Abstract
Increasing evidence suggests that inflammation/cytokines may modulate hypothalamic responses to leptin, which is a key regulator of energy homeostasis and inflammatory/stress responses. We investigated a possible role of TNF-α, a key early mediator of inflammation, in regulating the expression and trafficking of the long-isoform leptin receptor (LEPRb), the primary mediator of leptin signaling, in cultured cells. We found that TNF-α in a wide range of concentrations up-regulated LEPRb protein level and soluble LEPR (sLEPR) release via ectodomain shedding of LEPRb in multiple cell types, including neuronal cells. TNF-α also acutely increased LEPRb cell surface expression and leptin-induced STAT3 phosphorylation. In contrast, TNF-α had no significant effects on the protein level or cell surface expression of several other transmembrane proteins, including the transferrin receptor and cadherin. The stimulatory effects of TNF-α on LEPRb cell surface expression and sLEPR release were not dependent on de novo protein synthesis or functional lysosomes but were blocked by brefeldin A, suggesting that an intact Golgi or continuous endoplasmic reticulum to Golgi transport of newly synthesized proteins is required for these effects. However, TNF-α did not increase the half-life of cell surface LEPRb. Protein kinase C (PKC) inhibitor GF109203X abrogated the effects of TNF-α, whereas the pan-PKC activator phorbol 12-myristate 13-acetate mimicked the TNF-α effects. Taken together, our results suggest that TNF-α, via activation of PKC, regulates anterograde trafficking and/or degradation of LEPRb in the biosynthetic pathway, leading to concomitant increases in LEPRb protein level, cell surface expression, and sLEPR production. The finding that LEPRb cell surface expression and sLEPR production, key modulators of leptin sensitivity and bioavailability, are direct targets of TNF-α signaling could have a potentially important implication in the regulation of leptin signaling activity in different pathophysiological conditions as diverse as obesity and sepsis.
Leptin, a peptide hormone produced predominantly in adipocytes, regulates many physiological functions, including energy homeostasis, reproduction, and immunity (1–7). Leptin signaling is also critically important in modulating the course and outcome of sepsis and other critical illnesses (8, 9). The leptin receptor (LEPR), encoded by a single gene in mammals, belongs to the type I cytokine receptor superfamily (10). Different membrane-bound LEPR isoforms, generated through alternative splicing, share a common extracellular domain but differ in the sequence of their cytoplasmic tails (10–13). LEPRb, the isoform with the longest cytoplasmic tail, is highly expressed in the brain and plays a key role in mediating leptin signaling via the Janus kinases (JAK) and signal transducer and activator of transcription (STAT) pathway (14–16). Soluble leptin receptor (sLEPR) is the main circulating high-affinity leptin-binding protein (17) and may be involved in modulating leptin signaling, clearance, and transport (18–21). sLEPR can be generated through alternative splicing and/or through ectodomain shedding (11–13), a proteolytic process in which the extracellular domain of membrane-bound LEPR, including LEPRb, is released from the membrane as sLEPR (22, 23).
Although LEPRb is crucial in mediating leptin signaling, regulation of LEPRb protein level and cell surface expression are poorly understood. LEPRb protein level and residence at the cell surface are very low even in transfected cells in which Leprb cDNA transcription is under the control of a strong viral promoter (24). LEPRb is a fast-turnover protein, with a half-life of approximately 1.5 h in HeLa cells (24). The majority of cellular LEPRb is localized in the perinuclear region in cultured cells and in vivo (24–28). Both ligand-dependent and ligand-independent internalization of Leprb have been observed (24, 25, 29). However, targeting internalized LEPRb for degradation in lysosomes is a relatively slow process (25, 30) and is regulated by endospanin-1 and endospanin-2, two small integral membrane proteins that localize, respectively, to endosomes and the trans-Golgi network (31). Additionally, there is little evidence that internalized LEPR is recycled back to the cell surface (25, 30, 31). Taken together, these observations suggest that in addition to endocytosis/lysosome-mediated degradation of LEPRb proteins, other posttranslational degradation mechanisms may also play an important role in regulating LEPR protein level.
TNF-α is an important early mediator of inflammatory responses and plays a pivotal role in orchestrating the cytokine cascade in many inflammatory diseases (32, 33). Aberrant TNF-α production and signaling are associated with the pathogenesis of many diseases, including sepsis, cancer, arthritis, obesity, and diabetes (32, 33). Multiple studies have shown that inflammation is associated with increased plasma levels of leptin and sLEPR in both humans and animals (34–36) and that plasma sLEPR levels are positively correlated with plasma TNF-α levels in patients in late stages of heart failure (37). These observations suggest that TNF-α may regulate LEPR/sLEPR expression.
To elucidate this potential novel mechanism for the interplay between inflammation and leptin signaling and to gain better understanding of posttranslational regulations of LEPRb expression and trafficking, we investigated effects of TNF-α on protein level, cell surface expression, and ectodomain shedding of LEPRb in cultured cells and the cellular mechanism underlying these effects. Our results indicate that TNF-α acutely up-regulates LEPRb protein level and cell surface expression, which leads to increased cellular response to leptin and sLEPR production through ectodomain shedding. Our data further suggest that TNF-α exerts these effects by affecting anterograde trafficking and intracellular degradation of LEPRb in the biosynthetic pathway through a protein kinase C (PKC)-dependent mechanism.
Materials and Methods
Reagents and chemicals
DuoSet ELISA development kit for mouse LEPR (catalog item DY497) was purchased from R&D Systems (Minneapolis, MN). MAPK inhibitors SP600125, SB202190, PD98059, and U0126 and PKC inhibitor GF109203X, human recombinant TNF-α, anti-ADAM10 (a disintegrin and metalloproteinase domain containing protein 10), and anti-ADAM17 antibodies were from Calbiochem (La Jolla, CA). The sources of antibodies to the following proteins are as follows: STAT3, Santa Cruz Biotechnology (Santa Cruz, CA); matrix metalloproteinase 14 (MMP14), Cedarlane Labs (Ontario, Canada); pan-cadherin, Abcam (Cambridge, MA); all other antibodies, Cell Signaling Technology (Woburn, MA; catalog item 9926). Nucleofector Kit V (catalog item VCA-1003) was from Lonza Group Ltd. (Basel, Switzerland). Lipofectamine 2000 and all tissue culture reagents were from Invitrogen (Carlsbad, CA). Human transferrin receptor (hTfR) expression vector has been previously described (38). EZ-Link Sulfo-NHS-LC-Biotin and high-capacity NeutrAvidin beads were from Thermo Fisher Scientific (Rockford, IL). All other chemicals were from Sigma-Aldrich (St. Louis, MO) unless otherwise specified.
Construction of dual-tagged LEPRb
pcDNA3 vector carrying the cDNA encoding the full-length mouse LEPRb (mLEPRb) with MycHis tag at the C terminus has been previously described (23). A FLAG tag (DYKDDDDK) was inserted after the signal sequence between Gly22 and Ser23 using QuikChange II XL site-directed mutagenesis kit (Agilent Technologies, Santa Clara, CA) to generate a dual-tagged LEPRb. The sequence of the mutagenesis primer was 5′-GTGATAGCTGCACTTGGATCTGACTACAAGGACGACGATGACAAGAACCTGGCATATCC-3′. The expression of the tagged LEPRb was under the control of the immediate early promoter of cytomegalovirus (pCMV) in the pcDNA3 vector.
Generation of recombinant adenoviruses encoding the dual-tagged LEPRb, the native mLEPRb, and enhanced green fluorescent protein (GFP)
AdEasy Recombinant Adenovirus Vector System (Agilent Technologies) was used to create recombinant adenovirus expressing LEPRb, mLEPRb (from Dr. Louis Tartaglia at Millennium Pharmaceuticals, Cambridge, MA) (11), and enhanced GFP (Clontech, Mountain View, CA) following the manufacturer's instructions. The cDNAs were inserted downstream of the pCMV promoter in the pShuttle-CMV vector. Recombinant adenoviruses were twice purified by CsCl banding and sterilized by passing through a 0.22-μm filter as previously described (39).
Cell culture, transfection, adenovirus infection, TNF-α treatment, and sample preparation
All cells were grown in DMEM supplemented with 10% fetal bovine serum, 100 IU penicillin/100 μg/ml streptomycin, and 2 nm l-glutamine. Nucleofection was used to transfect HepG2 cells, and Lipofectamine 2000 was used to transfect HEK293 and N2a cells according to the manufacturer's recommendation or as previously described (40). To transduce HEK293, HepG2, and GT1-7 cells with the recombinant adenovirus, cells were incubated with recombinant adenoviruses at a multiplicity of infection of approximately 5, 10, and 50, respectively, in the culture media without antibiotics for 16–24 h. The TNF-α treatment condition of each experiment is described in the corresponding figure legend. In general, before TNF-α treatment, cells were made quiescent by 15 h serum starvation in serum-free Opti-MEM for TNF-α treatment less than 6 h or 5 h serum starvation for TNF-α treatment more than 12 h. For the time-course study, cells were serum starved for 15 h before TNF-α treatment. All pharmacological treatments were carried out in serum-free Opti-MEM. Cell lysates were prepared by sonication in a Nonidet P-40 lysis buffer, and protein concentrations were determined as previously described (41).
TCA precipitation, SDS-PAGE, and Western blot analysis
TCA (trichloroacetic acid) precipitation was performed by mixing conditioned medium (CM) with equal volumes of 20% TCA (wt/vol solution in water) on ice for more than 30 min. TCA precipitates were washed twice with cold acetone, air dried, and solubilized in 0.1 n NaOH. Solubilized TCA precipitates and cell lysates were analyzed by SDS-PAGE and Western blotting using enhanced chemiluminescence as previously described (41). Unless otherwise indicated, LEPRb in cell lysates and sLEPR in CM were detected by anti-Myc and anti-FLAG antibodies, respectively. Quantification of Western blots was performed by densitometry of scanned films using Quantity One Software (Bio-Rad, Hercules, CA).
ELISA analysis of mouse sLEPR
ELISA analyses of sLEPR levels in CM using a DuoSet ELISA development kit from R&D Systems were carried according to manufacturer's instructions. This ELISA kit detects sLEPR and other LEPR molecules that contain the ectodomain of LEPR.
Analysis of cell surface expression of LEPRb by indirect immunofluorescence microscopy and fluorescence-activated cell sorting (FACS)
Cells seeded on poly-l-lysine-coated chamber slides were fixed with freshly prepared 4% paraformaldehyde in PBS for 15 min at room temperature. For permeabilization, fixed cells were incubated with 0.1% Triton X-100 for 5 min. Permeabilized or unpermeabilized cells were blocked for 30 min in PBS/1% BSA/5% normal control serum (from the species in which the fluorescence-conjugated secondary antibody was made) and incubated with primary antibody diluted in PBS/1% BSA overnight at 4 C, followed by fluorescence-conjugated secondary antibody for 2 h at room temperature. Vehicle- and TNF-α-treated cells were stained in parallel and photographed using the same exposure setting. For FACS analysis of cell surface expression of LEPRb, unpermeabilized cells were fixed and labeled with anti-FLAG antibody, followed by fluorescein isothiocyanate-conjugated secondary antibody as described above. FACS analyses were carried out using a BD FACS Aria IIu ROU fluorescence-activated cell sorter. Intact cells were selected based on the size of particles, and more than 15,000 intact cells per sample were analyzed. The specific staining of cell surface LEPRb was calculated as the mean cell surface fluorescence intensity of experimental cells expressing LEPRb minus that of the control cells (not expressing LEPRb) that had been subject to the same treatment to control for any nonspecific background staining.
Biotinylation of cell surface proteins and analysis of the half-life of cell surface LEPRb
To biotinylate cell surface proteins, cells were washed with PBS and labeled with EZ-Link Sulfo-NHS-LC-Biotin at a concentration of 1 mg/ml for 4 h at 4 C with gentle rotation as recommended by the manufacturer. Biotinylated surface proteins were captured with NeutrAvidin beads before analysis by SDS-PAGE and Western blotting. To determine the half-life of cell surface LEPRb, HEK293 cells expressing LEPRb were labeled with biotin EZ-Link Sulfo-NHS-LC-Biotin for 1 h. Biotinylation was terminated by removing the labeling solution and neutralization in Opti-MEM containing, in addition, 10 mm glutamine for 10 min at 4 C. The biotinylated cells were then treated with vehicle or TNF-α (50 ng/ml) for 2 and 4 h at 37 C in a CO2 incubator. Biotinylated proteins were captured by NeutrAvidin beads and analyzed by SDS-PAGE and Western blotting.
Statistical analysis
All experiments were carried out in duplicate or triplicate and repeated at least three times. Data are shown as means ± sem of a typical set of triplicate samples from a single experiment (for ELISA and FACS results) or of multiple sets of duplicate samples (for Western blot results). For statistical analysis of Western blot results, the data, e.g. LEPR expression levels (LEPRb to α-tubulin ratios), in each experiment were first normalized to the appropriate control from the same experiment before they were pooled. Differences between control and experimental groups were examined using ANOVA, and P < 0.05 was considered statistically significant.
Results
Analysis of protein expression, plasma membrane localization, signaling activity, and ectodomain shedding of the dual-tagged mLEPRb in cultured cells
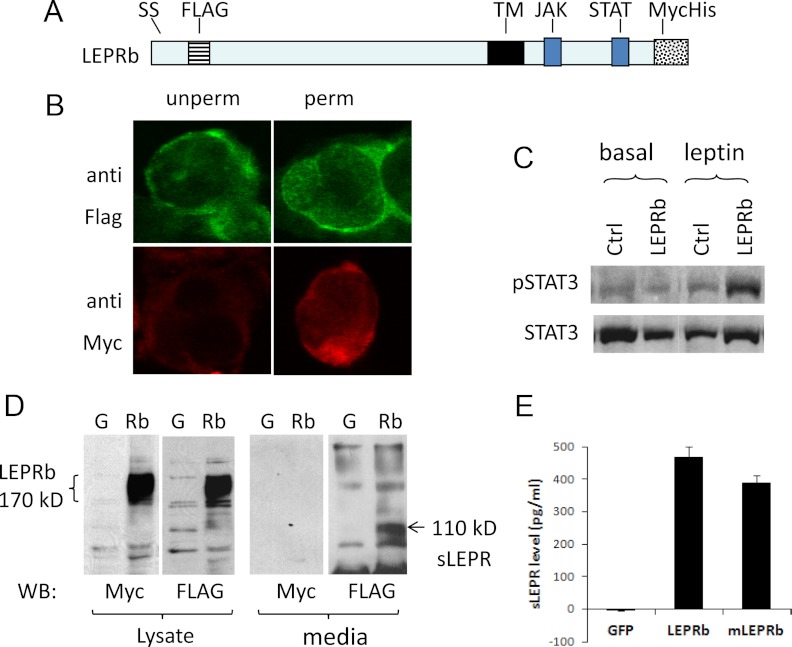
In this study, we used a mLEPRb construct tagged with the FLAG epitope inserted just after the signal sequence and the MycHis6 epitope at the carboxy terminus (Fig. 1A). These tags do not interfere with the proper orientation of the protein with respect to the plasma membrane because, in HEK293 cells infected with recombinant adenovirus expressing the tagged LEPRb (Ad-LEPRb), anti-FLAG antibody detected LEPRb in both permeabilized and unpermeabilized cells, whereas anti-Myc antibody detected LEPRb only in permeabilized cells (Fig. 1B). Expression of the tagged LEPRb markedly increased leptin-induced STAT3 phosphorylation in HepG2 cells (Fig. 1C), indicating that the tagged LEPRb is signaling competent. This tagged LEPRb construct was used in all experiments in this study unless otherwise indicated, and it will be referred to as LEPRb hereafter for simplicity.
Fig. 1.
Analysis of the expression, signaling activity, and ectodomain shedding of the dual-tagged LEPRb. Panel A, Schematic illustration of the dual-tagged mLEPRb construct showing positions of the FLAG and MycHis6 tags relative to the signal sequence (SS), transmembrane domain (TM), and Janus kinase (JAK), and STAT3 binding sites. Panel B, Confocal images of Ad-LEPRb-infected HEK293 cells stained with anti-FLAG or anti-Myc antibody under permeabilized (perm) or unpermeabilized (unperm) conditions. Panel C, Western blot of phospho-STAT3 (P-STAT3) and STAT3 levels in HepG2 cells transfected with LEPRb or empty vector [control (Ctrl)]. Cells were stimulated for 30 min with 20 ng/ml leptin before the analysis. Panel D, Western blot (WB) of LEPRb in cell lysates and sLEPR in CM from HEK293 cells infected with Ad-LEPRb (Rb) or Ad-GFP (G). Lysates and CM were collected after they were incubated for 24 h in serum-free Opti-MEM. Protein bands were visualized with anti-FLAG and anti-Myc antibody as indicated. Bands corresponding to full-length LEPRb (∼170 kDa) and sLEPR (110 kDa) are indicated. Panel E, ELISA analysis of mouse sLEPR in HepG2 cells infected with Ad-GFP, Ad-LEPRb, or Ad-mLEPRb (mLEPRb). CM was harvested for ELISA after cells were incubated for 16 h in serum-free Opti-MEM.
A broad band of approximately 170 kDa was detected by Western blotting using either Myc or FLAG antibody in Ad-LEPRb-infected HEK293 cells. This band was absent in uninfected HEK293 cells (data not shown) or in cells infected with Ad-GFP (Fig. 1D), suggesting that the full-length LEPRb protein corresponds to the 170-kDa band. Apparent spreading of the band likely reflects variations in the degree of glycosylation (42, 43). A FLAG-positive, Myc-negative band with molecular mass of approximately 110 kDa was detected in CM of Ad-LEPRb-infected cells but not in CM of Ad-GFP-infected cells (Fig. 1D), suggesting that the 110-kDa band represents the extracellular domain of LEPRb generated through ectodomain shedding. No full-length LEPRb was detected by Western blot in CM (data not shown). The sizes of the full-length LEPRb and the extracellular shedding product observed here are similar to those previously reported (24, 44). The presence of the ectodomain of LEPRb in the CM of cells expressing LEPRb was independently confirmed by ELISA specific to mouse sLEPR, where sLEPR was detected only in CM of HepG2 cells expressing LEPRb but not in those expressing GFP (Fig. 1E). We also detected sLEPR in CM of HepG2 cells infected with recombinant adenovirus expressing the native mLEPRb (Fig. 1E), indicating that both native and tagged mLEPRb molecules could produce sLEPR through ectodomain shedding.
TNF-α up-regulates LEPRb protein level and ectodomain shedding in multiple cell types
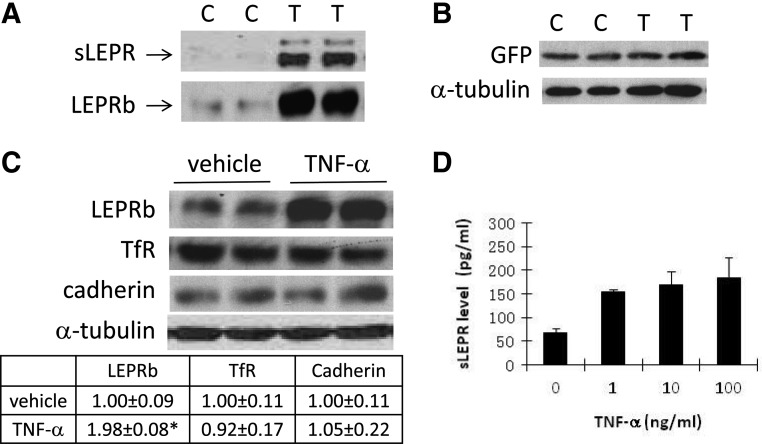
In Ad-LEPRb-infected HEK293 cells, treatment cells with 20 ng/ml TNF-α for 15 h increased LEPRb protein level in cell lysates and sLEPR in CM by 6.6 ± 0.7- and 6.1 ± 1.1-fold, respectively (both P < 0.01) (Fig. 2A). In contrast to the robust stimulation of LEPRb protein level, TNF-α had little effect on GFP protein levels in Ad-GFP-infected HEK293 cells (Fig. 2B), suggesting that the effect of TNF-α on LEPRb protein level is not related to the activity of pCMV promoter per se, which controls the expression of both LEPRb and GFP transgenes. To further investigate whether TNF-α has a similar effect on protein levels of other membrane proteins, we compared protein levels of LEPRb and the endogenous TfR and cadherin in vehicle- and TNF-α-treated HEK293 cells. TfR is a recycling receptor, and its recycling pathway is not shared by LEPRb (28). We found that although TNF-α treatment (50 ng/ml for 6 h) increased LEPRb protein level by 89%, but it had no significant effect on TfR or cadherin level (Fig. 2C). Increased sLEPR release by TNF-α was also confirmed by ELISA, which shows that TNF-α at 1 ng/ml is as effective as at 10–100 ng/ml in stimulating sLEPR release (Fig. 2D).
Fig. 2.
TNF-α up-regulates LEPRb protein level and sLEPR releases through ectodomain shedding of LEPRb. Panel A, Western blot analysis of LEPRb in cell lysates and sLEPR in CM. Ad-LEPRb-infected HEK293 cells were treated for 15 h with 20 ng/ml TNF-α (T) or vehicle control (C) before the analysis. Panel B, Western blot analysis of GFP and a-tubulin levels in cell lysates. HEK 293 cells infected with Ad-GFP were treated with TNF-α (T) or vehicle (C) the same as in A before the analysis. Panel C, Western blot analysis of LEPRb, the endogenous TfR, cadherin, and α-tubulin levels. Ad-LEPRb-infected HEK293 cells were treated for 6 h with vehicle or 50 ng/ml TNF-α before the analysis. The table below the gel image shows the levels of LEPRb, TfR, and cadherin, normalized by the respective α-tubulin levels of the cells, and relative to those of vehicle-treated cells as determined by densitometry. *, P < 0.05. Panel D, ELISA analysis of sLEPR levels in CM. Ad-LEPRb-infected HEK293 cells were treated for 6 h with the indicated concentrations of TNF-α. CM were collected for ELISA analysis.
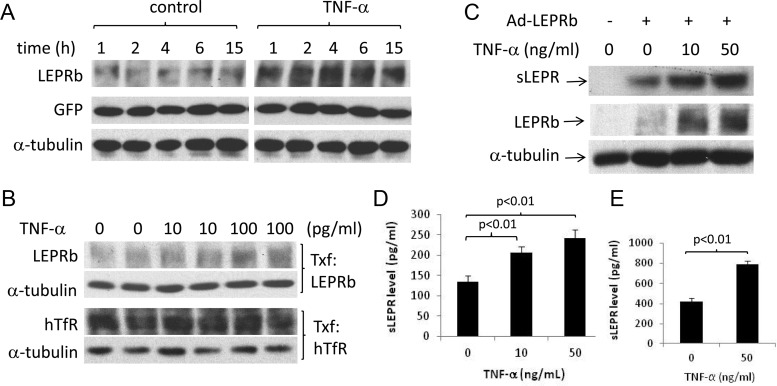
We further investigated whether TNF-α is effective in stimulating LEPRb protein expression and sLEPR in other cell types. Because LEPRb is expressed mainly in neurons in vivo and neuronal LEPRb is the main mediator of the metabolic effect of leptin (11, 16, 45), we investigated whether TNF-α is able to stimulate LEPRb expression in cells of neuronal origin. In N2a neuroblastoma cells transfected with LEPRb, TNF-α at 1 ng/ml increased LEPRb protein level after 1 h of treatment, and the effect persisted up to 15 h (Fig. 3A). TNF-α is also able to stimulate LEPRb protein level at lower concentrations, albeit to more modest degrees. One-hour TNF-α treatments at 10 and 100 pg/ml increased LEPRb protein levels by 12.8 and 37.0% in N2a cells (Fig. 3B). No significant effect on the protein level of human TfR transgene was observed under the same condition. Longer treatment with higher TNF-α concentrations (10–50 ng/ml for 6 h) resulted in more robust increases in LEPRb protein levels (1.29- to 1.62-fold above the controls) and sLEPR release (0.71- to 0.90-fold above the controls) (Fig. 3C). In Ad-LEPRb-infected GT1-7 mouse hypothalamic neuronal cells, TNF-α treatment (10 and 50 ng/ml for 16 h) also led to 53.6 and 81.5% increases in sLEPR levels in CM (both P < 0.01) (Fig. 3D). Similarly, TNF-α treatment nearly doubled sLEPR levels in CM of LEPRb-transfected HepG2 cells (P < 0.01) (Fig. 3E). Taken together, these results suggest that TNF-α up-regulates LEPRb protein level and sLEPR release in multiple cell types and that the effects of TNF-α appear to be specific to LEPRb.
Fig. 3.
TNF-α stimulates LEPRb protein expression and sLEPR release in multiple cell types. A, Western blot analysis of LEPRb, GFP, and α-tubulin levels in cell lysates. N2a cells were cotransfected with pcDNA plasmids carrying LEPRb and GFP cDNA. Transfected cells were then treated with vehicle or 1 ng/ml TNF-α for 1–15 h before the analysis. B, Western blot analysis of LEPRb, hTfR, and α-tubulin levels in cell lysates. N2a cells were transfected with the LEPRb expression plasmid or with the hTfR expression plasmid. Transfected cells were then treated with TNF at 0, 10, or 100 pg/ml for 1 h. LEPRb-transfected cells were analyzed for LEPRb and α-tubulin, and hTfR-transfected cells for hTfR and α-tubulin as indicated. C, Western blot analysis of LEPRb and α-tubulin in cell lysates and sLEPR in CM. LEPRb-transfected N2a cells were treated for 6 h with 0, 10, and 50 ng/ml TNF-α before the analysis. Lysates and CM from untransfected N2a cells were analyzed in parallel as controls. D, ELISA analysis of sLEPR levels in the CM of GT1-7 hypothalamic neuronal cells. Ad-LEPRb-infected GT1-7 cells were incubated for 16 h with 0, 10, and 50 ng/ml TNF-α before CM were collected for ELISA analysis. Endogenously produced mouse sLEPR, if any, was not detected by ELISA analysis in the CM of uninfected GT1-7 cells. E, ELISA analysis of sLEPR levels in the CM of HepG2 cells. LEPRb-transfected HepG2 cells were treated for 15 h with vehicle or with 50 ng/ml TNF-α before CM were analyzed by ELISA. No sLEPR in the CM of untransfected HepG2 cells was detected by ELISA.
TNF-α acutely increases LEPRb cell surface expression and cellular responses to leptin
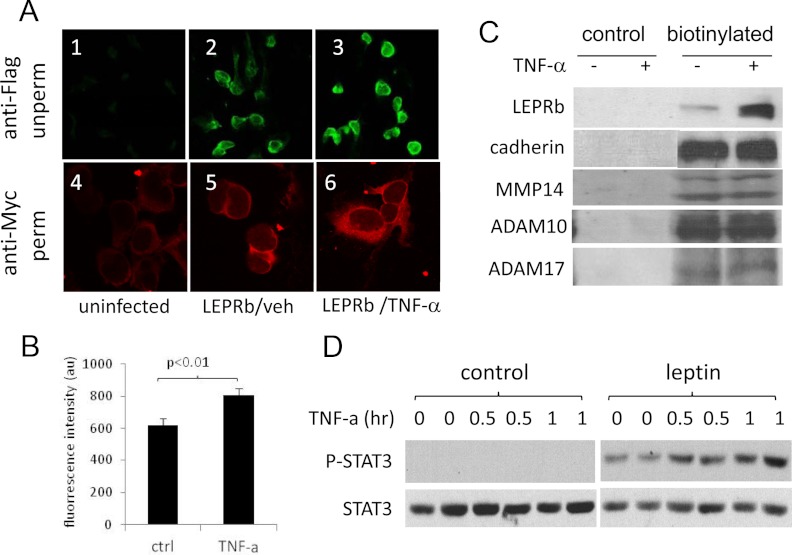
Total and cell surface LEPRb staining by indirect immunofluorescence microscopy appeared more intense in TNF-α-treated cells (Fig. 4A, panels 1–3) than in vehicle-treated cells (Fig. 4A, panels 4–6). We quantified acute effect of TNF-α on LEPRb cell surface expression using FACS after cells were treated with TNF-α (2 h, 50 ng/ml) and labeled by indirect immunofluorescence staining with anti-FLAG antibody. The data indicated that the TNF-α treatment led to a 29.9% increase in cell surface LEPRb level, 622 ± 41.3 (vehicle) vs. 805 ± 41.8 (TNF-α) (P < 0.01) (Fig. 4B). Biotinylation also showed that LEPRb cell surface levels were increased by approximately 7.5-fold after 20 h TNF-α treatment (20 ng/ml) (Fig. 4C). In contrast, no significant increase in cell surface expression of cadherin or three membrane-associated metalloproteinases, MMP14, ADAM10, and ADAM17, was observed under the same conditions, suggesting that TNF-α does not universally stimulate cell surface expression of type I plasma membrane proteins.
Fig. 4.
TNF-α up-regulates cell surface expression of LEPRb and cellular response to leptin. A, Confocal images of HEK293 cells stained for LEPRb. Ad-LEPRb-infected cells were treated for 6 h with vehicle (LEPRb/veh, panels 2 and 5) or with 100 ng/ml of TNF-α (LEPRb/TNF-α, panels 3 and 6). Cell surface LEPRb were stained by indirect immunofluorescence with anti-FLAG antibody in the unpermeabilized (unperm) condition (panels 1–3; ×20 objective), or permeabilized (perm) and stained by indirect immunofluorescence with anti-Myc antibody (panels 4–6; ×100 objective). Uninfected cells (panels 1 and 4) were included as controls for nonspecific staining. B, FACS analysis of cell surface LEPRb levels. Ad-LEPRb-infected HEK293 cells were treated for 2 h with vehicle control (ctrl) or 50 ng/ml TNF-α. After the treatment, cell surface LEPRb was labeled by indirect immunofluorescence staining with anti-FLAG antibody in the unpermeabilized condition. Fluorescence intensity was quantified by FACS. C, Western blot analysis of cell surface expression of LEPRb and the endogenous cadherin, MMP14, ADAM10, and ADAM17 genes. Ad-LEPRb-infected HEK293 cells were treated for 20 h with vehicle (−) or 20 ng/ml TNF-α (+). Cell surface proteins were biotinylated with EZ-Link Sulfo-NHS-LC-Biotin and captured with NeutrAvidin beads and analyzed by Western blot using anti-Myc antibody. Controls were treated and analyzed the same in parallel except that they were not biotinylated. D, Western blot of phospho-STAT3 (P-STAT3) and STAT3. Quiescent LEPRb-transfected HEK293 cells were treated with 1 ng/ml TNF-α for 0, 0.5, and 1 h as indicated. Without removing TNF-α, cells were then stimulated with or without 50 ng/ml leptin for 15 min. Cell lysates were analyzed for phospho-STAT3 and STAT3 by Western blotting.
We further investigated whether the up-regulation of LEPRb cell surface expression by TNF-α affects cellular response to leptin. As shown in Fig. 4D, compared with the vehicle, TNF-α treatment (1 ng/ml) for 0.5 and 1 h led to 37% (P < 0.05) and 104% (P < 0.01) increases, respectively, in leptin-stimulated STAT3 phosphorylation. No significant effect on the basal phospho-STAT3 (P-STAT3) level was observed. The high leptin concentration and short-duration condition (50 ng/ml for 15 min) was used here to ensure that the level of STAT3 phosphorylation was not limited by leptin availability or affected by potential feedback suppression. Our results suggest that TNF-α could increase leptin signaling activity by increasing cell surface expression of LEPRb given that free leptin concentration, which could be affected by sLEPR, is not a limiting factor.
Up-regulation of LEPRb cell surface expression and sLEPR release by TNF-α requires continuous endoplasmic reticulum (ER) to Golgi transport of LEPRb but is independent of de novo protein synthesis or lysosomal function
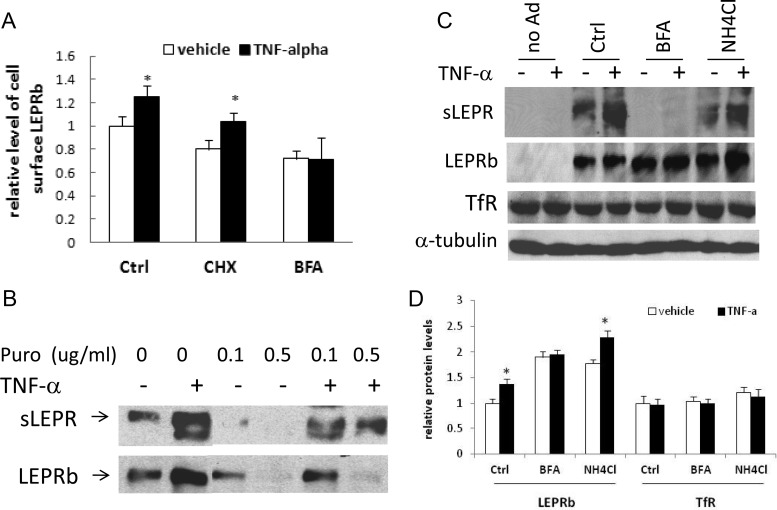
To investigate mechanisms underlying the stimulatory effects of TNF-α, we examined the acute effect of TNF-α on LEPRb cell surface expression in the presence of the protein synthesis inhibitor cycloheximide or brefeldin A, which impairs Golgi function and inhibits ER to Golgi transport of the newly synthesized proteins. Cell surface receptors were labeled by indirect immunofluorescence staining and quantified by FACS. Cycloheximide decreased the fluorescence intensity in both vehicle- and TNF-α-treated cells (50 ng/ml for 2 h) by approximately 20% but had little effect on TNF-α-mediated stimulation (28.8%) compared with a 25.3% stimulation in the absence of cycloheximide (Fig. 5A). In contrast, brefeldin A decreased LEPRb cell surface level by 29 and 44% in vehicle- and TNF-α-treated cells, respectively, and completely abolished the stimulatory effect of TNF-α (Fig. 5A). These results suggest that the acute up-regulation of cell surface expression of LEPRb by TNF-α is independent of de novo protein synthesis but requires continuous ER to Golgi transport of LEPRb in the biosynthetic pathway.
Fig. 5.
Stimulation of LEPRb cell surface expression and sLEPR release by TNF-α is blocked by brefeldin A, but not by inhibitors of protein synthesis or lysosomal function. A, FACS analysis of cell surface LEPRb levels. Ad-LEPRb-infected HEK293 cells were pretreated for 15 min with 20 μg/ml cycloheximide (CHX), 10 μg/ml brefeldin A (BFA), or neither [control (Ctrl)] followed by treatment for 2 h with vehicle or 50 ng/ml TNF-α in the continuous presence of cycloheximide, brefeldin, or neither. After the treatment, cell surface LEPRb proteins were labeled with anti-FLAG antibody and quantified by FACS. *, P < 0.05, vehicle- vs. TNF-α-treated cells. B, Western blot analysis of LEPRb levels in cell lysates and sLEPR levels in CM. Ad-LEPRb-infected HEK293 cells were pretreated for 30 min with puromycin (Puro) at concentrations of 0, 0.1, and 0.5 μg/ml and then treated for 6 h with vehicle (−) or with 50 ng/ml TNF-α (+) in the continuous presence of puromycin. Cell lysates and CM were analyzed by Western blot. C, Western blot analysis of sLEPR levels in CM and LEPRb, and the endogenous TfR and α-tubulin in the lysates of Ad-LEPRb-infected HEK293 cells. Cells were pretreated for 30 min with brefeldin A (BFA, 10 μg/ml), NH4Cl (20 mm), or neither [control (Ctrl)] followed by 6 h treatment with vehicle (−) or 50 ng/ml TNF-α (+) in the presence or absence of BFA or NH4Cl. Uninfected cells were included as a control (no Ad). D, Average LEPRb and TfR levels of a triplicate set of samples, determined by densitometry and expressed as ratios of LEPRb to α-tubulin and TfR to α-tubulin. *, P < 0.05, vehicle- vs. TNF-α-treated cells. The gel image of one of the three sets of samples is shown in C.
We investigated whether the up-regulation of sLEPR release by TNF-α is similarly affected by protein synthesis inhibitors and brefeldin. In HEK293 cells expressing LEPRb, TNF-α (20 ng/ml for 6 h) markedly increased sLEPR levels in the presence of another protein synthesis inhibitor, puromycin. The stimulations of sLEPR release were robust despite the complete inhibition of LEPRb protein synthesis in the presence of 0.1–0.5 μg/ml puromycin (Fig. 5B). Similarly, cycloheximide failed to block the stimulatory effect of TNF-α on sLEPR release (Supplemental Fig. 1, published on The Endocrine Society's Journals Online web site at http://endo.endojournals.org). These results suggest that the stimulation of sLEPR release by TNF-α is also mediated through a posttranslational mechanism. Brefeldin A completely blocked sLEPR release in both vehicle- and TNF-α-treated cells (Fig. 5C), which again suggests that continuous transport of LEPRb from ER to Golgi is essential for sLEPR release and TNF-α-mediated stimulation. Additionally, although brefeldin A treatment abolished the incremental (stimulatory) effect of TNF-α on LEPRb protein level, it nearly doubled LEPRb protein levels in both vehicle- and TNF-α-treated cells (Fig. 5, C and D). We also observed that the size of LEPRb in brefeldin-treated cells was smaller than that in untreated cells, suggesting that ER to Golgi transport of LEPRb and Golgi-mediated modifications of oligosaccharides on LEPRb were indeed inhibited. These results suggest that in the normal condition (without brefeldin), a significant fraction of newly synthesized LEPRb is degraded in a post-ER compartment and that TNF-α may suppress this degradation. On the other hand, treatment of cells with the lysosomal inhibitor ammonium chloride (NH4Cl) had no significant effect on sLEPR release in vehicle- or TNF-α-treated cells relative to control cells (Fig. 5C) but resulted in parallel increases in cellular LEPRb protein levels in both vehicle- and TNF-α-treated cells without attenuating the stimulatory effect of TNF-α (Fig. 5, C and D). In contrast, TNF-α had little effect on the protein level of the endogenous TfR with or without brefeldin or ammonium chloride (Fig. 5, C and D). Chloroquine, another lysosomal inhibitor, had similar effects on LEPRb protein levels (Supplemental Fig. 2). These results suggest that lysosomal function is involved in regulating cellular LEPRb protein level as previously reported (24, 25, 30) but is not required for the up-regulation of cellular LEPRb protein level and sLEPR release by TNF-α.
TNF-α does not increase the half-life of cell surface LEPRb
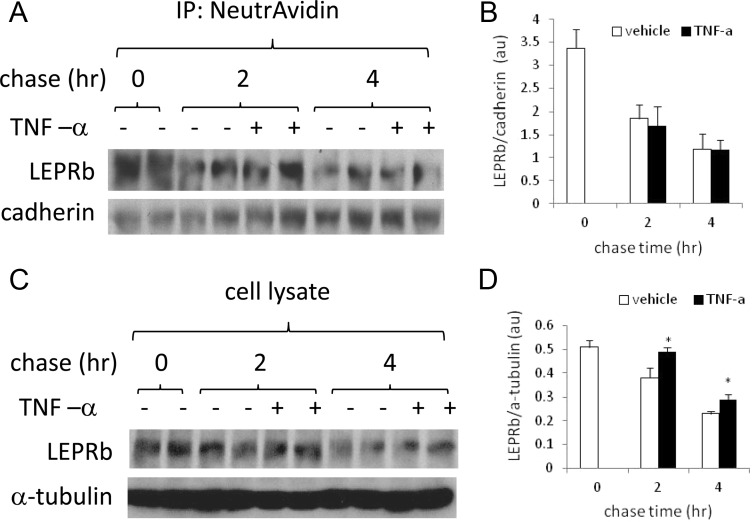
Posttranslational up-regulation of LEPRb cell surface expression may result from any or a combination of the following processes: increased anterograde trafficking and/or decreased degradation of LEPRb in the biosynthetic pathway, decreased endocytosis and/or degradation of internalized LEPRb, and decreased ectodomain shedding. The action of the two latter mechanisms is expected to increase the half-life of cell surface LEPRb, whereas the action of the former is not. To distinguish among these possibilities, we measured the effect of TNF-α on the half-life of cell surface LEPRb. We labeled cell surface proteins by biotinylation and then determined the levels of biotinylated LEPRb that remained after various periods of chase in the presence or absence of TNF-α (Fig. 6). Because TNF-α has little effect on cell surface cadherin levels as shown earlier (Fig. 3B), we used the ratio of biotinylated LEPRb to cadherin to normalize LEPRb levels. In Ad-LEPRb-infected HEK293 cells, levels of biotinylated LEPRb were not significantly different between vehicle- and TNF-α-treated cells during a 4-h chase. The LEPRb to cadherin ratio decreased from 3.37 ± 0.41 at the zero time point to 1.85 ± 0.29 and 1.70 ± 0.40 in vehicle-treated and TNF-α-treated cells, respectively, after a 2-h chase and further decreased to 1.19 ± 0.33 and 1.17 ± 0.25, respectively, after a 4-h chase, indicating that the half-life of cell surface LEPRb is approximately 2 h and is not increased by TNF-α treatment. As a control, we examined the effect of TNF-α on total cellular LEPRb protein level in these cells. Although the overall LEPRb protein level was decreased during the 4-h chase, probably due to manipulations related to biotinylation, TNF-α treatment increased cellular LEPRb protein levels (expressed as ratios to α-tubulin) by 28.9 and 26.1%, respectively, after a 2- and 4-h chase, relative to the vehicle-treated cells (both P < 0.05). Taken together, these results suggest that TNF-α increases LEPRb cell surface expression mainly by affecting the export/degradation of LEPRb in the biosynthetic pathway rather than by affecting the internalization/degradation of cell surface LEPRb.
Fig. 6.
TNF-α does not increase the half-life of cell surface LEPRb. A, Ad-LEPRb-infected HEK293 cells were made quiescent by overnight serum starvation. Cell surface proteins were labeled with EZ-Link Sulfo-NHS-LC-Biotin and chased at 37 C for 2 and 4 h in media containing vehicle or 50 ng/ml TNF-α. The 0-h chase corresponds to the biotinylated cells that were not chased at 37 C. Biotinylated proteins in cell lysates were captured with NeutrAvidin beads and analyzed for LEPRb and cadherin by Western blotting. IP, Immunoprecipitation. B, The average ratios of LEPRb to cadherin in two duplicate sets of samples, one of which is shown in A, C, Cell lysates of the same set of samples as describe in A were analyzed for total cellular LEPRb and α-tubulin by Western blotting. D, The average ratios of LEPRb to α-tubulin in two duplicate sets of samples, one of which is shown in C. *, P < 0.05, vehicle- vs. TNF-α-treated cells.
PKC is a key mediator of the effects of TNF-α on LEPRb protein levels and sLEPR release
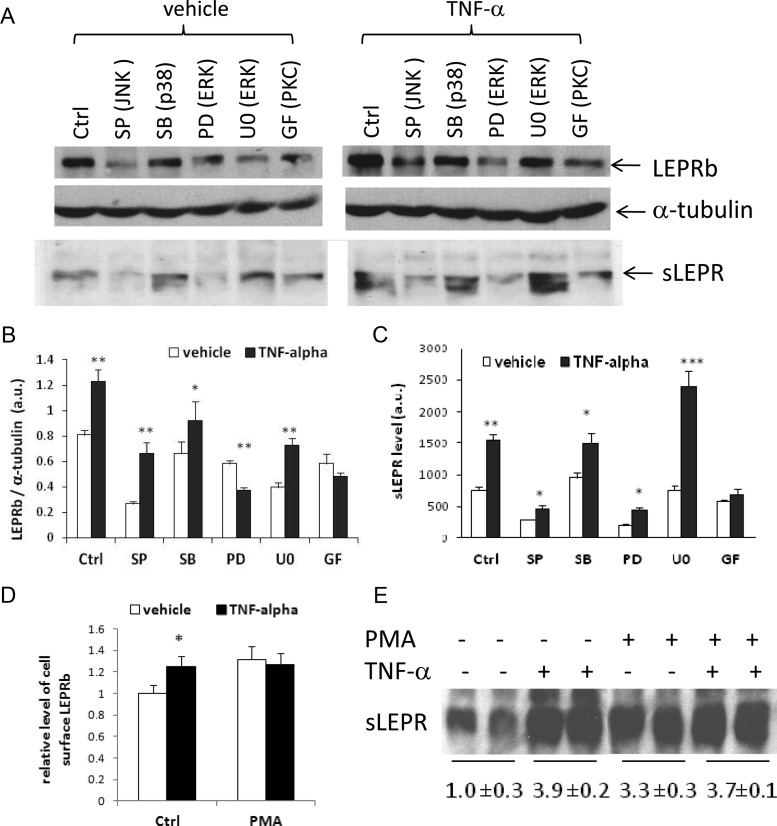
TNF-α is capable of activating multiple intracellular signaling pathways, including PKC and MAPKs (46–48). To investigate which of these signaling pathways mediates the TNF-α effects on LEPRb protein expression/sLEPR release, we examined the effects of known inhibitors of these signaling pathways on LEPR responses to TNF-α. As expected, TNF-α, at concentrations that stimulated LEPRb protein expression and sLEPR release, increased the phosphorylation of c-Jun N-terminal kinase (JNK), p38 MAPK, and ERK1/2 (Supplemental Fig. 3). However, TNF-α-induced stimulation of LEPRb protein levels and sLEPR release were not affected by the presence of inhibitors specific to the MAPK pathway: SP600125 (JNK inhibitor), SB202190 (p38 MAPK inhibitor), and PD98059 and U0126 (MEK/ERK inhibitors) (Fig. 7, A–C). We note, however, that SP600125 and PD98059 significantly inhibited the overall LEPRb protein levels and sLEPR release in both vehicle- and TNF-α-treated cells. In contrast, treatment of cells with the PKC inhibitor GF109203X completely blocked the stimulatory effects of TNF-α on both LEPRb protein level and sLEPR release, suggesting that PKC is the primary mediator of the TNF-α effects on LEPRb protein expression and sLEPR release (Fig. 7, A–C). To further examine the role of PKC in regulating LEPRb cell surface expression and sLEPR release, we tested the pan-PKC activator phorbol 12-myristate 13-acetate (PMA). A 2-h treatment with PMA acutely increased cell surface expression of LEPRb by 32%, which was comparable to the effect of TNF-α alone (27%) (Fig. 7D). Importantly, treatment of cells with the combination of TNF-α and PMA did not result in additional stimulation beyond the level induced by PMA or TNF-α alone (Fig. 7D), suggesting that TNF-α and PMA stimulate cell surface expression via the same PKC-related pathway. Similarly, PMA also significantly increased cellular LEPRb protein levels (Supplemental Fig. 4) and increased sLEPR release to a degree similar to those induced by TNF-α or the combination of both (Fig. 7E). These results suggest that activation of PKC is primarily responsible for the stimulatory effects of TNF-α on LEPRb protein expression and sLEPR release.
Fig. 7.
PKC activity is required for the stimulatory effects of TNF-α on LEPRb expression and sLEPR release and activation of PKC by PMA mimics the TNF-α effect. A, Western blot analysis of LEPRb levels in cell lysates and sLEPR levels in CM. Ad-LEPRb-infected HEK293 were pretreated with the specific inhibitors, 50 μm SP600125 (SP) (JNK), 10 μm SB202190 (SB) (p38 MAPK), 50 μm PD98059 (PD) and 5 μm U0126 (U0) (ERK), and 10 μm GF109203X (GF) (PKC) for 30 min followed by vehicle (−) or 50 ng/ml TNF-α (+) treatment for 6 h before Western blot analysis. B, Average LEPRb levels in the cell lysates shown in A and a duplicate set of samples, determined by densitometry and expressed as ratios of LEPRb to α-tubulin. *, P < 0.05; **, P < 0.01, vehicle- vs. TNF-α-treated cells, respectively. C, Average sLEPR levels in the CM shown in A and a duplicate set of samples, determined by densitometry and expressed in arbitrary units. *, P < 0.05; **, P < 0.01; ***, P < 0.001, vehicle- vs. TNF-α-treated cells, respectively. D, FACS analysis of cell surface LEPRb levels. Ad-LEPRb-infected HEK293 cells were treated with TNF-α (50 ng/ml), PMA (1 μg/ml), both, or neither for 2 h. Cell surface LEPRb proteins were labeled with anti-FLAG antibody followed by fluorescein isothiocyanate-conjugated secondary antibody, and quantified by FACS. Average cell surface LEPRb levels of a triplicate set of samples are shown. E, Western blot analysis of sLEPR levels in CM. Ad-LEPRb-infected HEK293 cells were treated with TNF-α (50 ng/ml), PMA (1 μg/ml), both, or neither for 14 h. CM were collected for Western blotting analysis. The average sLEPR levels of two duplicate sets of samples, including one set for which a gel image is not shown, relative to that of the control samples (untreated with TNF-α or PMA), are shown below the gel image. Ctrl, control.
Discussion
The regulation of protein level, cell surface expression, and ectodomain shedding of LEPRb by TNF-α and its potential physiological relevance
The effects of TNF-α on LEPRb protein level and cell surface expression in cultured cells are swift and persistent. Increases in LEPRb protein level can be detected after 1 h of TNF-α treatment in N2a cells, and increases in cellular response to leptin, as a measure of cell surface LEPRb levels, can be observed after 30 min TNF-α treatment. The stimulatory effect of TNF-α on LEPRb protein level persists up to 15 h in the continuous presence of TNF-α. The magnitude of the effect appears to depend in part on the duration of TNF-α treatment. Cellular response to leptin was increased by 37.5 and 104% after 0.5 and 1 h of TNF-α treatment, respectively. Similarly, cell surface LEPRb levels were increased by 29.9% and 7.5-fold after 2 and 20 h of TNF-α treatment, respectively. TNF-α is effective in stimulating LEPRb protein levels in a wide range of concentrations (10 pg/ml to 100 ng/ml), although the effects are modest at 10–100 pg/ml TNF-α but reach a maximal level around 1–10 ng/ml. Plasma TNF-α concentrations vary greatly in different pathophysiological conditions. In the obese state, which is associated with low-grade inflammation, plasma TNF-α concentrations are mildly elevated, reaching approximately 10 pg/ml (49, 50). However, local TNF-α levels at the site of inflammation, such as adipose tissue, liver, and hypothalamus, could be considerably higher than plasma TNF-α levels (51–53). On the other hand, plasma TNF-α concentrations could reach more than 20 ng/ml in sepsis (54, 55). Together, these results suggest that TNF-α could potentially modulate LEPRb expression and leptin signaling in a wide variety of pathophysiological conditions. However, we would also like to point out that our findings are based on studies in cultured cells in vitro; thus, their physiological relevance should be interpreted with caution. Although the study was not intended to model any particular in vivo conditions, we hope the findings can serve to elucidate this novel regulatory mechanism in cultured cells.
TNF-α also stimulates the release of sLEPR through ectodomain shedding of LEPRb, although it appears that only a small fraction of LEPRb undergoes ectodomain shedding at any given time. By Western blotting, we estimated that the amount of sLEPR in 15–20 h CM is about one fifth to 1/10 of the amount of LEPRb in the corresponding cell lysates. Because of the low sLEPR release rate, we cannot reliably detect sLEPR in CM with less than 4 h incubation time, and long incubation periods (>6 h) are often necessary to determine the effect of TNF-α on sLEPR release. In TNF-α-treated cells, increased ectodomain shedding of LEPRb may be due to increased LEPRb protein expression at the cell surface, increased sheddase activity, or both. The findings that sLEPR levels are increased in parallel with LEPRb cell surface levels in TNF-α-treated cells and are similarly affected by brefeldin and inhibitors of protein synthesis and lysosomal function suggest that the latter is likely an important contributing factor to the former. The overall LEPR expression level also appears to be an important determinant of plasma sLEPR level in vivo (44, 56, 57). On the other hand, it is possible that TNF-α may also increase putative LEPR sheddase activities (58, 59), contributing to the increase in sLEPR release. The finding that TNF-α up-regulates sLEPR release in cultured cells offers a plausible molecular mechanism for the increases in plasma sLEPR level associated with various inflammatory conditions in humans and animal models (34–37). Because sLEPR plays an important role in regulating leptin bioavailability (18–21), regulating sLEPR production is yet another potential mechanism by which TNF-α could modulate leptin signaling.
Regulation of trafficking and/or degradation of LEPRb in the biosynthetic pathway are key mechanisms in determining LEPRb protein level and cell surface expression in response to TNF-α stimulation
Our data have shown that the acute increases in LEPRb cell surface expression and sLEPR release by TNF-α cannot be blocked by protein synthesis inhibitors cycloheximide and puromycin. TNF-α does not up-regulate the protein levels of the GFP and hTfR transgenes in HEK293 and N2a cells or increase the protein levels of the endogenous TfR and cadherin in HEK293 cells. Furthermore, cell surface levels of cadherin, ADAM10, ADAM17, and MMP14 are not increased in HEK293 cells after 20 h TNF-α treatment, which increases cell surface LEPRb level by approximately 7.5-fold. Together, these results suggest that TNF-α specifically increases the protein level and cell surface expression of LEPRb through a posttranslational mechanism.
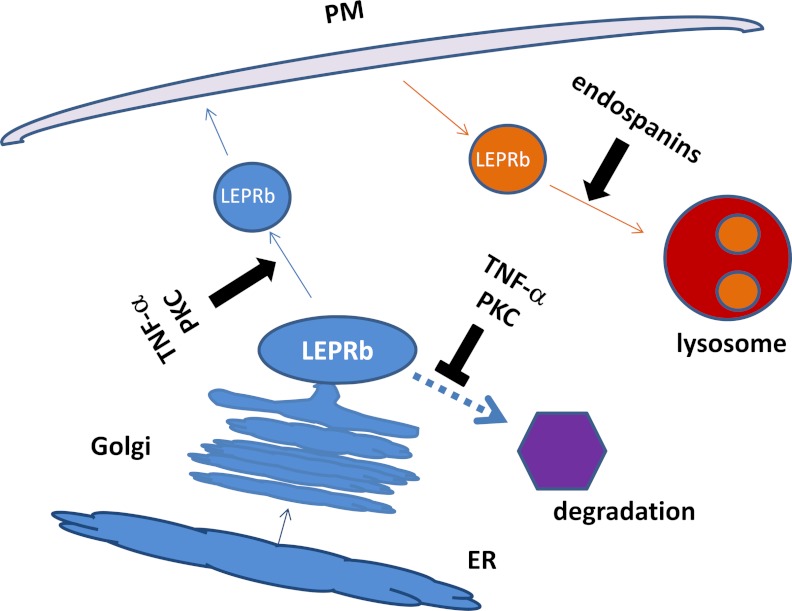
Decreased protein degradation must account at least in part for the posttranslational up-regulation of LEPRb protein level and cell surface expression induced by TNF-α. Because sLEPR release through ectodomain shedding is increased in TNF-α-treated cells, decreased ectodomain shedding of LEPRb as a main contributing factor can be ruled out. The observations that Golgi function and/or continuous transport of the newly synthesized LEPRb from ER to Golgi are necessary for the TNF-α effect and that blocking these functions by brefeldin leads to marked increases in total cellular LEPRb protein levels in both vehicle- and TNF-α-treated cells suggest that decreased degradation of the newly synthesized LEPRb in a post-ER compartment could be an important contributing factor to the TNF-α-mediated up-regulation of LEPRb protein level and cell surface expression. This notion is also supported by previous studies showing that although LEPRb is a fast turnover protein, it is expressed at low levels on the cell surface and undergoes endocytosis and lysosomal-mediated degradation at slow rates (24, 25, 29). We have further shown that TNF-α has no significant effect on the half-life of cell surface LEPRb and that lysosomal inhibitors do not block the stimulatory effects of TNF-α on LEPRb protein level or sLEPR release, suggesting that decreased endocytosis of cell surface LEPRb proteins and/or their degradation in lysosomes are unlikely to be responsible for the increased LEPRb protein levels in TNF-α-treated cells. Based on the above results/data, the following model is proposed (Fig. 8): newly synthesized LEPRb is sorted in the Golgi compartment or the trans-Golgi network, which either directs LEPRb to a compartment destined for the cell surface or targets it for fast degradation by a nonlysosomal mechanism, the nature of which is yet to be determined; this sorting process is regulated by TNF-α/PKC, which favors intracellular accumulation and export of LEPRb protein over degradation, leading to concomitant increases in LEPRb protein level and cell surface expression, which in turn contributes to the increased ectodomain shedding/sLEPR release.
Fig. 8.
Proposed model for TNF-α/PKC-mediated up-regulation of LEPRb protein level and cell surface expression. LEPRb in the biosynthetic pathway (blue) is sorted in the Golgi or trans-Golgi network (the blue oval labeled LEPRb), which directs LEPRb either to vesicles (the blue circle labeled LEPRb) destined to the plasma membrane (PM, light pink) or to a yet-to-be defined degradation compartment (purple hexagon). TNF-α/PKC activation increases the sorting of LEPRb toward the vesicles destined to the plasma membrane and decreases the sorting of LEPRb toward degradation compartment, resulting in concomitant increases in cellular protein level and cell surface expression of LEPRb. Endocytosis of LEPRb and trafficking of endocytic vesicles carrying LEPRb (the orange circle labeled LEPRb) to lysosomes (red and orange circles), which is regulated by endospanins (31), are not affected by TNF-α/PKC signaling.
In summary, we have presented the data to show that TNF-α in a wide range of concentrations stimulates LEPRb protein level and cell surface expression, which leads to increased cellular response to leptin and contributes to increased sLEPR release in cultured cells, and that TNF-α, through activation of PKC, exerts these effects by increasing anterograde transport and/or decreasing degradation of LEPRb in the biosynthetic pathway. These results suggest that TNF-α could potentially influence leptin signaling activity by modulating LEPRb cell surface expression and sLEPR production.
Supplementary Material
Acknowledgments
We thank Matthew Zimmer and David Kahler for performing FACS and assistance in FACS data analysis, Stuart Fischer for critical reading of the manuscript, Cai Li for providing pCDNA3 LEPRbMcyHis plasmid, and Joshua Feinberg, Jun Hou, and Pei Yi Kwan for technical assistance. We also thank our colleagues in the Naomi Berrie Diabetes Center for helpful discussions and suggestions.
This work was supported by grants from the National Institutes of Health, R01DK063034 (to Y.Z.), R01DK52431 (to R.L.L.), P30 DK26687, and P30 DK63068. L.G. was supported by a grant from the National Natural Science Foundation of China (30971082).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- ADAM
- A disintegrin and metalloproteinase domain containing protein
- Ad-LEPRb
- recombinant adenovirus expressing LEPRb
- CM
- conditioned medium
- ER
- endoplasmic reticulum
- FACS
- fluorescence-activated cell sorting
- GFP
- green fluorescent protein
- hTfR
- human transferrin receptor
- JNK
- c-Jun N-terminal kinase
- LEPR
- leptin receptor
- mLEPRb
- native LEPRb
- MMP14
- matrix metalloproteinase 14
- pCMV
- immediate early promoter of cytomegalovirus
- PKC
- protein kinase C
- PMA
- phorbol 12-myristate 13-acetate
- sLEPR
- soluble LEPR
- STAT
- signal transducer and activator of transcription
- TCA
- trichloroacetic acid.
References
- 1. Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. 1994. Positional cloning of the mouse obese gene and its human homologue. Nature 372:425–432 [DOI] [PubMed] [Google Scholar]
- 2. Leibel RL. 2008. Molecular physiology of weight regulation in mice and humans. Int J Obes (Lond) 32:S98–S108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Myers MG, Jr, Leibel RL, Seeley RJ, Schwartz MW. 2010. Obesity and leptin resistance: distinguishing cause from effect. Trends Endocrinol Metab 21:643–651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lord GM, Matarese G, Howard JK, Baker RJ, Bloom SR, Lechler RI. 1998. Leptin modulates the T-cell immune response and reverses starvation-induced immunosuppression. Nature 394:897–901 [DOI] [PubMed] [Google Scholar]
- 5. Guo X, Roberts MR, Becker SM, Podd B, Zhang Y, Chua SC, Jr, Myers MG, Jr, Duggal P, Houpt ER, Petri WA., Jr 2011. Leptin signaling in intestinal epithelium mediates resistance to enteric infection by Entamoeba histolytica. Mucosal Immunol 4:294–303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Heiman ML, Ahima RS, Craft LS, Schoner B, Stephens TW, Flier JS. 1997. Leptin inhibition of the hypothalamic-pituitary-adrenal axis in response to stress. Endocrinology 138:3859–3863 [DOI] [PubMed] [Google Scholar]
- 7. Elefteriou F, Ahn JD, Takeda S, Starbuck M, Yang X, Liu X, Kondo H, Richards WG, Bannon TW, Noda M, Clement K, Vaisse C, Karsenty G. 2005. Leptin regulation of bone resorption by the sympathetic nervous system and CART. Nature 434:514–520 [DOI] [PubMed] [Google Scholar]
- 8. Takahashi N, Waelput W, Guisez Y. 1999. Leptin is an endogenous protective protein against the toxicity exerted by tumor necrosis factor. J Exp Med 189:207–212 [PMC free article] [PubMed] [Google Scholar]
- 9. Tschöp J, Nogueiras R, Haas-Lockie S, Kasten KR, Castañeda TR, Huber N, Guanciale K, Perez-Tilve D, Habegger K, Ottaway N, Woods SC, Oldfield B, Clarke I, Chua S, Jr, Farooqi IS, O'Rahilly S, Caldwell CC, Tschöp MH. 2010. CNS leptin action modulates immune response and survival in sepsis. J Neurosci 30:6036–6047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Tartaglia LA, Dembski M, Weng X, Deng N, Culpepper J, Devos R, Richards GJ, Campfield LA, Clark FT, Deeds J, Muir C, Sanker S, Moriarty A, Moore KJ, Smutko JS, Mays GG, Wool EA, Monroe CA, Tepper RI. 1995. Identification and expression cloning of a leptin receptor, OB-R. Cell 83:1263–1271 [DOI] [PubMed] [Google Scholar]
- 11. Chen H, Charlat O, Tartaglia LA, Woolf EA, Weng X, Ellis SJ, Lakey ND, Culpepper J, Moore KJ, Breitbart RE, Duyk GM, Tepper RI, Morgenstern JP. 1996. Evidence that the diabetes gene encodes the leptin receptor: identification of a mutation in the leptin receptor gene in db/db mice. Cell 84:491–495 [DOI] [PubMed] [Google Scholar]
- 12. Lee GH, Proenca R, Montez JM, Carroll KM, Darvishzadeh JG, Lee JI, Friedman JM. 1996. Abnormal splicing of the leptin receptor in diabetic mice. Nature 379:632–635 [DOI] [PubMed] [Google Scholar]
- 13. Chua SC, Jr, Koutras IK, Han L, Liu SM, Kay J, Young SJ, Chung WK, Leibel RL. 1997. Fine structure of the murine leptin receptor gene: splice site suppression is required to form two alternatively spliced transcripts. Genomics 45:264–270 [DOI] [PubMed] [Google Scholar]
- 14. Bates SH, Stearns WH, Dundon TA, Schubert M, Tso AW, Wang Y, Banks AS, Lavery HJ, Haq AK, Maratos-Flier E, Neel BG, Schwartz MW, Myers MG., Jr 2003. STAT3 signalling is required for leptin regulation of energy balance but not reproduction. Nature 421:856–859 [DOI] [PubMed] [Google Scholar]
- 15. Schwartz MW, Seeley RJ, Campfield LA, Burn P, Baskin DG. 1996. Identification of targets of leptin action in rat hypothalamus. J Clin Invest 98:1101–1106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. de Luca C, Kowalski TJ, Zhang Y, Elmquist JK, Lee C, Kilimann MW, Ludwig T, Liu SM, Chua SC., Jr 2005. Complete rescue of obesity, diabetes, and infertility in db/db mice by neuron-specific LEPR-B transgenes. J Clin Invest 115:3484–3493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lammert A, Kiess W, Bottner A, Glasow A, Kratzsch J. 2001. Soluble leptin receptor represents the main leptin binding activity in human blood. Biochem Biophys Res Commun 283:982–988 [DOI] [PubMed] [Google Scholar]
- 18. Huang L, Wang Z, Li C. 2001. Modulation of circulating leptin levels by its soluble receptor. J Biol Chem 276:6343–6349 [DOI] [PubMed] [Google Scholar]
- 19. Yang G, Ge H, Boucher A, Yu X, Li C. 2004. Modulation of direct leptin signaling by soluble leptin receptor. Mol Endocrinol 18:1354–1362 [DOI] [PubMed] [Google Scholar]
- 20. Zastrow O, Seidel B, Kiess W, Thiery J, Keller E, Böttner A, Kratzsch J. 2003. The soluble leptin receptor is crucial for leptin action: evidence from clinical and experimental data. Int J Obes Relat Metab Disord 27:1472–1478 [DOI] [PubMed] [Google Scholar]
- 21. Tu H, Kastin AJ, Hsuchou H, Pan W. 2008. Soluble receptor inhibits leptin transport. J Cell Physiol 214:301–305 [DOI] [PubMed] [Google Scholar]
- 22. Maamra M, Bidlingmaier M, Postel-Vinay MC, Wu Z, Strasburger CJ, Ross RJ. 2001. Generation of human soluble leptin receptor by proteolytic cleavage of membrane-anchored receptors. Endocrinology 142:4389–4393 [DOI] [PubMed] [Google Scholar]
- 23. Ge H, Huang L, Pourbahrami T, Li C. 2002. Generation of soluble leptin receptor by ectodomain shedding of membrane-spanning receptors in vitro and in vivo. J Biol Chem 277:45898–45903 [DOI] [PubMed] [Google Scholar]
- 24. Belouzard S, Delcroix D, Rouillé Y. 2004. Low levels of expression of leptin receptor at the cell surface result from constitutive endocytosis and intracellular retention in the biosynthetic pathway. J Biol Chem 279:28499–28508 [DOI] [PubMed] [Google Scholar]
- 25. Barr VA, Lane K, Taylor SI. 1999. Subcellular localization and internalization of the four human leptin receptor isoforms. J Biol Chem 274:21416–21424 [DOI] [PubMed] [Google Scholar]
- 26. Baskin DG, Schwartz MW, Seeley RJ, Woods SC, Porte D, Jr, Breininger JF, Jonak Z, Schaefer J, Krouse M, Burghardt C, Campfield LA, Burn P, Kochan JP. 1999. Leptin receptor long-form splice-variant protein expression in neuron cell bodies of the brain and co-localization with neuropeptide Y mRNA in the arcuate nucleus. J Histochem Cytochem 47:353–362 [DOI] [PubMed] [Google Scholar]
- 27. Diano S, Kalra SP, Horvath TL. 1998. Leptin receptor immunoreactivity is associated with the Golgi apparatus of hypothalamic neurons and glial cells. J Neuroendocrinol 10:647–650 [DOI] [PubMed] [Google Scholar]
- 28. Lundin A, Rondahl H, Walum E, Wilcke M. 2000. Expression and intracellular localization of leptin receptor long isoform-GFP chimera. Biochim Biophys Acta 1499:130–138 [DOI] [PubMed] [Google Scholar]
- 29. Uotani S, Bjørbaek C, Tornøe J, Flier JS. 1999. Functional properties of leptin receptor isoforms: internalization and degradation of leptin and ligand-induced receptor downregulation. Diabetes 48:279–286 [DOI] [PubMed] [Google Scholar]
- 30. Wilcke M, Walum E. 2000. Characterization of leptin intracellular trafficking. Eur J Histochem 44:325–334 [PubMed] [Google Scholar]
- 31. Séron K, Couturier C, Belouzard S, Bacart J, Monté D, Corset L, Bocquet O, Dam J, Vauthier V, Lecœur C, Bailleul B, Hoflack B, Froguel P, Jockers R, Rouillé Y. 2011. Endospanins regulate a postinternalization step of the leptin receptor endocytic pathway. J Biol Chem 286:17968–17981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Sethi G, Sung B, Aggarwal BB. 2008. TNF: a master switch for inflammation to cancer. Front Biosci 13:5094–5107 [DOI] [PubMed] [Google Scholar]
- 33. Moller DE. 2000. Potential role of TNF-α in the pathogenesis of insulin resistance and type 2 diabetes. Trends Endocrinol Metab 11:212–217 [DOI] [PubMed] [Google Scholar]
- 34. Orbak Z, Ertekin V, Akçay F, Ozkan B, Ors R. 2003. Serum leptin levels in neonatal bacterial septicemia. J Pediatr Endocrinol Metab 16:727–731 [DOI] [PubMed] [Google Scholar]
- 35. Loffreda S, Yang SQ, Lin HZ, Karp CL, Brengman ML, Wang DJ, Klein AS, Bulkley GB, Bao C, Noble PW, Lane MD, Diehl AM. 1998. Leptin regulates proinflammatory immune responses. FASEB J 12:57–65 [PubMed] [Google Scholar]
- 36. Voegeling S, Fantuzzi G. 2001. Regulation of free and bound leptin and soluble leptin receptors during inflammation in mice. Cytokine 14:97–103 [DOI] [PubMed] [Google Scholar]
- 37. Schulze PC, Kratzsch J, Linke A, Schoene N, Adams V, Gielen S, Erbs S, Moebius-Winkler S, Schuler G. 2003. Elevated serum levels of leptin and soluble leptin receptor in patients with advanced chronic heart failure. Eur J Heart Fail 5:33–40 [DOI] [PubMed] [Google Scholar]
- 38. McGraw TE, Greenfield L, Maxfield FR. 1987. Functional expression of the human transferrin receptor cDNA in Chinese hamster ovary cells deficient in endogenous transferrin receptor. J Cell Biol 105:207–214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Duffy AM, O'Doherty AM, O'Brien T, Strappe PM. 2005. Purification of adenovirus and adeno-associated virus: comparison of novel membrane-based technology to conventional techniques. Gene Ther 12 Suppl 1:S62–S72 [DOI] [PubMed] [Google Scholar]
- 40. Zhang M, Guller S, Huang Y. 2007. Method to enhance transfection efficiency of cell lines and placental fibroblasts. Placenta 28:779–782 [DOI] [PubMed] [Google Scholar]
- 41. Guo K, McMinn JE, Ludwig T, Yu YH, Yang G, Chen L, Loh D, Li C, Chua S, Jr, Zhang Y. 2007. Disruption of peripheral leptin signaling in mice results in hyperleptinemia without associated metabolic abnormalities. Endocrinology 148:3987–3997 [DOI] [PubMed] [Google Scholar]
- 42. Haniu M, Arakawa T, Bures EJ, Young Y, Hui JO, Rohde MF, Welcher AA, Horan T. 1998. Human leptin receptor. Determination of disulfide structure and N-glycosylation sites of the extracellular domain. J Biol Chem 273:28691–28699 [DOI] [PubMed] [Google Scholar]
- 43. Kamikubo Y, Dellas C, Loskutoff DJ, Quigley JP, Ruggeri ZM. 2008. Contribution of leptin receptor N-linked glycans to leptin binding. Biochem J 410:595–604 [DOI] [PubMed] [Google Scholar]
- 44. Cohen P, Yang G, Yu X, Soukas AA, Wolfish CS, Friedman JM, Li C. 2005. Induction of leptin receptor expression in the liver by leptin and food deprivation. J Biol Chem 280:10034–10039 [DOI] [PubMed] [Google Scholar]
- 45. Campfield LA, Smith FJ, Burn P. 1996. The OB protein (leptin) pathway: a link between adipose tissue mass and central neural networks. Horm Metab Res 28:619–632 [DOI] [PubMed] [Google Scholar]
- 46. Saitoh M, Nishitoh H, Fujii M, Takeda K, Tobiume K, Sawada Y, Kawabata M, Miyazono K, Ichijo H. 1998. Mammalian thioredoxin is a direct inhibitor of apoptosis signal-regulating kinase (ASK) 1. EMBO J 17:2596–2606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Zhang HH, Halbleib M, Ahmad F, Manganiello VC, Greenberg AS. 2002. Tumor necrosis factor-α stimulates lipolysis in differentiated human adipocytes through activation of extracellular signal-related kinase and elevation of intracellular cAMP. Diabetes 51:2929–2935 [DOI] [PubMed] [Google Scholar]
- 48. Clarke CJ, Guthrie JM, Hannun YA. 2008. Regulation of neutral sphingomyelinase-2 (nSMase2) by tumor necrosis factor-α involves protein kinase C-delta in lung epithelial cells. Mol Pharmacol 74:1022–1032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Olszanecka-Glinianowicz M, Zahorska-Markiewicz B, Janowska J, Zurakowski A. 2004. Serum concentrations of nitric oxide, tumor necrosis factor (TNF)-α and TNF soluble receptors in women with overweight and obesity. Metabolism 53:1268–1273 [DOI] [PubMed] [Google Scholar]
- 50. Winkler G, Lakatos P, Salamon F, Nagy Z, Speer G, Kovács M, Harmos G, Dworak O, Cseh K. 1999. Elevated serum TNF-α level as a link between endothelial dysfunction and insulin resistance in normotensive obese patients. Diabet Med 16:207–211 [DOI] [PubMed] [Google Scholar]
- 51. Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW., Jr 2003. Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest 112:1796–1808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Obstfeld AE, Sugaru E, Thearle M, Francisco AM, Gayet C, Ginsberg HN, Ables EV, Ferrante AW., Jr 2010. C-C chemokine receptor 2 (CCR2) regulates the hepatic recruitment of myeloid cells that promote obesity-induced hepatic steatosis. Diabetes 59:916–925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Thaler JP, Yi CX, Schur EA, Guyenet SJ, Hwang BH, Dietrich MO, Zhao X, Sarruf DA, Izgur V, Maravilla KR, Nguyen HT, Fischer JD, Matsen ME, Wisse BE, Morton GJ, Horvath TL, Baskin DG, Tschöp MH, Schwartz MW. 2012. Obesity is associated with hypothalamic injury in rodents and humans. J Clin Invest 122:153–162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Damas P, Reuter A, Gysen P, Demonty J, Lamy M, Franchimont P. 1989. Tumor necrosis factor and interleukin-1 serum levels during severe sepsis in humans. Crit Care Med 17:975–978 [DOI] [PubMed] [Google Scholar]
- 55. Calandra T, Baumgartner JD, Grau GE, Wu MM, Lambert PH, Schellekens J, Verhoef J, Glauser MP. 1990. Prognostic values of tumor necrosis factor/cachectin, interleukin-1, interferon-α, and interferon-γ in the serum of patients with septic shock. Swiss-Dutch J5 Immunoglobulin Study Group. J Infect Dis 161:982–987 [DOI] [PubMed] [Google Scholar]
- 56. Sun Q, Cornelis MC, Kraft P, Qi L, van Dam RM, Girman CJ, Laurie CC, Mirel DB, Gong H, Sheu CC, Christiani DC, Hunter DJ, Mantzoros CS, Hu FB. 2010. Genome-wide association study identifies polymorphisms in LEPR as determinants of plasma soluble leptin receptor levels. Hum Mol Genet 19:1846–1855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Cohen SE, Kokkotou E, Biddinger SB, Kondo T, Gebhardt R, Kratzsch J, Mantzoros CS, Kahn CR. 2007. High circulating leptin receptors with normal leptin sensitivity in liver-specific insulin receptor knock-out (LIRKO) mice. J Biol Chem 282:23672–23678 [DOI] [PubMed] [Google Scholar]
- 58. Lehmann W, Edgar CM, Wang K, Cho TJ, Barnes GL, Kakar S, Graves DT, Rueger JM, Gerstenfeld LC, Einhorn TA. 2005. Tumor necrosis factor α (TNF-α) coordinately regulates the expression of specific matrix metalloproteinases (MMPS) and angiogenic factors during fracture healing. Bone 36:300–310 [DOI] [PubMed] [Google Scholar]
- 59. Bzowska M, Jura N, Lassak A, Black RA, Bereta J. 2004. Tumour necrosis factor-α stimulates expression of TNF-α converting enzyme in endothelial cells. Eur J Biochem 271:2808–2820 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.