Abstract
Apolipoprotein AIV (apo AIV) and cholecystokinin (CCK) are gastrointestinal satiation signals that are stimulated by fat consumption. Previous studies have demonstrated that peripheral apo AIV cannot cross the blood-brain barrier. In the present study, we hypothesized that peripheral apo AIV uses a CCK-dependent system and intact vagal nerves to relay its satiation signal to the hindbrain. To test this hypothesis, CCK-knockout (CCK-KO) mice and Long-Evan rats that had undergone subdiaphragmatic vagal deafferentation (SDA) were used. Intraperitoneal administration of apo AIV at 100 or 200 μg/kg suppressed food intake of wild-type (WT) mice at 30, 60, and 90 min. In contrast, the same dose did not reduce food intake in the CCK-KO mice. Blockade of the CCK 1 receptor by lorglumide, a CCK 1 receptor antagonist, attenuated apo AIV-induced satiation. Apo AIV at 100 μg/kg reduced food intake in SHAM rats but not in SDA rats. Furthermore, apo AIV elicited an increase in c-Fos-positive cells in the nucleus of the solitary tract (NTS), area postrema, dorsal motor nucleus of the vagus, and adjacent areas of WT mice but elicited only an attenuated increase in these same regions in CCK-KO mice. Apo AIV-induced c-Fos positive cells in the NTS and area postrema of WT mice were reduced by lorglumide. Lastly, apo AIV increased c-Fos positive cells in the NTS of SHAM rats but not in SDA rats. These observations imply that peripheral apo AIV requires an intact CCK system and vagal afferents to activate neurons in the hindbrain to reduce food intake.
According to the National Health and Nutrition Examination Survey, obesity is an epidemic in the United States with a prevalence rate of more than 30% for adults, as reported in 2007–2008 (1). Obesity increases the risk of type 2 diabetes mellitus, hypertension, coronary heart disease, and cancers of the breast and prostate (2). One of the key contributors to obesity is a high-fat diet, which promotes excess energy intake and leads to the development of obesity and insulin resistance (3). Gastrointestinal peptides such as cholecystokinin (CCK) and apolipoprotein AIV (apo AIV) contribute to the control of food intake and energy homeostasis (4). CCK is released from duodenal I cells via a chylomicron-dependent pathway after the consumption of dietary lipids (5, 6). The biological functions of CCK include stimulation of pancreatic enzyme release and gall bladder contraction as well as suppression of food intake, i.e. peripheral administration of exogenous CCK reduces food intake (7–9). Conversely, the application of either CCK1 receptor (CCK1R) antagonists or vagal deafferentation abolishes the satiation effect induced by peripheral administration of CCK (7, 10). The generally accepted model is that the inhibitory action of peripheral CCK on food intake is mediated via CCK1R on vagal afferent nerves passing from the wall of the intestine to the hindbrain.
Apo AIV is a major protein constituent of lymphatic triglyceride-rich lipoproteins, and its secretion by enterocytes lining the small intestine is mediated by chylomicron formation in response to a lipid meal (11). Apo AIV is associated with circulating high-density lipoproteins and plays a role in cholesterol transport and lipid metabolism (12). Apo AIV is also synthesized in the hypothalamus and the hindbrain (13, 14). Exogenous administration of apo AIV acts peripherally as well as centrally to suppress food intake (13, 15, 16). Therefore, dietary lipid-induced apo AIV is an important endogenous satiating signal. Peripheral coadministration of apo AIV and CCK has an additive effect in inhibiting food intake via CCK1R (17). However, the precise interaction of CCK and apo AIV in the control of food intake remains unknown. Because circulating apo AIV cannot cross the blood-brain barrier (14), ip-administered apo AIV must have a peripheral site of action. Therefore, we hypothesized that apo AIV interacts with intestinal CCK and that such interaction requires intact vagal afferents to relay the satiating message to the hindbrain. The aims of the present experiments were to determine whether: 1) peripheral apo AIV requires CCK to function as a satiating signal; and 2) peripheral apo AIV-induced satiation is mediated via CCK1R and vagal afferents.
Materials and Methods
Animals
Male CCK-knockout (CCK-KO) and wild-type (WT) mice (C57BL/6J background) were generated in an Association Assessment and Accreditation of Laboratory Animal Care-accredited facility under conditions of controlled illumination (12 h light, 12 h dark cycle, lights on from 0600 to 1800 h). The CCK-KO mice were backcrossed for more than 10 generations onto a C57BL/6J genetic background, and all the mice were genotyped by PCR analysis of the tail DNA (18). Male Long-Evans rats weighing 200 g were obtained from Harlan Sprague Dawley, Inc. (Indianapolis, IN). The rats had free access to pelleted chow (7002 Teklad 6% fat mouse/rat diet; Harlan Teklad, Madison, WI) and water. All animals were transferred to clean cages and deprived of food for 17 h (1700–1000 h) before each experiment. All animal protocols were approved by the University of Cincinnati Institutional Animal Care and Use Committee.
Subdiaphragmatic vagal deafferentation (SDA) surgery
Twenty-four Long-Evans rats (Harlan, Indianapolis, IN, 190–200 g) were housed in a facility for 14 d before surgery. Rats consumed a nutritionally complete liquid diet (Fortify; Kroger Co., Cincinnati, OH) for 4 d before surgery. All rats then underwent either SDA (n = 12) or sham surgery in which the same procedure was followed but the deafferentation was not made (SHAM, n = 12), according to our previous protocol (19). Briefly, left-side intracranial vagal rhizotomy and transection of the dorsal (left) subdiaphragmatic trunk of the vagus were performed in SDA rats, resulting in complete subdiaphragmatic vagal deafferentation (20). SHAM rats had a similar surgery exposing the vagal rootlets and dorsal subdiaphragmatic vagus, but all afferent and efferent nerves were left intact. For postsurgery care, rats continued to receive the liquid diet for 2 d and then a semiliquid diet for 4 d. After a 14-d recovery, functional verification of complete SDA was performed on 4-h fasted rats. Rats received 4 μg/kg CCK-8 (Sigma, St. Louis, MO) ip, and food was returned just before dark onset (1800 h). Food intake was measured after 30 min. The reduction in food intake due to CCK-8 was 70.2 ± 7.6% in SHAM rats and 4.9 ± 2.2% in SDA rats (Table 1). It is known that CCK satiating signals are relayed to the brain via the CCK1R on vagal afferent fibers (10). Therefore, the verification results are consistent with previous reports (10, 21) because the SDA rats deprived of abdominal vagal afferent input had no reliable reduction in food intake in response to ip CCK-8. In addition, based on previous analyses (19), SDA rats with successful elimination of vagal afferent nerves have less than 30% reduction in food intake by CCK administration, which is consistent with the present findings.
Table 1.
Body weight and verification in SDA and SHAM rats
| Surgery performed | SDA | SHAM |
|---|---|---|
| Initial BW (g) | 242.5 ± 2.18 | 233.8 ± 2.18 |
| Final BW (g) | 339.5 ± 7.47 | 346.2 ± 7.47 |
| 30-min reduction of food intake by CCK-8 | 4.9 ± 2.2% | 70.2 ± 7.6% |
| Verified number | 9 | 9 |
Data are body weight and number of SDA and SHAM Long-Evans rats. The CCK exclusion criterion for SDA rats was that the reduction of food intake greater than 30% in rats after ip administration of CCK-8 at 4 μg/kg. Data represent means ± sem. BW, Body weight.
Food intake in mice and rats
To determine meal patterns of CCK-KO and WT mice after the ip administration of mouse recombinant apo AIV, at 20 wk of age, all mice (n = 8/group) were acclimatized to individual metabolic cages for 3 d before the start of data collection. Mice had free access to powdered chow (7002 Teklad 6% fat mouse/rat diet; Harlan Teklad), and food intake was recorded for 3 d using the DietMax consumption system (Accuscan Instruments Inc., Columbus, OH). Body weight and water consumption were measured by weighing animals and water bottles (±0.01 g, Adenturer SL; Ohaus Corp., Pine Brook, NJ). In experiment 1, after an injection of 0.1 ml of either mouse recombinant apo AIV (50,100, or 200 μg/kg) or vehicle (saline) to 17-h fasted mice, food was returned and intake was assessed in the DietMax system at single-minute intervals for 24 h. In experiment 2, lorglumide was used to block CCK1Rs (17). On the test day, 17-h fasted WT mice (n = 8 per group) were administered two injections. The injections contained saline (0.1 ml) + saline (0.1 ml), saline (0.1 ml) + apo AIV (200 μg/kg in 0.1 ml saline), or lorglumide (0.3 mg/kg in 0.1 ml saline) + apo AIV (200 μg/kg in 0.1 ml saline). The first injection (saline or lorglumide) occurred 15 min before the second injection (saline or apo AIV), and food was returned immediately after the second injection. Intake was monitored in the DietMax system. Recombinant mouse apo AIV was produced by a bacterial expression system and the detailed procedure is described in a previous report (13).
Lastly, to determine the necessity of vagal afferent nerves to mediate the effect of apo AIV ip on food intake, fasted SDA and SHAM rats (n = 9 per group) received 0.2 ml of either recombinant apo AIV (100 μg/kg, an effective dose) or vehicle (saline) following a 17-h fast (17). Food was immediately returned, and food intake was assessed after 30, 60, and 90 min.
Neuronal activation in the hindbrain
To determine neuronal action in the hindbrain induced by peripheral apo AIV, 17-h fasted WT and CCK-KO mice (five to six mice per group) were killed after ip administration of either saline (0.1 ml) or apo AIV at 200 μg/kg in 0.1 ml saline. In the CCK1R study, 17 h-fasted WT mice (four mice per group) received two ip injections of saline (0.1 ml) + saline (0.1 ml), saline (0.1 ml) + apo AIV (200 μg/kg in 0.1 ml saline), or lorglumide (0.3 mg/kg in 0.1 ml saline) + AIV (200 μg/kg in 0.1 ml saline). In the SDA study, SDA and SHAM rats (three to four rats per group) were fasted for 17 h and killed after an ip injection of either saline (0.2 ml) or rat recombinant apo AIV at 100 μg/kg in 0.2 ml saline. After 90 min, the brain was perfused through the left ventricle of the heart with 0.9% saline, followed by perfusion of 4% paraformaldehyde to fix the brain (22, 23). The hindbrains were postfixed in 4% paraformaldehyde and in 30% sucrose-phosphate buffer. Regions of the hindbrain were identified according to the information provided by the mouse/rat brain stereotaxic atlas of Paxinos and Franklin (24). The frozen brain was sliced with a microtome (cut 4055; Olympus, Tokyo, Japan) and brain sections (30 μm) were stained with diluted c-Fos Ab-5 (1:30,000; Calbiochem, Gibbstown, NJ) and biotinylated goat antirabbit secondary antibody (dilution 1:200) in normal goat serum plus 0.3% Triton X-100 buffer. The sections were incubated with horseradish peroxidase avidin-biotin complex (dilution 1:200) and developed by diaminobenzidine with nickel sulfate. As negative controls, sections were incubated with nonimmune rabbit serum instead of the primary antibody to test for nonspecific staining.
Data analysis and statistical analysis
Quantitative assessment of the c-Fos data were achieved by counting the number of c-Fos-positive cells per slide in the hindbrain (23). All slides were numbered without the treatment information to avoid bias in counting, and each hindbrain was bilaterally counted in at least 10 different sections for rats and five different sections for mice. c-Fos-positive neurons were identified according to the photomicrographs depicted in nucleus of the solitary tract (NTS) from Bregma −13.24 to −14.6 mm for rats and from Bregma −7.08 to −8.0 mm for mice according to the rat/mouse brain in stereotaxic coordinates (24). Cells with distinct brown nuclear c-Fos-like immunoreactivity staining in various nuclei of NTS were manually counted under light microscopy with low magnification (×40) with the aid of a 1-mm2 ruler (23). When counting in adjacent squares, c-Fos-positive cells laid on a borderline between the 1-mm2 square markers were not repeatedly counted. Data are presented as means ± sem of the average number of cells per slide. An increase in the percentage of c-Fos-positive cells in response to apo AIV was calculated as the average number of c-Fos-positive cells for each animal after apo AIV treatment minus the average number of c-Fos-positive cells after saline treatment divided by the average number of c-Fos cells after saline treatment. All values are presented as means ± se. The parametric statistical analyses, one-way ANOVA, and two-way ANOVA were performed using GraphPad Prism (version 5.0; San Diego, CA), followed by post hoc Newman-Keuls or Bonferroni test, respectively. All differences were considered significant if the values were P < 0.05.
Results
Experiment 1. Peripheral apo AIV reduction of food intake requires an intact CCK system
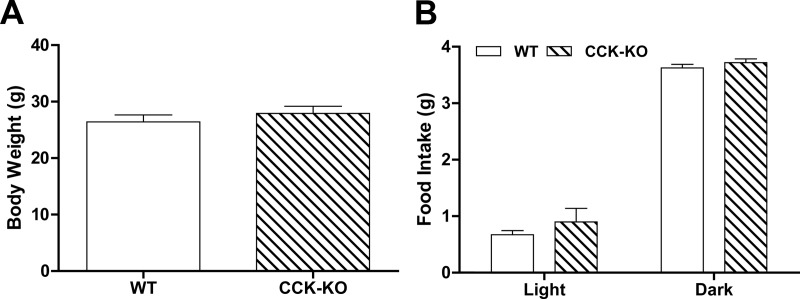
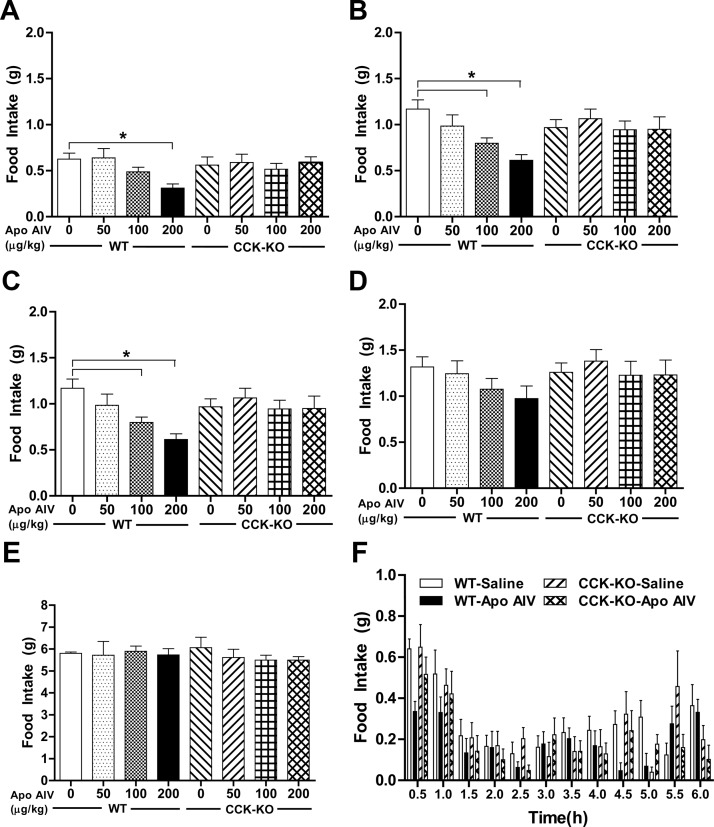
To test the hypothesis that peripheral apo AIV reduces food intake via a CCK-dependent system, we used a CCK-KO model. CCK-KO and WT mice maintained on chow had comparable body weight and food intake in the dark and light periods (Fig. 1, A and B). After the lowest dose of CCK-8 (50 μg/kg), neither WT nor CCK-KO mice had decreased food intake within our assessment period (0.5–2 h), suggesting that this dose is subthreshold for the reduction of food intake in these mice. At 30 min, only the highest dose of ip apo AIV (200 μg/kg) significantly reduced food intake in WT mice relative to saline-treated mice (Fig. 2A, P < 0.01). At 60 and 90 min, WT mice, but not CCK-KO mice, had a significant reduction in food intake compared with saline-treated animals after ip administration of apo AIV above 100 μg/kg (Fig. 2, B and C, P < 0.01). At 2 h, no significant differences in food intake were found in either WT mice or CCK-KO mice (Fig. 2D). Importantly, at no dose of apo AIV, and at no time point, was there a reduction of food intake in CCK-KO animals. The 24-h cumulative food intakes in WT and CCK-KO mice after different apo AIV treatments were comparable with those of the saline-treated groups (Fig. 2E). Relative to the saline-treated controls, WT mice treated with 200 μg/kg apo AIV had decreased meal size over the 6-h assessment period (Fig. 2F). In contrast, apo AIV-treated CCK-KO mice had comparable meal size to that of saline-treated CCK-KO mice. The data suggest that the decreased food intake by apo AIV in WT mice is due to a reduction of meal size and that this inhibitory effect requires an intact CCK system.
Fig. 1.
Body weight and daily food intake in CCK-KO and WT mice. Body weight (A) and daily food intake (B) were determined using the DietMax food system (Accuscan Instruments) after 5 d acclimatization. Data are expressed as mean ± sem for 10 animals per group.
Fig. 2.
Effect of apo AIV on the control of food intake in CCK-KO and WT mice. Fasted mice were injected ip with either Apo AIV (50, 100, and 200 μg/kg in saline) or saline. Food intake was determined at 30 min (A), 60 min (B), 90 min (C), 120 min (D), and 24 h (E) in mice. Meal size (F) in mice was monitored over the 6-h assessment period. Data are expressed as mean ± sem (n = 10). Values with asterisks represent significant differences relative to the WT mice (P < 0.05).
To confirm that apo AIV is less efficacious at inhibiting food intake in CCK-KO mice than in WT mice, we calculated the percentage change relative to a saline injection for each mouse after each dose of apo AIV at three time points (30, 60, and 90 min). A two-away ANOVA followed by post hoc tests revealed that WT mice had a significantly greater reduction of food intake than CCK-KO mice in response to apo AIV (200 μg/kg) at 60 and 90 min (P < 0.05). There were no interactions between strains and treatments at 60 or 90 min. These findings indicate that mice lacking the CCK gene have reduced satiating sensitivity in response to peripheral apo AIV treatment.
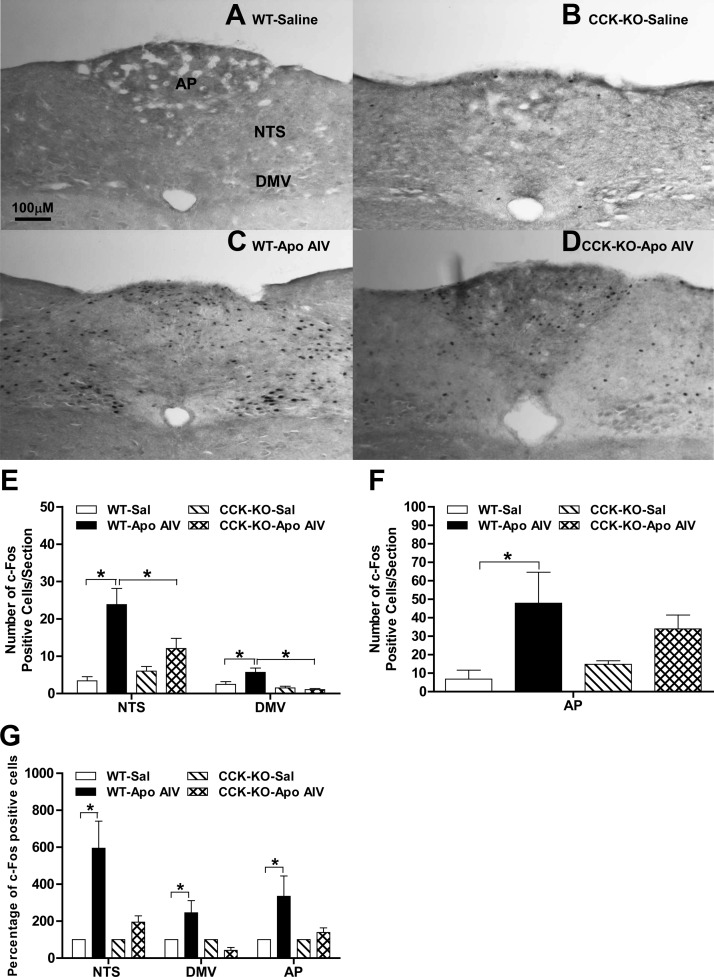
The immunoreactive protein, c-Fos, is commonly used as a marker of neuronal activity in brains (25). Due to greater reduction of food intake in WT mice after apo AIV ip at 200 μg/kg at 90 min, brains were collected after apo AIV (200 μg/kg) ip at this time point. As depicted in Fig. 3, A–F, apo AIV significantly increased the number of c-Fos-positive cells in the area postrema (AP) and the NTS (NTS/AP) and in the dorsal motor nucleus of the vagus (DMV) in WT mice relative to saline-treated groups (P < 0.05). In contrast, apo AIV-treated CCK-KO mice had significantly fewer c-Fos-positive cells in these same areas (P < 0.05). A two-way ANOVA revealed that significant differences in the percentage of c-Fos-positive cells in both the NTS and AP were also found between strains and treatments (Fig. 3G). In the DMV, there was a significant difference in the percentage of c-Fos cells between strains and a significant interaction. These findings suggest that peripheral apo AIV activates neurons in the NTS, DMV, and AP and that activation in these nuclei is mediated via a CCK-dependent pathway.
Fig. 3.
Effect of apo AIV on c-Fos expression in CCK-KO and WT mice. Fasted mice received either 0.1 ml of ip saline (A and B) or apo AIV at 200 μg/kg (C and D) for 90 min. The images of diaminobenzidine-stained c-Fos proteins in the NTS are as follows: WT mice (A and C); CCK-KO mice (B and D); number of c-Fos cells in the NTS and DMV (E) and the AP (F); and the percentage of c-Fos cells in the NTS (G). Data are expressed as mean ± sem for five to six animals per group, and values with asterisks represent significant differences relative to the WT mice (P < 0.05).
Experiment 2. CCK1Rs are necessary for apo AIV-induced satiation
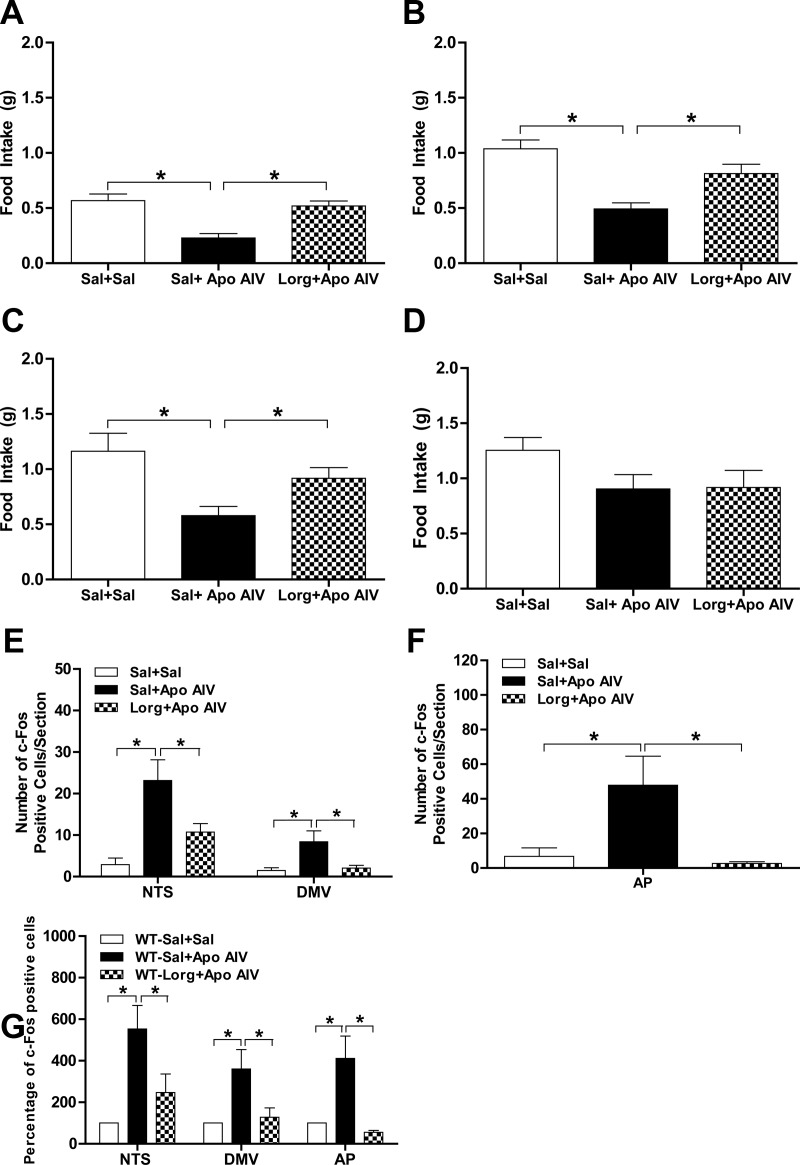
To determine whether apo AIV-induced satiation is CCK1R dependent, combinations of a CCK1R antagonist, lorglumide, and apo AIV were applied to WT mice. After saline plus apo AIV at 200 μg/kg, mice had a significant reduction of food intake relative to saline-treated controls at 30, 60, and 90 min (Fig. 4, A–C). In contrast, mice with lorglumide plus apo AIV had comparable food intake as saline-treated mice at 30, 60, and 90 min (Fig. 4, A–C). At 2 h, mice treated with either apo AIV plus saline or apo AIV plus lorglumide had comparable food intake relative to saline-treated animals (Fig. 4D). These findings indicate that the pharmacological blockade of CCK1R attenuates apo AIV-induced inhibition of food intake. As depicted in Fig. 4, E and F, the combination of lorglumide plus apo AIV significantly decreased the number of c-Fos-positive cells in the NTS, DMV, and AP relative to apo AIV-treated mice (P < 0.05). The combination of apo AIV plus saline increased the percentage of c-Fos-positive cells in the NTS, DMV, and AP significantly more than apo AIV plus lorglumide (Fig. 4G, P < 0.05). These findings suggest that CCK1Rs are essential for peripheral apo AIV-induced satiation and neuronal activation in the NTS, DMV, and AP.
Fig. 4.
Blockade of CCK1R attenuated apo AIV-induced satiation and c-Fos-positive cells. Fasted mice were injected ip with either the combination of apo AIV (200 μg/kg in saline) and saline, apo AIV (200 μg/kg in saline) and lorglumide (0.3 mg/kg in saline) or two injections of saline. Food intake was monitored at 30 min (A), 60 min (B), 90 min (C), and 120 min (D) in WT mice (n = 7–8 per group). The number of c-Fos cells in the NTS and DMV (E), in the AP (F), and the percentage of c-Fos positive cells (G) in the NTS were determined in mice (n = 4 per group). Data are expressed as mean ± sem and asterisks indicate significant differences from the other group (P < 0.05). Sal, Saline; Lorg, lorglumide.
Experiment 3. Peripheral apo AIV requires intact vagal afferent nerves to elicit satiation
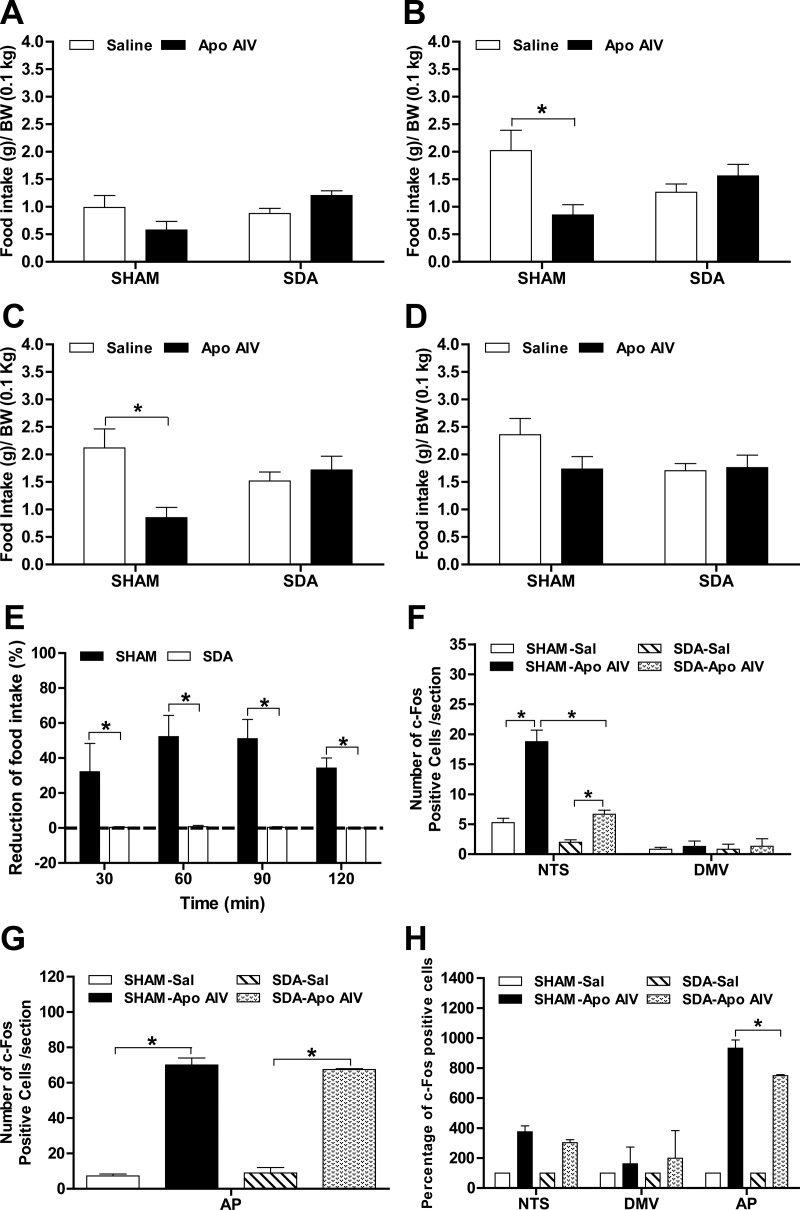
Before surgery, SDA and SHAM rats had comparable body weights (Table 1). After surgery, SDA rats gained slightly less body weight than SHAM rats, but the difference did not reach significance (Table 1). Fasted SDA rats consumed comparable amounts of food relative to SHAM rats in response to refeeding (34.3 ± 3.8 and 41.3 ± 1.8 g, respectively, P = NS). To determine whether the effect of peripheral apo AIV on food intake is mediated via vagal afferents, SDA and SHAM rats received either saline or rat recombinant apo AIV at 100 μg/kg ip, an effective dose, in suppressing food intake in normal rats (17). At 30 min, apo AIV did not decrease food intake in SHAM rats (Fig. 5A). At 60 and 90 min, apo AIV significantly reduced food intake in SHAM rats (Fig. 5, B and C, P < 0.05). In contrast, apo AIV had no significant effect on food intake in SDA rats at 30, 60, or 90 min. At 2 h, all treated groups had comparable food intake (Fig. 5D). In Fig. 5E, SHAM rats had significantly greater reduction of food intake at 30, 60, 90, and 120 min than SDA rats (Fig. 5E, P < 0.01). A significant difference was found between surgical groups and treatments, and there was a significant interaction at these time points (P < 0.05). Thus, exogenous apo AIV at 100 μg/kg does not reduce food intake in rats lacking intact vagal afferent nerves, implying that the afferent vagus is necessary for apo AIV's satiating action.
Fig. 5.
Effect of Apo AIV on food intake and c-Fos-positive cells in the NTS of rats. Fasted rats received ip apo AIV (100 μg/kg in saline) or saline. Food intake was monitored at 30 min (A), 60 min (B), 90 min (C), and 120 min (D) in SDA and SHAM rats. The reduction of food intake (E) was calculated in SDA and SHAM rats. The number of c-Fos cells in the NTS and DMV (F), in the AP (G), and the percentage of c-Fos-positive cells (H) in the NTS were determined in the SDA and SHAM rats. The reduction in food intake (percentage) is calculated as 100 minus the percentage change relative to a saline injection for each rat after apo AIV treatments at four time points (30, 60, 90, and 120 min). Data are expressed as mean ± sem (n = 9). Values with asterisks represent significant differences relative to the WT mice (P < 0.05). After a 17-h fast, rats (n = 3–4) received apo AIV at 100 μg/kg or saline, and the hindbrains were collected after 90 min. Data are expressed as means ± sem and asterisks indicate significant differences from the other group (P < 0.05).
After ip apo AIV (100 μg/kg) administration, the number of c-Fos-positive cells increased in the NTS, DMV, and AP of SHAM rats relative to administration of the vehicle (Fig. 5, F and G, P < 0.05). Apo AIV administration also increased the number of c-Fos cells in the AP and NTS in SDA rats relative to that in saline-treated SDA rats (P < 0.05). Peripheral apo AIV induced fewer c-Fos positive cells in the NTS of SDA rats than of SHAM rats (P < 0.05). No significant differences in the number of c-Fos cells were found between surgical groups and treatments in DMV. As depicted in Fig. 5H, a significant difference in the percentage of c-Fos cells in the AP was found between surgical groups and treatments, and there was a significant interaction. However, there were no significant differences in the percentage of c-Fos-positive cells in the NTS between surgical groups or treatments and no interaction. These data suggest that SDA attenuates apo AIV-induced neuronal activation in the NTS and AP.
Discussion
Peripheral administration of CCK-8 reduces food intake, and the satiation signals are relayed via CCK1R on vagal afferent nerves (10, 26, 27). CCK-induced satiating signals are attenuated by CCK1R antagonists (28, 29). CCK-KO mice were used to determine whether peripheral administration of apo AIV at effective doses requires an intact CCK system to suppress food intake. CCK-KO mice do not produce any functional CCK and have comparable body weight, food intake, and fat mass as WT mice when maintained on chow (18, 30). The CCK-KO mice have slightly increased food intake during the light period but have normal total food intake (30). In addition, when maintained on low-fat diets, CCK-KO mice have normal plasma glucose, leptin, and insulin (31) as well as plasma lipid content (our unpublished data) compared with WT mice. In the present study, CCK-KO mice had comparable body weight and food intake as WT mice. Apo AIV administration suppressed food intake in WT mice by decreasing meal size, and this satiating effect lasted for 90 min. In contrast, the ability of apo AIV to reduce intake in CCK-KO mice was greatly attenuated. Recently we reported that fasted apo AIV KO mice have a greater satiating response to CCK than their WT controls, indicating that endogenous apo AIV and exogenous CCK interact to inhibit food intake in vivo (32).
These findings suggest that the combination of apo AIV and CCK might co-dependently reduce food intake via CCK1R, which appears not to be consistent with our previous report of an additive interaction of CCK and apo AIV to inhibit food intake (17). In the previous study of apo AIV KO mice, the increased sensitivity in response to CCK might have been due to increased CCK1R expression in the nodose ganglia and/or NTS (32). CCK-KO mice have normal CCK1R in pancreas (18) and secrete comparable amounts of plasma apo AIV as determined by immunoblots (our unpublished data). It is thus possible that the level of CCK1R in CCK-KO mice might be responsible for abolishing the satiating response to exogenous apo AIV in the present study. Further experiments in determining CCK1R expression in nodose ganglia and the NTS in CCK-KO mice might be required to pursue the mechanisms for the abolished apo AIV-induced satiation.
Because the CCK-KO mouse is a global knockout model, the involvement of peripheral vs. central CCK in the apo AIV-induced satiating effect remains unclear in the present study. Circulating apo AIV does not cross the blood-brain barrier (14) but is able to increase CCK-elicited activity in vagal afferent fibers, which discharge via a CCK1R-dependent pathway in vitro (33). In experiment 2, we used a pharmacological approach to determine the involvement of CCK1R in apo AIV's anorectic action in WT mice. Lorglumide alone and lorglumide plus CCK caused comparable food intake in mice relative to saline-treated controls, indicating that this CCK1R antagonist itself does not increase intake and attenuates CCK-induced satiation (34). In the present study, blockade of CCK1R using lorglumide significantly attenuated apo AIV-induced satiation in WT mice, suggesting that CCK1R play an important role in relaying apo AIV's anorectic signals to the brain. Therefore, we sought to investigate whether vagal afferent fibers are an important route in relaying apo AIV-induced satiating signals to the hindbrain.
SDA is a surgical procedure that eliminates all neuronal signals mediated via vagal afferent fibers from the upper gut, including the liver, but leaves half of the vagal efferent fibers intact (20). In the present study, the SDA rats had a slightly lower body weight gain that was secondary to a lower food intake, but these minor differences did not reach significance relative to the SHAM rats. Vagotomy attenuates the suppression of food intake induced by nutrients and reduces vagal afferent activity stimulated by jejunal long-chain fatty acids (35, 36). A major finding of the present study is that peripheral apo AIV does not reduce food consumption in rats after SDA, i.e. apo AIV significantly decreased food intake in SHAM rats but not in SDA rats, suggesting that the SDA-reduced baseline food intake does not mask the ability of apo AIV to inhibit food intake. The important point is that the inhibitory effect of peripherally administered apo AIV on food intake appears to be mediated via vagal afferent nerves.
The hindbrain solitary complex, including the NTS and DMV, is an important site that receives satiating signals elicited by peripheral signals (37–40). Previous studies indicate that lower neuronal activation occurs in the NTS of apo AIV KO than in WT mice in response to dietary lipids (41); thus, peripheral apo AIV is important in transmitting lipid-induced satiating signals to the hindbrain (41). In the present study, peripheral apo AIV activated more neurons in the NTS and DMV of WT mice than in CCK-KO mice. These observations imply that peripheral apo AIV induces neuronal activation in the hindbrain and that this requires an intact CCK-dependent pathway and possibly vagal nerves. Blockade of CCK1R using the CCK1R antagonist lorglumide attenuated neuronal activation in the NTS and DMV, the implication being that CCK1R on vagal nerves are involved in the apo AIV-induced neuronal activation in the NTS. Our recent report that fasted apo AIV KO mice have up-regulated Cck1r gene expression in the nodose ganglia and NTS implies that endogenous apo AIV mediates CCK sensitivity by influencing CCK1R in the nodose ganglia and NTS (32). These findings suggest that apo AIV and CCK interact at several levels to provide signals to the NTS and that this activation is mediated via CCK1R.
In the present study, SDA significantly reduced the number of c-Fos-positive cells in the NTS/AP in both saline-treated and apo AIV treated rats. These data are consistent with the food intake results in which the satiating response to apo AIV was attenuated in SDA rats. In the NTS and AP, the percentage of apo AIV-induced c-Fos-positive cells in SDA rats was comparable with that in SHAM rats, suggesting that the SDA-reduced baseline in neuronal activation might have masked an apo AIV-increased percentage of c-Fos cells in the NTS. Alternatively, apo AIV-induced neuronal activation in NTS may be partially mediated via vagal nerves. The key finding is that subdiaphragmatically originating vagal afferents and a CCK-dependent system are important for the transduction of apo AIV-induced satiating signals from the digestive tract to the hindbrain.
The AP is a hindbrain circumventricular organ and is adjacent to the NTS (42, 43). The present findings demonstrate that peripheral apo AIV significantly increases c-Fos expression in the AP of SHAM and SDA rats, whereas it tended to increase c-Fos expression in the AP of WT and CCK-KO mice. This difference in neuronal activation in the AP of mice and rats may be due to species variation in response to circulating apo AIV. Systemic CCK has a direct effect on the activation of neurons in the AP area in which a high concentration of binding sites for CCK is present (38, 44, 45). Blockade of CCK1R attenuated neuronal activation in the AP induced by peripherally administered apo AIV, implying that circulating apo AIV interacts with CCK1R to activate neuronal activation in the AP. However, neither CCK nor vagal nerves appear to directly activate neurons in the AP. Consistent with this, it has also been reported that the AP does not mediate CCK-induced satiation (46–48). There was no relationship between apo AIV-induced neuronal activation in the AP and the satiating effect in the present study. Our present data are in agreement with the working model proposed by Raybould and colleagues (41) that apo AIV release induced by lipid absorption results in the stimulation of CCK release, followed by activation of the vagal nerves via a CCK1R-dependent pathway. In conclusion, systemic apo AIV requires an intact CCK system and CCK1R on vagal afferent nerves to inhibit food intake.
Conclusion
Peripheral administration of exogenous apo AIV inhibits food intake and increases neuronal activation in the NTS, and these are mediated via a CCK-dependent pathway. Furthermore, peripheral apo AIV appears to activate neurons and relays its satiating signals to the NTS via vagal afferent fibers.
Acknowledgments
This work was supported in part by the National Institutes of Health Grants DK83550, DK017844-35, DK078201-04, DK092779, DK095440, DK076928, DK092138, and DK059630.
Disclosure Summary: No conflicts of interest, financial or otherwise, are declared by the authors.
Footnotes
- AP
- Area postrema
- apo AIV
- apolipoprotein AIV
- CCK
- cholecystokinin
- CCK1R
- CCK1 receptor
- DMV
- dorsal motor nucleus of the vagus
- NTS
- nucleus of the solitary tract
- SDA
- subdiaphragmatic vagal deafferentation
- WT
- wild type.
References
- 1. Flegal KM, Carroll MD, Ogden CL, Curtin LR. 2010. Prevalence and trends in obesity among U.S. adults, 1999–2008. JAMA 303:235–241 [DOI] [PubMed] [Google Scholar]
- 2. Kopelman PG. 2000. Obesity as a medical problem. Nature 404:635–643 [DOI] [PubMed] [Google Scholar]
- 3. Kahn BB, Flier JS. 2000. Obesity and insulin resistance. J Clin Invest 106:473–481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Strader AD, Woods SC. 2005. Gastrointestinal hormones and food intake. Gastroenterology 128:175–191 [DOI] [PubMed] [Google Scholar]
- 5. Green GM, Taguchi S, Friestman J, Chey WY, Liddle RA. 1989. Plasma secretin, CCK, and pancreatic secretion in response to dietary fat in the rat. Am J Physiol 256:G1016–G1021 [DOI] [PubMed] [Google Scholar]
- 6. Raybould HE, Meyer JH, Tabrizi Y, Liddle RA, Tso P. 1998. Inhibition of gastric emptying in response to intestinal lipid is dependent on chylomicron formation. Am J Physiol 274:R1834–R1838 [DOI] [PubMed] [Google Scholar]
- 7. Gibbs J, Young RC, Smith GP. 1973. Cholecystokinin decreases food intake in rats. J Comp Physiol Psychol 84:488–495 [DOI] [PubMed] [Google Scholar]
- 8. Moran TH. 2009. Gut peptides in the control of food intake. Int J Obes (Lond) 33(Suppl 1):S7–S10 [DOI] [PubMed] [Google Scholar]
- 9. Dockray GJ. 2012. Cholecystokinin. Curr Opin Endocrinol Diabetes Obes 19:8–12 [DOI] [PubMed] [Google Scholar]
- 10. Moran TH, Baldessarini AR, Salorio CF, Lowery T, Schwartz GJ. 1997. Vagal afferent and efferent contributions to the inhibition of food intake by cholecystokinin. Am J Physiol 272:R1245–R1251 [DOI] [PubMed] [Google Scholar]
- 11. Hayashi H, Nutting DF, Fujimoto K, Cardelli JA, Black D, Tso P. 1990. Transport of lipid and apolipoproteins A-I and A-IV in intestinal lymph of the rat. J Lipid Res 31:1613–1625 [PubMed] [Google Scholar]
- 12. Dvorin E, Gorder NL, Benson DM, Gotto AM., Jr 1986. Apolipoprotein A-IV. A determinant for binding and uptake of high density lipoproteins by rat hepatocytes. J Biol Chem 261:15714–15718 [PubMed] [Google Scholar]
- 13. Liu M, Maiorano N, Shen L, Pearson K, Tajima D, Zhang DM, Woods SC, Seeley RJ, Davidson WS, Tso P. 2003. Expression of biologically active rat apolipoprotein AIV in Escherichia coli. Physiol Behav 78:149–155 [DOI] [PubMed] [Google Scholar]
- 14. Shen L, Pearson KJ, Xiong Y, Lo CM, Tso P, Woods SC, Davidson WS, Liu M. 2008. Characterization of apolipoprotein A-IV in brain areas involved in energy homeostasis. Physiol Behav 95:161–167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fujimoto K, Cardelli JA, Tso P. 1992. Increased apolipoprotein A-IV in rat mesenteric lymph after lipid meal acts as a physiological signal for satiation. Am J Physiol 262:G1002–G1006 [DOI] [PubMed] [Google Scholar]
- 16. Fujimoto K, Fukagawa K, Sakata T, Tso P. 1993. Suppression of food intake by apolipoprotein A-IV is mediated through the central nervous system in rats. J Clin Invest 91:1830–1833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lo CM, Zhang DM, Pearson K, Ma L, Sun W, Sakai RR, Davidson WS, Liu M, Raybould HE, Woods SC, Tso P. 2007. Interaction of apolipoprotein AIV with cholecystokinin on the control of food intake. Am J Physiol Regul Integr Comp Physiol 293:R1490–R1494 [DOI] [PubMed] [Google Scholar]
- 18. Lacourse KA, Swanberg LJ, Gillespie PJ, Rehfeld JF, Saunders TL, Samuelson LC. 1999. Pancreatic function in CCK-deficient mice: adaptation to dietary protein does not require CCK. Am J Physiol 276:G1302–G1309 [DOI] [PubMed] [Google Scholar]
- 19. Arnold M, Mura A, Langhans W, Geary N. 2006. Gut vagal afferents are not necessary for the eating-stimulatory effect of intraperitoneally injected ghrelin in the rat. J Neurosci 26:11052–11060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Norgren R, Smith GP. 1994. A method for selective section of vagal afferent or efferent axons in the rat. Am J Physiol 267:R1136–R1141 [DOI] [PubMed] [Google Scholar]
- 21. Smith GP, Jerome C, Norgren R. 1985. Afferent axons in abdominal vagus mediate satiety effect of cholecystokinin in rats. Am J Physiol 249:R638–R641 [DOI] [PubMed] [Google Scholar]
- 22. Verbalis JG, Stricker EM, Robinson AG, Hoffman GE. 1991. Cholecystokinin activates C-fos expression in hypothalamic oxytocin and corticotropin-releasing hormone neurons. J Neuroendocrinol 3:205–213 [DOI] [PubMed] [Google Scholar]
- 23. Lo CM, Ma L, Zhang DM, Lee R, Qin A, Liu M, Woods SC, Sakai RR, Raybould HE, Tso P. 2007. Mechanism of the induction of brain c-Fos-positive neurons by lipid absorption. Am J Physiol Regul Integr Comp Physiol 292:R268–R273 [DOI] [PubMed] [Google Scholar]
- 24. Paxinos G, Franklin KBJ. 2004. The mouse/rat brain in stereotaxic coordinates. San Diego: Elsevier Science [Google Scholar]
- 25. Morgan JI, Curran T. 1991. Stimulus-transcription coupling in the nervous system: involvement of the inducible proto-oncogenes fos and jun. Annu Rev Neurosci 14:421–451 [DOI] [PubMed] [Google Scholar]
- 26. Li BH, Rowland NE. 1995. Effects of vagotomy on cholecystokinin- and dexfenfluramine-induced Fos-like immunoreactivity in the rat brain. Brain Res Bull 37:589–593 [DOI] [PubMed] [Google Scholar]
- 27. Sayegh AI, Ritter RC. 2000. Vagus nerve participates in CCK-induced Fos expression in hindbrain but not myenteric plexus. Brain Res 878:155–162 [DOI] [PubMed] [Google Scholar]
- 28. Hayes MR, Savastano DM, Covasa M. 2004. Cholecystokinin-induced satiety is mediated through interdependent cooperation of CCK-A and 5-HT3 receptors. Physiol Behav 82:663–669 [DOI] [PubMed] [Google Scholar]
- 29. Reidelberger RD, Hernandez J, Fritzsch B, Hulce M. 2004. Abdominal vagal mediation of the satiety effects of CCK in rats. Am J Physiol Regul Integr Comp Physiol 286:R1005–R1012 [DOI] [PubMed] [Google Scholar]
- 30. Lo CM, Samuelson LC, Chambers JB, King A, Heiman J, Jandacek RJ, Sakai RR, Benoit SC, Raybould HE, Woods SC, Tso P. 2008. Characterization of mice lacking the gene for cholecystokinin. Am J Physiol Regul Integr Comp Physiol 294:R803–R810 [DOI] [PubMed] [Google Scholar]
- 31. Lo CM, Obici S, Dong HH, Haas M, Lou D, Kim DH, Liu M, D'Alessio D, Woods SC, Tso P. 2011. Impaired insulin secretion and enhanced insulin sensitivity in cholecystokinin-deficient mice. Diabetes 60:2000–2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Yoshimichi G, Lo CC, Tamashiro KL, Ma L, Lee DM, Begg DP, Liu M, Sakai RR, Woods SC, Yoshimatsu H, Tso P. 2012. Effect of peripheral administration of cholecystokinin on food intake in apolipoprotein AIV knockout mice. Am J Physiol Gastrointest Liver Physiol 302:G1336–G1342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Glatzle J, Darcel N, Rechs AJ, Kalogeris TJ, Tso P, Raybould HE. 2004. Apolipoprotein A-IV stimulates duodenal vagal afferent activity to inhibit gastric motility via a CCK1 pathway. Am J Physiol Regul Integr Comp Physiol 287:R354–R359 [DOI] [PubMed] [Google Scholar]
- 34. Kaltwasser MT, Petrack B, Crawley JN. 1987. Potency of CR 1409, a new proglumide analog, on cholecystokinin-mediated behaviors and receptor binding. Neurochem Int 10:547–553 [DOI] [PubMed] [Google Scholar]
- 35. Yox DP, Stokesberry H, Ritter RC. 1991. Fourth ventricular capsaicin attenuates suppression of sham feeding induced by intestinal nutrients. Am J Physiol 260:R681–R687 [DOI] [PubMed] [Google Scholar]
- 36. Cox JE, Kelm GR, Meller ST, Randich A. 2004. Suppression of food intake by GI fatty acid infusions: roles of celiac vagal afferents and cholecystokinin. Physiol Behav 82:27–33 [DOI] [PubMed] [Google Scholar]
- 37. Palkovits M, Kiss JZ, Beinfeld MC, Williams TH. 1982. Cholecystokinin in the nucleus of the solitary tract of the rat: evidence for its vagal origin. Brain Res 252:386–390 [DOI] [PubMed] [Google Scholar]
- 38. Mönnikes H, Lauer G, Arnold R. 1997. Peripheral administration of cholecystokinin activates c-fos expression in the locus coeruleus/subcoeruleus nucleus, dorsal vagal complex and paraventricular nucleus via capsaicin-sensitive vagal afferents and CCK-A receptors in the rat. Brain Res 770:277–288 [DOI] [PubMed] [Google Scholar]
- 39. Smith GP. 2000. The controls of eating: a shift from nutritional homeostasis to behavioral neuroscience. Nutrition 16:814–820 [DOI] [PubMed] [Google Scholar]
- 40. Berthoud HR, Neuhuber WL. 2000. Functional and chemical anatomy of the afferent vagal system. Auton Neurosci 85:1–17 [DOI] [PubMed] [Google Scholar]
- 41. Whited KL, Lu D, Tso P, Kent Lloyd KC, Raybould HE. 2005. Apolipoprotein A-IV is involved in detection of lipid in the rat intestine. J Physiol 569:949–958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Leslie RA, Gwyn DG, Hopkins DA. 1982. The central distribution of the cervical vagus nerve and gastric afferent and efferent projections in the rat. Brain Res Bull 8:37–43 [DOI] [PubMed] [Google Scholar]
- 43. Shapiro RE, Miselis RR. 1985. The central organization of the vagus nerve innervating the stomach of the rat. J Comp Neurol 238:473–488 [DOI] [PubMed] [Google Scholar]
- 44. Zarbin MA, Innis RB, Wamsley JK, Snyder SH, Kuhar MJ. 1983. Autoradiographic localization of cholecystokinin receptors in rodent brain. J Neurosci 3:877–906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Moran TH, Robinson PH, Goldrich MS, McHugh PR. 1986. Two brain cholecystokinin receptors: implications for behavioral actions. Brain Res 362:175–179 [DOI] [PubMed] [Google Scholar]
- 46. Edwards GL, Ritter RC. 1981. Ablation of the area postrema causes exaggerated consumption of preferred foods in the rat. Brain Res 216:265–276 [DOI] [PubMed] [Google Scholar]
- 47. Ritter RC, Edwards GL. 1984. Area postrema lesions cause overconsumption of palatable foods but not calories. Physiol Behav 32:923–927 [DOI] [PubMed] [Google Scholar]
- 48. Edwards GL, Ritter RC. 1986. Area postrema lesions: cause of overingestion is not altered visceral nerve function. Am J Physiol 251:R575–R581 [DOI] [PubMed] [Google Scholar]