Abstract
The kisspeptin receptor (KISS1R) is a Gαq/11-coupled seven-transmembrane receptor activated by a group of peptides referred to as kisspeptins (Kps). The Kp/KISS1R signaling system is a powerful regulator of GnRH secretion, and inactivating mutations in this system are associated with hypogonadotropic hypogonadism. A recent study revealed that Kp triggers prolonged signaling; not from the inability of the receptor to undergo rapid desensitization, but instead from the maintenance of a dynamic and active pool of KISS1R at the cell surface. To investigate this further, we hypothesized that if a dynamic pool of receptor is maintained at the cell surface for a protracted period, chronic Kp-10 treatment would trigger the sustained activation of Gαq/11 as evidenced through the prolonged activation of phospholipase C, protein kinase C, and prolonged mobilization of intracellular Ca2+. Through single-cell analyses, we tested our hypothesis in human embryonic kidney (HEK) 293 cells and found that was indeed the case. We subsequently determined that prolonged KISS1R signaling was not a phenomenon specific to HEK 293 cells but is likely a conserved property of KISS1R-expressing cells because evidence of sustained KISS1R signaling was also observed in the GT1–7 GnRH neuronal and Chinese hamster ovary cell lines. While exploring the regulation of prolonged KISS1R signaling, we identified a critical role for extracellular Ca2+. We found that although free intracellular Ca2+, primarily derived from intracellular stores, was sufficient to trigger the acute activation of a major KISS1R secondary effector, protein kinase C, it was insufficient to sustain chronic KISS1R signaling; instead extracellular Ca2+ was absolutely required for this.
KISS1R (kisspeptin receptor) is a seven-transmembrane receptor (7TMR) activated by a group of peptides referred to as kisspeptins (Kps) (1). Activated KISS1R couples to Gαq/11 and triggers the activation of the primary effector, phospholipase C (PLC), which in turn hydrolyzes phosphatidylinositol 4,5-bisphosphate (PIP2) into the secondary messengers inositol 1,4,5 trisphosphate (InsP3) and diacylglycerol (DAG). InsP3 diffuses into the cell and binds its receptors on the endoplasmic reticulum, thereby releasing Ca2+, another major secondary effector, into the cell (1–4). Presumably, KISS1R activation then results in the activation of conventional and novel protein kinase C (PKC) isoforms that are regulated by Ca2+ and DAG and by DAG only, respectively. KISS1R activation has also been demonstrated to trigger arachidonic acid formation and ERK 1/2, p38, and phosphatidylinositol-3-kinase/Akt activation (5–9).
Since its discovery, as reported by four independent groups in 2001 (6–8, 10), the kisspeptin receptor (KISS1R) is now established as a powerful regulator of GnRH secretion (11, 12). KISS1R signaling is also reported to regulate placentation (13), kidney formation (14), insulin secretion (15), and tumor cell metastasis (16). Given these KISS1R-regulated roles, particularly GnRH secretion, we continue to study the mechanisms underlying KISS1R signaling. To this end, we recently defined clear and strong roles for G protein-coupled receptor kinase 2 (GRK2) and β-arrestin-1 and -2 in mediating KISS1R desensitization and internalization (17). In this study we demonstrated that following Kp-10 treatment, KISS1R signaling was desensitized in a phosphorylation-independent but GRK2-dependent manner. GRK2-dependent desensitization was coupled to β-arrestin recruitment to the receptor and its subsequent internalization via clathrin-coated pits. We further determined that, in addition to regulating KISS1R G protein-dependent signaling, β-arrestins also mediate KISS1R G protein-independent signaling (17, 18). It is likely that such signaling occurs both at the plasma membrane and upon internalization because we observed that KISS1R is constitutively associated with β-arrestin and that both molecules cointernalized on clathrin-coated vesicles.
In a recent study, Bianco et al. (19) confirmed our findings that KISS1R undergoes rapid desensitization and internalization but in addition, through the use of radioligand-binding studies, the authors clearly demonstrated that a dynamic pool of KISS1R is maintained at the cell surface. This dynamic pool of cell surface KISS1R, which is believed to be derived from the continuous recycling of desensitized/resensitized receptors as well as from a pool of nonrecycling receptors, accounts for the source of the prolonged Kp-dependent signaling. Whereas the Pampillo et al. (17) and Bianco et al. (19) studies were conducted in heterologous cell systems, taken together, our findings are consistent with previously reported observations that reveal that, in the mouse hypothalamus, Kiss1r signaling is initially prolonged but does eventually desensitize (20–25).
Because chronic Kp administration can potentially be developed as a major clinical therapy to treat a number of disorders such as central precocious puberty, prostate cancer, and endometriosis, a goal of this study was to explore the molecular aspects of KISS1R activity further in the continued presence of Kp. To this end, based on the study by Bianco et al. (19), we hypothesized that upon chronic Kp-10 treatment, if a dynamic pool of receptor is maintained at the cell surface for a prolonged period, Kp will trigger the sustained activation of the Gαq/11-coupled signaling pathway. To test this, using molecular reporters we conducted single-cell analysis in HEK 293 cells and determined the spatiotemporal properties of PLC and PKC activation, as well as the prolonged mobilization of intracellular Ca2+. Our results clearly revealed that Kp triggers chronic signaling in KISS1R-expressing cells and that prolonged KISS1R signaling was entirely dependent on extracellular Ca2+, a major secondary effector that regulates numerous developmental and physiological events.
Materials and Methods
For single-cell confocal imaging assays
Human embryonic kidney cells (HEK 293) were from the American Tissue Culture Collection (Manassas, VA). The GT1–7 hypothalamic GnRH neuronal cell line was the kind gift of Dr. Pamela Mellon, University of California, San Diego. The calcium indicator, Oregon Green 488 BAPTA-1 AM, and calcium chelator BAPTA-AM were obtained from Cedarlane Laboratories (Burlington, Ontario, Canada). Kp-10 was purchased from Tocris Cookson, Inc. (Ellisville, MO); thapsigargin was obtained from Calbiochem (La Jolla, CA), and EGTA was purchased from Sigma (St. Louis, MO). All other biochemical reagents were acquired from Sigma, Fisher Scientific (Pittsburgh, PA), and VWR Scientific (Mississauga, Ontario, Canada). The plasmid constructs, green fluorescent protein (GFP)-PHPLC-δ1, and GFP-PKC-βII were described previously (26).
For Ca2+ assays
Construction of human KISS1R vector and the generation of Chinese hamster ovary (CHO)-KISS1R cells were previously described (16, 27). Cell culture medium was obtained from Mediatech, Inc. (Manassas, VA), and Kp-10 was synthesized by Tufts Medical Center Core Facility (Boston, MA); all other chemicals were from Sigma-Aldrich (St. Louis, MO).
Confocal microscopy
HEK 293 cells were cultured in MEM supplemented with 10% (vol/vol) fetal bovine serum, 1% (vol/vol) nonessential amino acids, and gentamicin (5 μg/ml). After transfection cells were reseeded on collagen-coated 35-mm glass-bottomed culture dishes. Before visualization, the cells were washed three times with HEPES-buffered salt solution, Hank's balanced salt solution (1.2 mm KH2PO4, 5 mm NaHCO3, 20 mm HEPES, 11 mm glucose, 116 mm NaCl, 4.7 mm KCl, 1.2 mm MgSO4, 2.5 mm CaCl2, pH 7.4) and incubated for 1 h at 37 C in Hank's balanced salt solution.
Confocal analysis was performed on an Olympus Fluoview 1000 laser-scanning confocal microscope (Olympus Corp., Lake Success, NY) using the 60X Plan Apochromat 1.42 oil objective. Enhanced GFP and Oregon Green 488 BAPTA fluorescence was visualized with excitation at 488 nm and emission 515- to 540-nm emission filter set. For calcium imaging by confocal microscopy, following the manufacturer's instructions for preparing Oregon Green 488 BAPTA-1 AM, cells were preloaded for 30 min with 10 μm Oregon Green 488 BAPTA-1 AM. Fluorescent signals were collected sequentially every 6 to 12 sec using the Olympus LSM software time scan function. Thapsigargin and BAPTA-AM were dissolved in dimethylsulfoxide and diluted in PBS just before use to a final concentration of 1 μm and 50 μm, respectively. EGTA was used at a final concentration of 2.5 mm.
Image analysis
Regions of interest were identified in cells, and the fluorescence intensity (arbitrary units) at a given region was monitored over time. The change in intensity over time was then presented graphically. Each experiment was conducted five independent times. However, representative images from one independent experiment are presented.
Calcium assays
CHO-KISS1R cells (1 × 104/well) were plated into black-walled, clear-bottomed 96-well plates. The next day, the cells were washed with DMEM/F12 and incubated in serum-free DMEM/F12 for 2 h at 37 C. The cells were then treated with 100 nm Kp-10, and the Fluo-4 Direct Kit (Invitrogen, Carlsbad, CA) was used to measure intracellular Ca2+ according to the manufacturer's protocol. Parental CHO cells were also treated with Kp-10 as a negative control, and there was no response to Kp-10 stimulation (data not shown). The change in fluorescence intensity before and after addition of ligand was measured using a POLARstar OPTIMA multifunction plate reader (BMG Labtech, Durham, NC). Ca2+ measurements were made 5 sec after addition of Kp-10 and then repeated every 15 sec for the indicated time. The intracellular Ca2+ response was determined by measuring relative fluorescence intensity with a POLARstar OPTIMA plate reader.
Results
Chronic Kp administration triggers the prolonged activation of PLC
For 7TMRs, the hydrolysis of PIP2 and formation of InsP3 and DAG represent receptor-proximal events upstream of the release of Ca2+ from intracellular stores and activation of the DAG/Ca2+-dependent conventional PKC isoforms (PKCα, -βI, -βII, and -γ). Thus, PLC activation strongly reflects Gαq/11 activation. Activation of PLC, hydrolysis of PIP2, and formation of InsP3 can be measured in single cells using the pleckstrin homology (PH) domain of PLC-δ1 fused to GFP (GFP-PH-PLC-δ1) as a fluorescent indicator (28, 29). Therefore, we used GFP-PH-PLC-δ1 to investigate whether chronic stimulation of KISS1R triggered prolonged activation of Gαq/11. We did this by coexpressing FLAG-KISS1R and GFP-PH-PLC-δ1 in HEK 293 cells and determining the spatiotemporal properties of GFP-PH-PLC-δ1 under basal and Kp-stimulated conditions.
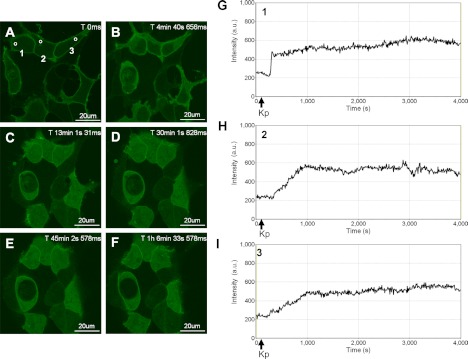
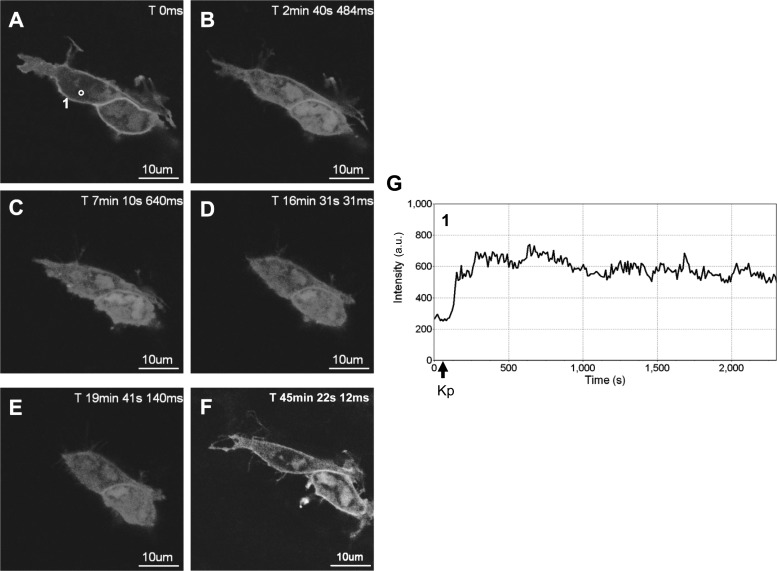
In the absence of KISS1R activation, GFP-PH-PLC-δ1 was localized at the plasma membrane (Fig. 1A) due to the interaction of the PLC-δ1 PH domain with membrane PIP2 (30). In response to Kp-10 treatment (∼ 1 min after imaging began), GFP-PH-PLC-δ1 was released from the plasma membrane and accumulated in the cytosol of the cell (Fig. 1, panels B–F and G–I). The release of GFP-PH-PLC-δ1 from the plasma membrane is the consequence of both PIP2 hydrolysis and the high affinity of the PLC-δ1 PH domain for InsP3 (29). As we had hypothesized, in response to KISS1R activation, GFP-PH-PLC-δ1 remained in the cytosol for a prolonged period of time generally exceeding 1 h (Fig. 1, B–F and G–I) before beginning to slowly redistribute back to the plasma membrane (this was generally observed to occur within 90 min; data not shown), presumably due to the eventual desensitization and internalization of a major fraction of plasma membrane-bound KISS1R. To determine whether the temporal properties of activated GFP-PH-PLC-δ1 were unique to the HEK 293 cell system, we conducted the above experiment in the GnRH-secreting hypothalamic neuronal cell line, GT1–7. Here we observed that in GT1–7 cells, activated GFP-PH-PLC-δ1 remained in the cytosol for more than 30 min (Fig. 2, A–E and G) before beginning to slowly redistribute back to the plasma membrane, as clearly observed after 45 min (Fig. 2F).
Fig. 1.
Spatiotemporal characteristics of GFP-PH-PLC-δ1 in HEK 293 cells in response to KISS1R activation. A–F, Representative images selected from a time series of laser-scanning confocal microscopic images showing the plasma membrane and cytosolic localization of GFP-PH-PLC-δ1 in KISS1R-expressing HEK 293 cells in the absence of agonist (0 s) and in response to 100 nm Kp-10 treatment approximately 1 min later (see solid black arrows on x-axis of panels G–I). Cytosolic regions of interest in three cells (shown by small white circles in panel A) were analyzed quantitatively by determining the changes in GFP fluorescence over time. These changes are presented graphically (G–I). These studies were conducted five independent times, and representative images from one independent experiment are shown. ms, Millisecond; s, second.
Fig. 2.
Spatiotemporal characteristics of GFP-PH-PLC-δ1 in GT1–7 cells in response to KISS1R activation. A–F, Representative images selected from a time series of laser-scanning confocal microscopic images showing the plasma membrane and cytosolic localization of GFP-PH-PLC-δ1 in KISS1R-expressing GT1-T cells in the absence of agonist (0 s) and in response to 100 nm Kp-10 treatment approximately 1 min later (see solid black arrows on x-axis of panel G). A cytosolic region of interest in one cell (shown by small white circle in panel A) was analyzed quantitatively by determining the changes in GFP fluorescence over time. These changes are presented graphically (panel G). Note: although cells were imaged for approximately 1 h, due to their movement over this period, a given region of interest could only be analyzed for subperiods of time. As a result, we present a region of interest that represents the first 37 min of imaging (panel G). These studies were conducted five independent times, and representative images from one independent experiment are shown. ms, Millisecond; s, second.
Chronic Kp administration triggers the prolonged mobilization of Ca2+
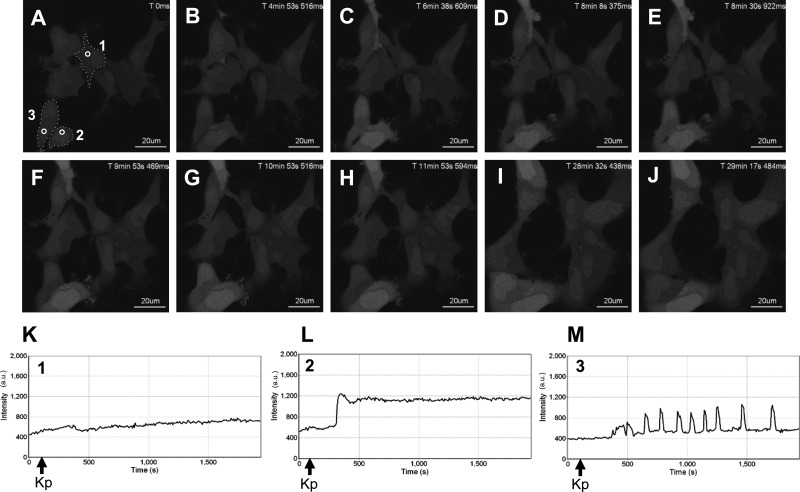
Activation of Gαq/11-coupled 7TMRs triggers the release of Ca2+ from intracellular stores whereas desensitization prevents it. Consequently, free cytosolic Ca2+ levels decline rapidly due to the reuptake by the intracellular stores. Because Kp-10 treatment triggers a prolonged activation of KISS1R and PLC, we wanted to determine what effect this had on the spatiotemporal properties of Ca2+ release. To do this, HEK 293 cells expressing FLAG-KISS1R were preloaded with Oregon Green 488 BAPTA-1-AM, and live-cell confocal imaging was conducted. About 1 min later, 100 nm Kp-10 was added, and live-cell imaging was continued for 1 h.
After 100 nm Kp-10 treatment we observed three distinct responses. 1) In the majority of cells (∼70%) we observed a gradual rise in free intracellular Ca2+ (Fig. 3A–K; cell number 1). 2) In another well-represented group of cells (∼25%) we observed a rapid rise in free intracellular Ca2+ (Fig. 3, A–J and L; cell number 2). In both responses, the increase in Ca2+ was sustained for the entire period for which the cells were imaged (∼1 h). 3) Finally, in approximately 5% of all cells we found that the increase in Ca2+ was initially sustained for varying periods of time, and this was followed by Ca2+ oscillations (Fig. 3, A–J and M; cell number 3) that sometimes reverted to a sustained phase of free intracellular Ca2+ (data not shown). We considered that the three response patterns we observed were due to different levels of resting Ca2+, but as seen, the relative levels of resting Ca2+ were almost identical in all cell types (see relative intensity values on the y-axis, Fig. 3, K–M).
Fig. 3.
Spatiotemporal characteristics of Ca2+ in HEK 293 cells in response to KISS1R activation. A–J, Representative images selected from a time series of laser-scanning confocal microscopic images showing the cytosolic and nuclear levels of free intracellular Ca2+ (as assessed by Oregon Green 488 BAPTA-1-AM fluorescence) in KISS1R-expressing HEK 293 cells in the absence of agonist (0 s) and in response to 100 nm Kp-10 treatment approximately 1 min later (see solid black arrows on x-axis of panels K–M). Cytosolic regions of interest in three cells (shown by small white circles in panel A) were analyzed quantitatively by determining the changes in fluorescence over time. These changes are presented graphically (K–M). These studies were conducted five independent times, and representative images from one independent experiment are shown. ms, Millisecond; s, second.
Some studies have suggested that sustained levels of Ca2+ are linked to the use of supramaximal stimuli (31). Given the possibility that 100 nm Kp-10 is a supramaximal stimulus, we determined whether the sustained response in Ca2+ mobilization would be diminished at lower Kp-10 concentrations (10 nm, 1 nm, and 0.1 nm) in KISS1R-expressing cells. Specifically, we analyzed KISS1R-expressing cells that were derived from the same dish of transfected cells to control for variations in receptor expression between Kp treatments. At 10 and 1 nm Kp-10, sustained levels of Ca2+ were again observed (data not shown). However, no visible Ca2+ mobilization was detected with 0.1 nm Kp-10 (data not shown).
Chronic Kp administration triggers the prolonged activation of PKC
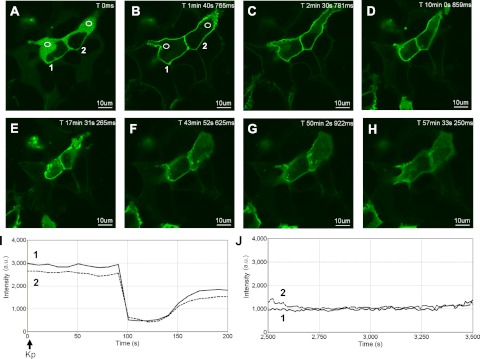
Activation of Gαq/11-coupled 7TMRs triggers the activation of conventional and novel PKCs. This can be detected visually by the translocation of fluorescently tagged PKC isoforms from the cytosol to the plasma membrane (26, 32) where PKC can participate in an array of signaling events. To determine whether activation of KISS1R would result in the prolonged activation of PKC, FLAG-KISS1R and GFP-PKC-βII were coexpressed in HEK 293 cells and treated with Kp-10. This resulted in the rapid translocation of cytosolic GFP-PKC-βII to the plasma membrane (Fig. 4, A, B, and I). Immediately after localizing to the membrane, approximately half of this membrane-bound pool returned to the cytosol (Fig. 4, A–D and I). This was in contrast to the strong and prolonged translocation of GFP-PH-PLC-δ1 into the cytosol (Fig. 1). Additionally, compared with GFP-PH-PLC-δ1, which took about 90 min to begin returning to the plasma membrane, a significant fraction of plasma membrane-bound GFP-PKC-βII returned to the cytosol in about 45 min (Fig. 4, F–H), presumably due to the desensitization and internalization of a major fraction of cell-surface KISS1R. Finally, it should be noted that although it was an infrequent event (∼5% of all cells), as were the Ca2+ oscillations previously observed, GFP-PKC-βII began oscillating repetitively between the plasma membrane and cytosol after varying periods of time at the plasma membrane (data not shown).
Fig. 4.
Spatiotemporal characteristics of GFP-PKC-βII in HEK 293 cells in response to KISS1R activation. A–H, Representative images selected from a time series of laser-scanning confocal microscopic images showing the plasma membrane and cytosolic localization of GFP-PKC-βII in KISS1R-expressing HEK 293 cells in the absence of agonist (0 s) and in response to 100 nm Kp-10 treatment approximately 10 sec later (see solid black arrow on x-axis of panel I). Cytosolic regions of interest in two cells (shown by small white circles in panels A and B) were analyzed quantitatively by determining the changes in GFP fluorescence over time. These changes are presented graphically (panels I and J, cell number 1: solid line; cell number 2: dashed line). Note: although cells were imaged for approximately 1 h, due to their movement over this period, given regions of interest could only be analyzed for subperiods of time. As a result, we present regions of interest that were quantified over two successive periods [0–200 sec (I) and 2500–3500 sec (J)]. These studies were conducted five independent times, and representative images from one independent experiment are shown. ms, Millisecond; s, second.
Free intracellular Ca2+ is not required for PKC activation and prolonged KISS1R signaling
Ca2+ is a major secondary effector that regulates numerous developmental and physiological events. Given its importance in the downstream propagation of 7TMR signals, we assessed the relative contribution of intracellular and extracellular Ca2+ in regulating prolonged KISS1R signaling. The prolonged KISS1R/Ca2+-dependent signaling event we examined was PKC activity. Specifically, we examined the effect of Ca2+ on the initial activation and continued activity of PKC.
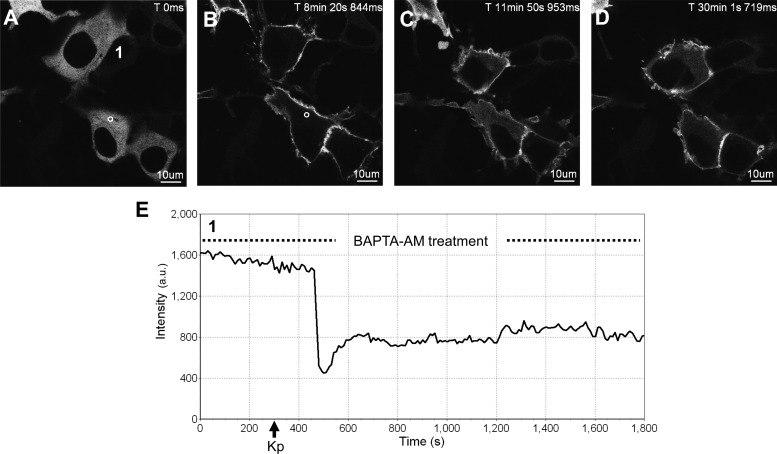
To determine the effect of free intracellular Ca2+ on the initial activation of PKC, cytosolic Ca2+ was chelated using BAPTA-AM before Kp-10 treatment of HEK 293 cells coexpressing FLAG-KISS1R and GFP-PKC-βII. Before and after Kp-10 treatment, we characterized the spatiotemporal properties of GFP-PKC-βII relative to Kp-treated but BAPTA-AM-untreated control cells. When cells were pretreated for 5 min with BAPTA-AM, GFP-PKC-βII remained localized to the cytosol (Fig. 5, A and E) and was indistinguishable from that in untreated control cells (Fig. 4A). After Kp-10 treatment, in the continued presence of BAPTA-AM, GFP-PKC-βII translocated rapidly to the plasma membrane where greater than half of it persisted (Fig. 5, B–E) for more than 1 h before gradually returning to the cytosol. Again, this was identical to the Kp-10-treated but BAPTA-AM-untreated control cells (Fig. 4).
Fig. 5.
The effect of BAPTA-AM pretreatment on the spatiotemporal characteristics of GFP-PKC-βII in HEK 293 cells in response to KISS1R activation. A–D, Representative images selected from a time series of laser-scanning confocal microscopic images showing the plasma membrane and cytosolic localization of GFP-PKC-βII in KISS1R-expressing HEK 293 cells pretreated with 50 μm BAPTA-AM (see dashed line in panel E) followed by 100 nm Kp-10 approximately 250 sec later (see solid black arrow on x-axis of panel E) in the continued presence of BAPTA-AM (see dashed line in panel E). Cytosolic region of interest in a cell (shown by small white circle in panel A) was analyzed quantitatively by determining the changes in GFP fluorescence over time. These changes are presented graphically (panel E). Note: although cells were imaged for approximately 1 h, due to their movement over this period, a given region of interest could only be analyzed for subperiods of time. As a result, we present a region of interest that represents the first 30 min of imaging (panel G). These studies were conducted five independent times, and representative images from one independent experiment are shown. ms, Millisecond; s, second.
Next, we examined the effect of intracellular Ca2+ on the continued activity of PKC. This was done by first treating cells coexpressing FLAG-KISS1R and GFP-PKC-βII with Kp-10. Immediately after the rapid translocation of GFP-PKC-βII to the plasma membrane, cells were treated with BAPTA-AM, and the spatiotemporal properties of GFP-PKC-βII were assessed relative to Kp-10-treated but BAPTA-AM-untreated control cells. One hour later, GFP-PKC-βII was still visible at the plasma membrane in both control and experimental cells.
Extracellular Ca2+ is required for the prolonged activation of PKC
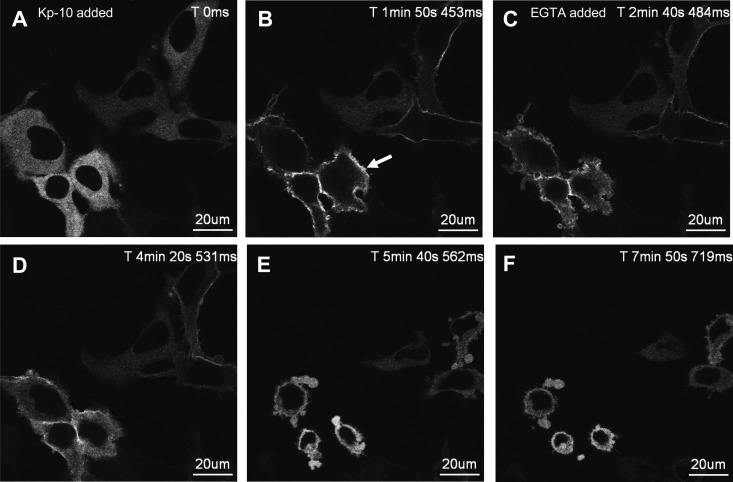
Next, we examined the effect of extracellular Ca2+ on the initial activation and continued activity of PKC. To determine the effect of extracellular Ca2+ on the initial activation of PKC, extracellular Ca2+ was chelated using EGTA before Kp-10 treatment of HEK 293 cells coexpressing FLAG-KISS1R and GFP-PKC-βII. Before and after Kp-10 treatment, we characterized the spatiotemporal properties of GFP-PKC-βII relative to Kp-treated but EGTA-untreated control cells. When cells were pretreated for approximately 5 min with EGTA, GFP-PKC-βII was localized to the cytosol (Fig. 6, A and B) and was indistinguishable from untreated control cells (Fig. 4). After Kp-10 treatment (Fig. 6C), in the continued presence of EGTA, GFP-PKC-βII translocated rapidly to the plasma membrane (Fig. 6D) and unlike control cells, rapidly returned to the cytosol (Fig. 6, E–F vs. Fig. 4, B–H). Thus, chelation of extracellular Ca2+ cannot block the initial activation of PKC but it does rapidly block the continued activity of PKC.
Fig. 6.
The effect of EGTA pretreatment on the spatiotemporal characteristics of GFP-PKC-βII in HEK 293 cells in response to KISS1R activation. A–F, Representative images selected from a time series of laser-scanning confocal microscopic images showing the plasma membrane and cytosolic localization of GFP-PKC-βII in KISS1R-expressing HEK 293 cells pretreated with 2.5 mm EGTA followed by 100 nm Kp-10 approximately 4 min later in the continued presence of EGTA. White solid arrow shows transient translocation of GFP-PKC-βII (panel D). Prolonged exposure to EGTA caused cells to lift due to the chelation of extracellular Ca2+; as a result, cells could only be imaged for up to 10–25 min after EGTA treatment. These studies were conducted five independent times, and representative images from one independent experiment are shown. ms, Millisecond; s, second.
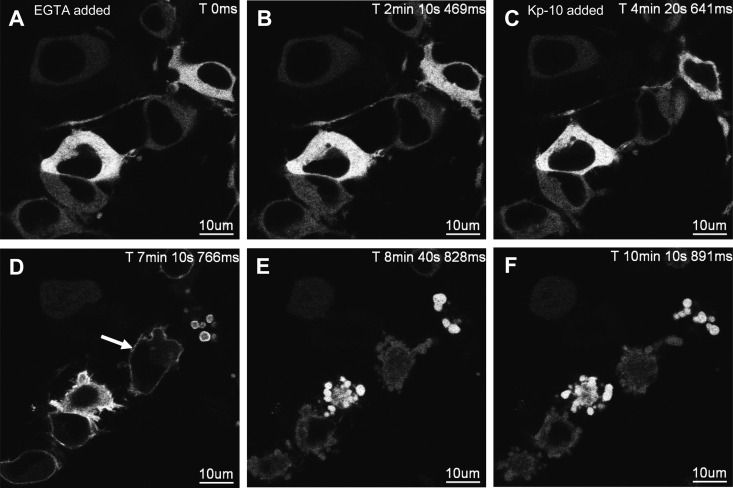
The latter finding was explored further by first treating cells coexpressing FLAG-KISS1R and GFP-PKC-βII with Kp-10 (Fig. 7A). Immediately after the rapid translocation of GFP-PKC-βII to the plasma membrane (Fig. 7B), cells were treated with EGTA, and the spatiotemporal properties of GFP-PKC-βII were assessed relative to Kp-10-treated only control cells (Fig. 4). In EGTA-treated cells, GFP-PKC-βII rapidly returned to the cytosol (Fig. 7, C–F); however, in the control cells most of the GFP-PKC-βII persisted at the plasma membrane for more than 1 h (Fig. 4).
Fig. 7.
The effect of EGTA treatment on the spatiotemporal characteristics of GFP-PKC-βII in HEK 293 cells after KISS1R activation. A–F, Representative images selected from a time series of laser-scanning confocal microscopic images showing the plasma membrane and cytosolic localization of GFP-PKC-βII in KISS1R-expressing HEK 293 cells after 100 nm Kp-10 treatment. Approximately 4 min Kp-activated cells were treated with 2.5 mm EGTA. White solid arrow shows translocation of GFP-PKC-βII (panel B). Prolonged exposure to EGTA caused cells to lift due to the chelation of extracellular Ca2+; as a result, cells could only be imaged for up to 10–25 min after EGTA treatment. These studies were conducted five independent times, and representative images from one independent experiment are shown. ms, Millisecond; s, second.
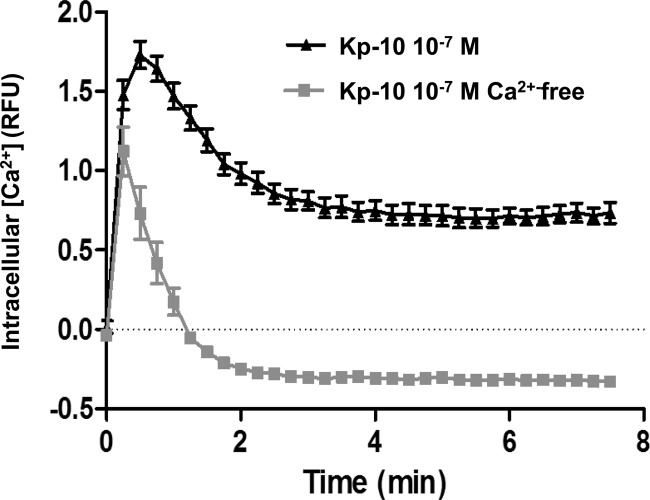
To further demonstrate that extracellular Ca2+ is required for maintaining the prolonged Kp-dependent activity of PKC, using a suspended population of CHO cells stably expressing KISS1R, we determined what effect an absence of extracellular Ca2+ had on Kp-10-induced free intracellular Ca2+ levels. Our results showed that, in the presence of extracellular Ca2+, there was a biphasic response in the mobilization of intracellular Ca2+ in response to Kp-10 treatment (Fig. 8). Specifically, there was a rapid and acute rise in free intracellular Ca2+, which plateaued out into a prolonged phase representing about 50% of the initial maximum response (Fig. 8). When the experiment was conducted in Ca2+-free media, the acute phase of intracellular Ca2+ mobilization, although smaller in amplitude and shorter in duration, was again observed. However, notably, the sustained Ca2+ response was completely abolished, and free intracellular Ca2+ dropped rapidly below the pretest basal level where it was subsequently maintained for the duration of the experiment (Fig. 8). These results suggest that the Kp-10-stimulated acute mobilization of Ca2+ is primarily the result of release of intracellular Ca2+ stores with a smaller contribution from the influx of extracellular Ca2+, whereas the sustained Ca2+ response is derived primarily from the influx of extracellular Ca2+.
Fig. 8.
The presence of extracellular Ca2+ is essential for a sustained Kp-10-induced Ca2+ response. CHO-KISS1R cells were treated with 100 nm Kp-10 in the presence or absence of extracellular calcium in the medium. The mobilization of intracellular Ca2+ was measured as relative fluorescence units (RFU) every 15 sec for 7.5 min. A single representative experiment is shown, which was repeated three times with similar results. The values shown represent the ratio of the difference between Kp-10-stimulated and basal values divided by the basal value at each time point.
Either stored intracellular or extracellular Ca2+ is sufficient for triggering the initial activation of PKC
Activation of conventional PKC-βII is dependent on the presence of DAG and Ca2+. However, neither BAPTA-AM alone, which chelates free intracellular Ca2+, nor EGTA alone, which chelates extracellular Ca2+, blocked the initial activation of PKC. Hence, we reasoned it must have been the Kp-10-dependent release of stored Ca2+ from the endoplasmic reticulum that was driving the initial activation of PKC, a conclusion strengthened by our previous data derived from examining Kp-induced Ca2+ mobilization in KISS1R-expressing CHO cells (Fig. 8). To test this idea, in HEK 293 cells coexpressing FLAG-KISS1R and GFP-PKC-βII, we simultaneously depleted intracellular Ca2+ stores using thapsigargin and chelated extracellular Ca2+ using EGTA and then determined the effect of Kp-10 treatment on PKC translocation. We began by examining the combined thapsigargin/EGTA treatment rather than thapsigargin treatment only. We reasoned that if thapsigargin blocked the initial activation of PKC, the eventual entry of extracellular Ca2+ into the cell would have still led to the activation of PKC and would not have allowed us to delineate a role for stored Ca2+ in the activation of PKC and KISS1R signaling.
We thus characterized the spatiotemporal properties of GFP-PKC-βII before and after Kp-treatment relative to thapsigargin/EGTA-untreated control cells. When cells were pretreated for 5 min with thapsigargin and EGTA, GFP-PKC-βII was localized to the cytosol (Supplemental Fig. 1, A and B) and was indistinguishable from untreated control cells (Fig. 4). After Kp-10 treatment, GFP-PKC-βII translocation to the plasma membrane in thapsigargin/EGTA-treated cells was barely detectable (Supplemental Fig. 1, C–F) but careful analysis revealed very weak translocation in some cells (see arrow, Supplemental Fig. 1, D–F). This was in direct contrast to control cells in which GFP-PKC-βII translocated rapidly to the plasma membrane and persisted for more than 1 h (Fig. 4) and to cells pretreated with EGTA in which GFP-PKC-βII translocated rapidly to the plasma membrane and returned rapidly to the cytosol (Fig. 6). Interestingly, when cells were pretreated with thapsigargin only, GFP-PKC-βII translocated rapidly to the plasma membrane where it persisted for a prolonged period in excess of 45 min (Supplemental Fig. 2) before slowly returning to the cytosol in a manner indistinguishable from control cells (Fig. 4) and cells treated with BAPTA-AM (Fig. 5).
Discussion
Recently, studies from our laboratories (17, 19) have provided detailed mechanistic insight into the desensitization of wild-type KISS1R as well as the underlying molecular defects in the naturally occurring KISS1R loss-of-function R331X (17) and gain-of-function R386P (19, 33) mutations. With respect to the wild-type receptor, both studies showed that KISS1R undergoes ligand-induced desensitization and rapid internalization. Although activated KISS1R does undergo rapid desensitization (17, 19), Bianco et al. (19) demonstrated that, mostly through the continuous recycling of the desensitized/resensitized receptor, the cell maintains a dynamic and active population of KISS1R at the cell surface, thus allowing for a protracted period of KISS1R signaling. Furthermore, in the case of R386P, the authors demonstrated that through reduced degradation of the mutant receptor, there is a larger population of mutant vs. wild-type receptor at the cell surface, thereby leading to further prolongation in responsiveness to Kp.
Given the potential clinical importance of KISS1R in treating an array of sex hormone-dependent disorders by either decreasing or increasing the rate of desensitization in KISS1R, we further explored the major findings of Bianco et al. (19). Here we hypothesized that upon chronic Kp-10 treatment, if a dynamic pool of receptor is maintained at the cell surface for a prolonged period, Kp will trigger the sustained activation of PLC, PKC, and prolonged mobilization of free intracellular Ca2+. As hypothesized, an analysis of PLC activation, a receptor-proximal event that strongly reflects Gαq/11 activation revealed that Kp does trigger prolonged signaling by KISS1R-expressing cells. Although we used the HEK 293 and to a lesser extent the GT1–7 GnRH neuronal cell models transiently expressing KISS1R, the temporal characteristics of prolonged Kp-dependent signaling in both cell types fits well with that described by Bianco et al. (19) for CHO cells stably expressing KISS1R. Overall, the recapitulation of findings in various cellular systems suggests that sustained KISS1R signaling is perhaps a more widespread property associated with cells that express the receptor than previously recognized.
Cells use Ca2+ to regulate a wide array of physiological processes, from the subsecond release of synaptic neurotransmitters to the regulation of gene expression. They do so by using both intracellular and extracellular Ca2+ sources to generate signals that transduce exogenous stimulation into physiological responses. It is well documented that prolonged elevations of Ca2+ lead to cell damage or death and accordingly, cells generally limit the spatiotemporal extent of their intracellular Ca2+ rises (34). It is thus not surprising that Ca2+ oscillations have emerged as an ubiquitous paradigm for cellular signal transduction (31, 35). By using brief pulses of Ca2+, instead of tonic rises, cells avoid the deleterious effects of sustained cytosolic Ca2+ levels. Furthermore, it has been suggested that because frequency modulation is more faithfully recapitulated, compared with amplitude modulation, cells would benefit more from Ca2+ oscillations in assuring signaling fidelity in response to a given stimulus (31, 34).
With this in mind, when we found that Kp-triggered a prolonged mobilization of free intracellular Ca2+, we considered that this was a physiologically irrelevant response to the 100 nm Kp-10 stimulus (31). However, even after a 100-fold reduction in agonist concentration, the prolonged mobilization of Ca2+ was still the predominant response. Furthermore, we noted that we are not the first group to report on the sustained rise in intracellular Ca2+ after Kp treatment. As early as 2008, using perforated-patch electrophysiology coupled to real-time Ca2+ imaging in coronal brain slices from adult female GnRH-Pericam mice, Liu et al. (23) demonstrated that 100 nm Kp evoked an approximately 10% increase in intracellular Ca2+ levels in GnRH neurons that lasted for 2–3 min before returning to pretest Ca2+ levels. More recently, in primary cultures of rat GnRH neurons, Kroll et al. (36) demonstrated that 100 nm Kp-10 triggered a rise in cytosolic Ca2+, and this was maintained not only during the chronic perfusion of Kp but even after the washout period. Based on these findings, we suggest that because similar responses can be induced in brain slices and primary cell cultures, our findings may also be relevant in a physiological context.
Another hallmark of Gαq/11-coupled 7TMR activation is the activation of the conventional PKC isoforms (α, βI, βII, and γ), which are regulated by Ca2+ and DAG and the novel PKC isoforms (δ, ϵ, η, and θ), which are regulated by DAG, but not Ca2+. We have previously used GFP-PKC-βII as a reporter molecule to successfully probe the activity of the Gαq/11-coupled metabotropic glutamate receptors in HEK 293 cells (26, 32) and have done the same here to assess KISS1R activity and downstream signaling in response to chronic Kp treatment. Based on the rapid redistribution of GFP-PKC-βII from the cytosol to the plasma membrane and its prolonged presence at the membrane in response to Kp treatment, we confirm that the prolonged activation of KISS1R translates into prolonged downstream signaling events as initially suggested by sustained activation of PLC and sustained levels of cytosolic Ca2+. Although it is assumed that the activation of a Gαq/11-coupled 7TMR would result in the activation of conventional and novel PKC isoforms, to the best of our knowledge, this is the first report confirming that Kp triggers PKC activation. The KISS1R-dependent signaling events that PKC regulates are currently unknown but they might include regulation of the KISS1R-coupled MAPK cascades. PKC might also play major roles in regulating the homologous desensitization of KISS1R (17, 37) and perhaps even the heterologous desensitization of KISS1R-trans-activated signaling systems (14).
In our study, we find that the spatiotemporal properties of PKC activation accurately reflected changes in cytosolic Ca2+ after Kp treatment. Compared with the Kp-dependent activation of GFP-PH-PLC-δ1, we also made a consistent and interesting finding regarding Kp-triggered GFP-PKC-βII activation. Here we found that although Kp triggered a rapid and prolonged activation of PKC, a substantial fraction (close to ∼50%) of the activated PKC rapidly desensitized and returned to the cytosol. This was in marked contrast to PLC activation. The reason for this is presently unknown, but we would suggest that as a fraction of membrane-bound KISS1R undergoes rapid desensitization, so does a fraction of the activated PKC population that was rapidly recruited to the plasma membrane.
Finally, we assessed the relative contribution of intracellular and extracellular Ca2+ in regulating prolonged KISS1R signaling. Specifically, we examined the roles of these two sources on GFP-PKC-βII activation. Our results clearly revealed that both intracellular and extracellular Ca2+ contribute to KISS1R-dependent activation of PKC. However, intracellular Ca2+, which is released downstream of Gαq/11-coupled 7TMR activation, appeared sufficient to trigger the initial activation of PKC because Kp, in the presence of EGTA, triggered a rapid and transient redistribution of GFP-PKC-βII in the cell. Interestingly, although intracellular Ca2+ mostly triggered the initial activation of PKC, it was not absolutely required because thapsigargin and BAPTA-AM pretreatment failed to block PKC activation. Instead, extracellular Ca2+ appeared sufficient to trigger the initial activation of PKC and more noteworthy was absolutely required for maintaining its prolonged activation. In an independent approach, these results, which were based on the analysis of single adherent HEK 293 cells transiently expressing KISS1R, were fully supported through the analysis of intracellular Ca2+ mobilization in a suspended population of CHO cells stably expressing KISS1R in the presence and absence of extracellular Ca2+. Our findings on the major role of extracellular Ca2+ in regulating the prolonged signaling downstream of KISS1R activation is supported by the findings of Kroll et al. (36) made in cultured rat GnRH neurons. In their study, the authors clearly demonstrated that the removal of Ca2+ from the external medium completely abolished the Kp-induced rise in Ca2+.
Initially, prolonged KISS1R signaling proved to be difficult for us to reconcile given that we had previously shown KISS1R undergoes rapid desensitization and internalization in HEK 293 cells (17). However, in light of the findings by Bianco et al. (19), it is now clear that prolonged signaling is not the result of an inability of individual KISS1R molecules to undergo desensitization, but instead it is the result of the cell maintaining a dynamic and active pool of receptors at the cell surface while KISS1R undergoes cycles of activation and desensitization. Thus, whereas the receptor undergoes Kp-dependent rapid desensitization, the receptor-expressing system (i.e. the cell) does not. This model would therefore adequately explain the results of a study by d'Anglemont de Tassigny et al. (22) in which the authors found that chronically Kp-stimulated hypothalamic explants took about 6 h to return to baseline GnRH secretion. It would also explain more recent clinical observations in which, despite chronically infusing Kp-10 for 22.5 h in healthy male subjects, KISS1R, as determined by LH secretion, did not show signs of desensitization (38). Given what we now know, it might be better stated that KISS1R-expressing cells, rather than KISS1R, failed to undergo Kp-dependent desensitization within that period of chronic infusion.
In conclusion, our results show that upon chronic stimulation, cells expressing KISS1R display a prolonged activation of the Gαq/11 signaling pathway as determined by assessing the activation of PLC, PKC, and mobilization of free intracellular Ca2+ in a nonneuronal and GnRH-secreting-neuronal cell line. These in vitro findings are well aligned with recent in vivo and ex vivo studies (22, 23, 36, 38) and, together with the findings from Bianco et al. (19), inform us that in some systems chronic Kp administration might not prove to be an effective therapy to rapidly desensitize the HPG axis, as once proposed. It also tells us that chronic infusion instead has the potential of being used to activate the axis without concerns about rapid desensitization, as was initially considered. Finally, although our findings might not impact directly upon the development of novel Kp/KISS1R-based therapies to treat conditions such as delayed and precocious puberty, it provides a greater understanding of how this important signaling system functions in diverse cell types.
Supplementary Material
Acknowledgments
This work was supported by the Natural Sciences and Engineering Research Council of Canada RGPIN/327334–2011 (to A.V.B.); Canadian Institutes of Health Research MOP 107972 (to M.B.); Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD), National Institutes of Health (NIH), through cooperative agreement U54 HD028138 as part of the Specialized Cooperative Centers Program in Reproduction and Infertility Research (to U.B.K.), by NIDDK/NIH T32 DK007529 (to L.M.) and by NICHD/NIH K08HD070957 (to L.M.).
Disclosure Summary: The authors have nothing to declare.
Footnotes
- CHO
- Chinese hamster ovary
- DAG
- diacylglycerol
- GFP
- green fluorescent protein
- GRK2
- G protein-coupled receptor kinase 2
- HEK
- human embryonic kidney
- InsP3
- inositol 1,4,5 trisphosphate
- KISS1R
- kisspeptin receptor
- Kp
- kisspeptin
- PH
- pleckstrin homology
- PIP2
- phosphatidylinositol 4,5-bisphosphate
- PKC
- protein kinase C
- PLC
- phospholipase C
- 7TMR
- seven-transmembrane receptor.
References
- 1. Kirby HR, Maguire JJ, Colledge WH, Davenport AP. 2010. International Union of Basic and Clinical Pharmacology. LXXVII. Kisspeptin receptor nomenclature, distribution, and function. Pharmacol Rev 62:565–578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Neer EJ. 1995. Heterotrimeric G proteins: organizers of transmembrane signals. Cell 80:249–257 [DOI] [PubMed] [Google Scholar]
- 3. Hamm HE. 1998. The many faces of G protein signaling. J Biol Chem 273:669–672 [DOI] [PubMed] [Google Scholar]
- 4. Hepler JR, Gilman AG. 1992. G proteins. Trends Biochem Sci 17:383–387 [DOI] [PubMed] [Google Scholar]
- 5. Lee DK, Nguyen T, O'Neill GP, Cheng R, Liu Y, Howard AD, Coulombe N, Tan CP, Tang-Nguyen AT, George SR, O'Dowd BF. 1999. Discovery of a receptor related to the galanin receptors. FEBS Lett 446:103–107 [DOI] [PubMed] [Google Scholar]
- 6. Kotani M, Detheux M, Vandenbogaerde A, Communi D, Vanderwinden JM, Le Poul E, Brézillon S, Tyldesley R, Suarez-Huerta N, Vandeput F, Blanpain C, Schiffmann SN, Vassart G, Parmentier M. 2001. The metastasis suppressor gene KiSS-1 encodes kisspeptins, the natural ligands of the orphan G protein-coupled receptor KISS1R. J Biol Chem 276:34631–34636 [DOI] [PubMed] [Google Scholar]
- 7. Muir AI, Chamberlain L, Elshourbagy NA, Michalovich D, Moore DJ, Calamari A, Szekeres PG, Sarau HM, Chambers JK, Murdock P, Steplewski K, Shabon U, Miller JE, Middleton SE, Darker JG, Larminie CG, Wilson S, Bergsma DJ, Emson P, Faull R, Philpott KL, Harrison DC. 2001. AXOR12, a novel human G protein-coupled receptor, activated by the peptide KiSS-1. J Biol Chem 276:28969–28975 [DOI] [PubMed] [Google Scholar]
- 8. Ohtaki T, Shintani Y, Honda S, Matsumoto H, Hori A, Kanehashi K, Terao Y, Kumano S, Takatsu Y, Masuda Y, Ishibashi Y, Watanabe T, Asada M, Yamada T, Suenaga M, Kitada C, Usuki S, Kurokawa T, Onda H, Nishimura O, Fujino M. 2001. Metastasis suppressor gene KiSS-1 encodes peptide ligand of a G-protein-coupled receptor. Nature 411:613–617 [DOI] [PubMed] [Google Scholar]
- 9. Castaño JP, Martínez-Fuentes AJ, Gutiérrez-Pascual E, Vaudry H, Tena-Sempere M, Malagón MM. 2009. Intracellular signaling pathways activated by kisspeptins through GPR54: do multiple signals underlie function diversity? Peptides 30:10–15 [DOI] [PubMed] [Google Scholar]
- 10. Clements MK, McDonald TP, Wang R, Xie G, O'Dowd BF, George SR, Austin CP, Liu Q. 2001. FMRFamide-related neuropeptides are agonists of the orphan G-protein-coupled receptor GPR54. Biochem Biophys Res Commun 284:1189–1193 [DOI] [PubMed] [Google Scholar]
- 11. de Roux N, Genin E, Carel JC, Matsuda F, Chaussain JL, Milgrom E. 2003. Hypogonadotropic hypogonadism due to loss of function of the KiSS1-derived peptide receptor KISS1R. Proc Natl Acad Sci USA 100:10972–10976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Seminara SB, Messager S, Chatzidaki EE, Thresher RR, Acierno JS, Jr, Shagoury JK, Bo-Abbas Y, Kuohung W, Schwinof KM, Hendrick AG, Zahn D, Dixon J, Kaiser UB, Slaugenhaupt SA, Gusella JF, O'Rahilly S, Carlton MB, Crowley WF, Jr, Aparicio SA, Colledge WH. 2003. The GPR54 gene as a regulator of puberty. N Engl J Med 349:1614–1627 [DOI] [PubMed] [Google Scholar]
- 13. Bilban M, Ghaffari-Tabrizi N, Hintermann E, Bauer S, Molzer S, Zoratti C, Malli R, Sharabi A, Hiden U, Graier W, Knöfler M, Andreae F, Wagner O, Quaranta V, Desoye G. 2004. Kisspeptin-10, a KiSS-1/metastin-derived decapeptide, is a physiological invasion inhibitor of primary human trophoblasts. J Cell Sci 117:1319–1328 [DOI] [PubMed] [Google Scholar]
- 14. Yi T, Tan K, Cho SG, Wang Y, Luo J, Zhang W, Li D, Liu M. 2010. Regulation of embryonic kidney branching morphogenesis and glomerular development by KISS1 receptor (Gpr54) through NFAT2- and Sp1-mediated Bmp7 expression. J Biol Chem 285:17811–17820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Silvestre RA, Egido EM, Hernández R, Marco J. 2008. Kisspeptin-13 inhibits insulin secretion without affecting glucagon or somatostatin release: study in the perfused rat pancreas. J Endocrinol 196:283–290 [DOI] [PubMed] [Google Scholar]
- 16. Zajac M, Law J, Cvetkovic DD, Pampillo M, McColl L, Pape C, Di Guglielmo GM, Postovit LM, Babwah AV, Bhattacharya M. 2011. GPR54 (KISS1R) transactivates EGFR to promote breast cancer cell invasiveness. PLoS One 6:e21599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Pampillo M, Camuso N, Taylor JE, Szereszewski JM, Ahow MR, Zajac M, Millar RP, Bhattacharya M, Babwah AV. 2009. Regulation of GPR54 signaling by GRK2 and β-arrestin. Mol Endocrinol 23:2060–2074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Szereszewski JM, Pampillo M, Ahow MR, Offermanns S, Bhattacharya M, Babwah AV. 2010. GPR54 regulates ERK1/2 activity and hypothalamic gene expression in a Gα(q/11) and β-arrestin-dependent manner. PLoS One 5:e12964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bianco SD, Vandepas L, Correa-Medina M, Gereben B, Mukherjee A, Kuohung W, Carroll R, Teles MG, Latronico AC, Kaiser UB. 2011. KISS1R intracellular trafficking and degradation: effect of the Arg386Pro disease-associated mutation. Endocrinology 152:1616–1626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Han SK, Gottsch ML, Lee KJ, Popa SM, Smith JT, Jakawich SK, Clifton DK, Steiner RA, Herbison AE. 2005. Activation of gonadotropin-releasing hormone neurons by kisspeptin as a neuroendocrine switch for the onset of puberty. J Neurosci 25:11349–11356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Messager S, Chatzidaki EE, Ma D, Hendrick AG, Zahn D, Dixon J, Thresher RR, Malinge I, Lomet D, Carlton MB, Colledge WH, Caraty A, Aparicio SA. 2005. Kisspeptin directly stimulates gonadotropin-releasing hormone release via G protein-coupled receptor 54. Proc Natl Acad Sci USA 102:1761–1766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. d'Anglemont de Tassigny X, Fagg LA, Carlton MB, Colledge WH. 2008. Kisspeptin can stimulate gonadotropin-releasing hormone (GnRH) release by a direct action at GnRH nerve terminals. Endocrinology 149:3926–3932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Liu X, Lee K, Herbison AE. 2008. Kisspeptin excites gonadotropin-releasing hormone neurons through a phospholipase C/calcium-dependent pathway regulating multiple ion channels. Endocrinology 149:4605–4614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Pielecka-Fortuna J, Chu Z, Moenter SM. 2008. Kisspeptin acts directly and indirectly to increase gonadotropin-releasing hormone neuron activity and its effects are modulated by estradiol. Endocrinology 149:1979–1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zhang C, Roepke TA, Kelly MJ, Rønnekleiv OK. 2008. Kisspeptin depolarizes gonadotropin-releasing hormone neurons through activation of TRPC-like cationic channels. J Neurosci 28:4423–4434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Dale LB, Babwah AV, Bhattacharya M, Kelvin DJ, Ferguson SS. 2001. Spatial-temporal patterning of metabotropic glutamate receptor-mediated inositol 1,4,5-triphosphate, calcium, and protein kinase C oscillations: protein kinase C-dependent receptor phosphorylation is not required. J Biol Chem 276:35900–35908 [DOI] [PubMed] [Google Scholar]
- 27. Kuohung W, Burnett M, Mukhtyar D, Schuman E, Ni J, Crowley WF, Glicksman MA, Kaiser UB. 2010. A high-throughput small molecule ligand screen targeted to agonists and antagonists of the G-protein-coupled receptor GPR54. J Biomol Screen 15:508–517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Stauffer TP, Ahn S, Meyer T. 1998. Receptor-induced transient reduction in plasma membrane PtdIns(4,5)P2 concentration monitored in living cells. Curr Biol 8:343–346 [DOI] [PubMed] [Google Scholar]
- 29. Hirose K, Kadowaki S, Tanabe M, Takeshima H, Iino M. 1999. Spatiotemporal dynamics of inositol 1,4,5-trisphosphate that underlies complex Ca2+ mobilization patterns. Science 284:1527–1530 [DOI] [PubMed] [Google Scholar]
- 30. Lemmon MA, Ferguson KM, Schlessinger J. 1996. PH domains: diverse sequences with a common fold recruit signaling molecules to the cell surface. Cell 85:621–624 [DOI] [PubMed] [Google Scholar]
- 31. Dupont G, Combettes L, Bird GS, Putney JW. 2011. Calcium oscillations. Cold Spring Harb Perspect Biol 3:a004226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Babwah AV, Dale LB, Ferguson SS. 2003. Protein kinase C isoform-specific differences in the spatial-temporal regulation and decoding of metabotropic glutamate receptor1a-stimulated s messenger responses. J Biol Chem 278:5419–5426 [DOI] [PubMed] [Google Scholar]
- 33. Teles MG, Bianco SD, Brito VN, Trarbach EB, Kuohung W, Xu S, Seminara SB, Mendonca BB, Kaiser UB, Latronico AC. 2008. A GPR54-activating mutation in a patient with central precocious puberty. N Engl J Med 358:709–715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Thul R, Bellamy TC, Roderick HL, Bootman MD, Coombes S. 2008. Calcium oscillations. Adv Exp Med Biol 641:1–627 [DOI] [PubMed] [Google Scholar]
- 35. Putney JW, Jr, Poggioli J, Weiss SJ. 1981. Receptor regulation of calcium release and calcium permeability in parotid gland cells. Philos Trans R Soc Lond B Biol Sci 296:37–45 [DOI] [PubMed] [Google Scholar]
- 36. Kroll H, Bolsover S, Hsu J, Kim SH, Bouloux PM. 2011. Kisspeptin-evoked calcium signals in isolated primary rat gonadotropin-releasing hormone neurones. Neuroendocrinology 93:114–120 [DOI] [PubMed] [Google Scholar]
- 37. Hauger RL, Olivares-Reyes JA, Braun S, Catt KJ, Dautzenberg FM. 2003. Mediation of corticotropin releasing factor type 1 receptor phosphorylation and desensitization by protein kinase C: a possible role in stress adaptation. J Pharmacol Exp Ther 306:794–803 [DOI] [PubMed] [Google Scholar]
- 38. George JT, Veldhuis JD, Roseweir AK, Newton CL, Faccenda E, Millar RP, Anderson RA. 2011. Kisspeptin-10 is a potent stimulator of LH and increases pulse frequency in men. J Clin Endocrinol Metab 96:E1228–E1236 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.