Abstract
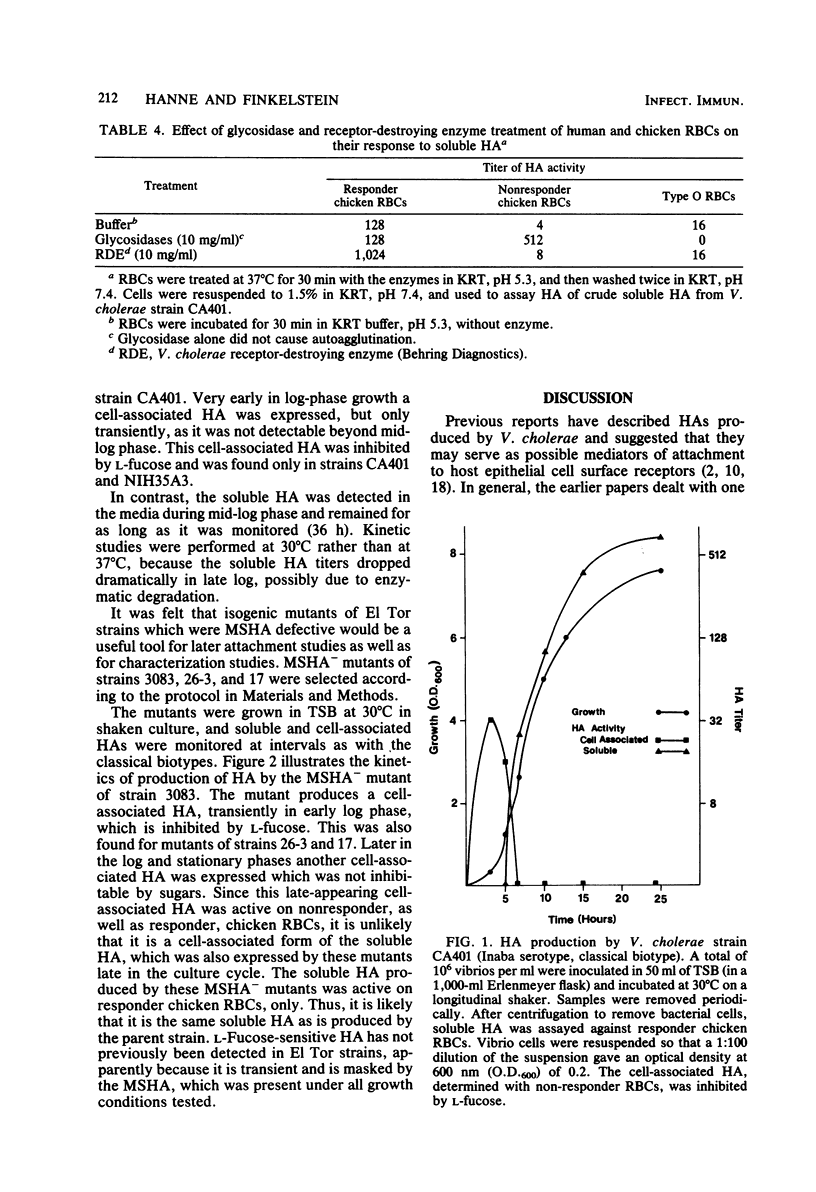
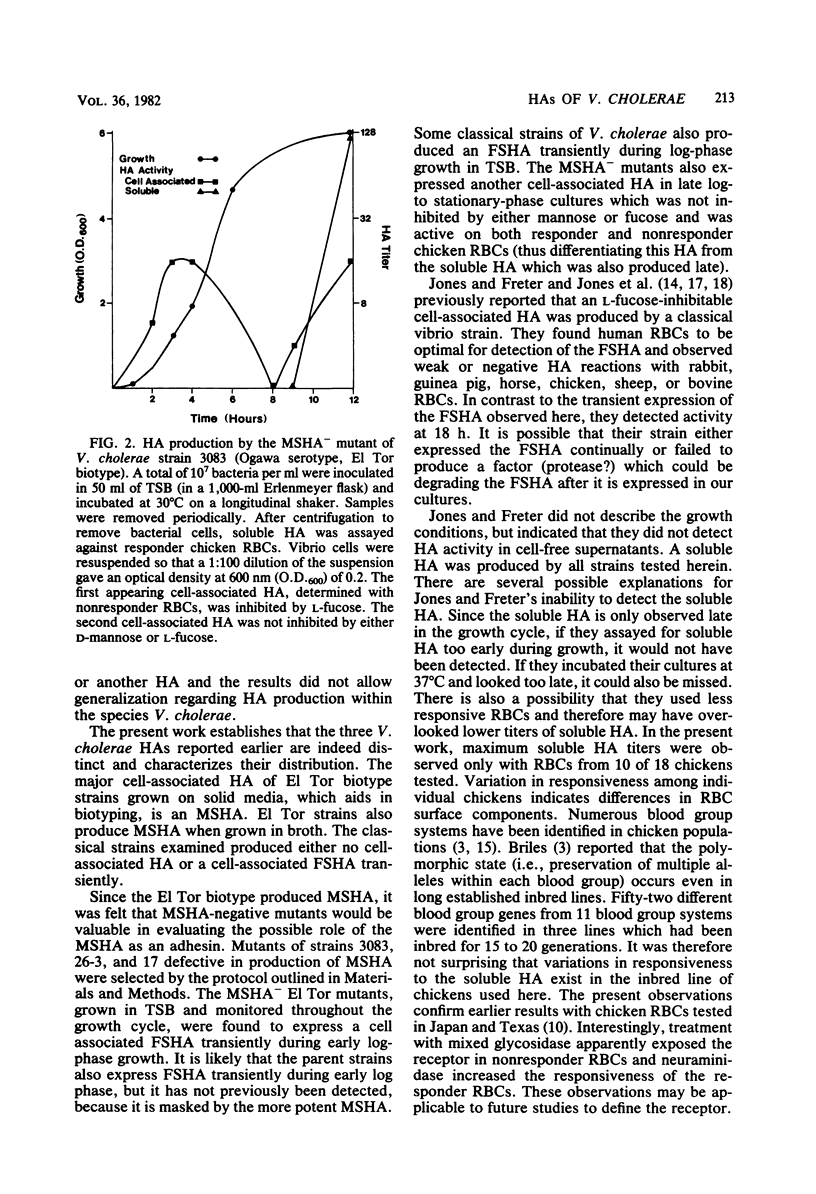
Examination of the distribution of cell-associated and soluble hemagglutinins (HA) produced by Vibrio cholerae revealed the existence of four different HAs. A cell-associated mannose-sensitive HA (MSHA) was produced only by the El Tor biotype. This was evident with all El Tor strains examined. It appears to be responsible for the HA biotyping differentiation of El Tor from classical biotype V. cholerae. The MSHA had no apparent divalent ion requirement; it was inhibited by D-mannose and D-fructose; and it was active on all human (A, B, O) and all chicken erythrocytes tested. Spontaneous MSHA- mutants of El Tor strains were selected by cosedimentation of MSHA+ parent bacteria with erythrocytes. An L-fucose-sensitive HA was detected transiently in early log-phase growth with two of the four classical strains examined and with MSHA- mutants of El Tor biotype strains 3083, 26-3, and 17. MSHA- mutants also expressed another cell-associated HA in late log-phase cultures. A "soluble" HA was detected in late log-phase cultures of all strains tested. This HA was not inhibitable by any sugars tested; it required CA2+ ions for maximum activity; and it was active on some chicken erythrocytes but not others.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abrams G. D., Bishop J. E. Effect of the normal microbial flora on the resistance of the small intestine to infection. J Bacteriol. 1966 Dec;92(6):1604–1608. doi: 10.1128/jb.92.6.1604-1608.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BURROWS W., HUSAIN S. S. Studies on immunity to Asiatic cholera. VIII. The virulence of strains of Vibrio cholerae for the mouse. J Infect Dis. 1956 Jul-Aug;99(1):90–102. doi: 10.1093/infdis/99.1.90. [DOI] [PubMed] [Google Scholar]
- Bhattacharjee J. W., Srivastava B. S. Mannose-sensitive haemagglutinins in adherence of Vibrio cholerae eltor to intestine. J Gen Microbiol. 1978 Aug;107(2):407–410. doi: 10.1099/00221287-107-2-407. [DOI] [PubMed] [Google Scholar]
- Burrows M. R., Sellwood R., Gibbons R. A. Haemagglutinating and adhesive properties associated with the K99 antigen of bovine strains of Escherichia coli. J Gen Microbiol. 1976 Oct;96(2):269–275. doi: 10.1099/00221287-96-2-269. [DOI] [PubMed] [Google Scholar]
- Chaicumpa W., Atthasishtha N. The study of intestinal immunity against V. cholerae: purification of V. cholerae El Tor haemagglutinin and the protective role of its antibody in experimental cholera. Southeast Asian J Trop Med Public Health. 1979 Mar;10(1):73–80. [PubMed] [Google Scholar]
- Chaicumpa W., Atthasisiha N. Study of intestinal immunity against V. cholerae: role of antibody to V. cholerae haemagglutinin in intestinal immunity. Southeast Asian J Trop Med Public Health. 1977 Mar;8(1):13–18. [PubMed] [Google Scholar]
- DIXON J. M. The fate of bacteria in the small intestine. J Pathol Bacteriol. 1960 Jan;79:131–140. doi: 10.1002/path.1700790116. [DOI] [PubMed] [Google Scholar]
- Evans D. G., Evans D. J., Jr New surface-associated heat-labile colonization factor antigen (CFA/II) produced by enterotoxigenic Escherichia coli of serogroups O6 and O8. Infect Immun. 1978 Aug;21(2):638–647. doi: 10.1128/iai.21.2.638-647.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FINKELSTEIN R. A., NORRIS H. T., DUTTA N. K. PATHOGENESIS EXPERIMENTAL CHOLERA IN INFANT RABBITS. I. OBSERVATIONS ON THE INTRAINTESTINAL INFECTION AND EXPERIMENTAL CHOLERA PRODUCED WITH CELL-FREE PRODUCTS. J Infect Dis. 1964 Jun;114:203–216. doi: 10.1093/infdis/114.3.203. [DOI] [PubMed] [Google Scholar]
- Finkelstein R. A., LoSpalluto J. J. Pathogenesis of experimental cholera. Preparation and isolation of choleragen and choleragenoid. J Exp Med. 1969 Jul 1;130(1):185–202. doi: 10.1084/jem.130.1.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freter R., Jones G. W. Adhesive properties of Vibrio cholerae: nature of the interaction with intact mucosal surfaces. Infect Immun. 1976 Jul;14(1):246–256. doi: 10.1128/iai.14.1.246-256.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones G. W., Abrams G. D., Freter R. Adhesive properties of Vibrio cholerae: adhesion to isolated rabbit brush border membranes and hemagglutinating activity. Infect Immun. 1976 Jul;14(1):232–239. doi: 10.1128/iai.14.1.232-239.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones G. W., Freter R. Adhesive properties of Vibrio cholerae: nature of the interaction with isolated rabbit brush border membranes and human erythrocytes. Infect Immun. 1976 Jul;14(1):240–245. doi: 10.1128/iai.14.1.240-245.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones G. W., Rutter J. M. The association of K88 antigen with haemagglutinating activity in porcine strains of Escherichia coli. J Gen Microbiol. 1974 Sep;84(1):135–144. doi: 10.1099/00221287-84-1-135. [DOI] [PubMed] [Google Scholar]
- Moon H. W., Isaacson R. E., Pohlenz J. Mechanisms of association of enteropathogenic Escherichia coli with intestinal epithelium. Am J Clin Nutr. 1979 Jan;32(1):119–127. doi: 10.1093/ajcn/32.1.119. [DOI] [PubMed] [Google Scholar]
- Nelson E. T., Clements J. D., Finkelstein R. A. Vibrio cholerae adherence and colonization in experimental cholera: electron microscopic studies. Infect Immun. 1976 Aug;14(2):527–547. doi: 10.1128/iai.14.2.527-547.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tweedy J. M., Park R. W., Hodgkiss W. Evidence for the presence of fimbriae (pili) on vibrio species. J Gen Microbiol. 1968 Apr;51(2):235–244. doi: 10.1099/00221287-51-2-235. [DOI] [PubMed] [Google Scholar]
- Watanabe Y., Verwey W. F. Protective antigens from El Tor vibrios. 1. The preparation and properties of a purified protective antigen from an El Tor vibrio (Ogawa subtype). Bull World Health Organ. 1965;32(6):809–821. [PMC free article] [PubMed] [Google Scholar]


