Abstract
Administration of the glucagon-like peptide-1 (GLP-1) receptor agonists GLP-1 and exendin-4 (Ex-4) directly into the central nervous system decreases food intake. But although Ex-4 potently suppresses food intake after peripheral administration, the effects of parenteral GLP-1 are variable and not as strong. A plausible explanation for these effects is the rapid inactivation of circulating GLP-1 by dipeptidyl peptidase-4 (DPP-4), an enzyme that does not alter Ex-4 activity. To test this hypothesis, we assessed the relative potency of Ex-4 and GLP-1 under conditions in which DPP-4 activity was reduced. Outbred rats, wild-type mice, and mice with a targeted deletion of DPP-4 (Dpp4−/−) were treated with GLP-1 alone or in combination with the DPP-4 inhibitor vildagliptin, Ex-4, or saline, and food intake was measured. GLP-1 alone, even at high doses, did not affect feeding in wild-type mice or rats but did reduce food intake when combined with vildagliptin or given to Dpp4−/− mice. Despite plasma clearance similar to DPP-4-protected GLP-1, equimolar Ex-4 caused greater anorexia than vildagliptin plus GLP-1. To determine whether supraphysiological levels of endogenous GLP-1 would suppress food intake if protected from DPP-4, rats with Roux-en-Y gastric bypass and significantly elevated postprandial plasma GLP-1 received vildagliptin or saline. Despite 5-fold greater postprandial GLP-1 in these animals, vildagliptin did not affect food intake in Roux-en-Y gastric bypass rats. Thus, in both mice and rats, peripheral GLP-1 reduces food intake significantly less than Ex-4, even when protected from DPP-4. These findings suggest distinct potencies of GLP-1 receptor agonists on food intake that cannot be explained by plasma pharmacokinetics.
Glucagon-like peptide-1 (GLP-1) is secreted from L cells in the ileum and colon during meal ingestion and is essential for normal glucose tolerance (1, 2). The finding that GLP-1 potently lowers blood glucose in type 2 diabetic subjects has launched a broad effort to harness the GLP-1 signaling system for therapeutic use. Because circulating GLP-1 is rapidly inactivated in vivo by the serine protease dipeptidyl peptidase-4 (DPP-4) (3–5), alternative strategies for targeting the GLP-1 system have been developed, including GLP-1 receptor (GLP-1r) agonists that are resistant to DPP-4 (6) and DPP-4 inhibitors that prolong the circulating half-life of endogenous GLP-1 (7). These approaches have been effective, and several new compounds are now available to treat patients with type 2 diabetes (8, 9).
In addition to a role in the regulation of blood glucose, GLP-1 also affects feeding behavior in rodents and humans (10–12). The GLP-1r is expressed in brain areas involved in the control of food intake, including the hypothalamus and the caudal brainstem (13, 14), and administration of GLP-1 directly into the central nervous system (CNS) reduces short-term food intake in rats and mice (15–17). Moreover, CNS administration of GLP-1r antagonists increases food intake and body weight in rats, supporting a role for endogenous GLP-1 as a physiological regulator of satiation (15, 18). Although systemic administration of the DPP-4-resistant GLP-1r agonist exendin-4 (Ex-4) consistently lowers food intake in animals (19, 20), the effects of peripherally administered native GLP-1 to suppress feeding are not as consistent (11, 15, 19, 21–23). Turton and colleagues (15) noted no effect of peripherally administered GLP-1 (up to 500 μg ip) on food intake in rats, representative of negative results from a number of laboratories. However, there have also been several reports of iv or ip GLP-1 causing anorexia in animals (19, 21–25), although the amounts of peptide used in these studies is far greater than the doses of Ex-4 that induce satiety. One plausible explanation for the difference in potency between Ex-4 and native GLP-1 is that Ex-4 is not metabolized by DPP-4 and, therefore, has a significantly longer plasma half-life than bioactive GLP-1 (9, 11). However, DPP-4 inhibitors do not cause weight loss in clinical or animal studies (9, 26), an outcome that has been attributed to levels of endogenous GLP-1 that even when protected from inactivation by DPP-4 are much lower than those attained by GLP-1r agonists like Ex-4 (9, 27). Thus, the common explanation for the greater anorectic effects of GLP-1r agonists compared with DPP-4 inhibitors includes both increased peptide concentration and decreased peptide metabolism (25). Although there have been detailed comparisons of the binding affinity and biological activity of GLP-1r agonists suggesting the potential for modest and, in some cases, species-specific differences (28–30), there have been just a few studies of the relative anorectic potencies of native GLP-1 and synthetic GLP-1r agonists (19, 31).
We have recently demonstrated reduced anorectic potency of GLP-1 compared with Ex-4 when delivered directly into the CNS (16). A clinical implication of this study is that treatment approaches based on the endogenous peptide might not have the same effects on food intake as other GLP-1r agonists. We hypothesized that peripherally administered GLP-1 would cause equivalent anorexia as Ex-4 if given in an equivalent dose and protected from metabolism by DPP-4. We used two different experimental paradigms to extend the circulating half-life of exogenously administered GLP-1 for comparison with Ex-4: 1) pharmacological DPP-4 inhibition in mice and rats using vildagliptin and 2) mice lacking a functional gene encoding DPP-4. These studies were extended to rats with Roux-en-Y gastric bypass (RYGB), a model in which endogenous GLP-1 concentrations are elevated (32, 33), to determine whether protection of the high levels of GLP-1 by DPP-4 inhibition affects food intake.
Materials and Methods
Animals
Male Long-Evans rats were obtained from Harlan Laboratories (Indianapolis, IN) at 275–300 g. Male Dpp4−/− mice and wild-type (WT) C57BL/6 control mice were obtained from Taconic (Germantown, NY) at age 6–8 wk. The generation of Dpp4−/− mice has been described previously; experimental cohorts were generated by breeding Dpp4+/− heterozygotes on the C57BL6/J background to obtain littermate WT and Dpp4−/− for comparisons in the experiments. The Dpp4−/− animals have normal growth, development, and reproductive capacity but elevated plasma levels of intact GLP-1 and improved glucose tolerance compared with WT controls (34, 35). All rats and mice were individually housed in a temperature-controlled room under a 12-h light, 12-h dark cycle (lights on from 1200–2400 h), and fed standard pelleted or powdered chow (7012 Teklad LM-485; Harlan Laboratories). Drinking water was available ad libitum. All animal procedures were approved by the Institutional Animal Care and Use Committee at the University of Cincinnati.
Genotyping
The genotyping of the Dpp4−/− mouse line was performed using RT-PCR to generate either a 630-bp amplicon containing exons 1 and 2 of the DPP4 gene (forward 5′-TGA CTT CTG CCT GCG CTC AAG-3′; reverse 5′-GCT CAG CAG AAC TAT TGG CAC-3′; PCR protocol: 94 C for 5 min; 40 cycles of 94 C for 30 sec, 55 C for 30 sec, and 72 C for 1 min; and then 72 C for 5 min final elongation) or a 233-bp amplicon of the Neo gene (forward 5′-GTC TTG TCG ATC AGG ATG ATC TG-3′; reverse 5′-CAA TAT CAC GGG TAG CCA ACG C-3′; PCR protocol: 94 C for 5 min; 35 cycles of 94 C for 30 sec, 57 C for 30 sec, and 72 C for 1 min; and then 72 C for 5 min final elongation).
Drugs
GLP-1(7–36NH2) (21st Century Biochemicals, Marlboro, MA), vildagliptin (kindly provided by Dr. Bryan Burkey, Novartis, Cambridge, MA), and Ex-4 (Amylin, Inc., La Jolla, CA) were reconstituted in saline containing 0.25% (wt/vol) BSA (Sigma-Aldrich, St. Louis, MO), aliquoted, and stored at −20 C. All peptides were administered ip in a volume of 100 μl.
Food intake studies in mice (experiments 1–3)
Experiment 1: anorectic effects of GLP-1 with or without vildagliptin in WT mice.
To determine the effects of exogenous GLP-1 on food intake and the role of metabolism by DPP-4 on this process, WT mice received ip injections of 1) GLP-1 (500 μg) or saline and 2) GLP-1 (500 μg) in combination with the DPP-4 inhibitor vildagliptin (150 μg) or vildagliptin (150 μg) alone.
Experiment 2: anorectic effects of GLP-1 and Ex-4 in Dpp4−/− mice
To investigate whether targeted deletion of the mouse Dpp4 gene would increase the anorectic effect of GLP-1 compared with that of Ex-4, Dpp4−/− mice received ip injections of GLP-1 (5, 50, and 500 μg), Ex-4 (1.25, 2.5, and 10 μg), or saline.
Experiment 3: comparison of the anorectic effects of GLP-1 and Ex-4 in WT mice
To compare the anorectic effects of equivalent doses of GLP-1 and Ex-4 on food intake, WT mice were administered vildagliptin alone (150 μg), GLP-1 (2 μg; 600 pmol) in combination with vildagliptin, Ex-4 (2.5 μg; 600 pmol), or saline.
All food intake studies in mice were performed with powdered chow. On the day of experiments, mice were placed in cages with fresh bedding, and food was removed 2 h before lights off. The mice were injected ip at the beginning of the dark phase when food was returned to the cages. Food intake was measured after 1, 2, 4, 6, and 24 h. The data from mice that spilled food in the bottom of the cage were not included in the final dataset. Each of the three food intake experiments in mice included two to four separate experiments that were averaged for final results. Treatments were randomly assigned and given in a counterbalanced design, such that each treatment was repeated equally (to the greatest extent possible with uneven animal numbers) on each experimental day with 1 wk allocated between treatments. Each mouse received a given treatment once.
Food intake studies in rats (experiments 4–7)
These experiments used the same protocol as experimetns 1–3 above with the exception that rats were fed pelleted chow.
Experiment 4: anorectic effects of GLP-1 with or without vildagliptin in rats
To test the effects of exogenous GLP-1 on food intake and the role of metabolism by DPP-4 to limit this process, rats received ip injections of GLP-1 (10, 100, or 500 μg) with or without vildagliptin (10 mg) or vildagliptin alone.
Experiment 5: anorectic effects of Ex-4 in rats
Rats were given doses of Ex-4 0.4–16 μg or saline ip before food intake measurements.
Experiment 6: anorectic effects of GLP-1
The anorectic effects of GLP-1 (10 μg; 3 nmol), with and without vildagliptin (10 mg), were compared with those of an equimolar dose of Ex-4 (12.5 μg; 3 nmol).
Experiment 7: effect of DPP-4 inhibition in rats with RYGB surgery
To determine whether increased activity of endogenous GLP-1 has an anorectic effect when protected from rapid inactivation, a cohort of Long-Evans rats who had either RYGB or sham surgery were tested after a standard test meal. The rats had had surgery 6 months before testing. The animals were placed in cages with fresh bedding and fasted overnight. At 30 min before the onset of the dark, vildagliptin (10 mg) or saline was injected ip. Pelleted chow was provided at dark onset, and food intake was measured 1, 2, 4, 6, and 24 h later. One week later, the animals were tested again and received the opposite treatment (vildagliptin or saline) from their first test. Treatments were randomly assigned and given in a counterbalanced fashion, such that each treatment was repeated equally (to the greatest extent possible with uneven animal numbers) on each experimental day.
To assess plasma GLP-1, the RYGB and sham rats were trained to consume a liquid diet (Ensure; Abbott Ross, Columbus, OH) and studied after approximately 1 wk of stable oral intake and weight maintenance. Rats were fasted overnight and allowed to drink 5 ml of Ensure timed with the initiation of the light cycle the following day. All animals consumed the liquid meal within 5 min, and tail blood was obtained for measurement of GLP-1 at 0, 15, and 30 min.
Roux-en-Y gastric bypass
Gastric bypass surgery in rats was performed as described previously (36). In brief, for this specific cohort, male Long-Evans rats (Harlan Laboratories; 250–300 g) were fed a high-fat butter oil-based diet (Research Diets, New Brunswick, NJ; D12451: 41% fat, 4.54 kcal/g) for 8 wk before surgery until they became obese and hyperinsulinemic. RYGB or sham procedure was performed (36), and animals were allowed to recover from surgery, ultimately establishing the typical stable weight differences before experiments.
Measurement of GLP-1, Ex-4, and DPP-4 activity in plasma
Three experiments were performed in mice that had been fasted for 4 h: 1) Dpp4−/− and WT mice received 100 μg GLP-1, 2) WT mice were given GLP-1 (100 μg) either alone or in combination with vildagliptin (150 μg), and 3) WT mice were given GLP-1 (10 μg) in combination with vildagliptin (150 μg) or Ex-4 (2.5 μg). The drugs were administered ip, and blood was sampled before and 30 min after for experiments 1 and 2 and at 0, 30, 120, and 180 min in experiment 3. Blood was taken by orbital sinus puncture during isoflurane anesthesia. Blood for peptide assay was collected in Eppendorf tubes prefilled with a cocktail of EDTA (0.5 m), heparin (800 U/ml), aprotinin (0.28 mm), and diprotin A (0.066 mm); 50 μl of the cocktail was used for 500 μl blood. For measurement of DPP-4 activity, blood was sampled in EDTA tubes (Sarstedt, Nürnbrecht, Germany). Blood samples were immediately placed on ice and centrifuged (1100 × g for 10 min at 4 C) within 60 min. Plasma samples were stored at −20 C until assayed.
Peptide clearance study
Male Long-Evans rats were anesthetized with ketamine (24 mg/kg, ip) and xylazine (3 mg/kg, ip) and had catheters placed in the jugular vein (silicone tubing, 0.64 mm inner diameter × 1.19 mm outer diameter; Technical Products, Inc. of Georgia, Lawrenceville, GA) and carotid artery (polyethylene tubing, 0.58 mm inner diameter × 0.97 mm outer diameter; Instech Solomon, San Antonio, TX). The recovery period was at least 1 wk; only rats that had regained more than 90% of presurgery body weight were used. Rats were randomly divided into four groups: 1) saline ip and GLP-1 iv, 2) vildagliptin ip and GLP-1 iv, 3) saline ip and Ex-4 iv, and 4) vildagliptin ip and Ex-4 iv. After an overnight fast (from 1700 h the day before the experiment), rats were weighed and placed individually in cages where they were studied conscious and unrestrained. The venous catheter was connected to an infusion system [fluorescein isothiocyanate (FITC)-inulin and GLP-1/Ex-4 precalibrated infusion pumps], whereas the arterial catheter was connected to tubing for blood sampling. After 30 min for habituation, rats were injected ip with vildagliptin (10 mg in 1 ml) or saline. The experiment started with a blood sample at 0 min and a bolus of FITC-inulin (20 mg/ml; 18 μl/kg·h; Sigma-Aldrich) and either GLP-1 (8 μg/kg·h) or Ex-4 (16 μg/kg·h) was given followed by a continuous infusion of FITC-inulin (4.5 μl/kg·h) and GLP-1 (2 μg/kg·h) or Ex-4 (4 μg/kg·h) for 150 min. Arterial blood was sampled at 10, 30, 60, 90, 120, 140, and 160 min for measurements of plasma FITC-inulin and at 120, 140, and 160 min for determination of plasma GLP-1 or Ex-4 (blood was collected in tubes containing EDTA-aprotinin-diprotin A). Plasma samples for FITC-inulin determinations were refrigerated and protected from light until assayed or frozen for measurement of intact and total GLP-1 or Ex-4.
Plasma assays
Plasma concentrations of intact and total GLP-1 were measured using mouse/rat active GLP-1(7–36NH2) and total GLP-1 assay kits (Meso Scale Discovery, Gaithersburg, MD) using a SECTOR 2400 imager (Meso Scale Discovery). Plasma Ex-4 was measured by an enzyme immunoassay kit according to the instructions of the manufacturer (Phoenix Pharmaceuticals, Burlingame, CA). Plasma DPP-4 activity was measured by incubating 40 μl plasma with 60 μl PBS (pH 8.0) and 100 μl 1.0 mm Gly-Pro-p-nitroanilide hydrochloride (pNA; Sigma-Aldrich) as enzyme substrate for 60 min at 37 C. After incubation, 200 μl PBS was added to all wells. The blank contained 100 μl 1.0 mm pNA, 260 μl PBS, and 40 μl plasma, which was added to the well after incubation. The standard curve was generated using 4-nitroaniline (Sigma-Aldrich), which is the cleavage product of pNA. Enzymatic activities were measured spectrophotometrically (SpectraMax Plus; Molecular Devices, Sunnyvale, CA) at 405 nm. Plasma inulin concentrations were determined by fluorescent detection at 485 and 538 nm.
Calculations and statistical analysis
For each rat, plasma clearance of inulin was calculated by dividing the inulin infusion rate with the inulin plasma concentration measured at steady state. In the same way, the plasma clearance of total and intact GLP-1 and Ex-4 were computed by dividing the infusion rate of peptides by their steady-state plasma concentrations. The plasma clearances of the peptides were then normalized for inulin clearance (i.e. plasma clearance of peptide divided with plasma clearance of inulin) in each rat. Comparisons of total and intact GLP-1 and Ex-4, with and without vildagliptin, were made with unpaired t tests. In experiments 1–7, food intake was compared among groups using two- or three-way ANOVA; the factors used in these analyses were GLP-1 or saline treatment (a GLP-1 factor), vildagliptin or saline treatment (a vildagliptin factor), Dpp4−/− or WT strain (strain factor), and time. When significant interactions between experimental factors were demonstrated, post hoc tests were made at each time point using one-way ANOVA followed by Dunnett's multiple-comparison test. Group differences in plasma GLP-1 and DPP-4 activity levels were analyzed using one-way ANOVA followed by Tukey's multiple-comparison test. Three-way ANOVAs were performed with Statistica version 10 (Statsoft, Tulsa, OK) and one- and two-way ANOVAs using GraphPad Prism version 5.01 (GraphPad Software, Inc., San Diego, CA). Data are presented as mean ± sem. P < 0.05 was considered statistically significant.
Results
At the initiation of these studies, Dpp4−/− mice and WT mice were approximately 10 wk old and had similar body weights (25.5 ± 0.3 and 25.0 ± 0.4 g, respectively). At the conclusion of these studies, the body weights of the Dpp4−/− mice were slightly less than those of the WT mice (33.0 ± 0.7 and 35.2 ± 1.1 g, respectively; P = 0.0814). Rats weighed 300–350 g at the time of food intake studies. The RYGB and sham surgery animals were studied at body weights of 478 ± 120 and 609 ± 82 g, respectively.
Plasma levels of GLP-1 and DPP-4 activity in Dpp4−/− and WT mice
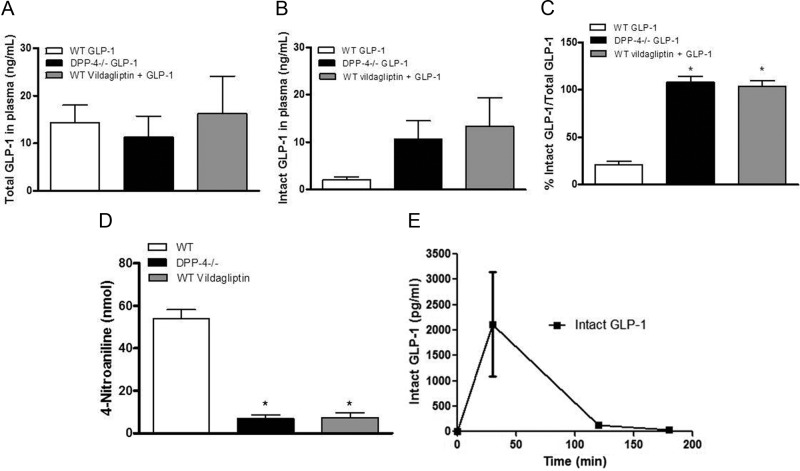
To evaluate the role of DPP-4 activity to inactivate systemically administered GLP-1, Dpp4−/− and WT mice were injected ip with GLP-1 (100 μg) alone or in combination with vildagliptin (150 μg) 30 min before collection of blood. Total GLP-1 levels, comprising intact GLP-1(7–36NH2) and its DPP-4 metabolite GLP-1(9–36NH2), were similar in Dpp4−/− mice and WT mice with or without vildagliptin (Fig. 1A). The concentrations of intact GLP-1 in plasma were comparable in the plasma of Dpp4−/− mice and WT mice given vildagliptin (Fig. 1B). In contrast, WT mice injected with GLP-1 alone had significantly lower plasma levels of intact GLP-1. This is most apparent in Fig. 1C where the percentage of intact to total GLP-1 in plasma is presented for each group. The results of the intact and total GLP-1 measures are supported by the plasma DPP-4 activity measured in Dpp4−/−, WT, and WT plus vildagliptin mice (Fig. 1D); these data indicate minimal DPP-4 activity in the animals with the gene disruption and those receiving the pharmacological inhibitor. After injection of 10 μg GLP-1 in combination with vildagliptin (150 μg) or Ex-4 (2.5 μg) to WT mice, plasma concentrations of intact GLP-1 had returned to basal levels by 180 min (Fig. 1E), as had concentrations of Ex-4 (data not shown). These experiments demonstrate that vildagliptin and Dpp4−/− gene deletion had similar short-term effects on plasma levels of exogenous GLP-1 and that after ip injection, even relatively large doses of GLP-1 and Ex-4 were cleared within 2–3 h.
Fig. 1.
A–C, Plasma concentrations of total GLP-1 (A), intact GLP-1(7–36NH2) (B), and ratios of intact to total GLP-1 (C) in Dpp4−/− and WT mice 30 min after injection 100 μg GLP-1; D, plasma DPP-4 activity measured in the same mice; E, serial measures of intact GLP-1 concentrations after ip injection of GLP-1 (10 μg) plus vildagliptin (150 μg). Data are mean ± sem. *, P < 0.05 vs. WT (n = 8–18).
Plasma clearance of GLP-1 and Ex-4 in rats
During the infusion studies, plasma concentrations of FITC-inulin, Ex-4, and GLP-1 reached steady state within 30 min of starting the infusion. Figure 2 shows the plasma clearances of total GLP-1, intact GLP-1, and Ex-4. Clearance of total GLP-1 was similar after both the saline and vildagliptin treatments, 7.5 ± 0.6 and 8.3 ± 1.0 ml/min, respectively. However, clearance of intact GLP-1 was significantly greater with saline treatment and uninhibited DPP-4 activity compared with protection from DPP-4 with vildagliptin treatment (35.2 ± 7.1 and 6.6 ± 1.0 ml/min, P < 0.05). Clearance of Ex-4 was not affected by vildagliptin treatment, averaging 5.0 ± 0.3 ml/min, and was slightly but significantly less than the clearance of total GLP-1 (P < 0.05).
Fig. 2.
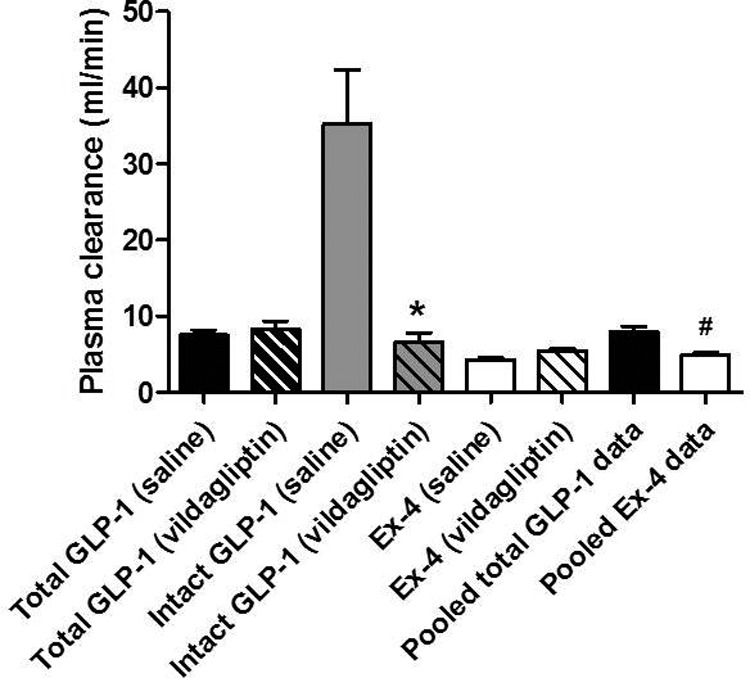
Plasma clearance (milliliters per minute) of total GLP-1, intact GLP-1, and Ex-4 in rats pretreated with saline or vildagliptin. Because clearance of total GLP-1 and Ex-4 was similar after both the saline and vildagliptin treatments, the data were pooled. Data are mean ± sem. *, P < 0.05 vs. intact GLP-1 (saline); #, P < 0.05 vs. pooled total GLP-1 (n = 5–7).
Anorectic effects of GLP-1 with or without DPP-4 inhibition
The anorectic effect of systemically administered GLP-1, given either alone or in combination with the DPP-4 inhibitor vildagliptin to WT mice over 24 h, is depicted in Fig 3A. There were highly significant interactions between the GLP-1 and vildagliptin factors (P = 0.004) and the vildagliptin factor, GLP-1 factor, and time (P = 0.02). Based on post hoc comparisons, ip GLP-1 did not affect food intake in a dose of 500 μg (∼15,000 μg/kg) compared with saline (Fig. 3A). In contrast, GLP-1 in combination with vildagliptin significantly reduced food intake in the WT mice over the first 2, 4, and 6 h (P < 0.001). Thus, pharmacological protection from metabolism by DPP-4 permitted anorectic effects of GLP-1 in WT mice.
Fig. 3.

A–C, Food intake after ip GLP-1 (500 μg) or saline alone or in combination with vildagliptin (150 μg) in WT mice (A); GLP-1 (5, 50, 500 μg) in WT (B) or Dpp4−/− (C) mice. Data are mean ± sem. *, P < 0.05 vs. saline (n = 10–12).
Graded doses of GLP-1 (5, 50, or 500 μg) were administered ip to WT (Fig. 3B) and Dpp4−/− mice (Fig. 3C). When the two strains were compared, there was a significant interaction between the GLP-1 and strain factors (P = 0.016) and a significant main effect of time (P < 0.001) on food intake. Post hoc comparisons showed the effect of vildagliptin treatment; 500 μg GLP-1 reduced food intake at 2, 4, and 6 h (P < 0.05) in Dpp4−/− mice. Neither 5 nor 50 μg GLP-1 had a significant effect on food intake in either strain.
The effects of GLP-1 on food intake in rats were similar to those observed in mice. There was a significant interaction of the GLP-1 and vildagliptin factors (P = 0.007) and of time and the GLP-1 and vildagliptin factors (P = 0.002). Intraperitoneal GLP-1, even in high doses, did not affect food consumption (Fig. 4A). In contrast, when given with vildagliptin, all three doses of GLP-1 (10, 100, and 500 μg) reduced intake (P < 0.05), with a dose effect apparent at 24 h (Fig. 4B).
Fig. 4.

A and B, Food intake in rats after ip GLP-1 (10, 100, 500 μg) (A) or GLP-1 (10, 100, 500 μg) (B) in combination with vildagliptin (10 mg). Data are mean ± sem. *, P < 0.05 vs. saline (n = 10 per group).
Anorectic effects of Ex-4 in Dpp4−/− and WT mice and in rats
Ex-4 reduced food intake comparably in both strains of mice. There were significant interactions of time and the GLP-1 factor in both groups (P < 0.001). There was significant suppression of intake by the 2.5- and 10-μg doses of Ex-4 for up to 24 h compared with saline-treated controls (Fig 5, A and B; P < 0.05). Even the lowest dose of Ex-4 (1.25 μg) reduced food intake significantly at 1, 2, and 4 h relative to saline (P < 0.05 for each comparison). Similar findings were observed in rats where there was also a time by treatment interaction (P < 0.001) and a clear dose-responsive suppression of chow consumption lasting for 24 h (P < 0.05; Fig. 5C).
Fig. 5.

A–C, Effect of ip Ex-4 on food intake in WT mice (A), Dpp4−/− mice (B), and rats (C). Data are mean ± sem. *, P < 0.05 vs. saline (n = 10 mice; n = 12 rats).
Comparison of the anorectic effects of DPP-4-protected GLP-1 and Ex-4
When given in equimolar amounts, Ex-4 was a more potent inhibitor of food intake than the combination of GLP-1 and vildagliptin in both rats and mice. There was a significant time by treatment interaction in each strain (P < 0.001). Compared with saline-treated animals, neither vildagliptin nor GLP-1 alone had any anorectic effect in WT mice or rats. In mice, 2 μg (600 pmol) of DPP-4-protected GLP-1 did not affect food intake significantly, whereas 600 pmol Ex-4 (2.5 μg) potently decreased food intake for up to 24 h relative to saline (P = 0.0002; Fig 6A). In rats, 10 μg (3 nmol) GLP-1 plus vildagliptin reduced food intake compared with saline. However, the effect was significantly less than that of 12.5 μg (3 nmol) of Ex-4 at 4 and 24 h (P < 0.05; Fig. 6B).
Fig. 6.

A and B, Effects of vildagliptin and GLP-1 given separately, GLP-1 in combination with vildagliptin, Ex-4, or saline on food intake in WT mice (A) and rats (B). Data are mean ± sem. *, P < 0.05 vs. saline (n = 10–13 mice; n = 20–21 rats).
Anorectic effects of vildagliptin in RYGB rats with elevated GLP-1
To test the hypothesis that elevated amounts of endogenously released GLP-1 would have anorectic actions if protected from DPP-4, rats with RYGB were compared with sham-operated controls. Rats with RYGB had significantly elevated plasma total GLP-1 concentrations compared with the controls, with peak levels roughly 7-fold greater (P < 0.05; Fig. 7A). However, treatment with vildagliptin did not affect food intake in the RYGB or sham groups compared with saline treatment; there was no interaction between time, treatment, and surgery (Fig. 7B).
Fig. 7.

A, Plasma total GLP-1 response to Ensure in RYGB (n = 7) and sham rats (n = 7); B, food intake in RYGB and sham rats after ip injection of vildagliptin (10 mg) or saline. Data are mean ± sem.
Discussion
The ability of exogenous long-acting GLP-1r agonists to reduce food intake is well established (37), whereas the satiating effects of endogenous GLP-1 are more variable and challenging to elicit (10, 15, 19, 22–25, 31, 38, 39). This difference has generally been ascribed to factors such as relative plasma concentrations and rates of metabolism rather than to inherent differences in potency among compounds. However, definitive studies directly comparing the anorectic actions of GLP-1 and long-acting GLP-1r agonists under conditions of similar circulating concentrations have not been performed. Our findings, in contrast to the prevailing view, indicate that peripherally administered Ex-4 is unequivocally a more potent inhibitor of food intake on a molar basis than DPP-4-protected GLP-1. Moreover, even in rats with RYGB and significantly elevated levels of endogenous GLP-1, protection from DPP-4 did not elicit an anorectic response. These findings indicate that pharmacological amounts of intact GLP-1 may be necessary to suppress feeding. These results point to factors beyond plasma pharmacokinetics causing the relative actions of circulating GLP-1r ligands to suppress food intake and suggest that there are inherent differences in the capacity of Ex-4 and GLP-1 to activate the neural systems regulating feeding behavior.
We used pharmacological and genetic methods to reduce DPP-4 activity and extend the half-life of native GLP-1. Based on the comparisons of bioactive GLP-1 in mice given exogenous peptide and direct measurements of functional DPP-4 activity, both vildagliptin and the Dpp4 gene deletion reduced the metabolism of GLP-1 substantially. The measurement of intact to total GLP-1 and DPP-4 activity was only made at 30 min after injection of GLP-1 such that the complete duration of vildagliptin activity was not determined. However, previous studies in rats in which direct measurement of DPP-4 activity was assessed indicate that ip vildagliptin provides protection from DPP-4 over at least 4 h (40, 41), well within the time that exogenous GLP-1 was present in the circulation. Thus, we presume that in the presence of vildagliptin, the predominant GLP-1 species in the circulation of our mice and rats was the bioactive, intact form, GLP-1(7–36).
In WT mice and rats, GLP-1 was rapidly inactivated by DPP-4. This is clearly evident in the comparison of intact vs. total GLP-1 in the plasma of untreated and vildagliptin-treated mice and the clearance of intact GLP-1 with and without vildagliptin in rats. In keeping with the rapid metabolism of GLP-1 demonstrated here and similar to a previous report (15), we found that doses up to 500 μg given ip did not affect food intake in normal rodents. In contrast, 500 μg GLP-1 administered in combination with vildagliptin reduced food intake for up to 6 h in WT and Dpp4−/− mice and 24 h in outbred in rats. Thus, protection from metabolism by DPP-4 is able to promote the anorectic effect of peripherally administered GLP-1. Similar to the response to Ex-4, the anorectic effect far outlived the elevation of plasma GLP-1 in the circulation. From these studies, it appears that 2 h or less of elevated GLP-1r agonist is sufficient for long-lasting biological actions to reduce food intake. Although our data do not directly address the mechanism by which this occurs, current models of GLP-1-induced satiety include effects of circulating GLP-1r agonists to activate specific regions of the CNS (42–44) and for peptide released by L cells into the submucosa to stimulate afferent neural signals to the hindbrain (45, 46). We presume that the ip treatments used in this study could activate either of these systems.
Ex-4 is not degraded by DPP-4 and therefore has a longer plasma half-life than intact GLP-1 (9). Despite extending the plasma pharmacokinetics of GLP-1 with DPP-4 inhibition, our data indicate that peripherally injected Ex-4 is a more potent inhibitor of food intake on a molar basis than GLP-1. In WT mice, 2 μg GLP-1 given in combination with vildagliptin did not decrease food intake, whereas an equimolar amount of Ex-4 (2.5 μg) potently suppressed food intake for up to 24 h. A similar relative anorectic potency of the two GLP-1r agonists was seen in rats. Importantly, this difference in potency is not likely to be explained by insufficient DPP-4 inhibition and relatively lower circulating levels of intact GLP-1(7–36NH2). First, we used a dose of vildagliptin that is above maximal pharmacological potency (40, 41),and that resulted in an intact to total GLP-1 ratio of almost 100%, a profile comparable to that of Dpp4−/− mice. Second, both Ex-4 and GLP-1 were cleared from the circulation within 2 h, well within the effective period of DPP-4 inhibition by vildagliptin (40, 41). Finally, the relative effect of vildagliptin-protected GLP-1 to suppress food intake was not enhanced early in the feeding studies as would be expected if the activity of vildagliptin was relatively short-lived. Based on these findings, it seems improbable that differences in circulating levels of intact GLP-1 and Ex-4 explain the differential potency of the GLP-1r ligands on food intake.
The observation that peripherally administered Ex-4 more potently inhibited food intake than DPP-4-protected GLP-1 suggests that Ex-4 and GLP-1 have differential actions to activate the key circuits in the CNS that control feeding. Previous work by our group and others support possibilities such as differential activation of visceral afferent nerves (44, 47, 48) or circumventricular organs (43) by circulating peptide, variable transport across the blood-brain barrier (49), or differences in the mechanism of action of native GLP-1 compared with Ex-4 (16). It is not likely that Ex-4 acts independently of the GLP-1r to reduce feeding because peptide administered into the brain in Glp1r−/− mice had no effect on food intake (16). However, we have recently demonstrated that the anorectic action of Ex-4 administered centrally is less susceptible to GLP-1r antagonism with Ex-9 (16). GLP-1 and Ex-4 have similar affinity and potency for the rodent and human GLP-1r (50, 51). However, recent findings indicate that Ex-4 and GLP-1 have distinct interactions with the extracellular portions of the GLP-1r (30). This, taken together with the above finding, raises the possibility for differential potency in key sites related to feeding behavior. Regardless, the greater potency of Ex-4 compared with GLP-1 to reduce food intake when given in the CNS is consistent with the conclusion that relative plasma pharmacokinetics is not the principle cause of differential effects on food intake.
In recent years, GLP-1 has been the focus of considerable research on the mechanisms of weight loss and improved metabolism after bariatric surgery. Patients having either RYGB or vertical sleeve gastrectomy have postprandial plasma levels of GLP-1 that are 5- to 10-fold elevated compared with subjects without a surgical procedure (52, 53). Elevated plasma levels of GLP-1 account for at least some of the enhanced insulin secretion seen after RYGB (32, 33, 54), suggesting that the peptide mediates some of the metabolic effects of surgery. There is no direct evidence that GLP-1 mediates the changes in food intake occurring after RYGB. However, treatment with a GLP-1r antagonist blocked the anorectic effect of enteric lipid infusions (55), an experimental treatment that mimics the increased nutrient delivery to the distal small bowel after RYGB. The plasma levels of GLP-1 observed in our rats after RYGB suggest that this model shares key features of human bariatric surgery. However, treatment with vildagliptin to extend the activity of the supraphysiological GLP-1 levels occurring after RYGB did not affect short-term food intake. This suggests either that the anorectic effects of GLP-1 in RYGB rats are near maximal even without DPP-4 inhibitor treatment or that plasma GLP-1 does not have a major impact on the acute feeding response after surgery. Although a recent study has demonstrated that Ex-4 reduces food intake in rats with RYGB (56), more work will be required to determine whether there is a contribution of endogenous GLP-1 to satiety after this procedure.
In summary, our findings indicate that peripherally administered Ex-4 more potently inhibits food intake than DPP-4-protected GLP-1 despite similar exposure from the circulating plasma. These observations indicate that Ex-4 and GLP-1 affect feeding differentially and that mechanisms in addition to DPP-4 are involved in the pharmacodynamics of this response. The results of these studies add to a growing body of work indicating that different GLP-1r agonists may have distinct properties beyond pharmacokinetics and raise the likelihood that greater understanding in this area can lead to improved therapeutic developments.
Acknowledgments
We thank Dr. Bei B. Zhang and Merck Research Laboratories for providing the Dpp4−/− mice, and Dr. Didier Marguet for permission to use these animals for our study. We also thank Nilika Chaudhary for help with the GLP-1 assays and Ronald D. Bitner for technical assistance in Dpp-4 colony genotyping. Drs. Darleen Sandoval and Stephen Benoit provided valuable statistical help. Vildagliptin was a kind gift from Dr. Bryan Burke at Novartis.
This work was supported by National Institutes of Health Grants PHS DK057900 and DK56863 and a research grant from Amylin Pharmaceuticals.
Disclosure Summary: L.J., B.A.A., J.H., K.R., E.P.S., T.M.G., and S.C.W. have nothing to declare. R.J.S. consults for Amylin Pharmaceuticals, Johnson & Johnson, and Roche; is a scientific advisory board member for Eli Lilly, Johnson & Johnson, Zafgen Inc., and Merck; is on the speaker's bureau for Amylin Pharmaceuticals, Eli Lilly, Novo Nordisk, and Merck; and has stock options in Zafgen Inc. and research grants from Amylin Pharmaceuticals, Johnson & Johnson, Zafgen Inc., Roche, and Mannkind. D.A.D. consults for Amylin, Lilly, Merck, Novo Nordisk, Takeda, and Zealand and has research grants from MannKind Pharmaceuticals, Sanofi Aventis, and Ethicon Endosurgery.
Footnotes
- CNS
- Central nervous system
- DPP-4
- dipeptidyl petidase-4
- Ex-4
- exendin-4
- FITC
- fluorescein isothiocyanate
- GLP-1
- glucagon-like peptide-1
- GLP-1r
- GLP-1 receptor
- pNA
- Gly-Pro-p-nitroanilide hydrochloride
- RYGB
- Roux-en-Y gastric bypass
- WT
- wild type.
References
- 1. Vahl TP, D'Alessio DA. 2004. Gut peptides in the treatment of diabetes mellitus. Expert Opin Investig Drugs 13:177–188 [DOI] [PubMed] [Google Scholar]
- 2. Drucker DJ. 2006. The biology of incretin hormones. Cell Metab 3:153–165 [DOI] [PubMed] [Google Scholar]
- 3. Deacon CF, Nauck MA, Toft-Nielsen M, Pridal L, Willms B, Holst JJ. 1995. Both subcutaneously and intravenously administered glucagon-like peptide I are rapidly degraded from the NH2-terminus in type II diabetic patients and in healthy subjects. Diabetes 44:1126–1131 [DOI] [PubMed] [Google Scholar]
- 4. Lambeir AM, Durinx C, Scharpé S, De Meester I. 2003. Dipeptidyl-peptidase IV from bench to bedside: an update on structural properties, functions, and clinical aspects of the enzyme DPP IV. Crit Rev Clin Lab Sci 40:209–294 [DOI] [PubMed] [Google Scholar]
- 5. Mentlein R, Gallwitz B, Schmidt WE. 1993. Dipeptidyl-peptidase IV hydrolyses gastric inhibitory polypeptide, glucagon-like peptide-1(7–36) amide, peptide histidine methionine and is responsible for their degradation in human serum. Eur J Biochem 214:829–835 [DOI] [PubMed] [Google Scholar]
- 6. Madsbad S, Kielgast U, Asmar M, Deacon CF, Torekov SS, Holst JJ. 2011. An overview of once-weekly glucagon-like peptide-1 receptor agonists: available efficacy and safety data and perspectives for the future. Diabetes Obes Metab 13:394–407 [DOI] [PubMed] [Google Scholar]
- 7. Raz I, Hanefeld M, Xu L, Caria C, Williams-Herman D, Khatami H. 2006. Efficacy and safety of the dipeptidyl peptidase-4 inhibitor sitagliptin as monotherapy in patients with type 2 diabetes mellitus. Diabetologia 49:2564–2571 [DOI] [PubMed] [Google Scholar]
- 8. Drucker DJ. 2007. Dipeptidyl peptidase-4 inhibition and the treatment of type 2 diabetes: preclinical biology and mechanisms of action. Diabetes Care 30:1335–1343 [DOI] [PubMed] [Google Scholar]
- 9. Drucker DJ, Nauck MA. 2006. The incretin system: glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors in type 2 diabetes. Lancet 368:1696–1705 [DOI] [PubMed] [Google Scholar]
- 10. Verdich C, Flint A, Gutzwiller JP, Näslund E, Beglinger C, Hellström PM, Long SJ, Morgan LM, Holst JJ, Astrup A. 2001. A meta-analysis of the effect of glucagon-like peptide-1 (7–36) amide on ad libitum energy intake in humans. J Clin Endocrinol Metab 86:4382–4389 [DOI] [PubMed] [Google Scholar]
- 11. Barrera JG, Sandoval DA, D'Alessio DA, Seeley RJ. 2011. GLP-1 and energy balance: an integrated model of short-term and long-term control. Nat Rev Endocrinol 7:507–516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Barrera JG, Jones KR, Herman JP, D'Alessio DA, Woods SC, Seeley RJ. 2011. Hyperphagia and increased fat accumulation in two models of chronic CNS glucagon-like peptide-1 loss of function. J Neurosci 31:3904–3913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Merchenthaler I, Lane M, Shughrue P. 1999. Distribution of pre-pro-glucagon and glucagon-like peptide-1 receptor messenger RNAs in the rat central nervous system. J Comp Neurol 403:261–280 [DOI] [PubMed] [Google Scholar]
- 14. Shimizu I, Hirota M, Ohboshi C, Shima K. 1987. Identification and localization of glucagon-like peptide-1 and its receptor in rat brain. Endocrinology 121:1076–1082 [DOI] [PubMed] [Google Scholar]
- 15. Turton MD, O'Shea D, Gunn I, Beak SA, Edwards CM, Meeran K, Choi SJ, Taylor GM, Heath MM, Lambert PD, Wilding JP, Smith DM, Ghatei MA, Herbert J, Bloom SR. 1996. A role for glucagon-like peptide-1 in the central regulation of feeding. Nature 379:69–72 [DOI] [PubMed] [Google Scholar]
- 16. Barrera JG, D'Alessio DA, Drucker DJ, Woods SC, Seeley RJ. 2009. Differences in the central anorectic effects of glucagon-like peptide-1 and exendin-4 in rats. Diabetes 58:2820–2827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Tang-Christensen M, Vrang N, Larsen PJ. 2001. Glucagon-like peptide containing pathways in the regulation of feeding behaviour. Int J Obes Relat Metab Disord 25(Suppl 5):S42–S47 [DOI] [PubMed] [Google Scholar]
- 18. Meeran K, O'Shea D, Edwards CM, Turton MD, Heath MM, Gunn I, Abusnana S, Rossi M, Small CJ, Goldstone AP, Taylor GM, Sunter D, Steere J, Choi SJ, Ghatei MA, Bloom SR. 1999. Repeated intracerebroventricular administration of glucagon-like peptide-1-(7–36) amide or exendin-(9–39) alters body weight in the rat. Endocrinology 140:244–250 [DOI] [PubMed] [Google Scholar]
- 19. Rodriquez de Fonseca F, Navarro M, Alvarez E, Roncero I, Chowen JA, Maestre O, Gómez R, Muñoz RM, Eng J, Blázquez E. 2000. Peripheral versus central effects of glucagon-like peptide-1 receptor agonists on satiety and body weight loss in Zucker obese rats. Metabolism 49:709–717 [DOI] [PubMed] [Google Scholar]
- 20. Scott KA, Moran TH. 2007. The GLP-1 agonist exendin-4 reduces food intake in nonhuman primates through changes in meal size. Am J Physiol Regul Integr Comp Physiol 293:R983–R987 [DOI] [PubMed] [Google Scholar]
- 21. Chelikani PK, Haver AC, Reidelberger RD. 2005. Intravenous infusion of glucagon-like peptide-1 potently inhibits food intake, sham feeding, and gastric emptying in rats. Am J Physiol Regul Integr Comp Physiol 288:R1695–R1706 [DOI] [PubMed] [Google Scholar]
- 22. Dakin CL, Small CJ, Batterham RL, Neary NM, Cohen MA, Patterson M, Ghatei MA, Bloom SR. 2004. Peripheral oxyntomodulin reduces food intake and body weight gain in rats. Endocrinology 145:2687–2695 [DOI] [PubMed] [Google Scholar]
- 23. Baumgartner I, Pacheco-López G, Rüttimann EB, Arnold M, Asarian L, Langhans W, Geary N, Hillebrand JJ. 2010. Hepatic-portal vein infusions of glucagon-like peptide-1 reduce meal size and increase c-Fos expression in the nucleus tractus solitarii, area postrema and central nucleus of the amygdala in rats. J Neuroendocrinol 22:557–563 [DOI] [PubMed] [Google Scholar]
- 24. Neary NM, Small CJ, Druce MR, Park AJ, Ellis SM, Semjonous NM, Dakin CL, Filipsson K, Wang F, Kent AS, Frost GS, Ghatei MA, Bloom SR. 2005. Peptide YY3–36 and glucagon-like peptide-17–36 inhibit food intake additively. Endocrinology 146:5120–5127 [DOI] [PubMed] [Google Scholar]
- 25. Williams DL, Baskin DG, Schwartz MW. 2009. Evidence that intestinal glucagon-like peptide-1 plays a physiological role in satiety. Endocrinology 150:1680–1687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Raun K, von Voss P, Gotfredsen CF, Golozoubova V, Rolin B, Knudsen LB. 2007. Liraglutide, a long-acting glucagon-like peptide-1 analog, reduces body weight and food intake in obese candy-fed rats, whereas a dipeptidyl peptidase-IV inhibitor, vildagliptin, does not. Diabetes 56:8–15 [DOI] [PubMed] [Google Scholar]
- 27. DeFronzo RA, Okerson T, Viswanathan P, Guan X, Holcombe JH, MacConell L. 2008. Effects of exenatide versus sitagliptin on postprandial glucose, insulin and glucagon secretion, gastric emptying, and caloric intake: a randomized, cross-over study. Curr Med Res Opin 24:2943–2952 [DOI] [PubMed] [Google Scholar]
- 28. Mann RJ, Nasr NE, Sinfield JK, Paci E, Donnelly D. 2010. The major determinant of exendin-4/glucagon-like peptide 1 differential affinity at the rat glucagon-like peptide 1 receptor N-terminal domain is a hydrogen bond from SER-32 of exendin-4. Br J Pharmacol 160:1973–1984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. López de Maturana R, Willshaw A, Kuntzsch A, Rudolph R, Donnelly D. 2003. The isolated N-terminal domain of the glucagon-like peptide-1 (GLP-1) receptor binds exendin peptides with much higher affinity than GLP-1. J Biol Chem 278:10195–10200 [DOI] [PubMed] [Google Scholar]
- 30. Donnelly D. 2012. The structure and function of the glucagon-like peptide-1 receptor and its ligands. Br J Pharmacol 166:27–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hayes MR, Kanoski SE, Alhadeff AL, Grill HJ. 2011. Comparative effects of the long-acting GLP-1 receptor ligands, liraglutide and exendin-4, on food intake and body weight suppression in rats. Obesity 19:1342–1349 [DOI] [PubMed] [Google Scholar]
- 32. Chambers AP, Stefater MA, Wilson-Perez HE, Jessen L, Sisley S, Ryan KK, Gaitonde S, Sorrell JE, Toure M, Berger J, D'Alessio DA, Sandoval DA, Seeley RJ, Woods SC. 2011. Similar effects of roux-en-Y gastric bypass and vertical sleeve gastrectomy on glucose regulation in rats. Physiol Behav 105:120–123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Cummings DE. 2009. Endocrine mechanisms mediating remission of diabetes after gastric bypass surgery. Int J Obes (Lond) 33(Suppl 1):S33–S40 [DOI] [PubMed] [Google Scholar]
- 34. Conarello SL, Li Z, Ronan J, Roy RS, Zhu L, Jiang G, Liu F, Woods J, Zycband E, Moller DE, Thornberry NA, Zhang BB. 2003. Mice lacking dipeptidyl peptidase IV are protected against obesity and insulin resistance. Proc Natl Acad Sci USA 100:6825–6830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Marguet D, Baggio L, Kobayashi T, Bernard AM, Pierres M, Nielsen PF, Ribel U, Watanabe T, Drucker DJ, Wagtmann N. 2000. Enhanced insulin secretion and improved glucose tolerance in mice lacking CD26. Proc Natl Acad Sci USA 97:6874–6879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Chambers AP, Jessen L, Ryan KK, Sisley S, Wilson-Pérez HE, Stefater MA, Gaitonde SG, Sorrell JE, Toure M, Berger J, D'Alessio DA, Woods SC, Seeley RJ, Sandoval DA. 2011. Weight-independent changes in blood glucose homeostasis after gastric bypass or vertical sleeve gastrectomy in rats. Gastroenterology 141:950–958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Baggio LL, Drucker DJ. 2007. Biology of incretins: GLP-1 and GIP. Gastroenterology 132:2131–2157 [DOI] [PubMed] [Google Scholar]
- 38. Long SJ, Sutton JA, Amaee WB, Giouvanoudi A, Spyrou NM, Rogers PJ, Morgan LM. 1999. No effect of glucagon-like peptide-1 on short-term satiety and energy intake in man. Br J Nutr 81:273–279 [PubMed] [Google Scholar]
- 39. Rüttimann EB, Arnold M, Geary N, Langhans W. 2010. GLP-1 antagonism with exendin (9–39) fails to increase spontaneous meal size in rats. Physiol Behav 100:291–296 [DOI] [PubMed] [Google Scholar]
- 40. Thomas L, Eckhardt M, Langkopf E, Tadayyon M, Himmelsbach F. 2008. (R)-8-(3-amino-piperidin-1-yl)-7-but-2-ynyl-3-methyl-1-(4-methyl-quinazolin-2-ylmethyl)-3,7-dihydro-purine-2,6-dione (BI 1356), a novel xanthine-based dipeptidyl peptidase 4 inhibitor, has a superior potency and longer duration of action compared with other dipeptidyl peptidase-4 inhibitors. J Pharmacol Exp Ther 325:175–182 [DOI] [PubMed] [Google Scholar]
- 41. Burkey BF, Li X, Bolognese L, Balkan B, Mone M, Russell M, Hughes TE, Wang PR. 2005. Acute and chronic effects of the incretin enhancer vildagliptin in insulin-resistant rats. J Pharmacol Exp Ther 315:688–695 [DOI] [PubMed] [Google Scholar]
- 42. Punjabi M, Arnold M, Geary N, Langhans W, Pacheco-López G. 2011. Peripheral glucagon-like peptide-1 (GLP-1) and satiation. Physiol Behav 105:71–76 [DOI] [PubMed] [Google Scholar]
- 43. Yamamoto H, Kishi T, Lee CE, Choi BJ, Fang H, Hollenberg AN, Drucker DJ, Elmquist JK. 2003. Glucagon-like peptide-1-responsive catecholamine neurons in the area postrema link peripheral glucagon-like peptide-1 with central autonomic control sites. J Neurosci 23:2939–2946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kanoski SE, Fortin SM, Arnold M, Grill HJ, Hayes MR. 2011. Peripheral and central GLP-1 receptor populations mediate the anorectic effects of peripherally administered GLP-1 receptor agonists, liraglutide and exendin-4. Endocrinology 152:3103–3112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Holst JJ, Deacon CF. 2005. Glucagon-like peptide-1 mediates the therapeutic actions of DPP-IV inhibitors. Diabetologia 48:612–615 [DOI] [PubMed] [Google Scholar]
- 46. D'Alessio D, Lu W, Sun W, Zheng S, Yang Q, Seeley R, Woods SC, Tso P. 2007. Fasting and postprandial concentrations of GLP-1 in intestinal lymph and portal plasma: evidence for selective release of GLP-1 in the lymph system. Am J Physiol Regul Integr Com Physiol 293:R2163–R2169 [DOI] [PubMed] [Google Scholar]
- 47. Vahl TP, Tauchi M, Durler TS, Elfers EE, Fernandes TM, Bitner RD, Ellis KS, Woods SC, Seeley RJ, Herman JP, D'Alessio DA. 2007. Glucagon-like peptide-1 (GLP-1) receptors expressed on nerve terminals in the portal vein mediate the effects of endogenous GLP-1 on glucose tolerance in rats. Endocrinology 148:4965–4973 [DOI] [PubMed] [Google Scholar]
- 48. Zhao S, Kanoski SE, Yan J, Grill HJ, Hayes MR. 2012. Hindbrain leptin and glucagon-like-peptide-1 receptor signaling interact to suppress food intake in an additive manner. Int J Obes (Lond) 10.1038/ijo.2011.265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Kastin AJ, Akerstrom V, Pan W. 2002. Interactions of glucagon-like peptide-1 (GLP-1) with the blood-brain barrier. J Mol Neurosci 18:7–14 [DOI] [PubMed] [Google Scholar]
- 50. Thorens B, Porret A, Bühler L, Deng SP, Morel P, Widmann C. 1993. Cloning and functional expression of the human islet GLP-1 receptor. Demonstration that exendin-4 is an agonist and exendin-(9–39) an antagonist of the receptor. Diabetes 42:1678–1682 [DOI] [PubMed] [Google Scholar]
- 51. Mann R, Nasr N, Hadden D, Sinfield J, Abidi F, Al-Sabah S, de Maturana RL, Treece-Birch J, Willshaw A, Donnelly D. 2007. Peptide binding at the GLP-1 receptor. Biochem Soc Trans 35:713–716 [DOI] [PubMed] [Google Scholar]
- 52. Peterli R, Wölnerhanssen B, Peters T, Devaux N, Kern B, Christoffel-Courtin C, Drewe J, von Flüe M, Beglinger C. 2009. Improvement in glucose metabolism after bariatric surgery: comparison of laparoscopic Roux-en-Y gastric bypass and laparoscopic sleeve gastrectomy: a prospective randomized trial. Ann Surg 250:234–241 [DOI] [PubMed] [Google Scholar]
- 53. Korner J, Inabnet W, Febres G, Conwell IM, McMahon DJ, Salas R, Taveras C, Schrope B, Bessler M. 2009. Prospective study of gut hormone and metabolic changes after adjustable gastric banding and Roux-en-Y gastric bypass. Int J Obes 33:786–795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Salehi M, Prigeon RL, D'Alessio DA. 2011. Gastric bypass surgery enhances glucagon-like peptide 1-stimulated postprandial insulin secretion in humans. Diabetes 60:2308–2314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Dailey MJ, Moghadam AA, Moran TH. 2011. Jejunal linoleic acid infusions require GLP-1 receptor signaling to inhibit food intake: implications for the effectiveness of Roux-en-Y gastric bypass. Am J Physiol Endocrinol Metab 301:E1184–E1190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Fenske WK, Bueter M, Miras a D, Ghatei M a, Bloom SR, le Roux CW. 2012. Exogenous peptide YY3–36 and Exendin-4 further decrease food intake, whereas octreotide increases food intake in rats after Roux-en-Y gastric bypass. Int J Obes (Lond) 36:379–384 [DOI] [PubMed] [Google Scholar]