Abstract
At puberty, neurokinin B (NKB) and kisspeptin (Kiss1) may help to amplify GnRH secretion, but their precise roles remain ambiguous. We tested the hypothesis that NKB and Kiss1 are induced as a function of pubertal development, independently of the prevailing sex steroid milieu. We found that levels of Kiss1 mRNA in the arcuate nucleus (ARC) are increased prior to the age of puberty in GnRH/sex steroid-deficient hpg mice, yet levels of Kiss1 mRNA in wild-type mice remained constant, suggesting that sex steroids exert a negative feedback effect on Kiss1 expression early in development and across puberty. In contrast, levels of Tac2 mRNA, encoding NKB, and its receptor (NK3R; encoded by Tacr3) increased as a function of puberty in both wild-type and hpg mice, suggesting that during development Tac2 is less sensitive to sex steroid-dependent negative feedback than Kiss1. To compare the relative responsiveness of Tac2 and Kiss1 to the negative feedback effects of gonadal steroids, we examined the effect of estradiol (E2) on Tac2 and Kiss1 mRNA and found that Kiss1 gene expression was more sensitive than Tac2 to E2-induced inhibition at both juvenile and adult ages. This differential estrogen sensitivity was tested in vivo by the administration of E2. Low levels of E2 significantly suppressed Kiss1 expression in the ARC, whereas Tac2 suppression required higher E2 levels, supporting differential sensitivity to E2. Finally, to determine whether inhibition of NKB/NK3R signaling would block the onset of puberty, we administered an NK3R antagonist to prepubertal (before postnatal d 30) females and found no effect on markers of pubertal onset in either WT or hpg mice. These results indicate that the expression of Tac2 and Tacr3 in the ARC are markers of pubertal activation but that increased NKB/NK3R signaling alone is insufficient to trigger the onset of puberty in the mouse.
Emerging evidence suggests that neurokinin B (NKB) and kisspeptin (Kiss1) play important roles in the regulation of gonadotropin secretion and reproductive function, although the cellular and molecular mechanisms of action remain largely unresolved. NKB has been implicated in the regulation of gonadotropin secretion by virtue of its elevated expression in a discrete population of neurons in the infundibulum/arcuate nucleus (ARC) of postmenopausal women, suggesting a link between gonadal sex steroid-negative feedback and NKB in the regulation of GnRH secretion (1, 2). Kisspeptin, a potent GnRH secretagogue (3), was subsequently colocalized with NKB in certain cells within the ARC, in which expression of Kiss1 mRNA was similarly elevated under conditions of low sex steroids (4, 5). The coexpression of Tac2, encoding NKB, and Kiss1, encoding kisspeptin, within the neurons of the ARC is conserved across species, having been confirmed in the human, monkey, rat, mouse, sheep, and goat (4, 6–10). Additionally, these neurons coexpress the NKB receptor (NK3R/Tacr3), suggesting that autosynaptic modulation of Kiss1 neurons by NKB may play a role in the regulation of kisspeptin and subsequently GnRH secretion (9, 11). Notably, the Kiss1/NKB-expressing cells in the ARC of the rodent also express estrogen receptor-α and androgen receptor (12). Because Kiss1 and NKB are both negatively regulated by gonadal steroids in the ARC, this could plausibly constitute a major regulatory circuit in the feedback control of the hypothalamic-pituitary-gonadal axis (13).
In addition to Kiss1/NKB neurons in the ARC, a separate Kiss1 neuronal population found in the anteroventral periventricular nucleus (AVPV)/periventricular nucleus of mice does not coexpress NKB (9). This Kiss1 population in the AVPV is sexually dimorphic, with far greater numbers of these cells evident in females, and exhibits positive regulation by estradiol (E2) (12, 14, 15). The AVPV Kiss1 neurons are thought to contribute to the E2-induced GnRH/LH surge mechanism for ovulation after becoming identifiable in females with the onset of puberty (16, 17). By contrast, the developing ARC populations of both males and females have similar increases in Kiss1 expression patterns. The increase is independent of sex steroids and advocates that the onset of puberty is primarily initiated through activation of the Kiss1/NKB neurons in the ARC, perhaps with ancillary contributions of AVPV Kiss1, postulated to accelerate puberty in females (18).
The identification of patients with GnRH deficiency (idiopathic hypogonadotropic hypogonadism) who harbor mutations in TAC3 or TACR3 (the human genes encoding NKB and its cognate receptor) highlighted an apparent role for NKB in the regulation of GnRH during the pubertal transition in primate species (19–21). Studies in rodents, sheep, and monkeys have demonstrated stimulatory effects of NK3R agonists on LH secretion, perhaps acting through a Kiss1- and GnRH-dependent pathway (7, 22–24). However, these effects are complex and can sometimes be reversed by the action of sex steroids (9, 10, 22–26). We argued that if NKB in the ARC were important for pubertal maturation and if its contributions to GnRH/LH secretion were linked to sex steroid status, then to tease this apart, we must develop a strategy to isolate the effects of sex steroids from the sex steroid-independent events involved in the development of NKB/NK3R signaling in the brain.
The primary purpose of this study was to test the hypothesis that NKB in the ARC plays a role in initiating the onset of puberty in the mouse. To this end, we first investigated whether levels of Tac2 and Tacr3 mRNA in the ARC increase in association with pubertal development. To isolate the spontaneous developmental events that happen in the brain from actions influenced by sex steroids during puberty, developmental Tac2, Tacr3, Kiss1, and Kiss1r expression was compared across pubertal development [postnatal day (P) 10 to P60] in intact wild-type (WT) mice and in a GnRH/sex steroid-deficient model, the hpg mouse (27). The resulting failure of the hpg mouse to undergo puberty provides an excellent model to study the onset and maturation of Tac2 and Kiss1 expression without confounding actions of pubertal changes in gonadal sex steroids and other reproductive hormones. This eliminated the need for early surgical gonadectomy, associated recovery time, and clearance of endogenous hormones before the earliest age tested (P10). Second, to investigate whether the steroid milieu distinctly influences the expression of Tac2 and Kiss1 in the ARC, we examined the sensitivity of each to E2-negative feedback in vivo. Finally, to test the hypothesis that amplification of NKB/NK3R signaling triggers the onset of puberty, we examined the effects of an NK3R antagonist on pubertal maturation.
Materials and Methods
Animals
All experiments were approved by the Harvard Medical Area Standing Committee on Animals in the Harvard Medical School Center for Animal Resources and Comparative Medicine. Mice were maintained in a 12-h light, 12-h dark cycle and were fed a standard rodent diet. Female hypogonadal (hpg) mice possessing the Gnrh1 gene deletion (HPG-Gnrh1hpg) and WT littermates were bred and genotyped as previously described (18), with details in Supplemental Materials, published on The Endocrine Society's Journals Online web site at http://endo.endojournals.org. NK3R antagonist studies were also conducted in WT CD-1 females (juveniles starting at P10) obtained from Charles River Labs (Wilmington, MA) and maintained with the dam until weaning (P21).
Hormone assays
Circulating gonadotropins and E2 were measured in sera separated from whole blood collected from the submandibular cheek pouch. Sera were stored at −80 C until assayed. LH and FSH were measured with Milliplex MAP, rat pituitary panel assay (catalog no. RPT86K; EMD Chemicals, Inc., Billerica, MA), using kit standards and measured on a Luminex 100/200 instrument (Luminex Corp., Austin, TX). The intra- and interassay variance (%CV) for LH and FSH were less than 9 and 15%, respectively, and the average minimum detectable concentrations were 3.28 and 7.62 pg/ml, respectively. Serum E2 levels were assayed by the ImmunChem double-antibody 17β-estradiol RIA kit (MP Biomedicals, Inc., Solon, OH), and the minimum detectable concentration was 7.2 pg/ml.
Real-time quantitative RT-PCR (qRT-PCR)
For real-time qRT-PCR, ARC tissues from intact female hpg mice and WT littermates aged P10, P12, P15, P18, P22, P27, P30, P45, and P60 (n = 5 per group) were collected and prepared as described previously (18). Details of tissue dissection, sample preparation and analysis are provided in the Supplemental Materials. Briefly, total RNA was reverse transcribed, and real-time PCR was performed with an ABI 7300 real-time PCR system using the Taqman detection system and gene expression assays with primer sequences specific for Kiss1 (identification no. MM00617576_M1), Tac2 (tachykinin2) (identification no. MM01160363_M1), Kiss1r (identification no. MM00475045_M1), and Tacr3 (identification no. Mm00445346_M1) (Applied Biosystems, Inc., Foster City, CA).
In situ hybridization (ISH)
Coronal cryosections (20 μm) through the ARC of WT and hpg female (P12, P30, and P60; n = 5) were thaw mounted onto SuperFrost Plus slides (VWR Scientific, West Chester, PA) and analyzed by radioactive ISH with a protocol previously described (28). The detection of Tac2 mRNA in the ARC of these mice was performed with a validated riboprobe sequence (29). Details of probe generation, ISH procedure and quantification are described in Supplemental Materials. The number of cells reported represents the cells within sections containing the ARC nucleus for a single set (one of five), not the total number of cells in the ARC.
Adult steroid hormone replacement
Neuroendocrine responses to E2-negative feedback were evaluated in P60 female hpg, juvenile (P13) female hpg, and ovariectomized (OVX) adult (P60) WT mice (n = 10–13 per group). Bilateral OVX was performed under pentobarbital anesthesia. Hormone treatments in adult WT were administered at time of OVX and to intact hpg adult and hpg juvenile mice. Details of E2 capsule preparation and treatments are described in Supplemental Materials. All mice were killed 7 d after treatment and the brains were prepared for qRT-PCR of ARC neuropeptides. Results were analyzed as above and normalized to vehicle (VEH)-treated, WT adult levels. At harvest, uterine weights were recorded and sera were processed for circulating E2 measurements. This experiment was repeated in OVX pubertal (P30) WT mice (n = 4–6 per group) following the identical protocol above.
Inhibition of NKB signaling during puberty
Blockade of NKB signaling during pubertal development (P10 to P30) was tested with SB222200 [(S)-(2)-N-(a-ethylbenzyl)-3-methyl-2-phenylquinoline-4-carboxamide; Sigma, St. Louis, MO], a selective NK3R antagonist with an approximately 60-fold selectivity vs. NK2R and greater than 100,000-fold selectivity vs. NK1R (30). WT CD-1 mouse pups, standardized to eight pups per nursing female, were administered daily 30 μl sc injections of SB222200 (5 mg/kg; solubilized in ethanol and diluted in 60% polyethylene glycol), or VEH from ages P10 to P30 (n = 25 per group) (30, 31). At P21, females were weaned and monitored daily for vaginal opening (VO). After VO, the age of first estrus (between P27 and P37) was monitored by daily vaginal lavage and cytology, which continued after the treatment.
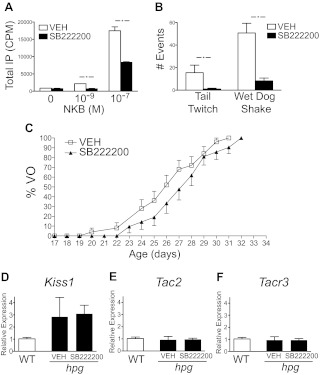
Separately, two additional tests were performed to examine whether NK3R blockade alters Kiss1 expression identified in hpg females. The first experiment was daily treatment with SB222200 (5 mg/kg) or VEH from P12 to P24 in female hpg mice, and the second was conducted with higher daily doses of SB222200 (10 mg/kg) in older hpg females from P27 to P30. Two hours after the final SB222200 injection, the hpg females were killed, and the ARC mRNA was processed for qRT-PCR as above. To ensure efficacy of SB222200, the compound was validated by the following two assays. In COS-7 cells transfected with an expression vector encoding human NK3R, pretreatment with SB222200 (1.4E-7 m), a concentration calculated from surface area equivalent doses used for 15-g mice, was tested for the ability to block NKB-generated total inositol phosphate (IP) accumulation using an IP assay previously described (21). Next, in adult WT mice, the central pharmacological blockade of NK3R-evoked characteristic behaviors (tail whips and wet dog shakes) was tested to ensure that the in vivo protocol inhibited NK3R signaling in the brain (32). The inhibitor SB222200 (5 mg/kg, sc), or VEH, was administered 30 min before injection of an NK3R agonist, senktide (1 mg/kg, in 0.1 ml PBS, sc; Tocris, Ellisville, MO). Quantification of tail whip and wet dog shake behaviors were conducted over 20 min after senktide by continuously observing treated mice in a perspex box (13 × 23.5 × 14.5 cm) and manually counting the number of specific behaviors exhibited (30).
Data analysis
Multiple comparisons were analyzed by ANOVA or two-way ANOVA followed by appropriate post hoc analyses. Parametric statistics were used with data that satisfied Bartlett tests for equal variances. Otherwise, transformed or nonparametric statistics were used. Suitable results were analyzed by unpaired t test for the comparison of mean differences between two different genotypes of similar age. Survival analysis (log rank, Mantel-Cox test) was used to determine effects of the SB222200 treatment on age of VO. Data were analyzed using Prism statistics software (GraphPad, Inc., San Diego, CA) when possible. All data are presented as the mean ± sem. Differences were considered significant when P < 0.05.
Results
Changes in serum gonadotropin hormones during postnatal development
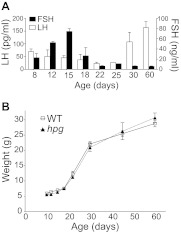
Serum LH and FSH levels in WT female mice decreased to a nadir between P18 and P25 followed by increases in LH (Fig. 1A), consistent with previous reports (33). LH and FSH were undetectable in female hpg mice at corresponding ages (data not shown). Weights of WT and hpg females were recorded across development as an index of pubertal maturation (Fig. 1B). Between P10 and P60, no differences in weights between genotypes were detected (despite the absence of gonadal maturation in hpg mice). Growth rate escalated between P18 and P30 in both WT and hpg females, corresponding to increases in LH in WT mice.
Fig. 1.
Developmental changes in body weight and serum gonadotropin profiles in female mice. A, Serum LH (white bars) and FSH (black bars) levels in WT female mice across postnatal development, with a significant variation across age for both LH (P < 0.0029) and FSH (P < 0.0001; ANOVA). B, Growth rate as reflected by body mass across postnatal development from P10 to P60 in WT and hpg female mice, with significant variance across age (P < 0.0001), without effect of genotype (P > 0.05) or interaction between age and genotype (P > 0.05; two way ANOVA).
Expression of Kiss1, Tac2, and their receptors in the ARC of WT and hpg females as markers of pubertal activation
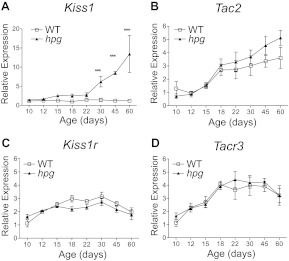
Developmental profiling of Kiss1 and Tac2 mRNA in the ARC was examined by qRT-PCR in intact WT and hpg mice at ages P10, P12, P15, P18, P22, P30, P45, and P60 focusing on the mouse ages considered juvenile to prepubertal (P10, P12, P15, P18, and P22), peripubertal (P30), and postpubertal or adult (P45-P60) (16). In agreement with our previous results, no significant changes in ARC Kiss1 mRNA were found in intact WT mice during maturation, as predicted due to the influence of gonadal sex steroid negative feedback (P > 0.05; ANOVA, Neuman Keuls post hoc) (18). In contrast, ARC Kiss1 mRNA in hpg mice increased across maturation, with Kiss1 mRNA becoming elevated by the age of P30 compared with P10 (P < 0.05; ANOVA, Dunnett's post hoc; Fig. 2A). ARC Kiss1 mRNA levels in hpg mice continued to increase and remained significantly higher than in WT from P30 onward (P < 0.05; two way ANOVA, Bonferroni post hoc; Fig. 2A). There was an early trend toward a developmental increase in Kiss1 expression in hpg mice over that in WT by P15, which did not reach statistical significance but occurred before the WT pubertal LH increase at P30. Additionally, Kiss1 mRNA levels between the genotypes (hpg and age matched WT females), age, and the interaction between age and genotype were all found to be significantly different by two-way ANOVA (P < 0.001; Table 1).
Fig. 2.
Expression of Kiss1, Tac2, and their receptors in the ARC of intact WT and hpg females across postnatal development. Developmental profile analysis of mRNA levels in the ARC as measured by qRT-PCR of WT and hpg females for Kiss1 (A), Tac2 (B), Kiss1r (C) and Tacr3 (D). Values are mean ± sem (n = 4–6). WT, white squares; hpg, black triangles. ***, Significant differences between genotypes (P < 0.001, ANOVA, Neuman-Keuls post hoc). Statistical analysis of effects of age and genotype were compared by two-way ANOVA and are summarized in Table 1.
Table 1.
Significant sources of variation in expression profiles
| Kiss1 | Tac2 | Kiss1r | Tacr3 | |
|---|---|---|---|---|
| Age | Yes, P < 0.0001 | Yes, P < 0.0001 | Yes, P < 0.0001 | Yes, P < 0.0001 |
| Genotype | Yes, P < 0.0001 | No | No | No |
| Interaction | Yes, P < 0.0001 | No | No | No |
Two-way ANOVA results from qRT-PCR gene expression analysis in the ARC of female mice at P10 to P60. The source of variation was tested for age, genotype, and interaction between both. Significance is P < 0.05.
In contrast to the distinct Kiss1 expression patterns between the gonad-intact WT mice and the hypogonadal hpg mice, Tac2 expression in the ARC increased similarly in both WT and hpg mice across early development (Fig. 2B). The age-dependent increases in Tac2 mRNA levels were highly significant in both WT and hpg mice, without influence from genotype (two way ANOVA, P < 0.001; Table 1). This unexpected increase in ARC Tac2 expression in intact WT mice suggested the absence of suppression by gonadal sex steroids. Notably, the rate of significant increase in Tac2 expression in the ARC that was evident in both WT and hpg mice was diminished after P30 but only in WT mice, suggesting that Tac2 expression might become negatively influenced with age in intact WT mice compared with the sex steroid-deficient hpg mice (Fig. 2B; P < 0.05, regression analysis).
Expression patterns of Kiss1r and Tacr3 mRNA were similar in the ARC for WT and hpg mice, suggesting minimal influence of gonadal sex steroids on the expression of either Kiss1r or Tacr3 (Fig. 2, C and D). An age-dependent increase in Kiss1r mRNA was evident in both WT and hpg mice (Table 1), which peaked at P30 and then declined at P45 and P60 (Fig. 2C). Tacr3 mRNA levels also changed with age (Table 1), with significant increases beginning at P15 for both genotypes (Fig. 2D; P < 0.05, ANOVA, post hoc tests). These data reveal a significant increase in expression of both Tac2 and Tacr3 in the ARC before puberty in the intact WT animal, in contrast to the expression pattern of Kiss1 in the ARC.
Tac2 Expression Patterns Suggest that Negative Feedback by Sex Steroids Occurs Only in Adult Mice
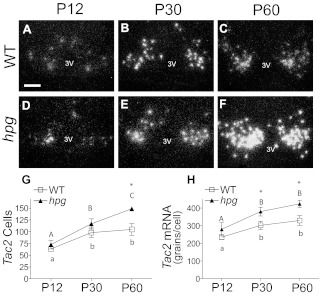
To more specifically characterize the developmental increases in Tac2 expression that suggested differences from Kiss1 in negative feedback sensitivity to gonadal sex steroids, Tac2 mRNA was measured at the cellular level by in situ hybridization (ISH) in the WT and hpg female ARC at specific ages during development: P12 (juvenile), P30 (peripubertal), and P60 (adult) (Fig. 3, A–F). In this region, approximately 50% of Tac2 mRNA-positive neurons have been previously identified to coexpress Kiss1 [and prodynorphin (pdyn)] and are referred to as kisspeptin, NKB, dynorphin A (KNDy) neurons (9). Tac2 mRNA increased from P12 to P30 in the ARC of both WT and hpg mice (Fig. 3, A, B, D, and E). There was little change in the Tac2 signal in WT mice between P30 and P60, whereas the Tac2 mRNA signal continued to increase beyond P30 in hpg mice and appeared more intense than in the WT at P60 (Fig. 3, C and F). Quantification confirmed that significant increases in Tac2 mRNA-positive cells continued between P30 and P60 in hpg mice, with no significant increase in Tac2-positive cells beyond P30 in WT mice (Fig. 3G; P < 0.05, ANOVA, Neumann-Keuls). hpg Tac2 mRNA silver grains within individual cells did not increase significantly beyond P30, indicative of maximum signal saturation in the P60 hpg ARC (Fig. 3H). Together these data establish a pattern of Tac2 expression in intact WT mice that stabilized between P30 and P60 but continued to increase in hpg mice, suggesting either the developmental attainment of responsiveness to negative feedback actions of sex steroids or the requirement of higher levels of estrogens found in adults to suppress Tac2 gene expression.
Fig. 3.
Postpubertal increases in ARC Tac2 expression by in situ hybridization in hpg mice but not in intact WT mice. Representative dark-field images of silver grain Tac2 mRNA signal as measured by ISH in coronal sections of the ARC of WT and hpg females at juvenile age (P12; A and D), pubertal age (P30; B and E), and adult age (P60; C and F). 3V, Third ventricle. Scale bar, 100 μm. G, Quantification of the number of neurons in the ARC positive for Tac2 mRNA by ISH in hpg mice compared with WT mice. H, Mean of representative Tac2 mRNA silver grain signals per neuron in the ARC of WT and hpg mice (mean signal intensity). Values are mean ± sem (n = 5). WT, white squares; hpg, black triangles. Different letters denote significant differences (P < 0.05). *, Significant differences between genotypes.
Differential sensitivity of Tac2 and Kiss1 expression to estrogen negative feedback
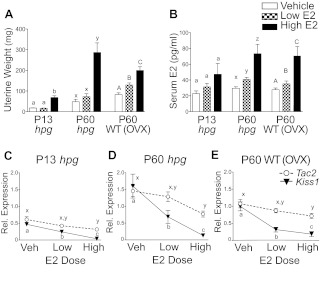
Juvenile hpg mice (P13), adult hpg mice (P60), and adult OVX WT mice (P60) were treated with varying levels of E2 via capsule implants to determine whether the differences in Tac2 and Kiss1 expression in the ARC were due to differential sensitivity to negative feedback regulation by gonadal sex steroids or whether activation of estrogen negative feedback for Tac2 is acquired later in development. As a bioassay to confirm physiological effects of the E2 treatments (vehicle, low E2, and high E2), responses of reproductive tissues (uteri, and, if intact, with ovaries) were evaluated by differences in weight (Fig. 4A). In OVX WT mice, significant increases in uterine weights occurred between vehicle (83 ± 8 mg) and low-dose E2 (129 ± 10 mg; P < 0.05) and between low and high doses of E2 (200 ± 18 mg; P < 0.05). Only high-dose E2 significantly increased uterine weight in juvenile hpg (vehicle, 18 ± 1 mg; 1ow E2, 17 ± 1 mg; high E2, 69 ± 8 mg) and adult hpg (vehicle, 48 ± 12 mg; low E2, 73 ± 14 mg; high E2, 287 ± 46 mg; P < 0.05). Serum E2 levels were also measured to confirm that capsule treatments of low and high doses of E2 approximated ranges found across diestrus to proestrus in mice (30–70 pg/ml) (34) (Fig. 4B).
Fig. 4.
Differential sensitivity of Tac2 and Kiss1 to negative feedback inhibition by physiological levels of E2. A, Uterine weights in response to increasing levels of E2 in juvenile (P13) and adult (P60) hpg mice and in OVX adult (P60) WT mice. Sesame oil (VEH), low-dose E2 (LOW; 8.3 μg/kg) or high-dose E2 (HIGH; 167 μg/kg) were administered in SILASTIC brand capsules (Dow Corning Corp., Midland, MI) at the ages indicated. B, Serum E2 levels achieved with the varying E2 dosages. C–E, Differences in Tac2 (open circles) and Kiss1 (black triangles) mRNA levels in the ARC in response to vehicle (VEH), low-dose E2 (LOW), and high-dose E2 (HIGH) in juvenile (P13) hpg (C), adult (P60) hpg(D), and adult (P60) WT females (E). Values are normalized to adult WT VEH levels (n = 10–13). Among dose responses to E2 for Kiss1 or Tac2, different letters denote significant differences (P < 0.05).
Kiss1 and Tac2 expression in the ARC was measured from vehicle- and E2-treated mice to study their negative feedback responsiveness. At all ages, Kiss1 mRNA was significantly reduced with low-dose E2 treatment, indicating a robust repressive action of E2 on ARC Kiss1 expression. In contrast, low-dose E2 treatment did not significantly reduce Tac2 mRNA levels; only high-dose E2 significantly reduced Tac2 expression compared with vehicle treatment, reflecting a lower responsiveness of Tac2 expression to negative feedback effects of E2 than Kiss1. These findings provide a possible explanation for the increase in Tac2 over Kiss1 expression at the levels of circulating E2 found during the pubertal transition (up to P30), with Tac2 expression continuing to increase until the higher circulating E2 levels of the cycling adult female are achieved. A subsequent experiment was performed in OVX pubertal (P30) WT mice (n = 4–6 per group) using the same protocol (Supplemental Fig. 1). The results at P30 again demonstrated a lack of Tac2 sensitivity to E2 negative regulation, whereas Kiss1 expression was significantly repressed at low-dose E2 treatment.
Effects of blockade of NK3R signaling on markers of pubertal onset
Before P30, Tac2 expression increased in the ARC of both gonad-intact WT mice and hypogonadal hpg mice during postnatal development. To determine whether the NKB/NK3R system has a stimulatory role on pubertal maturation, the effects of pharmacologic blockade with an NK3R antagonist (SB222200) across pubertal onset (P10 to P30) were tested (7). Additional validation of SB222200 effectiveness in blocking NK3R-mediated signaling was conducted with the following: 1) an IP assay using COS-7 cells transfected with human NK3R and treated with NKB (Fig. 5A), and 2) assays in mice treated with senktide, an NK3R agonist that elicits specific characteristic behaviors mediated through the central nervous system (Fig. 5B). In both assays, SB222200 resulted in a significant inhibition of NK3R signaling effects. Repeated daily administration of SB222200 (5 mg/kg, sc) or vehicle to intact WT mice for 20 d (P10 to P30; n = 25) resulted in a modest delay in the mean age of VO in the NK3R antagonist-treated mice by 2 d compared with the vehicle-treated cohort, although the delay did not reach statistical significance (VEH, 26 d; SB222200, 28 d; survival analysis; P = 0.1604) (Fig. 5C). Also, the age of first vaginal estrus between vehicle- (30.8 ± 0.6 d) and SB222200-treated mice (31.5 ± 0.6 d) demonstrated a delay with the NK3R antagonist, which also did not reach statistical significance (data not shown).
Fig. 5.
Blockade of NK3R signaling during development. A, Effects of an NK3R antagonist, SB222200, on NKB-stimulated IP accumulation in COS-7 cells transiently transfected with an expression vector encoding the human NK3R. *, P < 0.05. B, Effects of sc injection of SB222200 (5 mg/kg) on Senktide-induced tail whips and wet dog shakes (n = 4). *, P < 0.05. C, Comparison of timing of VO in mice treated with daily sc injections of SB222200 (5 mg/kg) or VEH. The cumulative percentage of mice with VO as a function of age is shown (n = 25; VEH; open squares; and n = 21; SB222200; black triangles). D--F, qRT-PCR analysis of Kiss1, Tac2, and Tacr3 expression in the ARC of hpg females at age P24 (black bars) treated daily with SB222200 (5 mg/kg) or VEH beginning at age P12, compared with vehicle-treated WT littermates (white bars).
The effects of NK3R signaling blockade with SB222200 on the robust increases of Kiss1 and Tac2 mRNA in the ARC measured in hpg females (see Fig. 2) were performed as an additional test to examine actions of NKB signaling upstream of GnRH. SB222200 (5 mg/kg, sc) or vehicle was injected daily in hpg female mice from P12 to P24. SB222200 treatment had no detectable effect on ARC Kiss1, Tac2, or Tacr3 expression (Fig. 5, D–F). This experiment was repeated with older hpg female mice, at higher daily doses of SB222200 (10 mg/kg) for shorter intervals (P27 to P30) but with similar results, showing no significant differences in ARC Kiss1 expression (data not shown). Together these results showed that SB222200 can block central NK3R signaling but with little effect on age of vaginal opening or first estrus in WT mice and without detectable changes in Kiss1 expression in the ARC of hpg mice.
Discussion
Although many lines of evidence support a key role for kisspeptin signaling in pubertal development (35–37), the possible role of NKB, coexpressed with kisspeptin in the ARC, is less certain. In humans, NKB and its receptor NK3R seem to be essential, given recent reports of GnRH deficiency and hypogonadotropic hypogonadism in patients bearing loss-of-function mutations of either TAC3 or its receptor, TACR3 (19–21, 38, 39). Curiously, in a subset of these adult patients with either TAC3 or TACR3 mutations, the reversal of GnRH deficiency and restoration of reproductive function have occurred spontaneously, or more commonly after steroid treatment for infertility (21, 40). The existence of such a reversal in adulthood suggests potential redundancy of NKB/NK3R signaling or developmental actions no longer necessary after puberty (21). Nonetheless, if NKB acts as an essential upstream regulator that drives pubertal GnRH release, mediated perhaps by kisspeptin, then to understand this developmental relationship, differences among species, sex, and influence of gonadal status during pubertal onset need to be considered (11, 41). Genetic knockout mouse models of NKB signaling deficiency are fertile with only minor reproductive deficits, underscoring the uncertainty of NKB as a putative driver of central reproduction (42, 43). The goal of this work was to investigate the functional contribution and timing of NKB signaling in reproductive maturation and to gain a better understanding of the influence of sex steroids on Tac2 and Kiss1 expression during pubertal development.
To address these questions and to isolate the spontaneous developmental events in the brain from those that are triggered by the action of sex steroids at puberty, the expression of Tac2, Tacr3, Kiss1, and Kiss1r was evaluated across development in WT and hpg mice, an animal with a profound deficiency in GnRH (thus having low circulating levels of sex steroids) (27). Although caveats may exist in extending findings from GnRH-deficient mice to WT mice, we have previously shown in the hpg mouse model that the GnRH deficiency itself does not affect GnRH neuronal development or influence (directly or indirectly) GnRH neurophysiology (44). Here we found that the expression of Tac2 and Tacr3 in the ARC increases at normal pubertal ages in both WT and hpg mice (P12 to P30). These findings indicate that there is little influence of gonadal status on Tac2/Tacr3 expression at the time of puberty and identify Tac2 and Tacr3 as key central markers corresponding to pubertal onset.
The escalating expression of Tac2 and Tacr3 across development was distinct from that of Kiss1 in the ARC, which remained nearly constant in intact WT mice across puberty (in the face of increasing sex steroids). In other words, during development in WT mice, increasing gonadal sex steroids levels do not appear to substantially inhibit (or restrain) the expression of Tac2 and Tacr2, thus challenging the notion that NKB and NK3R are always negatively regulated by E2 (9, 45, 46). Here Tac2 expression was shown to be less sensitive to E2 than Kiss1, as reflected by the unique expression patterns of Kiss1 and Tac2 in WT vs. hpg mice during development. This was shown by qRT-PCR and corroborated by in situ hybridization, ensuring the Tac2 expression changes were only measured in the area of the KNDy neuron population. These findings were validated in vivo by the measured repression of Tac2 expression by high (adult) levels of E2, whereas suppression of Kiss1 expression in the ARC required only low doses of E2. From these findings, the discrete expression patterns and responses to E2 suggested the independent regulation of Kiss1 and Tac2, at least at the mRNA level, potentially implicating separate functions for kisspeptin and NKB.
The temporal increases of Kiss1 and Tac2 mRNA in the ARC of hpg mice found at pubertal ages (P12 to P30) were associated with age-dependent changes in circulating concentrations of gonadotropins found in intact WT mice. This finding supports the model that the expression of Tac2 and Kiss1 is physiologically initiated early in development (∼P15), with Kiss1 expression being maintained thereafter at a steady state by progressively increasing concentration of E2 during sexual maturation. In contrast, the expression of Tac2 continues to increase throughout the pubertal transition (P12 to P30), until the negative feedback effects of E2 become manifest in adulthood. Thus, the preponderance of physiological changes associated with the pubertal transition, including rapid growth and increased serum LH levels, are subsequent to the measurable increases in Tac2 (and Tacr3) expression in the ARC, and occur in the setting of unchanging expression of Kiss1.
The next experiments determined whether the developmental changes and distinct expression patterns of Tac2 and Kiss1 in the ARC resulted from differential sensitivity to sex steroid regulation. Possible differences between E2 action on Kiss1 and Tac2 were postulated to be one, or a combination, of the following. Higher concentrations of E2 may be required to suppress Tac2 expression, whereas lower E2 concentrations are sufficient to suppress Kiss1 expression. Naturally, E2 concentrations become higher during pubertal development, and this increase may subsequently suppress Tac2 expression at ages already under negative Kiss1 gene regulation by E2. Another possibility is that components of the transcriptional mechanism regulating Tac2 may develop at later ages than the components required for Kiss1 to be repressed by E2. These possibilities were tested by using an E2 treatment regimen designed to achieve low and high concentrations of circulating E2 in OVX WT adult female mice alongside intact juvenile hpg and adult hpg females. Results showed that the sensitivity to E2 necessary to trigger suppression of Kiss1 and Tac2 expression did not develop with age because the juvenile (P13) hpg mice had Kiss1 and Tac2 responses to E2 that were similar to those of both adult (P60) hpg mice and WT mice. Rather, at both juvenile (P13) and adult (P60) ages, high doses of E2 were required to suppress Tac2 expression, whereas low doses were sufficient to inhibit Kiss1 expression. As such, this inquiry into the pubertal role of NKB indicated that as ovarian E2 production matures, Tac2 expression increases through the juvenile to adult transition, whereas Kiss1 expression in the ARC is already under E2-negative regulation. Confirmation that the differences in Kiss1 and Tac2-negative feedback responsiveness were consistent at P30 in OVX WT females provided further support that a relative lack of steroid sensitivity contributes to increased Tac2 expression during puberty.
The early pubertal (P12 to P16) rise in Tac2 expression that increases through puberty (P30) lends support to the concept that NKB itself is an essential early regulator of pubertal onset (21). To test the hypothesis that NKB signaling is required for the entry into puberty, the NK3R antagonist, SB222200, was used to specifically inhibit NK3R signaling before puberty. The daily injections of SB222200 in juvenile WT mice resulted only in a modest trend of delayed VO that was not statistically significant and argues against a straightforward role of NKB in pubertal development. Although this antagonist efficiently crosses the blood-brain barrier, it is possible that the daily mode of administration may have produced only a limited duration of blockade (13, 30, 31). In rats, SB222200 administered by repeated chronic intracerebroventricular infusion also produced a modest delay in VO as we observed; however, the SB222200 treatment did not significantly alter serum LH (26, 47). Here the effectiveness of the antagonist was further validated in mice to demonstrate the efficient blockade of stereotypical behaviors mediated by central NK3R signaling in mice (32). However, the lack of significant effect on VO by blocking NK3R would be considered consistent with a report of knockout mice lacking the Tac2 and Tacr3 genes that displayed normal timing of VO (although disrupted estrous cycles and other reproductive phenotypes were found in the Tac2−/− and Tac3r−/− mice) (43).
The administration of the NK3R antagonist to hpg mice across early pubertal ages (P12 to P24) was also ineffective in altering the developmental increases of both Kiss1 and Tac2 expression that normally occur in the ARC of hpg mice at these ages. Therefore, if the inhibitor did disrupt NK3R signaling in the ARC, then at least in the hpg background, these results do not directly support NKB/NK3R in stimulating prepubertal Kiss1 expression. Together the pharmacological experiments with SB222200 aimed to address the causal role of NK3R signaling in the onset of puberty yielded essentially negative results and are therefore consistent with the view derived from knockout studies that in mice NKB signaling is not obligatory for normal timing of puberty (43). Alternatively, the antagonism of NK3R signaling could be compensated by activation of other neuropeptide systems (40, 48, 49). Recently in rats, individual subtype-specific neurokinin receptor antagonists failed to alter circulating LH, whereas an antagonist for all three receptors (NK1R, NK2R, and NK3R) suppressed circulating LH levels and pulse frequency, suggesting multiple neurokinin receptor pathways may redundantly contribute to GnRH/LH secretion (50).
The finding that the NK3R antagonist resulted in minimal change in the age of VO or Kiss1 expression in the ARC needs to be considered in light of the emerging model that links autocrine NKB signaling in KNDy neurons to GnRH secretion. The proposed circuit consists of NKB acting autosynaptically to synchronize the pulsatile secretion of kisspeptin, which potently elicits GnRH release (9, 24). NKB/NK3R signaling is not yet known to regulate Kiss1 expression, which appears to be activated independently of NKB. However, evidence continues to support the opposing actions of NKB on GnRH/LH secretion, a stimulatory role via kisspeptin (11, 51) and a possible inhibitory role via dynorphin A (26). Without sufficient evidence otherwise, we surmise that the actions of NKB possibly describe an intrinsic neuromodulator, its cotransmission with kisspeptin and dynorphin A essential to stabilize the KNDy network and prevent it from becoming overmodulated and nonfunctional, with few effects of NKB signaling itself found under basal conditions (52).
In summary, we have shown that the developmental pattern of Tac2 expression and its regulation by gonadal steroids are distinct from those of Kiss1 and mark Tac2 and Tacr3 as indices of pubertal maturation. Although our understanding of the functional contributions of NKB/NK3R to reproduction is still emerging, the prospects of a central role of NKB in puberty are bolstered by its early developmental expression. The distinct thresholds to E2-negative feedback between Tac2 and Kiss1 expression in the ARC provide a mechanism that further discriminates the regulation of these neuropeptides, with repression of Kiss1 in the ARC being more sensitive to E2 than that of Tac2. These differences provide greater insight into the independent and cooperative actions of kisspeptin and NKB during pubertal onset in the context of a changing E2 milieu yet underscore the complexity of the multiple neuroendocrine parameters regulating the central initiation of reproductive function.
Supplementary Material
Acknowledgments
We thank Darlene Vaughn (Harvard Center for Comparative Medicine) for her dedicated care of the mice, and Dr. Amy Oakley and Simina Popa (University of Washington) for their assistance in preparing this work.
This work was supported by the National Institutes of Health/Office of Research on Women's Health/Eunice Kennedy Shriver National Institute of Child Health and Human Development (Grant K12 HD051959), National Institutes of Health/Eunice Kennedy Shriver National Institute of Child Health and Human Development (Grant HD061577), National Institutes of Health/Eunice Kennedy Shriver National Institute of Child Health and Human Development (Grant HD049651) and the Eunice Kennedy Shriver National Institute of Child Health and Human Development/National Institutes of Health through Cooperative Agreements U54 HD28138 and U54 HD12629.
Disclosure Summary: R.A.S. and U.B.K. received research grants totaling more than $10,000. The other authors have nothing to declare.
Footnotes
- ARC
- Arcuate nucleus
- AVPV
- anteroventral periventricular nucleus
- E2
- estradiol
- IP
- inositol phosphate
- ISH
- in situ hybridization
- Kiss1
- kisspeptin
- NKB
- neurokinin B
- NK3R
- NKB receptor
- OVX
- ovariectomized
- P
- postnatal day
- qRT-PCR
- quantitative RT-PCR
- VEH
- vehicle
- VO
- vaginal opening
- WT
- wild type.
References
- 1. Rance NE, Young WS., 3rd 1991. Hypertrophy and increased gene expression of neurons containing neurokinin-B and substance-P messenger ribonucleic acids in the hypothalami of postmenopausal women. Endocrinology 128:2239–2247 [DOI] [PubMed] [Google Scholar]
- 2. Akesson TR, Sternini C, Micevych PE. 1991. Continuous estrogen decreases neurokinin B expression in the rat arcuate nucleus. Mol Cell Neurosci 2:299–304 [DOI] [PubMed] [Google Scholar]
- 3. Seminara SB, Kaiser UB. 2005. New gatekeepers of reproduction: GPR54 and its cognate ligand, KiSS-1. Endocrinology 146:1686–1688 [DOI] [PubMed] [Google Scholar]
- 4. Goodman RL, Lehman MN, Smith JT, Coolen LM, de Oliveira CV, Jafarzadehshirazi MR, Pereira A, Iqbal J, Caraty A, Ciofi P, Clarke IJ. 2007. Kisspeptin neurons in the arcuate nucleus of the ewe express both dynorphin A and neurokinin B. Endocrinology 148:5752–5760 [DOI] [PubMed] [Google Scholar]
- 5. Rometo AM, Krajewski SJ, Voytko ML, Rance NE. 2007. Hypertrophy and increased kisspeptin gene expression in the hypothalamic infundibular nucleus of postmenopausal women and ovariectomized monkeys. J Clin Endocrinol Metab 92:2744–2750 [DOI] [PubMed] [Google Scholar]
- 6. Hrabovszky E, Ciofi P, Vida B, Horvath MC, Keller E, Caraty A, Bloom SR, Ghatei MA, Dhillo WS, Liposits Z, Kallo I. 2010. The kisspeptin system of the human hypothalamus: sexual dimorphism and relationship with gonadotropin-releasing hormone and neurokinin B neurons. Eur J Neurosci 31:1984–1998 [DOI] [PubMed] [Google Scholar]
- 7. Ramaswamy S, Seminara SB, Ali B, Ciofi P, Amin NA, Plant TM. 2010. Neurokinin B stimulates GnRH release in the male monkey (Macaca mulatta) and is colocalized with kisspeptin in the arcuate nucleus. Endocrinology 151:4494–4503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Xu J, Kirigiti MA, Grove KL, Smith MS. 2009. Regulation of food intake and gonadotropin-releasing hormone/luteinizing hormone during lactation: role of insulin and leptin. Endocrinology 150:4231–4240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Navarro VM, Gottsch ML, Chavkin C, Okamura H, Clifton DK, Steiner RA. 2009. Regulation of gonadotropin-releasing hormone secretion by kisspeptin/dynorphin/neurokinin B neurons in the arcuate nucleus of the mouse. J Neurosci 29:11859–11866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wakabayashi Y, Nakada T, Murata K, Ohkura S, Mogi K, Navarro VM, Clifton DK, Mori Y, Tsukamura H, Maeda K, Steiner RA, Okamura H. 2010. Neurokinin B and dynorphin A in kisspeptin neurons of the arcuate nucleus participate in generation of periodic oscillation of neural activity driving pulsatile gonadotropin-releasing hormone secretion in the goat. J Neurosci 30:3124–3132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ramaswamy S, Seminara SB, Plant TM. 2011. Evidence from the agonadal juvenile male rhesus monkey (Macaca mulatta) for the view that the action of neurokinin B to trigger gonadotropin-releasing hormone release is upstream from the kisspeptin receptor. Neuroendocrinology 94:237–245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Smith JT, Dungan HM, Stoll EA, Gottsch ML, Braun RE, Eacker SM, Clifton DK, Steiner RA. 2005. Differential regulation of KiSS-1 mRNA expression by sex steroids in the brain of the male mouse. Endocrinology 146:2976–2984 [DOI] [PubMed] [Google Scholar]
- 13. Rance NE, Krajewski SJ, Smith MA, Cholanian M, Dacks PA. 2010. Neurokinin B and the hypothalamic regulation of reproduction. Brain Res 1364:116–128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Smith JT, Cunningham MJ, Rissman EF, Clifton DK, Steiner RA. 2005. Regulation of Kiss1 gene expression in the brain of the female mouse. Endocrinology 146:3686–3692 [DOI] [PubMed] [Google Scholar]
- 15. Herbison AE. 2008. Estrogen positive feedback to gonadotropin-releasing hormone (GnRH) neurons in the rodent: the case for the rostral periventricular area of the third ventricle (RP3V). Brain Res Rev 57:277–287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Clarkson J, Herbison AE. 2006. Postnatal development of kisspeptin neurons in mouse hypothalamus; sexual dimorphism and projections to gonadotropin-releasing hormone neurons. Endocrinology 147:5817–5825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kauffman AS, Gottsch ML, Roa J, Byquist AC, Crown A, Clifton DK, Hoffman GE, Steiner RA, Tena-Sempere M. 2007. Sexual differentiation of Kiss1 gene expression in the brain of the rat. Endocrinology 148:1774–1783 [DOI] [PubMed] [Google Scholar]
- 18. Gill JC, Wang O, Kakar S, Martinelli E, Carroll RS, Kaiser UB. 2010. Reproductive hormone-dependent and -independent contributions to developmental changes in kisspeptin in GnRH-deficient hypogonadal mice. PLoS One 5:e11911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Topaloglu AK, Reimann F, Guclu M, Yalin AS, Kotan LD, Porter KM, Serin A, Mungan NO, Cook JR, Ozbek MN, Imamoglu S, Akalin NS, Yuksel B, O'Rahilly S, Semple RK. 2009. TAC3 and TACR3 mutations in familial hypogonadotropic hypogonadism reveal a key role for neurokinin B in the central control of reproduction. Nat Genet 41:354–358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Young J, Bouligand J, Francou B, Raffin-Sanson ML, Gaillez S, Jeanpierre M, Grynberg M, Kamenicky P, Chanson P, Brailly-Tabard S, Guiochon-Mantel A. 2010. TAC3 and TACR3 defects cause hypothalamic congenital hypogonadotropic hypogonadism in humans. J Clin Endocrinol Metab 95:2287–2295 [DOI] [PubMed] [Google Scholar]
- 21. Gianetti E, Tusset C, Noel SD, Au MG, Dwyer AA, Hughes VA, Abreu AP, Carroll J, Trarbach E, Silveira LF, Costa EM, de Mendonça BB, de Castro M, Lofrano A, Hall JE, Bolu E, Ozata M, Quinton R, Amory JK, Stewart SE, Arlt W, Cole TR, Crowley WF, Kaiser UB, Latronico AC, Seminara SB. 2010. TAC3/TACR3 mutations reveal preferential activation of gonadotropin-releasing hormone release by neurokinin B in neonatal life followed by reversal in adulthood. J Clin Endocrinol Metab 95:2857–2867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Billings HJ, Connors JM, Altman SN, Hileman SM, Holaskova I, Lehman MN, McManus CJ, Nestor CC, Jacobs BH, Goodman RL. 2010. Neurokinin B acts via the neurokinin-3 receptor in the retrochiasmatic area to stimulate luteinizing hormone secretion in sheep. Endocrinology 151:3836–3846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Corander MP, Challis BG, Thompson EL, Jovanovic Z, Loraine Tung YC, Rimmington D, Huhtaniemi IT, Murphy KG, Topaloglu AK, Yeo GS, O'Rahilly S, Dhillo WS, Semple RK, Coll AP. 2010. The effects of neurokinin B upon gonadotrophin release in male rodents. J Neuroendocrinol 22:181–187 [DOI] [PubMed] [Google Scholar]
- 24. Navarro VM, Castellano JM, McConkey SM, Pineda R, Ruiz-Pino F, Pinilla L, Clifton DK, Tena-Sempere M, Steiner RA. 2011. Interactions between kisspeptin and neurokinin B in the control of GnRH secretion in the female rat. Am J Physiol Endocrinol Metab 300:E202–E210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sandoval-Guzmán T, Rance NE. 2004. Central injection of senktide, an NK3 receptor agonist, or neuropeptide Y inhibits LH secretion and induces different patterns of Fos expression in the rat hypothalamus. Brain Res 1026:307–312 [DOI] [PubMed] [Google Scholar]
- 26. Kinsey-Jones JS, Grachev P, Li XF, Lin YS, Milligan SR, Lightman SL, O'Byrne KT. 2012. The inhibitory effects of neurokinin B on GnRH pulse generator frequency in the female rat. Endocrinology 153:307–315 [DOI] [PubMed] [Google Scholar]
- 27. Charlton H. 2004. Neural transplantation in hypogonadal (hpg) mice—physiology and neurobiology. Reproduction 127:3–12 [DOI] [PubMed] [Google Scholar]
- 28. Han SK, Gottsch ML, Lee KJ, Popa SM, Smith JT, Jakawich SK, Clifton DK, Steiner RA, Herbison AE. 2005. Activation of gonadotropin-releasing hormone neurons by kisspeptin as a neuroendocrine switch for the onset of puberty. J Neurosci 25:11349–11356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Gottsch ML, Cunningham MJ, Smith JT, Popa SM, Acohido BV, Crowley WF, Seminara S, Clifton DK, Steiner RA. 2004. A role for kisspeptins in the regulation of gonadotropin secretion in the mouse. Endocrinology 145:4073–4077 [DOI] [PubMed] [Google Scholar]
- 30. Sarau HM, Griswold DE, Bush B, Potts W, Sandhu P, Lundberg D, Foley JJ, Schmidt DB, Webb EF, Martin LD, Legos JJ, Whitmore RG, Barone FC, Medhurst AD, Luttmann MA, Giardina GA, Hay DW. 2000. Nonpeptide tachykinin receptor antagonists. II. Pharmacological and pharmacokinetic profile of SB-222200, a central nervous system penetrant, potent and selective NK-3 receptor antagonist. J Pharmacol Exp Ther 295:373–381 [PubMed] [Google Scholar]
- 31. Nwaneshiudu CA, Unterwald EM. 2009. Blockade of neurokinin-3 receptors modulates dopamine-mediated behavioral hyperactivity. Neuropharmacology 57:295–301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Nordquist RE, Ballard TM, Algeyer B, Pauly-Evers M, Ozmen L, Spooren W. 2010. Pharmacological characterization of senktide-induced tail whips. Neuropharmacology 58:259–267 [DOI] [PubMed] [Google Scholar]
- 33. Michael SD, Kaplan SB, Macmillan BT. 1980. Peripheral plasma concentrations of LH, FSH, prolactin and GH from birth to puberty in male and female mice. J Reprod Fertil 59:217–222 [DOI] [PubMed] [Google Scholar]
- 34. Porter KL, Chanda S, Wang HQ, Gaido KW, Smart RC, Robinette CL. 2002. 17β-estradiol is a hormonal regulator of mirex tumor promotion sensitivity in mice. Toxicol Sci 69:42–48 [DOI] [PubMed] [Google Scholar]
- 35. Seminara SB, Messager S, Chatzidaki EE, Thresher RR, Acierno JS, Jr, Shagoury JK, Bo-Abbas Y, Kuohung W, Schwinof KM, Hendrick AG, Zahn D, Dixon J, Kaiser UB, Slaugenhaupt SA, Gusella JF, O'Rahilly S, Carlton MB, Crowley WF, Jr, Aparicio SA, Colledge WH. 2003. The GPR54 gene as a regulator of puberty. N Engl J Med 349:1614–1627 [DOI] [PubMed] [Google Scholar]
- 36. de Roux N, Genin E, Carel JC, Matsuda F, Chaussain JL, Milgrom E. 2003. Hypogonadotropic hypogonadism due to loss of function of the KiSS1-derived peptide receptor GPR54. Proc Natl Acad Sci USA 100:10972–10976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Roa J, Navarro VM, Tena-Sempere M. 2011. Kisspeptins in reproductive biology: consensus knowledge and recent developments. Biol Reprod 85:650–660 [DOI] [PubMed] [Google Scholar]
- 38. Guran T, Tolhurst G, Bereket A, Rocha N, Porter K, Turan S, Gribble FM, Kotan LD, Akcay T, Atay Z, Canan H, Serin A, O'Rahilly S, Reimann F, Semple RK, Topaloglu AK. 2009. Hypogonadotropic hypogonadism due to a novel missense mutation in the first extracellular loop of the neurokinin B receptor. J Clin Endocrinol Metab 94:3633–3639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Francou B, Bouligand J, Voican A, Amazit L, Trabado S, Fagart J, Meduri G, Brailly-Tabard S, Chanson P, Lecomte P, Guiochon-Mantel A, Young J. 2011. Normosmic congenital hypogonadotropic hypogonadism due to TAC3/TACR3 mutations: characteriaztion of neuroendocrine phenotypes and novel mutations. PLoS One 6:e25614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Topaloglu AK, Semple RK. 2011. Neurokinin B signalling in the human reproductive axis. Mol Cell Endocrinol 346:57–64 [DOI] [PubMed] [Google Scholar]
- 41. Kauffman AS. 2010. Coming of age in the kisspeptin era: sex differences, development, and puberty. Mol Cell Endocrinol 324:51–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kung TT, Crawley Y, Jones H, Luo B, Gilchrest H, Greenfeder S, Anthes JC, Lira S, Wiekowski M, Cook DN, Hey JA, Egan RW, Chapman RW. 2004. Tachykinin NK3-receptor deficiency does not inhibit pulmonary eosinophilia in allergic mice. Pharmacol Res 50:611–615 [DOI] [PubMed] [Google Scholar]
- 43. Yang JJ, Caligioni CS, Chan YM, Seminara SB. 2012. Uncovering novel reproductive defects in neurokinin B receptor null mice: closing the gap between mice and men. Endocrinology 153:1498–1508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Gill JC, Wadas B, Chen P, Portillo W, Reyna A, Jorgensen E, Mani S, Schwarting GA, Moenter SM, Tobet S, Kaiser UB. 2008. The gonadotropin-releasing hormone (GnRH) neuronal population is normal in size and distribution in GnRH-deficient and GnRH receptor-mutant hypogonadal mice. Endocrinology 149:4596–4604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Dellovade TL, Merchenthaler I. 2004. Estrogen regulation of neurokinin B gene expression in the mouse arcuate nucleus is mediated by estrogen receptor α. Endocrinology 145:736–742 [DOI] [PubMed] [Google Scholar]
- 46. Kauffman AS, Navarro VM, Kim J, Clifton D, Steiner RA. 2009. Sex differences in the regulation of Kiss1/NKB neurons in juvenile mice: implications for the timing of puberty. Am J Physiol Endocrinol Metab 297:1212–1221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Navarro VM, Ruiz-Pino F, Sánchez-Garrido MA, García-Galiano D, Hobbs SJ, Manfredi-Lozano M, León S, Sangiao-Alvarellos S, Castellano JM, Clifton DK, Pinilla L, Steiner RA, Tena-Sempere M. 2012. Role of neurokinin B in the control of female puberty and its modulation by metabolic status. J Neurosci 32:2388–2397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Pintado CO, Pinto FM, Pennefather JN, Hidalgo A, Baamonde A, Sanchez T, Candenas ML. 2003. A role for tachykinins in female mouse and rat reproductive function. Biol Reprod 69:940–946 [DOI] [PubMed] [Google Scholar]
- 49. Almeida TA, Rojo J, Nieto PM, Pinto FM, Hernandez M, Martin JD, Candenas ML. 2004. Tachykinins and tachykinin receptors: structure and activity relationships. Curr Med Chem 11:2045–2081 [DOI] [PubMed] [Google Scholar]
- 50. Noritake K, Matsuoka T, Ohsawa T, Shimomura K, Sanbuissho A, Uenoyama Y, Maeda K, Tsukamura H. 2011. Involvement of neurokinin receptors in the control of pulsatile luteinizing hormone secretion in rats. J Reprod Dev 57:409–415 [DOI] [PubMed] [Google Scholar]
- 51. García-Galiano D, van Ingen Schenau D, Leon S, Krajnc-Franken MA, Manfredi-Lozano M, Romero-Ruiz A, Navarro VM, Gaytan F, van Noort PI, Pinilla L, Blomenröhr M, Tena-Sempere M. 2012. Kisspeptin signaling is indispensable for neurokinin B, but not glutamate, stimulation of gonadotropin secretion in mice. Endocrinology 153:316–328 [DOI] [PubMed] [Google Scholar]
- 52. Harris-Warrick RM, Johnson BR. 2010. Checks and balances in neuromodulation. Front Behav Neurosci 4:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.