Abstract
During spermatogenesis, spermiogenesis that releases sperm into the tubule lumen and restructuring of the blood-testis barrier (BTB) that accommodates the transit of preleptotene spermatocytes take place simultaneously, but at the opposite ends of the seminiferous epithelium. These events are tightly regulated and coordinated; however, neither the underlying mechanism(s) nor the involving molecules are known. Herein, the Scribble/Lgl (Lethal giant larvae)/Dlg (Discs large) polarity complex was shown to regulate spermatid polarity during spermiogenesis and tight junction (TJ)-permeability barrier via changes in protein distribution at the apical ectoplasmic specialization and the BTB during the epithelial cycle, respectively. Scribble, Lgl2, and Dlg1 were found to be expressed by Sertoli and germ cells. Scribble also displayed stage-specific expression at the BTB, being highest at stages VII–VIII, colocalizing with TJ proteins occludin and ZO-1. Unlike components of other polarity complex modules, such as partitioning-defective 6, the knockdown of which by RNA interference was found to impede Sertoli cell TJ barrier, a knockdown of the Scribble complex (i.e. simultaneous knockdown of Scribble, Lgl and Dlg or Lgl alone; but not Scribble or Dlg alone) both in vitro and in vivo promoted the TJ integrity. This was mediated by reorganizing actin filament network at the Sertoli cell-cell interface, which, in turn, affected changes in the localization and/or distribution of occludin and/or β-catenin at the BTB. These knockdowns also perturbed F-actin organization at the Sertoli cell-spermatid interface, thereby modulating spermatid adhesion and polarity at the apical ectoplasmic specialization. In summary, the Scribble/Lgl/Dlg complex participates in the regulation of BTB dynamics and spermatid adhesion/polarity in the testis.
In the mammalian testis, the blood-testis barrier (BTB) divides the seminiferous epithelium into the basal and the adluminal compartment, so that meiosis I/II and postmeiotic spermatid development take place in the adluminal compartment, segregated from the systemic circulation (1, 2). Although the BTB is one of the tightest blood-tissue barriers, it undergoes cyclic restructuring to facilitate the transit of preleptotene spermatocytes from the basal to the adluminal compartment (3, 4). Interestingly, spermatids derived from meiosis in the adluminal compartment that adhere to the Sertoli cell also undergo cyclic restructuring so that round spermatids can develop into elongated spermatids via 19 steps in the rat during spermiogenesis and migrate to the luminal edge for their release into the lumen at spermiation (2, 5). A testis-specific anchoring junction known as ES (ectoplasmic specialization) is prominently detected at the BTB and at the Sertoli-spermatid (steps 8–19) interface, known as basal and apical ES, respectively. Both basal and apical ES share similar ultrastructural features in which bundles of actin filaments that lie perpendicular to the apposing Sertoli-Sertoli and Sertoli-spermatid plasma membranes, respectively, are sandwiched in between cisternae of endoplasmic reticulum and the plasma membrane (1). It is also this unique actin filament bundles that confer the unusual adhesive strength to the ES (1, 2, 4, 6, 7). Because the basal ES and the apical ES undergo cyclic restructuring during spermatogenesis (1, 2, 8), the actin filament bundles at both sites must be cyclically remodeled, yet the underlying mechanism(s) and the regulatory molecule(s) remain unknown. Herein, the Scribble/Lgl (lethal giant larvae)/Dlg (discs large) polarity complex was shown to participate in the restructuring of the ES via their effects on the actin filament network, which, in turn, modulates the distribution, localization, and/or recruitment of cell adhesion protein complexes during the seminiferous epithelial cycle of spermatogenesis.
The Scribble polarity complex is composed of the Scribble, the Lgl (four mammalian homologs of Lgl1–4 are known, with Lgl2 being the predominant form in the testis), and the Dlg (five mammalian homologs of Dlg 1–5 are known, the predominant form in the testis is Dlg1), which is restricted to the basolateral region in a cell epithelium. Component proteins of the Scribble complex display mutually exclusive distribution pattern vs. the partitioning-defective (Par)-based and the Crumbs-based polarity complexes, with these latter two complexes located at the apical region of an epithelium (9–11). Because component proteins in each of these protein complexes can recruit their own partners, thereby creating distinctly different complexes, this thus confers apicobasal polarity necessary for epithelial homeostasis (9, 10). Recent studies have shown that these protein complexes, in addition to cell polarity, are crucial to regulate cell adhesion, cell cycle progression, cell signaling, and protein trafficking (2, 9–12). However, few reports are found in the literature investigating the role of these polarity proteins in spermatogenesis. Herein, we report the involvement of the Scribble/Lgl2/Dlg1complex in Sertoli cell BTB function and spermatid polarity.
Materials and Methods
Animals and antibodies
The use of Sprague Dawley rats (Charles River Laboratories, Kingston, NY) was approved by the Rockefeller University Institutional Animal Care and Use Committee with Protocol nos, 06018 and 12506. Antibodies used for various experiments were obtained commercially from different vendors or prepared in our laboratory as described in Supplemental Table 1 published on The Endocrine Society's Journals Online web site at http://endo.endojournals.org.
Primary Sertoli cell cultures
Sertoli cells were isolated from 20-d rat testes, cultured in serum-free F12/DMEM in a humidified atmosphere of 95% air/5% CO2 (vol/vol) at 35 C with supplements (13). In brief, Sertoli cells were plated on Matrigel (BD Biosciences, San Jose, CA)-coated culture dishes, bicameral units, or microscopic coverslips at different cell densities depending on their use for subsequent experiments: 1) 12-well culture dishes at 0.5×106 cells/cm2 containing 3-ml F12/DMEM per well for lysate preparation or RNA extraction; 2) microscopic (round) coverslips at 0.05 × 106 cells/cm2 and placed in six-well dishes containing 5-ml F12/DMEM per well for dual-labeled immunofluorescence analysis; and 3) Millicell bicameral units [Millipore, 12-mm (diameter) inserts, ∼0.6 cm2 surface area with inserts placed in 24-well dishes; the apical and the basal chamber each contained 0.5 ml of F12/DMEM] at 1.2 × 106 cells/cm2 for transepithelial electrical resistance (TER) measurement to assess the Sertoli cell TJ (tight junction)-permeability barrier function. These cell densities for different studies were selected based on pilot experiments so that sufficient proteins or RNA/DNA could be obtained for lysate preparation for immunoblotting or for nucleic acid extraction, fluorescence microscopy could be performed to visualize protein distribution at the evenly spaced cell-cell interface, or Sertoli cell TJ-barrier assembly or changes in TJ barrier function could be quantified. On d 2 after isolation, a brief hypotonic treatment of 2 min using 10 mm Tris, pH 7.4, at 22 C, was performed to lyse residual germ cells (14), so that these cultures were more than 98% pure with negligible contaminations of Leydig, myoid, and germ cells (15). Sertoli cells obtained from 20-d-old rats were differentiated and ceased to divide (16). It was noted that these Sertoli cells were morphologically and functionally similar to Sertoli cells isolated from adult rat testes (17, 18). Furthermore, ultrastructures, such as TJ, basal ES, gap junction, and desmosome were found in these cultures under electron microscopy (19, 20), analogous to the Sertoli cell BTB in vivo (2, 6). For each experiment involving Sertoli cell cultures, triplicate culture dishes/coverslips/bicameral units were used per time point in each treatment vs. control group, and each experiment was repeated at least three times, excluding pilot experiments that assessed optimal experimental conditions.
Assessment of Sertoli cell TJ barrier function
Functional TJ barrier was assessed by quantifying TER across the Sertoli cell epithelium with cells cultured on Matrigel-coated bicameral units (13, 21, 22). Each time point had triplicate bicameral units, and each experiment was repeated at least three times using different cell preparations.
Transient transfection of small interfering RNA (siRNA) duplexes in cultured Sertoli cells
On d 3 or d 4, when an intact cell epithelium was established, Sertoli cells cultured on Matrigel-coated culture plates, Millicell bicameral units or coverslips were transfected with ON- TARGET siRNA duplexes (Dharmacon, Lafayette, CO; Thermo Fisher Scientific, Pittsburgh, PA) that specifically targeted Scribble (J-080977-12), Lgl2 (J-096480-09), or Dlg1 (J-090298-10) vs. nontargeting siRNA control (D-001810-10) (see Table 1), using RiboJuice siRNA transfection reagent (Novagen, Madison, WI; EMD Biosciences, San Diego, CA) in a final reaction volume of 1 ml F12/DMEM per well (containing ∼4 μl RiboJuice) in 12-well dishes or 2.5 ml F12/DMEM per well (containing 8 μl RiboJuice) in six-well dishes with cells on coverslips, and 0.5 ml F12/DMEM (containing ∼3–4 μl RiboJuice) in the 12-mm bicameral unit (apical chamber) with each unit seated in a well of 24-well dishes which contained 0.5 ml F12/DMEM (basal chamber). For single gene knockdown experiments, the concentration of siRNA duplexes used was 100 nm for immunoblot, 200 nm for TER assay, and 80 nm for dual-labeled immunofluorescence analysis. For triple knockdown of Scribble, Lgl2, and Dlg1, the total siRNA concentration was at 120 nm for immunoblot, 200 nm for TER assay, and 90 nm for immunofluorescence analysis, with equal amounts from each siRNA duplex. These concentrations of siRNA duplexes were selected based on pilot experiments because of differences in cell densities that yielded optimal phenotypes without detectable cytotoxicity based on XTT (2,3-bis-(2-methoxy-4-nitro-5-sulfophenyl)-2H-tetrazolium-5-carboxanilide) assay (19). After transfection for 24 h, cells were rinsed and replenished with fresh F12/DMEM and incubated for another 48 h before being fixed for dual-labeled immunofluorescence analysis, or 72 h before termination for lysate preparation or RNA extraction for subsequent immunoblotting or RT-PCR/quantitative real-time RT-PCR (qPCR) analysis. For dual-labeled immunofluorescence analysis, Sertoli cells were transfected with 1 nm siGLO red transfection indicator (catalog no. D-001630-02; Dharmacon; Thermo Scientific) together with the siRNA duplexes to monitor transfection efficiency.
Table 1.
siRNA duplexes used for different RNAi experiments in this report
| Target gene | Gene ID | siRNA sequence (5′-3′) | Catalog no. |
|---|---|---|---|
| Scribble | 362938 | Sense: UAGCGGUAAAUCUCCUCUGtt | J-080977 |
| Antisence: CAGAGGAGAUUUACCGCUAtt | |||
| Lgl2a | 116458 | Not available | J-096480 |
| Dlg1 | 25252 | Sense: UAAUCGGGCUCGUUCUUUCtt | J-090298 |
| Antisence: GAAAGAACGAGCCCGAUUAtt |
The specific targeting siRNA duplexes and nontargeting control siRNA duplexes used in this study were purchased from Thermo Scientific Dharmacon (Lafayette, CO).
Sequences of siRNA duplexes targeting at Lgl2 (rat) and nontargeting siRNA (D-001810-10) are not available from the manufacturer; thus, they are not listed herein.
Treatment of adult rats and Sertoli cells with adjudin, 1-(2,4-dichlorobenzyl)-1H-indazole-3-carbohydrazide
Adjudin is a male contraceptive under investigations known to induce germ cell loss from the epithelium (23). At 50 mg/kg body weight (b.w.), a single dose by gavage was found to induce reversible infertility in male rats (23, 24). Adjudin also up-regulated the expression of adhesion proteins at the BTB (e.g. occludin, claudin-11, JAM-A, N-cadherin, γ-catenin, and coxsackievirus and adenovirus receptor) during anchoring junction disruption (24, 25); in this manner, Sertoli cell TJ barrier was tightened (25). Thus, anchoring junction restructuring induced by adjudin would not elicit BTB disruption (25) because of an up-regulation of BTB constituent proteins, which was used to tighten the barrier function. In fact, the BTB was found to remain intact by 2 wk after adjudin treatment when virtually all germ cells, except spermatogonia, were depleted from the epithelium (24). Thus, this is a unique mechanism in the testis to protect the BTB from being disrupted during anchoring junction restructuring associated with the release of sperm at spermiation (26). In short, this is a valuable model to assess whether there is a down-regulated expression of Scribble during adjudin-induced BTB tightening in vivo. Adult male rats (∼300 g b.w.) were treated with a single dose of adjudin (50 mg/kg b.w.) by gavage to induce germ cell loss from the epithelium (23, 27). At selected time point vs. controls with n = 3 rats per time point, rats were euthanized by CO2 asphyxiation. One of the testes from each rat were fixed in Bouin's fixative and embedded in paraffin for histological analysis; the other testis of the same rat was frozen in liquid nitrogen, stored at −80 C, and used for either lysate preparation or to obtain frozen sections. For cultured Sertoli cells, adjudin stock (1 μg/μl in ethanol) was added to cells cultured alone for 4 d to a final concentration of 1 μg/ml from d 4 to d 7. Thereafter, cells were harvested in Nonidet P-40 lysis buffer (28) for immunoblot analysis, or in TRIzol reagent to extract RNA for RT-PCR and qPCR.
Knockdown in adult rat testes in vivo
Nontargeting control and specific siRNA duplexes against either Scribble, Lgl2 or Scribble, Lgl2, and Dlg1 (SLD) in transfection mix was administered into adult rat testes daily, with a total of three injections in three consecutive days using a 28-gauge needle. For each testis in single-gene silencing group, the transfection mix containing 100 nm siRNA duplexes (the testis volume was assumed to be ∼1.6 ml) and 7.5 μl RiboJuice transfection medium was suspended in Opti-MEM (Invitrogen, Carlsbad, CA) to a final volume of 200 μl per testis. For the triple-silencing group, the concentration of siRNA duplex mix was 120 nm and composed with equal amounts of each siRNA duplex. Nontargeting control siRNA and silencing duplex mix were administered to the left and right testis of the same rat, respectively, and each time point in each treatment and control group had at least three rats. Two days after the last treatment, rats were euthanized by CO2 asphyxiation, and testes were snap frozen in liquid nitrogen and stored at −80 C until used.
RNA extraction, RT-PCR, and qPCR
TRIzol reagent (Invitrogen) was used for RNA extraction from testes, Sertoli cells, and germ cells (29). RNA samples were treated with RNase-free DNase I (Invitrogen) to eliminate contaminating genomic DNA before their use for RT-PCR with Go TaqDNA polymerase (Promega Corp., Madison, WI) (29, 30). Primers used for RT-PCR were listed in Supplemental Table 2. The authenticity of the PCR products was confirmed by direct nucleotide sequencing at Genewiz. To further confirm the efficacy of RNA silencing or adjudin treatment in vivo and in vitro on the expression of Lgl2 and Dlg1, qPCR was performed as detailed elsewhere (31, 32) because specific antibodies against Lgl2 and Dlg1 were not satisfactory after several commercially available antibodies were tested. Primers that were used for RT-PCR were also used for qPCR (see Supplemental Table 2).
Lysate preparation and immunoblot analysis
Lysates of testes, Sertoli cells, and germ cells were prepared in Nonidet P-40 lysis buffer [50 mm Tris (pH 8.0) at 22 C, containing 150 mm NaCl, 2 mm EGTA, 10% glycerol (vol/vol), and 1% Nonidet P-40 Alternative (vol/vol; Calbiochem, La Jolla, CA), with protease inhibitor mixture and phosphatase inhibitor cocktails 1 and 2 (Sigma-Aldrich, St. Louis, MO) freshly added to the lysis buffer just before its use for protein extraction] as described elsewhere (20, 29). Protein concentrations were quantified by using the DC protein assay kit (Bio-Rad Laboratories, Hercules, CA). Approximately 80 μg of protein lysates from testes, and 30 and 100 μg of protein lysates from Sertoli and germ cells, respectively, were used per lane for immunoblotting (20, 29) with specific antibodies (Supplemental Table 1), and actin served as a loading control. Enhanced chemiluminescence was performed using kits prepared in our laboratory as described previously (33).
Immunohistochemistry (IHC) and dual-labeled immunofluorescence analysis
IHC was performed as described elsewhere (34). For immunofluorescence, Sertoli cells plated on coverslips at 0.05 × 106 cells/cm2 and frozen sections of testes (∼7 μm) on poly-l-lysine-coated slides were fixed for 4 min in 4% paraformaldehyde (wt/vol, in PBS) or Bouin's fixative, respectively. Cells or sections were permeabilized in 0.1% Triton X-100 (vol/vol, in PBS) for 4 min, followed by blocking with 5% BSA (wt/vol, in PBS) for 30 min and incubated with primary antibodies at specified concentrations (Supplemental Table 1) overnight at 4 C (frozen sections) or at room temperature (Sertoli cells) and then, secondary antibodies (Supplemental Table 1) conjugated with CY3–555 (red) or fluorescein isothiocyanate (FITC)-488 (green) at room temperature for 1 h. For F-actin staining, rhodamine- or FITC-conjugated phalloidin (Invitrogen) was used. Sections or cells were mounted in Prolong Gold Antifade reagent (Invitrogen) containing DAPI (4′,6-iamidino-2-phenylindole). Images were visualized and captured by an Olympus BX61 fluorescence microscope with an Olympus DP71 digital camera (12.5 megapixel) using the Olympus MicroSuite FIVE software package (Version 1226) (Olympus America, Inc., Melville, NY). Fluorescence images were adjusted for brightness/contrast and images were merged to assess colocalization of proteins using Photoshop. Semiquantitative analysis of fluorescence images was performed by quantifying signal intensity using ImageJ 1.44I (35). All micrographs are representative results of three independent experiments.
Statistical analysis
Statistical analysis was performed using the GB-STAT software package (Version 7.0; Dynamic Microsystems). Statistical significance between treatment and control groups was analyzed with either Student's t test or one-way ANOVA followed by a two-tailed Dunnett's test.
Results
Expression of Scribble polarity complex components in the rat testis
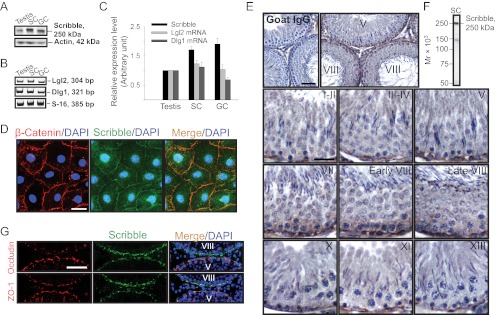
Expression of Scribble polarity protein complex components was examined in the rat testis (Fig. 1, A–E). Scribble was detected in adult rat testes and cultured Sertoli and germ cells (Fig. 1, A and C) by immunoblotting using a specific anti-Scribble antibody (Fig. 1, F; and Supplemental Table 1). It is noted that there are four isoforms of Lgl (Lgl1–4) and 5 isoforms of Dlg (Dlg1–5) in mammalian cells/tissues, but Lgl2 and Dlg1 are the predominant isoforms of Lgl and Dlg in the Scribble protein complex in the mammalian testis (11), which, therefore, were selected in our studies. Indeed, Lgl2 and Dlg1 were also detected by RT-PCR (Fig. 1, B and C) and qPCR (see below) using corresponding specific primer pairs (Supplemental Table 2), because specific antibodies against rat Lgl2 and Dlg1 from several vendors were shown to be unsatisfactory. Scribble was also found to localize at the Sertoli cell-cell interface in vitro, colocalizing with basal ES protein β-catenin (Fig. 1D), an integrated component of the TJ barrier at the Sertoli cell BTB, implicating Scribble as a BTB component.
Fig. 1.
Expression of components of the Scribble polarity complex in cultured Sertoli and germ cells and the testis, and stage-specific localization of Scribble at the BTB during the epithelial cycle of spermatogenesis. A, Immunoblotting of Scribble in lysates of adult rat testis, Sertoli cells (SC), and germ cells (GC) with actin served as a loading control (50 μg protein/lane). B, RT-PCR results depicting the relative steady-state mRNA levels of Lgl2 and Dlg1 in lysates of testis, SC, and GC, with S-16 serving as a control. C, Histogram summarizes results of immunoblotting and RT-PCR shown in panels A and B after normalizing each data point against actin (for immunoblot) or S-16 (for RT-PCR). Protein (Scribble) or mRNA (Lgl2 or Dlg1) level in the testis was arbitrarily set as 1. Each bar is the mean ± sd of n = 3. D, Colocalization of Scribble (green) and basal ES protein β-catenin (red) to the Sertoli cell-cell interface by dual-labeled immunofluorescence analysis in cells cultured for 4 d. Nuclei were stained with DAPI (blue). Scale bar, 5 μm, which applies to other micrographs. E, Stage-specific expression and localization of Scribble at the BTB in the epithelium during the epithelial cycle. Scribble was expressed predominantly at the basal region near the basement membrane, consistent with its localization at the BTB, with the highest level in stages VII–VIII, at the time of BTB restructuring to facilitate the transit of preleptotene spermatocytes at the site. Negative control was shown in which the anti-Scribble antibody was substituted by normal goat IgG; scale bar, 40 μm, which applies to the micrograph on the right. Scale bar, 20 μm, in the staged I–II tubule, which applies to all other micrographs. F, Immunoblot analysis illustrating the specificity of the goat anti-Scribble antibody using Sertoli cell (SC) lysates. G, Colocalization of Scribble (green) with tight junction proteins occludin (red) or ZO-1 (red), predominantly at the BTB. Nuclei were stained with DAPI (blue). Scale bar, 30 μm, which applies to other micrographs.
Stage-specific expression of Scribble at the BTB during the seminiferous epithelial cycle
Expression of Scribble in the testis was examined by IHC using cross-sections of paraffin-embedded normal rat testes (Fig. 1E) and a specific anti-Scribble antibody (Fig. 1F and Supplemental Table 1). Immunoreactive Scribble appearing as reddish-brown precipitate, not in the normal goat IgG control, was detected in the epithelium at the basolateral region near the basement membrane; this location is consistent with its localization at the BTB, but most predominantly at stage VII–VIII at the time of BTB restructuring to facilitate the transit of preleptotene spermatocytes (Fig. 1E), which suggests that a down-regulation of Scribble expression could promote BTB function. This stage-specific expression of Scribble was also noted by immunofluorescence analysis, colocalizing Scribble with TJ-proteins occludin and ZO-1 (Fig. 1G). Expression of occludin at the BTB was shown to be down-regulated in stage VIII tubules but not ZO-1 (Fig. 1G), consistent with an earlier report (36), but the expression of Scribble exhibited an opposite trend vs. occludin, in which Scribble had an up-regulated expression at the BTB in stage VIII tubules (Fig. 1, panel G vs. panel E), promoting BTB restructuring. In short, Scribble displayed stage-specific expression at the BTB, predominantly at stage VIII, colocalizing with occludin, ZO-1, and β-catenin (Fig. 1, D, E, and G).
Down-regulation of Scribble at the BTB is associated with a tighter barrier: a study using the adjudin model
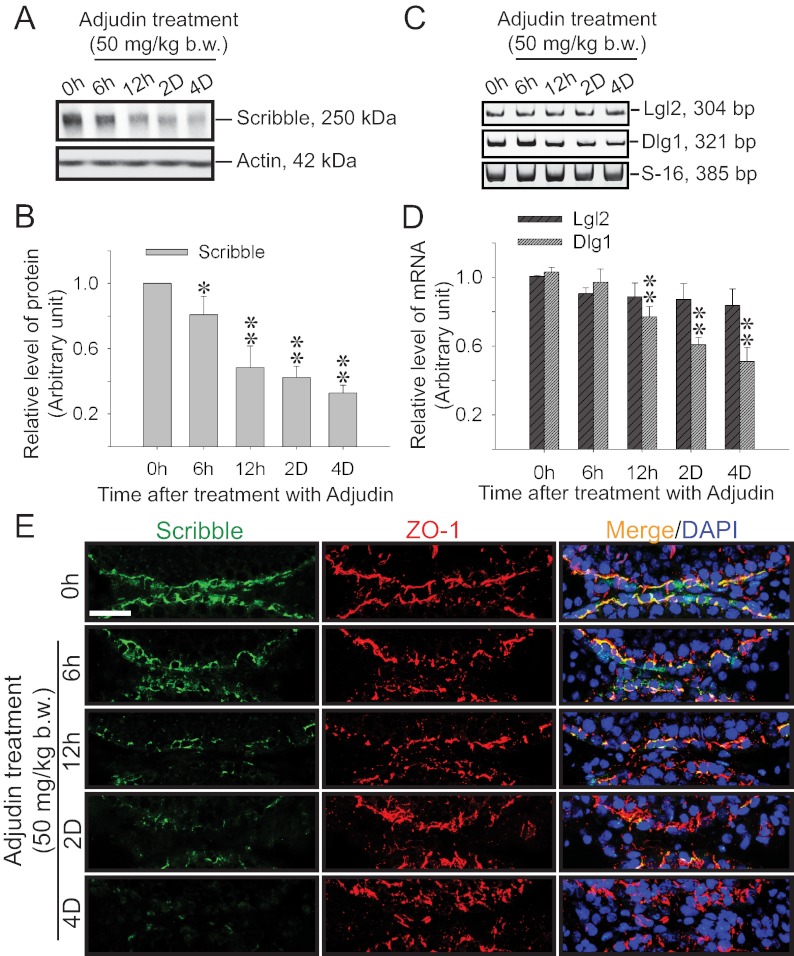
Using the adjudin model in which adjudin was found to induce germ cell loss in adult rat testes, while promoting the Sertoli cell BTB function, a time-dependent decline in the protein level of Scribble was detected by immunoblotting, and its expression was down-regulated by as much as 3-fold by approximately 2–4 d after adjudin treatment (Fig. 2, A and B); another Scribble component Dlg1 followed similar trend of down-regulation, but not Lgl2 (Fig. 2, C and D). Dual-labeled immunofluorescence analysis (Fig. 2E) also confirmed results of immunoblotting shown in Fig. 2, A and B, illustrating a time-dependent loss of Scribble signals at the BTB (Supplemental Fig. 2), and the adjudin-induced tightening of the BTB was associated with an increase in ZO-1 (a TJ-associated adaptor) (Fig. 2, panel E vs. panels A and B). The finding of the adjudin-induced up-regulation of ZO-1 at the BTB during germ cell loss from the epithelium (Fig. 2E) that tightens the Sertoli cell TJ-barrier (25) is consistent with two earlier reports (24, 25). The adjudin-induced down-regulation of Scribble expression at the BTB shown in Fig. 2E was further validated by IHC using paraffin sections of rat testes (Supplemental Fig. 1). These findings thus strengthen the notion that a down-regulation of Scribble promotes the BTB integrity whereas its up-regulation facilitates BTB restructuring (Fig. 2 and Supplemental Figs. 1 and 2 vs. Fig. 1E). To further validate the finding reported in Fig. 2 that the adjudin-induced down-regulation of Scribble was not entirely the result of germ cell loss from the epithelium because germ cells contribute significantly to the pool of Scribble in the testis or its lysates, Sertoli cells that were cultured in vitro alone for 4 d with an established TJ barrier that mimicked the Sertoli cell BTB in vivo were treated with adjudin. It was found that Scribble (Supplemental Fig. 3A) and its components Lgl2 and Dlg1 (Supplemental Fig. 3B) displayed time-dependent down-regulated expression in these Sertoli cell cultures (Supplemental Fig. 3), illustrating that data reported in Fig. 2 and Supplemental Fig. 2 are the result of adjudin-induced down-regulation of Scribble in Sertoli cells.
Fig. 2.
Changes in the expression and localization of Scribble and/or its partners during adjudin-induced junction restructuring in adult rat testes. Adjudin was administered to adult rats at 0 h (hr), and testes were obtained at specified time points for immunoblot analysis (A and B), RT-PCR (C), qPCR (D), dual-labeled immunofluorescence analysis (E), and IHC (Supplemental Fig. 1). A, Changes in the protein level of Scribble after adjudin treatment vs. actin, which served as a protein loading control. B, Histogram summarizes the result shown in panel A after normalizing each data point against actin. Protein level at 0 h was arbitrarily set at 1. Each bar, mean ± sd of n = 3 rats. *, P < 0.05; **, P < 0.01. C, RT-PCR results illustrating changes in the steady-state mRNA levels of Lgl2 and Dlg1 after adjudin treatment, with S-16 serving as a control. D, Results of qPCR on the steady-state mRNA levels of Lgl2 and Dlg1, validating the findings in panel C. Each bar, mean ± sd of n = 3 rats. **, P < 0.01. E, Changes in expression and colocalization of Scribble (green) with TJ-protein ZO-1 (red) after adjudin treatment. Nuclei were stained with DAPI (blue). Scale bar, 50 μm, which applies to all micrographs.
Scribble/Lgl2/Dlg1 protein complex is a crucial regulator of Sertoli cell TJ-permeability barrier
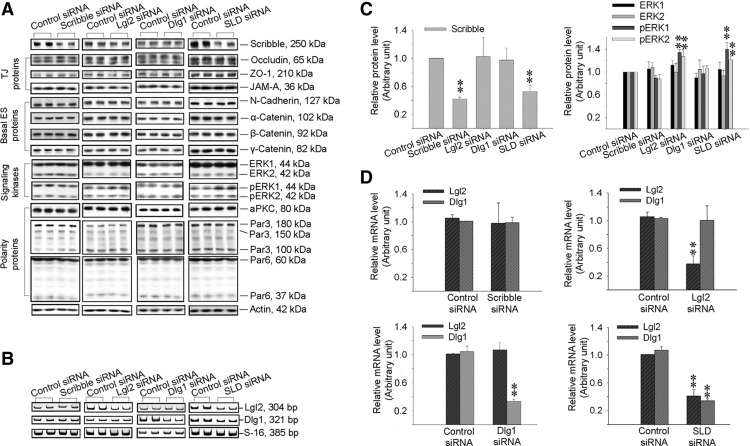
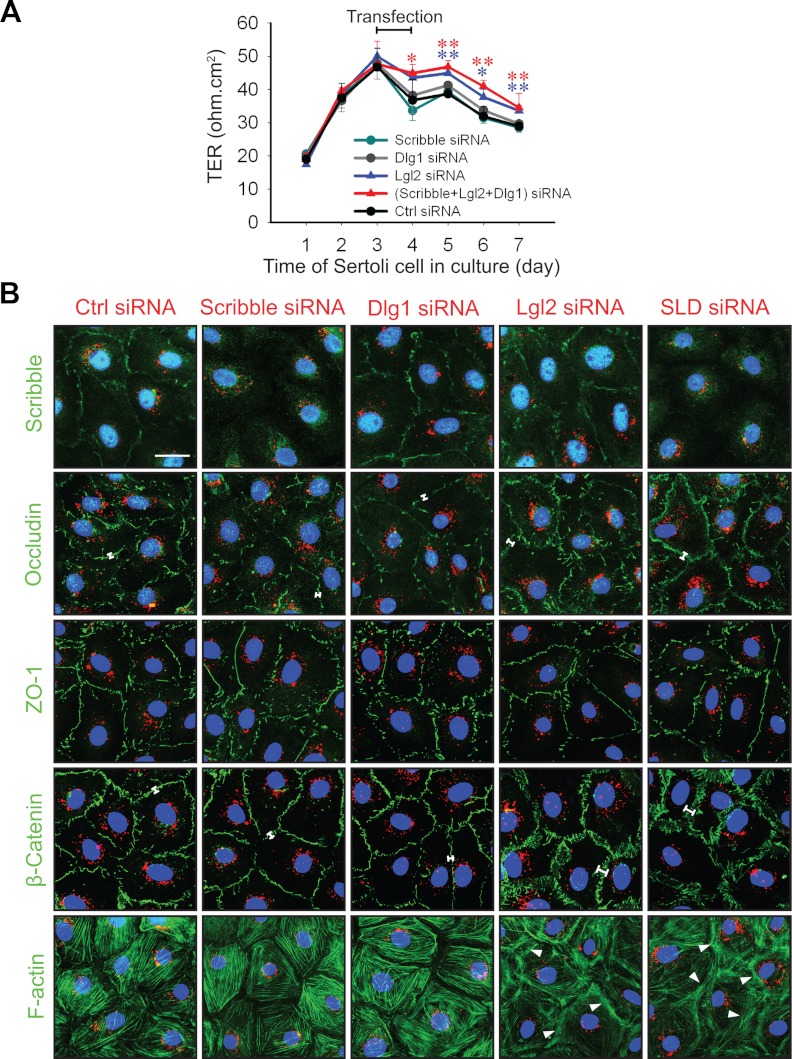
The role of Scribble, Lgl2, and Dlg1 in Sertoli cell BTB function was assessed by silencing these genes either separately (single silencing) or altogether (triple silencing) by RNA interference (RNAi) using primary Sertoli cells cultured in vitro. Primary Sertoli cells cultured in vitro is known to establish a functional TJ barrier and with ultrastructures of TJ, basal ES, gap junction, and desmosome (19, 20), which mimics the BTB in vivo (1), thus this method is widely used in the field to study BTB function. As shown in Fig. 3A-D, and Supplemental Fig. 4, the knockdown of either Scribble, Lgl2, or Dlg1 alone by approximately 60–70%, or the triple knockdown of Scribble, Lgl2, and Dlg1 (SLD) by about 55%, 60%, and 70%, respectively (Fig. 3), no off-target effects were detected using multiple BTB-associated protein markers, except that the knockdown of either Lgl2 alone (but not Scribble or Dlg1 alone) or SLD was associated with an up-regulation of pERK1/2 (Fig. 3). This finding is in agreement with earlier studies in which Scribble, a tumor suppressor, was shown to limit mammalian cancer cell invasion and mitotic proliferation by promoting ERK phosphorylation and its nuclear translocation (37, 38). It is noted that immunoblotting was not performed to monitor changes in the steady-state protein levels of either Lgl2 or Dlg1 to assess the efficacy of their knockdown in these RNAi experiments because satisfactory antibodies against these proteins were not available for rats commercially; instead, RT-PCR (Fig. 3B) and qPCR (Fig. 3C) using corresponding specific primers (Supplemental Table 2) were performed to assess the efficacy of their knockdown. Interestingly, although the knockdown of Scribble or Dlg1 alone did not promote or impede the Sertoli cell TJ barrier function, a triple SLD knockdown or Lgl2 knockdown alone was found to promote the Sertoli cell TJ barrier (Fig. 4, A and B) without any detectable off-target effects (Supplemental Fig. 4). These results are consistent with findings shown in Fig. 1 regarding the stage-specific expression of the Scribble protein complex and results from the adjudin model shown in Fig. 2 that a down-regulation of the Scribble protein complex promoted and tightened BTB integrity. It was also noted that after SLD triple- or Lgl2 single-, but not Scribble single- or Dlg1 single-, knockdown vs. nontargeting control, a tightening of the Sertoli cell TJ barrier was manifested by an enhanced localization of occludin and β-catenin (see white brackets) but not ZO-1, and an increase in F-actin bundles (see white arrowheads), at the cell-cell interface (Fig. 4B).
Fig. 3.
Changes in the steady-state protein or mRNA levels of Scribble complex components and BTB-associated proteins after single knockdown of either Scribble, Lgl2, or Dlg1 vs. triple knockdown of Scribble, Lgl2, and Dlg1 by RNAi. Sertoli cells cultured in vitro for 4 d with an established TJ barrier were transfected with specific siRNA duplexes targeting 1) Scribble, 2) Lgl2, 3) Dlg1, or 4) Scribble, Lgl2 and Dlg1 (SLD, triple knockdown) vs. nontargeting control for 24 h. On d 7 (i.e. 2 d after transfection), cultures were terminated for immunoblotting (A and C), RT-PCR (B), and qPCR (D) to assess the efficacy of the knockdown and any off-target effects. A, Immunoblotting showed a knockdown of Scribble by approximately 60% in Scribble single-silencing group and by about 55% in triple Scribble/Lgl2/Dlg1-silencing group (SLD) vs. nontargeting control. In Lgl2 single-silencing and SLD triple-silencing groups, levels of pERK1 and pERK2 were induced vs. control. No off-target effect was detected. Also, the single silencing of either Lgl2 or Dlg1 did not affect the steady-state protein level of Scribble. B, RT-PCR result illustrating the steady-state mRNA levels of Lgl2 and Dlg1 in the three single-silencing groups, and the triple-silencing group vs. the nontargeting control group. This experiment was done to assess the specificity of each knockdown because satisfactory antibodies against Lgl2 and Dlg1 were not available commercially. C, Histograms summarizing part of the data shown in panel A after normalizing each data point against actin (see Supplemental Fig. 4 for remaining data), illustrating specificity of the knockdown. Protein level in the nontargeting control siRNA group was arbitrarily set at 1. Each bar, mean ± sd of n = 3 experiments. **, P < 0.01. D, qPCR results that validated the RT-PCR findings shown in panel B. In Scribble single-silencing group, Lgl2 and Dlg1 mRNA levels were unaffected. In Lgl2 single-silencing group, the level of Lgl2 mRNA was knocked down by about 60% with no off-target effect on Dlg1. In Dlg1 single-silencing group, the level of Dlg1 mRNA was knocked down by approximately 70% with no off-target effect on Lgl2. In SLD triple-silencing group, the levels of Lgl2 and Dlg1 were knocked down by approximately 60% and 70%, respectively. Each bar, mean ± sd of n = 3 experiments. **, P < 0.01.
Fig. 4.
Changes in the Sertoli cell TJ-permeability barrier after knockdown of different components of the Scribble protein complex by RNAi. A, The TJ-barrier function was quantified by measuring TER across the Sertoli cell epithelium. Sertoli cells were plated on Matrigel-coated bicameral units at time 0, and on d 3, cells were transfected for 24 h with the corresponding siRNA duplexes. In SLD triple-silencing group, the transfection mix was composed of equal amount of the three siRNA duplexes targeting at Scribble, Lgl2, and Dlg1 at about 67 nm each to a total of 200 nm siRNA duplexes vs. 200 nm for the single knockdown of either Scribble, Lgl2, or Dlg1 and also nontargeting control cells. B, Changes in protein distribution at the cell-cell interface in Sertoli cells cultured on Matrigel-coated coverslips at 0.05 × 106 cells/cm2 after knockdown of different components of the Scribble complex vs. nontargeting control (Ctrl) siRNA duplexes. Transfection for 24 h using siRNA duplexes (at a final concentration of 80 nm for single silencing of Scribble, Dlg1, or Lgl2; or 90 nm for triple silencing of Scribble, Lgl2, and Dlg1) (SLD) with siGLO red transfection indicator (1 nm, to assess successful transfection) was performed on d 3 before immunofluorescence microscopy or F-actin staining (green, using FITC-conjugated phalloidin) on d 6. Single knockdown of Scribble or Dlg1 had no apparent effect on the distribution of BTB proteins (e.g. occludin, ZO-1, and β-catenin, all in green) vs. control at the Sertoli cell-cell interface, as well as F-actin filament organization. In contrast, single knockdown of Scribble or SDL triple knockdown, but not Dlg1- or Lgl2-single knockdown, indeed reduced the signal of Scribble (green) considerably at the Sertoli cell-cell interface, illustrating the efficacy and specificity of the knockdown. However, single knockdown of Lgl2 and triple SLD knockdown caused a more focused localization of occludin and β-catenin (annotated by white brackets), but not ZO-1 (green), at the cell-cell interface, concomitant with a redistribution of F-actin, which was more intensely localized at the cell-cell interface (see white arrowheads). Cell nuclei were stained with DAPI (blue). Scale bar, 5 μm, which applies to other micrographs.
Scribble protein complex regulates localization of proteins (e.g. occludin) at the BTB and spermatid polarity at the apical ES in a stage-specific manner
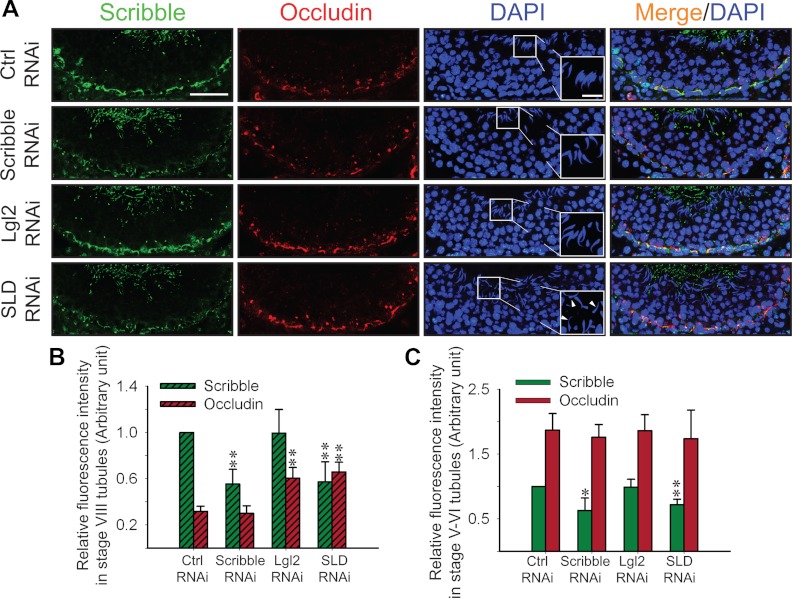
To further validate the physiological function of the Scribble/Lgl2/Dlg1 complex in regulating BTB dynamics, specific siRNA duplexes that were shown to silence these genes in vitro were used to transfect testes in vivo, and changes in phenotypes were investigated. However, Dlg1 knockdown was not included in the subsequent in vivo experiments shown in Figs. 5 and 6 because the single silencing of Dlg1 in Sertoli cells had no detectable effects on either the Sertoli cell TJ barrier function (Fig. 4A) or protein distribution and/or localization at the Sertoli cell-cell interface (Fig. 4B). Because the knockout of occludin, an integral TJ protein at the BTB, led to loss of fertility in occludin−/− mice by 30–60 wk (39) due to BTB disruption, and it displayed stage-specific expression in rats when it was down-regulated at the BTB in stage VIII tubules (36), it was selected for examination. After Scribble single or SLD triple knockdown, but not Lgl2 single knockdown, a considerable (Fig. 5A) and significant (Fig. 5B) loss of Scribble fluorescence signals at the BTB was detected. Interestingly, an increase in occludin at the BTB was also detected in stage VIII tubules in the triple SLD and Lgl2-single silencing group (Fig. 5, A and B), consistent with the in vitro findings shown in Fig. 4, A and B, that a triple SLD or Lgl2-single RNAi led to a tightening of the Sertoli cell TJ barrier and a concomitant increase in occludin accumulation at the Sertoli cell-cell interface (Fig. 5, A and B, vs. Fig. 4, A and B). Also, the polarity of the step 19 spermatids in these stage VIII tubules was also perturbed in the SLD-triple, not the Lgl2-single, silencing group, with their heads no longer pointing toward the basement membrane, but pointing randomly in different directions (see white arrowheads in Fig. 5A) (Table 2). Interestingly, the effects of SLD-triple and Lgl2-single RNAi appeared to be limited to stage VIII tubules (or stage VIII tubules were most susceptible to the SLD-triple and Lgl2-single knockdown) because occludin expression from tubules at stage V–VI (Fig. 5C and Supplemental Fig. 5) was not down-regulated and the spermatid polarity was not affected (Table 2) even though Scribble was knocked down in these tubules (Supplemental Fig. 5).
Fig. 5.
Changes in the distribution of TJ-protein occludin and spermatid polarity in the seminiferous epithelium after knockdown of Scribble complex components by RNAi in adult rat testes in vivo. Rats were treated with corresponding siRNA duplexes to silence different components of the Scribble complex vs. nontargeting controls. Control, Scribble-silenced, Lgl2-silenced, and Scribble/Lgl2/Dlg1 triple-silenced testes were immunostained for Scribble (panel A, green) and occludin (panel A, red) using frozen sections from rats 3 d after the last siRNA transfection mix injection. A, In stage VIII tubules, the signals of Scribble in the epithelium were considerably weakened in Scribble single- and SLD triple-silenced (but not in Lgl2 single-silenced group) groups vs. controls (Ctrl). The signal of occludin in Scribble single-silenced tubules was similar to controls; however, it became more intense in Lgl2 single-silenced and SLD triple-silenced tubules vs. control. Nuclei were stained with DAPI (blue). The boxed area in micrographs in the DAPI panel was enlarged and shown in insets. White arrowheads denote misoriented spermatids with incorrect polarity, only found in SLD triple-silenced group. Scale bar, 80 μm, which applies to all other micrographs; scale bar in inset, 20 μm, which applies to all other insets. B and C, Semiquantitative analysis of data shown in panel A and in Supplemental Fig. 5. Fluorescence intensity of Scribble and occludin in the epithelium was quantified by ImageJ 1.44I in each treatment group vs. control in stage VIII (B) or stage V–VI (C) tubules. The signal intensity of Scribble in the control group was arbitrarily set at 1. At least 50 staged tubules were randomly selected from three rats and quantified. Each bar, mean ± sd of n = 3 rats. *, P < 0.05; **, P < 0.01.
Fig. 6.
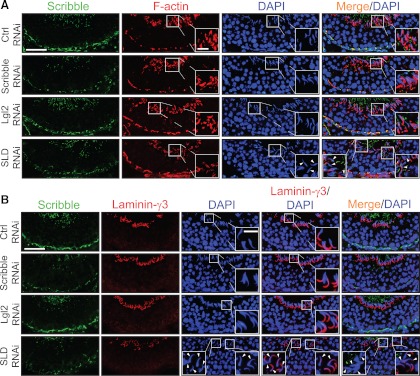
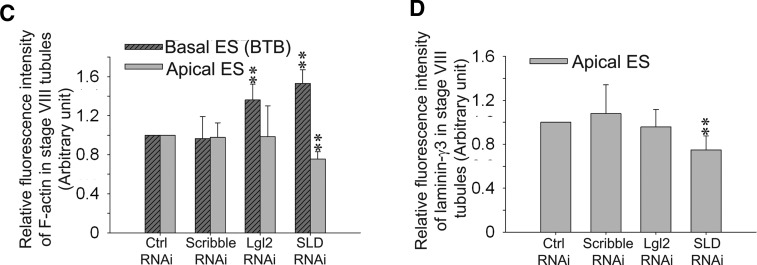
Changes in F-actin organization, apical ES protein distribution, and spermatid polarity in the seminiferous epithelium in stage VIII tubules after knockdown of components of Scribble complex in adult rat testes in vivo. A, F-actin (red) in the epithelium was visualized by using rhodamine-conjugated phalloidin in siRNA-transfected rat testes in nontargeting control (Ctrl) group vs. treatment groups. In both Lgl2 single- and SLD triple-silenced groups, F-actin staining at the BTB increased considerably in stage VIII tubules, but not in Scribble single-silenced group, when compared with the control group. Scribble (green) staining in the epithelium was found to diminish considerably in the Scribble single-silenced and SLD triple-silenced groups, but not the Lgl2 single-silenced group vs. the control group, because of the Scribble knockdown. Also, the organization of F-actin at the apical ES appeared to be disrupted as illustrated by the diffused and weakened F-actin signals at the site in the SLD triple-silenced group (but not in the single Scribble- or even the Lgl2-silenced group) vs. the control group, illustrating the actin filament bundles at the ES were reorganized, with these F-actin bundles being shifted from the apical to the basal ES at the BTB. Thus, cell polarity in the step 19 spermatids in stage VIII tubules from testes of the SLD triple-silenced group was found to be disrupted, possibly due to the diminished actin filament bundles at the apical ES, with these elongated spermatids no longer pointing toward the basement membrane and became misoriented, unlike the nontargeting or single-silenced Scribble and Lgl2 groups. Indeed, F-actin staining was considerably diminished in misoriented spermatids with a loss of cell polarity (see insets in SLD RNAi panel which magnified spermatids in boxed areas vs. control and other treatment groups). Cell nuclei were stained with DAPI (blue). White arrowheads denote mis-oriented spermatids that had lost their polarity in SLD triple-silenced group with diminished F-actin. B, Due to the loss of spermatid polarity as noted in panel A, apical ES protein laminin-γ3 (red) vs. Scribble (green) was also examined in the epithelium of these rat testes and shown herein. Distribution of laminin-γ3 at the apical ES surrounding the spermatid head in Scribble or Lgl2 single-silenced group remained unaltered, similar to the nontargeting control group, but laminin-γ3 was considerably weakened at the apical ES in SLD triple-silenced group. It was also noted that in step 19 spermatids that had lost their polarity and became misoriented, laminin-γ3 staining was either considerably weakened or virtually absent at the apical ES in elongated spermatids (see insets in the SLD RNAi panel wherein step 19 spermatids in boxed areas were magnified). White arrowheads denote misoriented spermatids that had lost the polarity in SLD triple-silenced group with either diminished or a virtual loss of laminin-γ3 staining. Scale bar, 80 μm in panels A and B, which applies to all micrographs in panels A and B; scale bar, 20 μm in inset, which applies to all insets in panels A and B. Imaging analysis of fluorescence signals of F-actin (C) and laminin-γ3 (D) from micrographs obtained from dual-labeled immunofluorescence analysis, which was used to investigate changes in the expression and localization of Scribble and F-actin in the seminiferous epithelium after knockdown of either Scribble, Lgl2, SLD vs. nontargeting control, such as those shown in Fig. 6 in stage VIII tubules. About 90 stage VIII tubules were randomly selected from cross-sections of three rat testes in different silencing groups vs. control group. A significant increase in F-actin fluorescence at the BTB was noted in the Lgl2 single-knockdown (but not the Scribble single-knockdown) and SDL triple-knockdown groups vs. nontargeting control group; however, a significant decrease in F-actin and laminin-γ3 was also noted at the apical ES in the SLD triple-knockdown group, but not in either single-knockdown group.**, P < 0.01, compared with nontargeting control (Ctrl) group.
Table 2.
Seminiferous tubules from rats displaying a loss of spermatid polarity at different stages of the seminiferous epithelial cycle after knockdown of different Scribble components by RNAi in vivoa
| Treatment | Seminiferous tubules (%) displaying loss of spermatid polarityb (mean ± sd) |
||
|---|---|---|---|
| I–IV | VII–VIII | IX–XIV | |
| Control RNAi | 2 ± 2 | 9.33 ± 3.06 | 3.25 ± 1.09 |
| Scribble RNAic | 3.33 ± 1.15 | 10.67 ± 2.31 (ns) | 2.67 ± 3.06 |
| Lgl2 RNAi | 3.15 ± 1.99 | 8.7 ± 0.61 (ns) | 4 ± 2 |
| SLD RNAic | 3.33 ± 3.06 | 22.67 ± 4.16** | 4 ± 2 |
ns, Not significantly different;
, P < 0.01 when compared with control RNAi group.
Testes from three animals in each treatment group were analyzed, with at least approximately 50–60 tubules randomly selected from the testes.
Tubules that contained more than 10 spermatids with loss of polarity were scored as damaged tubules.
In Scribble RNAi or SLD RNAi testes, only tubules with reduced Scribble expression as the result of Scribble silencing were selected for analysis.
Scribble protein complex regulates F-actin at the BTB and the apical ES during the epithelial cycle
To probe the mechanism underlying the changes in phenotypes after SLD-triple RNAi (Fig. 5, Supplemental Fig. 5, and Table 2), we next examined changes in F-actin network and the localization of laminin-γ3 chain, an integrated component of the apical ES (40), in the epithelium after different knockdowns (Fig. 6, A-D). When Scribble was silenced as indicated by a considerable loss of Scribble (green) signals in the epithelium, most notably at the BTB (Fig. 6A), a considerable (Fig. 6A) and statistically significant (Fig. 6C) increase or decrease in F-actin signals at the BTB or apical ES, respectively, was detected in the SLD-triple but not the Scribble-single RNAi animal group. In the Lgl2 single-silenced group, an increase in F-actin signals was detected at the BTB but no decline was detected at the apical ES (Fig. 6, A and C). The increased F-actin network at the BTB (Fig. 6A) thus supported better localization of occludin as shown in Fig. 5A. However, the declining F-actin at the apical ES (Fig. 6, A and C) in the SLD triple-silencing group failed to support proper spermatid polarity, and the heads of these step 19 spermatids were pointing randomly in different directions in the epithelium instead of toward the basement membrane (see white arrowheads) in the SLD-triple RNAi group vs. the control group. These findings are consistent with data shown in Fig. 6B, depicting a significant reduction in laminin-γ3 expression at the apical ES in early stage VIII tubules (see white arrowheads in Fig. 6B; see also Fig. 6D). Thus, a loss of F-actin at the apical ES failed to support proper localization of laminin-γ3 at the site in a stage-specific manner, leading to a loss of spermatid polarity, in SLD triple-silenced testes but not in the Scribble or Lgl2 single-silenced testes (Fig. 6 and Table 2).
Discussion
Scribble/Lgl2/Dlg1 complex promotes Sertoli cell BTB function via protein recruitment to the site
Earlier biochemical and genetic analysis in Drosophila and Caenorhabditis elegans have shown that the Scribble polarity complex, together with the Par-based and the Crumbs-based protein complexes, is crucial to confer apicobasal polarity in epithelia, critical for embryogenesis and development (10, 11). Herein, we report that in addition to the Par-based complex such as Par6 and 14-3-3 (22, 28), Scribble, Lgl2, and Dlg1 were found in Sertoli and germ cells of adult rat testes. More important, Scribble was found to localize in the seminiferous epithelium, most notably at the BTB, colocalizing with TJ/BTB proteins occludin and ZO-1, analogous to the intestinal epithelium using T84 and SK-CO15 cell monolayers (41). Scribble also displays a stage-specific expression pattern with highest expression at stage VIII of the epithelial cycle at the time of BTB restructuring to facilitate the transit of preleptotene spermatocytes, illustrating its enhanced expression correlates with BTB restructuring events. This reciprocal relationship between the expression of Scribble and BTB restructuring was supported by findings in the adjudin model in which adjudin-induced anchoring junction disruption in the adluminal compartment that associated with an increase in BTB integrity (25, 42, 43) was found to induce a time-dependent and significant decline in Scribble expression. In short, these findings illustrate the involvement of the Scribble complex in BTB dynamics. However, the knockdown of either Scribble or Dlg1 alone by RNAi in Sertoli cells cultured in vitro with a functional TJ barrier that mimicked the Sertoli cell BTB in vivo failed to modulate the TJ-permeability barrier function. However, the knockdown of either Lgl2 alone, but not Scribble or Dlg1 alone, or the SDL triple knockdown were found to promote the TJ-barrier function. These findings thus illustrate that Lgl2 is perhaps a more important regulatory member in the Scribble/Lgl2/Dlg1 complex in regulating Sertoli cell TJ-barrier function. More important, the knockdown of the entire Scribble complex or Lgl2 alone (but not Scribble or Dlg1 alone) was found to induce redistribution of TJ- (e.g. occludin) and basal ES- (e.g. β-catenin) proteins (but not ZO-1) at the BTB with more of these proteins being accumulated at the Sertoli cell-cell interface. Furthermore, F-actin bundles were also found to be accumulated at the cell-cell interface in Lgl2 single-silencing or SLD triple-silencing groups, apparently being used to reinforce the TJ barrier. These findings in vitro were confirmed in vivo when either the entire Scribble complex, Scribble alone, or Lgl2 alone was silenced by RNAi. Studies in vivo have also confirmed the notion that all three components of the Scribble complex are needed, and Lgl2 is likely a more important member, to regulate the BTB integrity because only in the triple-Scribble/Lgl2/Dlg1 knockdown and Lgl2 single-knockdown testes vs. Scribble single-knockdown and nontargeting control, consistent changes in phenotypes regarding BTB integrity were detected. For instance, the silencing of either Scribble/Lgl2/Dlg1 or Lgl2 alone, but not Scribble alone, in vivo promoted the localization of occludin at the BTB via an increase in F-actin at BTB. Such an increase in F-actin at the BTB, perhaps, is being used to retain or recruit integral membrane proteins (e.g, occludin) at the site to reinforce BTB integrity, consistent with the in vitro findings. However, these changes in vivo were restricted to stage VIII tubules (but not other stages such as stages V–VI tubules) when the endogenous expression of occludin was low at this stage (36). These findings thus illustrate that the regulation of BTB by Scribble/Lgl2/Dlg1 is a stage-specific event.
Role of Lgl2 in Sertoli cell BTB function
It is of interest to note that adjudin treatment of rats in vivo that down-regulated significantly the expression of Scribble and Dlg1, but only mildly and not significantly for Lgl2 (Fig. 2, A–D), was capable of promoting the BTB function (Fig. 2E). But single knockdown of Lgl2 alone was found to be sufficient to promote: 1) the Sertoli cell TJ barrier function and the localization or recruitment of occludin and β-catenin to the cell-cell interface in vitro, and 2) the distribution of F-actin at the BTB both in vitro and in vivo. It thus raises the question of how could these observations be accounted for regarding the functional role of Lgl2 at the BTB. Before this is addressed, two issues must be reiterated. First, using Sertoli cells cultured in vitro with an established TJ barrier, exposure of these cells (with a purity of >98%) to adjudin was found to down-regulate the expression of Scribble, Dlg1, and Lgl2 significantly (Supplemental Fig. 3) while the Sertoli TJ-barrier function was promoted (25), consistent with data shown in Fig. 4, A and B, when the TJ barrier was tightened (Fig. 4A), and occludin, β-catenin, and F-actin were recruited and localized to the cell-cell interface after Lgl2 single knockdown, similar to the SLD triple knockdown (Fig. 4B). Second, a single knockdown of Lgl2 in the testis in vivo that promoted the BTB via recruitment of F-actin and occludin was stage specific, restricted to stage VIII tubules (Figs. 5 and 6; Table 2), and stage VIII tubules represented approximately 7–10% of all 14 staged tubules in rats (44). Thus, the mild and nonstatistical significant decline of Lgl2 in adjudin-treated rats shown in Fig. 2, C and D, when the BTB function was promoted is likely due to the presence of multiple molecular targets of adjudin in the testis. For instance, peritubular myoid cells in the tunica propria in rodents, but not in primates, was shown to contribute significantly to BTB function (45), and adjudin may exert its BTB-promoting effects via the myoid cells in addition to the Sertoli cells, and its additive effects on these two cell types may account for the mild decline of Lgl2 in the testis in vivo vs. the significant decline of Lgl2 in the Sertoli cell cultures in vitro (Fig. 2, C and D, vs. Supplemental Fig. 3B). This possibility must be vigorously investigated using Sertoli-myoid cell cocultures in future experiments. Nonetheless, these findings support the notion that Lgl2 is crucial to Sertoli cell BTB function.
The Scribble/Lgl2/Dlg1 complex-regulated spermatid polarity is mediated via its effects on F-actin and protein expression (e.g. laminin-γ3) at the apical ES
Although the in vitro model using purified Sertoli cells failed to monitor changes in cell polarity, studies in vivo have clearly illustrated the role of the Scribble/Lgl2/Dlg1 in spermatid polarity consistent with earlier reports regarding the role of this complex on mammalian cell polarity (46, 47). In short, the triple silencing of Scribble/Lg12/Dlg1, but not either Scribble or Lgl2 alone, was found to alter the overall organization of the F-actin network at the apical ES in the seminiferous epithelium with more actin filaments localized to the BTB concomitant with its considerable reduction at the apical ES in stage VIII tubules. More importantly, the expression of apical ES adhesion proteins, such as laminin-γ3 chain at the BTB (40), was found to be down-regulated only in the triple, not the Lgl2 single, silencing group, perhaps also related to a loss of F-actin at the apical ES. Such a loss of actin filaments thus impedes the recruitment of laminin-γ3 to the site, which resulted in a weakened adhesion at the apical ES, leading to the loss of polarity in spermatids. In this context, it is of interest in future studies to determine whether Dlg1 plays any role in spermatid polarity in the testis.
Summary
Lgl2 is an important regulatory component of the Scribble/Lgl2/Dlg1 complex on Sertoli cell BTB function in a stage-specific manner. To maintain spermatid polarity, however, all three Scribble complex complexes are required.
Supplementary Material
Acknowledgments
This work was supported by grants from the National Institute of Child Health and Human Development R01 HD056034 (to C.Y.C.) and U54 HD029990, Project 5 (to C.Y.C.); and Grant 81100462 from the National Natural Science Foundation of China (to W.H.S.).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- BTB
- Blood-testis barrier
- b.w.
- body weight
- DAPI
- 4′,6-diamidino-2-phenylindole
- Dlg
- discs large
- ES
- ectoplasmic specialization
- FITC
- fluorescein isothiocyanate
- IHC
- immunohistochemistry
- Lgl
- lethal giant larvae
- qPCR
- quantitative real-time RT-PCR
- RNAi
- RNA interference; siRNA small interfering RNA
- SLD
- Scribble, Lgl2, and Dlg1
- TER
- transepithelial electrical resistance
- TJ
- tight junction.
References
- 1. Cheng CY, Mruk DD. 2012. The blood-testis barrier and its implication in male contraception. Pharmacol Rev 64:16–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Cheng CY, Mruk DD. 2010. A local autocrine axis in the testes that regulates spermatogenesis. Nat Rev Endocrinol 6:380–395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hess RA, de Franca L. 2008. Spermatogenesis and cycle of the seminiferous epithelium. Adv Exp Med Biol 636:1–15 [DOI] [PubMed] [Google Scholar]
- 4. Mruk DD, Silvestrini B, Cheng CY. 2008. Anchoring junctions as drug targets: role in contraceptive development. Pharmacol Rev 60:146–180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. O'Donnell L, Nicholls PK, O'Bryan MK, McLachlan RI, Stanton PG. 2011. Spermiation: the process of sperm release. Spermatogenesis 1:14–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Vogl AW, Vaid KS, Guttman JA. 2008. The Sertoli cell cytoskeleton. Adv Exp Med Biol 636:186–211 [DOI] [PubMed] [Google Scholar]
- 7. Wolski KM, Perrault C, Tran-Son-Tay R, Cameron DF. 2005. Strength measurement of the Sertoli-spermatid junctional complex. J Androl 26:354–359 [DOI] [PubMed] [Google Scholar]
- 8. Cheng CY, Mruk DD. 2011. Actin binding proteins and spermatogenesis. Some unexpected findings. Spermatogenesis 1:99–104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wong EW, Cheng CY. 2009. Polarity proteins and cell-cell interactions in the testis. Int Rev Cell Mol Biol 278:309–353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Iden S, Collard JG. 2008. Crosstalk between small GTPases and polarity proteins in cell polarization. Nat Rev Mol Cell Biol 9:846–859 [DOI] [PubMed] [Google Scholar]
- 11. Assémat E, Bazellières E, Pallesi-Pocachard E, Le Bivic A, Massey-Harroche D. 2008. Polarity complex proteins. Biochim Biophys Acta 1778:614–630 [DOI] [PubMed] [Google Scholar]
- 12. Cheng CY, Wong EW, Lie PP, Li MW, Mruk DD, Yan HH, Mok KW, Mannu J, Mathur PP, Lui WY, Lee WM, Bonanomi M, Silvestrini B. 2011. Regulation of blood-testis barrier dynamics by desmosome, gap junction, hemidesmosome and polarity proteins: an unexpected turn of events. Spermatogenesis 1:105–115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mruk DD, Cheng CY. 2011. An in vitro system to study Sertoli cell blood-testis barrier dynamics. Methods Mol Biol 763:237–252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Galdieri M, Ziparo E, Palombi F, Russo MA, Stefanini M. 1981. Pure Sertoli cell cultures: a new model for the study of somatic-germ cell interactions. J Androl 2:249–254 [Google Scholar]
- 15. Lee NP, Mruk DD, Conway AM, Cheng CY. 2004. Zyxin, axin, and Wiskott-Aldrich syndrome protein are adaptors that link the cadherin/catenin protein complex to the cytoskeleton at adherens junctions in the seminiferous epithelium of the rat testis. J Androl 25:200–215 [DOI] [PubMed] [Google Scholar]
- 16. Orth JM. 1982. Proliferation of Sertoli cells in fetal and postnatal rats: a quantitative autoradiographic study. Anat Rec 203:485–492 [DOI] [PubMed] [Google Scholar]
- 17. Li JC, Lee TW, Mruk TD, Cheng CY. 2001. Regulation of Sertoli cell myotubularin (rMTM) expression by germ cells in vitro. J Androl 22:266–277 [PubMed] [Google Scholar]
- 18. Lui WY, Lee WM, Cheng CY. 2003. Transforming growth factor-β3 regulates the dynamics of Sertoli cell tight junctions via the p38 mitogen-activated protein kinase pathway. Biol Reprod 68:1597–1612 [DOI] [PubMed] [Google Scholar]
- 19. Li MW, Mruk DD, Lee WM, Cheng CY. 2009. Disruption of the blood-testis barrier integrity by bisphenol A in vitro: is this a suitable model for studying blood-testis barrier dynamics? Int J Biochem Cell Biol 41:2302–2314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lie PP, Cheng CY, Mruk DD. 2010. Crosstalk between desmoglein-2/desmocollin-2/Src kinase and coxsackie and adenovirus receptor/ZO-1 protein complexes, regulates blood-testis barrier dynamics. Int J Biochem Cell Biol 42:975–986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Siu ER, Wong EW, Mruk DD, Porto CS, Cheng CY. 2009. Focal adhesion kinase is a blood-testis barrier regulator. Proc Natl Acad Sci USA 106:9298–9303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wong EW, Mruk DD, Lee WM, Cheng CY. 2008. Par3/Par6 polarity complex coordinates apical ectoplasmic specialization and blood-testis barrier restructuring during spermatogenesis. Proc Natl Acad Sci USA 105:9657–9662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Cheng CY, Mruk D, Silvestrini B, Bonanomi M, Wong CH, Siu MK, Lee NP, Mo MY. 2005. AF-2364 [1-(2,4-dichlorobenzyl)-1H-indazole-3-carbohydrazide] is a potential male contraceptive: a review of recent data. Contraception 72:251–261 [DOI] [PubMed] [Google Scholar]
- 24. Mok KW, Mruk DD, Lee WM, Cheng CY. 2012. Spermatogonial stem cells alone are not sufficient to re-initiate spermatogenesis in the rat testis following adjudin-induced infertility. Int J Androl 35:86–101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Su L, Cheng CY, Mruk DD. 2010. Adjudin-mediated Sertoli-germ cell junction disassembly affects Sertoli cell barrier function in vitro and in vivo. Int J Biochem Cell Biol 42:1864–1875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Xia W, Wong CH, Lee NP, Lee WM, Cheng CY. 2005. Disruption of Sertoli-germ cell adhesion function in the seminiferous epithelium of the rat testis can be limited to adherens junctions without affecting the blood-testis barrier integrity: an in vivo study using an androgen suppression model. J Cell Physiol 205:141–157 [DOI] [PubMed] [Google Scholar]
- 27. Cheng CY, Silvestrini B, Grima J, Mo MY, Zhu LJ, Johansson E, Saso L, Leone MG, Palmery M, Mruk DD. 2001. Two new male contraceptives exert their effects by depleting germ cells prematurely from the testis. Biol Reprod 65:449–461 [DOI] [PubMed] [Google Scholar]
- 28. Wong EW, Sun S, Li MW, Lee WM, Cheng CY. 2009. 14–3-3 protein regulates cell adhesion in the seminiferous epithelium of rat testes. Endocrinology 150:4713–4723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Yan HH, Mruk DD, Wong EW, Lee WM, Cheng CY. 2008. An autocrine axis in the testis that coordinates spermiation and blood-testis barrier restructuring during spermatogenesis. Proc Natl Acad Sci USA 105:8950–8955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Xia W, Mruk DD, Lee WM, Cheng CY. 2006. Differential interactions between transforming growth factor-β3/TβR1, TAB1, and CD2AP disrupt blood-testis barrier and Sertoli-germ cell adhesion. J Biol Chem 281:16799–16813 [DOI] [PubMed] [Google Scholar]
- 31. Xia W, Mruk DD, Cheng CY. 2007. C-type natriuretic peptide regulates blood-testis barrier dynamics in adult rat testes. Proc Natl Acad Sci USA 104:3841–3846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Xia W, Mruk DD, Lee WM, Cheng CY. 2007. Unraveling the molecular targets pertinent to junction restructuring events during spermatogenesis using the Adjudin-induced germ cell depletion model. J Endocrinol 192:563–583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Mruk DD, Cheng CY. 2011. Enhanced chemiluminescence (ECL) for routine immunoblotting. An inexpensive alternative to commercially available kits. Spermatogenesis 1:121–122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Li MW, Mruk DD, Lee WM, Cheng CY. 2009. Connexin 43 and plakophilin-2 as a protein complex that regulates blood-testis barrier dynamics. Proc Natl Acad Sci USA 106:10213–10218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Su L, Mruk DD, Lui WY, Lee WM, Cheng CY. 2011. P-glycoprotein regulates blood-testis barrier dynamics via its effects on the occludin/zonula occludens 1 (ZO-1) protein complex mediated by focal adhesion kinase (FAK). Proc Natl Acad Sci USA 108:19623–19628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Li MW, Xia W, Mruk DD, Wang CQ, Yan HH, Siu MK, Lui WY, Lee WM, Cheng CY. 2006. TNFα reversibly disrupts the blood-testis barrier and impairs Sertoli-germ cell adhesion in the seminiferous epithelium of adult rat testes. J Endocrinol 190:313–329 [DOI] [PubMed] [Google Scholar]
- 37. Dow LE, Elsum IA, King CL, Kinross KM, Richardson HE, Humbert PO. 2008. Loss of human Scribble cooperates with H-Ras to promote cell invasion through deregulation of MAPK signalling. Oncogene 27:5988–6001 [DOI] [PubMed] [Google Scholar]
- 38. Nagasaka K, Pim D, Massimi P, Thomas M, Tomaić V, Subbaiah VK, Kranjec C, Nakagawa S, Yano T, Taketani Y, Myers M, Banks L. 2010. The cell polarity regulator hScrib controls ERK activation through a KIM site-dependent interaction. Oncogene 29:5311–5321 [DOI] [PubMed] [Google Scholar]
- 39. Takehashi M, Kanatsu-Shinohara M, Miki H, Lee J, Kazuki Y, Inoue K, Ogonuki N, Toyokuni S, Oshimura M, Ogura A, Shinohara T. 2007. Production of knockout mice by gene targeting in multipotent germline stem cells. Dev Biol 312:344–352 [DOI] [PubMed] [Google Scholar]
- 40. Yan HH, Cheng CY. 2006. Laminin α3 forms a complex with β3 and γ3 chains that serves as the ligand for α6β1-integrin at the apical ectoplasmic specialization in adult rat testes. J Biol Chem 281:17286–17303 [DOI] [PubMed] [Google Scholar]
- 41. Ivanov AI, Young C, Den Beste K, Capaldo CT, Humbert PO, Brennwald P, Parkos CA, Nusrat A. 2010. Tumor suppressor scribble regulates assembly of tight junctions in the intestinal epithelium. Am J Pathol 176:134–145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Xia W, Cheng CY. 2005. TGF-β3 regulates anchoring junction dynamics in the seminiferous epithelium of the rat testis via the Ras/ERK signaling pathway: an in vivo study. Dev Biol 280:321–343 [DOI] [PubMed] [Google Scholar]
- 43. Lie PP, Xia W, Wang CQ, Mruk DD, Yan HH, Wong CH, Lee WM, Cheng CY. 2006. Dynamin II interacts with the cadherin- and occludin-based protein complexes at the blood-testis barrier in adult rat testes. J Endocrinol 191:571–586 [DOI] [PubMed] [Google Scholar]
- 44. Hess RA, Schaeffer DJ, Eroschenko VP, Keen JE. 1990. Frequency of the stages of the cycle of the seminiferous epithelium in the rat. Biol Reprod 43:517–524 [DOI] [PubMed] [Google Scholar]
- 45. Setchell BP. 2008. Blood-testis barrier, functional and transport proteins and spermatogenesis. Adv Exp Med Biol 636:212–233 [DOI] [PubMed] [Google Scholar]
- 46. Müsch A, Cohen D, Yeaman C, Nelson WJ, Rodriguez-Boulan E, Brennwald PJ. 2002. Mammalian homolog of Drosophila tumor suppressor lethal (2) giant larvae interacts with basolateral exocytic machninery in Madin-Darby canine kidney cells. Mol Biol Cell 13:158–168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Yamanaka T, Horikoshi Y, Izumi N, Suzuki A, Mizuno K, Ohno S. 2006. Lgl mediatres apical domain disassmbly by suppressing the PAR-3-aPKC-PAR-6 complex to orient apical membrane polarity. J Cell Sci 119:2107–2118 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.