Abstract
Although mastermind-like domain containing 1 (MAMLD1) (CXORF6) on human chromosome Xq28 has been shown to be a causative gene for 46,XY disorders of sex development with hypospadias, the biological function of MAMLD1/Mamld1 remains to be elucidated. In this study, we first showed gradual and steady increase of testicular Mamld1 mRNA expression levels in wild-type male mice from 12.5 to 18.5 d postcoitum. We then generated Mamld1 knockout (KO) male mice and revealed mildly but significantly reduced testicular mRNA levels (65–80%) of genes exclusively expressed in Leydig cells (Star, Cyp11a1, Cyp17a1, Hsd3b1, and Insl3) as well as grossly normal testicular mRNA levels of genes expressed in other cell types or in Leydig and other cell types. However, no demonstrable abnormality was identified for cytochrome P450 17A1 and 3β-hydroxysteroid dehydrogenase (HSD3B) protein expression levels, appearance of external and internal genitalia, anogenital distance, testis weight, Leydig cell number, intratesticular testosterone and other steroid metabolite concentrations, histological findings, in situ hybridization findings for sonic hedgehog (the key molecule for genital tubercle development), and immunohistochemical findings for anti-Müllerian hormone (Sertoli cell marker), HSD3B (Leydig cell marker), and DEAD (Asp-Glu-Ala-Asp) box polypeptide 4 (germ cell marker) in the KO male mice. Fertility was also normal. These findings imply that Mamld1 deficiency significantly reduces mRNA expression levels of multiple genes expressed in mouse fetal Leydig cells but permits normal genital and reproductive development. The contrastive phenotypic findings between Mamld1 KO male mice and MAMLD1 mutation positive patients would primarily be ascribed to species difference in the fetal sex development.
Mastermind-like domain containing 1 (MAMLD1) (alias CXORF6) on human chromosome Xq28 is a causative gene for 46,XY disorders of sex development (DSDs) with hypospadias as a salient clinical phenotype. Indeed, several pathologic nonsense and frameshift mutations (p.E124X, p.Q197X, p.R653X, and p.E109fsX121) have been identified in patients with various types of hypospadias with and without other associated genital abnormalities, such as micropenis and cryptorchidism (1–3). In addition, a specific polymorphism(s) and a haplotype of MAMLD1 appear to constitute a genetic risk factor for hypospadias (2, 4, 5).
To date, several important findings have been revealed for MAMLD1 and its mouse homolog Mamld1. First, the upstream region of MAMLD1/Mamld1 harbors a putative binding site for NR5A1 (alias SF-1 and AD4BP) (6) that regulates the transcription of a vast array of genes involved in sex development (7). Second, nuclear receptor subfamily 5, group A, member 1 protein can bind to the putative target site and exert a transactivation function for Mamld1 (6). Third, Mamld1 is clearly coexpressed with mouse Nr5a1 in fetal Leydig and Sertoli cells in the fetal testis (1). Fourth, transient Mamld1 knockdown using small interfering RNAs (siRNAs) significantly reduces Cyp17a1 expression (8) and testosterone (T) production in cultured mouse Leydig tumor cells (MLTCs) (6, 8). These findings imply that MAMLD1/Mamld1 is involved in the molecular network for T production probably via the transactivation of CYP17A1/Cyp17a1 under the regulation of NR5A1 and that MAMLD1 mutations result in 46,XY DSD phenotype with hypospadias primarily because of compromised, but not abolished, T production around the critical period for sex development.
However, the biological function of MAMLD1/Mamld1 during testis development remains to be elucidated. Thus, we examined testicular Mamld1 mRNA expression pattern in wild-type (WT) male mice and performed molecular and phenotypic analyses in Mamld1 knockout (KO) male mice.
Materials and Methods
WT and Mamld1 KO male mice
We examined WT male mice of the C57BL/6 strain purchased from Sankyo Labo Service Corp., Inc. (Tokyo, Japan) and Mamld1 KO male mice generated by Macrogen, Inc. (Seoul, Korea). This study was approved by the Animal Ethics Committee of National Research Institute for Child Health and Development.
Mamld1 KO male mice were produced by a standard gene-targeting procedure (9). In brief, a targeting vector was designed to replace Mamld1 exon 3, which harbors a translation start codon and approximately two thirds of the coding sequence, with a PGK-neo cassette (Fig. 1A). After transfection of the targeting vector into 129/Sv embryonic stem cells by electroporation, two clones of recombination-positive embryonic stem cells were selected by Southern blot analysis using probes at the 5′ and 3′ flanking regions of Mamld1 and injected into blastocysts. The blastocysts were then transferred into pseudopregnant ICR female mice, to generate chimeric male mice. The chimeric male mice were mated with C57BL/6 female mice, and germline transmission of the mutant gene was confirmed by Southern blot analysis. Subsequently, Mamld1 KO male mice were produced by mating heterozygous (+/−) female mice with WT male mice. The Mamld1 KO mouse strain was backcrossed with the C57BL/6 strain and maintained for multiple generations by cross-mating between heterozygous (+/−) female mice and WT male mice.
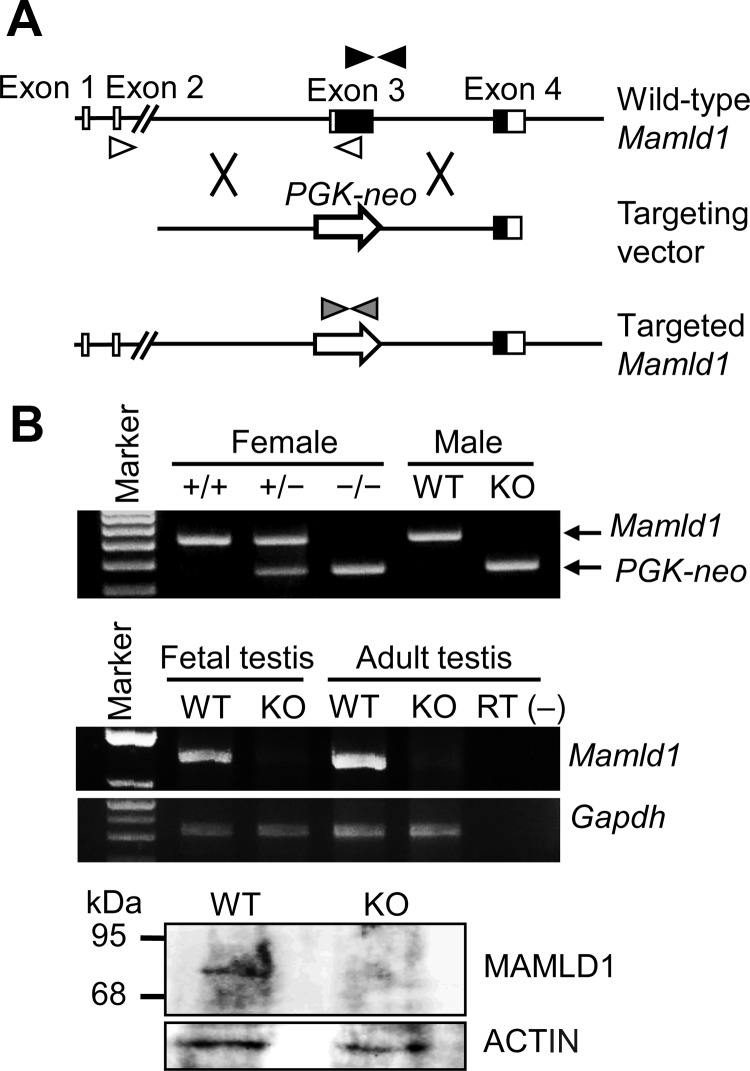
Fig. 1.
Generation of Mamld1 KO mice. A, Schematic representation of the gene targeting procedure. Exon 3 of WT Mamld1 was replaced by the PGK-neo cassette (PGK-neo) through homologous recombination indicated by cross symbols. The black and white boxes denote the coding regions and the untranslated regions, respectively. Paired black, white, and gray arrowheads indicate the primer set for amplification of WT Mamld1 genomic sequence, that for amplification of Mamld1 transcripts, and that for amplification of Neomycin-resistant gene. B, Confirmation of Mamld1 KO. Genotyping analysis (upper panel), RT-PCR analysis (middle panel), and Western blot analysis (lower panel) are consistent with successful Mamld1 KO. +/+, WT female mice; +/−, heterozygous female mice; −/−, homozygous female mice; RT (−), negative control without reverse transcriptase.
In this study, KO male mice of the ninth generation were examined. The noon of the day when a vaginal plug was observed was designated 0.5 d postcoitum (dpc). PCR-based genotyping analysis with tail tissue genomic DNA was performed for Mamld1, PGK-neo, and Sry, using primers shown in Supplemental Table 1, published on The Endocrine Society's Journals Online web site at http://endo.endojournals.org. Body weight and testis weight were measured at birth.
Genital and testicular sample preparation
In the male mice, androgen synthesis starts after approximately 13.5 dpc (10, 11), and morphological characteristics of the male external genitalia are established around 16.5 dpc (12, 13). Thus, genital and testicular samples were prepared from genotype- and embryonic day-matched KO male mice and their WT littermates in the latter half of the fetal life and at birth.
Real-time RT-PCR analyses
Testes from three mice were pooled in a single tube, and five tubes were prepared for each embryonic day. Total RNA was extracted from homogenized samples using ISOGEN (Nippongene, Tokyo, Japan), and cDNA was synthesized from 200 ng of total RNA using High Capacity cDNA Reverse Transcription kit (Life Technologies, Carlsbad, CA). Real-time RT-PCR was performed for Mamld1 and 17 genes involved in sex development and expressed in the fetal testis (Amh, Ar, Arx, Cyp11a1, Cyp17a1, Ddx4, Dhh, Dlx5, Dlx6, Gata4, Hsd17b3, Hsd3b1, Insl3, Nr5a1, Ptch1, Sox9, and Star) as well as Gapdh used as an internal control, using the ABI 7500 Fast real-time PCR system (Life Technologies) and TaqMan gene expression assay kit. Primers and probes used are shown in Supplemental Table 2.
Western blot analysis
Testes collected as described above were homogenized, diluted in Laemmli buffer, and heated at 95 C. Protein extracts were subjected to a standard SDS-PAGE (12% gel) and were hybridized with anti-MAMLD1-antibody (Ab), anti-cytochrome P450 17A1(CYP17A1)-Ab, and anti-3β-hydroxysteroid dehydrogenase (HSD3B)-Ab, as well as anti-ACTIN-Ab (A2066; Sigma, St. Louis, MO) used as an internal control. Anti-MAMLD1-Ab was generated against mouse MAMLD1 peptide (CGSESFLPGSSFAHE) using rabbits, anti-CYP17A1-Ab was purchased from Santa Cruz Biotechnology, Inc. (sc-46081; Santa Cruz, CA), and anti-HSD3B-Ab was as reported previously (14). Chemiluminescence signals were detected using ECL Plus Western Blot Detection kit (GE Healthcare UK Ltd., Buckinghamshire, UK), and signal densities were assessed using an Odyssey Infrared Imaging System (LI-COR Biosciences, Lincoln, NE).
Stereoscopic observation
Morphological findings of external and internal genital regions were examined, as were anogenital distance (AGD) (the distance between the anus and the penoscrotal junction) and AGD index (AGI) (AGD divided by body weight) as indicators for the androgen action during the embryonic period (15–17). Furthermore, whole mount in situ hybridization was performed for sonic hedgehog (Shh), one of the key molecules for the development of genital tubercle (18, 19), using an antisense cRNA fragment as a probe (GenBank accession no. BC063087; nucleotide position, 138-1499). Sense cRNA was used as a negative control. Hybridization was performed using the Wilkinson procedure (20), and signals were visualized with the BM Purple AP Substrate (Roche, Mannheim, Germany).
Histological and immunohistochemical examinations
Histological examination was performed for tissue samples that were fixed with 4% paraformaldehyde, dehydrated, and embedded in paraffin. Serial 6-μm sections were mounted on Superfrost slides, and every tenth section was stained with hematoxylin-eosin.
Immunohistochemical examination was carried out for the remaining section slides that were deparaffinized and incubated with 3% H2O2 in PBS to inactivate endogenous peroxidases. The slides were then incubated in blocking solution (Roche) and transferred into a new solution containing polyclonal primary Abs against anti-Mülerian hormone (sc-46081; Santa Cruz Biotechnology, Inc.) as a marker for Sertoli cells, HSD3B as a marker for Leydig cells, DEAD (Asp-Glu-Ala-Asp) box polypeptide 4 (ab13840; Abcam, Cambridge, UK) as a marker for germ cells, and proliferating cell nuclear antigen (PC10; Dako, Glostrup, Denmark) as a marker for proliferating cells. The samples were washed and incubated with secondary Abs conjugated with horseradish peroxidase (Santa Cruz Biotechnology, Inc.). The Simple Stain DAB Solution (Nichirei, Tokyo, Japan) was used for color development. Apoptotic cells were detected by terminal deoxynucleotidyl transferase 2′-deoxyuridine, 5′-triphosphate nick end labeling staining using an In Situ Apoptosis Detection kit (TaKaRa Bio, Shiga, Japan). Furthermore, HSD3B-positive cells in four randomly selected fields of each testis were counted, to estimate the number of Leydig cells.
Measurement of intratesticular T and steroid metabolites
Intratesticular T and steroid metabolites were measured at 18.5 dpc by liquid chromatography tandem mass spectrometry (ASKA Pharma Medical, Kanagawa, Japan) using samples stored at −80 C, because intratesticular T usually peaks at 18.5 dpc in normal mice (10, 11).
Cross-mating experiments
Cross-mating was performed between Mamld1 KO male mice and WT or heterozygous (+/−) female mice and between WT male mice and WT or heterozygous (+/−) female mice.
Statistical analysis
The data are expressed as the mean ± sem. Statistical significance of the mean between two groups was examined by Student's t test, and that of the frequency between two groups was examined by χ2 test. P < 0.05 was considered significant.
Results
Mamld1 expression in the fetal testis of WT male mice
Real-time RT-PCR analyses indicated a gradual and steady increase in the Mamld1 mRNA levels from 12.5 to 18.5 dpc (Fig. 2).
Fig. 2.
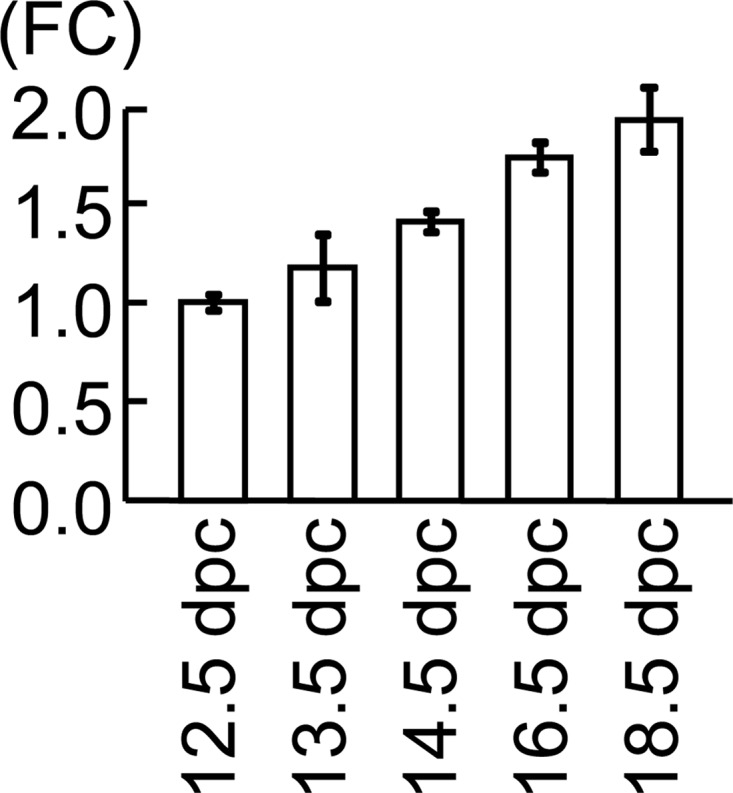
Testicular Mamld1 expression levels during the latter half of the fetal life in WT male mice. Figure indicates the data obtained by real-time RT-PCR analyses. Fold change (FC) represents relative mRNA levels of Mamld1 against Gapdh. The relative expression level of Mamld1 mRNA at 12.5 dpc was designated as 1.0.
Generation of Mamld1 KO male mice
Mamld1 KO male mouse was successfully produced. Mamld1 exon 3 was deleted from the genome of the KO mice, and neither Mamld1 mRNA nor MAMLD1 protein was identified in the testis of the KO mice (Fig. 1B). Body weight was comparable between the KO male mice and their WT littermates (Table 1).
Table 1.
Comparison between Mamld1 KO mice and their WT littermates
| KO | WT | P value | |
|---|---|---|---|
| Body weight (g) (at birth) | 1.48 ± 0.03 (n = 10) | 1.44 ± 0.03 (n = 10) | 0.40 |
| AGD (mm) (at birth) | 1.33 ± 0.02 (n = 10) | 1.32 ± 0.02 (n = 10) | 0.62 |
| AGI (mm/g) (at birth) | 0.90 ± 0.02 (n = 10) | 0.92 ± 0.02 (n = 10) | 0.55 |
| Leydig cells (HSD3B-stained cells) (number/HPF) (at 14.5 dpc) | 69.3 ± 8.2 (n = 3) | 75.1 ± 7.6 (n = 3) | 0.63 |
| Testis weight (mg) (at birth) | 1.46 ± 0.08 (n = 10) | 1.35 ± 0.08 (n = 10) | 0.34 |
| Intratesticular steroid metabolites (at 18.5 dpc) | |||
| Pregnenolone (pg/two testes) | 17.9 ± 4.0 (n = 4) | 15.4 ± 1.4 (n = 4) | 0.57 |
| Progesterone (pg/two testes) | 16.5 ± 4.6 (n = 4) | 15.0 ± 1.7 (n = 4) | 0.56 |
| 17-OH pregnenolone (pg/two testes) | 15.2 ± 2.9 (n = 4) | 15.4 ± 1.3 (n = 4) | 0.77 |
| 17-OH progesterone (pg/two testes) | 10.4 ± 1.7 (n = 4) | 13.5 ± 2.5 (n = 4) | 0.15 |
| Androstenedione (ng/two testes) | 0.44 ± 0.15 (n = 4) | 0.51 ± 0.07 (n = 4) | 0.25 |
| T (ng/two testes) | 2.31 ± 0.30 (n = 4) | 2.38 ± 0.31 (n = 4) | 0.89 |
Expressed as mean ± sem. HPF, High power field (234.1 × 175.5 μm).
Gene and protein expression pattern in the fetal testes of Mamld1 KO mice
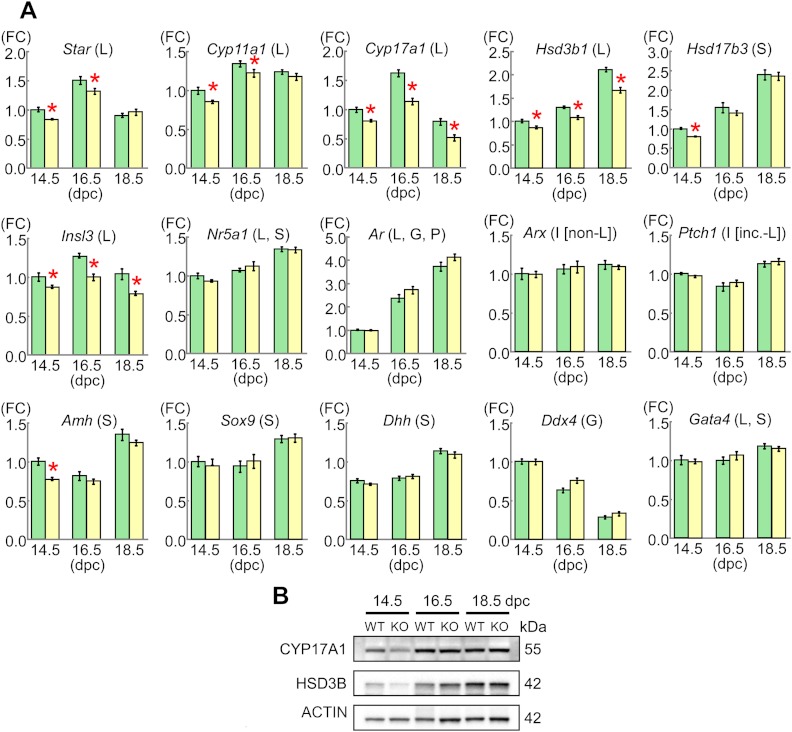
The results are shown in Fig. 3. Relative mRNA levels of Cyp17a1, Hsd3b1, and Insl3 mRNAs were mildly but significantly lower in the KO male mice than in their WT littermates at 14.5, 16.5, and 18.5 dpc, as were those for Star and Cyp11a1 at 14.5 and 16.5 dpc (65–80%) (Dlx5 and Dlx6 expression levels were extremely low). By contrast, relative mRNA levels of the remaining genes were comparable between the KO male mice and their WT littermates, except for relative mRNA levels of Hsd17b3 and Amh at 14.5 dpc. However, expression levels of CYP17A1 and HSD3B proteins were similar between the KO male mice and their WT littermates and were obviously higher at 16.5 and 18.5 dpc than at 14.5 dpc.
Fig. 3.
Gene and protein expression patterns in the fetal testes. A, Relative mRNA levels of examined genes against Gapdh. FC, Fold change; L, Leydig cells; S, Sertoli cells; G, germ cells; P, peritubular cells; I [non-L], interstitial cells excluding Leydig cells; I [inc.-L], interstitial cells including Leydig cells. The green and the yellow bars indicate the data obtained from WT male mice and Mamld1 KO male, respectively. For each gene, the relative expression level of mRNA in WT male mice at 14.5 dpc was designated as 1.0. Red asterisks indicate significant results (P < 0.05). B, Western blot analysis for CYP17A1 and HSD3B, as well as for ACTIN.
External genital findings of Mamld1 KO male mice
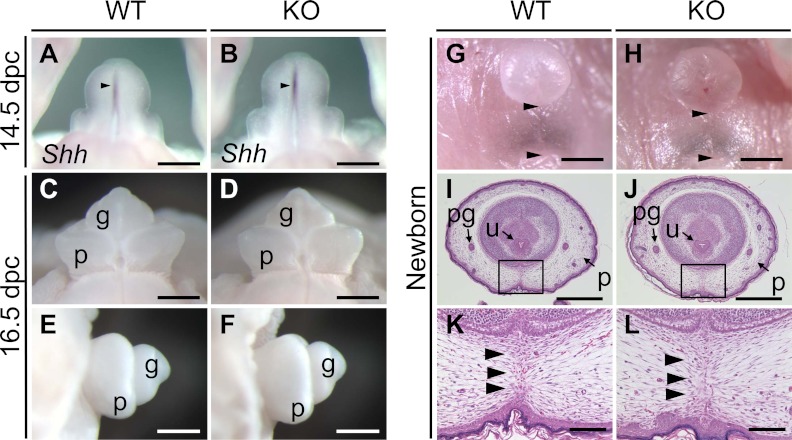
External genitalia were obviously normal in the Mamld1 KO male mice (Fig. 4 and Table 1). Shh was normally expressed in the urethral epithelium of the KO male mice at 14.5 dpc, and subsequent outgrowth of genital tubercle and fusion of the urethral folds at the ventral midline occurred in the KO male mice at the same embryonic stages as in their WT littermates. Furthermore, external genitalia were normally developed at birth, with the comparable AGD and AGI between the KO mice and their WT littermates.
Fig. 4.
External genitalia of WT and Mamld1 KO male mice. A and B, Whole mount in situ hybridization for Shh (arrowheads) in the developing genital region at 14.5 dpc. C–F, Appearance of the genital tubercle at 16.5 dpc. G and H, Appearance of the external genitalia at birth. The distance between the anus and the penoscrotal junction (arrowheads) represents the AGD. I–L, Histological findings of the external genitalia at birth. Arrowheads in K and L indicate the fused prepuce. g, Glans; p, prepuce; pg, preputal gland; u, urethra. Scale bars: 500 μm (A–F, I, and J), 1 mm (G and H), and 100 μm (K and L).
Internal genital findings of Mamld1 KO mice
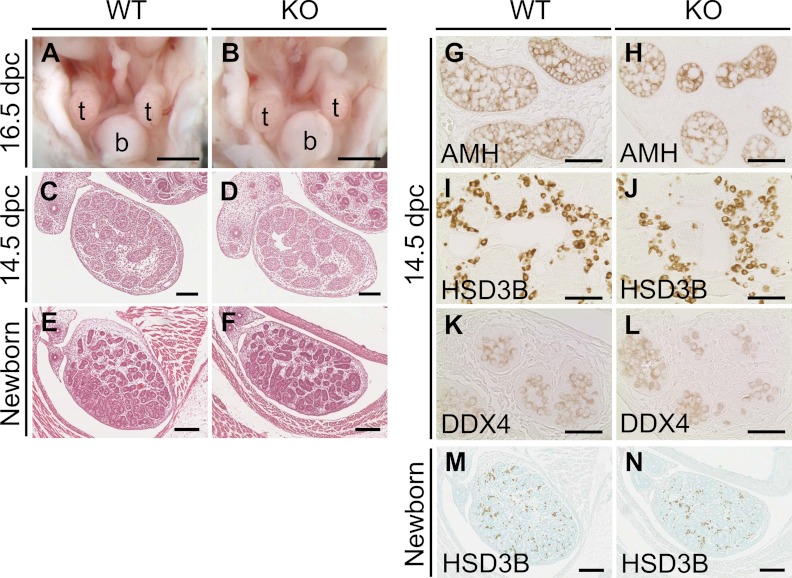
Internal genitalia of the Mamld1 KO male mice were also free from demonstrable abnormality (Fig. 5 and Table 1). Intraabdominal testicular descent, wolffian development, and müllerian regression were normally observed in the KO male mice at 16.5 dpc. Testicular histological findings were comparable between the KO mice and their WT littermates at 14.5 dpc and at birth. Immunohistochemical findings indicated the presence of similar numbers of Sertoli cells (anti-Müllerian hormone-stained cells), Leydig cells (HSD3B-stained cells), and germ cells [DEAD (Asp-Glu-Ala-Asp) box polyoeotide 4-stained cells] at 14.5 dpc as well as the presence of a similar number of Leydig cells (HSD3B-stained cells) at birth between the KO mice and their WT littermates. A relatively large number of mitotic cells (proliferating cell nuclear antigen-stained cells) was also identified in both the KO mice and their WT littermates, as were a small number of apoptotic cells (terminal deoxynucleotidyl transferase 2′-deoxyuridine, 5′-triphosphate nick end labeling-stained cells) (data not shown). In addition, testis weights at birth and intratesticular concentrations of T and other steroid metabolites at 18.5 dpc were also similar between the KO mice and their WT littermates.
Fig. 5.
Internal genitalia of WT and Mamld1 KO male mice. A and B, Appearance of internal genital organs at 16.5 dpc. C–F, Histological findings of testes at 14.5 dpc and birth. G–N, Immunohistochemical findings of testes at 14.5 dpc and birth. b, Bladder; t, testis. Scale bars: 1 mm (A and B), 100 μm (C and D), 200 μm (E, F, M, and N), and 50 μm (G–L).
Cross-mating experiments
The results are shown in Table 2. Mamld1 KO male mice produced offspring with WT and heterozygous (+/−) female mice, as did WT male mice. Furthermore, the frequency of littermate offspring [Mamld1 KO male mice, WT male mice, homozygous (−/−) female mice, heterozygous (+/−) female mice, and WT female mice] was in agreement with the expected Mendelian mode of inheritance.
Table 2.
Cross-mating experiments for Mamld1
| Offspring produced by cross-mating between Mamld1 KO male mice (n = 5) and WT female mice (n = 24) | |||||
| Sex and Mamld1 genotype | Male (−) | Male (+) | Female (−/−) | Female (+/−) | Female (+/+) |
| Number and frequency | n/o | 89 (45.6%) | n/o | 106 (54.4%) | n/o |
| Offspring produced by cross-mating between Mamld1 KO male mice (n = 14) and heterozygous female mice (n = 49) | |||||
| Sex and Mamld1 genotype | Male (−) | Male (+) | Female (−/−) | Female (+/−) | Female (+/+) |
| Number and frequency | 84 (23.6%) | 96 (27.0%) | 94 (26.4%) | 82 (23.0%) | n/o |
| Offspring produced by cross-mating between WT male mice (n = 6) and WT female mice (n = 12) | |||||
| Sex and Mamld1 genotype | Male (−) | Male (+) | Female (−/−) | Female (+/−) | Female (+/+) |
| Number and frequency | n/o | 58 (59.8%) | n/o | n/o | 39 (40.2%) |
| Offspring produced by cross-mating between WT male mice (n = 9) and heterozygous female mice (n = 46) | |||||
| Sex and Mamld1 genotype | Male (−) | Male (+) | Female (−/−) | Female (+/−) | Female (+/+) |
| Number and frequency | 86 (25.3%) | 85 (25.0%) | n/o | 84 (24.7%) | 85 (25.0%) |
WT or +, WT; KO or −, Mamld1 KO; n/o, not obtained.
Discussion
The Mamld1 mRNA expression was gradually and steadily increased from 12.5 to 18.5 dpc in the fetal testis of WT male mice. In this regard, intratesticular T has also been reported to increase in a similar manner in the mouse (10, 11). In addition, human study has also revealed clear MAMLD1 expression in the fetal testis. These findings would argue for a positive role of MAMLD1/Mamld1 in the T production in the fetal testis (1, 21).
We generated and studied Mamld1 KO male mice. The results are summarized as follows: 1) mRNA levels of genes exclusively expressed in Leydig cells (Star, Cyp11a1, Cyp17a1, Hsd3b1, and Insl3) were mildly but significantly reduced, whereas those of genes expressed in other cell types or in Leydig and other cell types grossly remained normal (Hsd17b3 is expressed in Sertoli cells of the fetal testis, although it is expressed in Leydig cells of the adult testis) (22, 23); 2) despite such mild reduction of mRNA levels, CYP17A1 and HSD3B proteins were sufficiently produced; 3) no demonstrable abnormality was identified by detailed studies for the external and internal genital regions; and 4) the Mamld1 KO male mice retained normal fertility. Collectively, these findings imply that Mamld1 deficiency reduces mRNA expression levels of multiple, if not all, genes expressed in mouse fetal Leydig cells but permits normal genital development and reproductive function. In support of this notion, such discrepancy between mRNA levels and protein levels as well as phenotypic consequences has been reported previously (24–26). Indeed, Greenbaum et al. (27) have proposed three possible explanations for the poor correlations between mRNA and protein expression levels: 1) there are many complicated and varied posttranscriptional mechanisms involved in turning mRNA into protein that are not yet sufficiently well defined; 2) proteins may differ substantially in their in vivo half lives; and 3) there may be a significant amount of error and noise in both protein and mRNA experiments that limit our ability to get a clear picture. These explanations would also apply to our results indicating normal expression of CYP17A1 and HSD3B proteins, in the presence of mildly but significantly reduced expression of Cyp17a1 and Hsd3b1 mRNAs. Furthermore, because CYP17A1 and HSD3B protein levels increased in a manner grossly similar to that reported for intratesticular T (10, 11) in both the Mamld1 KO male mice and their WT littermates, this would be consistent with the apparently normal testicular function of the Mamld1 KO male mice.
The normal phenotype in the Mamld1 KO male mice is contrastive to the DSD phenotype in the MAMLD1 mutation positive patients (1, 3). In this regard, it is notable that male genital development is primarily induced by testicular T that is produced via Δ5-pathway under the stimulation of chorionic gonadotropin during the first trimester in the human (28–31), whereas it is primarily carried out by testicular T that is produced via Δ4-pathway independently of the chorionic gonadotropin stimulation during the late gestational period in the mouse (10, 31, 32). Thus, although the detailed mechanism(s) remains to be clarified, such species difference in the fetal male sex development may underlie the phenotypic difference between the Mamld1 KO male mice and the MAMLD1 mutation positive patients. In addition, the bias that individuals with abnormal phenotypes only are usually examined in the human study may also be relevant to this matter.
The results of mRNA expression levels and intratesticular hormone concentrations in the Mamld1 KO male mice are different from those identified by transient Mamld1 knockdown experiments using siRNAs and MLTCs (6, 8), although the normal Leydig cell number of the Mamld1 KO male mice appears to be consistent with the sustained proliferation of siRNA-transfected MLTCs (8). Indeed, Mamld1 knockdown has predominantly affected Cyp17a1 expression (8) and significantly decreased T and other steroid metabolite after 17α-hydroxylation (6, 8). However, MLTCs are derived from adult Leydig tumor cells and are characterized by a markedly low 17α-hydroxylase activity and a well-preserved 17/20 lyase activity for both Δ4- and Δ5-pathways (33). Such unique properties of MLTCs may be relevant to the preferential impairment of Cyp17a1 expression and 17α-hydroxylation in siRNA-transfected MLTCs.
Two findings also appear to be worth pointing out in this study. First, Insl3 mRNA expression was significantly reduced and Amh mRNA expression was grossly normal, in the Mamld1 KO mice. Such mRNA expression patterns, if they also take place in the human, would be relevant to the frequent occurrence of cryptorchidism and the lack of müllerian derivatives in patients with MAMLD1 mutations (1). Second, Mamld1 KO male mice, WT male mice, homozygous (−/−) female mice, heterozygous (+/−) female mice, and WT female mice were born with frequencies consistent with the Mendelian mode of inheritance. Thus, although Mamld1 is ubiquitously expressed with strong expressions in the central nervous system (1), Mamld1 deficiency is unlikely to affect viability.
In summary, the present study implies that Mamld1 enhances mRNA expression levels of multiple genes exclusively expressed in fetal Leydig cells, although the effects of Mamld1 deficiency are insufficient to compromise the genital and reproductive development. Further studies will permit a better clarification of the biological function of MAMLD1/Mamld1.
Supplementary Material
Acknowledgments
This work was supported by the National Center for Child Health and Development Grant 23A-1; Grant for Research on Intractable Diseases from the Ministry of Health, Labor, and Welfare; Environment Research and Technology Development Fund C-0905 of the Ministry of Environment; Grants-in-Aid for Scientific Research (B) 23390249 and (S) 22227002 and for Young Scientists (B) 24790303 from the Japan Society for the Promotion of Science; and Grant-in-Aid for Scientific Research on Innovative Areas 22132004 from the Ministry of Education, Culture, Sports, Science, and Technology.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- Ab
- Antibody
- AGD
- anogenital distance
- AGI
- AGD index
- CYP17A1
- cytochrome P450 17A1
- dpc
- days postcoitum
- DSD
- disorder of sex development
- HSD3B
- 3β-hydroxysteroid dehydrogenase
- KO
- knockout
- MAMLD1
- mastermind-like domain containing 1
- MLTC
- mouse Leydig tumor cell
- Shh
- sonic hedgehog
- siRNA
- small interfering RNA
- T
- testosterone
- WT
- wild type.
References
- 1. Fukami M, Wada Y, Miyabayashi K, Nishino I, Hasegawa T, Nordenskjöld A, Camerino G, Kretz C, Buj-Bello A, Laporte J, Yamada G, Morohashi K, Ogata T. 2006. CXorf6 is a causative gene for hypospadias. Nat Genet 38:1369–1371 [DOI] [PubMed] [Google Scholar]
- 2. Kalfa N, Liu B, Klein O, Ophir K, Audran F, Wang MH, Mei C, Sultan C, Baskin LS. 2008. Mutations of CXorf6 are associated with a range of severities of hypospadias. Eur J Endocrinol 159:453–458 [DOI] [PubMed] [Google Scholar]
- 3. Ogata T, Laporte J, Fukami M. 2009. MAMLD1 (CXorf6): a new gene involved in hypospadias. Horm Res 71:245–252 [DOI] [PubMed] [Google Scholar]
- 4. Chen Y, Thai HT, Lundin J, Lagerstedt-Robinson K, Zhao S, Markljung E, Nordenskjöld A. 2010. Mutational study of the MAMLD1-gene in hypospadias. Eur J Med Genet 53:122–126 [DOI] [PubMed] [Google Scholar]
- 5. van der Zanden LF, van Rooij IA, Feitz WF, Franke B, Knoers NV, Roeleveld N. 2012. Aetiology of hypospadias: a systematic review of genes and environment. Hum Reprod Update 18:260–283 [DOI] [PubMed] [Google Scholar]
- 6. Fukami M, Wada Y, Okada M, Kato F, Katsumata N, Baba T, Morohashi K, Laporte J, Kitagawa M, Ogata T. 2008. Mastermind-like domain-containing 1 (MAMLD1 or CXorf6) transactivates the Hes3 promoter, augments testosterone production, and contains the SF1 target sequence. J Biol Chem 283:5525–5532 [DOI] [PubMed] [Google Scholar]
- 7. Lin L, Achermann JC. 2008. Steroidogenic factor-1 (SF-1, Ad4BP, NR5A1) and disorders of testis development. Sex Dev 2:200–209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Nakamura M, Fukami M, Sugawa F, Miyado M, Nonomura K, Ogata T. 2011. Mamld1 knockdown reduces testosterone production and Cyp17a1 expression in mouse Leydig tumor cells. PLoS One 6:e19123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hogan B, Beddington R, Costantini F, Lacy E. 1994. Manipulating the mouse embryo: a laboratory manual. New York: Cold Spring Harbor Laboratory Press [Google Scholar]
- 10. O'Shaughnessy PJ, Baker P, Sohnius U, Haavisto AM, Charlton HM, Huhtaniemi I. 1998. Fetal development of Leydig cell activity in the mouse is independent of pituitary gonadotroph function. Endocrinology 139:1141–1146 [DOI] [PubMed] [Google Scholar]
- 11. O'Shaughnessy PJ, Baker PJ, Johnston H. 2006. The foetal Leydig cell-differentiation, function and regulation. Int J Androl 29:90–95; discussion 105–108 [DOI] [PubMed] [Google Scholar]
- 12. Miyagawa S, Satoh Y, Haraguchi R, Suzuki K, Iguchi T, Taketo MM, Nakagata N, Matsumoto T, Takeyama K, Kato S, Yamada G. 2009. Genetic interactions of the androgen and Wnt/β-catenin pathways for the masculinization of external genitalia. Mol Endocrinol 23:871–880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Suzuki K, Ogino Y, Murakami R, Satoh Y, Bachiller D, Yamada G. 2002. Embryonic development of mouse external genitalia: insights into a unique mode of organogenesis. Evol Dev 4:133–141 [DOI] [PubMed] [Google Scholar]
- 14. Fatchiyah, Zubair M, Shima Y, Oka S, Ishihara S, Fukui-Katoh Y, Morohashi K. 2006. Differential gene dosage effects of Ad4BP/SF-1 on target tissue development. Biochem Biophys Res Commun 341:1036–1045 [DOI] [PubMed] [Google Scholar]
- 15. Graham S, Gandelman R. 1986. The expression of ano-genital distance data in the mouse. Physiol Behav 36:103–104 [DOI] [PubMed] [Google Scholar]
- 16. Kerin TK, Vogler GP, Blizard DA, Stout JT, McClearn GE, Vandenbergh DJ. 2003. Anogenital distance measured at weaning is correlated with measures of blood chemistry and behaviors in 450-day-old female mice. Physiol Behav 78:697–702 [DOI] [PubMed] [Google Scholar]
- 17. Swan SH, Main KM, Liu F, Stewart SL, Kruse RL, Calafat AM, Mao CS, Redmon JB, Ternand CL, Sullivan S, Teague JL. 2005. Decrease in anogenital distance among male infants with prenatal phthalate exposure. Environ Health Perspect 113:1056–1061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Haraguchi R, Mo R, Hui C, Motoyama J, Makino S, Shiroishi T, Gaffield W, Yamada G. 2001. Unique functions of Sonic hedgehog signaling during external genitalia development. Development 128:4241–4250 [DOI] [PubMed] [Google Scholar]
- 19. Miyagawa S, Matsumaru D, Murashima A, Omori A, Satoh Y, Haraguchi R, Motoyama J, Iguchi T, Nakagata N, Hui CC, Yamada G. 2011. The role of sonic hedgehog-Gli2 pathway in the masculinization of external genitalia. Endocrinology 152:2894–2903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wilkinson D. 1992. In situ hybridization: a practical approach. London: Oxford University Press [Google Scholar]
- 21. O'Shaughnessy PJ, Baker PJ, Monteiro A, Cassie S, Bhattacharya S, Fowler PA. 2007. Developmental changes in human fetal testicular cell numbers and messenger ribonucleic acid levels during the second trimester. J Clin Endocrinol Metab 92:4792–4801 [DOI] [PubMed] [Google Scholar]
- 22. Baker PJ, Sha JH, O'Shaughnessy PJ. 1997. Localisation and regulation of 17β-hydroxysteroid dehydrogenase type 3 mRNA during development in the mouse testis. Mol Cell Endocrinol 133:127–133 [DOI] [PubMed] [Google Scholar]
- 23. O'Shaughnessy PJ, Baker PJ, Heikkilä M, Vainio S, McMahon AP. 2000. Localization of 17β-hydroxysteroid dehydrogenase/17-ketosteroid reductase isoform expression in the developing mouse testis-androstenedione is the major androgen secreted by fetal/neonatal leydig cells. Endocrinology 141:2631–2637 [DOI] [PubMed] [Google Scholar]
- 24. Lehmann KP, Phillips S, Sar M, Foster PM, Gaido KW. 2004. Dose-dependent alterations in gene expression and testosterone synthesis in the fetal testes of male rats exposed to di (n-butyl) phthalate. Toxicol Sci 81:60–68 [DOI] [PubMed] [Google Scholar]
- 25. Thompson CJ, Ross SM, Hensley J, Liu K, Heinze SC, Young SS, Gaido KW. 2005. Differential steroidogenic gene expression in the fetal adrenal gland versus the testis and rapid and dynamic response of the fetal testis to di(n-butyl) phthalate. Biol Reprod 73:908–917 [DOI] [PubMed] [Google Scholar]
- 26. Weisser J, Landreh L, Söder O, Svechnikov K. 2011. Steroidogenesis and steroidogenic gene expression in postnatal fetal rat Leydig cells. Mol Cell Endocrinol 341:18–24 [DOI] [PubMed] [Google Scholar]
- 27. Greenbaum D, Colangelo C, Williams K, Gerstein M. 2003. Comparing protein abundance and mRNA expression levels on a genomic scale. Genome Biol 4:117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Flück CE, Miller WL, Auchus RJ. 2003. The 17, 20-lyase activity of cytochrome p450c17 from human fetal testis favors the δ5 steroidogenic pathway. J Clin Endocrinol Metab 88:3762–3766 [DOI] [PubMed] [Google Scholar]
- 29. Fowler PA, Bhattacharya S, Gromoll J, Monteiro A, O'Shaughnessy PJ. 2009. Maternal smoking and developmental changes in luteinizing hormone (LH) and the LH receptor in the fetal testis. J Clin Endocrinol Metab 94:4688–4695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Huhtaniemi IT, Korenbrot CC, Jaffe RB. 1977. HCG binding and stimulation of testosterone biosynthesis in the human fetal testis. J Clin Endocrinol Metab 44:963–967 [DOI] [PubMed] [Google Scholar]
- 31. Scott HM, Mason JI, Sharpe RM. 2009. Steroidogenesis in the fetal testis and its susceptibility to disruption by exogenous compounds. Endocr Rev 30:883–925 [DOI] [PubMed] [Google Scholar]
- 32. Baker PJ, O'Shaughnessy PJ. 2001. Role of gonadotrophins in regulating numbers of Leydig and Sertoli cells during fetal and postnatal development in mice. Reproduction 122:227–234 [DOI] [PubMed] [Google Scholar]
- 33. Panesar NS, Chan KW, Ho CS. 2003. Mouse Leydig tumor cells produce C-19 steroids, including testosterone. Steroids 68:245–251 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.