Abstract
Smoking is a major risk factor for diabetes and cardiovascular disease and may contribute to nonalcoholic fatty liver disease. We hypothesize that in the presence of nicotine, high-fat diet (HFD) causes more severe hepatic steatosis in obese mice. Adult C57BL6 male mice were fed a normal chow diet or HFD and received twice daily injections of nicotine (0.75 mg/kg body weight, ip) or saline for 10 wk. Light microscopic image analysis revealed significantly higher lipid accumulation in livers from mice on HFD plus nicotine (190 ± 19 μm2), compared with mice on HFD alone (28 ± 1.2 μm2). A significant reduction in the percent volume of endoplasmic reticulum (67.8%) and glycogen (49.2%) was also noted in hepatocytes from mice on HFD plus nicotine, compared with mice on HFD alone. The additive effects of nicotine on the severity of HFD-induced hepatic steatosis was associated with significantly greater oxidative stress, increased hepatic triglyceride levels, higher incidence of hepatocellular apoptosis, inactivation (dephosphorylation) of AMP-activated protein kinase, and activation of its downstream target acetyl-coenzyme A-carboxylase. Treatment with acipimox, an inhibitor of lipolysis, significantly reduced nicotine plus HFD-induced hepatic lipid accumulation. We conclude that: 1) greater oxidative stress coupled with inactivation of AMP-activated protein kinase mediate the additive effects of nicotine and HFD on hepatic steatosis in obese mice and 2) increased lipolysis is an important contributor to hepatic steatosis. We surmise that nicotine exposure is likely to exacerbate the metabolic abnormalities induced by high-fat intake in obese patients.
Cigarette smoking is the leading preventable cause of death and disability worldwide (1). It constitutes a major risk factor for cardiovascular disease, chronic obstructive pulmonary disease, and lung cancer (2–4). Mounting evidence supports the notion that smoking contributes to nonalcoholic fatty liver disease (NAFLD). NAFLD is the most common form of liver pathologies and includes the whole spectrum of fatty liver, ranging from simple steatosis to nonalcoholic steatohepatitis (NASH), which can progress to liver cirrhosis and hepatocellular carcinoma (5–7). Multiple logistic regression analysis from a recent retrospective follow-up study involving 2029 Japanese subjects over a 10-yr period demonstrated that cigarette smoking is an independent risk factor for NAFLD (adjusted odd ratio 1.91; 95% confidence interval 1.34–2.72) (8). A statistically significant (P < 0.0001) association between smoking history and severity of liver fibrosis has also been demonstrated in a large multicenter cohort of 1091 subjects with biopsy-proven NAFLD (9). Of further importance, the health risk associated with smoking, whether passive or active, is exaggerated by obesity, and smoking and obesity are the leading causes of morbidity and mortality worldwide (10, 11). The life expectancy of an obese smoker is 13 yr less than that of a normal-weight nonsmoker (10). Furthermore, smoking lowers the body weight and body mass index (BMI), which make many people reluctant to quit smoking (10).
In the United States, 72% of the adult male population is overweight or obese with 11% of which having a BMI of 35 kg/m2 and 4% with a BMI of at least 40 kg/m2 (12). Obese men are at a higher risk to develop atherosclerosis, coronary heart disease, diabetes, hypertension, dyslipidemia, and NAFLD (13, 14). NAFLD, in turn, is an independent risk factor of atherosclerosis and cardiovascular disease (15, 16). Currently, 34% of the general population and over 75% of the obese and extremely obese individuals are estimated to have hepatic steatosis (17). Hispanics have the highest prevalence of hepatic steatosis followed by Caucasians and then African-Americans (18).
In a recent study (19), we demonstrated that twice daily ip injections of nicotine [0.75 mg/kg body weight (BW)] blunted weight gain in mice fed with either a high-fat diet (HFD) or normal chow diet (NCD), although this effect was significantly greater in mice on a HFD relative to mice on a NCD. Nicotine treatment also led to a reduction in body fat on mice on a HFD as determined by both dual-energy x-ray absorption (DXA) and computed tomography scans. In that study, we further noted that the effect of nicotine on weight loss in mice on a HFD was completely blocked by mecamylamine, a nonselective nicotinic acetylcholine receptor antagonist, suggesting a direct role of nicotine in preventing HFD-induced weight gain and abdominal fat accumulation in mice (19). This, at first glance, suggests that nicotine may prevent HFD-induced obesity. However, adipose tissue has the unique function of storing triglycerides in lipid droplets and upon lipolysis, to provide free fatty acid (FFA) to other organs during time of energy shortage (20). In obesity and other conditions where cellular lipid homeostasis is perturbed, lipolysis can contribute to ectopic lipid accumulation (21). Accordingly, increased abdominal lipolysis triggered by combined treatment with nicotine and a HFD would be expected to result in ectopic lipid accumulation in tissues such as liver and muscle. Indeed, recent animal studies, using first- or second-hand smoke, have shown that nicotine further worsens HFD-induced hepatic steatosis in genetic models of obesity and NAFLD, such as Zucker rats or Apolipoprotein B transgenic mice (22, 23).
In the current study, we tested the emerging hypothesis that nicotine plus a HFD exacerbates HFD-induced hepatic steatosis in part because of increasing lipolysis. To this end, we fed C57BL/6J mice a HFD deriving 60% of calories from fat (19), a commonly used model of diet-induced obesity (24–27). Under these conditions, mice develop visceral adiposity, hyperglycemia, insulin and leptin resistance, as well as hepatic steatosis (25, 26).
Materials and Methods
Animal
Male 10-wk-old C57BL/6 mice weighing 22–24 g obtained from Taconic Farms (Germantown, NY) were used for all experiments. Mice were housed (two to four per cage) under controlled temperature (22 C) and photoperiod (12-h light, 12-h dark cycle) with free access to water and food. Mice were fed either a NCD with 5% fat (2.03 kcal/g; laboratory rodent diet no. 5001; Lab Diet, Richmond, IN) or HFD with 60% of calories derived from fat consisting of 26.2% protein, 26.3% carbohydrate, and 34.9% fat (5.24 kcal/g; D12492; Research Diets, New Brunswick, NJ) for 10 wk. Animal handling and experimentation were in accordance with the recommendation of the American Veterinary Medical Association and were approved by the Charles R. Drew University School of Medicine and Science Institutional Animal Care and Use Committee.
Mice on either diet received twice daily ip injections of nicotine (0.75 mg/kg BW) or saline for 10 wk. (−)Nicotine liquid was purchased from Sigma Life Science (St. Louis, MO) and was covered with aluminum foil during storage to prevent light exposure. To determine whether nicotine-induced abdominal fat lipolysis mediated the ectopic fat deposition in the liver, additional groups of mice on a HFD were given nicotine or saline in the presence or absence of acipimox (0.05% in the drinking water) for 12 wk.
Animals were weighed daily, and the weight change from baseline was calculated. The amount of food consumed per mouse was also determined daily. Before killing, mice underwent DXA scanning as described previously (19). Mice were fasted overnight before euthanasia with a lethal injection of sodium pentobarbital (200 mg/kg BW). Livers were removed and weighed. Portions of liver were snap frozen in liquid N2 and stored frozen for subsequent measurements of alanine aminotransferase (ALT), triglycerides, oxidative stress, kinase activation, and changes in protein expression. The remaining portions of liver were either fixed in 2.5% glutaraldehyde for high-resolution light and electron microscopy or 4% paraformaldehyde for routine histological and immunohistochemical or immunofluorescence studies.
Measurements of hepatic ALT and triglyceride levels
ELISA kit (Uscn Life Science, Inc., Wuhan, China) was used for quantitative determination of aminotransferase (ALT) levels in liver lysates as per the manufacturer's instruction. Hepatic triglyceride levels were measured by using Abcam's triglyceride quantitation kit according to manufacturer's protocol (Abcam, Cambridge, MA).
Measurements of oxidative stress
Hepatic reduced glutathione (GSH) to oxidized GSH (GSSG) ratio was measured using a commercial kit (BIOXYTECH GSH/GSSG-412 assay kit; OXISResearch, a division of Oxis Health Product, Inc., Portland, OR), as described previously (28–30). The GSH to GSSG ratio is inversely related to reactive oxygen species (ROS) levels. Further evaluation of oxidative stress was achieved by measuring 4-hydroxy-trans-2-nonenal (4-HNE) levels, a biomarker of oxidative stress (31, 32), using an ELISA kit as described previously (29).
Assessment of apoptosis
In situ detection of cells with DNA strand breaks was performed in paraformaldehyde-fixed, paraffin-embedded liver sections by the terminal deoxynucleotidyl transferase 2′-deoxyuridine, 5′-triphosphate nick end labeling (TUNEL) technique (28, 30, 33, 34) using an ApopTag-peroxidase kit (Chemicon International, Inc., San Francisco, CA). Enumeration of TUNEL-positive nuclei was carried out in liver sections using an American Optical Microscope with a ×40 objective and a pair of ×10 eyepieces (Scientific Instruments, Buffalo, NY). Methyl green was used as a counterstain to detect nonapoptotic nuclei. A square grid fitted within one eyepiece provided a reference of 62,500 μm2. The rate of hepatocellular apoptosis was expressed as the percentage of the TUNEL-positive apoptotic nuclei per total nuclei (apoptotic plus nonapoptotic) present within the reference area (30).
Liver pathology
Liver pathology was evaluated using conventional histological analysis on hematoxylin and eosin (H&E)-stained sections. Further evaluation of pathology was achieved by high-resolution light microscopy using glutaraldehyde-fixed, osmium tetroxide-postfixed, epoxy-embedded, and toluidine blue-stained sections (30, 35) and electron microscopy. For electron microscopic studies, thin sections from selected tissue blocks were sectioned with an LKB ultramichrotome, stained with uranyl acetate, and examined with a Hitachi 600 electron microscope (Hitachi, Indianapolis, IN). An observer who was unaware of the treatment assignment performed and analyzed the electron micrographs. From each treatment group, 80 micrographs (20 micrographs/mouse) were selected for ultrastructural analysis. The point-counting method (36, 37) was used to estimate the volume density (Vv) (the volume of a given cellular component per unit cell volume) of lipid droplets and various organelles by superimposing a transparent overlay bearing a double-lattice grid on electron micrographs of hepatocytes. The Vv was obtained by dividing the points on lipid droplets or mitochondria by the total number of points counted over the hepatocyte. Values were expressed as percentages of the hepatocyte volume (Vv%), obtained by multiplying Vvs by 100.
Immunofluorescence analyses
Activation of c-Jun-NH2-terminal kinase (JNK) in liver cells undergoing apoptosis was detected by confocal microscopy using double immunostaining for the phospho-JNK (red) and DNA fragmentation (green) as previously described (30, 34, 38). In situ detection of cells with DNA strand breaks was performed in paraformaldehyde-fixed, paraffin-embedded liver sections using an ApopTag Fluorescein kit (Chemicon International, Inc.). In brief, after fluorescein staining, slides were then incubated in a humidified chamber for overnight at 4 C with a rabbit polyclonal phospho-JNK, which detects JNK only when phosphorylated at threonine 183 and tyrosine 185 (1:100) from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA) followed by goat-antirabbit Texas Red-labeled secondary antibody for 45 min at room temperature, washed, and mounted in ProLong Antifade (Molecular Probes, Eugene, OR). For negative controls, sections were treated only with secondary antibody, and no signals were detected.
Western blotting
Western blotting was performed using liver lysates as described previously (28, 30, 33, 34). In brief, proteins (50–80 μg) were separated on a 4–12% SDS-PAGE with 2-(N-morpholine) ethane sulfonic acid or 3 (N-morpholino) propane sulfonic acid buffer purchased from Invitrogen (Carlsbad, CA) at 200 V. Gel was transferred on an Immun-Blot polyvinylidene fluoride membrane (Bio-Rad, Hercules, CA) overnight at 4 C. Membranes were blocked in blocking solution (0.3% Tween 20 in Tris-buffered saline and 10% nonfat dry milk) for 1 h at room temperature and then probed using rabbit polyclonal phospho-AMP-activated protein kinase (AMPK) (1:300), total-AMPK (1:300), phospho-acetyl-coenzyme A-carboxylase (ACC) (1:200), phospho-JNK (1:400), total-JNK (1:400), cleaved caspase 9 (1:100), and cleaved caspase 3 (1:200) antibodies from Cell Signaling Technology (Beverly, MA) and mouse monoclonal TNF-α (1:100) antibody from Abcam (San Francisco, CA) for 1 h at room temperature or overnight at 4 C with constant shaking. After three 10-min washes in Tris-buffered saline with Tween 20 buffer, membranes were then incubated in antirabbit or antimouse IgG secondary antibody (Amersham Biosciences, Piscataway, NJ) at a 1:2000 dilution. All antibodies were diluted in blocking buffer. For immunodetection, membranes were washed three times in Tris-buffered saline with Tween 20 wash buffer, incubated with enhanced chemiluminescence solutions per the manufacturer's specifications (Amersham Biosciences), and exposed to Hyperfilm enhanced chemiluminescence. The membranes were stripped and reprobed with a rabbit polyclonal glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (1:2000) for normalization of the loading. Band intensities were determined using Quantity One software from Bio-Rad.
Statistical analysis
Descriptive statistics was used to depict the data and to examine the distribution of the variables. Data were presented as mean ± sem. We used ANOVA to assess the statistically significant difference among various treatment groups. If overall ANOVA revealed significant differences, post hoc (pairwise) comparisons were performed using Tukey's tests. Differences were considered significant if P < 0.05. Statistical analyses were performed using the SigmaStat 2.0 Program (Jandel Corp., San Rafael, CA).
Results
Effects of nicotine plus HFD on body weight, fat mass, oxidative stress, and hepatic ALT and triglyceride levels
As expected from our previous studies (19), nicotine significantly (P < 0.01) reduced (by ∼20%) body weight in mice on a HFD relative to mice fed HFD alone. DXA scan showed a significant (P < 0.001) decrease in percent fat after combined treatments with nicotine and HFD (16.1 ± 1.2%) compared with mice on a HFD alone (8.5 ± 2.1%).
Effects of nicotine plus HFD on hepatic ALT and triglyceride levels, and GSH to GSSG ratio, are summarized in Table 1. No significant changes in hepatic ALT levels were noted among various treatment groups. However, compared with mice fed with NCD in the presence or absence of nicotine, mice on a HFD exhibited a significant (P < 0.05) increase in hepatic triglyceride levels. A further significant (P < 0.05) increase in hepatic triglyceride levels was noted after combined treatments with nicotine and HFD. To determine whether nicotine-induced fat lipolysis contributes to higher triglyceride levels in the liver, mice on HFD plus nicotine were treated with either acipimox (0.05% in drinking water) or water alone for 12 wk. Treatment with acipimox fully attenuated nicotine plus HFD-induced increase in hepatic triglyceride levels. Addition of acipimox to nicotine-treated mice on a HFD had no discernible effect on hepatic ALT levels when compared with mice treated with nicotine plus HFD. To test whether nicotine plus HFD causes greater oxidative stress, we measured hepatic GSH to GSSG ratio, which is inversely related to ROS levels (Table 1). When tested individually, nicotine and HFD induced a comparably significant rise (P < 0.01) in oxidative stress relative to NCD-fed mice treated with saline. When combined, nicotine plus HFD caused a further increase (P < 0.01) in oxidative stress. The combined effect of nicotine and HFD on oxidative stress was supported by a rise in 4-HNE, a lipid peroxidation marker (31, 32). Although HFD alone elevated 4-HNE levels (30 ± 1.5 vs. 20 ± 0.2 μg/mg protein in NCD-fed mice; P < 0.05), HFD plus nicotine induced a further significant increase in 4-HNE levels (42 ± 0.7 μg/mg protein; P < 0.05).
Table 1.
Hepatic ALT and triglyceride levels and GSH to GSSG ratio levels
| Treatment | ALT (ng/mg protein) | Triglyceride (nmol/mg protein) | GSH to GSSG ratio |
|---|---|---|---|
| NCD + saline | 187 ± 9a | 217 ± 21a | 63.5 ± 4.7a |
| NCD + nicotine | 265 ± 21a | 177 ± 5a | 12.5 ± 1.3b |
| HFD + saline | 265 ± 29a | 372 ± 35b | 14.3 ± 1.6b |
| HFD + nicotine | 258 ± 18a | 502 ± 42c | 9.1 ± 1.7c |
| HFD + nicotine + acipimox | 260 ± 50a | 390 ± 18b | ND |
Values are given as mean ± sem (n = 4–6). In each column, means with unlike superscripts are significantly (P < 0.05) different. ND, Not done.
Nicotine exacerbates HFD-induced hepatic steatosis in obese mice
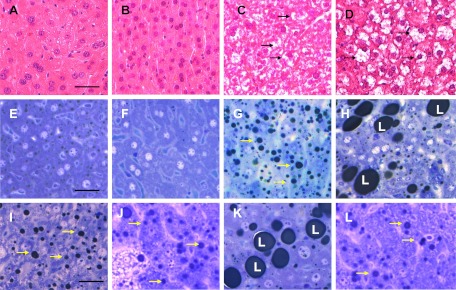
H&E-stained liver sections from mice fed NCD in the presence or absence of nicotine exhibited normal morphology (Fig. 1, A and B). Compared with mice on HFD plus saline, where a modest increase in lipid accumulation was detected (Fig. 1C), combined treatment with HFD and nicotine led to a marked increase in lipid accumulation in the liver (Fig. 1D). These results were confirmed by high-resolution light microscopy; using glutaraldehyde-fixed, osmium tetroxide-postfixed, epoxy-embedded, and toluidine blue-stained liver sections (Fig. 1, E–H). Glutaraldehyde fixation followed by osmium postfixation staining allows retention of fat that would have been normally washed out during tissue processing. Mice fed NCD in the absence (Fig. 1E) or presence of nicotine (Fig. 1F) exhibited normal histology with little or no lipid accumulation. In contrast, mice fed HFD showed accumulation of many smaller lipid droplets (Fig. 1G). Nicotine plus HFD, however, caused a striking increase in larger lipid droplets (Fig. 1H) compared with those from mice fed HFD and saline (Fig. 1G). Within the study paradigm, we did not find progression of liver pathology, including infiltration of inflammatory cells, inflammatory foci, and portal/lobular fibrosis beyond steatosis in nicotine-treated mice on a HFD.
Fig. 1.
Representative H&E-stained liver sections from mice fed with NCD without (A) or with (B) nicotine exhibit normal histological appearance. Compared with a mouse on a HFD, where a modest increase in lipid accumulation (arrow) is detected (C), combined treatment with nicotine and HFD causes a marked increase in lipid accumulation in the liver (D). E–H, Representative light microscopic images of glutaraldehyde-fixed, osmium tetroxide-postfixed, epoxy-embedded, and toluidine blue-stained liver sections from different treatment groups show nicotine plus a HFD (H) causes a striking increase in lipid accumulation of varying sizes in hepatocytes compared with those from mice on a HFD alone (G, arrow). Mice fed with NCD with (F) or without nicotine (E) have normal liver morphology. I–L, Lipid accumulation was similar between HFD (I) and HFD plus acipimox (J) groups. Acipimox treatment fully attenuated HFD plus nicotine-induced exacerbated hepatic steatosis (K and L). Scale bar, 25 μm. Data are representative of four mice in each group.
We next assessed the effect of acipimox on HFD plus nicotine-induced ectopic fat deposition in the liver. Fat accumulation was similar between HFD and saline (Fig. 1I) and acipimox plus HFD (Fig. 1J). As expected, nicotine plus HFD resulted in a marked increase in lipid accumulation compared with those from mice on HFD alone (Fig. 1K). Treatment with acipimox completely blocked the HFD plus nicotine-induced fat accumulation (Fig. 1L) to levels seen in mice on HFD and saline (Fig. 1I).
Quantitative image analysis further revealed a significant (P < 0.001) increase in intracellular lipid content after combined treatment with HFD and nicotine (190 ± 19 μm2) compared with mice on a HFD and saline (28 ± 1.2 μm2). Treatment with acipimox fully attenuated HFD plus nicotine-induced intracellular fat accumulation to levels seen in mice on a HFD and saline (30 ± 1.3 μm2). Acipimox plus HFD yielded similar fat accumulation as HFD plus saline (30 ± 1.3 vs. 28 ± 1.3 μm2).
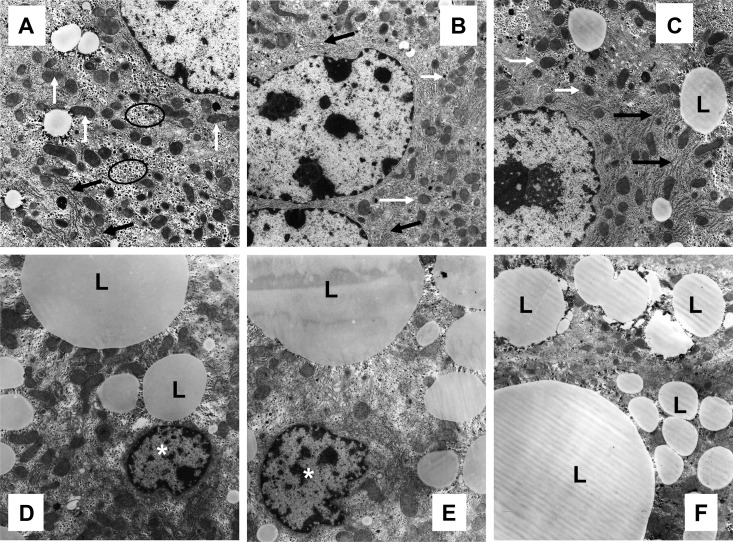
To further substantiate the light microscopic findings, we performed electron microscopic analysis. Hepatocytes from NCD-fed mice exhibited normal ultrastructure, characterized by numerous mitochondria, smooth and rough endoplasmic reticulum (ER), and glycogen deposition with minimal lipid accumulation (Fig. 2A). Nicotine-treated NCD mice had normal hepatocyte ultrastructure (Fig. 2B) similar to that seen in mice on a NCD plus saline (Fig. 2A). Hepatocytes from HFD-fed mice plus saline exhibited a modest lipid accumulation (Fig. 2C) compared with those from mice fed NCD plus saline in the absence (Fig. 2A) or presence of nicotine (Fig. 2B). Notably, addition of nicotine to HFD led to a striking increase in lipid accumulation of varying sizes in hepatocytes along with a decrease in the amount of ER and glycogen (Fig. 2, D--F) compared with those from mice fed NCD in the absence (Fig. 2A) or presence of nicotine (Fig. 2B) or to mice fed HFD alone (Fig. 2C). A distinct increase in the amount of hepatocyte apoptosis, characterized by nuclear condensation and fragmentation, was also noted after combined treatment with HFD and nicotine (Fig. 2, D and E).
Fig. 2.
Transmission electron micrographs of hepatocytes from mice fed with NCD without (A) or with nicotine (B) exhibit normal ultrastructure, characterized by numerous mitochondria (white arrow), ER (black arrow), and glycogen deposition (oval outline). A representative hepatocyte from a mouse on a HFD shows a modest increase in lipid (L) accumulation (C), compared with those from mice on a NCD (A) or nicotine plus NCD (B). Nicotine plus HFD caused a striking increase in lipid (L) accumulation of varying sizes in hepatocytes (D–F) compared with those from mice on a HFD alone (C). Also shown in D and E are two apoptotic hepatocyte nuclei (asterisk). Scale bar, 1 μm. Data are representative of four mice in each group.
Ultrastructural analysis of volumetric compositions of lipid droplets and various organelles in hepatocytes from mice in various treatment groups is summarized in Table 2. A significant (P < 0.01) increase (1.7-fold) in the Vv% of lipid droplets was noted after combined treatment with HFD and nicotine compared with mice on HFD and saline. However, the Vv% of ER and glycogen were significantly (P < 0.01) decreased in nicotine-treated HFD-fed mice compared with mice fed HFD alone. No significant changes in the Vv% of lipid droplets, ER, and glycogen were noted between nicotine- and saline-treated NCD-fed mice. There were no differences in Vv% of mitochondria among various treatment groups.
Table 2.
Morphometric data on volumetric composition (Vv%) of lipid droplets and various organelles in hepatocytes from mice on a NCD or HFD in the absence or presence of nicotine
| Treatment | Lipid droplets | Mitochondria | ER | Glycogen |
|---|---|---|---|---|
| NCD + saline | 5.6 ± 0.8a | 12.9 ± 1.5a | 7.1 ± 0.54a | 8.5 ± 1.0a |
| NCD + nicotine | 3.3 ± 0.3a | 15.1 ± 1.4a | 7.4 ± 0.6a | 9.6 ± 0.8a |
| HFD + saline | 9.5 ± 2.4b | 12.9 ± 1.5a | 6.2 ± 0.1a | 6.5 ± 1.2a |
| HFD + nicotine | 16.2 ± 2.7c | 11.3 ± 0.1a | 2.0 ± 0.3b | 3.3 ± 0.2b |
Values are given as mean ± sem of four mice per group. In each column, means with unlike superscripts are significantly (P < 0.01) different.
Nicotine plus HFD inactivates AMPK
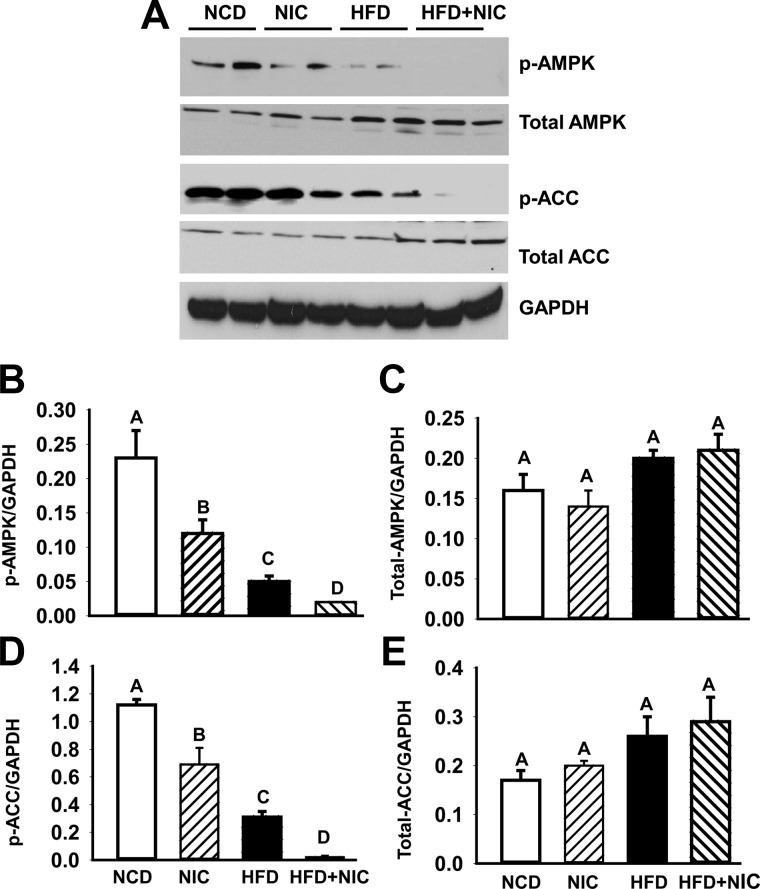
AMPK, a central regulator of cellular energy homeostasis, plays an important role in fatty acid metabolism through its ability to regulate key fatty acid biosynthetic pathway (6, 39). To investigate whether exacerbation of hepatic steatosis in HFD-fed mice by nicotine is associated with inactivation of APMK, we carried out Western blot analysis to test the phosphorylation state of AMPK in liver lysates (Fig. 3A). Compared with mice fed NCD with or without nicotine, HFD-fed mice exhibited a significant decrease in the phosphorylation of AMPK, as shown by densitometric analysis of phospho-AMPK (Fig. 3B). Combining nicotine with HFD completely dephosphorylated (inhibited) AMPK, as shown by the significant additional approximately 60% reduction in phospho-AMPK relative to HFD-fed mice (Fig. 3B). Consistently, HFD alone caused an approximately 72% decrease in the phosphorylation of ACC (Fig. 3D), a target of AMPK and a rate-limiting enzyme in fatty acid biosynthesis (6, 39), leading to its activation. Combined with nicotine, HFD caused an additional approximately 94% decrease in phospho-ACC (Fig. 3D). There was no significant (P > 0.05) difference in total-AMPK (Fig. 3C) or total-ACC (Fig. 3E) levels among various groups.
Fig. 3.
A, Western blot analysis shows that mice fed with HFD have decreased hepatic phospho-AMPK (p-AMPK) and phospho-ACC (p-ACC) levels compared with mice on a NCD with or without nicotine treatment. Addition of nicotine to HFD leads to complete dephosphorylation of AMPK and ACC. The gels are representative of two animals in each group from one of three separate experiments. GAPDH in the immunoblot is shown as a loading control. B–E, Quantitation of band intensities. Data for p-AMPK (B), total-AMPK (C), p-ACC (D), and total-ACC (E) were normalized to GAPDH. Values are means ± sem of four animals per group. Means with unlike superscripts are significantly (P < 0.05) different.
HFD in combination with nicotine induces hepatocellular apoptosis
Given that hepatocyte apoptosis plays a pivotal role in the pathogenesis of NAFLD (7, 40, 41), we next analyzed the incidence of hepatocellular apoptosis, expressed as the percentage of TUNEL-positive nuclei per total nuclei (apoptotic plus nonapoptotic nuclei). Nicotine treatment did not alter the incidence of hepatocellular apoptosis in NCD-fed mice (1.0 ± 0.3 vs. 0.9 ± 0.3; P > 0.05). In contrast, HFD induced apoptosis by approximately 3-fold (2.7 ± 0.2 vs. 0.9 ± 0.3; P < 0.05). Combined with nicotine, HFD induced a further significant increase (10.1 ± 0.1; P < 0.05) in the incidence of hepatocellular apoptosis.
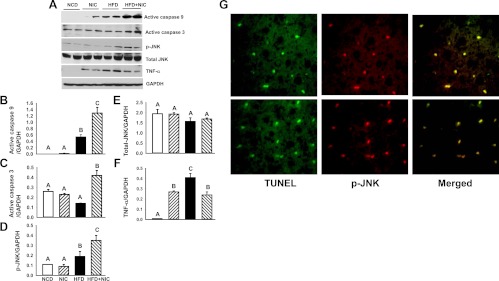
To further examine hepatocellular apoptosis, we carried out Western blot analysis of the initiator caspase 9 and the executioner caspase 3 (Fig. 4A). Densitometric analysis revealed that nicotine in NCD-treated mice did not induce activation of hepatic caspase 9 or caspase 3. In contrast, HFD induced a significant (P < 0.05) increase in active caspase 9 expression (Fig. 4B). Combined with nicotine, HFD caused an additional increase (by ∼2.4-fold) in active caspase 9 expression (Fig. 4B). Although HFD plus saline did not affect significantly active caspase 3 content, it induced its protein content by approximately 3-fold when combined with nicotine treatment in HFD-treated mice (Fig. 4C). Consistent with implication of the JNK signaling pathways in hepatocyte apoptosis in murine models of NAFLD (7, 30, 42), we observed induction of phospho-JNK content by HFD plus nicotine, in parallel to changes in caspase 9 and caspase 3 activation (Fig. 4, A and D). There was no significant (P > 0.05) difference in total-JNK levels (Fig. 4, A and E) among various groups. Furthermore, double immunofluorescence staining revealed costaining of phospho-JNK and TUNEL (Fig. 4G), indicating activation of JNK signaling in cells undergoing apoptosis. Consistent with TNF-α playing an important role in the pathogenesis of NAFLD (7, 42, 43) and in JNK activation, Western blot analysis indicated induction of hepatic protein content of this proinflammatory cytokine by all treatment groups with a more marked effect by HFD alone (Fig. 4, A and F). Together, the data suggest an induction in JNK-mediated apoptotic pathway.
Fig. 4.
A, Western blot analysis shows increased expression of hepatic active caspase 9, phospho-JNK (p-JNK), and TNF-α in mice on a HFD. Addition of nicotine to HFD further results in a decrease in TNF-α expression but in an increase in the expression of hepatic p-JNK, active caspase 9, and active caspase 3. The gels are representative of two animals in each group from one of three separate experiments. GAPDH in the immunoblot is shown as a loading control. B–F, Quantitation of band intensities. Data for active caspase 9 (B), active caspase 3 (C), p-JNK (D), total-JNK (E), and TNF-α (F) were normalized to GAPDH. Values are means ± sem of four animals per group. Means with unlike superscripts are significantly (P < 0.05) different. G, Confocal images of liver cells show TUNEL (green), phospho-JNK (red), and colocalization of TUNEL and phospho-JNK (yellow) in HFD plus nicotine-induced hepatocellular apoptosis. Scale bar, 25 μm.
Discussion
Steatosis can prime the liver to develop more progressive liver pathologies in response to additional metabolic and/or environmental stressors. Mechanistically, this is commonly mediated by the prevalent “two-hit” hypothesis that implies accumulation of triglycerides in hepatocytes (steatosis) in the first hit, followed by triggering progression to inflammation, oxidative stress, and apoptosis in the second hit (7, 41). In more advanced cases, fibrosis is also exacerbated, leading to the progressive form of NAFLD, known as NASH. Environmental stressors that can trigger progression to the second phase are HFD, cigarette smoke, drugs, or pollutants. Metabolic stressors include obesity, diabetes, hypertension, hypertriglyceridemia, and hypercholesterolemia. Nonetheless, the molecular underpinning of steatosis is not well understood. Oxidative stress coupled with hepatocyte apoptosis is believed to play a pivotal role in pathogenesis of NAFLD (7, 44). In fact, emerging data suggest that hepatocyte apoptosis plays a key component in the progression of simple steatosis to NASH (7, 44).
In this study, we used the model of diet-induced obesity in C57BL6J mice to study the underlying mechanisms underlying the detrimental effects of two common lifestyle factors, such as nicotine and HFD, in the development of fatty liver disease. We elected to use a single drug (nicotine) as opposed to first- or second-hand smoke to eliminate the confounding effects of other components involved in cigarette smoking. We also purposely used shorter (10 wk) duration to examine the synergistic effects of these two insults in the initiation of NAFLD, because a longer exposure to HFD alone results in extensive steatosis (45) and systemic inflammation (46).
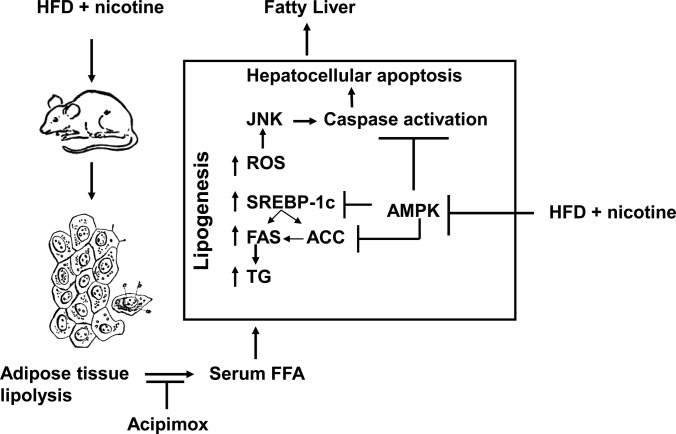
The first report of hepatic steatosis after nicotine treatment was by Valenca et al. (47). In the current study, we found that nicotine alone did not lead to hepatic steatosis but that nicotine caused steatosis only when combined with HFD. The results of the present study confirm and extend earlier studies involving first- or second-hand smoke and genetic models of NAFLD such as Zucker rats (22) or apolipoprotein B transgenic mice (23) by demonstrating that nicotine exacerbates HFD-induced hepatic steatosis. This study is unique in showing that nicotine leads to hepatic steatosis in a diet-induced obesity model and also in providing first evidence that abdominal lipolysis is an important contributor to nicotine-induced exacerbation of HFD-induced hepatic steatosis. Moreover, we found that this additive effect of nicotine is associated with greater oxidative stress, increased hepatic triglyceride levels, higher incidence of hepatocellular apoptosis, inactivation (dephosphorylation) of AMPK, and activation of its downstream target ACC, leading to increased hepatic lipogenesis, as depicted in the model (Fig. 5).
Fig. 5.
Model illustrating how nicotine plus a HFD could exacerbate HFD-induced hepatic steatosis in obese mice. Nicotine plus a HFD promotes abdominal lipolysis, resulting in FFA release from adipose tissue into the circulation, thereby contributing to the buildup of lipids as triglyceride (TG) in the liver. In addition, nicotine plus a HFD may also promote de novo lipogenesis through inactivation of AMPK and activation of its downstream target ACC, leading to the development of hepatic steatosis. Inactivation of AMPK can also stimulate lipogenesis through up-regulation of key genes in the lipogenic pathway such as fatty acid synthase (FAS) and ACC by activating the transcription factor sterol regulatory element binding protein-1c (SREBP-1c). Intrahepatic lipid accumulation can also trigger hepatocellular apoptosis through generation of oxidative stress coupled with activation of JNK-mediated apoptotic signaling. AMPK inactivation could further sensitize liver cells to nicotine plus HFD-induced apoptosis.
Elevated ROS levels trigger a state of oxidative stress, a critical step in the pathogenesis of NAFLD (7, 41, 44). This study showed that nicotine initiated oxidative stress, with a higher extent when combined with HFD than alone. This is consistent with previous data showing that both HFD and nicotine are capable of generating oxidative stress in various tissues, including liver (7, 48–52). Thus, it is likely that generation of severe oxidative stress could aggravate liver lesions in the combined treatment group through the formation of reactive and biologically active lipid peroxidation products such as 4-HNE (7, 41, 44, 53). The observed increase in hepatic 4-HNE levels in nicotine-treated mice on a HFD is consistent with this view. It is pertinent to note that in spite of a significant increase in oxidative stress, there was little or no liver damage in mice treated with nicotine or HFD alone. Therefore, it is possible that the oxidative stress generated by either individual treatment is not severe enough to induce liver damage. This is consistent with earlier reports indicating that the cellular responses of oxidative stress varies depending on the cell type, the levels of ROS achieved, and the duration of exposure (52). However, we cannot rule out the possibility that in addition to high levels of oxidative stress, other factors may have also contributed to hepatic steatosis caused by the combined treatment with nicotine and HFD.
AMPK is a central regulator of lipid homeostasis and mediates suppression of lipogenic gene expression, such as ACC and fatty acid synthase, through inhibition of sterol regulatory element binding protein-1c (39, 54). ACC is the rate-determining enzyme for the synthesis malonyl-coenzyme A, both a critical substrate for fatty acid biosynthesis and a potent inhibitor of fatty acid oxidation (39). AMPK can also phosphorylate and inactivate ACC leading to inhibition of de novo fatty acid and cholesterol synthesis (39). Consistent with a pivotal role for AMPK in lipid homeostasis, we here show that the additive effects of nicotine and HFD on the severity of hepatic steatosis were associated with complete inhibition of AMPK. The net effect of AMPK inactivation is decreased phosphorylation and activation of ACC, leading to de novo fatty acid and cholesterol synthesis in the liver. These results are consistent with our earlier reports as well as others linking inhibition of AMPK with NAFLD. For example, ApoE−/− mice fed HFD develop hepatic steatosis through inhibition of AMPK (30). Mice fed HFD for long term (8 months) also develop hepatic steatosis in association with inhibition of AMPK coupled with activation of ACC that was attenuated by betaine, a naturally occurring metabolite of choline and an essential biochemical component of the methionine-homocysteine cycle (55). Our findings are also in accord to a previous report indicating that second-hand smoke stimulates lipid accumulation in the liver of ApoB100 transgenic mice through inactivation of AMPK and activation of ACC (23). Collectively, nicotine in combination with HFD triggers hepatic lipid accumulation through inactivation of AMPK.
Oxidative stress has been implicated in apoptotic signaling in various cell types, including hepatocytes (7, 41, 53). One possible mechanism by which oxidative stress can induce hepatocyte apoptosis in response to HFD plus nicotine is through stimulation of JNK signaling, resulting in the activation of the mitochondria-dependent intrinsic pathway signaling (7, 30, 56, 57). JNK is activated in various animal models of obesity and also in patients with NASH, and its deletion results in attenuation of fatty liver (7). Consistent with a role of JNK in hepatocellular apoptosis, in the present study, we found a greater degree of JNK activation in the combined treatment group compared with that of mice on a HFD. Costaining for TUNEL and phospho-JNK further confirmed activation of JNK only in those cells undergoing apoptosis. Activation of JNK is further associated with stimulation of the mitochondria-dependent apoptotic pathway characterized by activation of the initiator caspase 9, the key caspase involved in the mitochondria-dependent pathway, and the executioner caspase 3. Of further interest, there have been recent studies implicating that activation of AMPK can attenuate cell death in different cell types triggered by various insults (58–61). Thus, it is possible that complete inactivation of AMPK could increase the susceptibility of hepatocytes to JNK-induced apoptosis in the combined treatment group.
Steatosis, the hallmark feature of NAFLD, can occur as a result of excess FFA delivery from lipolysis of adipose tissue, increased de novo lipogenesis, and reduced fat oxidation and export in the form of very low-density lipoprotein (6, 7). It is generally believed that FFA derived from adipose tissue lipolysis accounts for up to 60% of hepatic triglycerides and close to 30% for de novo lipogenesis (6, 7). We predict that similar mechanisms are also responsible for HFD plus nicotine-induced hepatic steatosis. Indeed, here, we show that lipolysis inhibitor acipimox attenuates nicotine plus HFD-induced hepatic steatosis, suggesting that nicotine-induced lipolysis is an important contributor to hepatic steatosis. At present, we are unable to determine the precise contribution of de novo lipogenesis to HFD plus nicotine-induced hepatic steatosis. Clearly, this merits further investigation.
It is worth noting here that nicotine has a direct effect on stimulating lipolysis in adipocytes (62, 63), which express nicotinic acetylcholine receptors (64). Consistent with the adipose tissue lipolytic effect of nicotine, we also found in our earlier report (19), as well in the present study, that nicotine significantly reduced body fat content in mice fed HFD. Thus, it is possible that nicotine may play a direct role in stimulating adipose tissue lipolysis in mice on a HFD.
One potential limitation of our study is that the mode of nicotine administration does not incorporate voluntary consumption and also that only twice a day peak of nicotine is nonphysiological, compared with how smokers receive nicotine. Self-administration paradigms are technically difficult (especially in mice) and are limited by a U-shaped dose response of nicotine. Our laboratory and others have used oral nicotine self-selection, but we found that rodents prefer vehicle to nicotine due to the aversive taste of nicotine. In pilot studies, we also attempted to use an Alzet minipump with nicotine alternating with mineral oil and found that the stress of implanting the minipump, even without nicotine, dramatically increased corticosterone levels, indicating a high degree of stress. Similarly, we also tried using mice with jugular cannulations and injected nicotine through the cannula. However, the vehicle-treated mice also had high corticosterone levels, indicating activation of the stress response by the cannula. Nicotine pellets are also available but suffer from continuous exposure to nicotine that is likely to lead to tolerance. For these reasons, as reported in our recent study (19), we decided to use nicotine delivered by twice daily ip injections of 0.75 mg/kg BW that is approximately equal to the amount of nicotine a two-pack a day smoker might be exposed in a whole day (65). This has also the advantage of avoiding tolerance. However, the possibility that enhanced nicotine exposure by twice daily injections of nicotine may cause hepatic steatosis seems unlikely, because nicotine alone did not lead to hepatic steatosis but caused severe steatosis only when combined with a HFD. Although we are confident that this model is a realistic choice to study the effects of nicotine on ectopic lipid accumulation in the liver in obese mice, we do recognize the limitation of this system of nicotine delivery that is clearly different from human situations.
In summary, we have provided insights into the molecular mechanisms by which nicotine exacerbates HFD-induced hepatic steatosis and emphasizes the suitability of this model to study NAFLD, with the aim to develop better therapeutic treatments for hepatic steatosis. We conclude that: 1) greater oxidative stress coupled with inactivation of AMPK is critical for the additive effects of nicotine and HFD on the severity of hepatic steatosis in obese mice and 2) adipose tissue lipolysis is an important contributor to lipid accumulation in liver. Although further mechanistic studies are needed, the data provide an in vivo demonstration of the detrimental effect of nicotine plus high-fat intake on metabolism in humans.
Acknowledgments
We thank the oxidative core laboratories for performing the GSH/GSSG, 4-HNE, ALT, and triglyceride assays and biostatistical core for statistical assistance, both of the National Institutes of Health Accelerating Excellence in Translational Science Grant U54MD007598 (formerly U54 MD007598).
This work was supported by National Institutes of Health Minority Institution Drug Abuse Research Program Grants R24DA017298 (to T.C.F. and A.P.S.-H.), R21 DA022342 (to T.C.F.), and R01 DK054254, R01 DK083850, and R01 HL112248 (to S.M.N.).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- ACC
- Acetyl-coenzyme A-carboxylase
- ALT
- alanine aminotransferase
- AMPK
- AMP-activated protein kinase
- BMI
- body mass index
- BW
- body weight
- DXA
- dual-energy x-ray absorption
- ER
- endoplasmic reticulum
- FFA
- free fatty acid
- GAPDH
- glyceraldehyde-3-phosphate dehydrogenase
- GSH
- glutathione
- GSSG
- oxidized GSH
- H&E
- hematoxylin and eosin
- HFD
- high-fat diet
- 4-HNE
- 4-hydroxy-trans-2-nonenal
- JNK
- c-Jun-NH2-terminal kinase
- NAFLD
- nonalcoholic fatty liver disease
- NASH
- nonalcoholic steatohepatitis
- NCD
- normal chow diet
- ROS
- reactive oxygen species
- TUNEL
- terminal deoxynucleotidyl transferase 2′-deoxyuridine, 5′-triphosphate nick end labeling
- Vv
- volume density
- Vv%
- percentage of the hepatocyte volume.
References
- 1. He J, Gu D, Wu X, Reynolds K, Duan X, Yao C, Wang J, Chen CS, Chen J, Wildman RP, Klag MJ, Whelton PK. 2005. Major causes of death among men and women in China. N Engl J Med 353:1124–1134 [DOI] [PubMed] [Google Scholar]
- 2. Barnes PJ. 2003. New concepts in chronic obstructive pulmonary disease. Ann Rev Med 75:19–37 [DOI] [PubMed] [Google Scholar]
- 3. Hudson NL, Mannino DM. 2010. Tobacco use: a chronic illness? J Community Health 35:549–553 [DOI] [PubMed] [Google Scholar]
- 4. Zaher C, Halbert R, Dubios R, George D, Nonikov D. 2004. Smoking-related diseases: the importance of COPD. Int J Tuberc Lung 8:1423–1428 [PubMed] [Google Scholar]
- 5. Farrell GC, Teoh NC, McCuskey RS. 2008. Hepatic microcirculation in fatty liver disease. Anat Rec 291:684–692 [DOI] [PubMed] [Google Scholar]
- 6. Postic C, Girard J. 2008. Contribution of de novo fatty acid synthesis to hepatic steatosis and insulin resistance: lessons from genetically engineered mice. J Clin Invest 118:829–838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Trauner M, Arrese M, Wagner M. 2010. Fatty liver and lipotoxicity. Biochem Biophys Acta 1801:299–310 [DOI] [PubMed] [Google Scholar]
- 8. Hamabe A, Uto H, Imamura Y, Kusano K, Mawatari S, Kumagai K, Kure T, Tamai T, Moriuchi A, Sakiyama T, Oketani M, Ido A, Tsubouchi H. 2011. Impact of cigarette smoking on onset of nonalcoholic fatty liver disease over a 10-year period. J Gastroenterol 46:769–778 [DOI] [PubMed] [Google Scholar]
- 9. Zein CO, Unalp A, Colvin R, Liu YC, McCullough AJ. 2011. Smoking and severity of hepatic fibrosis in nonalcoholic fatty liver disease. J Hepatol 54:753–759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chiolero A, Faeh D, Paccaud F, Cornuz J. 2008. Consequences of smoking for body weight, body fat distribution, and insulin resistance. Am J Clin Nutr 87:801–809 [DOI] [PubMed] [Google Scholar]
- 11. Haslam DW, James WP. 2005. Obesity. Lancet 366:1197–1209 [DOI] [PubMed] [Google Scholar]
- 12. Flegal KM, Carroll MD, Ogden CL, Curtin LR. 2010. Prevalence and trends in obesity among US adults, 1999–2008. JAMA 303:235–241 [DOI] [PubMed] [Google Scholar]
- 13. Friedman JM. 2004. Modern science versus the stigma of obesity. Nat Med 10:563–569 [DOI] [PubMed] [Google Scholar]
- 14. Schindhelm RK, Diamant M, Dekker JM, Tushuizen ME, Teerlink T, Heine RJ. 2006. Alanine aminotransferase as a marker of non-alcoholic fatty liver disease in relation to type 2 diabetes mellitus and cardiovascular disease. Diabetes Metab Res Rev 22:437–443 [DOI] [PubMed] [Google Scholar]
- 15. Hamaguchi M, Kojima T, Takeda N, Nagata C, Takeda J, Sarui H, Kawahito Y, Yoshida N, Suetsugu A, Kato T, Okuda J, Ida K, Yoshikawa T. 2007. Nonalcoholic fatty liver disease is a novel predictor of cardiovascular disease. World J Gastroenterol 13:1579–1584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Targher G, Bertolini L, Poli F, Rodella S, Scala L, Tessari R, Zenari L, Falezza G. 2005. Nonalcoholic fatty liver disease and risk of future cardiovascular events among type 2 diabetic patients. Diabetes 54:3541–3546 [DOI] [PubMed] [Google Scholar]
- 17. Browning JD, Szczepaniak LS, Dobbins R, Nuremberg P, Horton JD, Cohen JC, Grundy SM, Hobbs HH. 2004. Prevalence of hepatic steatosis in an urban population in the United States: impact of ethnicity. Hepatology 40:1387–1395 [DOI] [PubMed] [Google Scholar]
- 18. Browning JD, Horton JD. 2004. Molecular mediators of hepatic steatosis and liver injury. J Clin Invest 114:147–152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mangubat M, Lutfy K, Lee ML, Pulido L, Stout D, Davis R, Shin CS, Shahbazian M, Seasholtz S, Sinha-Hikim A, Sinha-Hikim I, O'Dell LE, Lyzlov A, Liu Y, Friedman TC. 2012. Effect of nicotine on body composition in mice. J Endocrinol 212:317–326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ahmadian M, Duncan RE, Sul HS. 2009. The skinny on fat: lipolysis and fatty acid utilization in adipocytes. Trends Endocrinol Metab 20:424–428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. DeFronzo RA. 2010. Insulin resistance, lipotoxicity, type 2 diabetes and atherosclerosis: the missing links. The Claude Bernard Lecture 2009. Diabetologia 53:1270–1287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Azzalini L, Ferrer E, Ramalho LN, Moreno M, Domínguez M, Colmenero J, Peinado VI, Barberà JA, Arroyo V, Ginès P, Caballería J, Bataller R. 2010. Cigarette smoking exacerbates nonalcoholic fatty liver disease in obese rats. Hepatology 51:1567–1576 [DOI] [PubMed] [Google Scholar]
- 23. Yuan H, Shyy JY, Martins-Green M. 2009. Second-hand smoke stimulates lipid accumulation in the liver by modulating AMPK and SREBP-1. J Hepatol 51:535–547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Behan JW, Avramis VI, Yun JP, Louie SG, Mittelman SD. 2010. Diet-induced obesity alters vincristine pharmacokinetics in blood and tissues of mice. Pharmacol Res 61:385–390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Collins S, Martin TL, Surwit RS, Robidoux J. 2004. Genetic vulnerability to diet-induced obesity in the C57BL/6J mouse: physiological and molecular characteristics. Physiol Behav 81:243–248 [DOI] [PubMed] [Google Scholar]
- 26. de Meijer VE, Le HD, Meisel JA, Akhavan Sharif MR, Pan A, Nosé V, Puder M. 2010. Dietary fat intake promotes the development of hepatic steatosis independently from excess caloric consumption in a murine model. Metabolism 59:1092–1105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Patsch JM, Kiefer FW, Varga P, Pail P, Rauner M, Stupphann D, Resch H, Moser D, Zysset PK, Stulnig TM, Pietschmann P. 2011. Increased bone resorption and impaired bone microarchitecture in short-term and extended high-fat diet-induced obesity. Metabolism 60:243–249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sinha-Hikim I, Shen R, Nzenwa I, Gelfand R, Mahata SK, Sinha-Hikim AP. 2011. Minocycline suppresses oxidative stress and attenuates fetal cardiac myocyte apoptosis triggered by in utero cocaine exposure. Apoptosis 16:563–573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sinha-Hikim I, Shen R, Paul Lee WN, Crum A, Vaziri ND, Norris KC. 2010. Effects of a novel cystine-based glutathione precursor on oxidative stress in vascular smooth muscle cells. Am J Physiol Cell Physiol 299:C638–C642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sinha-Hikim I, Sinha-Hikim AP, Shen R, Kim HJ, Kim H, French SW, Vaziri ND, Vaziri ND, Crum AC, Crum A, Rajavashisth TB, Norris KC. 2011. A novel cystine based antioxidant attenuates oxidative stress and hepatic steatosis in diet-induced obese mice. Exp Mol Pathol 91:419–428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kohen R, Nyska A. 2002. Oxidation of biological systems: oxidative stress phenomena, antioxidants, redox reactions, and methods for their quantification. Toxicol Pathol 30:620–650 [DOI] [PubMed] [Google Scholar]
- 32. Tam NN, Gao Y, Leung YK, Ho SM. 2003. Androgenic regulation of oxidative stress in the rat prostate: involvement of NAD(P)H oxidases and antioxidant defense machinery during prostatic involution and regrowth. Am J Pathol 163:2513–2522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Jia Y, Castellanos J, Wang C, Sinha-Hikim I, Lue Y, Swerdloff RS, Sinha-Hikim AP. 2009. Mitogen-activated protein kinase signaling in male germ cell apoptosis in the rat. Biol Reprod 80:771–780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Vera Y, Erkkilä K, Wang C, Nunez C, Kyttänen S, Lue Y, Dunkel L, Swerdloff RS, Sinha Hikim AP. 2006. Involvement of p38 mitogen-activated protein kinase and inducible nitric oxide synthase in apoptotic signaling of murine and human male germ cells after hormone deprivation. Mol Endocrinol 20:1597–1609 [DOI] [PubMed] [Google Scholar]
- 35. Deng QG, She H, Cheng JH, French SW, Koop DR, Xiong S, Tsukamoto H. 2005. Steatohepatitis induced by intragastric overfeeding in mice. Hepatology 42:905–914 [DOI] [PubMed] [Google Scholar]
- 36. Cruz-Orive LM, Weibel ER. 1990. Recent stereological methods for cell biology: a brief survey. Am J Physiol 258:L148–L156 [DOI] [PubMed] [Google Scholar]
- 37. Mahapatra NR, O'Connor DT, Vaingankar SM, Hikim AP, Mahata M, Ray S, Staite E, Wu H, Gu Y, Dalton N, Kennedy BP, Ziegler MG, Ross J, Mahata SK. 2005. Targeted ablation of chromogranin A gene: elevated blood pressure rescued by human homolog. J Clin Invest 115:1942–1952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Hikim AP, Lue Y, Yamamoto CM, Vera Y, Rodriguez S, Yen PH, Soeng K, Wang C, Swerdloff RS. 2003. Key apoptotic pathways for heat-induced programmed germ cell death in the testis. Endocrinology 144:3167–3175 [DOI] [PubMed] [Google Scholar]
- 39. Zhang BB, Zhou G, Li C. 2009. AMPK: an emerging drug target for diabetes and the metabolic syndrome. Cell Metab 9:407–416 [DOI] [PubMed] [Google Scholar]
- 40. Alkhouri N, Gornicka A, Berk MP, Thapaliya S, Dixon LJ, Kashyap S, Schauer PR, Feldstein AE. 2010. Adipocyte apoptosis, a link between obesity, insulin resistance, and hepatic steatosis. J Biol Chem 285:3428–3438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Mantena S, King A, Andringa K, Eccleston H, Bailey S. 2008. Mitochondrial dysfunction and oxidative stress in the pathogenesis of alcohol- and obesity-induced fatty liver diseases. Free Radic Biol 44:1259–1272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kanuri G, Spruss A, Wagnerberger S, Bischoff SC, Bergheim I. 2011. Role of tumor necrosis factor α (TNFα) in the onset of fructose-induced nonalcoholic fatty liver disease in mice. J Nutr Biochem 22:527–534 [DOI] [PubMed] [Google Scholar]
- 43. Kudo H, Takahara T, Yata Y, Kawai K, Zhang W, Sugiyama T. 2009. Lipopolysaccharide triggered TNF-α-induced hepatocyte apoptosis in a murine non-alcoholic steatohepatitis model. J Hepatol 51:168–175 [DOI] [PubMed] [Google Scholar]
- 44. Kojima H, Sakurai S, Uemura M, Fukui H, Morimoto H, Tamagawa Y. 2007. Mitochondrial abnormality and oxidative stress in nonalcoholic steatohepatitis. Alcohol Clin Exp Res 31:S61–S66 [DOI] [PubMed] [Google Scholar]
- 45. Zhang W, Kudo H, Kawai K, Fujisaka S, Usui I, Sugiyama T, Tsukada K, Chen N, Takahara T. 2010. Tumor necrosis factor-α accelerates apoptosis of steatotic hepatocytes from a murine model of non-alcoholic fatty liver disease. Biochem Biophys Res Commun 391:1731–1736 [DOI] [PubMed] [Google Scholar]
- 46. Kim F, Pham M, Maloney E, Rizzo NO, Morton GJ, Wisse BE, Kirk EA, Chait A, Schwartz MW. 2008. Vascular inflammation, insulin resistance, and reduced nitric oxide production precede the onset of peripheral insulin resistance. Arterioscler Thromb Vasc Biol 28:1982–1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Valenca SS, Gouveia L, Pimenta WA, Porto LC. 2008. Effects of oral nicotine on rat liver stereology. Int J Morphol 26:1013–1022 [Google Scholar]
- 48. Aragno M, Tomasinelli CE, Vercellinatto I, Catalano MG, Collino M, Fantozzi R, Danni O, Boccuzzi G. 2009. SREBP-1c in nonalcoholic fatty liver disease induced by Western-type high-fat diet plus fructose in rats. Free Radic Biol Med 47:1067–1074 [DOI] [PubMed] [Google Scholar]
- 49. El-Sokkary GH, Cuzzocrea S, Reiter RJ. 2007. Effect of chronic nicotine administration on the rat lung and liver: beneficial role of melatonin. Toxicology 239:60–67 [DOI] [PubMed] [Google Scholar]
- 50. Sreekala S, Indira M. 2009. Effects of exogenous selenium on nicotine-induced oxidative stress in rats. Biol Trace Elem Res 130:62–71 [DOI] [PubMed] [Google Scholar]
- 51. Taysi S, Gumustekin K, Demircan B, Aktas O, Oztasan N, Akcay F, Suleyman H, Akar S, Dane S, Gul M. 2010. Hippophae rhamnoides attenuates nicotine-induced oxidative stress in rat liver. Pharm Biol 48:488–493 [DOI] [PubMed] [Google Scholar]
- 52. Videla LA. 2010. Cytoprotective and suicidal signaling in oxidative stress. Biol Res 43:363–369 [PubMed] [Google Scholar]
- 53. Serviddio G, Bellanti F, Sastre J, Vendemiale G, Altomare E. 2010. Targeting mitochondria: a new promising approach for the treatment of liver diseases. Curr Med Chem 17:2325–2337 [DOI] [PubMed] [Google Scholar]
- 54. Mihaylova MM, Shaw RJ. 2011. The AMPK signalling pathway coordinates cell growth, autophagy and metabolism. Nat Cell Biol 13:1016–1023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Kathirvel E, Morgan K, Nandgiri G, Sandoval BC, Caudill MA, Bottiglieri T, French SW, Morgan TR. 2010. Betaine improves nonalcoholic fatty liver and associated hepatic insulin resistance: a potential mechanism for hepatoprotection by betaine. Am J Physiol Gastrointest Liver Physiol 299:G1068–G1077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Nakagawa H, Maeda S, Hikiba Y, Ohmae T, Shibata W, Yanai A, Sakamoto K, Ogura K, Noguchi T, Karin M, Ichijo H, Omata M. 2008. Deletion of apoptosis signal-regulating kinase 1 attenuates acetaminophen-induced liver injury by inhibiting c-Jun N-terminal kinase activation. Gastroenterology 135:1311–1321 [DOI] [PubMed] [Google Scholar]
- 57. Singh R, Wang Y, Schattenberg JM, Xiang Y, Czaja MJ. 2009. Chronic oxidative stress sensitizes hepatocytes to death from 4-hydroxynonenal by JNK/c-Jun overactivation. Am J Physiol Gastrointest Liver Physiol 297:G907–G917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Cacicedo JM, Benjachareonwong S, Chou E, Yagihashi N, Ruderman NB, Ido Y. 2011. Activation of AMP-activated protein kinase prevents lipotoxicity in retinal pericytes. Invest Ophthalmol Vis Sci 52:3630–3639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Jia F, Wu C, Chen Z, Lu G. 2011. AMP-activated protein kinase inhibits homocysteine-induced dysfunction and apoptosis in endothelial progenitor cells. Cardiovasc Drugs Ther 25:21–29 [DOI] [PubMed] [Google Scholar]
- 60. Meares GP, Hughes KJ, Jaimes KF, Salvatori AS, Rhodes CJ, Corbett JA. 2010. AMP-activated protein kinase attenuates nitric oxide-induced β-cell death. J Biol Chem 285:3191–3200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Yen CY, Lin MH, Liu SY, Chiang WF, Hsieh WF, Cheng YC, Hsu KC, Liu YC. 2011. Arecoline-mediated inhibition of AMP-activated protein kinase through reactive oxygen species is required for apoptosis induction. Oral Oncol 47:345–351 [DOI] [PubMed] [Google Scholar]
- 62. Andersson K, Arner P. 2001. Systemic nicotine stimulates human adipose tissue lipolysis through local cholinergic and catecholaminergic receptors. Int J Obes Relat Metab Disord 25:1225–1232 [DOI] [PubMed] [Google Scholar]
- 63. An Z, Wang H, Song P, Zhang M, Geng X, Zou MH. 2007. Nicotine-induced activation of AMP-activated protein kinase inhibits fatty acid synthase in 3T3L1 adipocytes: a role of oxidant stress. J Biol Chem 282:26793–26801 [DOI] [PubMed] [Google Scholar]
- 64. Liu RH, Mizuta M, Matsukura S. 2004. The expression and functional role of nicotinic acetylcholine receptors in rat adipocytes. J Pharmacol Exp Ther 310:52–58 [DOI] [PubMed] [Google Scholar]
- 65. Matta SG, Balfour DJ, Benowitz NL, Boyd RT, Buccafusco JJ, Caggiula AR, Craig CR, Collins AC, Damaj MI, Donny EC, Gardiner PS, Grady SR, Heberlein U, Leonard SS, Levin ED, Lukas RJ, Markou A, Marks MJ, McCallum SE, Parameswaran N, Perkins KA, Picciotto MR, Quik M, Rose JE, Rothenfluh A, Schafer WR, Stolerman IP, Tyndale RF, Wehner JM, Zirger JM. 2007. Guidelines on nicotine dose selection for in vivo research. Psychopharmacology 190:269–319 [DOI] [PubMed] [Google Scholar]