GH, Kim K. 2011. Adrenal peripheral oscillator in generating the circadian glucocorticoid rhythm. Ann NY Acad Sci 1220:71–81 [DOI] [PubMed] [Google Scholar]
- 54. Chung S, Son GH, Kim K. 2011. Circadian rhythm of adrenal glucocorticoid: its regulation and clinical implications. Biochim Biophys Acta 1812:581–591 [DOI] [PubMed] [Google Scholar]
- 55. Marti O, Gavaldà A, Jolin T, Armario A. 1993. Effects of regularity of exposure to chronic immobilization stress on the circadian pattern of pituitary adrenal hormones, growth hormone, and thyroid stimulating hormone in the adult male rat. Psychoneuroendocrinology 18:67–77 [DOI] [PubMed] [Google Scholar]
- 56. Kant GJ, Bauman RA, Pastel RH, Myatt CA, Closser-Gomez E, D'Angelo CP. 1991. Effects of controllable vs. uncontrollable stress on circadian temperature rhythms. Physiol Behav 49:625–630 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.