Abstract
Previous immunohistochemical and in situ hybridization studies of sheep, goats, and rodents indicated that kisspeptin (KP), neurokinin B (NKB), and dynorphin A (DYN) are extensively colocalized in the hypothalamic arcuate nucleus, thus providing a basis for the KP/NKB/DYN (KNDy) neuron concept; in both sexes, KNDy neuropeptides have been implicated in the generation of GnRH neurosecretory pulses and in the negative feedback effects of sexual steroids to the reproductive axis. To test the validity and limitations of the KNDy neuron concept in the human, we carried out the comparative immunohistochemical analysis of the three neuropeptides in the infundibular nucleus (Inf; also known as arcuate nucleus) and stalk of young male human individuals (<37 yr). Results of quantitative immunohistochemical experiments established that the regional densities of NKB immunoreactive (IR) perikarya and fibers, and the incidence of afferent contacts they formed onto GnRH neurons, were about 5 times as high as those of the KP-IR elements. Dual-immunofluorescent studies confirmed that considerable subsets of the NKB-IR and KP-IR cell bodies and fibers are separate, and only about 33% of NKB-IR perikarya and 75% of KP-IR perikarya were dual labeled. Furthermore, very few DYN-IR cell bodies could be visualized in the Inf. DYN-IR fibers were also rare and, with few exceptions, distinct from the KP-IR fibers. The abundance and colocalization patterns of the three immunoreactivities showed similar trends in the infundibular stalk around portal blood vessels. Together these results indicate that most NKB neurons in the Inf do not synthesize detectable amounts of KP and DYN in young male human individuals. These data call for a critical use of the KNDy neuron terminology when referring to the putative pulse generator system of the mediobasal hypothalamus. We conclude that the functional importance of these three neuropeptides in reproductive regulation considerably varies among species, between sexes, and at different ages.
Information accumulated from immunohistochemical and in situ hybridization studies has recently formed the basis for the kisspeptin/neurokinin B/dynorphin A (KNDy) neuron concept and terminology (1–4). As shown first for the sheep (5), many of these neurons with cell bodies located in the hypothalamic arcuate nucleus [ARC; called infundibular nucleus in humans (Inf)] cosynthesize kisspeptin (KP), neurokinin B (NKB), and dynorphin A (DYN). They have been implicated in negative sex steroid feedback to GnRH neurons (5–7) and proposed to also serve as pacemakers for the GnRH neurosecretory pulses (3, 4, 8, 9). Recent models of the GnRH pulse generator (3, 4, 8) suggest that KNDy neurons communicate with one another via NKB and its receptor, NK3, and possibly, also DYN and its receptor, KOR. In ovariectomized goats, central NKB increases and DYN decreases the frequencies of multiunit activity volleys and LH secretory pulses (8). Pulse generator cells, in turn, appear to communicate with GnRH neurons primarily via KP/KP receptor (KISS1R) signaling. GnRH neurons express KISS1R (10–12), and the majority of GnRH neurosecretory pulses show temporal association with KP pulses in the median eminence of monkeys (13).
The general consensus that KP, NKB, DYN, NK3, and KOR are expressed by the same neurons relies on combined neuroanatomical data from sheep (1, 5), rats (14), mice (3, 4, 15), goats (8), monkeys (16), and humans (17, 18). However, closer analysis of these reports, in retrospect, reveals that neuropeptide and receptor colocalizations are often only partial and also variable in the different studies, species, sexes, and age groups. Notably, in our recent immunohistochemical study of aged human individuals, we have detected robust sex differences in the abundance of KP-immunoreactive (IR) (17, 18) and NKB-IR (18) neuronal elements in the Inf. These studies have also revealed that the incidences of NKB-IR cell bodies, fibers, and appositions onto GnRH neurons exceed severalfold those of KP-IR elements, with particularly robust differences in males (18). These results suggested that NKB-IR neurons and their fibers are partly distinct from the KP-IR elements in these human models, thus challenging the universal validity of the KNDy neuron concept. Moreover, in triple-immunofluorescent studies of aged human males, GnRH neurons tended to receive primarily single-labeled afferent inputs from these peptidergic systems, with the KP/NKB double-labeled axons representing only 10.2% of all KP-IR afferents and the NKB/KP double-labeled axons about 8.8% of all NKB-IR afferents (18). From the above findings and preliminary observations indicating that KP immunolabeling is even weaker in young than in aged men, we predicted that the degree of overlap between the KNDy neuropeptides is much lower in young male humans than was suggested earlier for female sheep (1, 5), goats (8), or mice (3).
In the present study, we investigated the universal validity of the KNDy neuron concept via the parallel immunohistochemical analysis of NKB-, KP-, and DYN immunoreactivities in the Inf and the infundibular stalk (InfS) of young men. Specifically, 1) we compared the immunoreactive perikaryon and fiber densities in the Inf and the InfS, 2) we addressed the colocalization of KP with NKB and KP with DYN in perikarya and fibers, and finally, 3) we compared quantitatively the incidences of NKB-IR vs. KP-IR afferent contacts onto GnRH-IR neurons.
Materials and Methods
Human subjects
Human hypothalamic samples were obtained from autopsies at the Forensic Medicine Department of the University of Debrecen with permission from the Regional Committee of Science and Research Ethics of the University of Debrecen (DEOEC RKEB/IKEB: 3183-2010). Selection criteria included sudden causes of death, lack of a history of neurological and endocrine disorders, and postmortem delay less than 36 h. Tissue specimens from six young male individuals (aged 21–37 yr) were used.
Section preparation
After dissection, the hypothalamic tissue blocks were rinsed with running tap water and then immersion fixed with 4% formaldehyde in 0.1 m PBS (pH 7.4) for 7–14 d. After fixation, the blocks were trimmed in a way to include the optic chiasma rostrally, the mammillary bodies caudally and the anterior commissure dorsally (17–19). Bilateral sagittal cuts were placed 2 cm lateral from the midline. The blocks were bisected into right and left halves and then infiltrated with 20% sucrose for 5 d at 4 C. The right hemihypothalami were sectioned coronally at 30 μm with a Leica SM 2000R freezing microtome (Leica Microsystems, Nussloch GmbH, Germany). All experiments were performed on every 24th hemihypothalamic section from each subject.
Pretreatments
The tissues were permeabilized and endogenous peroxidase activity reduced using a mixture of 0.2% Triton X-100 and 0.5% H2O2 in PBS for 30 min. Antigen epitopes were unmasked by incubating sections in 0.1 m citrate buffer (pH 6.0) at 80 C for 30 min (18). Dual-immunofluorescent experiments also used a Sudan black pretreatment against autofluorescence (17, 18).
Immunohistochemical detection of KP
To detect KP immunoreactivity, sections were incubated in a sheep polyclonal antiserum against human kisspeptin-54 (GQ2; 1:200,000). This antiserum recognizes human KP-54, KP-14, and KP-10, exhibits less than 0.01% cross-reactivity in vitro with other related human RF amide peptides (20), and was used successfully in previous immunohistochemical experiments on primate hypothalami (16–18, 21). Incubation in the primary antibodies for 48 h at 4 C was followed by biotinylated secondary antibodies (Jackson ImmunoResearch Laboratories, West Grove, PA; 1:500) and the ABC Elite reagent (Vector Laboratories, Burlingame, CA; 1:1000) for 60 min each. The peroxidase reaction was visualized with nickel-intensified diaminobenzidine chromogen (19) and then postintensified with silver-gold (22).
Immunohistochemical detection of NKB and DYN
Another two parallel series of sections were used to visualize NKB and DYN, respectively. NKB neurons were detected with rabbit polyclonal antibodies against the C-terminal 28 amino acids of human pro-NKB (IS-682, P. Ciofi; 1:100,000) (17), whereas DYN neurons were labeled with rabbit polyclonal antibodies against amino acids 1–17 of porcine (and human) DYN (T-4268; Peninsula Laboratories; San Carlos, CA; 1:100,000). The primary antibodies were reacted with biotinylated antirabbit IgG (Jackson ImmunoResearch Laboratories; 1:500; 1 h) and then ABC Elite reagent (1:1000; 1 h). The peroxidase signal was developed with silver-gold-intensified nickel-diaminobenzidine. For control purposes, the mapping studies of DYN were replicated using a previously characterized second antiserum (IS-35; 1:200,000) against a different prodynorphin-derived peptide, dynorphin B (23).
Dual-immunoperoxidase detection of KP and GnRH or NKB and GnRH
Two section series were processed for the immunohistochemical detection of KP and NKB, respectively, as above. Then they were processed to detect GnRH using a guinea pig primary antiserum (no. 1018; 1:5000) (18), which was reacted with biotinylated antiguinea pig IgG (Jackson ImmunoResearch Laboratories; 1:500; 1 h) and ABC Elite solution (Vector Laboratories; 1:1000; 1 h). The peroxidase signal was visualized with brown diaminobenzidine.
Fluorescent immunohistochemistry
Other series of sections were processed for the dual-immunofluorescent studies of the colocalization between NKB and KP or DYN and KP. Incubation in a cocktail of primary antibodies (rabbit anti-NKB, 1:1000; and sheep anti-KP, 1:1000; or rabbit anti-DYN, 1:1000; and sheep anti-KP, 1:1000; 48 h; 4 C) was followed by a cocktail of fluorochrom-conjugated secondary antibodies [Jackson ImmunoResearch; antirabbit-fluorescein isothiocyanate (FITC), 1:250; antisheep-Cy3, 1:1000] for 5 h at room temperature.
To maximize sensitivity, other dual-immunofluorescent studies used tyramide signal amplification. In these experiments KP was detected first using sequential incubations in KP antibodies (1:30,000; 48 h; 4 C), biotinylated antigoat IgG (Jackson ImmunoResearch Laboratories; 1:500; 1 h), the ABC Elite reagent (Vector Laboratories; 1:1,000; 1 h), biotin tyramide working solution [1:1,000, in 0.05 m Tris-HCl buffer (pH 7.6), containing 0.003% H2O2; 30 min] (24), and finally, avidin-Cy-3 (Jackson ImmunoResearch; 1:1,000; 1 h). Then the sections were treated for 30 min with 0.5% H2O2 and 0.1% sodium azide in PBS to inactivate horseradish peroxidase. To detect NKB or DYN, the rabbit primary antibodies were used at 1:50,000 (48 h; 4 C) and reacted with antirabbit-peroxidase (Jackson ImmunoResearch; 1:500; 1 h). Then FITC-tyramide (24) [diluted 1:500 with 0.05 m Tris-HCl buffer (pH 7.6), containing 0.003% H2O2; 30 min] was deposited on the peroxidase sites. Control experiments included the omission of the NKB and DYN primary antibodies. Lack of FITC labeling in these control sections indicated that no FITC-tyramide deposition is caused by residual peroxidase activity on KP-IR sites.
Section mounting and coverslipping
After section mounting, immunoperoxidase-labeled sections were coverslipped with DPX (Fluka Chemie, Buchs, Switzerland) and immunofluorescent specimens with Mowiol.
Digital photography
The light and fluorescent microscopic images were scanned with an AxioCam MRc 5 digital camera mounted on a Zeiss AxioImager M1 microscope using the AxioVision 4.6 software (Carl Zeiss, Göttingen, Germany). Confocal images were prepared with an inverted Nikon Eclipse Ti-E microscope equipped with an A1R confocal system (Nikon, Vienna, Austria). The digital images were processed with the Adobe Photoshop CS software (Adobe Systems, San José, CA) at a 300-dpi resolution. Quantitative data were expressed as mean ± sem and statistical comparisons used one-way ANOVA.
Experiment 1. Studies of the incidences and overlaps of IR cell bodies in the Inf
The number of immunoreactive cell bodies was counted at 100 × magnification within a 0.25-mm2 counting area with the aid of a 5 × 5 ocular grid, as described previously (17, 18). Each subject was characterized by the maximal number of immunoreactive perikarya in this counting area (determined from two to six sections.)
The overlaps between NKB and KP immunoreactivities and between DYN and KP immunoreactivities were first assessed in confocal images of dual-immunofluorescent specimens. The percentages of single-labeled and double-labeled NKB-IR and KP-IR perikarya were also determined quantitatively from the specimens in which the tyramide signal amplification was used, using one to three representative confocal images per subject.
Experiment 2. Studies of the regional abundance of IR fibers in the Inf
The regional density of immunoreactive fibers was determined as described recently (18). First, digital images were taken from the bulk of KP-IR and NKB-IR neurons in the Inf. The files were opened with the Adobe Photoshop CS software. The immunolabeled cell bodies were erased (eraser tool) from the photomicrographs. The remaining images were compiled into TIF files and opened with the ImageJ software (public domain at http://rsbweb.nih.gov/ij/download.html). The regional fiber density in each photograph was defined as the area occupied by immunoreactive fibers/total area. For each subject, the mean fiber density was derived from one to three digital images. The overlap between NKB-IR and KP-IR axons or DYN-IR and KP-IR axons was also studied qualitatively in confocal images of dual-immunofluorescent specimens.
Experiment 3. Studies of immunoreactive fibers in the InfS
Projections of NKB-IR, KP-IR, DYN-IR, and GnRH-IR axons around the portal blood vessels of the InfS were analyzed in this experiment. Based on previous immunohistochemical results in the median eminence of different species (16, 23), we assumed that fibers containing KNDy peptides around the portal vasculature arise mostly from the ARC/Inf. First, sections labeled with peroxidase-based immunohistochemistry were used to study the relationship of fibers with the superficial and deep capillary plexuses of the human postinfundibular eminence (25). Then the extents of overlap between NKB and KP immunoreactivities and between DYN and KP immunoreactivities were assessed from dual-immunofluorescent specimens.
Experiment 4. Studies of the incidences of KP-IR and NKB-IR appositions onto GnRH-IR neurons of the Inf
Dual-immunoperoxidase labeled sections were selected (one to two from each individual) to determine the number of axonal contacts along the outlines of GnRH-IR cell bodies and dendrites. Counting of the appositions was carried out using a ×63 oil-immersion objective and contacts defined using stringent criteria (18, 26, 27). For each subject, the mean number of contacts per GnRH soma and 100 μm GnRH dendrite were calculated (18).
Results
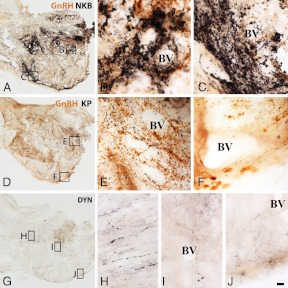
The comparative analysis of NKB, KP, and DYN immunoreactivities in immunoperoxidase-labeled sections of the Inf (Fig. 1) and the InfS (Fig. 2) revealed strikingly different labeling intensities for KNDy neuropeptides. In general, NKB-IR elements showed much higher abundance than KP-IR elements. DYN immunoreactivity, both in perikarya and fibers, was relatively sparse and weak.
Fig. 1.
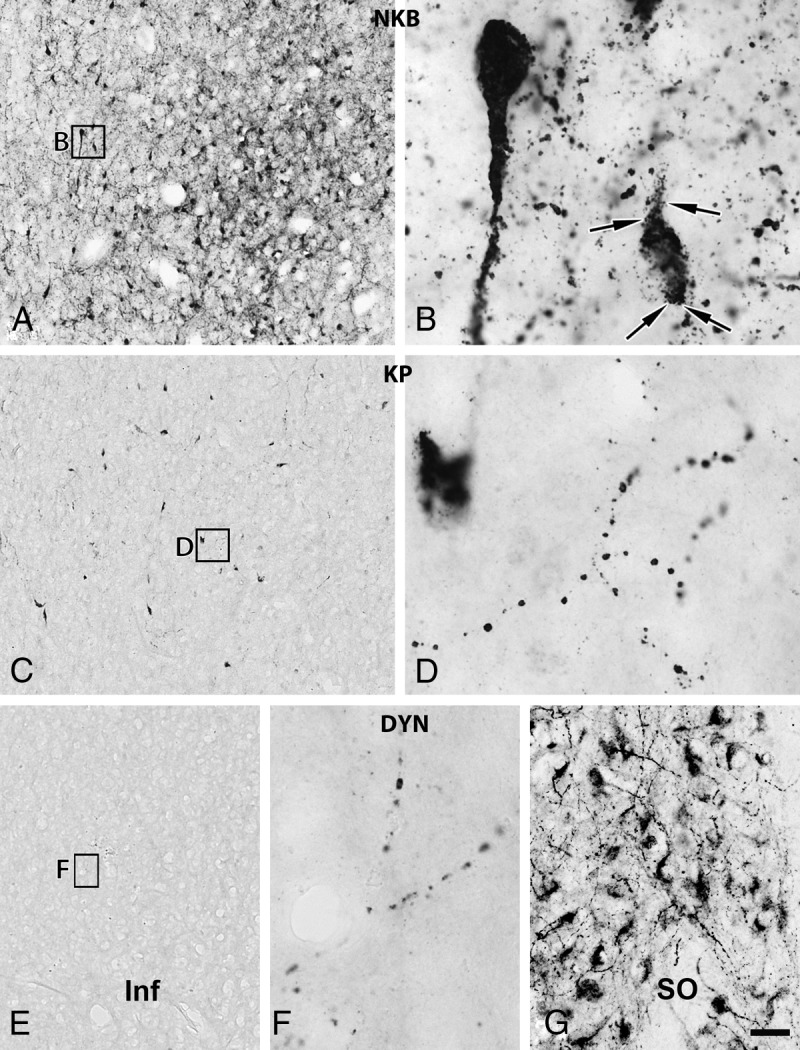
Relative abundances of NKB-IR, KP-IR, and DYN-IR cell bodies and fibers in the Inf of young male humans. The silver-gold intensified nickel-diaminobenzidine chromogen was used to visualize NKB (A and B), KP (C and D), and DYN (E–G) immunoreactivities in adjacent sections of the Inf from a 31-yr-old male subject. NKB-IR perikarya as well as nerve fibers (A and B) occur in much higher numbers than do KP-IR elements (C and D). For results of the quantitative analyses, which reveal about 5-fold differences for both perikaryon and fiber densities, see Fig. 5 and 6, respectively. Note that NKB-IR neurons receive numerous afferent contacts (arrows in B) from NKB-IR varicose axons; analogous juxtapositions in other species were proposed to underlie the main peptidergic signaling mechanism among the putative pulse-generating KNDy neurons. Few if any DYN-IR cell bodies are detectable in the Inf (none visible in this specific case), and only scattered DYN-IR fibers occur (E and F). This low level of the DYN signal does not appear to reflect a technical limitation of the immunohistochemical approach, given that IR perikarya and fibers are abundant elsewhere in the hypothalamus, including the supraoptic nucleus (SO; G). Scale bar, 100 μm for A, C, and E and 9 μm elsewhere.
Fig. 2.
Results of immunoperoxidase studies illustrating the differential innervation of the portal capillary plexus by NKB-IR, KP-IR, and DYN-IR fibers in a 31-yr-old man. A–I, The black silver-gold-intensified nickel-diaminobenzidine chromogen was used to detect NKB (A–C), KP (D--F), and DYN (G–J) immunoreactivities in adjacent sections of the InfS. Note that GnRH has also been visualized in A–F with brown diaminobenzidine. The postinfundibular eminence with its deep (B, E, and I) and superficial (C, F, and J) plexuses of portal blood vessels (BV) is surrounded by GnRH-IR hypophysiotropic axons (brown color in A–F). Of the axons immunoreactive for the three KNDy peptides (black color), those with NKB immunoreactivity represent the most frequently encountered phenotype (A) and densely innervate both the deep (B) and the superficial (C) portal capillaries; this innervation raises the possibility of NKB release into the hypophysial portal circulation. The KP-IR innervation of the portal BV is of much lower density (D--F). Although DYN-IR axons are readily detectable in the InfS (G) and contribute to the magnocellular axon tract (H), they occur only rarely around the portal BV (H and I). Scale bar, 100 μm for A, D, and G and 10 μm elsewhere.
Experiment 1. Incidence of IR perikarya in the Inf
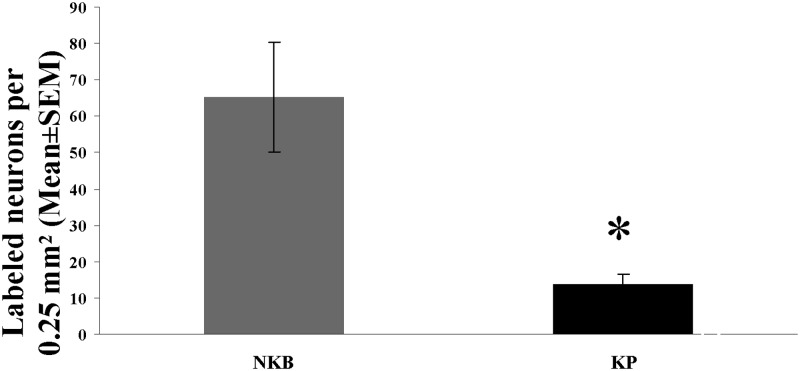
In peroxidase-based immunohistochemistry, many NKB-IR perikarya were identified in the Inf (Fig. 1, A and B). KP-IR cell bodies occurred in much lower numbers in neighboring sections (Fig. 1, C and D). Quantitative analysis showed that the density of KP neurons was about 5 times lower than that of NKB-IR perikarya (Fig. 5; P = 0.01 by ANOVA). DYN-IR perikarya were either entirely absent in some subjects (Fig. 1, E and F) or extremely rare in the Inf of others, preventing quantitative studies. In contrast, the supraoptic nucleus contained many labeled perikarya (Fig. 1G), making it unlikely that the low DYN signal in the Inf reflects technical limitations.
Fig. 5.
Regional abundance of NKB-IR and KP-IR neuronal perikarya in the Inf of young male humans. The maximal number of immunoreactive cell bodies per 0.25 mm2 counting frame (one to six per subject) was determined with the aid of an ocular frame and used as the index of the density of labeled perikarya. Results show that in young male human individuals, the mean incidence of NKB-IR cell bodies is about 5 times as high as the incidence of KP-IR cell bodies. *, P < 0.05 by ANOVA.
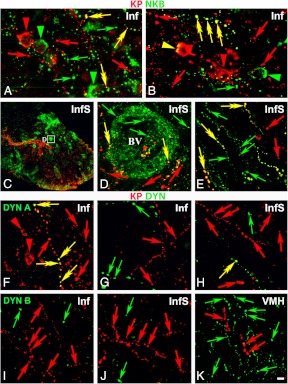
A surprising partial segregation of NKB-IR and KP-IR perikarya was revealed in dual-immunofluorescent specimens (Fig. 3, A and B). Tyramide signal amplification was crucial for sensitive detection of NKB/KP dual-labeled cell bodies which represented only 32.9 ± 4.7% of the NKB-IR and 75.2 ± 6.6% of the KP-IR perikarya (Fig. 3, A and B). Tyramide signal amplification was capable of visualizing only a few DYN-IR perikarya (not shown).
Fig. 3.
Results of immunofluorescent studies revealing a significant degree of mismatch between KP and NKB immunoreactivities (A–E) and KP and DYN (or dynorphin B) immunoreactivities (F–K) in the Inf (A, B, F, and G) and the InfS (C–E, and H). The dual-immunofluorescent visualization of NKB (green) and KP (red) immunoreactivities in the Inf (A and B) not only confirms the dominance of NKB-IR (green) over KP-IR (red) cell bodies (arrowheads) and axons (arrows) in the Inf but also reveals a considerable degree of segregation between the two different perikaryon (arrowheads) and fiber (arrows) populations. Yellow double arrows and arrowheads point to dual-labeled fibers and cell bodies, respectively. Single-labeled NKB-IR axons are also typical around the portal blood vessels (BV) of the postinfundibular eminence in the InfS (C–E), in addition to single-labeled KP-IR fibers (red) and NKB/KP dual-labeled (yellow) axons. The dual-immunofluorescent visualization of KP and DYN (F–H) illustrates the absence of DYN immunoreactivity from KP-IR cell bodies in the Inf (red arrowheads in F). With the exceptions of a few scattered dual-labeled fibers (yellow double arrows) in the Inf (F) and the InfS (H), the majority of KP-IR (red) and DYN-IR (green) axons are separate. Negative colocalization results are reproducible in the Inf (I) and the InfS (J) using an antiserum against a different prodynorphin cleavage product, dynorphin B. Note the high density of dynorphin B-IR fibers in the neighboring ventromedial nucleus (VMH) in K. High-power micrographs (A, B, and D–K) represent single optical slices (0.7 μm). Scale bar, 100 μm for C, 5 μm for D, and 10 μm for A, B, and E–K.
Experiment 2. Abundance of IR fibers in the Inf
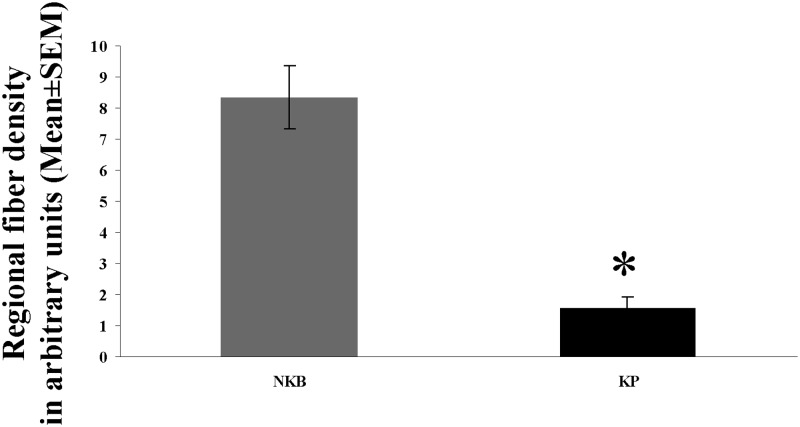
The incidence of immunolabeled fibers in the Inf followed a similar trend as shown by labeled perikarya. The most frequently encountered phenotype was, again, IR for NKB. These axons established many appositions (Fig. 1B) to NKB-IR cell bodies and their dendritic processes. Quantitative analysis of the area covered by immunohistochemical signal established that the mean incidence of NKB-IR fibers was about 5 times as high as that of KP-IR fibers (Fig. 6; P = 0.0001 by ANOVA). DYN-IR fibers were also detectable in the Inf, although less frequently than either NKB-IR or KP-IR axons (Fig. 1, E and F).
Fig. 6.
Density of NKB-IR and KP-IR fibers in the Inf of young men. The area covered by immunoreactive fibers (divided by the total area analyzed) was determined with the ImageJ software in digital photographs of the Inf and used as a fiber density measure. Areas that were occupied by labeled cell bodies and their thick proximal dendrites were deleted from the photographs with the eraser tool of the Adobe Photoshop software and thus excluded from the analysis. The density of NKB-IR fibers (expressed in arbitrary units) is 5.4 times as high as the density of KP-IR fibers. *, P < 0.0005 by ANOVA.
In immunofluorescent specimens, many NKB-IR fibers without KP immunolabeling as well as KP-IR fibers without NKB labeling could be seen in the Inf, in addition to dual-labeled axons (Fig. 3, A and B). DYN-IR fibers showed a high intensity of labeling only if the tyramide signal amplification approach was also used. Most of them were distinct from KP-IR axons, although dual-labeled KP/DYN-IR fibers occasionally occurred (Fig. 3, F and G). Similarly, the majority of KP-IR fibers were also devoid of dynorphin B immunoreactivity in the Inf and the InfS (Fig. 3, I and J), whereas this second antiserum also performed well in regions rich in DYN fibers, including the ventromedial nucleus (Fig. 3K).
Experiment 3. Abundance of immunoreactive fibers in the InfS
The InfS was associated with the superficial and the deep capillary plexuses of the postinfundibular eminence (25). Both were abundantly innervated by GnRH-IR axons (Fig. 2, A–F, brown color), suggesting they contribute to the GnRH supply of adenohypophysial gonadotropes. The relative abundance of the different types of labeled fibers around the two capillary plexuses matched what was seen in the Inf. Accordingly, portal blood vessels were surrounded by dense networks of NKB-IR fibers (Figs. 2, A–C) and innervated only moderately by KP-IR fibers (Figs. 2, D–F). Very few DYN-IR fibers occurred in the proximity of the portal capillaries (Figs. 2, G, I, and J). This low level of DYN signal did not reflect a technical limitation, considering that the magnocellular neurosecretory tract was immunolabeled heavily in the same sections (Fig. 2H).
The analysis of immunofluorescent specimens confirmed that NKB dominates over KP around the portal vasculature and NKB-IR fibers often lack KP labeling (Fig. 3, C–E). Similarly to the Inf, the InfS contained both single-labeled and double-labeled KP-IR fibers (Figs. 3, C–E). In sections dual labeled for KP and DYN, labeled fibers were mostly distinct, although rare colocalization cases were also detectable (Fig. 3H).
Experiment 4. Frequency of KP-IR and NKB-IR appositions onto GnRH-IR neurons
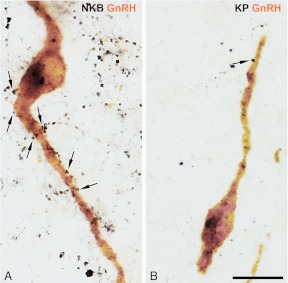
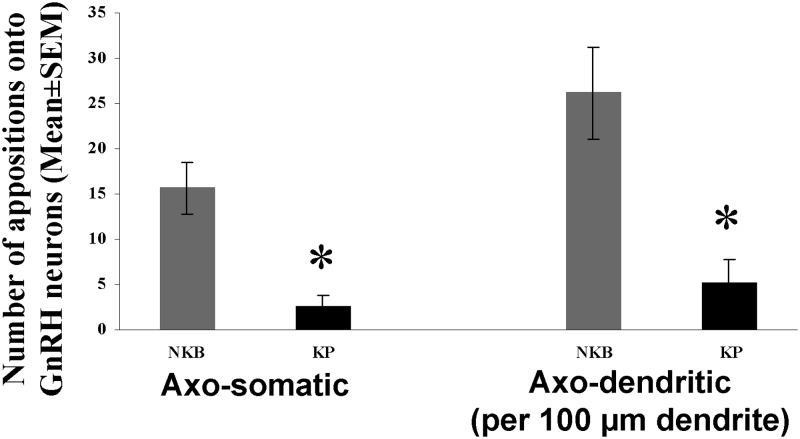
Sections double labeled with the silver-gold-intensified nickel-diaminobenzidine and diaminobenzidine chromogens were used to obtain quantitative estimates about NKB-IR and KP-IR inputs to GnRH-IR neurons. Microscopic analysis confirmed that NKB-IR (18) and KP-IR (17, 18) axons provide axosomatic and axodendritic inputs to GnRH neurons in the Inf (Fig. 4). Quantitative analysis (Fig. 7) established that GnRH-IR perikarya and dendrites, respectively, received 6 and 5 times heavier NKB-IR (Fig. 4A) than KP-IR (Fig. 4B) innervation (GnRH perikarya: P = 0.004; GnRH dendrites: P = 0.005, by ANOVA).
Fig. 4.
Different incidences of NKB-IR and KP-IR afferent contacts onto GnRH-IR neurons. NKB-IR axons (black color in A) show a much higher abundance in the Inf and establish considerably more axosomatic and axodendritic juxtapositions (arrows) onto GnRH-IR neurons (brown color) than do KP-IR axons (black color in B). For quantification of these results, see Fig. 7. Scale bar, 20 μm.
Fig. 7.
Incidences of NKB-IR and KP-IR contacts onto GnRH-IR neurons in the Inf in young men. High-power light microscopic analysis of sections, dual immunolabeled with the combined use of silver-gold-intensified nickel-diaminobenzidine and diaminobenzidine chromogens, was carried out to determine the relative incidences of NKB-IR and KP-IR neuronal appositions to the somata (left columns) and the dendrites (right columns) of GnRH-IR neurons. The counts were obtained from all GnRH-IR cell bodies and dendrites identified in one to three infundibular sections of each individual. The number of NKB-IR contacts is about 6 times as high on the somatic and 5 times as high on the dendritic compartment of GnRH neurons as those established by KP-IR axons. * P < 0.01 by ANOVA.
Discussion
Immunohistochemical results of this study provide evidence for limitations of the KNDy neuron terminology and concept. Specifically, our observations indicate that in young male humans the majority of NKB-IR neurons in the Inf, their processes and contacts onto GnRH neurons do not contain detectable amounts of KP immunoreactivity. Furthermore, KP-IR neuronal elements without NKB labeling also occur frequently in addition to NKB/KP dual-phenotype structures. Finally, we observed that most KP-IR neurons and fibers are devoid of DYN immunoreactivity in this human model.
Species differences in the colocalization of KNDy peptides
We propose that the different colocalization patterns in the present human study and in previous animal experiments partly reflect species differences in reproductive mechanisms (28). These may include the absence of DYN signal from most KP neurons and fibers in humans, which is in contrast with the extensive coexpression in the rodent (3, 4, 14), sheep (5), and goat (8) ARC. Another putative species difference is the large excess of NKB-IR over KP-IR perikarya in the present human study, as opposed to the 2-fold excess in the ARC of male mice (4).
Sex-dependent variations in the absolute and relative abundances of KP and NKB and their colocalization pattern
In a previous study of KP-IR and NKB-IR neurons, we identified a series of sex-dependent morphological differences between aged human males and females (18). In particular, KP immunoreactivity was highly sexually dimorphic; the number of KP-IR perikarya, the density of KP-IR fibers, and the incidence of KP-IR afferents on GnRH neurons were much higher in aged women compared with men (18). It is worth noting that the NKB to KP neuron density ratio also differed, being 2.18 in aged males (>50 yr) and 1.55 in postmenopausal females (>55 yr) (18). In further support of the idea that the KP/NKB colocalization pattern is sexually dimorphic, the ratio of dual-labeled NKB-IR as well as KP-IR afferent contacts onto GnRH-IR neurons was significantly higher in postmenopausal women (∼25–30%) than in aged men (∼8–10%) (18). We proposed that sex differences are due to either organizational effects of sex steroids during critical period(s) of sexual differentiation or alternatively to the loss of negative sex steroid feedback in postmenopausal women, unlike in aged men who maintain relatively high testosterone levels. In our previous dual-immunofluorescent study, we demonstrated a high degree of overlap between the KP and NKB systems of postmenopausal women; more than 80% of KP-IR perikarya and NKB-IR perikarya contained also the other neuropeptide (17). The degree of this colocalization is likely to be sexually dimorphic and much lower in aged males whose Inf contained more than twice as many NKB-IR as KP-IR perikarya (18).
It is worth noting that the sexual dimorphism of KP and NKB neurons in the ARC/Inf region is not unique to humans. The ARC of the sheep contains higher NKB (29) and KP (1) cell numbers in females than in males. In rats, sex differences were reported in the projection fields of NKB-IR axons within the infundibular area (23).
Aging-dependent variations in the absolute and relative abundances of KP and NKB
The human NKB and KP systems, at least in the male, also exhibit robust aging-dependent changes. Our preliminary data indicate that the enhancements of the absolute NKB and KP cell numbers coincide with a significantly enhanced percentage of KP-expressing NKB neurons in aged men (Molnár et al., manuscript in preparation).
Role of NKB in the regulation of the human reproductive axis
The tachykinin peptide NKB plays a crucial role in human reproduction and inactivating mutations of the NKB and NK3 encoding genes cause normosmic hypogonadotropic hypogonadism (30, 31). Although the first reports did not indicate fertility deficits in the NK3 mutant mice (32), later analysis focusing on the reproductive phenotype noticed subfertility (33), suggesting functional similarities in NKB/NK3 signaling between the human and mouse species.
Out of the three KNDy peptides, NKB provided the heaviest immunohistochemical signal in the Inf and the InfS of young men (present report), aged men (18), and aged women (18). In immunoperoxidase-based studies, the dominance of NKB over KP was highest in young men (present study) in which the density of NKB-IR perikarya and fibers were about 5 times as high as those of the KP-IR elements and NKB-IR axons established about 6 times as many axosomatic and 5 times as many axodendritic contacts onto GnRH neurons as did KP-IR axons.
Conflicting results of previous experiments suggest that the net effect of NKB on LH secretion depends on animal species and endocrine paradigms. Intraperitoneal or intracerebroventricular NKB administration to male mice had no effect on serum LH (34), whereas the intracerebroventricular injection of the selective NK3 agonist senktide reduced LH secretion in ovariectomized rats treated with a low dose of estradiol (35). Reduced LH secretion in response to senktide was also observed in ovariectomized and in ovariectomized and estradiol treated rats (36) and in ovariectomized mice (3), whereas another study on rats found stimulatory effect on LH secretion in the presence of physiological levels of estradiol (37). Senktide also stimulated LH secretion in castrated male monkeys (16) and in the follicular, but not in the luteal, phase in ewes (38), whereas reduced net LH secretion was observed in ovariectomized goats (8).
Multiunit activity recorded in the ARC is considered to be an electrophysiological correlate of the GnRH pulse generator activity. The coordinated bursts of neuronal firing occur in synchrony with the LH secretory pulses in various animal species (8, 39), including primates (40). Senktide dose dependently suppressed the frequency of pulsatile LH secretion and inhibited hypothalamic multiunit activity volleys in ovariectomized rats, independently of gonadal steroid levels (36). In contrast, a robust increase in the frequency of multiunit activity volleys was observed in ovariectomized goats (8).
NKB mainly acts upstream from GnRH neurons. For example, the stimulatory effect of intravenous NKB and senktide on LH secretion could be abolished by the GnRH receptor antagonist acyline (16). One major target site for NKB actions appears to be on NKB (KNDy) neurons of the ARC/Inf. Accordingly, 1) we found numerous NKB-IR afferent contacts on human NKB neurons, 2) similar contacts were reported previously in rats (14) and sheep (5, 41), 3) NK3 receptors are present on these cells in rodents and sheep (3, 14, 42), and 4) KP (KNDy) neurons in the ARC of male mice respond with c-Fos expression (4) and depolarization (4) to senktide.
There is little agreement regarding the possibility that NKB also influences GnRH neurons directly. In sheep, GnRH neurons do not express NK3 immunoreactivity (42). In mice, although single-cell microarray and RT-PCR studies provided proof for NK3 mRNA expression in GnRH neurons (43), in situ hybridization studies were unable to confirm this finding (4). Also, senktide did not activate mouse KNDy neurons in vitro (4). In rats, although immunohistochemical studies found evidence for NK3 immunoreactivity in only 16% of GnRH-IR cell bodies (44), the receptor was more abundant on GnRH-IR axon terminals in the median eminence (44) where frequent appositions between GnRH-IR and NKB-IR axons occurred (23, 44). Although NKB in itself did not alter GnRH release from hypothalamic explants of male rats, it abrogated the KP-induced release of GnRH, suggesting a complex mode of action, which is likely parallel with, and not only upstream from, the KP action (45). Recent functional evidence from KISS1R-knockout mice indicates that intact KP/KISS1R signaling is required for the suppression of LH secretion by senktide. This finding provides support for the concept that the dominant action of NKB is upstream from KP neurons, instead of being exerted directly on GnRH cells (45).
Results of the present and a previous (18) human study indicate that NKB-IR axons abundantly innervate human GnRH neurons and the incidence of these contacts is several times as high as those of KP-IR axons. It will require clarification whether this anatomical pathway uses NKB/NK3 signaling. Alternatively, neurotransmitter(s) other than NKB may act in this communication, which is not likely to be KP or DYN, in view of their relative paucity in young male individuals. In addition to innervating GnRH-IR cell bodies and dendrites, NKB-IR axons also represented the most abundant KNDy peptide around the portal capillary plexuses of the human postinfundibular eminence (25). This hypothalamic site that lies outside the blood-brain barrier may represent an important site of interaction between NKB-IR and GnRH-IR axons. It also remains possible that NKB is released into the hypophysial portal circulation to influence adenohypophysial functions. In vitro evidence from rats, indeed, indicates that NKB can induce prolactin secretion from perifused pituitary cells (46).
Role of KP in the regulation of the human reproductive axis
In humans, KP/KISSR1 signaling plays a pivotal role in reproduction. Loss of function mutations of the genes encoding KISSR1 (47–49) and KP (50) result in hypogonadotropic hypogonadism. In recent models of the GnRH pulse generator, KP was proposed to provide the main output signal of the pulse generator neuronal network toward GnRH neurons, whereas NKB and DYN seem to primarily account for the intranuclear communication of KNDy neurons via acting on NK3 and KOR, respectively (3, 8). It is generally believed that independently from the species, KP acts directly on GnRH neurons that express KISS1R mRNA (10–12) and, in mice, respond with depolarization to KP (11, 51, 52).
As in laboratory and domestic animals, KP increases LH secretion in men (20, 53, 54) and women (55, 56), most potently during the preovulatory phase of the menstrual cycle in the latter. It is interesting to note that the continuous intravenous infusion of KP enhanced the LH pulse frequency in men (53), indicating that KP not only acts on GnRH neurons but also upstream from the pulse generator network. This finding suggests a species difference from the mouse in which KNDy neurons do not appear to synthesize KISS1R (57) and express only NK3 (3) and KOR (3) mRNAs.
In humans, axosomatic, axodendritic and axoaxonal contacts may serve as communication pathways between KP and GnRH neurons (17). In previous studies we showed that the KP system of aged human individuals exhibits a robust sexual dimorphism (18) with postmenopausal women having several times higher densities of KP-IR cell bodies and fibers in the Inf and higher incidences of KP-IR afferent contacts onto GnRH neurons than aged men above 50 yr. Preliminary data that KP immunoreactivity is even much lower in the hypothalamus of young men (Molnár et al., manuscript in preparation) led us to carry out the present study to challenge the validity of the KNDy neuron terminology and concept for the young male human model. Our present immunohistochemical data indicate that the density of KP-IR perikarya and fibers in the Inf were approximately 5 times lower than those of the corresponding NKB-IR elements and the number of KP-IR appositions to GnRH-IR cell bodies and dendrites only reached about one fifth and one sixth, respectively, of those established by NKB-IR axons. The functional consequences of the surprisingly low level of KP immunoreactivity in young men (in somata as well as fibers) require clarification. In view that KP is thought to represent the main neurotransmitter output of the pulse generator system (3, 8), its low level in the mediobasal hypothalamus of young men is compatible with only a moderate stimulation of GnRH/LH secretion. Much higher raw numbers of the KP-IR cell bodies, fibers, and contacts onto GnRH neurons in aged male human subjects (18) and their highest incidence in postmenopausal women (18) are in accordance with the idea that KP immunoreactivity and serum LH levels are linked. A future challenge will be to correlate the immunohistochemical images of KP and the other KNDy peptides with the GnRH neurosecretory output at the different human age and sex groups.
Absence of DYN immunoreactivity from most KP neurons and their fiber projections
The opioid peptide DYN is rather ubiquitous and may have multiple sites of action upon the reproductive axis. These sites are likely upstream from the GnRH neuron that does not appear to express KOR in rats (58). DYN is critically involved in progesterone negative feedback to GnRH neurons in ewes; the majority of DYN cells in the ARC of ovariectomized ewes contain progesterone receptor (59), and progesterone treatment increases preprodynorphin mRNA expression in the ARC and DYN levels in the cerebrospinal fluid (60).
DYN is an important regulator of the pulse generator system. In sheep, KOR antagonists stimulate the episodic secretion of LH during the luteal phase (61). In ovariectomized goats, central administration of DYN decreases and KOR antagonist increases the frequencies of the multiunit activity volleys and of the LH secretory pulses (8). Opioid peptides also regulate negatively the pulsatile release of prolactin and LH in humans; this inhibitory tone can be suspended by the blockade of opioid receptors with naloxone (62, 63).
The concept and terminology of the KNDy neurons rely on the similar results of colocalization experiments from several animal species. DYN has been detected in NKB (and/or KP) neurons in the ARC of sheep (5, 41), mice (3, 4), rats (14, 23), and goats (8). Moreover, the DYN receptor KOR is present in subsets of KNDy neurons in the ARC of mice (3, 4). Our present immunohistochemical study to address the presence of DYN immunoreactivity in human KP neurons was also informed by previous reports in which preprodynorphin mRNA expression was detected in the human Inf (64) and the monkey ARC (65).
The somewhat unexpected absence of DYN immunoreactivity in most KP-IR somata and fibers of young male humans questions the universal importance of DYN peptides within NKB and KP neurons of the Inf and reveals an important difference from the rodent, sheep, and goat species (1–5, 8, 23). It is worth noting that species also vary considerably regarding the sex steroid regulation of DYN in the ARC/Inf. Preprodynorphin expressing neurons showed reduced numbers in postmenopausal women (64) and in ovariectomized ewes (60). In contrast, there was no change in mRNA expression in postmenopausal monkeys (65) and preprodynorphin mRNA was increased in the absence of sex steroids in mice (3).
The absence of DYN immunoreactivity from most KP-IR neurons and their fibers we report in this study is unlikely to be entirely caused by the limited sensitivity of the applied immunohistochemical method because of the following: 1) DYN-IR cell bodies (e.g. magnocellular perikarya in the supraoptic nucleus) and fibers (e.g. a dense fiber plexus in the ventromedial nucleus) were readily detectable elsewhere in the hypothalamus, 2) substantial colocalization with KP was also undetectable using the highly sensitive tyramide signal amplification method to visualize DYN, or 3) using an antiserum against a different prodynorphin cleavage product, dynorphin B.
Summary of neuroanatomical findings
First, the regional density of NKB-IR cell bodies, fibers, and contacts onto GnRH neurons exceed about 5-fold those of KP-IR neuronal elements in the Inf.
Second, in addition to NKB-IR cell bodies and processes (in both the Inf and the InfS) that are devoid of KP labeling, KP-IR elements lacking NKB immunoreactivity are also highly abundant, as established in dual-immunofluorescent studies. In this study only 32.9 ± 4.7% of the NKB-IR and 75.2 ± 6.6% of the KP-IR perikarya were dual labeled.
Third, DYN-IR cell bodies and fibers occur much less frequently than either NKB-IR or KP-IR elements; KP-IR axons in the Inf and the InfS contain DYN immunoreactivity only occasionally.
In conclusion, the immunohistochemical observations we made on hypothalamic tissue samples of young male human subjects question the universal validity of the KNDy neuron concept and terminology and suggest that the abundance of these peptides and their overlap are species, sex, and age dependent.
Acknowledgments
We thank Mr. László Barna (the Nikon Microscopy Center at Institute of Experimental Medicine), Nikon Austria GmbH, and Auro-Science Consulting Ltd. for kindly providing microscopy support. We also thank Ms. Hajni Bekó for expert technical assistance.
The research leading to these results has received funding from the National Science Foundation of Hungary (Grants OTKA K83710, K100722) and the European Community's Seventh Framework Program (FP7/2007–2013) under Grant 245009. W.S.D. is supported by a National Institute of Health Research Career Development Fellowship.
Disclosure Summary: All of the authors have nothing to disclose.
Footnotes
- ARC
- Arcuate nucleus
- DYN
- dynorphin A
- FITC
- fluorescein isothiocyanate
- Inf
- infundibular nucleus
- InfS
- infundibular stalk
- IR
- immunoreactive
- KISS1R
- KP receptor
- KNDy
- KP/NKB/DYN A
- KOR
- DYN receptor
- KP
- kisspeptin
- NK3
- NKB receptor
- NKB
- neurokinin B.
References
- 1. Cheng G, Coolen LM, Padmanabhan V, Goodman RL, Lehman MN. 2010. The kisspeptin/neurokinin B/dynorphin (KNDy) cell population of the arcuate nucleus: sex differences and effects of prenatal testosterone in sheep. Endocrinology 151:301–311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lehman MN, Coolen LM, Goodman RL. 2010. Minireview: kisspeptin/neurokinin B/dynorphin (KNDy) cells of the arcuate nucleus: a central node in the control of gonadotropin-releasing hormone secretion. Endocrinology 151:3479–3489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Navarro VM, Gottsch ML, Chavkin C, Okamura H, Clifton DK, Steiner RA. 2009. Regulation of gonadotropin-releasing hormone secretion by kisspeptin/dynorphin/neurokinin B neurons in the arcuate nucleus of the mouse. J Neurosci 29:11859–11866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Navarro VM, Gottsch ML, Wu M, García-Galiano D, Hobbs SJ, Bosch MA, Pinilla L, Clifton DK, Dearth A, Ronnekleiv OK, Braun RE, Palmiter RD, Tena-Sempere M, Alreja M, Steiner RA. 2011. Regulation of NKB pathways and their roles in the control of Kiss1 neurons in the arcuate nucleus of the male mouse. Endocrinology 152:4265–4275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Goodman RL, Lehman MN, Smith JT, Coolen LM, de Oliveira CV, Jafarzadehshirazi MR, Pereira A, Iqbal J, Caraty A, Ciofi P, Clarke IJ. 2007. Kisspeptin neurons in the arcuate nucleus of the ewe express both dynorphin A and neurokinin B. Endocrinology 148:5752–5760 [DOI] [PubMed] [Google Scholar]
- 6. Smith JT, Dungan HM, Stoll EA, Gottsch ML, Braun RE, Eacker SM, Clifton DK, Steiner RA. 2005. Differential regulation of KiSS-1 mRNA expression by sex steroids in the brain of the male mouse. Endocrinology 146:2976–2984 [DOI] [PubMed] [Google Scholar]
- 7. Smith JT, Clay CM, Caraty A, Clarke IJ. 2007. KiSS-1 messenger ribonucleic acid expression in the hypothalamus of the ewe is regulated by sex steroids and season. Endocrinology 148:1150–1157 [DOI] [PubMed] [Google Scholar]
- 8. Wakabayashi Y, Nakada T, Murata K, Ohkura S, Mogi K, Navarro VM, Clifton DK, Mori Y, Tsukamura H, Maeda K, Steiner RA, Okamura H. 2010. Neurokinin B and dynorphin A in kisspeptin neurons of the arcuate nucleus participate in generation of periodic oscillation of neural activity driving pulsatile gonadotropin-releasing hormone secretion in the goat. J Neurosci 30:3124–3132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gottsch ML, Popa SM, Lawhorn JK, Qiu J, Tonsfeldt KJ, Bosch MA, Kelly MJ, Rønnekleiv OK, Sanz E, McKnight GS, Clifton DK, Palmiter RD, Steiner RA. 2011. Molecular properties of Kiss1 neurons in the arcuate nucleus of the mouse. Endocrinology 152:4298–4309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Irwig MS, Fraley GS, Smith JT, Acohido BV, Popa SM, Cunningham MJ, Gottsch ML, Clifton DK, Steiner RA. 2004. Kisspeptin activation of gonadotropin releasing hormone neurons and regulation of KiSS-1 mRNA in the male rat. Neuroendocrinology 80:264–272 [DOI] [PubMed] [Google Scholar]
- 11. Han SK, Gottsch ML, Lee KJ, Popa SM, Smith JT, Jakawich SK, Clifton DK, Steiner RA, Herbison AE. 2005. Activation of gonadotropin-releasing hormone neurons by kisspeptin as a neuroendocrine switch for the onset of puberty. J Neurosci 25:11349–11356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Messager S, Chatzidaki EE, Ma D, Hendrick AG, Zahn D, Dixon J, Thresher RR, Malinge I, Lomet D, Carlton MB, Colledge WH, Caraty A, Aparicio SA. 2005. Kisspeptin directly stimulates gonadotropin-releasing hormone release via G protein-coupled receptor 54. Proc Natl Acad Sci USA 102:1761–1766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Keen KL, Wegner FH, Bloom SR, Ghatei MA, Terasawa E. 2008. An increase in kisspeptin-54 release occurs with the pubertal increase in luteinizing hormone-releasing hormone-1 release in the stalk-median eminence of female rhesus monkeys in vivo. Endocrinology 149:4151–4157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Burke MC, Letts PA, Krajewski SJ, Rance NE. 2006. Coexpression of dynorphin and neurokinin B immunoreactivity in the rat hypothalamus: morphologic evidence of interrelated function within the arcuate nucleus. J Comp Neurol 498:712–726 [DOI] [PubMed] [Google Scholar]
- 15. Kalló I, Vida B, Deli L, Molnár CS, Hrabovszky E, Caraty A, Ciofi P, Coen CW, Liposits Z. 2012. Co-localisation of kisspeptin with galanin or neurokinin B in afferents to mouse GnRH neurones. J Neuroendocrinol 24:464–476 [DOI] [PubMed] [Google Scholar]
- 16. Ramaswamy S, Seminara SB, Ali B, Ciofi P, Amin NA, Plant TM. 2010. Neurokinin B stimulates GnRH release in the male monkey (Macaca mulatta) and is colocalized with kisspeptin in the arcuate nucleus. Endocrinology 151:4494–4503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hrabovszky E, Ciofi P, Vida B, Horvath MC, Keller E, Caraty A, Bloom SR, Ghatei MA, Dhillo WS, Liposits Z, Kallo I. 2010. The kisspeptin system of the human hypothalamus: sexual dimorphism and relationship with gonadotropin-releasing hormone and neurokinin B neurons. Eur J Neurosci 31:1984–1998 [DOI] [PubMed] [Google Scholar]
- 18. Hrabovszky E, Molnar CS, Sipos M, Vida B, Ciofi P, Borsay BA, Sarkadi L, Herczeg L, Bloom SR, Ghatei MA, Dhillo WS, Kallo I, Liposits Z. 2011. Sexual dimorphism of kisspeptin and neurokinin B immunoreactive neurons in the infundibular nucleus of aged men and women. Front Endocrinol 2:80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hrabovszky E, Kalló I, Szlávik N, Keller E, Merchenthaler I, Liposits Z. 2007. Gonadotropin-releasing hormone neurons express estrogen receptor-β. J Clin Endocrinol Metab 92:2827–2830 [DOI] [PubMed] [Google Scholar]
- 20. Dhillo WS, Chaudhri OB, Patterson M, Thompson EL, Murphy KG, Badman MK, McGowan BM, Amber V, Patel S, Ghatei MA, Bloom SR. 2005. Kisspeptin-54 stimulates the hypothalamic-pituitary gonadal axis in human males. J Clin Endocrinol Metab 90:6609–6615 [DOI] [PubMed] [Google Scholar]
- 21. Ramaswamy S, Guerriero KA, Gibbs RB, Plant TM. 2008. Structural interactions between kisspeptin and GnRH neurons in the mediobasal hypothalamus of the male rhesus monkey (Macaca mulatta) as revealed by double immunofluorescence and confocal microscopy. Endocrinology 149:4387–4395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Liposits Z, Setalo G, Flerko B. 1984. Application of the silver-gold intensified 3,3′-diaminobenzidine chromogen to the light and electron microscopic detection of the luteinizing hormone-releasing hormone system of the rat brain. Neuroscience 13:513–525 [DOI] [PubMed] [Google Scholar]
- 23. Ciofi P, Leroy D, Tramu G. 2006. Sexual dimorphism in the organization of the rat hypothalamic infundibular area. Neuroscience 141:1731–1745 [DOI] [PubMed] [Google Scholar]
- 24. Hopman AH, Ramaekers FC, Speel EJ. 1998. Rapid synthesis of biotin-, digoxigenin-, trinitrophenyl-, and fluorochrome-labeled tyramides and their application for In situ hybridization using CARD amplification. J Histochem Cytochem 46:771–777 [DOI] [PubMed] [Google Scholar]
- 25. Duvernoy H, Koritké JG, Monnier G. 1971. [Vascularization of the posterior tuber in man and its relation to the tuber-hypophyseal vasculature]. J Neurovisc Relat 32:112–142 [DOI] [PubMed] [Google Scholar]
- 26. Turi GF, Liposits Z, Moenter SM, Fekete C, Hrabovszky E. 2003. Origin of neuropeptide Y-containing afferents to gonadotropin-releasing hormone neurons in male mice. Endocrinology 144:4967–4974 [DOI] [PubMed] [Google Scholar]
- 27. Hrabovszky E, Molnár CS, Nagy R, Vida B, Borsay BÁ, Rácz K, Herczeg L, Watanabe M, Kalló I, Liposits Z. 2012. Glutamatergic and GABAergic innervation of human gonadotropin-releasing hormone-I neurons. Endocrinology 153:2766–2776 [DOI] [PubMed] [Google Scholar]
- 28. Plant TM. 2012. A comparison of the neuroendocrine mechanisms underlying the initiation of the preovulatory LH surge in the human, Old World monkey and rodent. Front Neuroendocrinol 33:160–168 [DOI] [PubMed] [Google Scholar]
- 29. Goubillon ML, Forsdike RA, Robinson JE, Ciofi P, Caraty A, Herbison AE. 2000. Identification of neurokinin B-expressing neurons as an highly estrogen-receptive, sexually dimorphic cell group in the ovine arcuate nucleus. Endocrinology 141:4218–4225 [DOI] [PubMed] [Google Scholar]
- 30. Topaloglu AK, Reimann F, Guclu M, Yalin AS, Kotan LD, Porter KM, Serin A, Mungan NO, Cook JR, Ozbek MN, Imamoglu S, Akalin NS, Yuksel B, O'Rahilly S, Semple RK. 2009. TAC3 and TACR3 mutations in familial hypogonadotropic hypogonadism reveal a key role for neurokinin B in the central control of reproduction. Nat Genet 41:354–358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Guran T, Tolhurst G, Bereket A, Rocha N, Porter K, Turan S, Gribble FM, Kotan LD, Akcay T, Atay Z, Canan H, Serin A, O'Rahilly S, Reimann F, Semple RK, Topaloglu AK. 2009. Hypogonadotropic hypogonadism due to a novel missense mutation in the first extracellular loop of the neurokinin B receptor. J Clin Endocrinol Metab 94:3633–3639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kung TT, Crawley Y, Jones H, Luo B, Gilchrest H, Greenfeder S, Anthes JC, Lira S, Wiekowski M, Cook DN, Hey JA, Egan RW, Chapman RW. 2004. Tachykinin NK3-receptor deficiency does not inhibit pulmonary eosinophilia in allergic mice. Pharmacol Res 50:611–615 [DOI] [PubMed] [Google Scholar]
- 33. Yang JJ, Caligioni CS, Chan YM, Seminara SB. 2012. Uncovering novel reproductive defects in neurokinin B receptor null mice: closing the gap between mice and men. Endocrinology 153:1498–1508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Corander MP, Challis BG, Thompson EL, Jovanovic Z, Loraine Tung YC, Rimmington D, Huhtaniemi IT, Murphy KG, Topaloglu AK, Yeo GS, O'Rahilly S, Dhillo WS, Semple RK, Coll AP. 2010. The effects of neurokinin B upon gonadotrophin release in male rodents. J Neuroendocrinol 22:181–187 [DOI] [PubMed] [Google Scholar]
- 35. Sandoval-Guzmán T, Rance NE. 2004. Central injection of senktide, an NK3 receptor agonist, or neuropeptide Y inhibits LH secretion and induces different patterns of Fos expression in the rat hypothalamus. Brain Res 1026:307–312 [DOI] [PubMed] [Google Scholar]
- 36. Kinsey-Jones JS, Grachev P, Li XF, Lin YS, Milligan SR, Lightman SL, O'Byrne KT. 2012. The inhibitory effects of neurokinin B on GnRH pulse generator frequency in the female rat. Endocrinology 153:307–315 [DOI] [PubMed] [Google Scholar]
- 37. Navarro VM, Castellano JM, McConkey SM, Pineda R, Ruiz-Pino F, Pinilla L, Clifton DK, Tena-Sempere M, Steiner RA. 2011. Interactions between kisspeptin and neurokinin B in the control of GnRH secretion in the female rat. Am J Physiol Endocrinol Metab 300:E202–E210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Billings HJ, Connors JM, Altman SN, Hileman SM, Holaskova I, Lehman MN, McManus CJ, Nestor CC, Jacobs BH, Goodman RL. 2010. Neurokinin B acts via the neurokinin-3 receptor in the retrochiasmatic area to stimulate luteinizing hormone secretion in sheep. Endocrinology 151:3836–3846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. McGarvey C, Cates PA, Brooks A, Swanson IA, Milligan SR, Coen CW, O'Byrne KT. 2001. Phytoestrogens and gonadotropin-releasing hormone pulse generator activity and pituitary luteinizing hormone release in the rat. Endocrinology 142:1202–1208 [DOI] [PubMed] [Google Scholar]
- 40. O'Byrne KT, Knobil E. 1993. Electrophysiological approaches to gonadotrophin releasing hormone pulse generator activity in the rhesus monkey. Hum Reprod 8(Suppl 2):37–40 [DOI] [PubMed] [Google Scholar]
- 41. Foradori CD, Amstalden M, Goodman RL, Lehman MN. 2006. Colocalisation of dynorphin a and neurokinin B immunoreactivity in the arcuate nucleus and median eminence of the sheep. J Neuroendocrinol 18:534–541 [DOI] [PubMed] [Google Scholar]
- 42. Amstalden M, Coolen LM, Hemmerle AM, Billings HJ, Connors JM, Goodman RL, Lehman MN. 2010. Neurokinin 3 receptor immunoreactivity in the septal region, preoptic area and hypothalamus of the female sheep: colocalisation in neurokinin B cells of the arcuate nucleus but not in gonadotrophin-releasing hormone neurones. J Neuroendocrinol 22:1–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Todman MG, Han SK, Herbison AE. 2005. Profiling neurotransmitter receptor expression in mouse gonadotropin-releasing hormone neurons using green fluorescent protein-promoter transgenics and microarrays. Neuroscience 132:703–712 [DOI] [PubMed] [Google Scholar]
- 44. Krajewski SJ, Anderson MJ, Iles-Shih L, Chen KJ, Urbanski HF, Rance NE. 2005. Morphologic evidence that neurokinin B modulates gonadotropin-releasing hormone secretion via neurokinin 3 receptors in the rat median eminence. J Comp Neurol 489:372–386 [DOI] [PubMed] [Google Scholar]
- 45. García-Galiano D, van Ingen Schenau D, Leon S, Krajnc-Franken MA, Manfredi-Lozano M, Romero-Ruiz A, Navarro VM, Gaytan F, van Noort PI, Pinilla L, Blomenröhr M, Tena-Sempere M. 2012. Kisspeptin signaling is indispensable for neurokinin B, but not glutamate, stimulation of gonadotropin secretion in mice. Endocrinology 153:316–328 [DOI] [PubMed] [Google Scholar]
- 46. Henriksen JS, Saermark T, Vilhardt H, Mau SE. 1995. Tachykinins induce secretion of prolactin from perifused rat anterior pituitary cells by interactions with two different binding sites. J Recept Signal Transduct Res 15:529–541 [DOI] [PubMed] [Google Scholar]
- 47. de Roux N, Genin E, Carel JC, Matsuda F, Chaussain JL, Milgrom E. 2003. Hypogonadotropic hypogonadism due to loss of function of the KiSS1-derived peptide receptor GPR54. Proc Natl Acad Sci USA 100:10972–10976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Seminara SB, Messager S, Chatzidaki EE, Thresher RR, Acierno JS, Jr, Shagoury JK, Bo-Abbas Y, Kuohung W, Schwinof KM, Hendrick AG, Zahn D, Dixon J, Kaiser UB, Slaugenhaupt SA, Gusella JF, O'Rahilly S, Carlton MB, Crowley WF, Jr, Aparicio SA, Colledge WH. 2003. The GPR54 gene as a regulator of puberty. N Engl J Med 349:1614–1627 [DOI] [PubMed] [Google Scholar]
- 49. Semple RK, Achermann JC, Ellery J, Farooqi IS, Karet FE, Stanhope RG, O'Rahilly S, Aparicio SA. 2005. Two novel missense mutations in g protein-coupled receptor 54 in a patient with hypogonadotropic hypogonadism. J Clin Endocrinol Metab 90:1849–1855 [DOI] [PubMed] [Google Scholar]
- 50. Topaloglu AK, Tello JA, Kotan LD, Ozbek MN, Yilmaz MB, Erdogan S, Gurbuz F, Temiz F, Millar RP, Yuksel B. 2012. Inactivating KISS1 mutation and hypogonadotropic hypogonadism. N Engl J Med 366:629–635 [DOI] [PubMed] [Google Scholar]
- 51. Dumalska I, Wu M, Morozova E, Liu R, van den Pol A, Alreja M. 2008. Excitatory effects of the puberty-initiating peptide kisspeptin and group I metabotropic glutamate receptor agonists differentiate two distinct subpopulations of gonadotropin-releasing hormone neurons. J Neurosci 28:8003–8013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Pielecka-Fortuna J, Chu Z, Moenter SM. 2008. Kisspeptin acts directly and indirectly to increase gonadotropin-releasing hormone neuron activity and its effects are modulated by estradiol. Endocrinology 149:1979–1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. George JT, Veldhuis JD, Roseweir AK, Newton CL, Faccenda E, Millar RP, Anderson RA. 2011. Kisspeptin-10 is a potent stimulator of LH and increases pulse frequency in men. J Clin Endocrinol Metab 96:E1228–E1236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Chan YM, Butler JP, Pinnell NE, Pralong FP, Crowley WF, Jr, Ren C, Chan KK, Seminara SB. 2011. Kisspeptin resets the hypothalamic GnRH clock in men. J Clin Endocrinol Metab 96:E908–E915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Dhillo WS, Chaudhri OB, Thompson EL, Murphy KG, Patterson M, Ramachandran R, Nijher GK, Amber V, Kokkinos A, Donaldson M, Ghatei MA, Bloom SR. 2007. Kisspeptin-54 stimulates gonadotropin release most potently during the preovulatory phase of the menstrual cycle in women. J Clin Endocrinol Metab 92:3958–3966 [DOI] [PubMed] [Google Scholar]
- 56. Chan YM, Butler JP, Sidhoum VF, Pinnell NE, Seminara SB. 10 May 2012. Kisspeptin administration to women: a window into endogenous kisspeptin secretion and GnRH responsiveness across the menstrual cycle. J Clin Endocrinol Metab 10.1210/jc.2012-1282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. d'Anglemont de Tassigny X, Fagg LA, Carlton MB, Colledge WH. 2008. Kisspeptin can stimulate gonadotropin-releasing hormone (GnRH) release by a direct action at GnRH nerve terminals. Endocrinology 149:3926–3932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Sannella MI, Petersen SL. 1997. Dual label in situ hybridization studies provide evidence that luteinizing hormone-releasing hormone neurons do not synthesize messenger ribonucleic acid for μ, κ, or Δ opiate receptors. Endocrinology 138:1667–1672 [DOI] [PubMed] [Google Scholar]
- 59. Foradori CD, Coolen LM, Fitzgerald ME, Skinner DC, Goodman RL, Lehman MN. 2002. Colocalization of progesterone receptors in parvicellular dynorphin neurons of the ovine preoptic area and hypothalamus. Endocrinology 143:4366–4374 [DOI] [PubMed] [Google Scholar]
- 60. Foradori CD, Goodman RL, Adams VL, Valent M, Lehman MN. 2005. Progesterone increases dynorphin a concentrations in cerebrospinal fluid and preprodynorphin messenger ribonucleic acid levels in a subset of dynorphin neurons in the sheep. Endocrinology 146:1835–1842 [DOI] [PubMed] [Google Scholar]
- 61. Goodman RL, Coolen LM, Anderson GM, Hardy SL, Valent M, Connors JM, Fitzgerald ME, Lehman MN. 2004. Evidence that dynorphin plays a major role in mediating progesterone negative feedback on gonadotropin-releasing hormone neurons in sheep. Endocrinology 145:2959–2967 [DOI] [PubMed] [Google Scholar]
- 62. Yen SS, Quigley ME, Reid RL, Ropert JF, Cetel NS. 1985. Neuroendocrinology of opioid peptides and their role in the control of gonadotropin and prolactin secretion. Am J Obstet Gynecol 152:485–493 [DOI] [PubMed] [Google Scholar]
- 63. Snowden EU, Khan-Dawood FS, Dawood MY. 1986. Opioid regulation of pituitary gonadotropins and prolactin in women using oral contraceptives. Am J Obstet Gynecol 154:440–444 [DOI] [PubMed] [Google Scholar]
- 64. Rometo AM, Rance NE. 2008. Changes in prodynorphin gene expression and neuronal morphology in the hypothalamus of postmenopausal women. J Neuroendocrinol 20:1376–1381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Eghlidi DH, Haley GE, Noriega NC, Kohama SG, Urbanski HF. 2010. Influence of age and 17β-estradiol on kisspeptin, neurokinin B, and prodynorphin gene expression in the arcuate-median eminence of female rhesus macaques. Endocrinology 151:3783–3794 [DOI] [PMC free article] [PubMed] [Google Scholar]