Abstract
Dysregulated stress responsivity is a hallmark of neuropsychiatric disease. The regulation of stress activation and recovery involves tight coordination between neuronal and glial networks. At a certain threshold of sensitivity, stress exposure can evoke a neuroimmune response. Astrocytes are potential mediators of these effects because they are able to respond to neuroimmune effector molecules and regulate neuronal activity. Mice deficient in corticotropin-releasing factor receptor-2 display increased stress sensitivity and are therefore a useful model in which to examine the intersection of neuroimmune activation and stress pathway dysregulation. We hypothesized that a component of elevated stress reactivity may involve an engagement of neuroimmune effectors, including astrocytes. Therefore, we hypothesized that this phenotype may be rescued by concomitant nonsteroidal antiinflammatory drug (NSAID) treatment. To examine this, mice exposed to chronic stress were treated with NSAID in their drinking water, and changes in hypothalamic-pituitary-adrenal stress axis function were examined. As a correlate of altered astrocyte function, levels of glial fibrillary acidic protein were measured. Supportive of our hypothesis, NSAID treatment rescued the hypothalamic-pituitary-adrenal stress axis dysfunction in stress-sensitive corticotropin-releasing factor receptor-2−/− mice and also reversed the stress-induced increase in glial fibrillary acidic protein in stress-regulating brain regions including the paraventricular nucleus of the hypothalamus, ventral hippocampus, and prefrontal cortex. These findings support the local involvement of astrocytes in the exacerbation of stress pathway dysregulation. The specificity of these effects in a stress-sensitive genotype highlights the importance of utilizing a model of stress dysregulation in the examination of factors that may translate to neuropsychiatric disease.
Stress pathway dysregulation is one of the most pervasive symptoms in neuropsychiatric disease. Patients with stress-related affective disorders, such as anxiety, major depressive disorder, and posttraumatic stress disorder, often present with altered basal stress hormones, inappropriate feedback after stress exposure, and a failure to produce adaptive stress coping responses (1–3). Thus, the ability to appropriately respond and adapt to stress at the physiological, molecular, and cellular levels are necessary to prevent dysfunction and disease. Although complex regulatory mechanisms likely contribute to the development of neuropsychiatric disease, increasing evidence implicates inflammatory processes in their pathophysiology (4–6). Within the central nervous system, astrocytes function as immune effector cells capable of producing and responding to proinflammatory cytokines and are intricately involved in the integration of signals within neuronal networks (7–13). However, how such inflammatory processes intersect with stress reactivity is unknown.
Stress dysregulation and elevated neuroimmune activation commonly copresent in psychiatric patient populations, including major depressive disorder and posttraumatic stress disorder (14, 15). However, animal models relevant to neuropsychiatric disease rarely consider this dual phenotype. In healthy individuals, mild stress exposures do not typically produce neuropsychiatric disease symptoms, nor in healthy wild-type (WT) mice does mild stress result in substantial neuroimmune activation (16). In susceptible individuals, however, stressful life events can both precipitate disease onset and exacerbate symptoms (17). Thus, a copresentation of stress dysregulation and neuroimmune activation may be present only in susceptible individuals. Mice deficient in corticotropin-releasing factor receptor-2 (CRF2−/−) are hypersensitive to stress exposure, displaying augmented hypothalamic-pituitary-adrenal (HPA) stress axis corticosterone levels, increased anxiety-like behavior, and reduced ability to mount appropriate coping responses to stress exposure (18–20). These stress-sensitive mice are therefore a useful susceptible population in which to examine the intersection of neuroimmune activation and stress pathway dysregulation.
Therefore, we hypothesized that stress dysregulation, a significant factor in disease susceptibility, involves activation of neuroimmune factors in stress modulating brain regions. In addition, the ability to detect such changes may require an appropriate stress-sensitive animal model in which stress engages a neuroimmune response involving local astrocytes. The current studies examined changes in the astrocyte cytoskeletal protein, glial fibrillary acidic protein (GFAP), associated with exposure to chronic stress in brain regions central to the regulation of stress responsivity: the paraventricular nucleus of the hypothalamus (PVN), hippocampus, and medial prefrontal cortex (21–23). To determine whether genotypic differences in stress responsivity were related to differences in inflammatory processes, concomitant nonsteroidal antiinflammatory drug (NSAID) treatment was assessed for its ability to ameliorate stress dysregulation, as evidenced by the HPA stress axis production of corticosterone and parallel changes in astrocyte GFAP immunoreactivity in key stress-regulatory brain regions.
Materials and Methods
Animals
CRF2−/− mice were generated in-house on a mixed C57BL/6:129J background as described (18). Male WT and CRF2−/− littermates (14–17 wk) bred from heterozygous crosses were group housed under a 12-h light, 12-h dark cycle (lights on at 0600 h), with food and water available ad libitum. WT and CRF2−/− mice were randomly assigned to control (CTL; n = 7–9 per genotype) or chronic variable stress (CVS; n = 8–9 per genotype) treatment groups. To determine whether inflammation may be involved in the stress dysregulation found after exposure to chronic stress, an additional group received treatment with NSAID concomitant with CVS exposure (CVS + NSAID; n = 9 per genotype). All studies were performed in accordance with experimental protocols approved by the University of Pennsylvania Institutional Animal Care and Use Committee.
Chronic variable stress
Chronic variable stress was comprised of seven mild stressors presented in a randomized order for 4 wk beginning at 14–17 wk of age. Stressors were specifically selected to not directly perturb thermogenic, metabolic, or pain pathways, as described (20). Stressors included overnight wet cage bedding (damp but without standing water), 15 min acute restraint, cage change (three times in a single day), overnight exposure to novel objects (eight glass marbles) in cages, overnight novel noise using a white noise generator (Brookstone, Merrimack, NH), 15 min predator odor exposure (fox odor; Acros Organics, Fair Lawn, NJ; diluted 1:10,000 in mineral oil), and 36 h constant light exposure. The HPA stress axis assessment was administered to all mice and replaced the final stressor. Mice were killed 48 h after the HPA axis assessment, and brains were processed for immunofluorescence analysis of GFAP.
NSAID treatment
Beginning 1 wk before the onset of chronic variable stress and throughout the course of the study, CVS + NSAID WT and CRF2−/− mice received acetylsalicylic acid (CVS Caremark Corporation, Woonsocket, RI) at 162 mg/liter in their drinking water (available ad libitum), resulting in a dosage of approximately 480 μg/mouse·d (24). Acetylsalicylic acid has been shown to remain stable in water, by fluorescent polarization assay, for up to 48 h. Therefore, water (with or without acetylsalicylic acid) was replaced every 48 h (25).
HPA axis assessment
To examine the effects of chronic variable stress exposure and NSAID treatment on the HPA axis stress response, plasma corticosterone levels were determined following a 15-min restraint stress in WT and CRF2−/− mice (n = 7–9 per genotype/treatment group). Testing was administered between 0800 and 1100 h on the final day of CVS by placing mice in a 50-ml conical tube containing a 50-mm air hole. Tail blood samples (10 μl) were taken at onset and completion of restraint (0 and 15 min, respectively). To examine the stress recovery phase, additional samples were collected at 15 and 75 min after the culmination of the restraint (30 and 90 min, respectively). Samples were collected into 5 μl of 50 mm EDTA, centrifuged, and stored at − 80 C until analysis. Plasma corticosterone concentration was measured in duplicate using a 125I-corticosterone RIA kit (MP Biomedicals, Orangeburg, NY). The minimum detection limit of the assay was 7.7 ng/ml, and the intraassay coefficient of variation was 7.3%.
Immunofluorescence and analyses
Whole brains were removed from WT and CRF2−/− mice (n = 4) and frozen on dry ice 48 h after the HPA axis assessment test. Brains were stored at −80 C until cryostat sectioning. Frozen tissue was cut on a cryostat into 10-μm coronal sections containing the medial prefrontal cortex (bregma 1.78 to 1.42 mm), paraventricular nucleus of the hypothalamus (bregma −0.58 to −0.94 mm), dorsal hippocampus (bregma −1.46 to −1.70 mm), and ventral hippocampus (bregma −2.80 to −3.16 mm). Every third section was thaw mounted, air dried on charged slides (Colorfrost Plus; Fisher Scientific, Fair Lawn, NJ), and stored at −80 C. Sections were identified and anatomically matched using the Paxinos and Franklin mouse atlas (26). Immunofluorescence for the astrocyte-specific cytoskeletal protein, GFAP, was performed on three sections per animal per brain region. Slide-mounted fresh frozen sections were fixed for 10 min in ice cold acetone, washed three times in PBS, and then incubated in 3% normal goat serum and 0.25% Triton X-100 in PBS (NGS-PBST) to block and permeabilize, respectively. Sections were incubated overnight in rat anti-GFAP polyclonal antibody (1:250; Invitrogen, Carlsbad, CA; catalog no. 130300) in NGS-PBST. Sections were then washed and incubated for 1 h in goat antirat Alexa 568 fluorescent secondary antibody (1:500; Rockland Immunochemicals, Gilbertsville, PA; catalog no. A-11077) in NGS-PBST. Slides were mounted with ProLong gold antifade reagent containing 4′,6′-diamino-2-phenylindole (DAPI) to stain nuclei (Molecular Probes/Invitrogen, Carlsbad, CA) and then cured at room temperature overnight before image acquisition. Control sections were processed in parallel omitting either the primary or secondary antibodies.
Regions of interest were captured using a 10-bit cooled QICam digital camera (QImaging, Surrey, Canada) affixed to a Nikon Eclipse fluorescent microscope (Tokyo, Japan) at ×20 magnification (medial prefrontal cortex, hippocampus), or ×10 (paraventricular nucleus of the hypothalamus). Slides from each brain region were captured at a uniform exposure time; however, baseline differences in GFAP between brain regions necessitated different exposure times for different brain regions to remain within linear dynamic range for semiquantification. Semiquantitative fluorescence measurements were made within a defined region of interest to yield a mean intensity value, using IPLabs for Macintosh software (BD Biosciences/Scanalytics, Fairfax, VA).
Serum TNFα
Trunk blood was collected, mixed with 15 μl of 50 mm EDTA and centrifuged at 5000 rpm for 15 min. Plasma was collected and frozen at −80 C. Plasma TNFα levels were quantified by sandwich enzyme linked immunosorbent assay (R&D Systems, Minneapolis, MN) according to the manufacturer's instructions. The minimum detection limit of the assay was 7.21 pg/ml, and the intraassay coefficient of variation was 3.8%.
Statistical analyses
Corticosterone RIA
Corticosterone data were analyzed using a repeated-measures multivariate analysis of variance (genotype × treatment × time) with time as a within-subjects repeated measure. To assess the magnitude of the stress response, the area under the curve (AUC) was calculated separately for the stress response (0–30 min) and the recovery (30–90 min) phases. This approach yielded an integration of peak response with duration of stressor. A two-way ANOVA was performed on the AUC of the response phase and of the recovery phase. Main effects and interactions were further explored with Tukey honestly significantly difference post hoc test. Differences were identified at P < 0.05. Statistical analyses were performed with R software (R Foundation for Statistical Computing, Vienna, Austria). Data are reported as mean ± sem.
Immunofluorescence
In each brain region, the intensity of GFAP immunofluorescence within a defined region of interest was measured in three sections per animal. Models that included all possible combinations of predictor variables (genotype, treatment, animal) and interaction terms were fit to mean fluorescence intensity data and the best-fit model was determined according to Akaike's information criterion. The best-fit model across each brain region was a linear mixed model with different intercepts for each mouse. Confidence intervals that did not bound zero were considered to hold a measurable effect. Statistical analyses were performed with R software (R Foundation for Statistical Computing). P values were calculated using the pvals.fnc package (http://CRAN.R-project.org/package=languageR). Data were fit to the mixed models using lme4 package (http://CRAN.R-project.org/package=bbmle). Reported values are based on the parameter estimates of the best-fit model. Error bars represent the observed sd of the respective groups.
Results
HPA axis responses to acute restraint stress
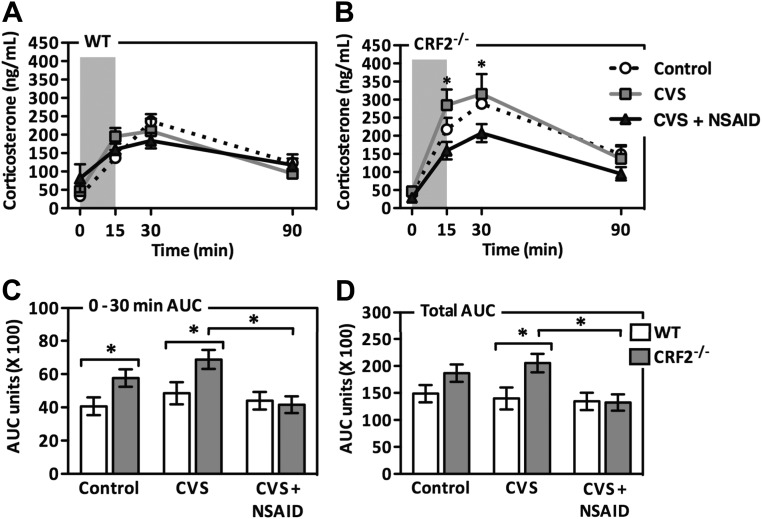
To assess HPA axis stress responsivity, corticosterone levels were measured after a 15-min restraint stress (Fig. 1). A repeated measures multivariate analysis of variance of corticosterone levels in response to an acute restraint stress revealed a main effect of genotype (CRF2−/− > WT) [F(1, 45) = 5.654; P = 0.02]. There was a significant interaction between genotype and treatment [F(2, 45) = 3.273; P = 0.048]. Post hoc analysis revealed this effect to be driven by augmented corticosterone secretion by CVS-treated CRF2−/− mice. NSAID treatment concomitant with CVS exposure prevented the augmented rise in corticosterone secretion. A two-way ANOVA of the AUC of the rise (0–30 min) in corticosterone production revealed a main effect of genotype (CRF2−/− > WT) [F(1,45) = 6.869; P = 0.012] and treatment (CVS > CTL; CVS > CVS + NSAID) [F(2,45) = 4.166; P = 0.023]. Analysis of AUC during the recovery phase (30–90 min) revealed the effects of genotype (CRF2−/− > WT) [F(1,45) = 3.898; P = 0.056] and treatment (CVS > CTL; CVS > CVS + NSAID) [F(2,45) = 2.657; P = 0.083] that did not reach statistical significance. As per our a priori objective, the AUC of corticosterone production during the recovery phase were compared between CVS and CVS + NSAID treatment groups. No significant effect was observed in WT mice; however, NSAID treatment significantly attenuated the augmented corticosterone response of CVS-exposed CRF2−/− mice.
Fig. 1.
Antiinflammatory treatment ameliorates chronic stress-induced corticosterone elevation in stress-sensitive CRF2−/− mice. Corticosterone levels were examined after a 15-min restraint stress (shaded column) in WT (A) and CRF2−/− (B) mice. Exposure to CVS enhanced corticosterone production in CRF2−/− mice, and this effect was ameliorated by NSAID treatment. C, Corticosterone production during the rise phase (0–30 min) was higher in the CRF2−/− mice and was further enhanced during CVS exposure. Concomitant NSAID treatment during CVS rescued this outcome. D, NSAID treatment reversed CVS-induced elevation in the total AUC of corticosterone production in CRF2−/− mice. Data are presented as mean values ± sem (n = 7–9). *, P < 0.05.
GFAP immunofluorescence
Paraventricular nucleus of the hypothalamus
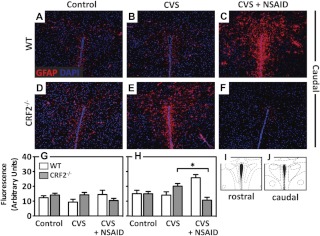
Immunofluorescence of GFAP was performed on sections from the caudal and rostral PVN (Fig. 2). In the caudal PVN, there was a significant interaction [T = −2.185, P = 0.033], whereby NSAID treatment decreased GFAP in CRF2−/− mice and increased GFAP in WT mice. GFAP did not significantly differ between genotype or treatment groups in the rostral PVN.
Fig. 2.
Antiinflammatory treatment prevents chronic stress-induced increases in GFAP in the PVN of stress-sensitive CRF2−/− mice. A–F, Representative immunofluorescence images (×10 magnification) of the astrocyte-specific cytoskeletal protein, GFAP (red), counterstained with DAPI (nuclei, blue) in the caudal PVN of (A–C) WT and (D–F) CRF2−/− mice for control (A and D), after exposure to CVS (B and E), or CVS + NSAID (C and F). Bar graphs illustrate semiquantitative analysis of GFAP immunofluorescence. G, No differences between groups were detected with CVS on GFAP in the rostral PVN. H, In the caudal PVN, NSAID treatment increased GFAP in WT but decreased GFAP in CRF2−/− mice. Atlas images illustrating the brain sections analyzed for rostral (I) and caudal (J) PVN, adapted from the mouse atlas (26). Data are presented as parameter estimates of the best-fit model + observed sd of respective groups (n = 3–4). *, P < 0.05.
Hippocampus
Immunofluorescence of GFAP was performed on sections from the dorsal and ventral CA1, CA3, and dentate gyrus of the hippocampus (Fig. 3). In both the CA1 and CA3 subregions of the ventral hippocampus, there was a significant interaction [T = 2.138–2.16, P = 0.034–0.037], whereby CVS increased GFAP selectively in CRF2−/− mice. The CVS-induced elevation in GFAP protein was prevented with concomitant NSAID treatment. No differences in GFAP were observed in WT animals across any treatment group in the ventral CA1, CA3, or dentate gyrus. GFAP did not significantly differ between genotype or treatment groups in any of the subregions of the dorsal hippocampus.
Fig. 3.
Antiinflammatory treatment prevents chronic stress-induced increases in GFAP in the ventral hippocampus of stress-sensitive CRF2−/− mice. A and B, Brain atlas images illustrating the brain regions analyzed for ventral (A) and dorsal (B) hippocampus, adapted from the mouse atlas (26). Boxes highlight the region corresponding to image acquisition. C–H, Representative immunofluorescence images (×10 magnification) of GFAP (red) counterstained with DAPI (nuclei, blue) in CA1 (C and F), CA3 (D and G), and dentate gyrus (E and H) of the ventral hippocampus of CVS exposed WT and CRF2−/− mice. I–K, Bar graphs illustrate semiquantitative analysis of GFAP immunofluorescence. CVS increased GFAP in both the CA1 (I) and CA3 (J) subregions of the ventral hippocampus selectively in stress-sensitive CRF2−/− mice. K, No differences were observed in the dentate gyrus. L–Q, Representative immunofluorescence images of the CA1 (L, O), CA3 (M and P), and dentate gyrus (N and Q) of the dorsal hippocampus of CVS treated WT and CRF2−/− mice. There were no significant effects of genotype or treatment on GFAP levels in the CA1 (R), CA3 (S), or dentate gyrus (T) subregions of the dorsal hippocampus. Data are presented as parameter estimates of the best-fit model + observed sd of respective groups (n = 3–4). *, P < 0.05.
Medial prefrontal cortex
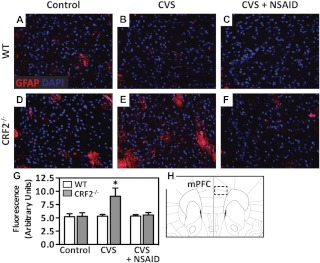
Immunofluorescence of GFAP was performed on sections from the medial prefrontal cortex (Fig. 4). There was a significant effect [T = 2.048, P = 0.045], whereby CVS increased GFAP selectively in CRF2−/− mice. The CVS-induced elevation in GFAP protein was prevented with concomitant NSAID treatment. No differences in GFAP were observed in WT animals across any treatment group.
Fig. 4.
Antiinflammatory treatment prevents chronic stress-induced increases in GFAP in the medial prefrontal cortex of stress-sensitive CRF2−/− mice. A–F, Representative immunofluorescence images (×20 magnification) of GFAP (red) counterstained with DAPI (nuclei, blue) in the medial prefrontal cortex (mPFC) of WT (A–C) and CRF2−/− (D--F) mice for control (A and D), after exposure to CVS (B and E), or CVS + NSAID (C and F). G, Bar graph illustrates semiquantitative analysis of GFAP immunofluorescence showing significantly elevated levels in the mPFC after CVS in CRF2−/− mice. Concomitant treatment with NSAID rescued this effect. H, Atlas image illustrating the brain section used for analysis, adapted from the mouse atlas (26). Data are presented as parameter estimates of the best-fit model + observed sd of respective groups (n = 3–4). *, P < 0.05.
Serum TNFα
All samples were at the minimal level of detection, between 0.056 and 0.128 pg/ml (data not shown). No significant differences in TNFα were found between genotypes [F(1, 38) = 0.02; P = 0.89] or prior exposure to chronic variable stress [F(2, 38) = 0.16; P = 0.85].
Discussion
The aim of the present study was to test the hypothesis that an underlying component of elevated stress responsivity involves an engagement of neuroimmune responses during chronic stress exposure. We propose that the ability to detect such changes requires an appropriately stress-sensitive model. Using CRF2−/− mice as a model of stress dysregulation, we uncovered several novel findings: 1) NSAID treatment ameliorated augmented HPA axis corticosterone production characteristic of CRF2−/− mice exposed to chronic stress; 2) in CRF2−/− mice, GFAP-positive astrocytes increased with chronic stress exposure in stress coordinating brain regions, paralleling changes in HPA axis corticosterone; and 3) NSAID treatment prevented stress-mediated changes in GFAP-positive astrocytes.
HPA stress axis activity is a physiological indicator of organismal stress state; exposure to stress ultimately results in the production of the primary stress hormone, corticosterone (27). Prior exposure to stress can modify HPA axis activity, augmenting or blunting responsivity to novel stressors (28, 29). Although it is necessary to maintain the ability to respond to a novel stressor, overactive HPA axis activity can be detrimental. We previously reported that exposure to chronic stress in CRF2−/− mice augmented HPA stress axis corticosterone levels, evidencing their heightened stress sensitivity (20). In our current study, NSAID treatment during chronic stress completely ameliorated this effect in CRF2−/− mice. These findings suggest an underlying neuroimmune involvement in the dysregulated stress response and may suggest a mechanism by which NSAID treatment may be efficacious in affective disorder treatment (30–33).
Astrocytes are a promising mediator of the neuroimmune consequence of stress exposure. In select environments, they can both produce and respond to neuroimmune effector molecules (reviewed in Ref. 7). Although numerous end-feet leave them elegantly poised to respond to and integrate signals within neuronal networks, perturbations to astrocytes have the potential for widespread impact, including changes in extracellular ionic homeostasis, local neurotransmitter regulation, and remodeling of neural circuits (9–13). Indeed, alterations in astrocyte structural morphology or function have been associated with neuropsychiatric illnesses, including depression and schizophrenia (34–36). Thus, altered astrocyte-mediated plasticity may explain why CRF2−/− mice are unable to mount an adaptive coping response to stress exposure (20). Therefore, we examined we examined GFAP immunoreactivity as a correlate of altered astrocyte function in the PVN, ventral hippocampus, and prefrontal cortex, stress-coordinating brain regions. Consistent with our hypothesis, GFAP immunoreactivity increased with chronic stress in CRF2−/− mice across these three brain regions. These stress-mediated changes were also prevented with concomitant NSAID treatment. Interestingly, GFAP immunoreactivity in the dorsal hippocampus was unaffected by stress exposure. Because astrocytes are widely heterogeneous in structure and function and play distinct roles across different brain regions, these findings point to a specificity of astrocyte changes that may parallel the specifics of dysregulation (37–39).
The PVN is a primary regulator of corticosterone production and is innervated by multiple limbic forebrain structures, including the prefrontal cortex and ventral hippocampus (40). These circuits play important roles in determining stressor intensity and in negative feedback (22, 23). A surprising finding in the PVN was the remarkable increase in GFAP positive astrocytes in the WT mice exposed to chronic stress and concomitant NSAID treatment. Although the explanation for this effect is not currently known, it demonstrates important genotypic differences in astrocyte plasticity following stress and may suggest a unique interaction of stress and neuroimmune activation in the normal healthy brain that may be beneficial in stress coping (16).
Corticotropin-releasing factor receptor-2 is expressed on peripheral immune cells (41, 42). Therefore, to ensure genotype effects detected here were not resultant from inflammatory cytokines produced in the periphery, we measured serum TNFα in these mice. Levels were below the minimal level of assay detection (7.7 pg/ml) for all animals. These findings are consistent with studies using similar models of chronic stress exposure that also failed to evoke a significant peripheral immune response in the absence of additional immune challenge (43, 44). These data do not preclude the possibility of peripheral inflammation at earlier time points or by other cytokines but do suggest that changes in neuroimmune modulation have arisen centrally rather than indirectly through activation of the peripheral immune system.
Neuroimmune effectors have been clearly documented to activate the stress response, and cytokine treatment can induce symptoms of depression (45, 46). Taken together, this suggests a potential role for immune effectors in stress dysregulation and related phenotypes found in neuropsychiatric disease. However, results of several investigations examining the neuroimmune response to stress exposure are conflicting; while in some models stress drives increases in cytokine production, other models show fewer changes (47–51). Our studies indicate that these differences may be driven by the sensitivity of the model used. In support of a neuroimmune contribution to the dysregulated stress state, NSAID treatment prevented dysregulation of HPA stress axis corticosterone secretion in a model of stress sensitivity. Furthermore, chronic stress resulted in the up-regulation of GFAP by astrocytes in the PVN, ventral hippocampus, and prefrontal cortex of CRF2−/− mice. The current studies provide evidence that not only may NSAID treatment be beneficial in the treatment of disorders associated with dysfunctional HPA stress axis but also that it may do so through either direct or indirect modification of astrocytes in brain regions involved in stress regulation. The specificity of these effects to stress-sensitive animals highlights the importance of using a model of stress dysregulation in the examination of factors that may translate to human disease.
Acknowledgments
We thank J. Fluharty for assistance with animal care and genotyping and Dr. C. Howerton for assistance with statistical analysis.
This work was supported by Grant MH073030 from the National Institutes of Health.
Disclosure Summary: The authors of this manuscript have nothing to declare.
Footnotes
- AUC
- Area under the curve
- CRF2−/−
- deficient in corticotropin-releasing factor receptor-2
- CTL
- control
- CVS
- chronic variable stress
- DAPI
- 4′,6′-diamino-2-phenylindole
- GFAP
- glial fibrillary acidic protein
- HPA
- hypothalamic-pituitary-adrenal
- NGS-PBST
- normal goat serum and Triton X-100 in PBS
- NSAID
- nonsteroidal antiinflammatory drug
- PVN
- paraventricular nucleus of the hypothalamus
- WT
- wild type.
References
- 1. Nestler EJ, Barrot M, DiLeone RJ, Eisch AJ, Gold SJ, Monteggia LM. 2002. Neurobiology of depression. Neuron 34:13–25 [DOI] [PubMed] [Google Scholar]
- 2. Breslau N, Davis GC. 1986. Chronic stress and major depression. Arch Gen Psychiatry 43:309–314 [DOI] [PubMed] [Google Scholar]
- 3. Koenig JI, Kirkpatrick B, Lee P. 2002. Glucocorticoid hormones and early brain development in schizophrenia. Neuropsychopharmacology 27:309–318 [DOI] [PubMed] [Google Scholar]
- 4. Dantzer R, O'Connor JC, Lawson MA, Kelley KW. 2011. Inflammation-associated depression: From serotonin to kynurenine. Psychoneuroendocrinology 36:426–436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Dowlati Y, Herrmann N, Swardfager W, Liu H, Sham L, Reim EK, Lanctôt KL. 2010. A meta-analysis of cytokines in major depression. Biol Psychiatry 67:446–457 [DOI] [PubMed] [Google Scholar]
- 6. Strous RD, Shoenfeld Y. 2006. Schizophrenia, autoimmunity and immune system dysregulation: a comprehensive model updated and revisited. J Autoimmun 27:71–80 [DOI] [PubMed] [Google Scholar]
- 7. Volterra A, Meldolesi J. 2005. Astrocytes, from brain glue to communication elements: the revolution continues. Nat Rev Neurosci 6:626–640 [DOI] [PubMed] [Google Scholar]
- 8. Theodosis DT, Poulain DA, Oliet SH. 2008. Activity-dependent structural and functional plasticity of astrocyte-neuron interactions. Physiological Reviews 88:983–1008 [DOI] [PubMed] [Google Scholar]
- 9. Allen NJ, Barres BA. 2005. Signaling between glia and neurons: focus on synaptic plasticity. Curr Opin Neurobiol 15:542–548 [DOI] [PubMed] [Google Scholar]
- 10. Haydon PG, Carmignoto G. 2006. Astrocyte control of synaptic transmission and neurovascular coupling. Physiol Rev 86:1009–1031 [DOI] [PubMed] [Google Scholar]
- 11. Schwarz JM, Bilbo SD. 22 February 2012. Sex, glia, and development: interactions in health and disease. Horm Behav 10.1016/j.yhbeh.2012.02.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Theodosis DT, Poulain DA. 1999. Contribution of astrocytes to activity-dependent structural plasticity in the adult brain. Adv Exp Med Biol 468:175–182 [DOI] [PubMed] [Google Scholar]
- 13. Ullian EM, Christopherson KS, Barres BA. 2004. Role for glia in synaptogenesis. Glia 47:209–216 [DOI] [PubMed] [Google Scholar]
- 14. Raison CL, Miller AH. 2011. Is depression an inflammatory disorder? Curr Psychiatry Rep 13:467–475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Pace TW, Heim CM. 2011. A short review on the psychoneuroimmunology of posttraumatic stress disorder: from risk factors to medical comorbidities. Brain Behav Immun 25:6–13 [DOI] [PubMed] [Google Scholar]
- 16. Munhoz CD, Lepsch LB, Kawamoto EM, Malta MB, Lima Lde S, Avellar MC, Sapolsky RM, Scavone C. 2006. Chronic unpredictable stress exacerbates lipopolysaccharide-induced activation of nuclear factor-κB in the frontal cortex and hippocampus via glucocorticoid secretion. J Neurosci 26:3813–3820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. MacMillan HL, Georgiades K, Duku EK, Shea A, Steiner M, Niec A, Tanaka M, Gensey S, Spree S, Vella E, Walsh CA, De Bellis MD, Van der Meulen J, Boyle MH, Schmidt LA. 2009. Cortisol response to stress in female youths exposed to childhood maltreatment: results of the youth mood project. Biol Psychiatry 66:62–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bale TL, Contarino A, Smith GW, Chan R, Gold LH, Sawchenko PE, Koob GF, Vale WW, Lee KF. 2000. Mice deficient for corticotropin-releasing hormone receptor-2 display anxiety-like behaviour and are hypersensitive to stress. Nat Genet 24:410–414 [DOI] [PubMed] [Google Scholar]
- 19. Bale TL, Vale WW. 2003. Increased depression-like behaviors in corticotropin-releasing factor receptor-2-deficient mice: sexually dichotomous responses. J Neurosci 23:5295–5301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. McEuen JG, Beck SG, Bale TL. 2008. Failure to mount adaptive responses to stress results in dysregulation and cell death in the midbrain raphe. J Neurosci 28:8169–8177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Keller-Wood ME, Dallman MF. 1984. Corticosteroid inhibition of ACTH secretion. Endocr Rev 5:1–24 [DOI] [PubMed] [Google Scholar]
- 22. Radley JJ, Sawchenko PE. 2011. A common substrate for prefrontal and hippocampal inhibition of the neuroendocrine stress response. J Neurosci 31:9683–9695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Jankord R, Herman JP. 2008. Limbic regulation of hypothalamo-pituitary-adrenocortical function during acute and chronic stress. Ann NY Acad Sci 1148:64–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Amateau SK, McCarthy MM. 2004. Induction of PGE2 by estradiol mediates developmental masculinization of sex behavior. Nat Neurosci 7:643–650 [DOI] [PubMed] [Google Scholar]
- 25. Bulckaen H, Prévost G, Boulanger E, Robitaille G, Roquet V, Gaxatte C, Garçon G, Corman B, Gosset P, Shirali P, Creusy C, Puisieux F. 2008. Low-dose aspirin prevents age-related endothelial dysfunction in a mouse model of physiological aging. Am J Physiol Heart Circ Physiol 294:H1562–H1570 [DOI] [PubMed] [Google Scholar]
- 26. Paxinos G, Franklin KB. 2003. The mouse brain in stereotaxic coordinates. 2nd ed London: Academic [Google Scholar]
- 27. Whitnall MH. 1993. Regulation of the hypothalamic corticotropin-releasing hormone neurosecretory system. Prog Neurobiol 40:573–629 [DOI] [PubMed] [Google Scholar]
- 28. Bhatnagar S, Dallman M. 1998. Neuroanatomical basis for facilitation of hypothalamic-pituitary-adrenal responses to a novel stressor after chronic stress. Neuroscience 84:1025–1039 [DOI] [PubMed] [Google Scholar]
- 29. Pfister HP, King MG. 1976. Adaptation of the glucocorticosterone response to novelty. Physiol Behav 17:43–46 [DOI] [PubMed] [Google Scholar]
- 30. Müller N, Schwarz MJ, Dehning S, Douhe A, Cerovecki A, Goldstein-Müller B, Spellmann I, Hetzel G, Maino K, Kleindienst N, Möller HJ, Arolt V, Riedel M. 2006. The cyclooxygenase-2 inhibitor celecoxib has therapeutic effects in major depression: results of a double-blind, randomized, placebo controlled, add-on pilot study to reboxetine. Mol Psychiatry 11:680–684 [DOI] [PubMed] [Google Scholar]
- 31. Mendlewicz J, Kriwin P, Oswald P, Souery D, Alboni S, Brunello N. 2006. Shortened onset of action of antidepressants in major depression using acetylsalicylic acid augmentation: a pilot open-label study. Int Clin Psychopharmacol 21:227–231 [DOI] [PubMed] [Google Scholar]
- 32. Brunello N, Alboni S, Capone G, Benatti C, Blom JM, Tascedda F, Kriwin P, Mendlewicz J. 2006. Acetylsalicylic acid accelerates the antidepressant effect of fluoxetine in the chronic escape deficit model of depression. Int Clin Psychopharmacol 21:219–225 [DOI] [PubMed] [Google Scholar]
- 33. Müller N, Riedel M, Schwarz MJ, Engel RR. 2005. Clinical effects of COX-2 inhibitors on cognition in schizophrenia. Eur Arch Psychiatry Clin Neurosci 255:149–151 [DOI] [PubMed] [Google Scholar]
- 34. Rajkowska G, Miguel-Hidalgo JJ, Wei J, Dilley G, Pittman SD, Meltzer HY, Overholser JC, Roth BL, Stockmeier CA. 1999. Morphometric evidence for neuronal and glial prefrontal cell pathology in major depression. Biol Psychiatry 45:1085–1098 [DOI] [PubMed] [Google Scholar]
- 35. Bowley MP, Drevets WC, Ongür D, Price JL. 2002. Low glial numbers in the amygdala in major depressive disorder. Biol Psychiatry 52:404–412 [DOI] [PubMed] [Google Scholar]
- 36. Toro CT, Hallak JE, Dunham JS, Deakin JF. 2006. Glial fibrillary acidic protein and glutamine synthetase in subregions of prefrontal cortex in schizophrenia and mood disorder. Neurosci Lett 404:276–281 [DOI] [PubMed] [Google Scholar]
- 37. Lee Y, Su M, Messing A, Brenner M. 2006. Astrocyte heterogeneity revealed by expression of a GFAP-LacZ transgene. Glia 53:677–687 [DOI] [PubMed] [Google Scholar]
- 38. Cahoy JD, Emery B, Kaushal A, Foo LC, Zamanian JL, Christopherson KS, Xing Y, Lubischer JL, Krieg PA, Krupenko SA, Thompson WJ, Barres BA. 2008. A transcriptome database for astrocytes, neurons, and oligodendrocytes: a new resource for understanding brain development and function. J Neurosci 28:264–278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kimelberg HK. 2004. The problem of astrocyte identity. Neurochem Int 45:191–202 [DOI] [PubMed] [Google Scholar]
- 40. Herman JP, Ostrander MM, Mueller NK, Figueiredo H. 2005. Limbic system mechanisms of stress regulation: hypothalamo-pituitary-adrenocortical axis. Prog Neuropsychopharmacol Biol Psychiatry 29:1201–1213 [DOI] [PubMed] [Google Scholar]
- 41. Lovenberg TW, Chalmers DT, Liu C, De Souza EB. 1995. CRF2α and CRF2β receptor mRNAs are differentially distributed between the rat central nervous system and peripheral tissues. Endocrinology 136:4139–4142 [DOI] [PubMed] [Google Scholar]
- 42. Baigent SM, Lowry PJ. 2000. mRNA expression profiles for corticotrophin-releasing factor (CRF), urocortin, CRF receptors and CRF-binding protein in peripheral rat tissues. J Mol Endocrinol 25:43–52 [DOI] [PubMed] [Google Scholar]
- 43. d'Audiffret AC, Frisbee SJ, Stapleton PA, Goodwill AG, Isingrini E, Frisbee JC. 2010. Depressive behavior and vascular dysfunction: a link between clinical depression and vascular disease? J Appl Physiol 108:1041–1051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Farooq RK, Isingrini E, Tanti A, Le Guisquet AM, Arlicot N, Minier F, Leman S, Chalon S, Belzung C, Camus V. 2012. Is unpredictable chronic mild stress (UCMS) a reliable model to study depression-induced neuroinflammation? Behav Brain Res 231:130–137 [DOI] [PubMed] [Google Scholar]
- 45. van der Meer MJ, Sweep CG, Pesman GJ, Tilders FJ, Hermus AR. 1996. Chronic stimulation of the hypothalamus-pituitary-adrenal axis in rats by interleukin 1β: central and peripheral mechanisms. Cytokine 8:910–919 [DOI] [PubMed] [Google Scholar]
- 46. Tsagarakis S, Gillies G, Rees LH, Besser M, Grossman A. 1989. Interleukin-1 directly stimulates the release of corticotrophin releasing factor from rat hypothalamus. Neuroendocrinology 49:98–101 [DOI] [PubMed] [Google Scholar]
- 47. Bartolomucci A, Palanza P, Parmigiani S, Pederzani T, Merlot E, Neveu PJ, Dantzer R. 2003. Chronic psychosocial stress down-regulates central cytokines mRNA. Brain Res Bull 62:173–178 [DOI] [PubMed] [Google Scholar]
- 48. You Z, Luo C, Zhang W, Chen Y, He J, Zhao Q, Zuo R, Wu Y. 2011. Pro- and anti-inflammatory cytokines expression in rat's brain and spleen exposed to chronic mild stress: involvement in depression. Behav Brain Res 225:135–141 [DOI] [PubMed] [Google Scholar]
- 49. Reyes TM, Walker JR, DeCino C, Hogenesch JB, Sawchenko PE. 2003. Categorically distinct acute stressors elicit dissimilar transcriptional profiles in the paraventricular nucleus of the hypothalamus. J Neurosci 23:5607–5616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Hueston CM, Barnum CJ, Eberle JA, Ferraioli FJ, Buck HM, Deak T. 2011. Stress-dependent changes in neuroinflammatory markers observed after common laboratory stressors are not seen following acute social defeat of the Sprague Dawley rat. Physiol Behav 104:187–198 [DOI] [PubMed] [Google Scholar]
- 51. Deak T, Bordner KA, McElderry NK, Barnum CJ, Blandino P, Jr, Deak MM, Tammariello SP. 2005. Stress-induced increases in hypothalamic IL-1: a systematic analysis of multiple stressor paradigms. Brain Res Bull 64:541–556 [DOI] [PubMed] [Google Scholar]