Abstract
The majority of cholesterol reduction therapies, such as the statin drugs, work primarily by inducing the expression of hepatic low-density lipoprotein receptors (LDLRs), rendering these therapeutics only partially effective in animals lacking LDLRs. Although thyroid hormones and their synthetic derivatives, often referred to as thyromimetics, have been clearly shown to reduce serum cholesterol levels, this action has generally been attributed to their ability to increase expression of hepatic LDLRs. Here we show for the first time that the thyroid hormone T3 and the thyroid hormone receptor-β selective agonists GC-1 and KB2115 are capable of markedly reducing serum cholesterol in mice devoid of functional LDLRs by inducing Cyp7a1 expression and stimulating the conversion and excretion of cholesterol as bile acids. Based on this LDLR-independent mechanism, thyromimetics such as GC-1 and KB2115 may represent promising cholesterol-lowering therapeutics for the treatment of diseases such as homozygous familial hypercholesterolemia, a rare genetic disorder caused by a complete lack of functional LDLRs, for which there are limited treatment options because most therapeutics are only minimally effective.
Familial hypercholesterolemia (FH) is a genetic disorder characterized by elevated levels of serum cholesterol and associated with early-onset cardiovascular disease. FH is a manifestation of a misfunctional allele encoding the low-density lipoprotein receptor (LDLR), the primary receptor responsible for clearing cholesterol-laden low-density lipoprotein (LDL) particles from the blood. Although FH, in its heterogenous form, affects one in 500 people (1), it can generally be treated with 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitors, a class of drugs collectively referred to as the statins. However, approximately one in 1 million people are affected by homozygous FH (hFH) and possess essentially no functional LDLRs. These patients manifest severe and widespread atherosclerosis leading to coronary events at ages as young as 1–2 yr, and untreated hFH generally results in death due to cardiovascular disease, commonly in the teen years. Although conventional treatment can extend the life expectancy of hFH patients, longer-term survival often requires unusual methods, such as liver transplantation or repeated plasmapheresis, which are generally not available to many patients. Because most common therapies used to reduce serum cholesterol levels, such as the statins and bile acid sequestrants, ultimately rely on a secondary induction of hepatic LDLRs to mediate the uptake of cholesterol containing lipoproteins from the plasma into the liver, their utility in treating hFH is limited. Thus, new therapeutic options for the treatment of hFH are sorely needed.
Although endogenous thyroid hormones (THs), such as T3, can lower serum cholesterol, their use in the treatment of hypercholesterolemia is limited due to deleterious cardiac and other side effects. This has led to development of thyromimetics, thyroid hormone analogs designed to maintain the beneficial effects of THs, such as cholesterol reduction and weight loss, without undesirable side effects (2). As a class, thyromimetics have been shown to lower serum cholesterol levels in rodents, monkeys, and humans. Although it is clear that THs and thyromimetics reduce serum cholesterol levels, the mechanism behind this reduction is less apparent. Thyroid hormone receptor (TR) agonists have been reported to reduce plasma cholesterol levels via induction of several different pathways that include increasing bile acid synthesis via the induction of Cyp7a1 (3–5) and increasing reverse cholesterol transport by increasing expression of the high-density lipoprotein (HDL) receptor scavenger receptor type B, class I (SR-BI) (3, 5). However, the most notable rationale has been that THs can stimulate expression of the LDLR (4, 6, 7). In support of this mechanistic view, all reports describing the use of T3 or thyromimetics in LDLR−/− mice have found the compounds to be ineffective at lowering plasma cholesterol levels in this genetic background (4, 5), despite the same compounds demonstrating efficacy in mice that possessed functional LDLRs.
GC-1 is a thyromimetic that has been studied extensively and has been shown to lower LDL cholesterol in multiple species (8, 9). A previous report described that GC-1 was able to reduce serum cholesterol by 25% in wild-type mice, with no accordant increase observed in LDLR levels, and this effect was attributed to increases in reverse cholesterol transport mediated by increased expression of the HDL receptor, SR-BI (3). Given that GC-1 was able to reduce serum cholesterol in the absence of any apparent increase in LDLR levels, it seemed reasonable to determine whether this compound retained at least partial efficacy in mice lacking functional LDLRs. Here we show that the thyroid hormone T3 and the thyromimetics GC-1 and KB2115 are potent and effective reducers of serum LDL cholesterol in LDLR−/− mice and present evidence to show that this effect is related to an increased conversion of cholesterol to bile acids and a subsequent increase in bile acid secretion. We propose that thyromimetics may represent a novel therapeutic approach to the treatment of hFH.
Materials and Methods
Animals
Male LDLR−/− and C57 mice were obtained from Jackson Labs (Bar Harbor, ME) and housed in a temperature-controlled environment with 12-h light, 12-h dark cycles and fed standard irradiated rodent chow ad libitum (TD.2918; Harlan Teklad, Indianapolis, IN). All mice used in the studies were male aged matched of 4 months of age. Before treatment mice were fed a Western diet containing 0.2% cholesterol (D12079B; Research Diets, New Brunswick, NJ) for 12 wk. Unless otherwise noted, at 6 wk old, mice were fed a Western diet containing 0.2% cholesterol (D12079B; Research Diets) for 12 wk. All animal experiments were approved by the Methodist Hospital Research Institute Institutional Animal Care and Use Committee.
Animal treatments
Triiodothyronine (T2877; Sigma, St. Louis, MO), KB2115 (10011054; Cayman Chemicals Co., Ann Arbor, MI), and atorvastatin (sc-337542; Santa Cruz Biotechnology, Santa Cruz, CA) dissolved in dimethylsulfoxide were administered by ip injection. GC-1 was administered by either ip injection or admixed in the diet (Western diet; Research Diets) and provided ad libitum. Details of each experiment are described in the figure legends. In all cases, animals in the control group received the appropriate vehicle solutions and diets identical with the treatment groups.
Lipid and lipoprotein analysis
For hepatic lipid analysis, liver tissue was homogenized and total lipids were extracted from liver using a Folch solution (chloroform-methanol, 2:1), precipitated with sodium chloride, dried, and resuspended in 2% Triton X-100 PBS. Plasma and tissue lipid extracts were assayed for cholesterol and triglycerides using commercial kits (Thermo Scientific, Waltham, MA). For lipoprotein profiling, pooled plasma was separated by fast protein liquid chromatography using two Superose 6 columns (GE Healthcare, Indianapolis, IN) linked in tandem, 300-ml fractions were collected, and lipid levels were analyzed in each fraction using a fluorescence enzymatic assay (Cayman).
Plasma 7α-hydroxy-4-cholesten-3-one (C4) levels
Plasma C4 levels were measured as described (8) with modifications. Deuterium-labeled C4 (Santa Cruz Biotechnology) internal standard was added to 10 μl of plasma, followed by ammonium sulfate and acetonitrile precipitation, and the supernatant was dried and resuspended in methanol. Plasma C4 levels were measured using liquid chromatography electrospray ionization-tandem mass spectrometry (12) on an Acquity UPLC system (Waters Corp., Milford, MA) and XevoTQ mass spectrometer (Waters Corp.) operated in the MRM mode using positive ion electrospray conditions. C4 levels were normalized to total cholesterol levels.
Fecal sterol analysis
Mice were acclimated to metabolic cages before a 72-h feces collection. Dried feces were boiled in alkaline methanol (1 m NaOH/methanol, 1:3) after addition of 5α-cholestane as an internal standard for neutral sterol analysis. After cooling to room temperature, neutral sterols were extracted using petroleum ether, and the combined organic layers were dried and redissolved in hexane for gas chromatography analysis. Total bile acids in the aqueous layer were measured using a spectrophotometric enzymatic assay (Diazyme, Poway, CA).
Protein extraction and Western blot analysis
Liver tissue was homogenized in tissue extraction reagent (Invitrogen, Carlsbad, CA) containing complete protease inhibitor (Roche Diagnostics, Indianapolis, IN). Equivalent amounts of total protein were separated by SDS-PAGE, transferred to polyvinylidene difluoride membranes, and probed with anti-SR-BI (ab3), anti-LDLR (ab52818), or anti-β-actin (ab6276) antibody (Abcam, Cambridge, MA) and secondary conjugated-horseradish peroxidase antirabbit or (sc-2004) antimouse (sc-2005) antibody (Santa Cruz Biotechnology). The proteins were visualized using Amersham enhanced chemiluminescence Western blotting detection regents (GE Healthcare) and autoradiography.
RNA isolation, reverse transcription, and real-time quantitative PCR
Total RNA was extracted and isolated using Qiazol and RNeasy minikit according to the manufacturer's protocol (QIAGEN, Valencia, CA). First-strand cDNA was transcribed from 2.5 mg of total RNA using SuperScript VILO cDNA synthesis kit (Invitrogen). TaqMan real-time PCRs were performed on a LightCycler 480 real-time PCR system (Roche), and relative mRNA levels were calculated by comparative the cycle threshold method using β-actin as the internal control. TaqMan primer/probe sets supplied by Applied Biosystems (Foster City, CA) were used (assay identifications are available upon request).
Hepatic triglyceride and apolipoprotein B (ApoB) secretion rate determination
Triglyceride and ApoB secretion rate was performed as previously described (10). Mice fasted for 4 h were injected with poloxamer 407 (1000 mg/kg) and 200 μCi of [35S]methionine. Blood samples were collected before the injection and at 60 and 120 min afterward. The concentration of serum triglycerides was measured using commercial kits. For ApoB secretion rate, plasma samples were separated on a 3–8% SDS-PAGE gel. The gel was dried under vacuum and exposed to x-ray film. Bands representing newly synthesized [35S]-labeled ApoB were excised and counted via liquid scintillation.
Statistical analysis
All results are expressed as mean ± sem. Statistical analysis was performed using GraphPad Prism software (GraphPad Inc., San Diego, CA). Comparisons of groups were performed using a Student's t test. A P < 0.05 was considered significant.
Results
GC-1 lowers serum cholesterol in LDLR−/− mice
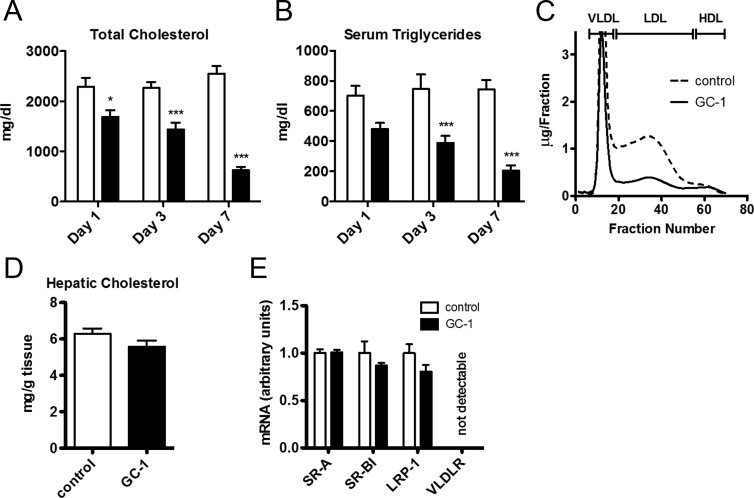
LDLR−/− mice fed a high-fat, high-cholesterol diet exhibited serum cholesterol levels that were elevated approximately 10-fold relative to wild-type mice fed a similar diet (Fig. 1A). Somewhat unexpectedly, supplementation of diets with the thyromimetic GC-1 triggered a rapid and marked decrease in serum cholesterol and triglycerides (Fig. 1, A and B), with both being reduced to about one third of their pretreatment levels in 7 d. Fast protein liquid chromatography fractionation of cholesterol containing lipoproteins indicated that the reduction in serum cholesterol resulted primarily from a reduction in LDL cholesterol (Fig. 1C) because little to no changes were apparent in the cholesterol content of very low-density lipoprotein (VLDL) and HDL fractions. Thus, GC-1 can induce dramatic LDL cholesterol lowering in the absence of LDL receptors in mice.
Fig. 1.
GC-1 reduces serum cholesterol and triglyceride levels in LDLR−/− mice. A and B, Male LDLR−/− mice were administered GC-1 (4.8 mg/kg diet) in a Western diet containing 0.2% cholesterol or a control diet lacking GC-1. Blood samples were collected at the times indicated and assayed for serum cholesterol and triglycerides (n = 6 per group). C, Size exclusion fractionation of serum lipoproteins from mice administered GC-1 as in A for 10 d (blood from five animals was pooled for each trace.) D, After 10 d of GC-1 treatment, mice were killed and hepatic cholesterol was extracted with chloroform and methanol and quantified (n = 5 per group). E, mRNA concentrations were measured by real-time quantitative PCR using total mRNA prepared from liver sections of mice treated as in D (n = 5 per group). *, P < 0.05; ***, P < 0.001.
GC-1 does not induce alternative lipoprotein receptors, including SR-BI
The majority of cholesterol reduction therapies, including the statins, work via an sterol regulatory element-binding protein (SREBP)-mediated increase in LDLR levels, which results in increased LDLR-dependent uptake of LDL cholesterol from the plasma compartment into the liver. For example, transgenic mice that overexpress SREBP-1a have reduced serum cholesterol levels despite an increased rate of cholesterol synthesis due to a 6-fold increase in liver cholesterol storage that is mediated via the LDLR (11). To assess whether GC-1 caused a similar net transfer of cholesterol from the serum into hepatocytes of LDLR−/− mice, perhaps via the induction of alternative hepatic lipoprotein receptors, liver cholesterol levels were measured. Total hepatic cholesterol content was unaffected by GC-1, and expression levels of alternative hepatic lipoprotein receptors were unchanged after GC-1 treatment (Fig. 1, D and E). Thus, the GC-1-dependent reduction in serum cholesterol was not due to a net increase in liver cholesterol storage.
Several reports have suggested that THs and GC-1 reduce serum cholesterol by increasing hepatic expression of the HDL receptor, SR-BI, and stimulating aspects of reverse cholesterol transport through the HDL pathway (10). However, GC-1 treatment did not induce expression of SR-BI at either the gene or protein level in LDLR−/− mice (Fig. 1E and Supplemental Fig. 1, published on The Endocrine Society's Journals Online web site at http://endo.endojournals.org). Given the absence of effects on HDL cholesterol levels and the HDL receptor, SR-BI, our data are largely inconsistent with the idea that marked GC-1-dependent reductions in LDL cholesterol levels in LDLR−/− mice are related to changes in HDL uptake.
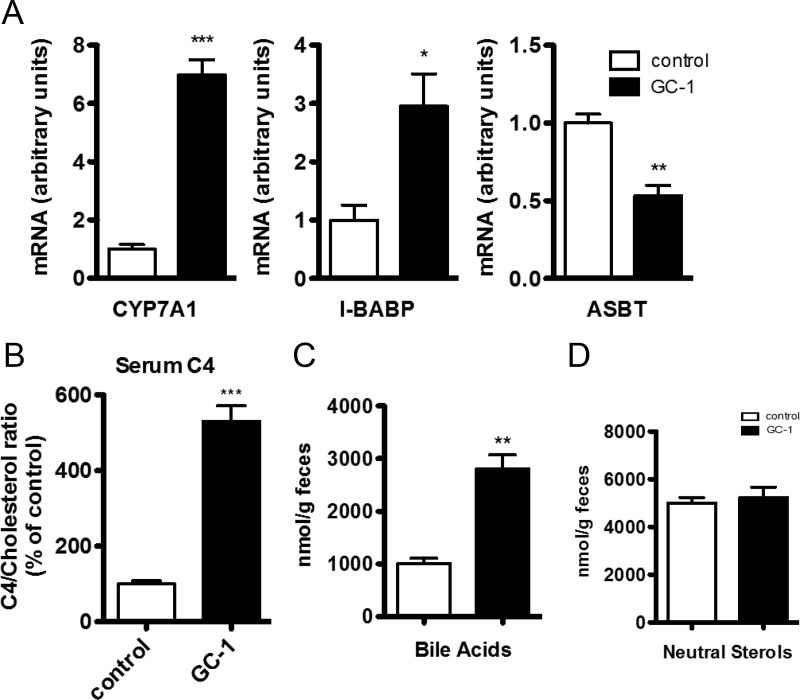
GC-1 strongly induces Cyp7a1, resulting in increased bile acid synthesis and secretion in LDLR−/− mice
Although we were unable to find mechanistic evidence of GC-1-dependent increases in cholesterol uptake into the liver, subsequent steps of reverse cholesterol transport, in particular numerous mediators of bile acid synthesis and excretion, were strongly affected by GC-1 treatment. Hepatic expression of Cyp7a1, the enzyme responsible for the rate-limiting step in the synthesis of bile acids from cholesterol, was increased 7-fold after GC-1 treatment (Fig. 2A). Consistent with the large increase in Cyp7a1 levels, serum levels of C4, a marker of bile acid synthesis and a physiological indicator of Cyp7a1 activity (12), were increased nearly 5-fold after GC-1 treatment (Fig. 2B), and fecal excretion of bile acids was increased 2.5-fold (Fig. 2C). In accord with increased synthesis of bile acids and an expansion of the bile acid pool, expression levels of ileal bile acid-binding protein, a reporter of intestinal bile acid levels (13), was increased approximately 3-fold (Fig. 2A). Apical socium-dependent bile acid transporter, which is responsible for reabsorption of bile acids in the ileum and whose inhibition has been shown to increase fecal bile acid excretion (14), was significantly reduced by GC-1 (Fig. 2A).
Fig. 2.
Effect of GC-1 on bile acid synthesis and fecal excretion. Male LDLR−/− mice were administered GC-1 (0.8 mg/kg diet) in a Western diet containing 0.2% cholesterol or control diet lacking GC-1. A, mRNA concentrations were measured by real-time quantitative PCR using total mRNA prepared from either liver (Cyp7a1) or ileum (I-BABP and ASBT) sections of mice treated with GC-1 for 10 d (n = 5 per group). B, Serum C4 levels were measured via mass spectrometry and normalized relative to serum cholesterol levels. C and D, Feces were collected from d 5 to d 8 of treatment and analyzed for bile acids and neutral sterols (n = 4–5 per group). *, P < 0.05; **P < 0.01; ***, P < 0.001.
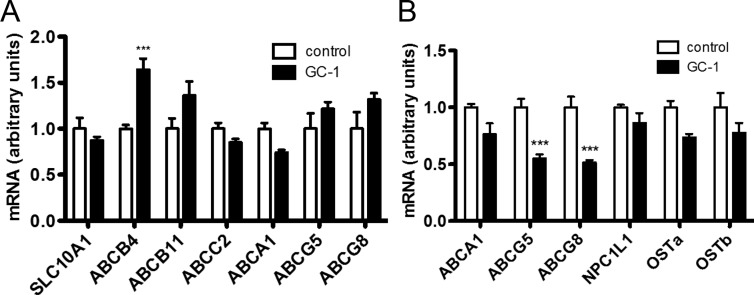
Although hepatic ATP-binding cassette, sub-family B, member 4, which is involved in bile acid and phospholipid excretion, was induced by GC-1, other hepatic transporters commonly implicated in cholesterol uptake and efflux were unaltered (Fig. 3A). Intestinal transporters including the liver X receptor and farnesoid X receptor target genes ABCG5/8 (15), and OSTa/b (16), whose induction has been linked to increased cholesterol and bile efflux were either unaltered or decreased (Fig. 3B). Although decreased expression of intestinal ABCG5/8 would be expected to increase the uptake of neutral sterols (15, 17), the net excretion of neutral sterols was unaltered (Fig. 2D), indicating that compensatory mechanisms, such as the actions of hepatic ABCG5/8 whose levels were unchanged, offset any increase in the uptake of neutral sterols. Collectively these data suggest that the induction of Cyp7a1 by GC-1 strongly affects bile acid synthesis and excretion pathways evoking a net flux of cholesterol from the plasma compartment into the bile pool as bile acids, which are then excreted in the feces.
Fig. 3.
Effect of GC-1 on expression of hepatic and intestinal transporters involved in cholesterol homeostasis. mRNA concentrations were measured by real-time quantitative PCR using total mRNA prepared from either liver (A) or ileum (B) sections of mice treated with GC-1 as in Fig. 1 for 10 d (n = 5 per group). ***, P < 0.001.
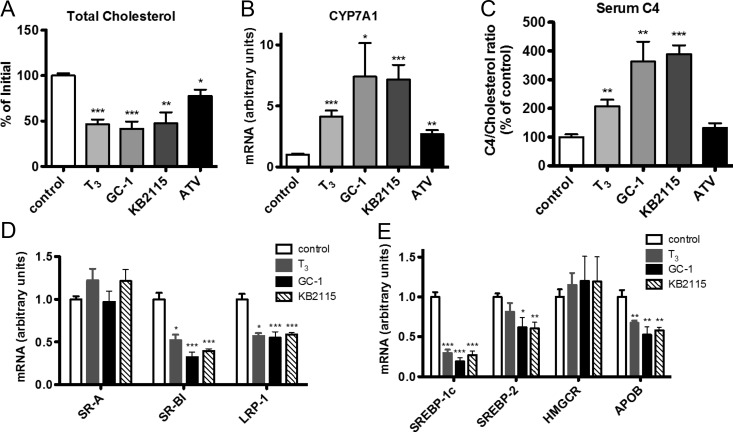
TR agonists reduce serum cholesterol more effectively than statins in LDLR−/− mice
To determine whether cholesterol reduction in LDLR−/− mice was a property unique to GC-1, we compared the efficacy of GC-1 with that of another thyromimetic, KB2115, and the thyroid hormone T3, as well as atorvastatin, the last of which represents one of the most powerful statin drugs (18). Mice treated with GC-1, KB2115, or T3 for 10 d exhibited similar levels of cholesterol reduction (Fig. 4A), indicating that the ability to significantly reduce serum cholesterol in the absence of functional LDLRs extends beyond GC-1 and is perhaps a general capability of all TR agonists. All three TR agonists increased Cyp7a1 expression between 4- and 7-fold and evoked a correspondent increase in bile acid synthesis, as indicated by increased plasma C4 levels (Fig. 4, B and C). Consistent with the idea that the statins and TR agonists use distinct mechanisms of cholesterol reduction, T3 and the thyromimetics were more effective than atorvastatin at reducing serum cholesterol in LDLR−/− mice, and only the TR ligands increased bile acid synthesis, as indicated by serum C4 levels. Expression levels of hepatic lipoprotein receptors and genes involved in cholesterol homeostasis were affected similarly by all TR agonists (Fig. 4, D and E). Notably, SR-BI was repressed by all treatments, further arguing against SR-BI-mediated reverse cholesterol transport playing a key role in the observed cholesterol reduction.
Fig. 4.
Effects of T3, thyromimetics, and statins on serum cholesterol in LDLR−/− mice. Changes in serum cholesterol levels (A), hepatic Cyp7a1 expression (B), serum C4 levels (C), and expression of hepatic of lipoprotein receptors (D) and regulatory genes (E) were measured from LDLR−/− mice fed a Western diet containing 0.2% cholesterol and treated with 20 mg/kg atorvastatin (ATV), 0.3 mg/kg T3, 0.3 mg/kg KB2115, 0.3 mg/kg GC-1, or vehicle administered via ip injection for 10 d (n = 5 per group). *, P < 0.05; **, P < 0.01; ***, P < 0.001.
TR agonists reduce plasma lipids despite increased hepatic VLDL and ApoB secretion
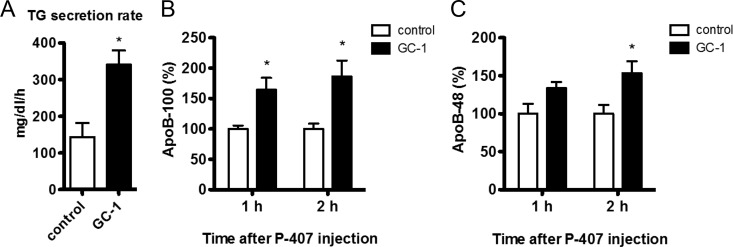
That hepatic cholesterol levels did not decrease despite markedly increased rates of bile acid synthesis and fecal excretion suggests that a compensatory mechanism must be responsible for preventing a reduction in hepatic cholesterol. Treatment with T3, GC-1, or KB2115 lowered hepatic expression of the lipogenic regulators SREBP-1c and SREBP-2 and decreased ApoB mRNA levels, leading us to hypothesize that the decreased hepatic secretion of ApoB-containing lipoproteins may account for the reduced levels of serum lipids as well as the maintenance of hepatic cholesterol. The rate of accumulation of triglycerides and nascent ApoB in the blood was determined after the coadministration of [35S]methionine and the lipoprotein lipase inhibitor poloxamer 407. Hepatic secretion of both triglycerides and [35S]methionine-labeled ApoB increased strongly after GC-1 treatment (Fig. 5). Although unexpected, these results are consistent with previous reports demonstrating that T3 treatment and Cyp7a1 overexpression increase hepatic ApoB and triglyceride secretion (19–21), despite both TH action and Cyp7a1 activity being clearly associated with net reductions in serum ApoB containing lipoproteins. Thus, the relationship between THs and their hepatic target Cyp7a1 with serum lipoprotein levels is likely complex.
Fig. 5.
Hepatic triglyceride and ApoB secretion rates. Male LDLR−/− mice were administered GC-1 (4.8 mg/kg diet) in a Western diet containing 0.2% cholesterol or control diet lacking GC-1 for 10 d. After a 4-h fast, mice were injected with poloxamer 407 (P-407) and [35S]methionine. Blood was collected at time 0, 1, and 2 h and assayed for triglycerides (TG) (A), ApoB-100 (B), and ApoB-48 (C) (n = 3–4 per group). *, P < 0.05.
GC-1 affects bile acid synthesis similarly in mice that possess functional LDLRs
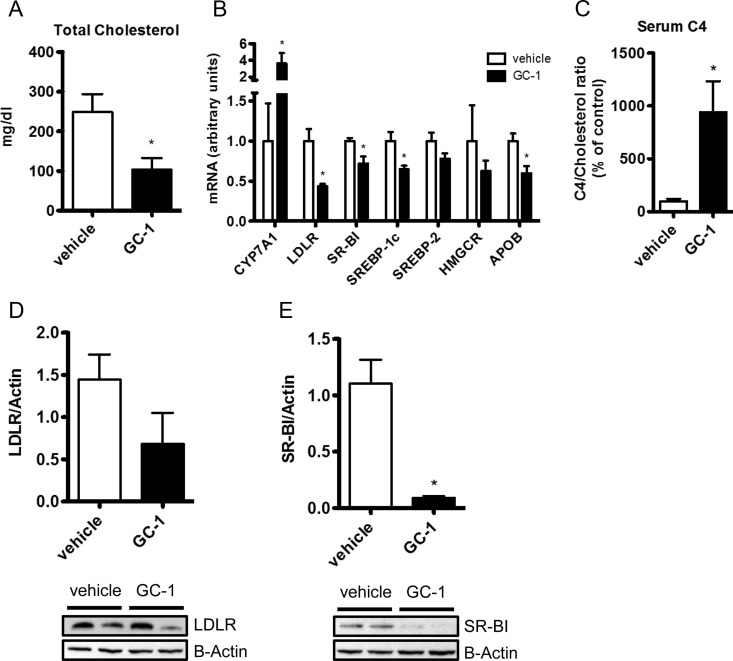
To assess whether TR agonists use distinct mechanisms of cholesterol reduction in LDLR−/− mice and wild-type mice that possess functional LDLRs, C57 mice fed a Western diet were treated with either GC-1 or T3. GC-1 treatment resulted in a complete normalization of serum cholesterol, markedly increased hepatic Cyp7a1 expression and rate of bile acid synthesis, and altered hepatic gene expression (Fig. 6, A–C) in a manner remarkably similar to that seen when LDLR−/− mice were administered GC-1 (Fig. 4). GC-1 led to a significant down-regulation of the hepatic lipoprotein receptors LDLR and SR-BI, at both the gene and protein level (Fig. 6, B, D, and E), making it unlikely that cholesterol uptake by either receptor was responsible for the decrease in serum cholesterol. Treatment with T3 at a level similar to that tolerated by LDLR−/− mice proved to be toxic to this strain, precluding detailed analysis of the data. Qualitatively, however, T3 appeared to act in a manner analogous to GC-1 (Supplemental Fig. 2). These data suggest that the hepatic induction of the lipoprotein receptors LDLR and SR-BI is likely unnecessary for the cholesterol lowering effects of TR activation.
Fig. 6.
GC-1 treatment elicits similar effects in mice with functional LDLRs. Male C57 mice fed a Western diet containing 0.2% cholesterol were administered 0.3 mg/kg/d GC-1 or vehicle via ip injection. Changes in serum cholesterol (A), hepatic gene expression (B), serum C4 levels (C), and LDLR (D) and SR-BI protein (E) were measured from mice treated with GC-1 for 10 d (n = 4–5 per group). *, P < 0.05.
Discussion
In this study, we report that the thyroid hormone T3 and two TRβ-selective thyromimetics, GC-1 and KB2115, bring about marked reductions in serum total and LDL cholesterol in LDLR−/− mice, which lack functional LDLRs. Our results suggest that cholesterol reduction in these mice is mediated primarily via the induction of Cyp7a1, leading to increased fecal excretion of cholesterol as bile acids, and does not require the action of alternative lipoprotein receptors such as the HDL receptor SR-BI. This finding suggests that current models, which generally suggest that the effects of THs and thyromimetics upon serum cholesterol are mediated principally by the increased expression of the hepatic lipoprotein receptors LDLR and SR-BI, are, at best, incomplete.
The actions of many commonly used cholesterol lowering therapeutics, such as the statins, bile sequestrants, and the NPC1L1 inhibitor ezetimibe, are mediated in large part by increased expression of LDLRs. Because THs have been shown to induce the LDLR similarly, it is not surprising that increased LDLR expression is the mechanism most common ascribed to cholesterol reduction by THs (22). Although several other mechanisms of cholesterol reduction by THs have been proposed, the lack of prior reports demonstrating that THs and thyromimetics can reduce serum cholesterol in the absence of LDLRs has led to the inference that cholesterol reduction by TR agonists is primarily an LDLR-dependent effect. Results of the current study reveal this assumption to be incorrect and demonstrate that TR activation can reduce serum cholesterol via an LDLR-independent mechanism.
Of the potential LDLR-independent mechanisms of cholesterol reduction by TR activation, the most commonly proposed is induction of the HDL receptor, SR-BI, leading to an increased rate of reverse cholesterol transport (3). Although several reports have indicated that THs can stimulate SR-BI expression, we do not believe that this effect plays an important role in cholesterol reduction in LDLR−/− mice. First, we did not observe changes in SR-BI expression, at either the transcript or protein level, in LDLR−/− or C57 mice. Second, cholesterol reduction in LDLR−/− mice is largely restricted to the LDL fraction, and there is little change in HDL cholesterol after thyromimetic treatment. Finally, although SR-BI induction and increased reverse cholesterol transport appears a quite reasonable mechanism by which THs could reduce cholesterol in most rodent species, which maintain the majority of their serum cholesterol as HDL cholesterol, it is difficult to rationalize how such a large decrease in LDL cholesterol levels could result from this mechanism in LDLR−/− mice, for which nearly all serum cholesterol is carried via LDL particles and have limited ability to transfer cholesterol between LDL and HDL particles due to their lack of cholesteryl ester transferase protein activity.
Instead, our results suggest that the TR agonists tested here reduce serum cholesterol levels in LDLR−/− mice by strongly inducing Cyp7a1, resulting in increased cholesterol to bile acid conversion and increased fecal bile acid excretion. That similar effects on Cyp7a1 expression and bile acid synthesis were observed in wild-type C57 mice, in the absence of LDLR or SR-BI induction, suggest to us that the reductions in serum cholesterol brought about by THs and TR activation may be mediated primarily via the induction of Cyp7a1. Indeed, the effects elicited by TR activation correspond nearly wholly with those seen in mice after hepatic overexpression of Cyp7a1. Increased Cyp7a1 activity has been demonstrated to be sufficient to lower plasma cholesterol levels in LDLR−/− mice and Watanabe heritable hyperlipidemia rabbits, both of which lack functional LDLRs (23–25). In accordance with our findings, this action did not require the induction of alternative hepatic lipoprotein receptors or hepatic cholesterol efflux transporters (24). The TR agonists studied did not reduce hepatic cholesterol levels despite strongly increasing hepatic excretion of bile acids. This is presumably due to increased hepatic synthesis of cholesterol because Cyp7a1 overexpression has been shown to evoke a compensatory increase in de novo cholesterol synthesis, preventing a decrease in hepatic cholesterol (25, 26) and T3 treatment has been shown to have similar effects on hepatic cholesterol synthesis (27).
The mechanism by which Cyp7a1 induction affects plasma lipoproteins appears to be multivariate and is not well understood. Consistent with Cyp7a1 being the primary mediator behind the lipid-lowering actions of TR activation, the manner by which the TR agonists tested reduce serum cholesterol and triglycerides appears to be correspondingly complex. Similar to prior reports, which have shown that T3 and Cyp7a1 overexpression increase hepatic triglyceride and VLDL secretion (19–21, 28), GC-1 treatment elicited an increase in the secretion of triglycerides and ApoB despite markedly decreasing total serum triglyceride and LDL cholesterol levels. This paradoxical effect of hepatic Cyp7a1 activation may help explain the divergent reports relating TR agonist treatment with serum triglyceride levels (3, 4, 8). Indeed, we have found that, in contrast to the results presented here, the treatment of nonhypertriglyceridemic mice with thyromimetics increases serum triglyceride levels (data not shown).
Because Cyp7a1 has been shown previously to be a TR-regulated gene in mice (29) and rats (30), it is perhaps not surprising that these compounds increased Cyp7a1 expression. However, the magnitude of the cholesterol reduction brought about by Cyp7a1 induction was quite unanticipated in light of the fact that the aforementioned studies demonstrating the ability of Cyp7a1 induction to reduce serum cholesterol used transgenic and viral overexpression methods. Thyromimetics could represent a novel avenue for treatment of defects in cholesterol metabolism associated with hFH. In accordance with animal studies, in which Cyp7a1 overexpression leads to reduced serum cholesterol and triglycerides and increased bile acid excretion, human deficiency in Cyp7a1 results in elevated serum cholesterol and triglycerides and an appreciably reduced rate of bile acid excretion (31), suggesting that increasing Cyp7a1 expression may be a viable LDLR-independent pharmacological strategy to reduce serum cholesterol in man. The regulation of Cyp7a1 has been reported to differ between rodents and humans (32), implying that these results may not translate to clinical studies. However, similar to our findings here, KB2115 has been shown to strongly stimulate bile acid synthesis in humans (33), increasing serum C4 levels by greater than 100%, indicating that a similar mechanism may play a role in the cholesterol-lowering effects of TR activation in man. This potential ability to reduce cholesterol via a mechanism distinct from the statin drugs may also provide a rationale behind the clinical efficacy of KB2115, which has been shown to act additively when administered concurrently with statins, further reducing total and LDL cholesterol levels when given to hypercholesterolemic patients already being treated with statins (34).
In conclusion, the thyroid hormone T3 and the thyromimetics, GC-1 and KB2115, reduce serum cholesterol via an LDLR-independent mechanism that appears to be mediated primarily by the induction of Cyp7a1 and increased bile acid synthesis, making it distinct from that of most other cholesterol-lowering therapies. This class of compounds may have value in treating high cholesterol, especially for diseases such as hFH, for which LDLR induction in minimally effective. Given the lack of effective treatment options for affected hFH patients and the severity of the disease, thyromimetics such as GC-1 and KB2115 may warrant evaluation for their ability to lower serum cholesterol in this population.
Supplementary Material
Acknowledgments
We thank Dr. David Engler from The Methodist Hospital Research Institute proteomics core for assistance with the plasma C4 analysis.
This work was supported by National Institutes of Health Grant RC4 DK090849 (to J.D.B. and P.W.) and Grant E-0004 from the Emerging Technology Fund of Texas and the Robert A. Welch Foundation. The University of Cincinnati Mouse Metabolic Phenotyping Center is supported by National Institutes of Health Grant U24 DK059630.
Disclosure Summary: J.Z.L., A.J.M., W.A.H., J.D.B., P.W., and K.J.P. have nothing to disclose. J.-Å.G. is a shareholder and consultant for KaroBio AB.
Footnotes
- ApoB
- Apolipoprotein B
- C4
- 7α-hydroxy-4-cholesten-3-one
- FH
- familial hypercholesterolemia
- HDL
- high-density lipoprotein
- hFH
- homozygous FH
- LDL
- low-density lipoprotein
- LDLR
- LDL receptor
- SR-BI
- scavenger receptor type B, class I
- SREBP
- sterol regulatory element-binding protein
- TH
- thyroid hormone
- TR
- thyroid hormone receptor
- VLDL
- very low-density lipoprotein.
References
- 1. Goldstein JL, Hobbs H., Brown MS. 2001. Familial hypercholesterolemia. In: The metabolic, molecular bases of inherited disease. New York: McGraw-Hill [Google Scholar]
- 2. Baxter JD, Webb P. 2009. Thyroid hormone mimetics: potential applications in atherosclerosis, obesity and type 2 diabetes. Nat Rev Drug Discov 8:308–320 [DOI] [PubMed] [Google Scholar]
- 3. Johansson L, Rudling M, Scanlan TS, Lundåsen T, Webb P, Baxter J, Angelin B, Parini P. 2005. Selective thyroid receptor modulation by GC-1 reduces serum lipids and stimulates steps of reverse cholesterol transport in euthyroid mice. Proc Natl Acad Sci USA 102:10297–10302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Erion MD, Cable EE, Ito BR, Jiang H, Fujitaki JM, Finn PD, Zhang BH, Hou J, Boyer SH, van Poelje PD, Linemeyer DL. 2007. Targeting thyroid hormone receptor-β agonists to the liver reduces cholesterol and triglycerides and improves the therapeutic index. Proc Natl Acad Sci USA 104:15490–15495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Tancevski I, Demetz E, Eller P, Duwensee K, Hoefer J, Heim C, Stanzl U, Wehinger A, Auer K, Karer R, Huber J, Schgoer W, Van Eck M, Vanhoutte J, Fievet C, Stellaard F, Rudling M, Patsch JR, Ritsch A. 2010. The liver-selective thyromimetic T-0681 influences reverse cholesterol transport and atherosclerosis development in mice. PLoS ONE 5:e8722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ness GC, Lopez D, Chambers CM, Newsome WP, Cornelius P, Long CA, Harwood HJ., Jr 1998. Effects of l-triiodothyronine and the thyromimetic l-94901 on serum lipoprotein levels and hepatic low-density lipoprotein receptor, 3-hydroxy-3-methylglutaryl coenzyme A reductase, and apo A-I gene expression. Biochem Pharmacol 56:121–129 [DOI] [PubMed] [Google Scholar]
- 7. Ness GC, Zhao Z. 1994. Thyroid hormone rapidly induces hepatic LDL receptor mRNA levels in hypophysectomized rats. Arch Biochem Biophys 315:199–202 [DOI] [PubMed] [Google Scholar]
- 8. Trost SU, Swanson E, Gloss B, Wang-Iverson DB, Zhang H, Volodarsky T, Grover GJ, Baxter JD, Chiellini G, Scanlan TS, Dillmann WH. 2000. The thyroid hormone receptor-β-selective agonist GC-1 differentially affects plasma lipids and cardiac activity. Endocrinology 141:3057–3064 [DOI] [PubMed] [Google Scholar]
- 9. Grover GJ, Egan DM, Sleph PG, Beehler BC, Chiellini G, Nguyen NH, Baxter JD, Scanlan TS. 2004. Effects of the thyroid hormone receptor agonist GC-1 on metabolic rate and cholesterol in rats and primates: selective actions relative to 3,5,3′-triiodo-l-thyronine. Endocrinology 145:1656–1661 [DOI] [PubMed] [Google Scholar]
- 10. Voyiaziakis E, Ko C, O'Rourke SM, Huang LS. 1999. Genetic control of hepatic apoB-100 secretion in human apoB transgenic mouse strains. J Lipid Res 40:2004–2012 [PubMed] [Google Scholar]
- 11. Shimano H, Horton JD, Hammer RE, Shimomura I, Brown MS, Goldstein JL. 1996. Overproduction of cholesterol and fatty acids causes massive liver enlargement in transgenic mice expressing truncated SREBP-1a. J Clin Invest 98:1575–1584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Honda A, Yamashita K, Numazawa M, Ikegami T, Doy M, Matsuzaki Y, Miyazaki H. 2007. Highly sensitive quantification of 7a-hydroxy-4-cholesten-3-one in human serum by LC-ESI-MS/MS. J Lipid Res 48: 458–464 [DOI] [PubMed] [Google Scholar]
- 13. Kanda T, Foucand L, Nakamura Y, Niot I, Besnard P, Fujita M, Sakai Y, Hatakeyama K, Ono T, Fujii H. 1998. Regulation of expression of human intestinal bile acid-binding protein in Caco-2 cells. Biochem J 330(Pt 1):261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bhat BG, Rapp SR, Beaudry JA, Napawan N, Butteiger DN, Hall KA, Null CL, Luo Y, Keller BT. 2003. Inhibition of ileal bile acid transport and reduced atherosclerosis in apoE−/− mice by SC-435. J Lipid Res 44:1614–1621 [DOI] [PubMed] [Google Scholar]
- 15. Yu L, Li-Hawkins J, Hammer RE, Berge KE, Horton JD, Cohen JC, Hobbs HH. 2002. Overexpression of ABCG5 and ABCG8 promotes biliary cholesterol secretion and reduces fractional absorption of dietary cholesterol. J Clin Invest 110:671–680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Rao A, Haywood J, Craddock AL, Belinsky MG, Kruh GD, Dawson PA. 2008. The organic solute transporter α-β, Ostα-Ostβ, is essential for intestinal bile acid transport and homeostasis. Proc Natl Acad Sci USA 105:3891–3896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Yu L, Hammer RE, Li-Hawkins J, Von Bergmann K, Lutjohann D, Cohen JC, Hobbs HH. 2002. Disruption of Abcg5 and Abcg8 in mice reveals their crucial role in biliary cholesterol secretion. Proc Natl Acad Sci USA 99:16237–16242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zadelaar S, Kleemann R, Verschuren L, de Vries-Van der Weij J, van der Hoorn J, Princen HM, Kooistra T. 2007. Mouse models for atherosclerosis and pharmaceutical modifiers. Arterioscler Thromb Vasc Biol 27:1706–1721 [DOI] [PubMed] [Google Scholar]
- 19. Davidson NO, Carlos RC, Drewek MJ, Parmer TG. 1988. Apolipoprotein gene expression in the rat is regulated in a tissue-specific manner by thyroid hormone. J Lipid Res 29:1511–1522 [PubMed] [Google Scholar]
- 20. Theriault A, Ogbonna G, Adeli K. 1992. Thyroid hormone modulates apolipoprotein B gene expression in HepG2 cells. Biochem Biophys Res Commun 186:617–623 [DOI] [PubMed] [Google Scholar]
- 21. Miyake JH, Doung XD, Strauss W, Moore GL, Castellani LW, Curtiss LK, Taylor JM, Davis RA. 2001. Increased production of apolipoprotein B-containing lipoproteins in the absence of hyperlipidemia in transgenic mice expressing cholesterol 7α-hydroxylase. J Biol Chem 276:23304–23311 [DOI] [PubMed] [Google Scholar]
- 22. Braverman LE, Utiger RD. 2005. Werner, Ingbar's the thyroid: a fundamental and clinical text. Philadelphia, PA: Lippincott Williams, Wilkins [Google Scholar]
- 23. Xu G, Salen G, Shefer S, Ness GC, Nguyen LB, Tint GS, Parker TS, Roberts J, Batta AK, Chen TS, Zhao Z, Kong X. 1996. Increasing hepatic cholesterol 7α-hydroxylase reduces plasma cholesterol concentrations in normocholesterolemic and hypercholesterolemic rabbits. Hepatology 24:882–887 [DOI] [PubMed] [Google Scholar]
- 24. Ratliff EP, Gutierrez A, Davis RA. 2006. Transgenic expression of CYP7A1 in LDL receptor-deficient mice blocks diet-induced hypercholesterolemia. J Lipid Res 47:1513–1520 [DOI] [PubMed] [Google Scholar]
- 25. Spady DK, Cuthbert JA, Willard MN, Meidell RS. 1998. Overexpression of cholesterol 7α-hydroxylase (CYP7A) in mice lacking the low density lipoprotein (LDL) receptor gene. J Biol Chem 273:126–132 [DOI] [PubMed] [Google Scholar]
- 26. Spady DK, Cuthbert JA, Willard MN, Meidell RS. 1995. Adenovirus-mediated transfer of a gene encoding cholesterol 7α-hydroxylase into hamsters increases hepatic enzyme activity and reduces plasma total and low density lipoprotein cholesterol. J Clin Invest 96:700–709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sample CE, Pendleton LC, Ness GC. 1987. Regulation of 3-hydroxy-3-methylglutaryl coenzyme A reductase mRNA levels by l-triiodothyronine. Biochemistry 26:727–731 [DOI] [PubMed] [Google Scholar]
- 28. Post SM, Groenendijk M, Solaas K, Rensen PC, Princen HM. 2004. Cholesterol 7α-hydroxylase deficiency in mice on an APOE*3-Leiden background impairs very-low-density lipoprotein production. Arterioscler Thromb Vasc Biol 24:768–774 [DOI] [PubMed] [Google Scholar]
- 29. Shin DJ, Plateroti M, Samarut J, Osborne TF. 2006. Two uniquely arranged thyroid hormone response elements in the far upstream 5′ flanking region confer direct thyroid hormone regulation to the murine cholesterol 7α hydroxylase gene. Nucleic Acids Res 34:3853–3861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ness GC, Lopez D. 1995. Transcriptional regulation of rat hepatic low-density lipoprotein receptor and cholesterol 7α hydroxylase by thyroid hormone. Arch Biochem Biophys 323:404–408 [DOI] [PubMed] [Google Scholar]
- 31. Pullinger CR, Eng C, Salen G, Shefer S, Batta AK, Erickson SK, Verhagen A, Rivera CR, Mulvihill SJ, Malloy MJ, Kane JP. 2002. Human cholesterol 7α-hydroxylase (CYP7A1) deficiency has a hypercholesterolemic phenotype. J Clin Invest 110:109–117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Drover VA, Agellon LB. 2004. Regulation of the human cholesterol 7α-hydroxylase gene (CYP7A1) by thyroid hormone in transgenic mice. Endocrinology 145:574–581 [DOI] [PubMed] [Google Scholar]
- 33. Berkenstam A, Kristensen J, Mellström K, Carlsson B, Malm J, Rehnmark S, Garg N, Andersson CM, Rudling M, Sjöberg F, Angelin B, Baxter JD. 2008. The thyroid hormone mimetic compound KB2115 lowers plasma LDL cholesterol and stimulates bile acid synthesis without cardiac effects in humans. Proc Natl Acad Sci USA 105:663–667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ladenson PW, Kristensen JD, Ridgway EC, Olsson AG, Carlsson B, Klein I, Baxter JD, Angelin B. 2010. Use of the thyroid hormone analogue eprotirome in statin-treated dyslipidemia. N Engl J Med 362:906–916 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.