Abstract
Cervical ripening is associated with loss of structural integrity and tensile strength, thus enabling the cervix to dilate at term. It is characterized by changes in glycosaminoglycan composition, increased water content, and a progressive reorganization of the collagen network. The peptide hormone relaxin via interaction with its receptor, relaxin family peptide receptor 1 (RXFP1), promotes tissue hydration and increases cervical hyaluronan (HA) concentrations, but the mechanisms that regulate these effects are not known. This study in relaxin mutant (Rln−/−) mice tested the hypothesis that relaxin regulates HA synthase and aquaporin (AQP) expression in the cervix. We also assessed expression of the RXFP1 protein by immunohistochemistry. Pregnant Rln−/− mice had lower Has2 and Aqp3 expression on d 18.5 of pregnancy and decreased cervical HA compared with wild-type Rln+/+ mice. Chronic infusion of relaxin for 4 or 6 d in pregnant Rln−/− mice reversed these phenotypes and increased Has2 and Aqp3 compared with placebo controls. Relaxin-treated mice also had lower Has1 and Aqp5. Changes in gene expression were paralleled by increases in cervical HA and variations in AQP3 and AQP5 protein localization in epithelial cells of Rln−/− cervices. Our findings demonstrate that relaxin alters AQP expression in the cervix and initiates changes in glycosaminoglycan composition through increased HA synthesis. These effects are likely mediated through RXFP1 localized to subepithelial stromal cells and epithelial cells. We suggest these actions of relaxin collectively promote water recruitment into the extracellular matrix to loosen the dense collagen fiber network.
Despite considerable advances in perinatal medicine, many women still experience prolonged labor or dystocia due to inadequate cervical ripening (1). In late pregnancy, the extracellular matrix (ECM) of the cervix is remodeled extensively so that it softens and dilates (2–4). Cervical ripening is a specific phase of remodeling associated with loss of structural integrity and tensile strength, which enables the cervix to dilate at the end of pregnancy (3, 5, 6). It is characterized by the realignment and loosening of the collagen fibers within the ECM, changes in collagen fibril structure, and increased tissue hydration. These processes are facilitated by an increase in glycosaminoglycans (GAGs) in the ECM, notably hyaluronan (HA) (6).
HA content in the cervix increases markedly in late pregnancy in several mammalian species, including rodents and humans (7–10). The function of HA is determined by its molecular weight and interactions with other HA-binding molecules. For example, during cervical ripening, there is an increase in large molecular weight HA, resulting in increased spacing between collagen fibers. Moreover, because HA is a hydrophilic macromolecule, it attracts water into these interfibrillar spaces, thereby contributing to the increase in cervical hydration (6, 11). HA synthesis is regulated by HA synthases (HASs), of which there are three isoforms (HAS1–HAS3) (12, 13). Has2 is the predominantly expressed isoform in the cervix of pregnant mice, and its transcriptional up-regulation in late pregnancy coincides with highest production of HA (10, 14). Similarly, Has2 mRNA transcripts are up-regulated in cervices of women in labor (10).
The influx of water molecules into the cervical ECM is also regulated by water channel molecules known as aquaporins (AQPs). AQP3, AQP4, AQP5, and AQP8 have been identified in the mouse cervix and are differently expressed and localized during pregnancy (15). The cervical epithelium is several layers thick and therefore is classified as a stratified epithelium. AQP3 localizes to inner cervical epithelial cells, whereas AQP4, AQP5, and AQP8 are in the outer epithelial cell layers closest to the lumen. Highest expression of Aqp8 occurs in early and midpregnancy, Aqp5 in midpregnancy, and Aqp3 in late pregnancy, with no change in Aqp4 (15).
To date, very little information is available on the regulation of Has, HA, and AQPs in the cervix. Expression of Has1, Has2, and Has3 in pregnant mouse cultured uterine cervical fibroblasts is significantly increased by IL-1β, whereas progesterone reduces Has1 and Has2 and increases Has3 (14). Similarly, administration of progesterone from d 17 of pregnancy in mice inhibits the increase in Has2 and decreases Has1 on d 18 (10). Moreover, the normal increase in Has2 and Aqp3 during late pregnancy does not occur in 5α reductase 1 knockout (Srd5a1−/−) mice, because their cervical progesterone levels remain elevated (10, 15). This implies a negative regulatory role for progesterone on HAS2 and AQP3. Numerous studies suggest that the peptide hormone relaxin also regulates cervical GAGs during pregnancy. First, circulating relaxin concentrations correlate with increases in HA content in the pregnant mouse cervix (16). Second, impaired cervical ripening in Srd5a1−/− mice is rescued by relaxin treatment (17). Third, cervices from ovariectomized (OVX) d 18 and 22 pregnant rats treated with progesterone and estrogen resemble those of early pregnant and nonpregnant (NP) cervices. Treatment of these OVX rats with relaxin significantly increases cervical HA concentrations (7). Similarly, the overall GAG to collagen ratio increases between d 80 and 110 of pregnancy in the pig cervix, and between control and OVX pigs with relaxin replacement (18). Relaxin is also reported to increase GAG synthesis in cultured human cervical stroma cells (19). However, no studies have investigated how relaxin treatment leads to an increase in HA or the potential effects of relaxin on cervical AQPs.
Relaxin mutant (Rln−/−) mice experience a high incidence of dystocia, a phenotype which is reversed by relaxin treatment for at least 3 d (20). This phenotype is largely due to impaired cervical ripening, characterized by dense collagen fiber bundles in the ECM, decreased wet weight and growth, and a cervical morphology essentially equivalent to NP mice (20–22). Similar observations were made in pregnant rats treated with a neutralizing monoclonal relaxin antibody (MCA1). The cervices from these MCA1-treated rats contained dense collagen fiber bundles in the ECM and were less distensible (23, 24). The cloning of the human relaxin family peptide receptor (RXFP1) (25), and the description of phenotypes in Rxfp1 gene knockout mice (26, 27), provided unequivocal evidence that relaxin receptors are present in the reproductive tract and that relaxin is essential for neonate survival. In the mouse, staining for Rxfp1-specific β-galactosidase activity was identified in the circular layer of the myometrium and below the basal layer of the vaginal and cervical epithelium of 8- to 10-wk-old female NP heterozygous Rxfp1 mutant mice (27, 28). However, differences in RXFP1 expression between Rln+/+ and Rln−/− mice have not been investigated, and RXFP1 protein has not been localized in the cervix of pregnant mice. In this study, we tested the hypothesis that abnormal cervical ripening in pregnant Rln−/− mice results from impaired HA synthesis and AQP expression in the cervix. The main objectives were to investigate in pregnant Rln−/− mice whether or not: 1) expression of Has1 and Has2 and HA content are decreased; 2) expression of AQPs is reduced; and 3) treatment with relaxin affects Has expression, HA content, and AQPs in the cervix. We also compared expression of RXFP1 in late pregnant Rln+/+ and Rln−/− mice by immunohistochemistry.
Materials and Methods
All animal experiments were approved by the University of Melbourne Animal Experimental Ethics Committee (AEEC 06-187) in accordance with the Australian Code of Practice and the National Health and Medical Research Council guidelines. This study used the original Rln−/− mouse (29) backcrossed on a C57/BLK6J background to the F14 generation and wild-type littermates (Rln+/+) of the same strain. Genotypes are confirmed by PCR analysis of genomic DNA from ear clips as previously described (29). Mice were housed in the Department of Zoology Animal Facility (University of Melbourne), maintained on an automated time cycle (10-h light, 14-h dark cycle) at 20 C, and had access to standard food pellets (Barastoc, Ridley AgriProducts, Melbourne, Victoria, Australia) and water ad libitum. Adult female mice aged between 2 and 3 months were mated with males of matching genotypes to obtain timed pregnancies. If a copulation plug was found the next morning, this was designated as d 0.5 of pregnancy. Delivery at term typically occurs between d 19 and 19.5 of pregnancy (20).
HAS, HA content, and AQPs in the cervix of Rln+/+ and Rln−/− mice
Mice were euthanized by isofluorane overdose on d 10.5, 14.5, 16.5, and 18.5 of pregnancy and 1 d postpartum (n = 5/group). An additional group of Rln+/+ mice (n = 5) was euthanized on d 19.5 of pregnancy, just before parturition. Rln−/− mice were not included in this group, because these mice are at risk of developing dystocia during labor (20). A group of 2-month-old virgin C57/BLK6J wild-type mice (n = 5) was included as the NP group. The length of the interpubic ligament was measured to confirm the genotype of the animal, because it is considered to be a reliable bioassay to demonstrate differences between Rln−/− and both Rln+/+ and Rln+/− pregnant mice (30). The cervix was cut away from the corpus region of the uterus and from the upper anterior region of the vagina. Cervix tissues were weighed, snap frozen, and stored at −80 C. Cervices from both genotypes on d 18.5 of pregnancy were fixed in 10% formalin (n = 2/group per genotype) for immunohistochemistry.
Effects of human recombinant relaxin (rhRLX) treatment on Has, HA content, and Aqps in the cervix of pregnant Rln−/− mice
A group of female Rln−/− mice was surgically implanted with Alzet osmotic minipumps (Model 1007D; flow rate, 0.5 μl/h; BioScientific Pty Ltd., Kirrawee, New South Wales, Australia) on d 12.5 of pregnancy to administer rhRLX (3.33 μg/h·kg; Corthera, Inc., San Mateo, CA) or placebo solution (0.9% saline) as a control (n = 5/group). This dose and duration of rhRLX was previously shown to prevent dystocia in pregnant Rln−/− mice (20). There were two periods of infusion, 4 and 6 d (n = 5/treatment per stage). The shorter infusion period was to investigate whether relaxin could stimulate changes in AQP expression 2 d before the normal onset of parturition. The longer infusion period was to reverse the impaired cervix phenotype of Rln−/− mice on the day of expected births (d 18.5 of pregnancy). Cervix tissues were collected as described above. Blood was collected by cardiac puncture to measure serum rhRLX concentrations using the Quantikine human H2 ELISA kit (R&D Systems, Inc., Minneapolis, MN) according to the manufacturer's instructions. Mouse plasma was diluted 1:250 with the Calibrator Diluent RD6-6 to obtain measurements in the linear range of the standard curve rhRLX standards (7.8–500 pg/ml). All samples were assayed on a single ELISA plate, and the limit of detection was 15.6 pg/ml.
Gene expression
Frozen cervix tissues were pulverized in prechilled capsules, containing steel balls, using a Digital Wig-L-Bug amalgamator (Dentsply Ltd., York, PA). Total RNA was extracted using TRI Reagent and treated with deoxyribonuclease I (Ambion, Applied Biosystems, Scoresby, Victoria, Australia) to remove any genomic DNA contaminants according to the manufacturer's instructions. RNA concentrations were measured using the NanoDrop ND-1000 Spectrophotometer (NanoDrop Technologies, Inc.; BioLab Pty Ltd., Scoresby, Victoria, Australia). First strand cDNA synthesis used a total of 300 ng of RNA in a 30-μl reaction volume containing 10 mm deoxynucleotide triphosphate mix, 50 ng/μl random hexamers, 6-μl 5× First Strand buffer, 0.1 m dithiothreitol, RNaseOUT, and 0.5 μl of Superscript III reverse transcriptase (Invitrogen, Mulgrave, Victoria, Australia). A separate reaction volume used 2 ng of RNA from each sample for ribosomal 18S (Rn18s) quantitative PCR (QPCR) reactions. All RNA samples used in each experimental study were reverse transcribed simultaneously to avoid variation during cDNA synthesis.
Relative quantification of Has1, Has2, Aqp3, Aqp5, and Aqp8 expression in cervix tissues used the comparative cycle threshold (2−ΔCT) method with Rn18s as the endogenous reference gene. Mouse-specific oligonucleotide primers and 6-carboxy fluorescent-labeled TaqMan probes (BioSearch Technologies, Inc., Novato, CA) for individual genes were specifically designed to span an exon/intron junction using the RealTimeDesign software. Primer and probe sequences are listed in Supplemental Table 1, published on The Endocrine Society's Journals Online web site at http://endo.endojournals.org. QPCR reactions were performed on the DNA Engine Opticon 2 machine in triplicate using 96-well optical reaction plates (MJ Research, Waltham, MA), in 20-μl reaction volumes consisting of 1 μl of cDNA, 10 μl of SensiMix dU (Quantace, Alexandria, New South Wales, Australia), 0.6 μl of forward and reverse primers (10 μm), 0.2 μl of probe, and 7.5 μl of nuclease-free water. The gene of interest and Rn18s were separately assessed. The experimental design ensured that samples for direct comparison (e.g. Rln+/+ and Rln−/− mice at the same stage) were included on the same plate.
Fluorophore-assisted carbohydrate electrophoresis (FACE) analysis
Cervices were lyophilized in a Speed-Vac for a minimum of 6 h to determine dry weight. Samples were then resuspended in 100 mm ammonium acetate and 0.0005% phenol red (pH 7.0) and prepared for analysis by FACE as described previously (Supplemental Materials and Methods) (10, 31). Values were quantified as picomoles HA and normalized to cervical dry weight.
Immunohistochemistry for AQP and relaxin receptors (RXFP1)
Cervix samples fixed in 10% formalin were embedded in paraffin wax, and 4-μm-thick sections were mounted onto Polysine (Menzel-Gläser, Braunschweig, Germany) coated slides (n = 2 sections/slide). Sections were immersed in antigen retrieval buffer [Tris/EDTA/EGTA (pH 9.0)] for 10 min at 90 C before cooling at room temperature (RT) for 20 min. Sections were then treated with 3% hydrogen peroxide for 1 h at RT. To localize AQP3 and AQP5, cervix tissue sections were incubated with Background Sniper (BioCare Medical, Concord, CA) for 20 min at RT to reduce nonspecific background staining. Sections were then blotted and incubated overnight at 4 C with rabbit anti-AQP3 and anti-AQP5 antibodies (both 0.4 mg/ml; Merck Pty Ltd., Kilsyth, Victoria, Australia) diluted 1:500 with Tris-buffered saline containing 0.5% BSA and Triton X-100. To localize RXFP1, cervix tissue sections were incubated with Rodent M blocker (BioCare Medical) for 30 min at RT to reduce nonspecific background staining. Sections were then blotted and incubated overnight at 4 C with a monoclonal antihuman RXFP1 antibody (M01, 0.36 mg/ml; Abnova; Sapphire Bioscience Pty Ltd., Waterloo, New South Wales, Australia) diluted 1:500 with Tris-buffered saline containing 0.5% BSA. This antibody detects an immunoreactive RXFP1 protein of approximately 75 kDa in the mouse cervix (32). Immunoreactivity was detected using the MACH 2 system (BioCare Medical) or Mouse-On-Mouse HRP-Polymer (BioCare Medical) and 3,3′-diaminobenzidine as the chromagen substrate (Vector Laboratories, Burlingame, CA). To enhance the signal, sections were incubated in 0.05 m sodium bicarbonate (pH 9.0) for 10 min and DAB Enhance (Vector Laboratories) for 20 sec. Negative controls were treated similarly, except they were incubated overnight with rabbit IgG or mouse IgG of equivalent concentration of the primary antibody used.
Statistical analysis
The ΔCT data from the QPCR of target genes and Rn18s were all normally distributed within groups (SPSS 19; IBM, White Plains, NY). Statistical differences in gene expression and HA content between NP, pregnancy, and postpartum stages in wild-type Rln+/+ mice were assessed by one-way ANOVA (SPSS 19) with Student-Newman-Keuls post hoc tests. Two-way ANOVA was used to determine whether there was a significant effect of genotype at each stage of pregnancy and 1 d postpartum with Student-Newman-Keuls post hoc analysis for pairwise comparisons. Independent t tests were also used to compare the gene expression and HA content between placebo and rhRLX groups in the infusion study. All statistical analysis was at the 95% confidence level.
Results
Has, HA content, and Aqps in the cervix of Rln+/+ and Rln−/− mice
Expression of the reference gene Rn18s did not differ significantly (P > 0.05) between NP, pregnancy stages, and 1 d postpartum in the cervix of Rln+/+ and Rln−/− mice and was therefore a valid reference gene for this tissue. There was no significant difference in Has1 expression between NP, d 14.5 and 16.5 of pregnancy, and 1 d postpartum in Rln+/+ mice. However, Has1 was significantly increased on d 18.5 of pregnancy, compared with d 14.5 of pregnancy and 1 d postpartum and also compared with Rln−/− mice on d 18.5 (Fig. 1A). There was no significant effect of genotype on Has1 expression at any other stage examined. In contrast, Has2 was significantly increased on d 18.5 of pregnancy compared with all other stages in Rln+/+ mice (Fig. 1B). Two-way ANOVA demonstrated a significant (F3,39 = 5.53, P < 0.01) decrease in Has2 on d 18.5 of pregnancy and an increase in Has2 1 d postpartum in the Rln−/− mice compared with Rln+/+ mice (Fig. 1B). To determine whether the differences in Has2 between genotypes correlate with changes in HA content, tissue HA levels were measured by FACE in NP, d 16.5 and 18.5 of pregnancy, and 1 d postpartum mice. Figure 1C shows a significant increase in HA content on d 18.5 of pregnancy and 1 d postpartum in the Rln+/+ mice and on d 18.5 of pregnancy in the Rln−/− mice compared with NP and d 16.5. However, HA content on d 18.5 and 1 d postpartum in Rln−/− mice was significantly (F2,26 = 3.93, P = 0.04) lower compared with Rln+/+ mice.
Fig. 1.
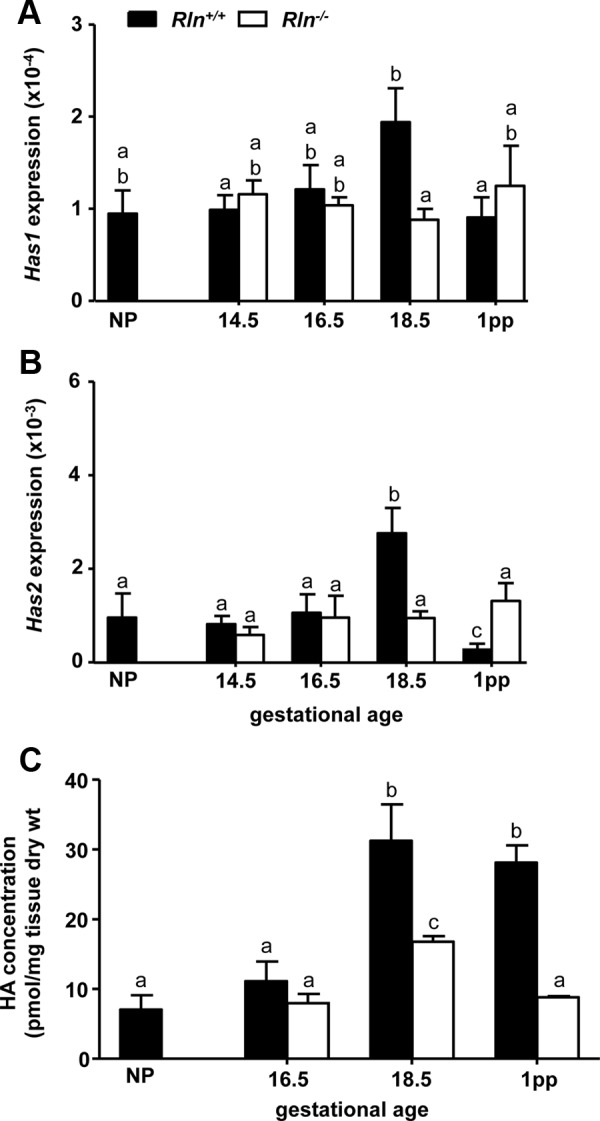
Has1 (A), Has2 (B), and HA (C) content in the cervix of NP, pregnant, and 1 d postpartum (1pp) Rln+/+ (black bars) and Rln−/− (white bars) mice. QPCR data are normalized to the reference gene Rn18S and presented as mean ± sem as calculated using the 2−ΔCT method (n = 5/group). Data labeled with different letters indicate significant differences (P < 0.05) between genotypes and reproductive stages.
The AQP genes were differentially expressed in the Rln+/+ mouse cervix during pregnancy. Aqp3 expression did not differ significantly between NP, d 14.5 and 16.5 of pregnancy, and 1 d postpartum in Rln+/+ mice. However, Aqp3 was significantly increased on d 18.5 of pregnancy compared with all other stages (Fig. 2A). In contrast, Aqp5 and Aqp8 expression was significantly higher at all pregnancy stages and 1 d postpartum compared with NP. Highest expression of Aqp5 was observed on d 16.5 of pregnancy (Fig. 2B). There was a similar profile of Aqp8 expression, with highest expression at all pregnancy stages and a sharp decrease on d 1 postpartum (Fig. 2C). The profile of Aqp3, Aqp5, and Aqp8 expression in the cervix of pregnant Rln−/− mice was essentially the same as Rln+/+ mice (Fig. 2). There were, however, significant differences in expression of all three AQPs in Rln−/− mice on 1 d postpartum compared with pregnancy stages. Aqp3 was higher, whereas Aqp5 and Aqp8 were lower. Two-way ANOVA demonstrated a significant (F3,39 = 7.95, P < 0.01) decrease in Aqp3 on d 18.5 of pregnancy in the Rln−/− mice and an increase in Aqp3 on d 1 postpartum compared with Rln+/+ mice (Fig. 2A). In contrast, there were no significant differences in Aqp5 and Aqp8 expression throughout pregnancy between Rln+/+ and Rln−/− mice. However, Aqp5 was significantly (F3,39 = 11.01, P < 0.01) lower in the Rln−/− mice compared with Rln+/+ mice on d 1 postpartum (Fig. 2B).
Fig. 2.
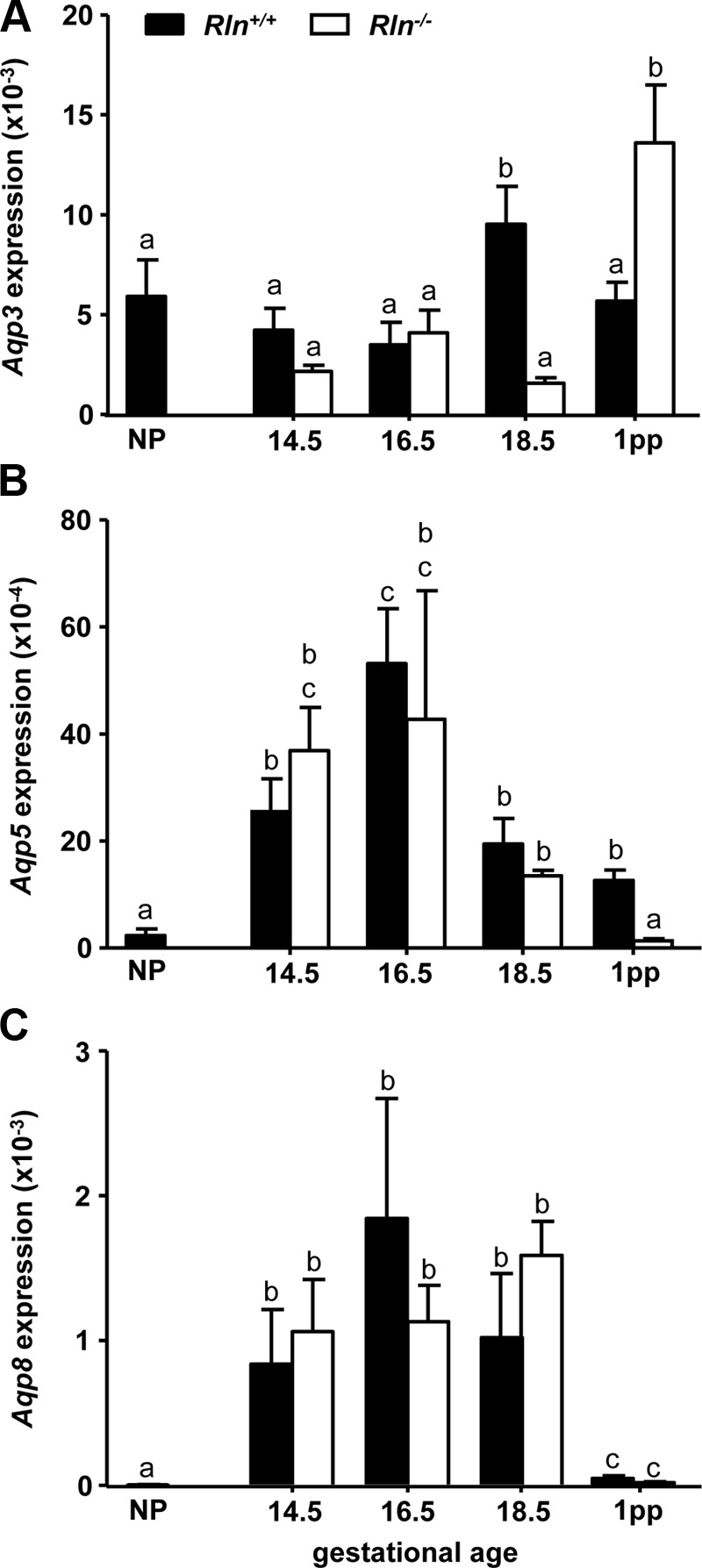
Aqp3 (A), Aqp5 (B), and Aqp8 (C) in the cervix of NP, pregnant, and 1 d postpartum (1pp) Rln+/+ (black bars) and Rln−/− (white bars) mice. QPCR data are normalized to the reference gene Rn18S and presented as mean ± sem as calculated using the 2−ΔCT method (n = 5/group). Data labeled with different letters indicate significant differences (P < 0.05) between genotypes and reproductive stages.
AQP3 and AQP5 localization in the late pregnant Rln+/+ and Rln−/− mouse cervix
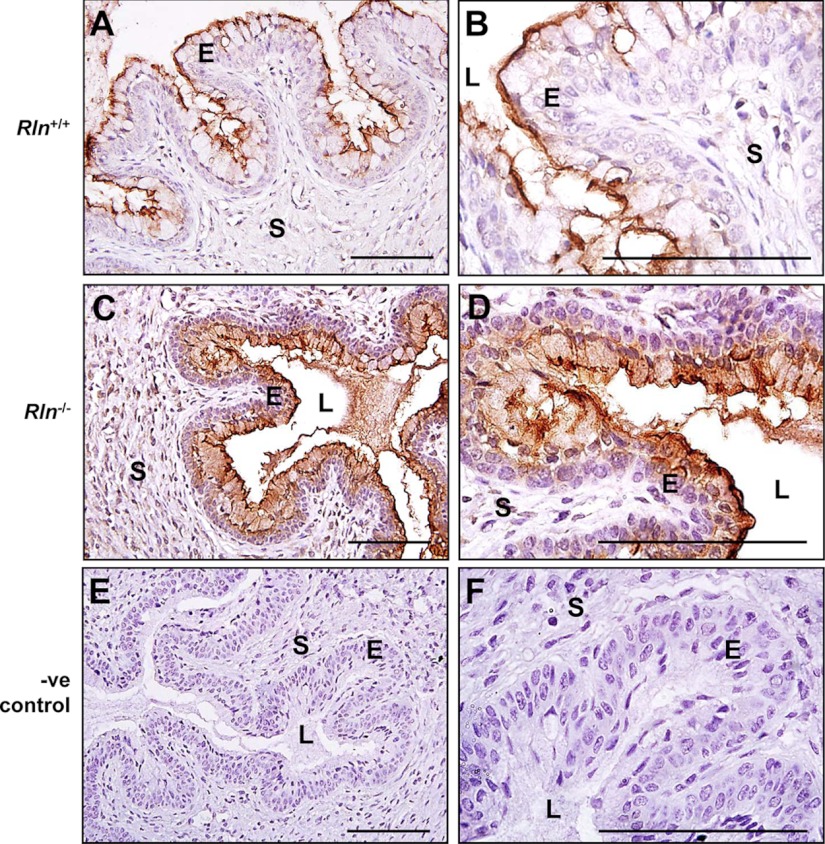
As reported in other studies (15), immunoreactive AQP3 was predominantly localized to the basal cell layers of the stratified epithelium lining the cervical lumen and stroma in both genotypes on d 18.5 of pregnancy (Fig. 3A). At higher magnification in the Rln+/+ mice, intense AQP3 immunostaining was observed in the cell membrane with more diffuse staining in the cytoplasm. Light immunostaining was also seen in the nuclei of cells in the stroma (Fig. 3B). Cell membrane localization of immunoreactive AQP3 was modestly reduced in the Rln−/− mouse cervix, with diffuse immunostaining of less intensity in the cytoplasm of the basal epithelial cells (Fig. 3, C and D). There was no immunostaining in the sections incubated with rabbit IgG as the negative control (Fig. 3, E and F).
Fig. 3.
Localization of AQP3 protein in the cervices of Rln+/+ (A and B) and Rln−/− (C and D) mice on d 18.5 of pregnancy by immunohistochemistry. Negative (-ve) controls used Rln+/+ cervix tissues incubated with rabbit IgG instead of the primary antiserum (E and F). S, Stroma; L, lumen; E, epithelium. Magnification bars, 500 μm.
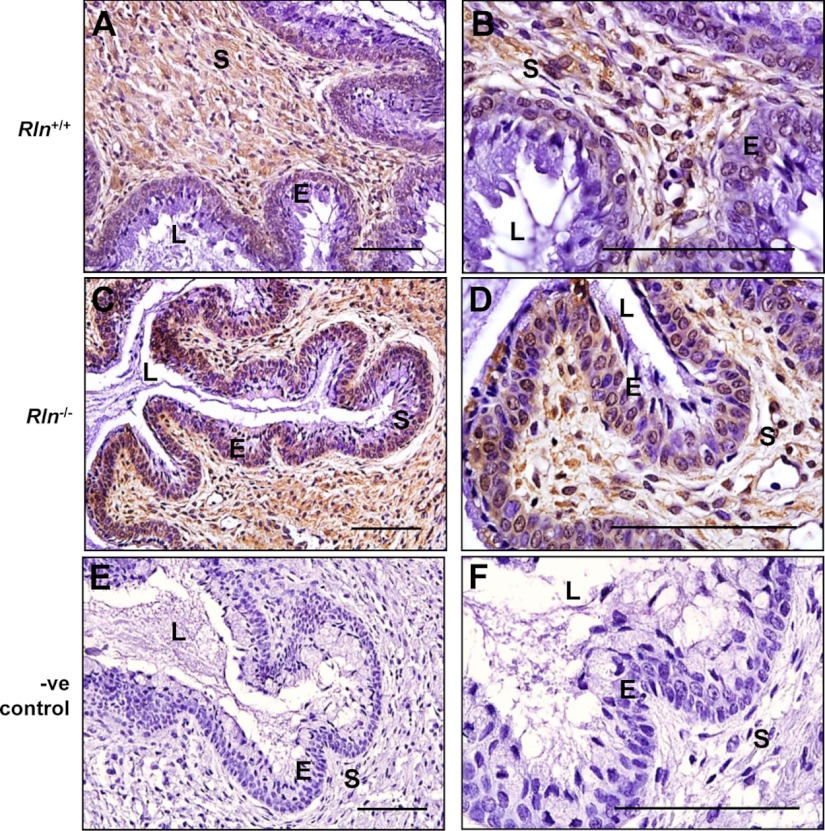
In contrast to the mRNA expression, there was more intense immunostaining for AQP5 in the cervix of Rln−/− on d 18.5 of pregnancy, compared with age-matched Rln+/+ controls (Fig. 4). In Rln+/+ mice, immunoreactive AQP5 was specifically detected in the apical layer of epithelial cells along the luminal surface, with no staining in the stroma (Fig. 4, A and B). In the Rln−/− mice, there was intense positive immunostaining for AQP5 in the apical cell membrane in addition to increased staining at the luminal surface. Staining was apparent on the surface of apical cells as well as diffuse staining in the cytoplasm (Fig. 4, B and D). Additionally, there was light and diffuse AQP5 immunostaining in the cytoplasm of some basal cells. There was no immunostaining in the sections incubated with rabbit IgG as the negative control (Fig. 4, E and F).
Fig. 4.
Localization of AQP5 protein in the cervices of Rln+/+ (A and B) and Rln−/− (C and D) mice on d 18.5 of pregnancy by immunohistochemistry. Negative (-ve) controls used Rln+/+ cervix tissues incubated with rabbit IgG instead of the primary antiserum (E and F). S, Stroma; L, lumen; E, epithelium. Magnification bars, 500 μm.
Effects of rhRLX treatment on Has1 and Has2 and Aqp mRNA expression and HA content
Plasma rhRLX concentrations after 4 and 6 d of infusion were 2.22 ± 0.75 and 2.57 ± 0.75 ng/ml, respectively. This is within the normal physiological range in pregnant mice (1.3–2.5 ng/ml) (16, 33). There was no rhRLX detected in the plasma of placebo-treated Rln−/− controls and no significant difference in Rn18s between Rln−/− placebo- and rhRLX-treated groups. Consistent with previous studies in rats, treatment with rhRLX in Rln−/− mice significantly increased cervix wet weight in both the 4-d (37.56 ± 2.56 mg) and 6-d (60.14 ± 3.06 mg) groups compared with placebo controls (22.48 ± 1.98 and 27.84 ± 4.60 mg, respectively). Similarly, rhRLX also significantly increased pubic symphysis length after 4 d (rhRLX 2.27 ± 0.21 mm vs. placebo 1.15 ± 0.29 mm) and 6 d (rhRLX 3.15 ± 0.11 mm vs. placebo 1.11 ± 0.23 mm) of infusion.
Within each treatment group, Has1 and Has2 expression did not differ between d 4 and 6. Infusion of rhRLX for 4 d significantly (t8 = 2.48, P = 0.04) decreased Has1 in the Rln−/− mouse cervix compared with placebo controls but not in the 6-d rhRLX-treated group (Fig. 5A). In contrast, rhRLX significantly increased Has2 after 4 d (t8 = 4.23, P < 0.01) and 6 d (t8 = 2.68, P = 0.03) (Fig. 5B). Despite the rhRLX-induced increase in Has2, HA content was only significantly (t6 = 2.661, P = 0.04) increased in animals treated with rhRLX for 6 d (Fig. 5C).
Fig. 5.
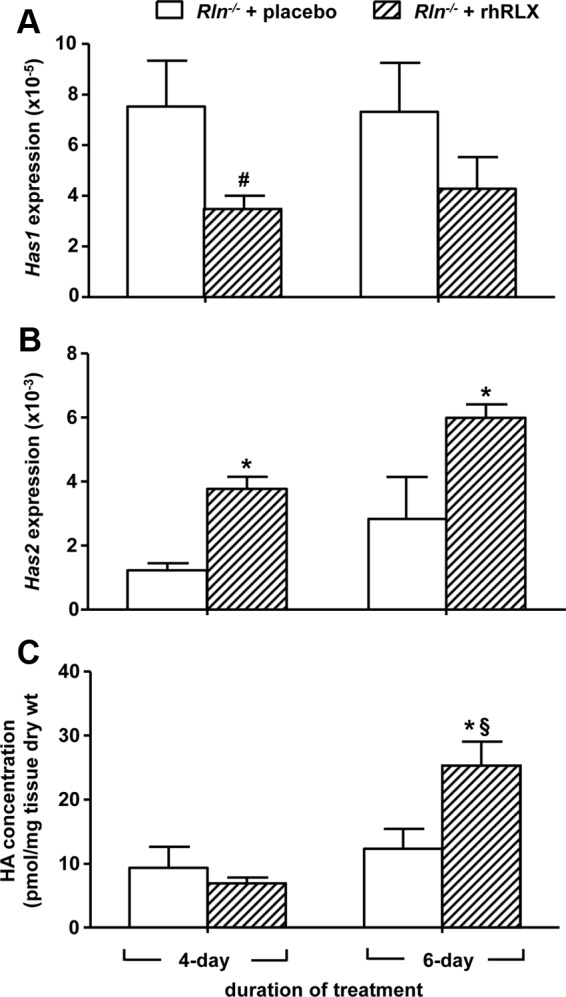
Has1 (A), Has2 expression (B), and HA content (C) in the cervix of pregnant Rln−/− mice treated with placebo (white bars) or rhRLX (hatched bars) for 4 and 6 d. QPCR data are normalized to the reference gene Rn18s and presented as mean ± sem as calculated using the 2−ΔCT method (n = 5/group). *, P < 0.05 higher and #, P < 0.05 lower vs. Rln−/− placebo (P < 0.05); §, P < 0.05 higher vs. 4-d Rln−/− rhRLX.
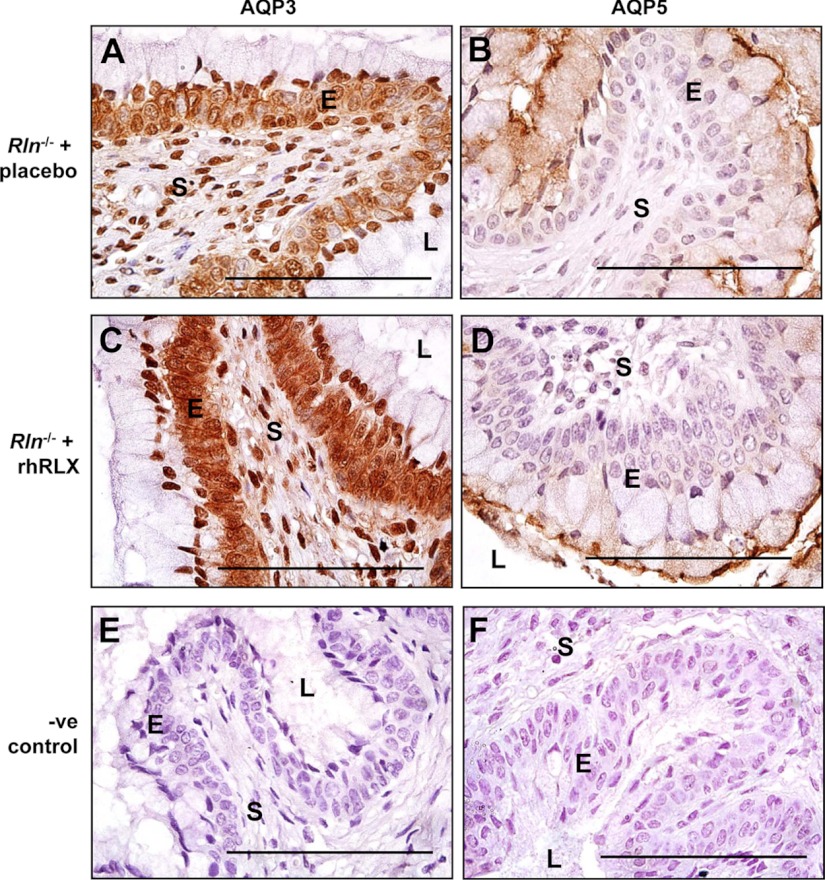
Treatment with rhRLX significantly increased Aqp3 in both the 4-d (t8 = 3.99, P < 0.01) and 6-d (t8 = 2.95, P = 0.02) infusion groups compared with placebo controls (Fig. 6A). In contrast, rhRLX treatment significantly (t8 = −2.63, P = 0.03) reduced Aqp5 in the 6-d infusion group but had no effect after 4 d of infusion (Fig. 6B). There was no effect of rhRLX on Aqp8 (Fig. 6C). The pattern of AQP3 and AQP5 immunostaining in the cervices of pregnant Rln−/− mice treated with placebo for 6 d was similar to that observed in Rln−/− mice on d 18.5 of pregnancy (Fig. 7, A and B). Treatment with rhRLX resulted in more intense AQP3 immunostaining in the basal epithelial cells and also around the cell membranes (Fig. 7C). It reduced the intensity of immunoreactive AQP5 in the cytoplasm of the apical cells, although immunostaining persisted in the cell membrane along the luminal surface (Fig. 7D). Immunoreactive AQP3 and AQP5 was absent in sections incubated with rabbit IgG (Fig. 7, E and F).
Fig. 6.
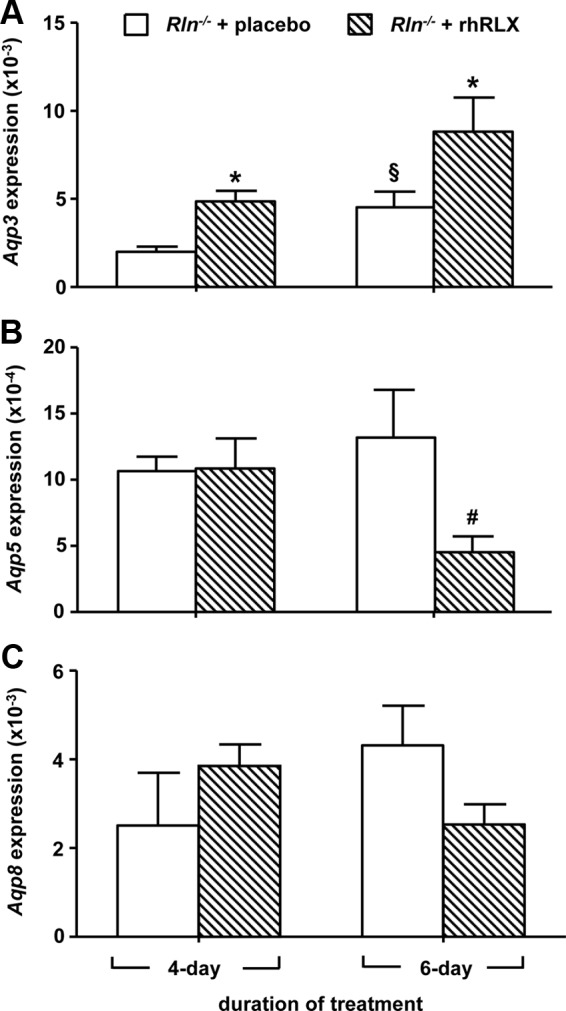
Aqp3 (A), Aqp5 (B), and Aqp8 (C) in the cervix of pregnant Rln−/− mice treated with placebo (white bars) or rhRLX (hatched bars) for 4 and 6 d. QPCR data are normalized to the reference gene Rn18s and presented as mean ± sem as calculated using the 2−ΔCT method (n = 5/group). *, P < 0.05 higher vs. Rln−/− placebo; #, P < 0.05 lower vs. Rln−/− placebo; §, P < 0.05 higher vs. 4-d Rln−/− rhRLX.
Fig. 7.
Immunoreactive AQP3 (A and C) and AQP5 (B and D) in the cervices of pregnant Rln−/− mice treated with placebo (A and B) or rhRLX (C and D) for 6 d. Negative (-ve) controls were rabbit IgG (E and F). S, Stroma; L, lumen; E, epithelium. Magnification bars, 500 μm.
Localization of RXFP1 protein in the pregnant mouse cervix
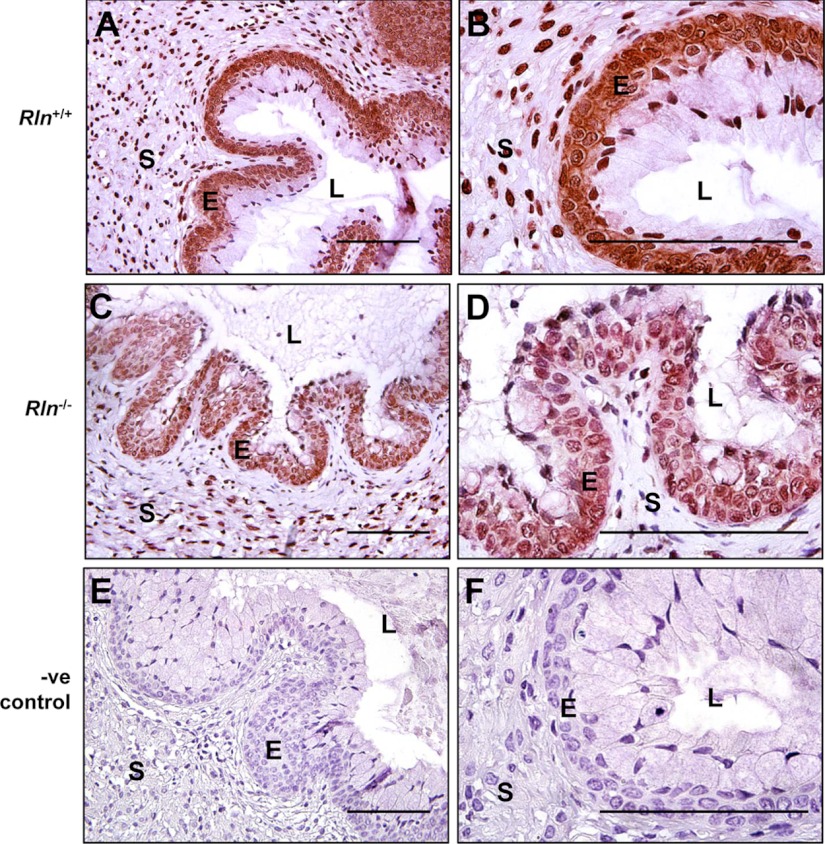
Immunoreactive RXFP1 was observed primarily in the cervical stroma of Rln+/+ mice, although faint immunostaining was also present in the basal layer of epithelial cells lining the lumen (Fig. 8, A and B). In the Rln−/− mice, the intensity of RXFP1 immunostaining was modestly higher in the stroma but particularly in the basal cell layers of the epithelium compared with the Rln+/+ mice (Fig. 8C). At higher magnification, the immunostaining appears to be in the cell cytoplasm and perinuclear (Fig. 8D). Some cells in the apical layer of the epithelium were also positive for RXFP1, particularly along the luminal surface. Incubation of mouse cervix tissues with mouse IgG resulted in no positive RXFP1 immunostaining (Fig. 8, E and F).
Fig. 8.
Localization of RXFP1 protein in the cervices of Rln+/+ (A and B) and Rln−/− (C and D) mice on d 18.5 of pregnancy by immunohistochemistry. Negative (-ve) controls used Rln+/+ cervix incubated with mouse IgG instead of the primary antisera (E and F). S, Stroma; L, lumen; E, epithelium. Magnification bars, 500 μm.
Discussion
This study first tested the hypothesis that abnormal cervical ripening in late pregnant Rln−/− mice results from impaired HA synthesis. Pregnant Rln−/− mice had lower Has1 and Has2 mRNA expression on d 18.5 of pregnancy and decreased cervical HA compared with Rln+/+ mice. Treatment of pregnant Rln−/− mice with relaxin partially reversed these phenotypes, by increasing Has2 and cervical HA content. However, Has1 expression was down-regulated. These changes in Has2, Has1, and HA content in the relaxin-treated Rln−/− mice were associated with an increase in cervix wet weight, dispersal of collagen fibers, and reduction in stromal ECM density reported previously in late pregnant, relaxin-treated Rln−/− mice (22).
The data reveal a regulatory action of relaxin on HAS enzymes, particularly Has2, which may explain the increase in HA content in the cervix at the end of pregnancy. To date, only two studies have profiled the expression of HAS isoforms in the cervix of pregnant mice (10, 14). The consistent finding is increased Has2 in late pregnancy. This also occurs in the human cervix, with more abundant Has2 mRNA transcripts in both the epithelium and stroma compared with Has1 (10, 34). Furthermore, Has2 is significantly elevated in cervical tissues from women in labor vs. nonlabor (10). Previous studies reported that OVX pregnant pigs and rats with steroid hormone replacement, but no circulating relaxin, have lower GAG content in the cervix, specifically HA and GAGs derived from the proteoglycan, decorin. Relaxin treatment of these OVX animal restores cervical HA to concentrations equivalent to intact pregnant controls (7, 18). Our findings support these early studies and demonstrate that a relaxin-mediated increase in Has2 is predominantly responsible for increased synthesis of cervical HA in late pregnancy, with reduced tissue HA in relaxin-deficient mice and increased HA in the cervices of relaxin-treated Rln−/− mice. Previous studies report low cervical Has1 expression and no change in late pregnant mice when relaxin levels are high. Although the current study showed that relaxin treatment did not increase Has1 synthesis, relaxin deficiency was associated with a decrease in Has1. There is no obvious explanation for this differential response to relaxin, but mice deficient in both Has1 and Has3 have normal parturition, suggesting that cervical HA synthesis is primarily regulated by Has2 (35). Has2 is capable of synthesizing HA of high molecular weight, with more hydrophilic regions to attract water and provide increased viscoelasticity to tissues. This is consistent with our hypothesis that the principle action of relaxin is to loosen and disperse the collagen fibers in the ECM, thereby contributing to cervical ripening in late pregnancy. With the loss of circulating relaxin immediately postpartum, expression of Has1 and Has2 also declined in wild-type mice postpartum. The observed lag between decreased Has expression and concentrations of HA in the cervix is likely due to the fact that activity of the HA-metabolizing enzyme, hyaluronidase, is not increased until postpartum as recently reported (31). In Rln−/− mice, there was a decrease in HA on d 1 postpartum compared with d 18.5 of pregnancy. This occurred in the absence of changes in Has gene expression and implies that activity of hyaluronidase enzymes may not be under regulation of relaxin in contrast to HA synthesis.
Our study also investigated a novel mechanism of relaxin action on cervical AQPs. Specifically, it revealed a new phenotype in late pregnant Rln−/− mice, with significantly reduced Aqp3 on d 18.5 of pregnancy and increased Aqp3 1 d postpartum. The increase in Aqp3 postpartum in Rln−/− mice was unexpected and may occur in response to reduced relaxin concentrations immediately postpartum. In the cervix of late pregnant Rln−/− mice, immunoreactive AQP3 appeared less intense compared with Rln+/+ mice, implying a reduction in cervical AQP3 protein in Rln−/− mice. Increased cervical Aqp3 coincides with maximal circulating relaxin in the mouse during late pregnancy, suggesting regulation by relaxin. This is clearly supported by an increase in cervical Aqp3 expression in late pregnant Rln−/− mice treated with rhRLX for 4 or 6 d. Intense immunostaining of AQP3 was observed in the cervix of Rln−/− mice treated with rhRLX compared with placebo controls and was equivalent to Rln+/+ mice. These findings indicate that rhRLX treatment increases AQP3 expression and restores the wild-type profile of AQP3 in the cervix of late pregnant Rln−/− mice. The stimulatory effects of relaxin on AQP3 may function to promote water uptake from the epithelium into the cervical stroma in late pregnancy and increase water content the cervix tissue. However, AQP3 is both a water- and glycerol-transporting protein that regulates cell membrane permeability. Loss of AQP3 in epidermal cells of the skin and corneal epithelium reduces cell migration and proliferation, in response to wounding or injury (36, 37). Given the established role of relaxin in modulating cervical cell proliferation during pregnancy (28, 38), we propose that this function of relaxin may also be mediated through an action on AQP3. Further studies are required to determine the mechanism by which AQP3 modulates cervical epithelial cell proliferation during pregnancy.
The intracellular mechanisms regulating AQP3 in the cervix are largely unknown, although several reports demonstrate an involvement of cAMP. Aqp3 is up-regulated in human amnion cells after exposure to a cAMP agonist and stimulator, Sp-diastereomer of cAMP and forskolin, respectively (39). In human colon epithelial cells, AQP3 gene and protein are also up-regulated by vasoactive intestinal polypeptide via a cAMP-dependent pathway to facilitate water transport in the gastrointestinal tract (40). Thus, AQP3 stimulation appears to be correlated with activation or increased cAMP activity. It is well established that relaxin increases cAMP levels in numerous target tissues (41). Therefore, we speculate that increased Aqp3 expression in the cervical epithelium of d 18.5 pregnant Rln+/+ mice and rhRLX-treated Rln−/− mice could be explained by a relaxin-mediated stimulation of cAMP.
Although there was no difference in Aqp5 mRNA expression between Rln+/+ and Rln−/− mice throughout pregnancy, relaxin administration to Rln−/− mice decreased Aqp5 expression, implying a negative regulation of AQP5 by relaxin. Compared with Rln+/+ mice, there was more intense AQP5 immunostaining in both apical and basal cells of the epithelium in Rln−/− mice. Increased Aqp5 gene expression in d 18.5 pregnant Rln−/− mice suggests that relaxin has an inhibitory effect on AQP5. This was confirmed in the treatment study; cervical Aqp5 expression was significantly decreased in Rln−/− mice after 6 d of rhRLX infusion, whereas immunoreactive AQP5 in the cervical epithelium was less intense compared with saline controls. These findings suggest that rhRLX treatment down-regulates Aqp5 expression and restricts AQP5 to the luminal apical region of the epithelium. In the mouse cervix, AQP5 is thought to increase in early pregnancy to permit the influx of water into the stroma and increase mucosal secretion in the epithelial cells (15). In late pregnancy, AQP5 decreases to prevent water efflux from the stroma. The inhibitory effects of relaxin on apical AQP5 may function to prevent water loss from the stroma to the lumen in late pregnancy and could partially contribute to the increase in tissue hydration by retaining fluid within the cervix tissue.
The observation that immunoreactive RXFP1 is localized to the stromal cells underlying the basal layer of epithelial cells of Rln+/+ mice is consistent with previous studies using RXFP1-specific β-galactosidase activity (26, 28) and in situ hybridization (42). However, we also found immunoreactive RXFP1 in the basal cell layer of the epithelium of late pregnant Rln+/+ mice. This epithelial staining was more intense in Rln−/− mice. The finding of RXFP1 in the cervical epithelium strengthens our hypothesis of a direct action of relaxin on HAS2, AQP3, and AQP5 expression, because all are localized predominantly to the epithelium. However, relaxin could also potentially regulate HAS2, AQP3, and AQP5 through paracrine cross talk signaling between stromal and basal layer epithelial cells. This concept was proposed by Yao et al. (28, 38), who demonstrated that relaxin promotes cell proliferation and inhibits apoptosis in the epithelium through activation of stromal RXFP1. Due to the close proximity of RXFP1 in the subepithelial stromal cells and the possible colocalization of RXFP1 and AQP3 in basal epithelial cells, it is possible that relaxin has a direct effect on AQP3. Because AQP5 and HAS2 are localized within the superficial terminally differentiated epithelium, the action of relaxin on these epithelial factors will likely involve paracrine signaling between stroma and epithelium, and downstream regulation via secondary intracellular pathways. Interestingly, HA is localized to ECM surrounding the stroma and to a lesser extent the epithelium (10), yet HAS2 is expressed in the epithelium. This is further evidence of cross talk between the epithelium and stroma to mediate changes in the composition and structure of the ECM in the cervix of pregnant mice.
In summary, we provide evidence that relaxin regulates HAS2 and specific AQPs in the cervix of late pregnant mice. The abnormal cervical ripening in Rln−/− mice results from a reduction in HA synthesis and changes in AQP expression in the cervix. These phenotypes can be reversed by relaxin treatment, which increases Has2 and Aqp3 expression and decreases Aqp5. Our findings suggest that a key role for relaxin in the cervix is to promote changes in the composition of GAGs and alter AQP expression to enhance water recruitment into the interfibrillar spaces. This increases spacing between collagen fibers, loosens the fiber bundles, and therefore, disrupts the dense organization of the ECM to enable cervical ripening.
Supplementary Material
Acknowledgments
We thank Dr. Dennis Stewart and Dr. Elaine Unemori (Corthera, Inc.) for providing the recombinant human H2 relaxin. We also thank Dr. Lenka Vodstrcil for her valuable contributions to this work and Tania Long and Darren Cipolla for their care of the mice and assistance with genotyping.
This work was funded by a University of Melbourne Research Collaboration grant (L.J.P.), National Institutes of Health Grants HL067937 (to K.P.C. and L.J.P.) and P0111149 (M.M.). Y.M.S. received a Department of Zoology Georgina Sweet Postgraduate scholarship and a Faculty of Science David Hay award (University of Melbourne).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- AQP
- Aquaporin
- 2−ΔCT
- comparative cycle threshold
- ECM
- extracellular matrix
- FACE
- fluorophore-assisted carbohydrate electrophoresis
- GAG
- glycosaminoglycan
- HA
- hyaluronan
- HAS
- HA synthase
- NP
- nonpregnant
- OVX
- ovariectomized
- QPCR
- quantitative PCR
- RFXP1
- relaxin family peptide receptor 1
- rhRLX
- human recombinant relaxin
- RT
- room temperature.
References
- 1. Shields SG, Ratcliffe SD, Fontaine P, Leeman L. 2007. Dystocia in nulliparous women. Am Fam Physician 75:1671–1678 [PubMed] [Google Scholar]
- 2. Leppert PC. 1995. Anatomy and physiology of cervical ripening. Clin Obstet Gynecol 38:267–279 [DOI] [PubMed] [Google Scholar]
- 3. Word RA, Li XH, Hnat M, Carrick K. 2007. Dynamics of cervical remodeling during pregnancy and parturition: mechanisms and current concepts. Semin Reprod Med 25:69–79 [DOI] [PubMed] [Google Scholar]
- 4. Timmons B, Akins M, Mahendroo M. 2010. Cervical remodeling during pregnancy and parturition. Trends Endocrinol Metab 21:353–361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Read CP, Word RA, Ruscheinsky MA, Timmons BC, Mahendroo MS. 2007. Cervical remodeling during pregnancy and parturition: molecular characterization of the softening phase in mice. Reproduction 134:327–340 [DOI] [PubMed] [Google Scholar]
- 6. Mahendroo M. 2012. Cervical remodeling in term and preterm birth: insights from an animal model. Reproduction 143:429–438 [DOI] [PubMed] [Google Scholar]
- 7. Downing SJ, Sherwood OD. 1986. The physiological role of relaxin in the pregnant rat. IV. The influence of relaxin on cervical collagen and glycosaminoglycans. Endocrinology 118:471–479 [DOI] [PubMed] [Google Scholar]
- 8. Rajabi MR, Solomon S, Poole AR. 1992. Activation of protein kinase C stimulates collagenase production by cultured cells of the cervix of the pregnant guinea pig. Am J Obstet Gynecol 167:194–200 [DOI] [PubMed] [Google Scholar]
- 9. El Maradny E, Kanayama N, Kobayashi H, Hossain B, Khatun S, Liping S, Kobayashi T, Terao T. 1997. The role of hyaluronic acid as a mediator and regulator of cervical ripening. Hum Reprod 12:1080–1088 [DOI] [PubMed] [Google Scholar]
- 10. Straach KJ, Shelton JM, Richardson JA, Hascall VC, Mahendroo MS. 2005. Regulation of hyaluronan expression during cervical ripening. Glycobiology 15:55–65 [DOI] [PubMed] [Google Scholar]
- 11. Ruscheinsky M, De la Motte C, Mahendroo M. 2008. Hyaluronan and its binding proteins during cervical ripening and parturition: dynamic changes in size, distribution and temporal sequence. Matrix Biol 27:487–497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Itano N, Kimata K. 1996. Expression cloning and molecular characterization of HAS protein, a eukaryotic hyaluronan synthase. J Biol Chem 271:9875–9878 [DOI] [PubMed] [Google Scholar]
- 13. Stern R. 2003. Devising a pathway for hyaluronan catabolism: are we there yet? Glycobiology 13:105R–115R [DOI] [PubMed] [Google Scholar]
- 14. Uchiyama T, Sakuta T, Kanayama T. 2005. Regulation of hyaluronan synthases in mouse uterine cervix. Biochem Biophys Res Commun 327:927–932 [DOI] [PubMed] [Google Scholar]
- 15. Anderson J, Brown N, Mahendroo MS, Reese J. 2006. Utilization of different aquaporin water channels in the mouse cervix during pregnancy and parturition and in models of preterm and delayed cervical ripening. Endocrinology 147:130–140 [DOI] [PubMed] [Google Scholar]
- 16. O'Byrne EM, Steinetz BG. 1976. Radioimmunoassay (RIA) of relaxin in sera of various species using an antiserum to porcine relaxin. Proc Soc Exp Biol Med 152:272–276 [DOI] [PubMed] [Google Scholar]
- 17. Mahendroo MS, Porter A, Russell DW, Word RA. 1999. The parturition defect in steroid 5α-reductase type 1 knockout mice is due to impaired cervical ripening. Mol Endocrinol 13:981–992 [DOI] [PubMed] [Google Scholar]
- 18. O'Day-Bowman MB, Winn RJ, Dziuk PJ, Lindley ER, Sherwood OD. 1991. Hormonal control of the cervix in pregnant gilts. III. Relaxin's influence on cervical biochemical properties in ovariectomized hormone-treated pregnant gilts. Endocrinology 129:1967–1976 [DOI] [PubMed] [Google Scholar]
- 19. Hwang JJ, Macinga D, Rorke EA. 1996. Relaxin modulates human cervical stromal cell activity. J Clin Endocrinol Metab 81:3379–3384 [DOI] [PubMed] [Google Scholar]
- 20. Parry LJ, Vodstrcil LA, Madden A, Amir SH, Baldwin K, Wlodek ME, Nicholas KR. 2009. Normal mammary gland growth and lactation capacity in pregnant relaxin-deficient mice. Reprod Fertil Dev 21:549–560 [DOI] [PubMed] [Google Scholar]
- 21. Zhao L, Samuel CS, Tregear GW, Beck F, Wintour EM. 2000. Collagen studies in late pregnant relaxin null mice. Biol Reprod 63:697–703 [DOI] [PubMed] [Google Scholar]
- 22. Parry LJ, McGuane JT, Gehring HM, Kostic IG, Siebel AL. 2005. Mechanisms of relaxin action in the reproductive tract: studies in the relaxin-deficient (Rlx−/−) mouse. Ann NY Acad Sci 1041:91–103 [DOI] [PubMed] [Google Scholar]
- 23. Hwang JJ, Sherwood OD. 1988. Monoclonal antibodies specific for rat relaxin. III. Passive immunization with monoclonal antibodies throughout the second half of pregnancy reduces cervical growth and extensibility in intact rats. Endocrinology 123:2486–2490 [DOI] [PubMed] [Google Scholar]
- 24. Lee AB, Hwang JJ, Haab LM, Fields PA, Sherwood OD. 1992. Monoclonal antibodies specific for rat relaxin. VI. Passive immunization with monoclonal antibodies throughout the second half of pregnancy disrupts histological changes associated with cervical softening at parturition in rats. Endocrinology 130:2386–2391 [DOI] [PubMed] [Google Scholar]
- 25. Hsu SY, Nakabayashi K, Nishi S, Kumagai J, Kudo M, Sherwood OD, Hsueh AJW. 2002. Activation of orphan receptors by the hormone relaxin. Science 295:671–674 [DOI] [PubMed] [Google Scholar]
- 26. Krajnc-Franken MA, van Disseldorp AJ, Koenders JE, Mosselman S, van Duin M, Gossen JA. 2004. Impaired nipple development and parturition in LGR7 knockout mice. Mol Cell Biol 24:687–696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kamat AA, Feng S, Bogatcheva NV, Truong A, Bishop CE, Agoulnik AI. 2004. Genetic targeting of relaxin and insulin-factor 3 receptors in mice. Endocrinology 145:4712–4720 [DOI] [PubMed] [Google Scholar]
- 28. Yao L, Agoulnik AI, Cooke PS, Meling DD, Sherwood OD. 2008. Relaxin acts on stromal cells to promote epithelial and stromal proliferation and inhibit apoptosis in the mouse cervix and vagina. Endocrinology 149:2072–2079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Zhao L, Roche PJ, Gunnersen JM, Hammond VE, Tregear GW, Wintour EM, Beck F. 1999. Mice without a functional relaxin gene are unable to deliver milk to their pups. Endocrinology 140:445–453 [DOI] [PubMed] [Google Scholar]
- 30. Steinetz BG, Beach VL, Kroc RL, Stasilli NR, Nussbaum RE, Nemith PJ, Dun RK. 1960. Bioassay of relaxin using a reference standard: a simple and reliable method utilizing direct measurement of interpubic ligament formation in mice. Endocrinology 67:102–115 [DOI] [PubMed] [Google Scholar]
- 31. Akgul Y, Holt R, Mummert M, Word A, Mahendroo M. 2012. Dynamic changes in cervical glycosaminoglycan composition during normal pregnancy and preterm birth. Endocrinology 153:3493–3503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Vodstrcil LA, Shynlova O, Verlander JW, Wlodek ME, Parry LJ. 2010. Decreased expression of the rat myometrial relaxin receptor (RXFP1) in late pregnancy is partially mediated by the presence of the conceptus. Biol Reprod 83:818–824 [DOI] [PubMed] [Google Scholar]
- 33. Ng SP, Steinetz BG, Lasano SG, Zelikoff JT. 2006. Hormonal changes accompanying cigarette smoke-induced preterm births in a mouse model. Exp Biol Med 231:1403–1409 [DOI] [PubMed] [Google Scholar]
- 34. Takemura M, Itoh H, Sagawa N, Yura S, Korita D, Kakui K, Kawamura M, Hirota N, Maeda H, Fujii S. 2005. Cyclic mechanical stretch augments hyaluronan production in cultured human uterine cervical fibroblast cells. Mol Hum Reprod 11:659–665 [DOI] [PubMed] [Google Scholar]
- 35. Mack JA, Feldman RJ, Itano N, Kimata K, Lauer M, Hascall VC, Maytin EV. 2012. Enhanced inflammation and accelerated wound closure following tetraphorbol ester application or full-thickness wounding in mice lacking hyaluronan synthases Has1 and Has3. J Invest Dermatol 132:198–207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hara-Chikuma M, Verkman AS. 2008. Aquaporin-3 facilitates epidermal cell migration and proliferation during wound healing. J Mol Med 86:221–231 [DOI] [PubMed] [Google Scholar]
- 37. Levin MH, Verkman AS. 2006. Aquaporin-3-dependent cell migration and proliferation during corneal re-epithelialization. Invest Ophthalmol Vis Sci 47:4365–4372 [DOI] [PubMed] [Google Scholar]
- 38. Yao L, Cooke PS, Meling DD, Shanks RD, Jameson JL, Sherwood OD. 2010. The effect of relaxin on cell proliferation in mouse cervix requires estrogen receptor α binding to estrogen response elements in stromal cells. Endocrinology 151:2811–2818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Wang S, Amidi F, Beall M, Gui L, Ross MG. 2006. Aquaporin 3 expression in human fetal membranes and its up-regulation by cyclic adenosine monophosphate in amnion epithelial cell culture. J Soc Gynecol Investig 13:181–185 [DOI] [PubMed] [Google Scholar]
- 40. Itoh A, Tsujikawa T, Fujiyama Y, Bamba T. 2003. Enhancement of aquaporin-3 by vasoactive intestinal polypeptide in a human colonic epithelial cell line. J Gastroenterol Hepatol 18:203–210 [DOI] [PubMed] [Google Scholar]
- 41. Halls ML, Bathgate RA, Summers RJ. 2007. Comparison of signaling pathways activated by the relaxin family peptide receptors, RXFP1 and RXFP2, using reporter genes. J Pharmacol Exp Ther 320:281–290 [DOI] [PubMed] [Google Scholar]
- 42. Parry LJ, Vodstrcil LA. 2007. Relaxin physiology in the female reproductive tract during pregnancy. Adv Exp Med Biol 612:34–48 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.