Abstract
Early exposure to the steroid sex hormone testosterone and its estrogen metabolite estradiol masculinize neural tissue during a developmental critical period. Many aspects of neuron anatomy and physiology are permanently altered, including later sensitivity to estradiol. Although it is well established that early hormone exposure alters neuronal responsiveness regarding classical estradiol actions (i.e. acting via nuclear estrogen receptors), it has not yet been determined whether it also alters neuronal processing of nonclassical estrogen receptor signaling, including the actions of membrane-associated estrogen receptors. Hence, we tested whether membrane estrogen receptor regulation of cAMP response element binding protein (CREB) phosphorylation observed in female (but not male) hippocampal pyramidal neurons is due to the lack of androgen and/or estrogen exposure in females during this critical period. Female rat neonates on postnatal d 0 and 1 were systemically injected with one of four compounds: vehicle, testosterone, the nonaromatizable androgen dihydrotestosterone, or estradiol. On postnatal d 2, primary hippocampal neuron cultures were generated from these animals. After 8–9 d in culture, we assessed whether estradiol affected CREB phosphorylation. Neurons from female neonates exposed to testosterone lacked estradiol signaling to CREB. In contrast, dihydrotestosterone injections of female neonates did not disrupt estradiol regulation of CREB. Estradiol injections of female neonates, however, eliminated estradiol signaling to CREB. These findings indicate that testosterone aromatization to estradiol leads to a masculinization/defeminization process whereby hippocampal neurons fail to exhibit rapid estradiol signaling to CREB. Broadly, these findings extend the organizational and aromatization hypotheses to rapid, nonclassical hormone action.
Early exposure to steroid sex hormones can masculinize/defeminize neural tissue. For example, in male rodents, the testes elevate plasma testosterone levels both in utero and in neonates. Testosterone can directly act on androgen receptors or can be metabolized to estradiol, activating estrogen receptors (ER). Stimulation of androgen and ER during development can masculinize neural substrates during a brief critical period; this is referred to as the organizational/aromatization hypothesis (1–3). This process, along with neurosteroids and genetic and environmental influences (4), creates sexually dimorphic neural tissue.
Neuron anatomy and physiology can be sexually dimorphic. This includes adult responsiveness to steroid sex hormones such as estradiol. Thus far, studies of development-induced sex differences in estradiol signaling have largely focused on the classical estradiol action of binding to nuclear-localized ER to directly affect gene expression. Although it is established that early hormone exposure can alter future responsiveness to classical estradiol action, it is unclear whether early hormone exposure can also impact future neuron responsiveness to nonclassical estradiol actions, due to membrane-associated ER (5, 6).
Here we test this hypothesis, using a well-studied, sexually dimorphic, nonclassical estradiol action in hippocampal neurons. In female but not male hippocampal pyramidal neurons, estradiol rapidly modulates cAMP response element binding protein (CREB) phosphorylation (7, 8). This occurs via direct coupling of membrane-associated ERα and -β to metabotropic glutamate receptors (mGluR), leading to estradiol-induced mGluR signaling (schematized in Ref. 6). Female rat neonates were injected once daily for 2 d with vehicle, testosterone, the nonaromatizable androgen dihydrotestosterone, or estradiol. Twenty-four hours after the second injection, hippocampal neuron cultures were generated. Using these cultures, we assessed whether hippocampal pyramidal neurons exhibited membrane ER/mGluR signaling to CREB. Neurons from female pups exposed to vehicle exhibited rapid estradiol action, whereas neurons from male pups exposed to vehicle did not. Neurons from female pups neonatally exposed to testosterone lacked rapid estradiol action. In contrast, neurons from female pups neonatally exposed to dihydrotestosterone exhibited rapid estradiol action. Neurons from female pups neonatally exposed to estradiol, however, lacked rapid estradiol action. Direct mGluR signaling to CREB was unaffected by neonatal hormone exposure. Collectively, these findings indicate that aromatization of testosterone to estradiol leads to a masculinization/defeminization process, whereby hippocampal neurons lose membrane-associated ER signaling. Broadly, these results demonstrate that early hormone exposure contributes to sex differences not only in nuclear ER but membrane ER signaling as well.
Materials and Methods
Animals
All protocols were approved by the Animal Care and Use Committee at the University of Minnesota. Female and male Sprague-Dawley rats were born from timed-pregnant females purchased from Harlan (Indianapolis, IN). Animals were housed with their littermates and dam. On postnatal d 0 and 1, following a well-established protocol (9), female pups received a sc injection (0.1 ml) of either cottonseed oil (vehicle) or oil containing 100 μg testosterone, estradiol, or dihydrotestosterone. Male pups received cottonseed oil. Each group contained two to four pups, and each experiment was replicated at least three times across different dams and litters.
Cell culture
On postnatal d 2, animals were killed, and hippocampal neurons were cultured using previously described techniques (8). Cultures were prepared in parallel from male and female pups, or oil- and hormone-injected pups, obtained from the same litter.
Drugs
Tetrodotoxin, d(−)-2-amino-5-phosphonopentanoic acid, (S)-3,5-dihydroxyphenylglycine (DHPG), 17β-estradiol, and (2R,4R)-4-aminopyrrolidine-2,4-dicarboxylate (APDC) were from Tocris (Ellisville, MO). Testosterone and dihydrotestosterone were from Steraloids (Newport, RI).
Immunocytochemistry
Immunocytochemistry protocols followed those described previously (8, 10). Neurons (8–9 d in vitro) were incubated in a Tyrode's solution containing tetrodotoxin (1 μm) and d(−)-2-amino-5-phosphonopentanoic acid (25 μm) at room temperature for 1.5–2.0 h. Cell stimulations were performed as follows: vehicle for 10 min, 1 nm estradiol for 5 min, 50 μm DHPG for 5 min, 10 μm APDC for 5 min, 20 mm K+ for 3 min, estradiol or APDC for 5 min, and then estradiol or APDC and 20 mm K+ for 3 min. Cells were fixed using ice-cold 4% paraformaldehyde (Electron Microscopy Sciences, Fort Washington, PA) in PBS containing 4 mm EGTA. Cells were washed in PBS, permeabilized with 0.1% Triton X-100 (VWR Scientific, West Chester, PA), washed, and blocked at 37 C for 30 min in PBS containing 1% BSA and 2% goat serum (Jackson ImmunoResearch, West Grove, PA). The cells were incubated at 37 C for 1 h in block containing a monoclonal antibody directed against serine 133 phosphorylated CREB (pCREB) (1:1000, 05-667; Upstate Biotechnology, Lake Placid, NY) and a polyclonal antibody targeting microtubule-associated protein 2 (MAP2) (1:1000, AB5622; Calbiochem, La Jolla, CA). Cells were washed and incubated for 1 h at 37 C in block solution containing fluorescein isothiocyanate- and cyanine 5 (Jackson ImmunoResearch) or Alexa Fluor 488-conjugated antirabbit and 635-conjugated antimouse (Invitrogen, Carlsbad, CA) secondary antibodies for visualization of MAP2 and pCREB, respectively. Cells were washed and mounted using Citifluor (Ted Pella, Redding, CA). Nuclear fluorescent intensities for pCREB were acquired using a Leica DM5500Q confocal system or a Yokogawa spinning-disc confocal system. Data acquired from the Yokogawa system were quantified using MetaMorph (version 6.0; Universal Imaging, Downingtown, PA). Data from the Leica were quantified with Leica LAS AF (version 1.9.0; Leica, Buffalo Grove, IL). The same antibodies and imaging system were used for all cells within an experiment. Experimental conclusions were not altered by these differences.
Following established protocols (8), the confocal excitation and detection settings for each experiment were determined using 20 mm K+-stimulated coverslips. Inter-coverslip variability was accounted for by subjecting two coverslips to each treatment. Neurons were selected randomly across both coverslips using MAP2 fluorescence, allowing blind acquisition of pCREB intensities. Data acquisition order was random. Images were captured through the approximate midline of each cell. During data analysis, MAP2 staining was used to draw a region of interest outlining the nucleus of each neuron, blinding analysis of pCREB intensity. The region of interest was then transferred to the pCREB image, and average fluorescence intensities within the nucleus were recorded. Background from a region of the image not containing pCREB fluorescence was subtracted from the average pCREB fluorescence.
Statistics
Experiments were analyzed using one-way ANOVAs and Tukey's post hoc test (Prism version 5.00; GraphPad Software, La Jolla, CA). Statistical differences between groups are depicted within each figure as different alphabetical characters. P values < 0.05 were considered a priori as significant. Data are presented as mean ± sem.
Results
Only hippocampal neurons from females exhibit rapid estradiol regulation of CREB phosphorylation
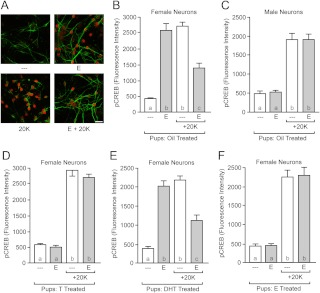
Estradiol rapidly phosphorylates CREB in female hippocampal pyramidal neurons, and preexposure to estradiol attenuates CREB phosphorylation induced by the depolarizing action of 20 mm K+ (8). These rapid estradiol effects are mediated through membrane-associated ER coupling to mGluR and are not observed in neurons from males (7, 8). For our first experiment, we verified that neonatal oil injections would not affect this outcome. We injected male and female littermate rats with vehicle on postnatal d 0 and 1 and then cultured hippocampal pyramidal neurons on d 2. After 8–9 d in culture, we assessed neuronal responsiveness to estradiol, examining changes in CREB phosphorylation using the following treatments: vehicle, estradiol, 20 mm K+, or estradiol with 20 mm K+ (Fig. 1A). In neurons from female animals [Fig. 1B; F(3,116) = 70.38, P < 0.0001], exposure to estradiol increased CREB phosphorylation compared with vehicle (P < 0.001). Estradiol also attenuated 20 mm K+-induced CREB phosphorylation compared with 20 mm K+ alone (P < 0.01). Neurons from male animals did not respond to estradiol either under baseline or 20 mm K+ conditions [Fig. 1C; F(3,118) = 47.12, P < 0.0001].
Fig. 1.
Rapid, nonclassical estradiol action on CREB phosphorylation is sex specific and is regulated by early hormone exposure. A, Example confocal images of cultured hippocampal neurons immunolabeled with MAP2 (green) and pCREB (red). Exposure to estradiol (E) rapidly increased CREB phosphorylation and decreased 20 mm K+-induced CREB phosphorylation in female neurons. Treatments were as follows: top left, vehicle; top right, estradiol; bottom left, 20 mm K+; bottom right, estradiol and 20 mm K+. Scale bar, 25 μm. B, Quantification of rapid estradiol modulation of CREB phosphorylation in female neurons. C, Estradiol exposure has no effect in male neurons. D, Neonatal exposure to testosterone (T) eliminates later responsiveness to rapid estradiol action in female neurons. E, Neonatal exposure to testosterone's nonaromatizable, androgenic metabolite dihydrotestosterone (DHT) does not eliminate later responsiveness to rapid estradiol action in female neurons. F, Neonatal exposure to testosterone's estrogenic metabolite estradiol eliminates later responsiveness to rapid estradiol action in female neurons. Letters within each bar indicate statistically significantly different groups; complete statistical information is in Results.
Neonatal exposure to testosterone eliminates rapid estradiol action
This sexually dimorphic sensitivity to estradiol could be organized early in life by hormone exposure, by a genetic program independent of early hormone exposure, or by environmental factors. To differentiate between these options, we followed the same experimental timeline as above, this time with neonatal injections of vehicle or testosterone. We reasoned that if in males neonatal exposure to masculinizing doses of testosterone eliminates later responsiveness to estradiol, then a similar treatment in females should also eliminate rapid estradiol action.
Neonatal testosterone exposure eliminated rapid estradiol action [Fig. 1D; F(3,117) = 155.0, P < 0.0001]. In neurons from females exposed to testosterone, estradiol did not increase CREB phosphorylation and did not attenuate 20 mm K+-induced CREB phosphorylation. This is in contrast to neurons from females neonatally exposed to vehicle [F(3,121) = 41.93, P < 0.0001]. These results support the hypothesis that membrane-associated ER signaling is lost upon early hormone exposure.
Neonatal exposure to dihydrotestosterone does not eliminate rapid estradiol action
In the brain, testosterone can act directly on androgen receptors or be metabolized into other hormones. These include dihydrotestosterone and estradiol. Dihydrotestosterone is a potent, nonaromatizable androgen that binds to androgen receptors, whereas estradiol binds to ER. To determine whether the effects of testosterone were due to activation of androgen receptors, we again followed the same experimental timeline, with neonatal injections of vehicle or dihydrotestosterone.
Neonatal exposure to dihydrotestosterone did not eliminate rapid estradiol action [Fig. 1E; F(3,118) = 72.97, P < 0.0001]. In neurons from females neonatally exposed to dihydrotestosterone, exposure to estradiol increased CREB phosphorylation compared with vehicle (P < 0.001) and attenuated 20 mm K+-induced CREB phosphorylation compared with 20 mm K+ alone (P < 0.001) Similar effects of estradiol were observed in neurons from vehicle-treated animals [F(3,116) = 70.38, P < 0.0001]. These results indicate that the organizational effect of early exposure to testosterone on hippocampal neuron estradiol sensitivity is not mediated via androgen receptors.
Neonatal exposure to estradiol eliminates rapid estradiol action
Given that testosterone can also be metabolized into estradiol, we next tested the role of early ER activation. We followed the same experimental timeline as above, with neonatal injections of vehicle or estradiol.
Neonatal estradiol exposure eliminated rapid estradiol action [Fig. 1F; F(3,128) = 57.87, P < 0.0001]. In neurons from females neonatally exposed to estradiol, exposure to estradiol did not increase CREB phosphorylation compared with vehicle (P > 0.05) and estradiol did not attenuate 20 mm K+-induced CREB phosphorylation compared with 20 mm K+ alone (P > 0.05). This is in contrast to the neurons obtained from females exposed to vehicle, which showed normal responses to estradiol [F(3,117) = 25.21, P < 0.0001]. These results support the hypothesis that sex differences in estradiol responsiveness are generated by activation of ER.
mGluR signaling is unaffected by neonatal exposure to testosterone
In both males and females, mGluR activation regulates CREB phosphorylation. Only in females, however, are membrane-associated ER functionally coupled to mGluR (7, 8). The hormone manipulations employed here eliminate this membrane-associated ER signaling in females. Our interpretation of these data is that the early hormone exposure is masculinizing/defeminizing the females by inducing a male-like phenotype: i.e. eliminating membrane-associated ER coupling to mGluR. If this interpretation is correct, then the direct mGluR pathway to CREB would remain intact in the masculinized/defeminized females.
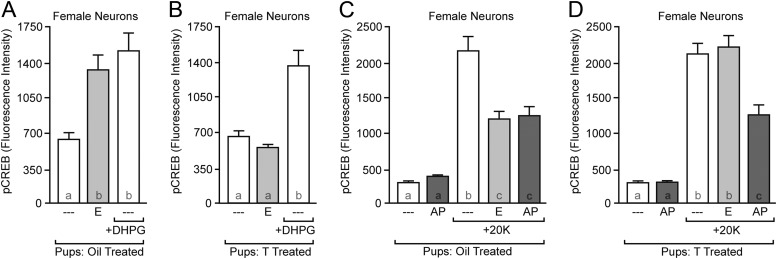
To test this hypothesis, we neonatally injected females with either vehicle or testosterone and then assessed neuronal sensitivity to the group I mGluR agonist DHPG and the group II mGluR agonist APDC on d 8 or 9. Neonatal exposure to vehicle did not eliminate ER or group I mGluR signaling [Fig. 2A; F(2,109) = 9.143, P = 0.0002]. In contrast, neonatal exposure to testosterone eliminated estradiol signaling without affecting group I mGluR signaling [Fig. 2B; F(2,108) = 17.34, P < 0.0001]. Similar results were obtained regarding group II mGluR signaling. Neonatal exposure to vehicle did not eliminate estradiol or group II mGluR signaling [Fig. 2C; F(4,146) = 35.80, P < 0.0001]. However, neonatal exposure to testosterone eliminated estradiol signaling without affecting group II mGluR signaling [Fig. 2D; F(4,160) = 47.77, P < 0.0001]. Overall, these data indicate that mGluR signaling to CREB is intact in masculinized/defeminized females, supporting the hypothesis that neonatal hormone exposure specifically modulates membrane ER signaling.
Fig. 2.
Neonatal exposure to testosterone (T) does not affect mGluR signaling. A, Neonatal exposure to oil does not affect group I mGluR signaling induced by the agonist DHPG or rapid estradiol (E) action in female neurons; B, neonatal exposure to testosterone does not eliminate group I mGluR signaling but does eliminate rapid estradiol action in female neurons; C, neonatal exposure to oil does not eliminate group II mGluR signaling induced by the agonist APDC (AP) in female neurons; D, neonatal exposure to testosterone does not affect group II mGluR signaling but does eliminate rapid estradiol action on pCREB intensity induced by 20 mm K+. Letters within each bar indicate statistically significantly different groups; complete statistical information is in Results.
Discussion
There are five principle findings of these experiments. First, rapid, nonclassical estradiol modulation of CREB phosphorylation occurs in female but not male hippocampal pyramidal neurons. Second, hippocampal pyramidal neurons from female neonates exposed to testosterone lacked estradiol signaling to CREB. Third, dihydrotestosterone injections of female neonates did not disrupt estradiol regulation of CREB. Fourth, estradiol injections of female neonates eliminated estradiol signaling to CREB. Finally, masculinization/defeminization does not affect mGluR signaling. Collectively, these experiments demonstrate that the organizational effects of early hormone exposure apply to rapid, nonclassical estradiol action.
Nonclassical estradiol action has been known since at least the 1960s, when Szego and Davis (11) demonstrated that within seconds, estradiol increased cAMP accumulation in uterine tissue. Later, in neurons, Kelly and colleagues (12) showed that estradiol could rapidly affect neuron electrophysiological properties. These discoveries were roughly in parallel with those delineating classical estradiol actions via ER acting in the nucleus to affect gene transcription via estrogen response elements (6, 13). Although initially controversial, today, it is well accepted that estradiol rapidly modulates neuronal electrophysiological properties (14–16). It is also definitive that estradiol can induce second messenger signaling pathways more commonly associated with G protein-coupled receptors to modify cellular physiology, gene transcription, and even anatomy (6, 13, 17–20). Indeed, nonclassical estradiol action can masculinize neural tissue (21). Here, we have chosen to work with rapid, nonclassical estradiol modulation of CREB phosphorylation in hippocampal pyramidal neurons (8). In female but not in male neurons, this modulation occurs via membrane-associated ERα and -β that couple to mGluR (schematized in Ref. 6). This coupling is widespread across the nervous system, including hippocampal (8, 22), striatal (23), cortical (24), arcuate (25), and dorsal root ganglion neurons (26) as well as hypothalamic astrocytes (27).
This association between membrane ER and mGluR, as well as other G proteins, occurs in brain regions that also express nuclear-localized ER that operate via the classical mechanism. This integration allows estradiol to not only slowly change gene expression but also exert influence over the functions typically ascribed to G protein-coupled receptors. This spectrum of estradiol action could thus potentially act in parallel to modulate neuron function (28). Examples of this include the arcuate nucleus/medial preoptic nucleus circuit, where rapid estradiol signaling facilitates classical nuclear ER action via mGluR stimulation (13, 25), experiments using the membrane-ER-knockout mouse, which found that normal development requires both membrane and nuclear ER (29), and teleosts, where rapid steroid hormone-specific modulation of a neural circuit controlling vocal behavior differs between sexes (30).
These complementary actions of classical and nonclassical estradiol signaling suggest that common developmental mechanisms underlie sexual dimorphisms in the diverse spectrum of estradiol action. Supporting this, use of the four core genotypes of mice (31) indicated that the sexually dimorphic nonclassical response of hypothalamic astrocytes to estradiol was dependent upon gonadal but not chromosomal sex (32). This implicates early hormone exposure, foreshadowing the conclusions presented here. It does not necessarily follow, however, that all sexually dimorphic sensitivity to nonclassical hormone signaling is organized by early hormone exposure. This is only one of several possible mechanisms, with others including neurosteroids and genetic and environmental influences (4). Thus, the developmental origin of sexually dimorphic phenomenon must continue to be evaluated on a case-by-case basis.
Acknowledgments
We acknowledge Dr. Jaclyn Schwarz for advice on neonatal hormone injections, Kyla Britson for technical assistance, and Dr. Robert Meisel and his laboratory for their support.
We are grateful for the following sources of funding: NIH F32 DA030828 (to J.M.), NIH T32 MH073129 (to D.D.G.), and NSF IOS-1146015 (to P.G.M.).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- APDC
- (2R,4R)-4-Aminopyrrolidine-2,4-dicarboxylate
- CREB
- cAMP response element binding protein
- DHPG
- (S)-3,5-dihydroxyphenylglycine
- ER
- estrogen receptors
- MAP2
- microtubule-associated protein 2
- mGluR
- metabotropic glutamate receptor
- pCREB
- phosphorylated CREB.
References
- 1. Breedlove SM, Hampson E. 2002. Sexual differentiation of the brain and behavior. In: Becker JB, Breedlove SM, Crews D, McCarthy MM, eds. Behavioral endocrinology. London: MIT Press; 75–114 [Google Scholar]
- 2. Feder HH, Whalen RE. 1965. Feminine behavior in neonatally castrated and estrogen-treated male rats. Science 147:306–307 [DOI] [PubMed] [Google Scholar]
- 3. Phoenix CH, Goy RW, Gerall AA, Young WC. 1959. Organizing action of prenatally administered testosterone propionate on the tissues mediating mating behavior in the female guinea pig. Endocrinology 65:369–382 [DOI] [PubMed] [Google Scholar]
- 4. McCarthy MM, Arnold AP. 2011. Reframing sexual differentiation of the brain. Nat Neurosci 14:677–683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Levin ER. 2011. Extranuclear steroid receptors: roles in modulation of cell functions. Mol Endocrinol 25:377–384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Meitzen J, Mermelstein PG. 2011. Estrogen receptors stimulate brain region specific metabotropic glutamate receptors to rapidly initiate signal transduction pathways. J Chem Neuroanat 42:236–241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Boulware MI, Kordasiewicz H, Mermelstein PG. 2007. Caveolin proteins are essential for distinct effects of membrane estrogen receptors in neurons. J Neurosci 27:9941–9950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Boulware MI, Weick JP, Becklund BR, Kuo SP, Groth RD, Mermelstein PG. 2005. Estradiol activates group I and II metabotropic glutamate receptor signaling, leading to opposing influences on cAMP response element-binding protein. J Neurosci 25:5066–5078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mong JA, Glaser E, McCarthy MM. 1999. Gonadal steroids promote glial differentiation and alter neuronal morphology in the developing hypothalamus in a regionally specific manner. J Neurosci 19:1464–1472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Meitzen J, Luoma JI, Stern CM, Mermelstein PG. 2011. β1-Adrenergic receptors activate two distinct signaling pathways in striatal neurons. J Neurochem 116:984–995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Szego CM, Davis JS. 1967. Adenosine 3′,5′-monophosphate in rat uterus: acute elevation by estrogen. Proc Natl Acad Sci USA 58:1711–1718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kelly MJ, Moss RL, Dudley CA. 1976. Differential sensitivity of preoptic-septal neurons to microelectrophoresed estrogen during the estrous cycle. Brain Res 114:152–157 [DOI] [PubMed] [Google Scholar]
- 13. Micevych PE, Mermelstein PG. 2008. Membrane estrogen receptors acting through metabotropic glutamate receptors: an emerging mechanism of estrogen action in brain. Mol Neurobiol 38:66–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Joëls M. 1997. Steroid hormones and excitability in the mammalian brain. Front Neuroendocrinol 18:2–48 [DOI] [PubMed] [Google Scholar]
- 15. Mermelstein PG, Becker JB, Surmeier DJ. 1996. Estradiol reduces calcium currents in rat neostriatal neurons via a membrane receptor. J Neurosci 16:595–604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Woolley CS. 2007. Acute effects of estrogen on neuronal physiology. Annu Rev Pharmacol Toxicol 47:657–680 [DOI] [PubMed] [Google Scholar]
- 17. Balthazart J, Ball GF. 2006. Is brain estradiol a hormone or a neurotransmitter? Trends Neurosci 29:241–249 [DOI] [PubMed] [Google Scholar]
- 18. Roepke TA, Qiu J, Bosch MA, Rønnekleiv OK, Kelly MJ. 2009. Cross-talk between membrane-initiated and nuclear-initiated oestrogen signalling in the hypothalamus. J Neuroendocrinol 21:263–270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Saldanha CJ, Remage-Healey L, Schlinger BA. 2011. Synaptocrine signaling: steroid synthesis and action at the synapse. Endocr Rev 32:532–549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Srivastava DP, Woolfrey KM, Liu F, Brandon NJ, Penzes P. 2010. Estrogen receptor β activity modulates synaptic signaling and structure. J Neurosci 30:13454–13460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Schwarz JM, Liang SL, Thompson SM, McCarthy MM. 2008. Estradiol induces hypothalamic dendritic spines by enhancing glutamate release: a mechanism for organizational sex differences. Neuron 58:584–598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Huang GZ, Woolley CS. 2012. Estradiol acutely suppresses inhibition in the hippocampus through a sex-specific endocannabinoid and mGluR-dependent mechanism. Neuron 74:801–808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Grove-Strawser D, Boulware MI, Mermelstein PG. 2010. Membrane estrogen receptors activate the metabotropic glutamate receptors mGluR5 and mGluR3 to bidirectionally regulate CREB phosphorylation in female rat striatal neurons. Neuroscience 170:1045–1055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Spampinato SF, Molinaro G, Merlo S, Iacovelli L, Caraci F, Battaglia G, Nicoletti F, Bruno V, Sortino MA. 2012. Estrogen receptors and type 1 metabotropic glutamate receptors are interdependent in protecting cortical neurons against β-amyloid toxicity. Mol Pharmacol 81:12–20 [DOI] [PubMed] [Google Scholar]
- 25. Dewing P, Boulware MI, Sinchak K, Christensen A, Mermelstein PG, Micevych P. 2007. Membrane estrogen receptor-α interactions with metabotropic glutamate receptor 1a modulate female sexual receptivity in rats. J Neurosci 27:9294–9300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Chaban V, Li J, McDonald JS, Rapkin A, Micevych P. 2011. Estradiol attenuates the adenosine triphosphate-induced increase of intracellular calcium through group II metabotropic glutamate receptors in rat dorsal root ganglion neurons. J Neurosci Res 89:1707–1710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Micevych P, Sinchak K. 2008. Synthesis and function of hypothalamic neuroprogesterone in reproduction. Endocrinology 149:2739–2742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Vasudevan N, Pfaff DW. 2008. Non-genomic actions of estrogens and their interaction with genomic actions in the brain. Front Neuroendocrinol 29:238–257 [DOI] [PubMed] [Google Scholar]
- 29. Pedram A, Razandi M, Kim JK, O'Mahony F, Lee EY, Luderer U, Levin ER. 2009. Developmental phenotype of a membrane only estrogen receptor α (MOER) mouse. J Biol Chem 284:3488–3495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Remage-Healey L, Bass AH. 2007. Plasticity in brain sexuality is revealed by the rapid actions of steroid hormones. J Neurosci 27:1114–1122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. De Vries GJ, Rissman EF, Simerly RB, Yang LY, Scordalakes EM, Auger CJ, Swain A, Lovell-Badge R, Burgoyne PS, Arnold AP. 2002. A model system for study of sex chromosome effects on sexually dimorphic neural and behavioral traits. J Neurosci 22:9005–9014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kuo J, Hamid N, Bondar G, Dewing P, Clarkson J, Micevych P. 2010. Sex differences in hypothalamic astrocyte response to estradiol stimulation. Biol Sex Differ 1:7. [DOI] [PMC free article] [PubMed] [Google Scholar]